Published online Feb 15, 1998. doi: 10.3748/wjg.v4.i1.52
Revised: October 12, 1997
Accepted: November 20, 1997
Published online: February 15, 1998
AIM: To study the distribution of arylsulfatase, β-galactosidase and lysozyme in gastric cancer cells, and its relationship to differentiation and invasion of gastric cancer cells.
METHODS: Histochemical, immunohistochemical and ruthenium red (RR) electrocytochemical technique for three types of hydrolases and proteoglycans in pericancerous matrix in 33 cases of gastric cancer were observed under light and electron microscopy.
RESULTS: The expression intensities of arylsulfatase, β-glactosidase and lysozyme in mucinous cell carcinomas were more intensive than those in well-differentiated and poorly-differentiated adenocarcinomas ( P < 0.05-0.01). The fibrous tissues smooth muscle and proteoglycans close to the cancer cells were degraded. They were found in the region far from the cancer cells. Expression of three enzymes mentioned above was low in adenocarcinoma cells, and fibrous tissues and RR granules were present and intact near the well-differentiated and poorly-differentiated adenocarcinoma cells.
CONCLUSION: Mucinous cell carcinoma may release various hydrolases into extracellular matrix, inducing degradation of pericancerous matrix and facilitating cancer cell invasion and metastasis.
- Citation: Yi YF, Huang YR. Arylsulfatase, β-galactosidase and lysozyme in gastric cancer cells and its relationship to invasion. World J Gastroenterol 1998; 4(1): 52-54
- URL: https://www.wjgnet.com/1007-9327/full/v4/i1/52.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i1.52
Tumor invasion and metastasis is a highly complex process. The mechanism of tumor invasion and metastasis is still not clear. In this paper the content and distribution of arylsulfatase β -galactosidase and lysozyme in three histological types of gastric cancer cells were studied by means of histochemical and immunohistochemical stains and the proteoglycans in pericancerous matrix by ruth enium red electrocytochemical stains respectively. The relationship between hydrolases and differentiation as well as invasion of gastric cancer cells were discussed.
Surgically resected specimens of 33 cases of gastric cancer were studied. Histologically there were 14 cases of mucinous cell carcinoma, 9 cases of well-differentiated adenocarcinoma, and 10 cases of poorly-differentiated adenocarcinoma. Each specimen was treated as follows: (1) The fresh tissues were fixed in 10% neutral buffered formalin, embedded in paraffin and cut into 5 μm section, and were stained by HE and lysozyme PAP immunohistochemical staining[1] (lysozyme antibody from Dako). (2) Fresh tissue were fixed in 3% glutaraldehyde solution for 18-20 h, and cut in a cryostat into 8 μm sections for arylsufatase and β-galactosidase histochemical staining[2,3]. Substrates were pnitrocatecholsulfate dipotassium salt and 5-bromo-4-chloro-3-indoxyl-β-galactoside (Sigma). (3) 0.1 mm3 tissues from 20 of 33 cases of gastric cancer (including 10 cases of mucinous cellcarcinoma, 5 cases of well-differentiated and 5 cases of poorly-differentiated adenocarcinoma) were fixed in 2% ruthenium red ( Merck ) mixed with 4% glutaralde hyde in 0.1 M Na-cacoaylate buffer (pH 7.4). The proteoglycans of the extra-cellular matrix and the ultrastructures of cancer cells were observed under a Hitachi-600 electronmicroscope[4].
The staining patterns in tissue sections were scored according to the relative intensity and extent of positive staining. Weak positive (+): the number of positive cells less than 1/3 of the total cells, mode-rate reaction (++): 1/3 to 2/3 of the total, strong positive (+++): more than 2/3 of the total.
The same enzyme staining grades from the tissues were compared by rank sum test.
