Published online Aug 21, 2025. doi: 10.3748/wjg.v31.i31.109857
Revised: June 26, 2025
Accepted: July 28, 2025
Published online: August 21, 2025
Processing time: 86 Days and 20.7 Hours
The number of tumor deposits (TDs) does not play a part in the current tumor node metastasis staging. Negative lymph node (NLN) status is associated with the prognosis of colorectal cancer (CRC), but its clear role in N1c stage remains to be defined.
To evaluate the combination of TDs and NLNs as potential prognostic indicators in N1c CRC.
We retrospectively identified 107 consecutive patients who had N1c CRC radically resected at China-Japan Friendship Hospital. The combination of TDs and NLNs was calculated by the formula NLNTD = NLN/(TD + 1). Cutoff values of NLNs and NLNTD were determined using the R package “survminer”. Disease-free survival (DFS), overall survival (OS) and cancer-specific survival (CSS) were determined using the Kaplan-Meier method to assess the impact of NLNTD on prognosis. Results were compared using the log-rank test.
The median follow-up time was 63.17 (45.33-81.37) months for DFS, with 33.64% (36/107) of patients experiencing recurrence during follow-up. Five-year DFS was 66.0% (57.3%-76.0%). There was no significant difference in prognosis between patients with > 12 and ≤ 12 NLNs (P = 0.058) for DFS. Similar results were seen according to the number of TDs. The definition of NLNTD = NLN/(TD + 1) with a cutoff value of 6 divided patients into two groups with different DFS (P = 0.005). Five-year DFS for patients with NLNTD > 6 was 73.5% (63.6%-85.0%), compared with 50.0% (35.7%-70.0%) for those with NLNTD ≤ 6. These two groups had different prognosis without perineural invasion (P = 0.012) or lymphovascular invasion (P = 0.002) even neither (P = 0.053). Similar results were seen for OS and CSS.
NLNTD could serve as important prognostic factor for outcomes in N1c CRC patients. These patients could be stratified for prognosis through NLNTD and the high-risk should be given more attention during treatment.
Core Tip: To evaluate the combination of tumor deposits (TDs) and negative lymph node (NLN) as potential prognostic indicators in N1c colorectal cancer (CRC). We retrospectively identified 107 consecutive patients who had CRC with N1c disease. There was no significant difference in prognosis between patients with more than 12 NLN or not for disease free survival, the same result could be seen according to the number of TD. The definition of combination of TD and NLN (NLNTD) = NLN/(TD + 1) with cutoff value as 6 could divide patients into two groups with different prognosis for disease free survival. These two groups had different prognosis without perineural invasion or lymphovascular invasion even neither.
- Citation: Sun ZG, Chen SX, Sun BL, Zhang DK, Zhong DR, Zhang TY, Hu YW, Han ZH, Wu WX, Hou ZY, Yao L, Zhang YJ, Sun HL, Jie JZ. Important role of tumor deposits and negative lymph nodes in prognosis of N1c colorectal cancer patients. World J Gastroenterol 2025; 31(31): 109857
- URL: https://www.wjgnet.com/1007-9327/full/v31/i31/109857.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i31.109857
In the United States, colorectal cancer (CRC) ranks as the second leading cause of cancer-related deaths[1]. CRC is among the most prevalent malignancies of the digestive system worldwide which poses a significant threat to human life and health due to its high incidence and mortality rates[2,3]. Tumor deposits (TDs) are defined as discrete tumor nodules within lymphatic drainage areas of the primary carcinoma, without identifiable lymph nodes, vascular or neural structures. CRC with N1c staging indicates the presence of TDs without direct evidence of lymph node metastasis, according to the eighth American Joint Committee on Cancer tumor node metastasis (TNM) staging system, which has been in use since 2017[4]. Stage N1c is associated with a significantly higher risk of tumor recurrence and distant metastasis[5,6].
However, the prognostic factors influencing outcomes in stage N1c patients remain unclear. The number of TDs does not play a role in the current TNM staging, regardless of N1c staging[7,8], which has been studied and improved by many researchers[9-11]. Negative lymph node (NLN) status is associated with the prognosis of CRC[12], but its clear role in N1c stage remains to be defined. The prognostic significance of NLN in stage III colon cancer remains unconfirmed[13]. The role of NLN status in N1c patients is needed via further verification.
This study retrospectively analyzed the clinicopathological information of patients with stage N1c CRC to explore the relationship between the combination of TDs and NLN status and prognosis. Our study sought to elucidate the prognostic factors associated with N1c-stage CRC, with the ultimate goal of facilitating collaborative advancements in CRC diagnosis and treatment.
This study enrolled consecutive patients with pathologically confirmed primary CRC who underwent curative surgical resection at China-Japan Friendship Hospital from July 2015 to July 2021. The inclusion criteria comprised: (1) Accessible information on TDs and NLNs in postoperative pathology reports; (2) Patients who underwent radical resection with postoperative pathological confirmation of malignancy; and (3) Availability of complete clinicopathological and follow-up data. The exclusion criteria were: (1) Receipt of neoadjuvant chemotherapy or chemoradiotherapy prior to surgery; (2) Presence of distant metastasis at initial diagnosis; (3) Patients with metastasis or mortality within 3 months postoperatively; (4) Follow-up duration < 2 years; (5) Unavailable TD count; and (6) Unavailable NLN count.
