INTRODUCTION
Ménétrier disease (MD) is a rare condition first described by the French pathologist Pierre Ménétrier in 1888[1]. MD is characterized by the presence of enlarged gastric mucosal folds predominantly in the proximal stomach, reduced gastric acid secretion, and a protein-losing enteropathy (PLE) leading to hypoalbuminemia[2]. It is also referred to as hypoproteinemic hypertrophic gastropathy or giant hypertrophic gastritis[1]. Given its rarity, MD is often misdiagnosed, necessitating a thorough understanding of its clinical presentation and management.
EPIDEMIOLOGY
MD is a rare condition, and its prevalence remains undefined due to limited epidemiological data. To date, fewer than 1000 cases have been reported. The disease predominantly affects adults, with most cases diagnosed in the fourth to sixth decades of life. Males are affected more than females. In adults, the condition is considered a premalignant disorder with a progressive clinical course[3]. One study reported 5- and 10-year survival rates of 72.7% and 65%, respectively, with 8.9% of patients developing gastric cancer over a 10-year follow-up period[4]. A pediatric variant of MD, which is linked to cytomegalovirus (CMV) infection, has been reported and is associated with a better prognosis[5].
PATHOPHYSIOLOGY
The exact pathogenesis of MD is unclear; however, evidence suggests that overexpression of transforming growth factor-alpha (TGF-α) leads to excessive activation of epidermal growth factor receptor (EGFR) signaling, which plays a pivotal role in the pathogenesis of the disease[6]. Through enhanced signaling to the EGFR, the overexpressed TGF-α stimulates foveolar mucus cell proliferation, enhances mucin secretion, and inhibits acid secretion. This explains the hypochlorhydria observed in this disease alongside normal or slightly increased levels of gastrin. TGF-α also contributes to the disruption of tight junctions between gastric epithelial cells, resulting in the leakage of serum proteins into the gastrointestinal lumen - a process known as PLE.
In this mechanism, CMV is thought to trigger the overexpression of TGF-α, subsequently leading to activation of the EGFR signaling pathway[3,7,8]. One proposed pathogenic mechanism in MD involves the abnormal accumulation of local TGF-α secondary to infection-related injury of the gastric mucosa. Specifically, CMV infection in the stomach has been shown to increase intracellular signaling pathways and activate proto-oncogenes, both of which contribute to upregulated TGF-α expression in gastric mucosal cells[9].
Helicobacter pylori (H. pylori) infection has also been proposed as a potential trigger in MD, particularly in adults. Although the exact mechanism remains unclear, several case reports have documented clinical and histologic improvement following H. pylori eradication which further supports a causative role in susceptible individuals[10,11].
The proposed pathogenesis centers on activation of the EGFR pathway. Overexpression of TGF-α, a ligand of EGFR, has been demonstrated in MD and reproduces the disease phenotype in transgenic mice[12]. H. pylori can upregulate this pathway by inducing TGF-α release via p38 mitogen-activated protein kinase and TACE (ADAM 17) pathway and by increasing gastric mucosal expression of EGFR and related growth factors[13]. However, unlike CMV, the evidence for direct molecular interaction with the EGFR-TGF-α axis is less robust, and more studies are needed to elucidate H. pylori's precise role in disease initiation and progression[14,15].
Other, less common infections have also been linked to MD, including herpes simplex virus, Giardia lamblia, and Mycoplasma pneumoniae, particularly in children[9].
Although most cases are sporadic, rare familial occurrences have been reported, suggesting a possible but limited genetic contribution. In one such family, a mutation in the SMAD4 gene was identified[16-18]. However, MD is most likely an acquired disease rather than an inherited one.
MD has also been observed in association with autoimmune conditions such as inflammatory bowel disease, sclerosing cholangitis, and ankylosing spondylitis, supporting the possibility of an underlying immunological component in its pathogenesis[12,19].
CLINICAL PRESENTATION
Patients with MD commonly present with nonspecific gastrointestinal symptoms such as epigastric pain, nausea, vomiting, and early satiety[20]. Rarely, they may present with gastroduodenal intussusception[21]. Chronic protein loss may lead to hypoalbuminemia, resulting in peripheral edema and ascites. MD can also present with recurrent venous thrombosis due to hypoalbuminemia[22,23]. Other symptoms include anorexia, weight loss, and gastrointestinal bleeding in severe cases.