In 14 cases of mucinous carcinoma, the cancer cells were diffusely distributed, some secreting a lot of mucin into extra-cellular matrix forming the mucin pool. There were no fibrous tissues and smooth muscle and lymphocyte infiltration in the pericancerous tissues. Nine cases of well-differentiated adenocarcinoma (including 2 cases of papillary carcinoma) displayed a tubular structure surroun ded by fibrous tissue proliferation or smooth muscle and lymphocyte infiltration. The cancer cells of 10 poorly-differentiated adenocarcinomas arranged in solid trabeculae of incomplete gland pattern accompanied by fibrous tissues and lymphocytes.
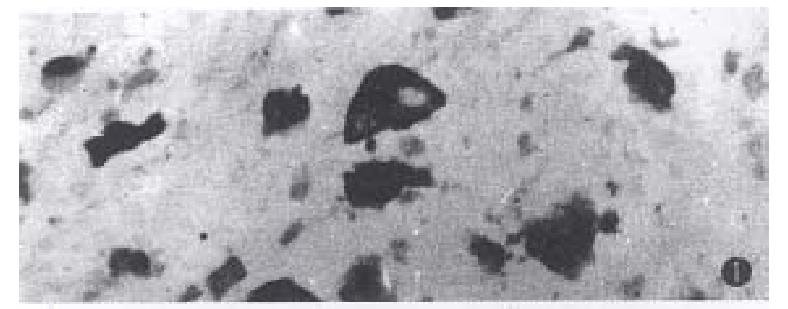
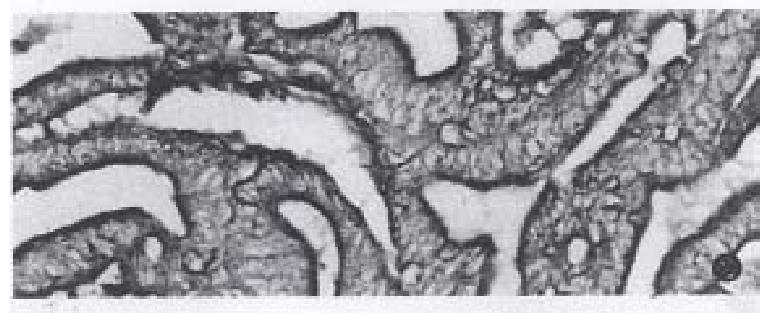
The positive reaction was dark-brown or yellow-brown in color. In cancer cells of mucinous carcinoma, the staining reaction was distributed in the cytoplasm and varied with the amount of cytoplasm, the more cytoplasm, the more staining intensity (Figure 1). There were light yellow-brown fine granules in the mucin pool. In well-differentiated adenocarcinomas the positive reaction was mostly located at the gland luminal border( Figure 2). In poorly-differentiated adenocarcinomas, most of cancer cells were negative, but were positive in the cytoplasm.
The enzyme-active sites were stained blue or turquoise. The reaction was most intense in mucinous cell carcinomas. The size of indigo particles was dependent on the cytoplasm, the particle was largest in abundant cytoplasm cells or signet-ring cells (Figure 3). Fine light blue granules were seen in the mucin-pool. In well-differentiated adenocarcinoma, the fine blue granules were mostly located in the apical cytoplasm of cancerous gland( Figure 4). In poorly-differentiated adenocarcinoma, some cancer cells were positive, and others were negative in reaction.
The positive reaction was yollow-brown. In mucinous cell carcinoma the positive product was diffusely distributed in the cytoplasm of most cancer cells, in signet-ring cell, the positive reaction was located on one side of the cytoplasm. In well-differentiated adenocarcinoma, positive product was located at the luminal side or in the cytoplasm in some cells, a portion of cancer cells were negative in reaction. In poorly-differentiated adenocarcinoma, most cancer cells were negative. The content and distribution of three type enzymes in gastric cancer cells are shown in Table 1.
These types of hydrolases in different types of gastric cancers were compared by rank sum test. There were significant differences between mucinous carcinoma and well-differentiated and poorly-differentiated adenocarcinoma (P < 0.01, P < 0.05). There were no significant differences between well-differentiated and poorly-differentiated adenocarcinoma (P > 0.05).