Clinical and pathological data were retrospectively extracted from electronic medical records within a prospectively maintained cohort. Baseline information included age, sex, surgical date, neoadjuvant treatment history, and distant metastasis. Preoperative clinical TNM staging was determined by two radiologists using computed tomography (CT) or magnetic resonance imaging (MRI). Pathological reports provided data on NLN count, TD count, lymphovascular invasion (LVI), perineural invasion (PNI), tumor differentiation, tumor size, depth of invasion, and pathological T stage.
Patient outcomes, including recurrence and survival status, were obtained via telephone interviews with patients or their families, supplemented by electronic medical records. Disease-free survival (DFS) was defined as the time from surgery to recurrence, last follow-up without recurrence, or death from other causes. Overall survival (OS) was calculated from surgery to death from any cause or the last follow-up for surviving patients. Cancer-specific survival (CSS) was measured from surgery to death due to cancer or the last follow-up for survivors.
All patients underwent radical surgery, including total mesorectal excision for rectal cancer and complete mesocolic excision for colon cancer. Adjuvant chemotherapy, using CAPOX (capecitabine + oxaliplatin) or FOLFOX (fluorouracil + oxaliplatin + leucovorin) regimens, was administered over six cycles, with chemoradiotherapy recommended as appropriate. Postoperative follow-up adhered to National Comprehensive Cancer Network guidelines, with visits every 