DIAGNOSTIC APPROACH
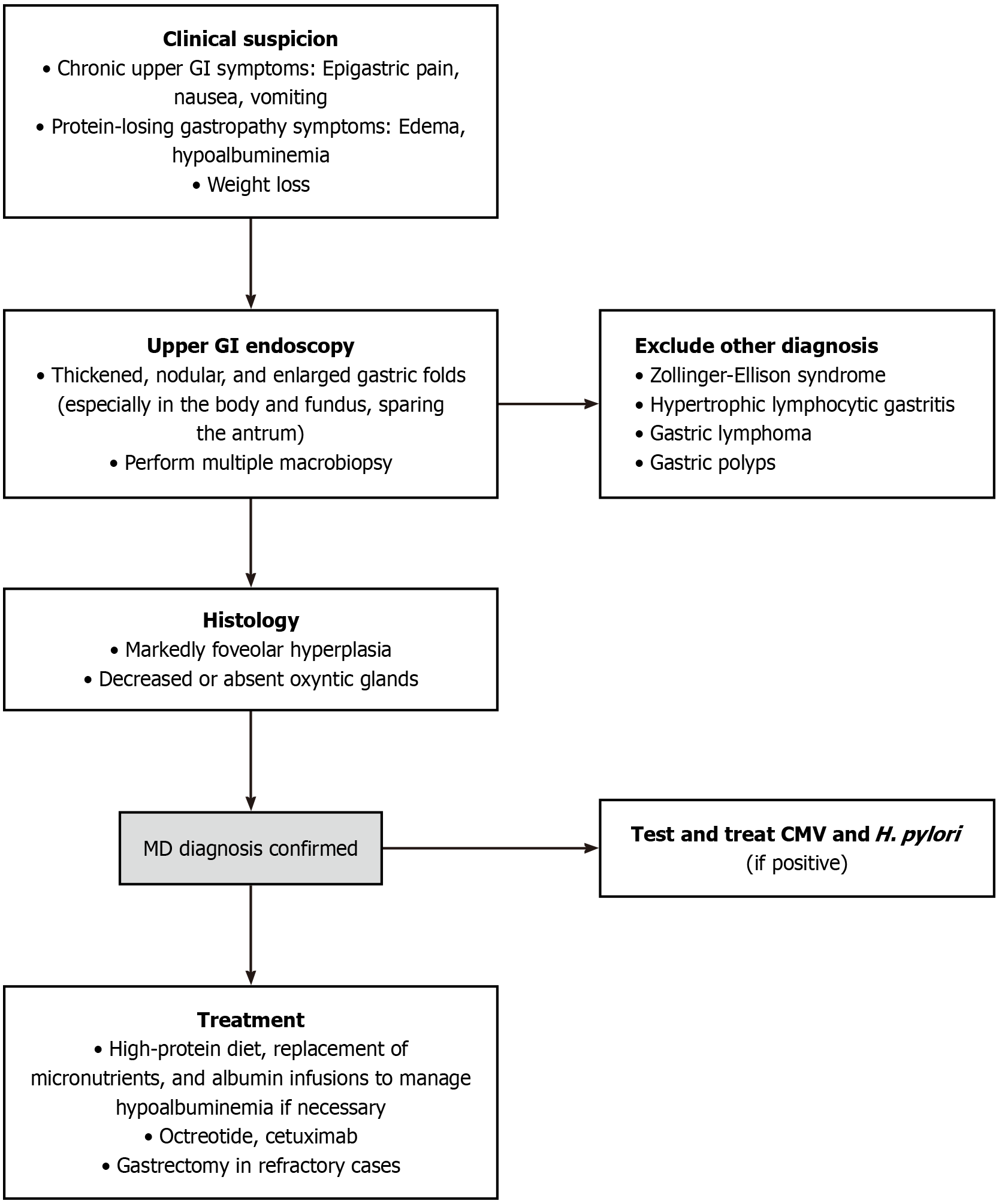
Due to the rarity of MD and the absence of well-defined diagnostic criteria, the diagnosis of MD is based on a combination of clinical, endoscopic, and histopathological findings (Figure 1).
Figure 1 Diagnostic algorithm.