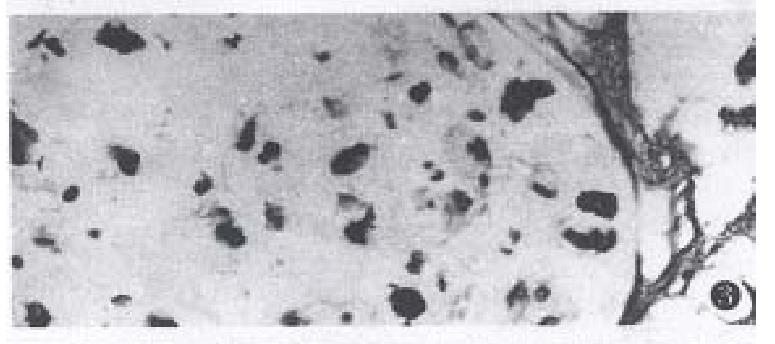
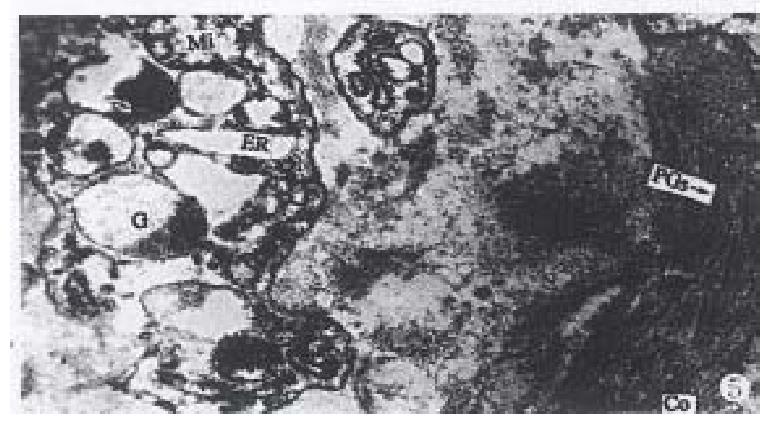
In mucinous carcinoma, the Golgi complex, endoplasmic reticulum, swollen mitochondria and secretory granules with various electron densities in the cytoplasm were easily found. RR granules and collagens close to the cancer cells of mucinous carcinoma were degraded but were observed in the region far from the cancer cells (Figure 5).
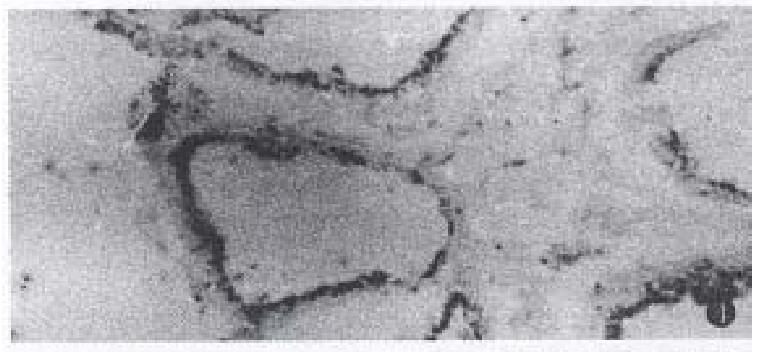
In well-differentiated adenocarcinoma, there were microvillus on the surface of luminal gland, and close connection and desmosomes between cancer cells. There were more swollen mitochondria, and smaller secretory granules with moderate density in the apical cytoplasm in some cancer cells. RR granules and collagens were present and intact near the adenocarcinoma. In poorly-differentiated adenocarcinoma, there were abundant ribosomes, a few secretory granules with moderate density in a few cancer cells. RR granules were found in surrounding cancer cells, which looked like short rods with 20 mm-50 mm in diameter with high electron density, and attached on the collagens or located between collagens.