3 months for the first 2 years, every 6 months for the next 3 years, and annually thereafter up to 10 years. Each visit included clinical history review, laboratory tests (e.g., carcinoembryonic antigen levels), and imaging assessments (abdominopelvic CT, chest CT, and pelvic MRI for rectal cancer patients) every 6 months.
Cutoff values were determined using the R package “survminer”. Survival differences in DFS, OS and CSS were evaluated using Kaplan-Meier curves and log-rank tests. Univariable and multivariable Cox proportional hazards models identified independent risk factors, with variables showing P < 0.01 in univariable analysis included in multivariable analysis. A two-sided P < 0.01 was considered statistically significant, with 95% confidence intervals (CIs). All analyses were performed using R version 4.0.5 (http://www.R-project.org/).
During the study period, 107 patients with stage N1c CRC underwent curative resection. The median follow-up time for DFS, OS and CSS was 63.17 (45.33-81.37), 60.83 (44.0-77.40) and 60.53 (42.83-77.40) months, respectively. The 5-year survival rates were 66.0% (57.3%-76.0%) for DFS, 79.5% (71.5%-88.4%) for OS, and 81.2% (73.4%-89.9%) for CSS. The clinicopathological characteristics of all patients are shown in Table 1. The cohort comprised 55 female (51.4%) and 52 male (48.6%) patients, with only 12 (11.2%) aged < 50 years. Pathological staging revealed pT2-3 disease in 97 patients (90.7%) and pT4a-b in 10 (9.3%). Mucinous adenocarcinoma was found in 15 (14.0%) patients. The most frequent number of TDs was one (73 patients, 68.2%). Eighty-nine (83.2%) of patients had > 12 NLNs obtained by postoperative pathology. PNI was detected in 48 (44.9%) patients and LVI in 23 (21.5%).