MD: Ménétrier disease; CMV: Cytomegalovirus; GI: Gastrointestinal; H. pylori: Helicobacter pylori.
Endoscopy
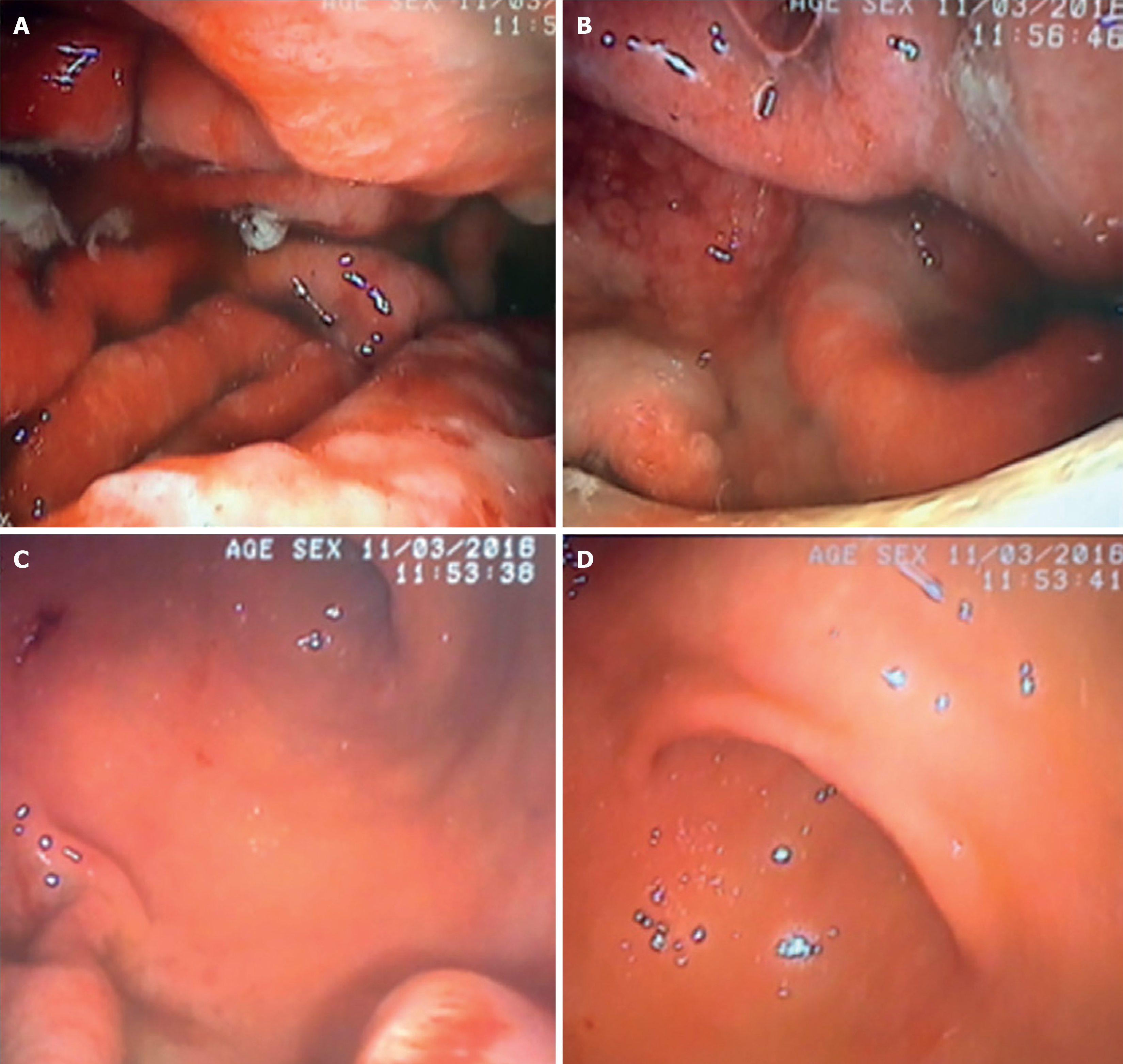
Esophagogastroduodenoscopy reveals thickened, nodular, and enlarged gastric folds, covered by abundant exudative fluid, predominantly affecting the body and fundus of the stomach (Figure 2A and B), while sparing the antrum (Figure 2C and D). These enlarged folds can resemble cerebral convolutions[24,25].
