Published online Feb 15, 1998. doi: 10.3748/wjg.v4.i1.38
Revised: May 28, 1997
Accepted: July 26, 1997
Published online: February 15, 1998
AIM: To detect glutathione S-transferase placental (GST-P) mRNA expression in hepatic preneoplastic lesions in rats.
METHODS: Using Solit-Farber model, the GST-P mRNA expression was observed in hepatic preneoplastic lesions induced by diethylnitrosamine (DEN) in rats and normal and regenerated hepatic tissues in the control group by in situ hybridization.
RESULTS: GST-P mRNA was mainly expressed in altered hepatic foci (AHF) and some of the oval cells in hepatic preneoplastic lesions and the extent of its expression was different among various foci or/and positive cells in the same focus whereas no expression was observed in normal and regenerated hepatic tissues.
CONCLUSION: Cells in AHF and oval cells may be the preneoplastic cells in the experimental hepatocellular carcinoma at the molecular level and heterogeneity exists in GST-P transcription levels.
- Citation: Zhu HZ, Zhang XL, Chen YS. Expression of glutathione S-transferase placental mRNA in hepatic preneoplastic lesions in rats. World J Gastroenterol 1998; 4(1): 38-40
- URL: https://www.wjgnet.com/1007-9327/full/v4/i1/38.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i1.38
Glutathione S-transferases (GSTs, EC2,5,1,18) are a family of dimeric proteins that may play important roles in both the intracellular transport of hydrophobic molecules and the metabolism of toxic compounds. The cytosolic GSTs can be divided into at least four different families, i.e. α , μ, π and θ based on N-terminal sequences, substrate specificities and affinity for non-substrate ligands in human. GST-P (the rat enzyme equivalent to human GST-π ) was first found in placenta, but later also in kidney, lung and testis[1]. Recently, it has been shown by Northern blot and Dot blot analysis that GST-P mRNA is present abundantly in preneoplastic lesions and hepatocellular carcinoma induced by genotoxic carcinogen[2]. However, in hepatic preneoplastic lesions, which cell type has GST-P mRNA expression has not been well elucidated. This study was to investigate the expression of GST-P mRNA in hepatic preneoplastic lesions in rats by in situ hybridization and reveal the significance and regulation mechanism of its expression.
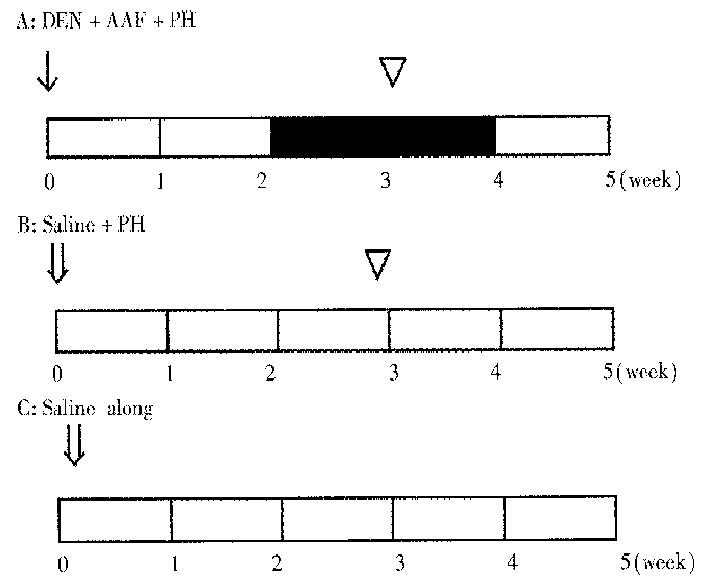
Seventy male Wistar rats, weighing 100 g-150 g, were randomly divided into experimental group (A) and control groups (B,C). Group A (30 rats), according to Solt-Farbar model[3], was initially given a single intraperitoneal injection of DEN at 200 mg/kg(Sigma product) dissolved in 0.9% NaCl to initiate hepatocarcinogenesis. After 2 weeks on basal diet, they were fed with 0.02% 2-acetylaminofluorene (AAF, Sigma product) for 2 weeks. All the rats were subjected to partial hepatectomy (PH) at the 3rd week. Group B (20 rats) were injected with 0.9% NaCl instead of DEN solution and then underwent PH at the 3rd week. Group C (20 rats) were injected with 0.9% NaCl only (Figure 1).