| Characteristics | No | Characteristics | No |
| Sex | cT stage | ||
| Female | 55 | 2 | 15 |
| Male | 52 | 3 | 64 |
| Age (years) | 4 | 2 | |
| ≤ 50 | 12 | cN stage | |
| > 50 | 95 | 0 | 19 |
| pT stage | 1 | 42 | |
| 2 and 3 | 97 | 2 | 20 |
| 4 | 10 | Poorly differentiated | |
| Mucinous adenocarcinoma | No | 96 | |
| No | 92 | Yes | 11 |
| Yes | 15 | PNI | |
| TD | No | 59 | |
| 1 | 73 | Yes | 48 |
| 2 | 16 | LVI | |
| > 2 | 18 | No | 84 |
| NLN | Yes | 23 | |
| > 12 | 89 | ||
| ≤ 12 | 18 |
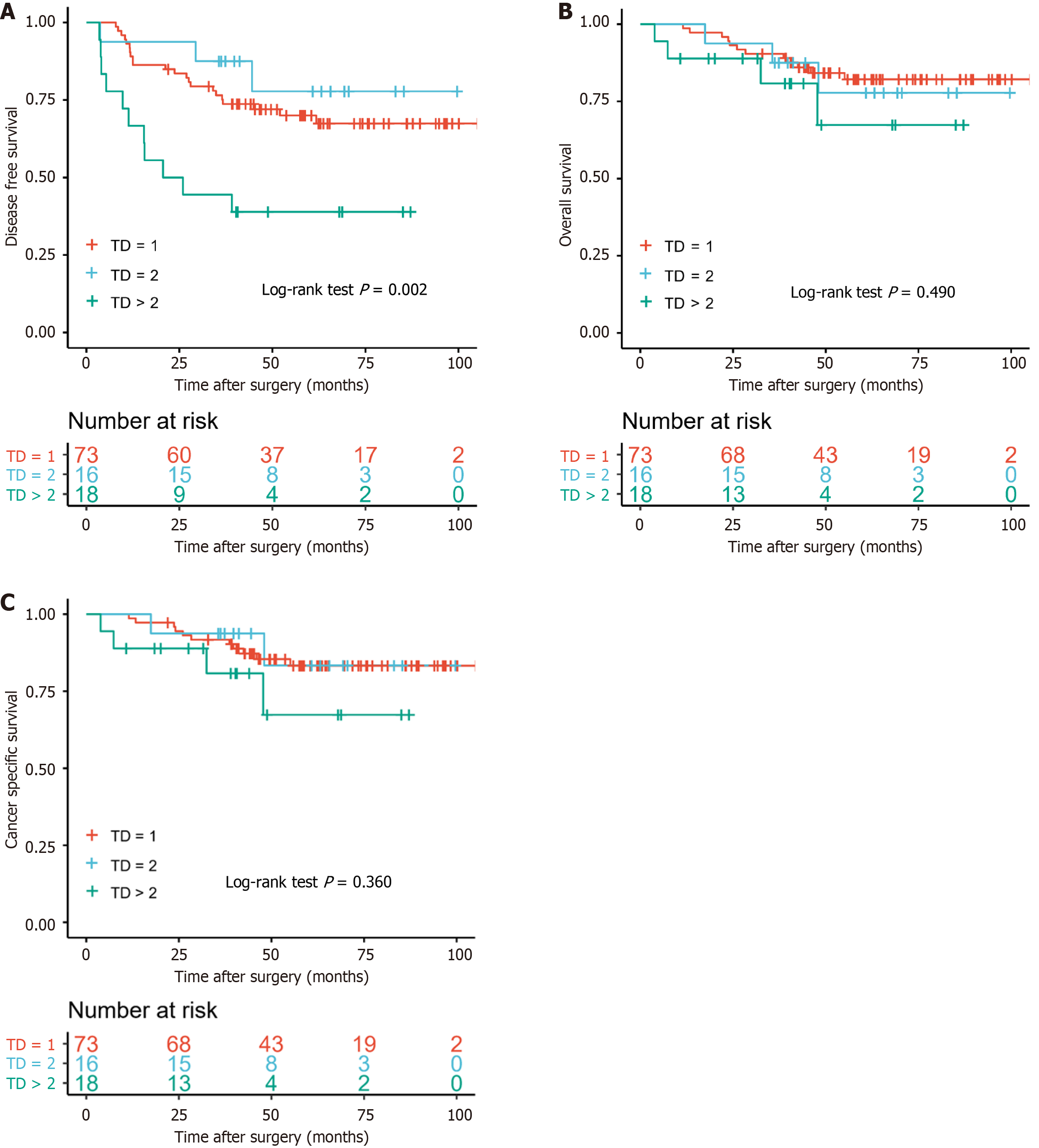
There was no significant difference in prognosis among patients with different numbers of TDs (TD = 1, = 2 and > 2) for DFS (P = 0.002), OS (P = 0.490) and CSS (P = 0.360) (Figure 1). The 5-year DFS of the TD = 1 group (70.0%) was less than that in the TD = 2 group (77.8%).
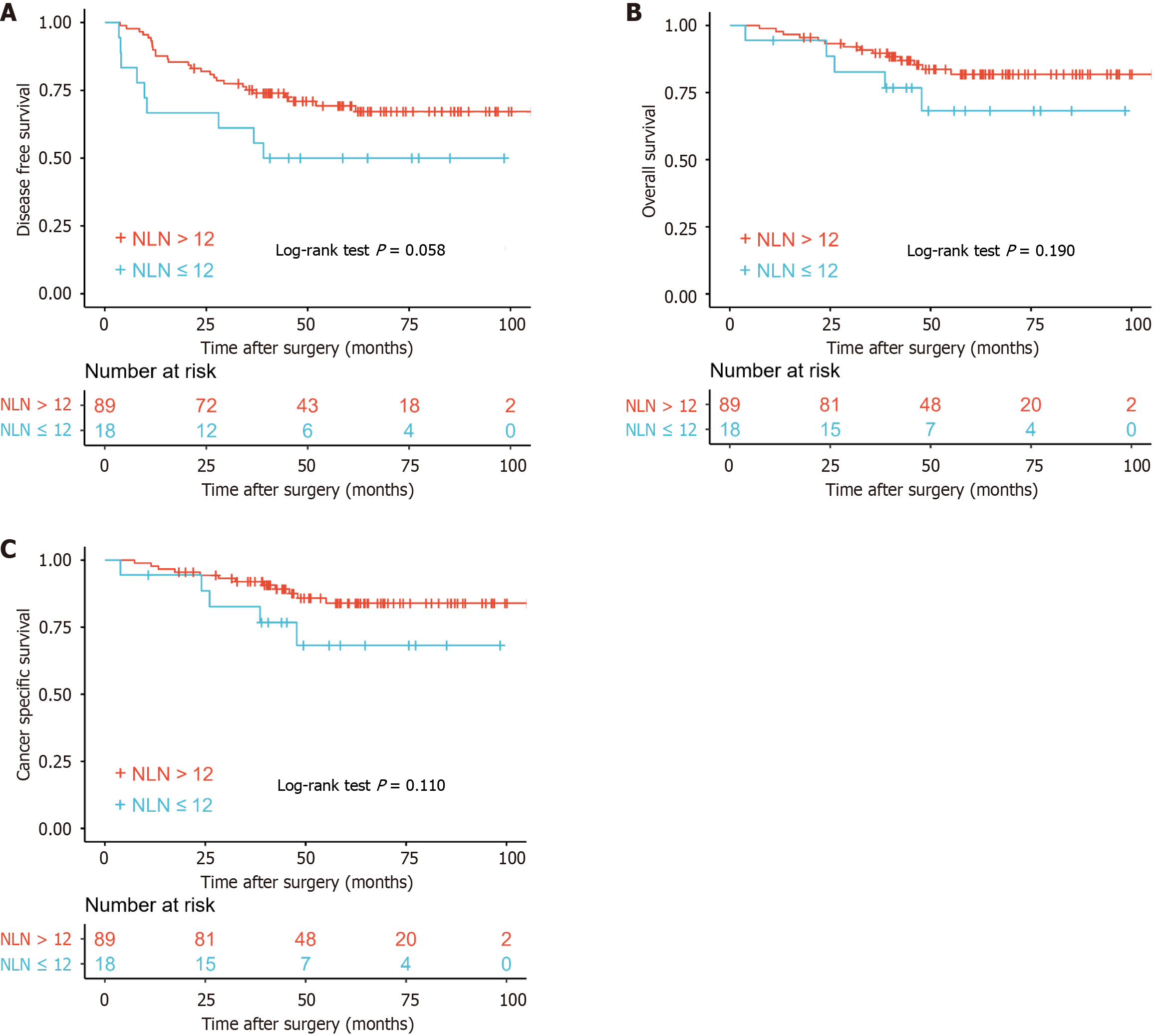
There was no significant difference in prognosis between patients with > 12 or < 12 NLNs for DFS (P = 0.058), OS (P = 0.190) and CSS (P = 0.110) (Figure 2).
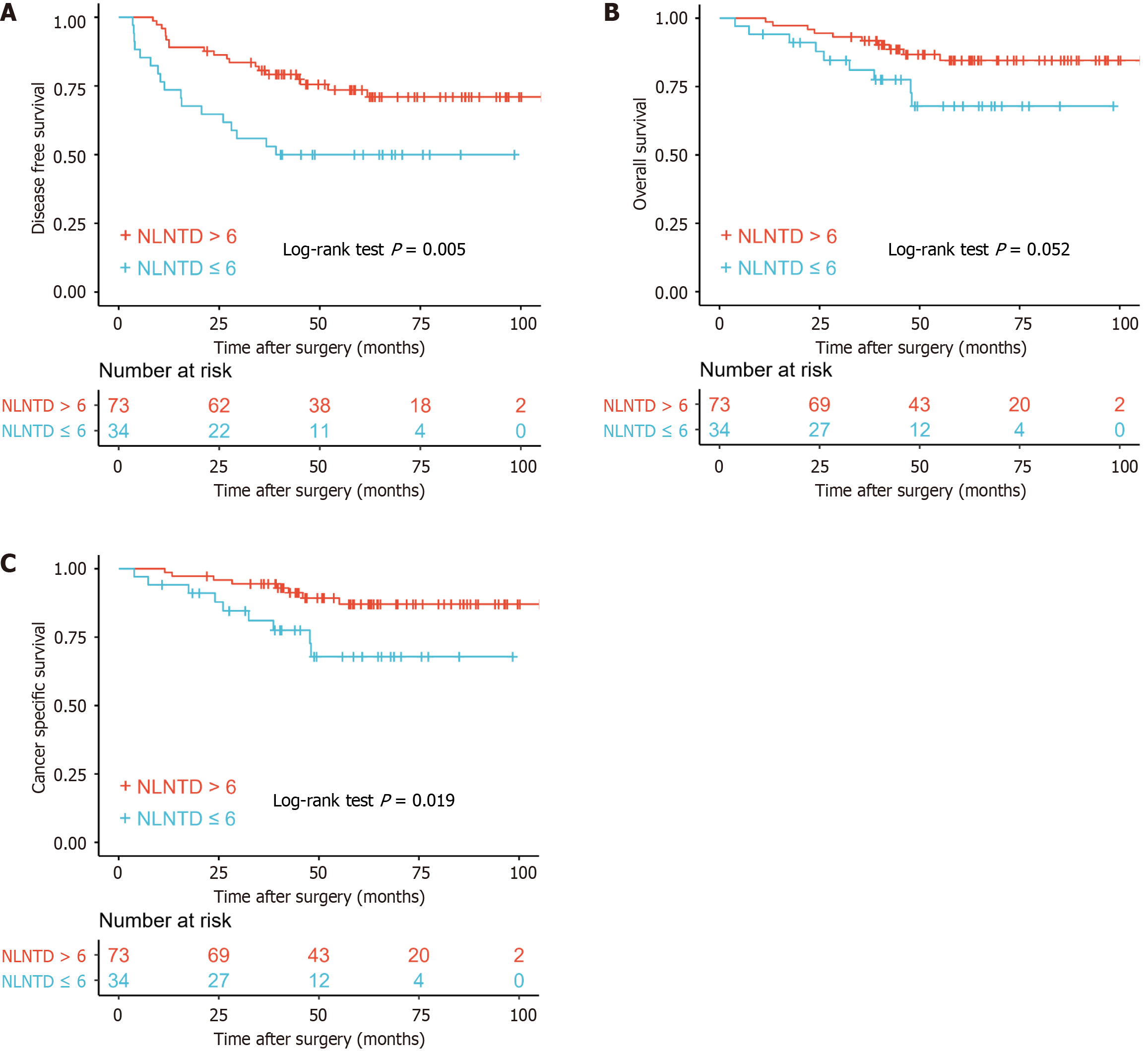
Although the effects of TDs and NLNs were not significant in different groups, the positive effect of NLNs was beyond doubt as was the negative effect of TDs. In order to better reflect the role of TDs and NLNs, we selected the new definition (combination of TD and NLN) named NLNTD. The formula of NLNTD = NLN/(TD + 1) combined the two factors that influence prognosis of patients.