Figure 2 Upper gastrointestinal endoscopy photographs showing marked congestive and thickened gastric folds, covered by a large amount of white exudative fluid that was partially washed and aspirated during examination[26].
A and B: These changes were found in the gastric body and fundus; C and D: Sparing the antrum. Citation: Nunes G, Barosa R, Patita M, Pinto-Marques P, Gonçalves D, Fonseca C, Alves de Matos A, Fonseca J. Ménétrier's disease: A case of successful treatment using long-acting octreotide. Acta Gastroenterol Belg 2019; 82: 429-432. Copyright© 2019, Acta Gastroenterol Belg. Published by Acta Gastro-Enterologica Belgica. The authors have obtained the permission for figure using from the Acta Gastro-Enterologica Belgica (Supplementary material).
Because accurate diagnosis of this disease requires examination of very thick gastric mucosa, it is recommended that a macrobiopsy be performed with a polypectomy snare that includes the muscularis mucosae[12]. MD patients often exhibit hypochlorhydria with relatively normal serum gastrin levels; therefore, gastric pH samples should be obtained during endoscopy[24].
Imaging
Endoscopic ultrasound (EUS) may show ascites, pleural effusion, and gastric wall thickening with mucosal expansion and intact submucosa[26].
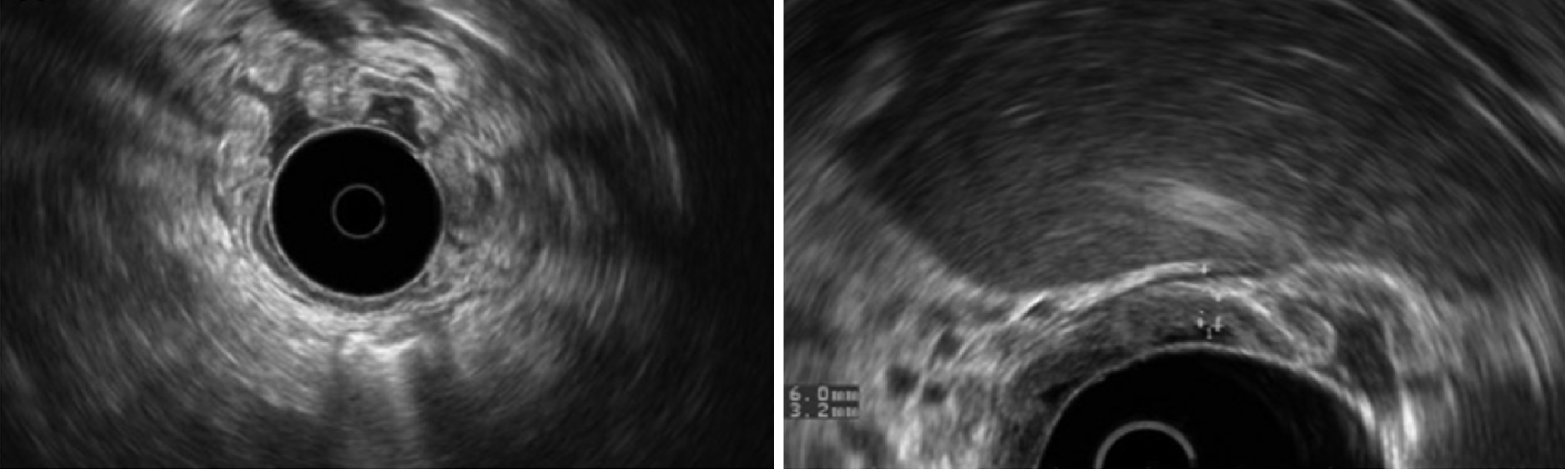
On EUS, patients might have giant gastric folds (Figure 3) that result from echogenic thickening of the mucosal layer with or without cystic components[27].
Figure 3 Endoscopic ultrasound photographs showing gastric wall thickening (6 mm) due to mucosal expansion (3.2 mm) with massive enlargement of gastric body great curvature folds[26].