Rats of each group were killed at the 1st, 2nd, 3rd, 4th and 5th week. The livers were removed. A portion of lesion tissues were stored at -80 °C. Frozen serial sections were made for in situ hybridization and histochemical demonstration. The remainders were fixed in 4% paraformaldehyde for routine staining with hematoxylin and eosin.
Pathomorphological observation and histochemical assay were made according to the National[4] and Rutenberg method[5]
The bacteria carrying the pUC18 plasmid containing full length 0.75 kb GST-P cDNA, PGP5 were gifts from Dr. Sugioka. The special restricted endunuclear enzymes cutting sites were EcoRI-SaLI. Probes were prepared according to J. Sambrook’s method[6] and labeled by digoxigenin (DIG) using DIG-labelling and detection kit (Boehringer Mannheim Inc).
In situ hybridization was done according to SU HC et al[7]. Frozen sections were fixed in 4% paraformaldehyde/PBS and then rinsed with PBS, glycine/PBS and Triton-100/PBS in turn. Hybridization buffer contained 110 μg/L DIG-GST cDNA probes, 5 × SSC, 50% formamide, 5 × Denhardts solution, 5% dextron sulfate and predenatured 0.5 g/L ssDNA. Optimal hybridization temperature was about 42 °C in humid chamber. As a negative control, hybridization buffer did not contain DIG-GST-P cDNA probes, and sections were digested with RNase at 37 °C before hybridization.
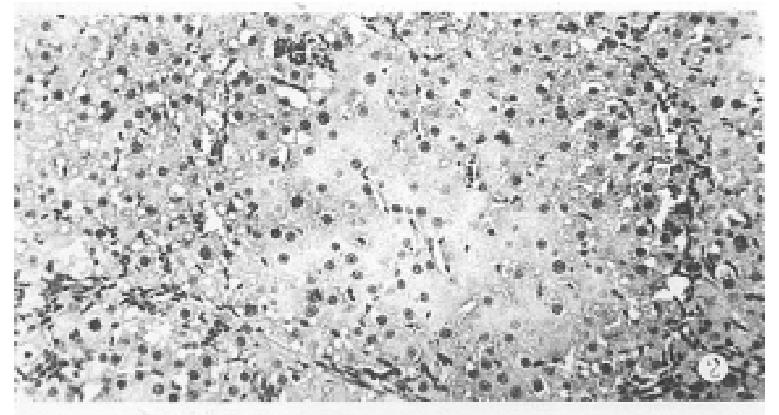
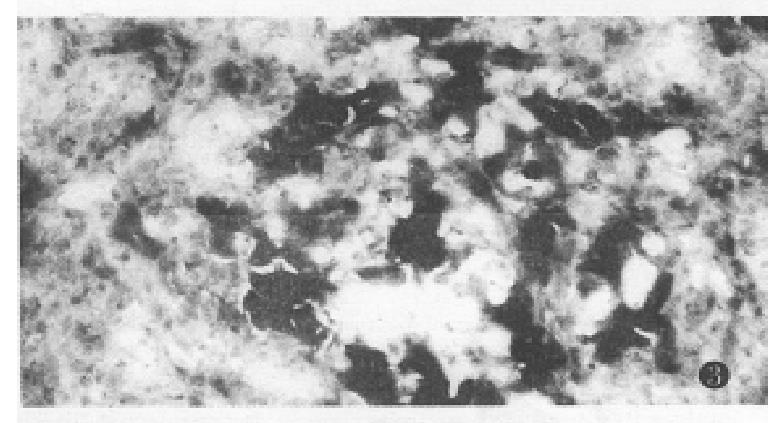
One week after PH, there was a grey granular appearance on gross inspection in experimental group rat liver. Histological examination of the liver tissues showed many AHF. These foci were mainly composed of basophilic cells. Proliferation of oval cells was found in portal areas (Figure 2). AHF was γ-GT positive (Figure 3).