The cutoff value of NLNTD was calculated as 6 by the R package “survminer” and was chosen for consideration of clinical application. When the number of TDs was 1 and the number of NLNs was 12, the NLNTD was calculated as 6. There was a significant difference in prognosis between patients divided according to NLNTD for DFS (P = 0.005), OS (P = 0.052) and CSS (P = 0.019) (Figure 3). Five-year DFS in patients with NLNTD > 6 was 73.5% (63.6%-85.0%) compared with 50.0% (35.7%-70.0%) in patients with NLNTD ≤ 6.
As we had previously published results showed[14], in univariable regression analysis, LVI [hazard ratio (HR) = 3.510, 95%CI: 1.785-6.902] and PNI (HR = 3.753, 95%CI: 1.830-7.70) were significantly associated with DFS. In multivariable regression analysis for DFS, LVI (HR = 3.368, 95%CI: 1.628-6.966) and PNI (HR = 3.055, 95%CI: 1.478-6.313) were confirmed as independent prognostic risk factors. The results for OS and CSS were similar.
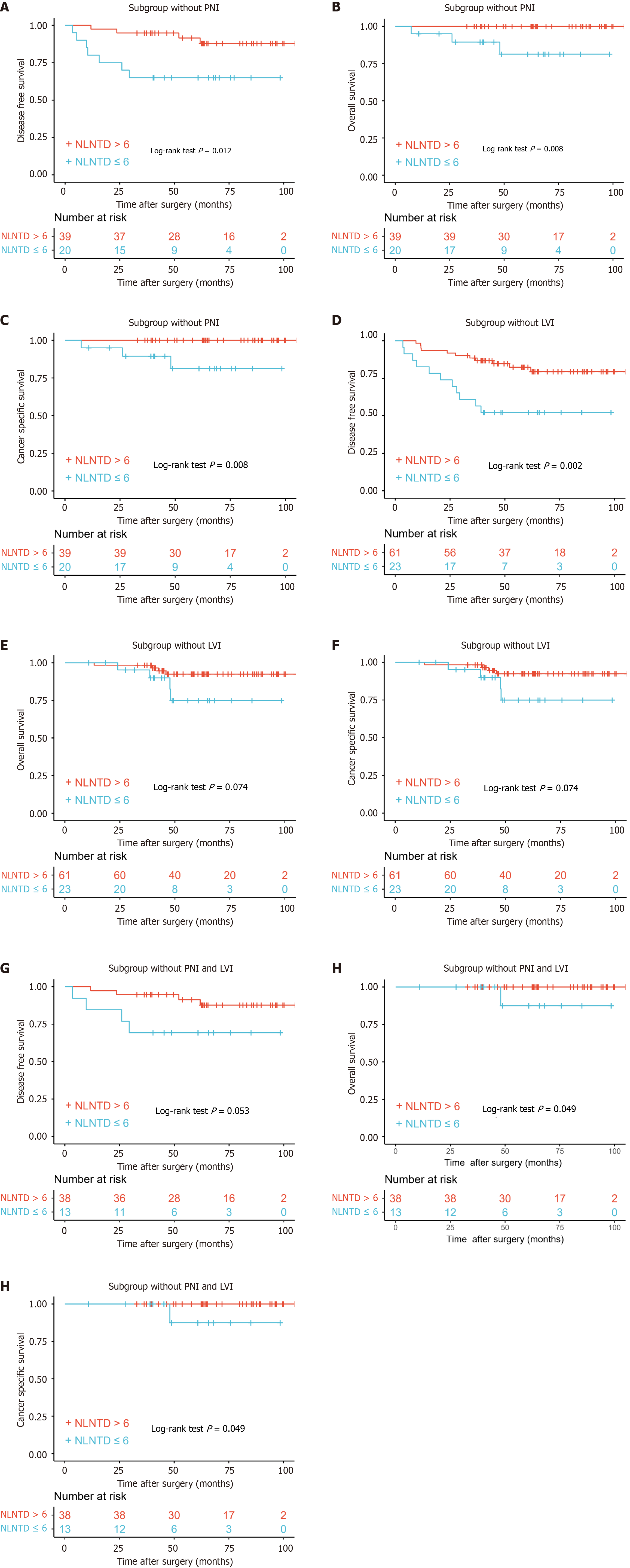
Kaplan-Meier survival curve analysis showed a significant difference in prognosis between patients divided according to NLNTD for DFS (P = 0.012), OS (P = 0.008) and CSS (P = 0.008) in the subgroup without PNI (Figure 4A-C). Similar results were seen in the subgroup without LVI (Figure 4D-F). When there was no LVI or PNI, the prognosis could be still differentiated by NLNTD (Figure 4G-I).