The submucosa was preserved, and the antrum was spared. Citation: Nunes G, Barosa R, Patita M, Pinto-Marques P, Gonçalves D, Fonseca C, Alves de Matos A, Fonseca J. Ménétrier's disease: A case of successful treatment using long-acting octreotide. Acta Gastroenterol Belg 2019; 82: 429-432. Copyright© 2019, Acta Gastroenterol Belg. Published by Acta Gastro-Enterologica Belgica. The authors have obtained the permission for figure using from the Acta Gastro-Enterologica Belgica (Supplementary material).
Contrast-enhanced computed tomography (CT) and magnetic resonance imaging may reveal diffuse thickened mucosa of the greater curvature and elongated mucosa of the antrum with remarkable enhancement[28].
Early and delayed fluorodeoxyglucose (FDG) positron emission tomography/CT may show increased FDG uptake in the thickened mucosa, which may be due to mucosal inflammation. Thus, MD should be included in the differential diagnosis of abnormal gastric FDG accumulation alongside tumor and nontumor processes[28].
Laboratory tests
Laboratory evaluation should include a complete blood count, metabolic panel, serum gastrin, and serology for H. pylori and CMV[3]. Hypoalbuminemia is a hallmark feature due to protein-losing gastropathy. CMV serology should be obtained in all patients, regardless of age, as cases of acute-onset, spontaneously resolving MD - typically linked to CMV infection - have been documented, predominantly in children but occasionally in adults. Testing for H. pylori is also recommended, given isolated reports of symptom remission following H. pylori eradication in affected individuals[12,13]. Gastric secretory analysis often reveals hypochlorhydria or achlorhydria, reflecting parietal cell loss, while serum gastrin levels tend to remain within normal limits[24,29].
Histology
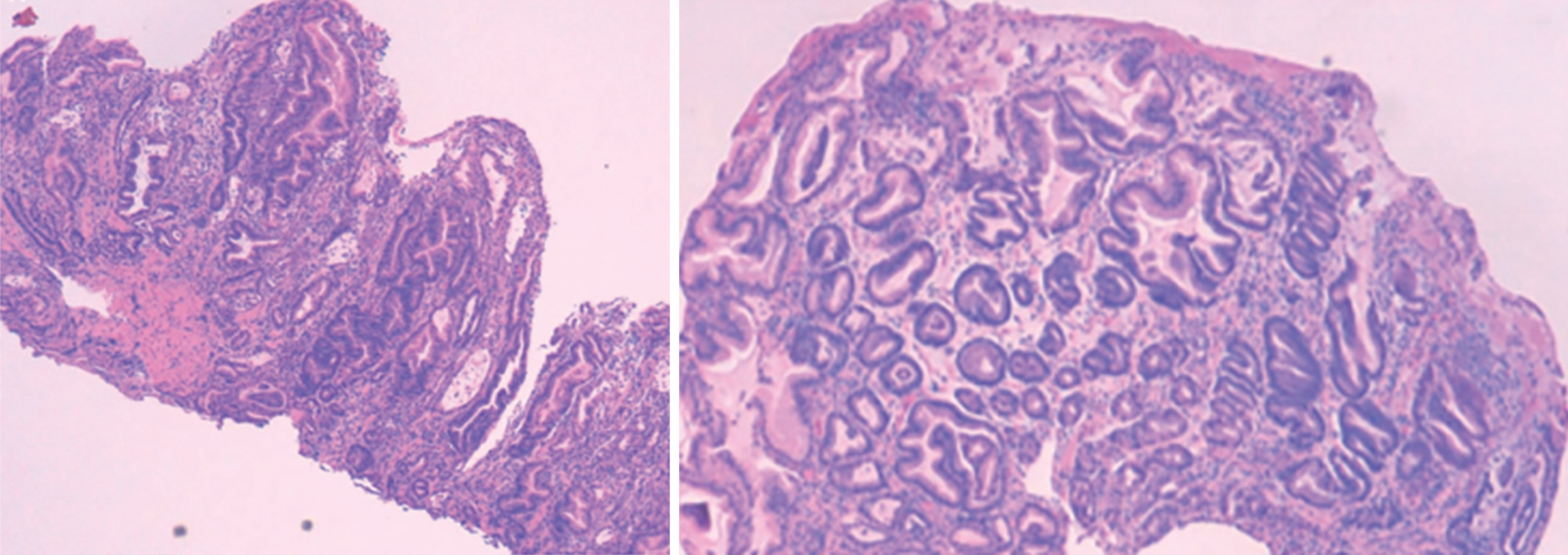
Gastric biopsies in MD characteristically reveal foveolar hyperplasia (Figure 4) along with a marked reduction or complete absence of oxyntic glands, typically with sparing of the antrum. In healthy gastric mucosa, the pit-to-gland ratio is approximately 1:4; however, in MD, this ratio is often reversed due to expansion of the surface mucous cell compartment, which may occupy nearly the full thickness of the mucosa. Additional histologic features include deep lateral glandular branching, lamina propria edema, surface erosions, and smooth muscle hyperplasia[20,24].