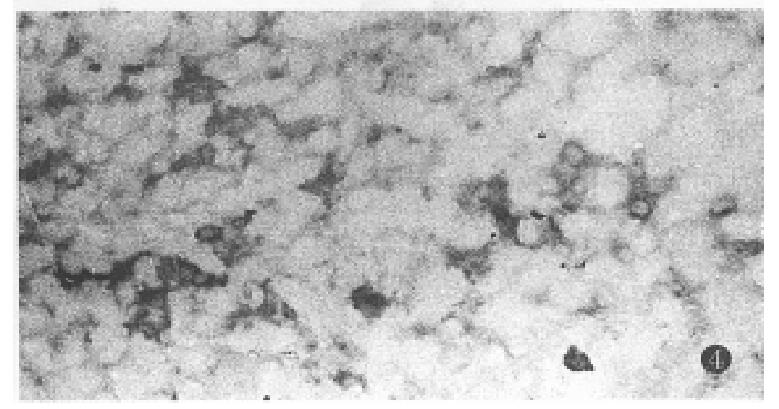
At the 3rd week, GST-P mRNA was expressed in a few clusters of oval and liver cells in preneoplastic lesions. At the 4th and the 5th week, GST-P mRNA was mainly detected in hyperplastic AHF and some of the oval cells. Hybridization signal positive granules were mainly distributed in the cytoplasm of altered hepatic cells (Figure 4). The extent of the GST-P mRNA expression was different among the foci or/and positive cells in the same focus. Results of negative control showed only background staining, indicating the specificity of hybridization. No positive signals were found in control groups.
The binding of electrophilia metabolites of carcinogens to macromolecules, especially the DNA, is a critical event in chemical carcinogenesis. GST can detoxify a number of carcinogenic electrophiles, including dioleposide metabolites of polycyclic aromatic hydrocarbons by catalysis of the conjugation with reduced glutathione. Four multigene families of GSTs were characterized in human and rats, i.e., α , μ, π and θ[1]. The expression of xenobiotic metabolizing enzymes in human hepatocellular carcinoma is complicated and a few findings support the proposal that GST-π could be used as a marker of hepatocellular carcinoma[8]. However, GST-P has been demonstrated to be a very useful marker enzyme, its mRNA is hardly detectable in normal rat livers, but is very strongly expressed in preneoplastic livers by induced genotoxic carcinogen by Northern blot and Dot blot hybridization methods[2]. There are many cell types in the liver tissues. Northern blot and Dot blot hybridization only showed an average level of the whole cell gene expression, not single cell gene expression and its characteristics. It provides the most accurate identification of RNA transcripts at single cell level by in situ hybridization technique. Our findings showed that GST-P mRNA was mainly expressed in AHF and some of the oval cells in hepatic preneoplastic lesions and the extent of its expession was different among foci or/and positive cells in the same focus whereas no expression was observed in normal and regenerated hepatic tissues. This study indicates that cells in AHF and oval cells may be the preneoplastic cells in experimental hepatocellular carcinoma at the molecular level and heterogeneity in GST-P transcript levels.