In our retrospective analysis of 107 consecutive patients with N1c CRC, the combined role of TDs and NLNs was clarified as a potential prognostic indicator in patients with N1c CRC. This study is significant given that the current TNM staging system does not incorporate TD counts[7,8,15], and the precise role of NLNs in the prognosis of N1c CRC remains to be investigated further. NLNTD, which combined TDs and NLNs, was first proposed in this study, and emerged as a significant factor influencing survival and disease progression in these patients. Given that LVI and PNI can affect the prognosis of N1c CRC patients[14], NLNTD could play a role in these patients without LVI or PNI.
TDs were initially defined as scattered nodules present in the adipose tissue surrounding the primary tumor, with histological evidence showing no residual lymphoid tissue within the nodules. TDs were defined as discrete tumor nodules located within the lymphatic drainage area of the primary carcinoma, lacking identifiable lymph node tissue or association with vascular/neural structures. This definition specifically excluded considerations of nodule size, shape, or contour characteristics[4]. TD-positive rates ranging from 9.5% to 26.9% have been reported[16,17]. Multiple investigations have consistently demonstrated that TD presence adversely impacts patient prognosis[18], independent of lymph node metastasis status[19-21] and neoadjuvant chemoradiotherapy[22].
Some researchers have indicated that there is no correlation between the number of TDs and prognosis[15]. From a biological perspective, TDs cannot be equated with LNs[23]. Although TDs are quantifiable, current N staging simply reports TDs as being present or absent, which results in a significant loss of information. Consequently, multiple studies have proposed modifying the existing N staging system to incorporate TD enumeration[9-11].
The prognostic significance of NLN count becomes particularly intriguing when considering its potential association with host immune response and survival outcomes. Current evidence demonstrates that tumor progression is critically mediated by an immunosuppressive tumor microenvironment, arising from complex interactions between malignant cells and various stromal components, including both immune and non-immune cell populations[24]. NLN status, which represents the absence of metastatic involvement in certain LNs, has been shown to correlate with CRC prognosis, albeit with varying degrees of significance across different stages[25,26].
Our results indicate that neither the number of NLNs alone nor the number of TDs alone significantly influenced the outcomes, including DFS, OS and CSS in our cohort. This finding underscores the complexity of CRC prognosis and highlights the potential limitations of considering these factors in isolation. We utilized a novel formula, NLNTD = NLN/(TD + 1), to assess the balance between the number of NLNs and TDs. This metric aims to capture the intricate interplay between the two variables, reflecting both tumor aggressiveness through TD count and the immune response or tumor-suppressing capacity suggested by NLN count. However, when combining NLN and TD using the NLNTD formula, we observed a significant difference in DFS, OS and CSS between patients with NLNTD values above and below the cutoff value. Specifically, patients with NLNTD > 6 had a significantly better 5-year DFS rate (73.5%) compared to those with NLNTD ≤ 6 (50.0%).
LVI and PNI status serve as robust predictors of DFS in CRC patients when used for risk stratification[27]. Chemotherapy may be protective specifically when LVI and PNI are present[28]. Our preliminary findings substantiate the significant association of LVI and PNI with poor prognosis in stage N1c CRC patients[14]. These findings align with prior studies establishing LVI and PNI as independent prognostic factors across multiple CRC stages[29,30]. NLNTD in prognosis was further validated across different subgroups, including those without PNI or LVI, suggesting that NLNTD may serve as a robust prognostic indicator irrespective of other aggressive tumor features.
The biological rationale behind the prognostic significance of NLNTD may lie in its ability to reflect the balance between tumor burden and host immune response. A higher NLNTD value suggests a greater number of NLNs compared to TDs, potentially indicating a more robust immune response capable of controlling tumor growth and spread. Conversely, a lower NLNTD value may signify a compromised immune status or a more aggressive tumor phenotype, leading to poorer prognosis. Our findings have important clinical implications. By incorporating NLNTD into the prognostic assessment of N1c CRC patients, clinicians can identify high-risk individuals who may benefit from more aggressive treatment strategies or closer follow-up. This personalized approach to care could lead to improved outcomes and quality of life for these patients.