Figure 4 Images from gastric tissue stained with hematoxylin & eosin, where signs of hypertrophic gastropathy are evident. Tortuous and dilated glands with corium edema are observed[26].
Oxyntic mucosa atrophy and foveolar hyperplasia are typical features of Ménétrier disease present in the patient's gastric mucosa. Citation: Nunes G, Barosa R, Patita M, Pinto-Marques P, Gonçalves D, Fonseca C, Alves de Matos A, Fonseca J. Ménétrier's disease: A case of successful treatment using long-acting octreotide. Acta Gastroenterol Belg 2019; 82: 429-432. Copyright© 2019, Acta Gastroenterol Belg. Published by Acta Gastro-Enterologica Belgica. The authors have obtained the permission for figure using from the Acta Gastro-Enterologica Belgica (Supplementary material).
DIFFERENTIAL DIAGNOSIS
MD should be distinguished from other hypertrophic gastropathies such as Zollinger-Ellison syndrome (ZES) and lymphocytic gastritis, all of which exhibit generalized rugal hypertrophy[25].
ZES is characterized by hypergastrinemia and increased acid secretion, unlike MD which presents with hypochlorhydria and normal serum gastrin levels. Additionally, ZES often presents with gastric or duodenal ulcers, which is not typical of MD[26].
Hypertrophic lymphocytic gastritis can present with giant mucosal folds in the fundus, resembling MD, and similarly demonstrates relative antral sparing. However, it is distinguished histologically by diffuse, severe inflammation and a marked increase in intraepithelial lymphocytes. In this condition, foveolar hyperplasia is typically limited to regions of active inflammation[12].
Gastric lymphoma can present with thickened gastric folds; however, there is no PLE as seen in MD; meanwhile, gastrointestinal stromal tumors typically present as localized masses rather than diffuse gastric hypertrophy.
Infectious diseases such as histoplasmosis, syphilis, tuberculosis, and infiltrative disorders like sarcoidosis can also mimic MD[3].
Finally, gastric polyps may present as focal hypertrophic gastropathy and, in the context of polyposis syndromes, can be numerous and diffusely distributed throughout the stomach, leading to a hypertrophic appearance. In one study, hyperplastic polyps and polyposis syndromes were the most frequent conditions mistaken for MD, with juvenile polyposis syndrome (JPS) being the pathology that most frequently mimics MD[24]. A severe gastric phenotype has been shown in SMAD4 mutations associated with JPS.
TREATMENT
There is currently no standardized treatment for MD, and management is based on the severity of the symptoms as well as the underlying pathophysiology. Furthermore, there is paucity of clinical trials for the disease, and pharmacologic treatments have demonstrated variable and often inconsistent efficacy. Nevertheless, supportive treatments include a high-protein diet, replacement of micronutrients, and albumin infusions to manage hypoalbuminemia if necessary.
Medical therapy
Patients with MD should be treated for H. pylori and CMV if the tests for these infections are positive. This is because it has been shown that eradicating H. pylori improves the disease conditions in some patients[12,13]. Similarly, treatment with ganciclovir is effective in certain CMV-related cases[3].
Antihistamines and anticholinergics are generally not considered first-line treatments for MD, and although they have been used in some cases, there is no concrete evidence their benefit in managing the disease[30]. Meanwhile, proton pump inhibitors reduce gastric acid secretion and alleviate the symptoms of MD[31].
Octreotide is a somatostatin analog that has shown significant benefits in case reports on MD[26,32-35]. It acts by modulating the TGF-α-EGFR signaling pathway, which underlies the disease’s pathogenesis. Reported dosing regimens are variable and not standardized and include 100-600 µg daily, administered subcutaneously or intravenously, or an octreotide depot formulation of 10-60 mg intramuscularly every 4 weeks[3,36]. Treatment duration has varied across reports, typically ranging from 3 to 15 months, depending on clinical response and tolerance. Case reports have demonstrated clinical, endoscopic, and histological improvement after a few months of therapy[26].