The results showed that Golgi complex, endoplasmic reticulum, secretory granules, and staining intensity of arylsulfatase, β-galactosidase and lysozyme were more intense in mucinous cell carcinoma and varied with cell differentiation. In well-differentiated adenocarcinoma, three kinds of hydrolases were mostly distributed in apical cytoplasm of luminal glands, electron-microscopically there were moderate electron density secretory granules in apical cytoplasm. In poorly-differentiated adenocarcinoma, there were few secretory granules, and, three types of enzymes were scanty. These results suggested that the content and distribution of these three enzymes are related to the differentiation and secretory function of cancer cells. The more differentiated of the secretory system, the more secretory granules in the cytoplasm and the more intensive enzymes staining reaction. Expression of three hydrolases in mucinous cell carcinoma was more intensive than those in well-differentiated and poorly-differentiated adenocarcinomas (P < 0.05). GERL (Golgi complex, endoplasmic reticulum and lysosome)[5] system was mature with a good secretory function, which may secrete various hydrolases.
Malignant tumors may produce various hydrolases, which degrade pericancerous matrix at different sites in favour of the invasion[6].
Arylsulfatase and β-glactosidase were lysosomal enzymes which degrade proteoglycans (PGs) in the extracellular matrix and basement membrane. PGs are very complex macromolecules containing a protein core, to which one or more glycosaminoglycans (GAGs) chain are covalently bound and the chain radiate out from central core protein[7], owing to the polyanionic on the chains which tend to separate from each other to form the network structure, which can hinder the cancer cell spread. Arylsulfatase is a key enzyme in hydrolysing PGs. Removal of sulfate is a key step. Only after the sulfate group of PG was degraded, PG may be further hydrolyzed by other lysosomal enzymes. β-galactosidase is another important hydrolase which degrades the PGs. β-galactosidase not only cleaves the linkages between β-N-acetylglucosamine and β-galactoside (R-GlcNAc-Gal) on the GAG chains, but also degrade oxosylserine residues of the carbohydrate-protein linkage region on PGs[8], resulting in macromolecular PGs hydrolyzation, cancer cell spreading in matrix. Lysozyme is a representative enzyme of macrophage cells. The functional mechanism of lysozyme is to cleave the linkage between N-acetylmuramic acid and N-acetylglucosamine of mucopeptide in cell wall of bacteria[8], but, whether it may degrade similar structure on PGs and hyaluronide in extracellular matrix of human body to the bacteria has not been reported in literature. In the present study, three hydrolases were abundant in mucinous cell carcinoma, the positive reaction was also found in the extracellular mucous pool. PGs and collagens and smooth muscle in pericancerous matrix close to the cancer cell disappeared, which suggest that the cancer cells may secrete various hydrolases to degrade the extracellular matrix in different sites. So the cancer cells can easily invade and metastasize.
Project supported by the National Science Foundation of China, No.386112487.
| 1. | Klockars M, Reitamo S. Tissue distribution of lysozyme in man. J Histochem Cytochem. 1975;23:932-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 196] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | Leznicki A, Bleszynski W. Histochemical localization of the soluble arylsulphatase activities in rat brain. Histochemie. 1971;24:251-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Lojda Z. Indigogenic methods for glycosidases. II. An improved method for beta-D-galactosidase and its application to localization studies of the enzymes in the intestine and in other tissues. Histochemie. 1970;23:266-288. [PubMed] |
| 4. | Luft JH. Ruthenium red and violet. II. Fine structural localization in animal tissues. Anat Rec. 1971;171:369-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 582] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 5. | Weiss L. The cells in leon veiss. Histology (cell and tissue biology). 5th ed. The Macmillan Press. 1983;1-90. |
| 6. | Yi F, Mia D. Hydrolases in malignant tumor and invasion. (review)Acta Univ Sci Med Chongqing. 1991;16:83-87. |
| 7. | Iozzo RV. Proteoglycans: structure, function, and role in neoplasia. Lab Invest. 1985;53:373-396. [PubMed] |
| 8. | Chipman DM, Sharon N. Mechanism of lysozyme action. Science. 1969;165:454-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 418] [Article Influence: 7.5] [Reference Citation Analysis (0)] |