The study was based on single-center retrospective data, small sample size, which may have introduced selection bias and limited the ability to establish causal relationships between NLNTD and prognostic outcomes. The cutoff value of 6 for NLNTD was determined based on the study cohort and lacked external validation, raising concerns about its reproducibility in other populations. Future studies should address these limitations through multicenter prospective designs, larger cohorts, and external validation to strengthen the clinical relevance of NLNTD as a prognostic marker. In conclusion, our study demonstrates that NLNTD, a novel combination of NLNs and TDs, serves as an important prognostic factor in N1c CRC patients. By stratifying patients based on NLNTD values, clinicians can better identify those at high risk of recurrence and tailor their treatment plans accordingly. Future large-scale, multicenter studies incorporating diverse patient cohorts are warranted to validate these findings and elucidate the potential mechanisms underlying the prognostic significance of NLNTD. With continued research, we may ultimately refine our understanding of CRC prognosis and improve patient outcomes.
NLNTD could serve as important prognostic factor for outcomes in N1c CRC patients. These patients could be stratified for prognosis through NLNTD and the high-risk should be given more attention during treatment.
We would like to express our gratitude to Zhang HZ, Feng L and Dong XS, for their invaluable guidance, patience and continuous support throughout this research project. Their expert advice, rigorous scrutiny and encouragement have been instrumental in shaping this work and fostering my academic growth.
| 1. | Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73:233-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1554] [Reference Citation Analysis (3)] |
| 2. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64562] [Article Influence: 16140.5] [Reference Citation Analysis (176)] |
| 3. | Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 9923] [Article Influence: 4961.5] [Reference Citation Analysis (2)] |
| 4. | Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2341] [Cited by in RCA: 4397] [Article Influence: 549.6] [Reference Citation Analysis (4)] |
| 5. | Nagtegaal ID, Knijn N, Hugen N, Marshall HC, Sugihara K, Tot T, Ueno H, Quirke P. Tumor Deposits in Colorectal Cancer: Improving the Value of Modern Staging-A Systematic Review and Meta-Analysis. J Clin Oncol. 2017;35:1119-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 195] [Article Influence: 21.7] [Reference Citation Analysis (1)] |
| 6. | Bouquot M, Creavin B, Goasguen N, Chafai N, Tiret E, André T, Flejou JF, Parc Y, Lefevre JH, Svrcek M. Prognostic value and characteristics of N1c colorectal cancer. Colorectal Dis. 2018;20:O248-O255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 7. | Benson AB, Venook AP, Adam M, Chang G, Chen YJ, Ciombor KK, Cohen SA, Cooper HS, Deming D, Garrido-Laguna I, Grem JL, Haste P, Hecht JR, Hoffe S, Hunt S, Hussan H, Johung KL, Joseph N, Kirilcuk N, Krishnamurthi S, Malla M, Maratt JK, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Shogan B, Skibber JM, Sofocleous CT, Tavakkoli A, Willett CG, Wu C, Jones F, Gurski L. NCCN Guidelines® Insights: Rectal Cancer, Version 3.2024. J Natl Compr Canc Netw. 2024;22:366-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 55] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 8. | Ness RM, Llor X, Abbass MA, Bishu S, Chen CT, Cooper G, Early DS, Friedman M, Fudman D, Giardiello FM, Glaser K, Gurudu S, Hall M, Huang LC, Issaka R, Katona B, Kidambi T, Lazenby AJ, Maratt J, Markowitz AJ, Marsano J, May FP, Mayer RJ, Olortegui K, Patel S, Peter S, Porter LD, Shafi M, Stanich PP, Terdiman J, Vu P, Weiss JM, Wood E, Cassara CJ, Sambandam V. NCCN Guidelines® Insights: Colorectal Cancer Screening, Version 1.2024. J Natl Compr Canc Netw. 2024;22:438-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 9. | Liu F, Zhao J, Li C, Wu Y, Song W, Guo T, Chen S, Cai S, Huang D, Xu Y. The unique prognostic characteristics of tumor deposits in colorectal cancer patients. Ann Transl Med. 2019;7:769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Chen H, Tang Z, Chen L, Li H, Wang X, Liu F, Sun Y. Evaluation of the impact of tumor deposits on prognosis in gastric cancer and a proposal for their incorporation into the AJCC staging system. Eur J Surg Oncol. 2018;44:1990-1996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Karamchandani DM, Chetty R, King TS, Liu X, Westerhoff M, Yang Z, Yantiss RK, Driman DK. Challenges with colorectal cancer staging: results of an international study. Mod Pathol. 2020;33:153-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Kuo YH, You JF, Hung HY, Chin CC, Chiang JM, Chang CH. Number of negative lymph nodes with a positive impact on survival of stage III colon cancer; a retrospective observation study for right side and left side colon. BMC Cancer. 2022;22:126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 13. | Johnson PM, Porter GA, Ricciardi R, Baxter NN. Increasing negative lymph node count is independently associated with improved long-term survival in stage IIIB and IIIC colon cancer. J Clin Oncol. 2006;24:3570-3575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 279] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 14. | Sun ZG, Chen SX, Sun BL, Zhang DK, Sun HL, Chen H, Hu YW, Zhang TY, Han ZH, Wu WX, Hou ZY, Yao L, Jie JZ. Important role of lymphovascular and perineural invasion in prognosis of colorectal cancer patients with N1c disease. World J Gastroenterol. 2025;31:102210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (4)] |