Cetuximab, an EGFR inhibitor, is a recombinant chimeric IgG1 monoclonal antibody that specifically binds to the extracellular domain of the EGF receptor, thereby blocking the binding of ligands such as TGF-α[37]. This monoclonal antibody has shown promise in reducing gastric mucosal hypertrophy and improving protein loss. Treatment typically consists of an initial loading dose of intravenous cetuximab (400 mg/m² body surface area), followed by three weekly intravenous infusions of 250 mg/m² for a 12-month period[37,38]. To date, only a single clinical trial has been conducted to formally evaluate pharmacologic therapy in MD. This phase I, single-arm, open-label study assessed the efficacy of cetuximab[37]. Nine adult patients with symptomatic MD received weekly infusions of cetuximab over four weeks. The trial demonstrated rapid and sustained clinical improvement, including resolution of hypoalbuminemia, reduction in peripheral edema, and weight gain. Endoscopic and histological assessments showed decreased rugal hypertrophy, reduced foveolar hyperplasia, lower epithelial proliferation (Ki-67), and partial restoration of gastric gland architecture. Cetuximab was well tolerated, with no serious adverse events. This remains the only prospective clinical study in MD and provides strong support for EGFR blockade as a disease-modifying strategy.
Other case report studies that analyzed the efficacy of cetuximab have demonstrated significant improvement both clinically (quality-of-life indices) and biochemically (increased parietal cell mass and gastric acidity) along with near-complete histological remission[12,39-41].
However, some case reports have documented a high relapse rate following the discontinuation of medical therapy. In selected cases, it may be reasonable to consider lifelong treatment in an effort to achieve sustained disease control and remission. This approach, particularly with cetuximab, remains investigational, and additional research is required to clarify the long-term safety and effectiveness of continuous therapy[38]. Emerging molecular therapies targeting the EGFR-TGF-α axis, which is implicated in the pathogenesis of MD, may offer novel treatment avenues. Agents such as EGFR tyrosine kinase inhibitors and ADAM17 inhibitors, which modulate this signaling pathway, are already under investigation or clinical use in malignancies like small cell lung cancer and could potentially be repurposed to interrupt the aberrant epithelial proliferation seen in MD[42,43].
Surgical intervention
In severe cases of MD that are refractory to medical therapy, with persistent and debilitating symptoms, such as refractory hypoalbuminemia or refractory gastrointestinal symptoms or suspected malignant transformation, a total or subtotal gastrectomy may be required[12,44,45]. Although MD primarily involves the fundus and body of the stomach, total gastrectomy is preferred over partial gastrectomy due to improved quality of life and fewer surgical complications. Additionally, it lowers the risk of future presentation of malignancy and eliminates the need for ongoing endoscopic surveillance[45].
PROGNOSIS AND FOLLOW UP
The prognosis of MD varies. Pediatric cases often resolve spontaneously, whereas adult cases may progress to severe malnutrition, with nearly 10% of patients potentially developing gastric cancer over time. Given the lack of data on whether current medical therapies reduce the long-term risk of malignancy, and in light of high relapse rates reported after treatment discontinuation - even in asymptomatic individuals - annual esophagogastroduodenoscopy should be considered in all patients as part of routine gastric cancer surveillance[4].
CONCLUSION
MD remains a rare but clinically significant disorder characterized by hypertrophic gastropathy, protein loss, and increased risk for gastric cancer. There is currently no standardized treatment; however, a better understanding of the role of EGFR signaling has led to the development of targeted therapies such as cetuximab, offering new hope for patients with the disease. Early diagnosis and tailored treatment approaches are crucial for optimal management and better patient outcomes.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: Portugal
Peer-review report’s classification
Scientific Quality: Grade A, Grade B, Grade C
Novelty: Grade B, Grade B, Grade C
Creativity or Innovation: Grade B, Grade C, Grade C
Scientific Significance: Grade A, Grade B, Grade C
P-Reviewer: Chisthi MM, MD, Professor, India; Li P, MD, PhD, Professor, China S-Editor: Li L L-Editor: A P-Editor: Zhang L