GST-P is known as a reliable tumor marker for chemical carcinogen-induced and spontaneously occurring preneoplastic lesions and hepatomas in rats. Thus, GST-P expression may be closely related to the process of hepatocarcinogenesis in rats. In multistep carcinogenesis, changes in the pattern of gene expression might be an important step in oncogene activation and/or inactivation of antioncogenes. To investigate possible alterations in gene expression pattern during neoplastic transformation, it may be useful to explore tumor marker gene expression. Why and how is the GST-P gene activated inevitably during the course of liver cell transformation Two possible mechanisms are considered: One is that the GST-P gene is located in close proximity to a gene that is directly involved in liver cell transformation and local chromosomal changes activate both genes simultaneously. The other is that those genes are not physically linked, but their expression is regulated by some common transcription factors. The GST-P gene has a strong enhancer element, GPE1, located at 2.5 kb upstream from the cap site. The enhancer consists of the TRE-like sequence with palindromical orientation. GPE1 is a major control element responsible for GST-P expression in preneoplastic lesions. The regulatory region of the GST-P gene has another TRE-like sequence located at 61bp upstream from the cap site. These TRE-like sequences are active as AP-1 (Jun/Fos) binding sites and TREs, at least in vitro DNA binding and transfection analyses. From these results, it is inferred that the GST-P gene is controlled, at least in part by AP-1. However, AP-1 is not the only factor activating GPE1, because the expression of Jun and Fos do not always correlate with GST-P expression. Recently, the consense Maf recognition element (MARE) sequence was identified and is similar to the TRE sequence. Since the TRE sequence of the proximal region of the GST-P gene is internal to a MARE, Maf too might regulate GST-P expression. Sakai found that peroxisome proliferator activated receptor α (PPARα ) can interact the Jun, not Maf, and inhibit the activation of GST-P expression[9-13].
Project supported by the National Natural Science Foundation ofChina, No. 39270303.
| 1. | Rushmore TH, Pickett CB. Glutathione S-transferases, structure, regulation, and therapeutic implications. J Biol Chem. 1993;268:11475-11478. [PubMed] |
| 2. | Sato K. Glutathione S-transferases and hepatocarcinogenesis. Jpn J Cancer Res. 1988;79:556-572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 93] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Solt DB, Farber E. New principle for the analysis of chemical carcinogenesis. Nature. 1976;263:702-703. |
| 5. | Rutenberg AM. Histochemical and ultrastructural demonstration of γ -GT activity. J Histochem Cytochem. 1969;17:517-519. |
| 6. | Sambrook J, Fristch EF, Maniatris T. Molecular cloning: a laboratory manual. 2nd ed. New York: Cold Spring Harbor Laboratory Press. 1989;16-34. |
| 7. | Su HC. In situ hybridization. Beijing: China Science and Technology Press. 1994;59-90. |
| 8. | Murray GI, Paterson PJ, Weaver RJ, Ewen SW, Melvin WT, Burke MD. The expression of cytochrome P-450, epoxide hydrolase, and glutathione S-transferase in hepatocellular carcinoma. Cancer. 1993;71:36-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Morimura S, Suzuki T, Hochi S, Yuki A, Nomura K, Kitagawa T, Nagatsu I, Imagawa M, Muramatsu M. Trans-activation of glutathione transferase P gene during chemical hepatocarcinogenesis of the rat. Proc Natl Acad Sci USA. 1993;90:2065-2068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Suzuki T, Imagawa M, Hirabayashi M, Yuki A, Hisatake K, Nomura K, Kitagawa T, Muramatsu M. Identification of an enhancer responsible for tumor marker gene expression by means of transgenic rats. Cancer Res. 1995;55:2651-2655. [PubMed] |
| 11. | Sakai M, Matsushima-Hibiya Y, Nishizawa M, Nishi S. Suppression of rat glutathione transferase P expression by peroxisome proliferators: interaction between Jun and peroxisome proliferator-activated receptor alpha. Cancer Res. 1995;55:5370-5376. [PubMed] |
| 12. | Sakai M, Muramatsu M, Nishi S. Suppression of glutathione transferase P expression by glucocorticoid. Biochem Biophys Res Commun. 1992;187:976-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Kataoka K, Noda M, Nishizawa M. Maf nuclear oncoprotein recognizes sequences related to an AP-1 site and forms heterodimers with both Fos and Jun. Mol Cell Biol. 1994;14:700-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 253] [Article Influence: 8.2] [Reference Citation Analysis (0)] |