| 15. | Tong LL, Gao P, Wang ZN, Song YX, Xu YY, Sun Z, Xing CZ, Xu HM. Is the seventh edition of the UICC/AJCC TNM staging system reasonable for patients with tumor deposits in colorectal cancer? Ann Surg. 2012;255:208-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Pyo DH, Kim SH, Ha SY, Yun SH, Cho YB, Huh JW, Park YA, Shin JK, Lee WY, Kim HC. Revised Nodal Staging Integrating Tumor Deposit Counts With Positive Lymph Nodes in Patients With Stage III Colon Cancer. Ann Surg. 2023;277:e825-e831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 17. | Delattre JF, Cohen R, Henriques J, Falcoz A, Emile JF, Fratte S, Chibaudel B, Dauba J, Dupuis O, Bécouarn Y, Bibeau F, Taieb J, Louvet C, Vernerey D, André T, Svrcek M. Prognostic Value of Tumor Deposits for Disease-Free Survival in Patients With Stage III Colon Cancer: A Post Hoc Analysis of the IDEA France Phase III Trial (PRODIGE-GERCOR). J Clin Oncol. 2020;38:1702-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (1)] |
| 18. | Jörgren F, Agger E, Lydrup ML, Buchwald P. Tumour deposits in colon cancer predict recurrence and reduced survival in a nationwide population-based study. BJS Open. 2023;7:zrad122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (1)] |
| 19. | Moon JY, Lee MR, Ha GW. Prognostic value of tumor deposits for long-term oncologic outcomes in patients with stage III colorectal cancer: a systematic review and meta-analysis. Int J Colorectal Dis. 2022;37:141-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 20. | Agger E, Jörgren F, Jöud A, Lydrup ML, Buchwald P. Negative Prognostic Impact of Tumor Deposits in Rectal Cancer: A National Study Cohort. Ann Surg. 2023;278:e526-e533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 21. | Lundström S, Agger E, Lydrup ML, Jörgren F, Buchwald P. Adverse impact of tumor deposits in lymph node negative rectal cancer - a national cohort study. Int J Colorectal Dis. 2023;38:66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (2)] |
| 22. | Wang Y, Zhang J, Zhou M, Yang L, Wan J, Shen L, Liang L, Yao Y, Zhang H, Zhang Z. Poor prognostic and staging value of tumor deposit in locally advanced rectal cancer with neoadjuvant chemoradiotherapy. Cancer Med. 2019;8:1508-1520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 23. | Sun Z, Wang ZN, Xu YY, Zhu GL, Huang BJ, Xu Y, Liu FN, Zhu Z, Xu HM. Prognostic significance of tumor deposits in gastric cancer patients who underwent radical surgery. Surgery. 2012;151:871-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Han PZ, Tan LC, Ouyang QS, Yu PC, Shi X, Hu JQ, Wei WJ, Lu ZW, Wang Y, Ji QH, Qu N, Mai HM, Wang YL. Development and validation of a gene model predicting lymph node metastasis and prognosis of oral squamous cell carcinoma based on single-cell and bulk RNA-seq analysis. J Oral Pathol Med. 2023;52:389-401. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 25. | Ogino S, Nosho K, Irahara N, Shima K, Baba Y, Kirkner GJ, Mino-Kenudson M, Giovannucci EL, Meyerhardt JA, Fuchs CS. Negative lymph node count is associated with survival of colorectal cancer patients, independent of tumoral molecular alterations and lymphocytic reaction. Am J Gastroenterol. 2010;105:420-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Dai X, Dai Z, Fu J, Liang Z, Du P, Wu T. Prognostic significance of negative lymph node count in microsatellite instability-high colorectal cancer. World J Surg Oncol. 2024;22:186. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 27. | Huh JW, Lee WY, Shin JK, Park YA, Cho YB, Kim HC, Yun SH. A novel histologic grading system based on lymphovascular invasion, perineural invasion, and tumor budding in colorectal cancer. J Cancer Res Clin Oncol. 2019;145:471-477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Skancke M, Arnott SM, Amdur RL, Siegel RS, Obias VJ, Umapathi BA. Lymphovascular Invasion and Perineural Invasion Negatively Impact Overall Survival for Stage II Adenocarcinoma of the Colon. Dis Colon Rectum. 2019;62:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (1)] |
| 29. | Al-Sukhni E, Attwood K, Gabriel EM, LeVea CM, Kanehira K, Nurkin SJ. Lymphovascular and perineural invasion are associated with poor prognostic features and outcomes in colorectal cancer: A retrospective cohort study. Int J Surg. 2017;37:42-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 30. | Chen PC, Yeh YM, Lin BW, Chan RH, Su PF, Liu YC, Lee CT, Chen SH, Lin PC. A Prediction Model for Tumor Recurrence in Stage II-III Colorectal Cancer Patients: From a Machine Learning Model to Genomic Profiling. Biomedicines. 2022;10:340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |