Published online Aug 21, 2025. doi: 10.3748/wjg.v31.i31.105665
Revised: March 27, 2025
Accepted: July 23, 2025
Published online: August 21, 2025
Processing time: 197 Days and 12.6 Hours
Recent research has increasingly highlighted the potential oncogenic effects of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection within the gastrointestinal tract. Growing evidence suggests that SARS-CoV-2 may contribute to the development of gastrointestinal malignancies through several mechanisms, including sustained chronic inflammation, disruption of normal cellular homeostasis, and potential viral integration into host cells. These pathological processes have the potential to dysregulate critical cellular pathways, thereby promoting cancer development in vulnerable populations. A thorough understanding of how SARS-CoV-2 interacts with the development of gastro
Core Tip: Investigating the potential oncological implications of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the gastrointestinal tract is crucial for a comprehensive understanding of the impact of the virus on humans. Recent research has identified a potential relationship between SARS-CoV-2 infection and the development of gastrointestinal cancers, sparking important discussions about the enduring health impacts of the virus. This review article highlighted the pressing need for ongoing research to elucidate the complex relationship between SARS-CoV-2 and gastrointestinal oncogenesis, providing valuable insights that can inform both coronavirus disease 2019 management strategies and cancer prevention efforts.
- Citation: Miteva DG, Gulinac M, Peruhova M, Velikova T. Exploring the oncogenic potential of SARS-CoV-2 in the gastrointestinal tract. World J Gastroenterol 2025; 31(31): 105665
- URL: https://www.wjgnet.com/1007-9327/full/v31/i31/105665.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i31.105665
The rapid spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has primarily caused respiratory distress but has also affected multiple organ systems, including the gastrointestinal (GI) tract[1]. The novel coronavirus responsible for the coronavirus disease 2019 (COVID-19) pandemic gains cellular entry by targeting specific receptors, particularly the angiotensin-converting enzyme 2 (ACE2) receptor. ACE2 receptors are abundantly expressed in the GI tract, presenting a plausible avenue for SARS-CoV-2 infection beyond the respiratory system[1].
Understanding the potential oncological implications of SARS-CoV-2 in the GI milieu requires a nuanced exploration of viral behavior in this anatomical domain[2]. Recent research has illuminated the viral tropism for GI tissues, raising intriguing questions about its impact on the delicate equilibrium of the GI ecosystem. Although COVID-19 primarily manifests as a respiratory illness, some patients also develop GI symptoms, highlighting the systemic impact of the virus and potential for long-term injuries[2].
The intricate interplay between SARS-CoV-2 and the GI tract involves a series of events, starting with viral entry and replication[2]. ACE2, the essential receptor for SARS-CoV-2, is expressed on the luminal surface of enterocytes in the small intestine and colon. Viral entry into host cells triggers a cascade of responses, potentially leading to direct cellular damage, inflammation, and immune modulation within the GI tract. These events pose crucial questions about the long-term consequences of SARS-CoV-2 infection on GI health, particularly regarding its oncogenic potential[2,3].
As the world grapples with the acute phases of the COVID-19 pandemic, a growing body of evidence suggests a plausible link between viral infections and cancer development. Several viruses, including hepatitis B and C, human papillomavirus, and Epstein-Barr virus, contribute to various malignancies. Given this context, the potential for SARS-CoV-2 to influence oncogenesis in the GI tract merits comprehensive investigation[3,4].
This comprehensive review aimed to synthesize existing knowledge regarding the interactions between SARS-CoV-2 and the GI environment with a focus on potential carcinogenic events. The molecular mechanisms governing viral entry, replication, and potential oncogenic effects were explored. Drawing insights from the available literature, the review delineated potential pathways linking SARS-CoV-2 to GI oncogenesis, critically assessing the existing evidence. Notably, we also aimed to identify gaps in the current understanding, emphasizing the need for further research to elucidate the complex relationships within the GI tract.
A comprehensive literature search was conducted to gather relevant articles for the review on the oncogenic potential of SARS-CoV-2 in the GI tract. The search was performed using multiple databases, including PubMed, Scopus, and Web of Science, to ensure a broad and inclusive collection of studies. The following key terms were used in the search: “SARS-CoV-2”, “oncogenic potential”, “gastrointestinal tract”, “cancer”, “GI malignancies”, “chronic inflammation”, “viral integration”, and “microbiome dysbiosis”. These terms were combined using Boolean operators as follows: (“SARS-CoV-2” OR “COVID-19”) AND (“oncogenic potential” OR “cancer” OR “GI malignancies”) AND (“gastrointestinal tract” OR “GI”) AND (“chronic inflammation” OR “inflammation”) AND (“viral integration” OR “viral genome”) AND (“microbiome dysbiosis” OR “gut microbiota dysbiosis”).
The inclusion criteria for selecting articles were: (1) Studies published in English; (2) Articles focusing on the GI tract and SARS-CoV-2 infection; (3) Research discussing the potential oncogenic effects of SARS-CoV-2, including mechanisms of inflammation, viral genome integration, and related pathways in GI malignancies; and (4) Clinical studies, systematic reviews, meta-analyses, and relevant preclinical studies. Exclusion criteria included studies unrelated to SARS-CoV-2 or the GI tract, articles not available in full text, or those not published in peer-reviewed journals, research not discussing the potential oncogenic effects of SARS-CoV-2 concerning the GI tract.
A total of 147 articles were initially identified, and after applying the inclusion and exclusion criteria, 131 articles were selected for detailed review. These articles provided the necessary evidence for evaluating the oncogenic implications of SARS-CoV-2 on the GI system. The search was last updated in November 2024, ensuring that the most current research was included in the review (n = 87 references cited in the paper).
Although SARS-CoV-2 primarily affects the respiratory system, the GI tract is also susceptible to infection[5]. SARS-CoV-2 internalizes in the host cell after ACE2 and/or TMPRSS2 receptors are recognized. SARS-CoV-2 demonstrates remarkable versatility in cellular entry mechanisms through diverse receptor families. These include proteoglycans (syndecan, glypican within the HSPG family), scavenger receptors (SR-B1), lectins (CD209 L/L-SIGN), and various transmembrane proteins such as CD147 (basigin), ASGR1, KREMEN1, NRP-1, AXL, and GRP78[6].
In addition to the role of virus entry, ACE2 is involved in the expression of antimicrobial peptides, the uptake of amino acids by intestinal epithelial cells, and the ecology of the gut microbiota[7]. According to recent studies of single-cell mRNA expression, enterocytes and mucus-producing cells have enriched expression of ACE2 and TMPRSS2. Moreover, ACE2 is highly expressed in the GI tract, particularly in the small intestine and pancreas[7].
The pathophysiological basis for infectious diarrhea and malabsorption disorders following SARS-CoV-2 infection lies in the dysregulation of intestinal ion transporters[8]. Studies also suggest that dysregulation of these transporters causes inflammation and GI symptoms[9]. In the GI system ACE2 serves critical physiological functions, particularly in nutrient absorption processes and the maintenance of ionic homeostasis. The receptor modulates sodium-coupled transport mechanisms for both amino acids and glucose at the enterocyte brush border, thereby contributing to osmotic regulation and electrolyte balance throughout the intestinal epithelium[10]. Consequently, the interaction of the virus with ACE2 receptors may compromise all these essential physiological processes.
Moreover, studies employing recombinant SARS-CoV-2 strains have shown that the virus can infect and replicate within human GI tissues[11]. Following cellular entry, viral replication and toxin-mediated cellular damage can trigger gastroenteritis-like manifestations, including diarrhea, nausea, vomiting, and abdominal pain[12]. Furthermore, the GI tract provides a favorable environment for SARS-CoV-2 replication due to the abundant expression of the ACE2 receptor found in mucosal glands and enterocytes[13]. Once the virus enters the GI cells, it can replicate and cause cell injury mediated by viral toxins.
The mechanism behind GI symptoms may involve disruption to the intestinal mucosal barrier and an increase in the generation of inflammatory substances. However, ACE2 receptors in the GI tract play a critical role in their genesis[14].
An early COVID-19 investigation involving 204 infected patients from Wuhan, China, who presented with characteristic respiratory manifestations, revealed that a significant proportion also experienced GI complications with diarrhea being the predominant symptom. The authors indicated that patients presenting with digestive manifestations demonstrated poorer clinical outcomes and required prolonged hospitalization compared with those without such symptoms[15]. The underlying cause of these symptoms is altered intestinal permeability and dysfunction of enterocytes[16].
In line with this elevated levels of fecal calprotectin in patients infected with SARS-CoV-2[17] indicate that SARS-CoV-2 triggers an inflammatory response in the gut. The mechanisms underlying GI manifestations in SARS-CoV-2 infection remain incompletely elucidated though evidence suggests compromised intestinal barrier function and alterations in gut microbial populations. Impairment of intestinal barrier integrity triggers the activation of both innate and adaptive immune responses, which release proinflammatory cytokines into the circulatory system, leading to systemic inflammation[17].
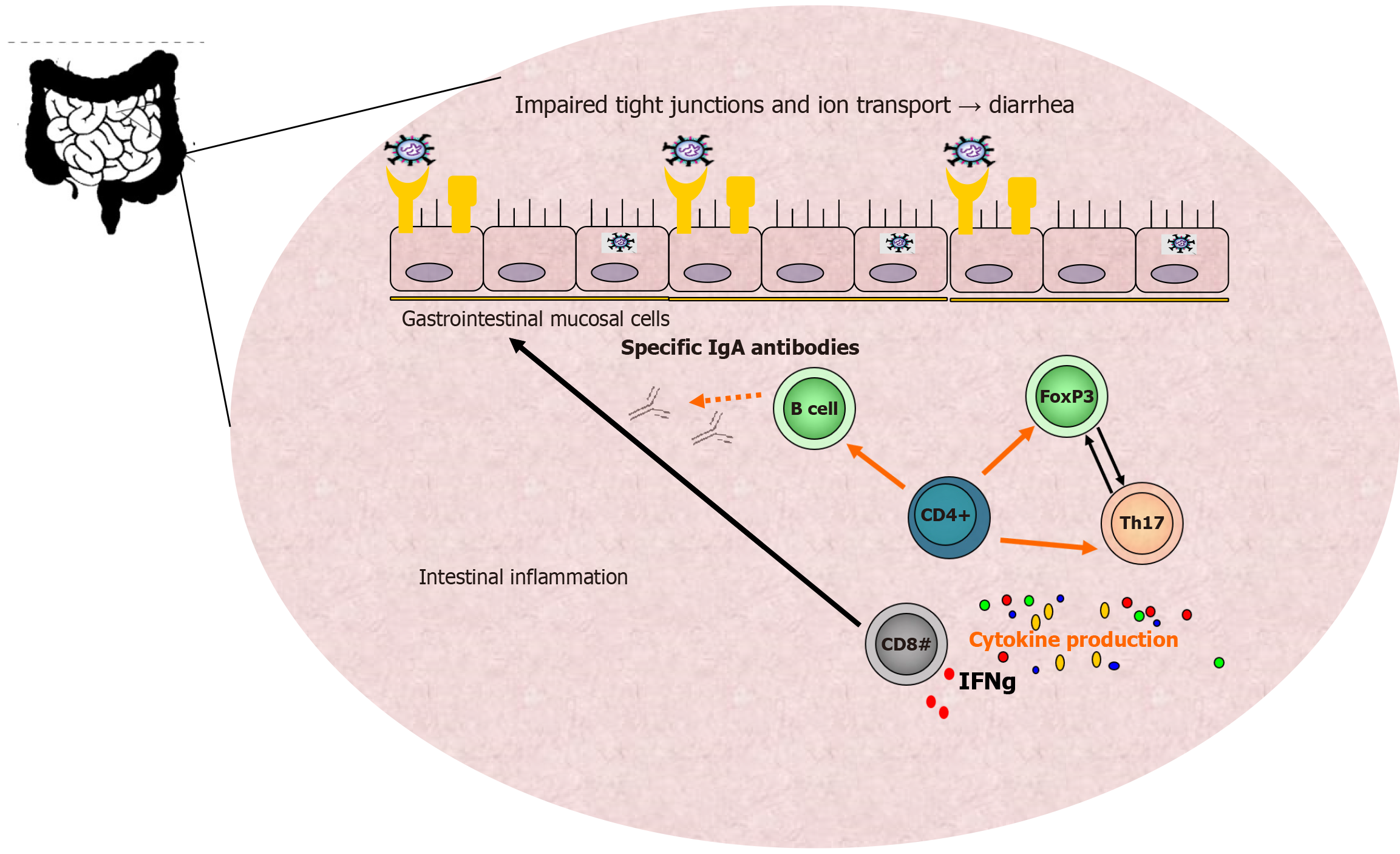
Various secondary signaling molecules prompt respiratory and GI mucosal cells to produce cytokines and display viral antigens through major histocompatibility complex class II molecules (human leukocyte antigens) to CD8+ cytotoxic T lymphocytes, which subsequently secrete additional cytokines, such as interferon (IFN)-gamma. Additionally, natural killer cells also aid in destroying infected host cells, alongside cytotoxic T cells, as part of the cellular immune response against every viral infection.
Through activation of B lymphocytes, T helper cells promote antibody production against specific SARS-CoV-2 antigens, contributing to the overall humoral immune response. Professional antigen-presenting cells, including macrophages and dendritic cells, display viral particles through human leukocyte antigen class II molecules and activate CD4+ T helper cells, which subsequently differentiate into various T helper subsets, including Th17 cells, that produce extensive cytokine arrays, resulting in the cytokine storm phenomena and acute respiratory distress syndrome[7,18].
The gut mucosal immune system plays a crucial role as the primary line of defense against physical and immunological threats. Additionally, GI involvement and symptoms in patients infected with SARS-CoV-2 have been associated with inferior clinical outcomes[18].
Studies show that GI symptoms occur in approximately 34% of patients with COVID-19. Loss of appetite and diarrhea are the predominant manifestations in adults while pediatric patients more commonly present with vomiting[19-21]. Loss of appetite, diarrhea, and nausea/vomiting represent the most frequently reported digestive tract symptoms[22].
A recent systematic review and meta-analysis involving 6686 patients with GI manifestations found that the overall prevalence is approximately 15% in SARS-CoV-2 infections. The same research indicated that reduced appetite occurred in a wide range of patients from 1% to 79% of cases[22]. The analysis also showed that the most common symptom was anorexia (26.8%), but the mechanism is still unknown. It is believed that the widespread disruption of taste and smell contribute to this phenomenon[23]. Hepatic damage has additionally been documented in certain patients, occurring in 39.6% to 43.4% of cases. The most commonly observed abnormalities include elevated levels of alanine aminotransferase and aspartate aminotransferase as well as reduced albumin concentrations[24].
In addition to the liver, SARS-CoV-2 can also affect other GI organs, including the small and large intestine, stomach, and pancreas. In line with this the COVID-19 pandemic has impacted the diagnosis of GI tumors. A nationwide survey by Buscarini et al[25] revealed a decline in GI cancer diagnoses with a 15.9% decrease in gastric cancer, 11.9% in colorectal cancer (CRC), and 9.9% in pancreatic cancer due to the pandemic[25].
Figure 1 summarizes the connection between the dysfunctional mucosa and some of the GI clinical symptoms of SARS-CoV-2 infection.
The error-prone RNA-dependent RNA polymerases of coronaviruses significantly cause viral genome mutations and recombination, which are strongly linked to viral adaptation[26]. Furthermore, evidence indicates that infectious agents contribute to cancer development and account for over 10% of human cancers[27,28]. SARS-CoV-2 infection may also serve as a risk factor for cancer development and recurrence. Research has demonstrated that SARS-CoV-2 infection and COVID-19 progression can significantly affect the immune system, inducing chronic inflammation and tissue damage and increasing the risk of cancer development[29,30]. Consequently, prolonged infection may contribute to both cancer emergence and progression. Accumulating data indicate that SARS-CoV-2 may function as a potential oncogenic risk factor. Available research on the carcinogenic influence of the virus encompasses immune evasion mechanisms, potential viral genome incorporation, genetic mutations, and epigenetic changes as well as sustained inflammation with associated oxidative stress[27-30].
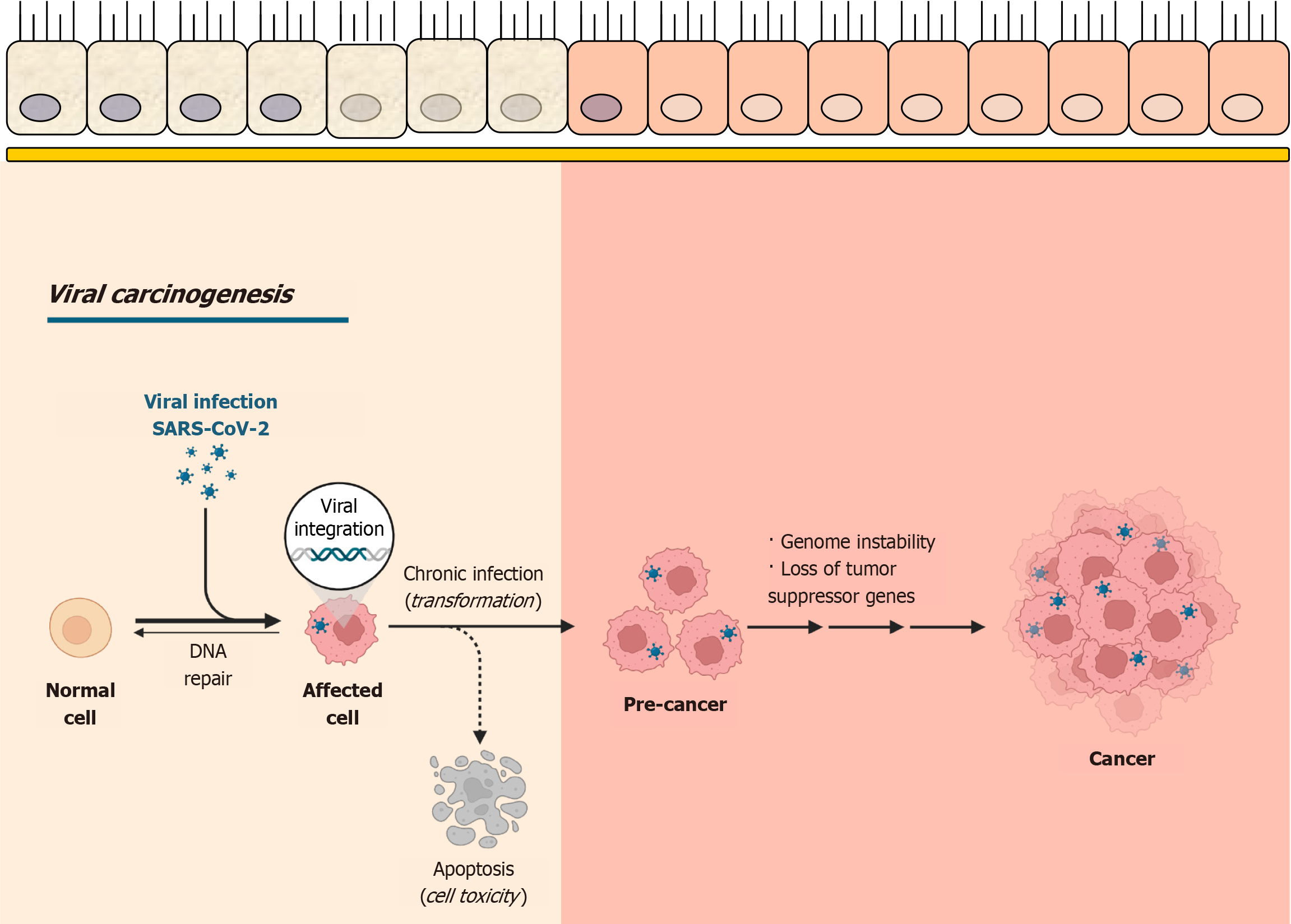
The integration of the viral genome (DNA and/or RNA) into the host cell DNA is well studied and induces mutations and the survival of the virus in cells[31]. In such altered and transformed cells, the cell cycle becomes disrupted, resulting in a state of uncontrolled cell division[32]. In addition to mutations epigenetic changes (such as DNA methylation, histone modification, and chromatin remodeling) occur due to viral integration, contributing to cancer development[33].
Data on SARS-CoV-2 integration into the human genome remain limited with only a few studies proposing potential hypotheses[34,35]. Some researchers have documented the prolonged presence of viral RNA in repeated PCR tests even after patients have recovered from COVID-19 and cannot be attributed to reinfection[36-38].
Viral RNA is reverse transcribed in human cells by reverse transcriptase from LINE elements[39]. Therefore, SARS-CoV-2 sequences may integrate into the host genome via LINE1 retrotransposition[34,40]. However, other studies suggest this is a hypothetical mechanism that rarely occurs in cells infected with the virus and does not occur in transfected cells[41,42].
The etiology of cancer is complex and involves both genetic and environmental factors[43]. Epigenetic modifications (i.e. DNA methylation, histone modifications, chromatin remodeling, and noncoding RNAs) include changes in gene expression without altering the underlying DNA sequence. Cancer cells exhibit changes in genomic information and the epigenome[44,45].
The epigenetic background contributes to changes in host susceptibility to SARS-CoV-2 infection and evasion of host immunity[46,47]. Since the ACE2 receptor plays a significant role in viral entry into host cells, the epigenetic regulation of ACE2 is crucial for infection spread, particularly given that the ACE2 receptor undergoes methylation[48,49].
Additionally, certain viral proteins interact with host enzymes, including histone deacetylases[50]. SARS-CoV-2 infection can also lead to the dysregulation of specific miRNAs and the suppression of toll-like receptor and IFN signaling pathways[51]. These findings suggest that SARS-CoV-2 infection may contribute to cancer development through epigenetic mechanisms that compromise immune responses.
Tumor dormancy is a critical phase in cancer development during which cells remain viable but stop proliferating[52]. Very often, dormant cancer cells (DCCs) can reactivate even though the initial treatment of the tumor was successful. Following SARS-CoV-2 infection, these dormant cells can become activated, leading to compromised innate immune cell function[53]. In severe COVID-19 there is pronounced neutrophil extracellular trap formation, which represents one of the primary DNA-based defense mechanisms against pathogens[54].
Certain cancers are associated with chronic inflammation, whereas others commonly develop from high cytokine concentrations[55,56]. A cytokine storm leads to systemic inflammation with tumor necrosis factor α and interleukin 6 as the primary participants, which may activate different oncogenic transcriptional factors[57-59]. Therefore, tumor necrosis factor α and interleukin 6 are the most effective inflammatory drivers for cancer growth. Neutrophil extracellular trap disruption and cytokine storms associated with SARS-CoV-2 infection may be responsible for the activation of DCC. Another possibility is that DCC activation may result from a prolonged host immune response to SARS-CoV-2, ultimately leading to immune system exhaustion[60].
The persistence of the virus in the evolutionary course depends on its ability to enter the host cell, gain access to the genetic material, and utilize its cellular machinery to generate new progeny viruses. Early in the COVID-19 pandemic, when the naïve host population was most significant, the virus had an evolutionary advantage over wild-type variants[61-63]. Subsequently, as immunity in the host population increased, new variants emerged with accumulated mutations that contributed to antigenic novelty and immune evasion[64-68]. These mutations are suggested to occur partly in the context of chronic infections[69].
Additionally, immunocompromised individuals exhibit prolonged shedding of SARS-CoV-2[70,71]. Based on amino acid substitutions observed in individuals with chronic infections, it has been suggested that such infections contribute to the emergence of SARS-CoV-2 variants[72]. Evidence supporting these observations includes the sets of mutations identified from chronic infection samples that are shared by variants of concern[73,74]. Viral mutations enable these pathogens to evade the human immune system and facilitate their replication[75]. Viral immune escape is an evo
Table 1 provides an overview of the key mechanisms by which SARS-CoV-2 may contribute to oncogenesis in the GI tract, summarizing the potential pathways involved, their biological implications, and associated clinical consequences.
| Key mechanisms | Description | Potential implications |
| SARS-CoV-2 viral entry and replication in GI tract | SARS-CoV-2 utilizes ACE2 receptors for cellular entry in the GI tract, triggering intracellular responses | Alteration of GI homeostasis, increased viral persistence, and systemic inflammation |
| Chronic inflammation and immune dysregulation | Persistent inflammation can lead to genetic instability, cell transformation, and increased cancer risk | Long-term tissue damage, enhanced susceptibility to malignancies |
| Potential viral integration into host DNA | Hypothetical integration of viral RNA into host DNA may cause genetic mutations and epigenetic alterations | Potential genomic instability, requiring further investigation |
| Gut microbiome dysbiosis and GI malignancy risk | Gut microbiota disruption linked to colorectal cancer progression due to immune suppression and chronic inflammation | Possible link between COVID-19 recovery and long-term cancer risk |
| Immune escape and oncogenesis | Mutations enabling immune escape may promote persistent viral presence and tumorigenesis | Emergence of new variants with oncogenic potential |
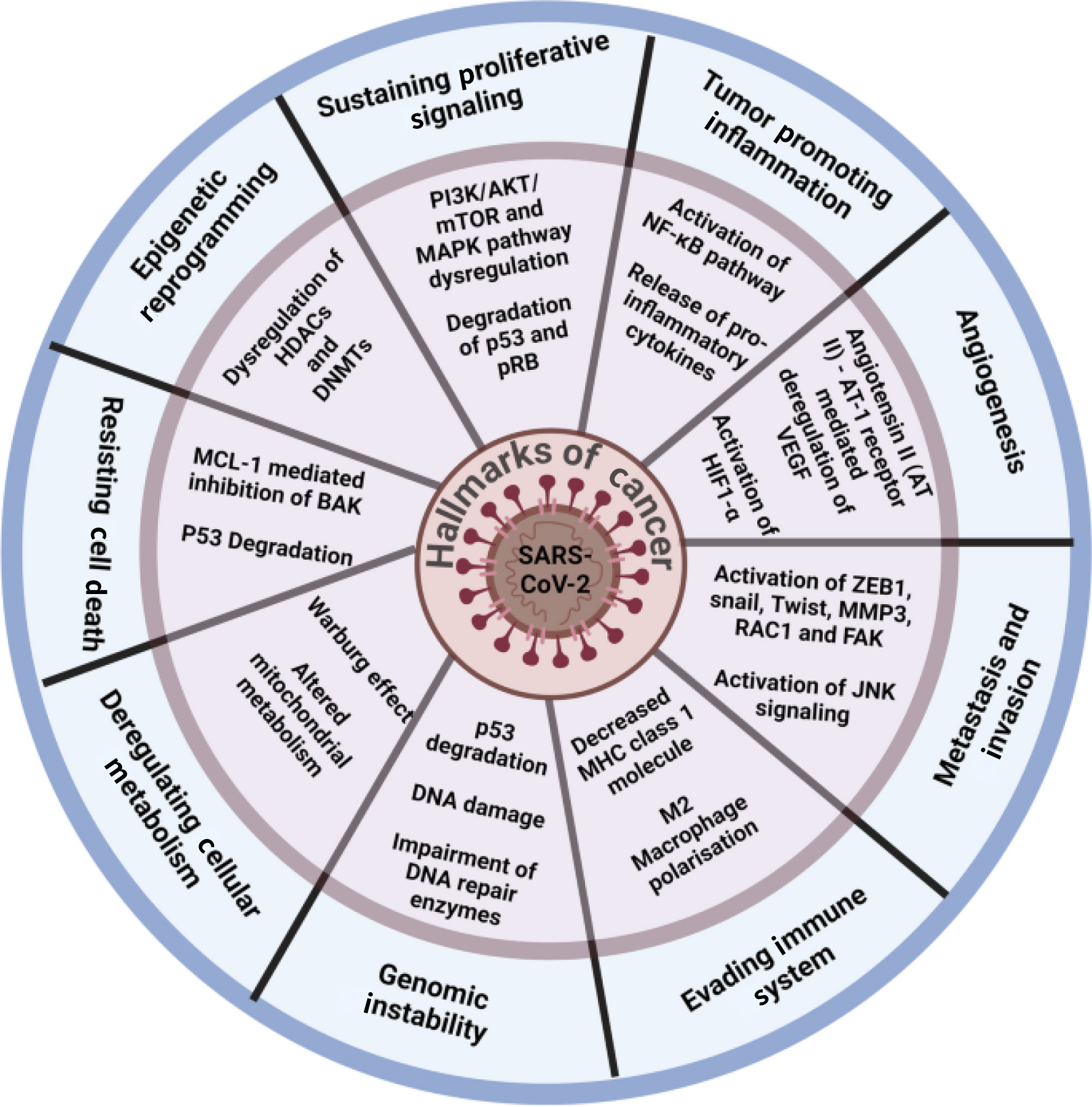
The latest research has shown that SARS-CoV-2 contributes to various hallmarks of cancer pathways, including the phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin pathway and the nuclear factor-κB pathway, among others[80].
SARS-CoV-2 influences all cancer-related hallmarks, such as genomic instability (by p53 degradation, DNA damage, impairment of DNA repair enzymes), evading immune system (i.e. decreasing major histocompatibility complex class I molecules, M2 macrophages polarization), metastasis and invasion (i.e. activation of ZEB1, snail, twist, MMP3, RAC1, FAK, activation of JNK signaling), angiogenesis [angiotensin II-angiotensin I receptor-mediated deregulation of VEGF, activation of HIF1-a], deregulated cellular metabolism (i.e. Warburg effect, altered mitochondrial metabolism), resisting cell death (MCL-1 mediated inhibition of BASSK, p53 degradation), epigenetic reprogramming (dysregulation of HDACs and DNMTs), sustaining proliferative signaling (phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin and MAPK pathway dysregulation, degradation of p53 and pRB), tumor promoting inflammation (activation of nuclear factor-kB pathway, release of proinflammatory cytokines), etc[80]. Figure 2 demonstrates the impact of SARS-CoV-2 on key cancer hallmarks involved in carcinogenic mechanisms.
As shown in Figure 3, SARS-CoV-2 infection can lead to viral integration, chronic infection, and cellular trans
Dysbiosis is defined as the overgrowth of pathogenic microbes and the loss of healthy commensal bacteria. It is linked to an increased risk of sepsis, multiorgan failure, and nosocomial infections[81]. Gut microbiota alterations in SARS-CoV-2 infection depend on the presence of the virus, COVID-19 medications, and GI symptoms of the disease[82,83]. Therefore, fecal samples from recovered patients should be monitored for at least 35 days after respiratory viral clearance since SARS-CoV-2 can remain detectable in stool specimens for prolonged periods[84].
Furthermore, evaluating intestinal microbiome composition and function is recommended to assess microbial homeostasis. Angelakis et al[85] demonstrated that prolonged treatment with doxycycline and hydroxychloroquine resulted in gut microbiome disruptions, characterized by substantial reductions in Bacteroidetes, Firmicutes, and Lactobacillus populations. Similar microbial imbalances may develop in patients with COVID-19, potentially leading to intestinal dysbiosis.
Howell et al[86] demonstrated that SARS-CoV-2-induced gut microbiota dysbiosis could be a risk factor for CRC development. Gut dysbiosis can be long-lasting, increasing the likelihood of future CRC diagnosis or exacerbating the disease in those already affected[86].
Furthermore, studies have demonstrated that SARS-CoV-2 infection alters the gut microbiota by reducing overall microbial diversity, increasing opportunistic pathogens (with complications such as Fusobacterium nucleatum bacteremia), and decreasing beneficial commensals, including butyrate-producing bacteria. Moreover, these alterations result in increased colonic inflammation, which in turn disrupts the gut barrier, expression of genes controlling CRC tumorigenesis, and tumor immunosuppression, all of which worsen the progression of CRC[86].
In line with this Odun-Ayo and Reddy[87] focused on CRC-associated gut dysbiosis. Recent research indicates that the immune-modulatory response to probiotics is shifting their beneficial use toward treating various diseases. The long-term impact of SARS-CoV-2-induced gut dysbiosis, mediated through microbiota-gut-lung interactions, may increase the risk of CRC or worsen existing cases[87].
While the current body of research provides valuable insights into the oncogenic potential of SARS-CoV-2, several limitations and gaps must be addressed to ensure a comprehensive understanding of this phenomenon. One of the most significant limitations is the relatively small sample sizes in many studies that restricts the generalizability of the findings. The majority of studies reviewed focused on specific populations or limited geographic regions, making it challenging to draw broad conclusions about the global impact of SARS-CoV-2 on cancer development.
Another common limitation is the presence of biases, particularly selection bias and publication bias. Many studies have been based on data from hospital settings or case reports, which may not accurately reflect the broader population. Additionally, there is often a lack of long-term follow-up data, which is essential for understanding the chronic effects of SARS-CoV-2 infection and its potential role in cancer development over time.
Furthermore, variations in research methodologies and diagnostic criteria across studies complicate the comparison of results. Differences in the definition of GI malignancies as well as the timing and type of SARS-CoV-2 exposure may contribute to inconsistent findings. The absence of standardized protocols for assessing oncogenic potential also adds to the complexity of synthesizing data from diverse studies.
Future research should address these limitations and focus on larger, multicenter studies with more diverse patient populations, standardized diagnostic criteria, and longer follow-up periods. These efforts would help clarify the long-term implications of SARS-CoV-2 infection on cancer risk and improve the accuracy and reliability of findings in this important area of research.
Future research on the oncogenic potential of SARS-CoV-2 should focus on addressing the gaps identified in the current literature, particularly by expanding the scope of studies to include a broader range of GI cancers. For example investigating the potential links between SARS-CoV-2 and other GI malignancies, such as pancreatic cancer, gastric cancer, and hepatocellular carcinoma, would significantly contribute to our understanding of the impact virus on different types of GI cancers. Furthermore, studies investigating the long-term effects of SARS-CoV-2 infection, particularly concerning cancer recurrence and progression, are necessary.
Research should also focus on elucidating the molecular mechanisms by which the virus may influence tumorigenesis, including its effects on immune modulation, inflammation, and microbiome dysbiosis. To strengthen the evidence base, future studies should include larger and more diverse patient cohorts and employ standardized methodologies to enhance the comparability and generalizability of findings. Such studies will enable researchers to comprehensively explore the oncogenic potential of SARS-CoV-2, ultimately advancing therapeutic strategies and preventive measures for patients at risk of developing virus-associated cancer.
Expanding research into the potential oncological implications of SARS-CoV-2 infection in the GI tract emphasizes the importance of ongoing investigation. Key mechanisms, including chronic inflammation, viral integration, and cellular dysregulation, offer insights into the link between COVID-19 and GI malignancies. As we continue to address the ongoing challenges of the pandemic, understanding these connections has significant implications for both immediate patient care and long-term health outcomes. This review emphasized the need for further research into this complex relationship to inform comprehensive approaches to patient management and illuminate the intricate mechanisms of SARS-CoV-2-associated oncogenesis. Moreover, this review advocated for a comprehensive understanding of the long-term effects of SARS-CoV-2 and encouraged future studies exploring the role of the virus in the progression of various GI cancers, including pancreatic, gastric, and hepatocellular carcinoma.
We want to thank Jelena Gulinac, M.Phil. (English), Faculty of Pedagogy, Paisii Hilendarski University of Plovdiv, Bulgaria, for her meticulous work in improving the language and style of this manuscript.
| 1. | Habibzadeh P, Dastsooz H, Eshraghi M, Łos MJ, Klionsky DJ, Ghavami S. Autophagy: The Potential Link between SARS-CoV-2 and Cancer. Cancers (Basel). 2021;13:5721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 2. | Amiama-Roig A, Pérez-Martínez L, Rodríguez Ledo P, Verdugo-Sivianes EM, Blanco JR. Should We Expect an Increase in the Number of Cancer Cases in People with Long COVID? Microorganisms. 2023;11:713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Stingi A, Cirillo L. SARS-CoV-2 infection and cancer: Evidence for and against a role of SARS-CoV-2 in cancer onset. Bioessays. 2021;43:e2000289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Ogarek N, Oboza P, Olszanecka-Glinianowicz M, Kocelak P. SARS-CoV-2 infection as a potential risk factor for the development of cancer. Front Mol Biosci. 2023;10:1260776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 5. | Velikova TV, Kotsev SV, Georgiev DS, Batselova HM. Immunological aspects of COVID-19: What do we know? World J Biol Chem. 2020;11:14-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 6. | Avdonin PP, Rybakova EY, Trufanov SK, Avdonin PV. SARS-CoV-2 Receptors and Their Involvement in Cell Infection. Biochem (Mosc) Suppl Ser A Membr Cell Biol. 2023;17:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 7. | Velikova T, Snegarova V, Kukov A, Batselova H, Mihova A, Nakov R. Gastrointestinal mucosal immunity and COVID-19. World J Gastroenterol. 2021;27:5047-5059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Das S, Jayaratne R, Barrett KE. The Role of Ion Transporters in the Pathophysiology of Infectious Diarrhea. Cell Mol Gastroenterol Hepatol. 2018;6:33-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 9. | Sueyoshi R, Ignatoski KM, Daignault S, Okawada M, Teitelbaum DH. Angiotensin converting enzyme-inhibitor reduces colitis severity in an IL-10 knockout model. Dig Dis Sci. 2013;58:3165-3177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Hashimoto T, Perlot T, Rehman A, Trichereau J, Ishiguro H, Paolino M, Sigl V, Hanada T, Hanada R, Lipinski S, Wild B, Camargo SM, Singer D, Richter A, Kuba K, Fukamizu A, Schreiber S, Clevers H, Verrey F, Rosenstiel P, Penninger JM. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 787] [Cited by in RCA: 988] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 11. | Lamers MM, Beumer J, van der Vaart J, Knoops K, Puschhof J, Breugem TI, Ravelli RBG, Paul van Schayck J, Mykytyn AZ, Duimel HQ, van Donselaar E, Riesebosch S, Kuijpers HJH, Schipper D, van de Wetering WJ, de Graaf M, Koopmans M, Cuppen E, Peters PJ, Haagmans BL, Clevers H. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020;369:50-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1245] [Cited by in RCA: 1311] [Article Influence: 262.2] [Reference Citation Analysis (0)] |
| 12. | Haga S, Yamamoto N, Nakai-Murakami C, Osawa Y, Tokunaga K, Sata T, Yamamoto N, Sasazuki T, Ishizaka Y. Modulation of TNF-alpha-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-alpha production and facilitates viral entry. Proc Natl Acad Sci U S A. 2008;105:7809-7814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 452] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 13. | Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS. Cryo-EM Structure of the 2019-nCoV Spike in the Prefusion Conformation. bioRxiv. 2020:2020.02.11.944462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 113] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 14. | Ye Q, Wang B, Zhang T, Xu J, Shang S. The mechanism and treatment of gastrointestinal symptoms in patients with COVID-19. Am J Physiol Gastrointest Liver Physiol. 2020;319:G245-G252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 114] [Article Influence: 22.8] [Reference Citation Analysis (2)] |
| 15. | Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, Li P, Hu B, Wang J, Hu C, Jin Y, Niu X, Ping R, Du Y, Li T, Xu G, Hu Q, Tu L. Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am J Gastroenterol. 2020;115:766-773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1160] [Cited by in RCA: 1205] [Article Influence: 241.0] [Reference Citation Analysis (0)] |
| 16. | Jin X, Lian JS, Hu JH, Gao J, Zheng L, Zhang YM, Hao SR, Jia HY, Cai H, Zhang XL, Yu GD, Xu KJ, Wang XY, Gu JQ, Zhang SY, Ye CY, Jin CL, Lu YF, Yu X, Yu XP, Huang JR, Xu KL, Ni Q, Yu CB, Zhu B, Li YT, Liu J, Zhao H, Zhang X, Yu L, Guo YZ, Su JW, Tao JJ, Lang GJ, Wu XX, Wu WR, Qv TT, Xiang DR, Yi P, Shi D, Chen Y, Ren Y, Qiu YQ, Li LJ, Sheng J, Yang Y. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69:1002-1009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 944] [Cited by in RCA: 870] [Article Influence: 174.0] [Reference Citation Analysis (0)] |
| 17. | Golonka RM, Saha P, Yeoh BS, Chattopadhyay S, Gewirtz AT, Joe B, Vijay-Kumar M. Harnessing innate immunity to eliminate SARS-CoV-2 and ameliorate COVID-19 disease. Physiol Genomics. 2020;52:217-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 18. | Miteva D, Peshevska-Sekulovska M, Snegarova V, Batselova H, Alexandrova R, Velikova T. Mucosal COVID-19 vaccines: Risks, benefits and control of the pandemic. World J Virol. 2022;11:221-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 19. | Lazova S, Tomov L, Miteva D, Tzotcheva I, Priftis S, Velikova T. Clinical and Laboratory Manifestation of Gastrointestinal Involvement in MIS-C: A Single-Center Observational Study. Gastroenterol Insights. 2023;14:236-248. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Lazova S, Alexandrova T, Gorelyova-Stefanova N, Atanasov K, Tzotcheva I, Velikova T. Liver Involvement in Children with COVID-19 and Multisystem Inflammatory Syndrome: A Single-Center Bulgarian Observational Study. Microorganisms. 2021;9:1958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Cheung KS, Hung IFN, Chan PPY, Lung KC, Tso E, Liu R, Ng YY, Chu MY, Chung TWH, Tam AR, Yip CCY, Leung KH, Fung AY, Zhang RR, Lin Y, Cheng HM, Zhang AJX, To KKW, Chan KH, Yuen KY, Leung WK. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples From a Hong Kong Cohort: Systematic Review and Meta-analysis. Gastroenterology. 2020;159:81-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1113] [Cited by in RCA: 1132] [Article Influence: 226.4] [Reference Citation Analysis (1)] |
| 22. | Mao R, Qiu Y, He JS, Tan JY, Li XH, Liang J, Shen J, Zhu LR, Chen Y, Iacucci M, Ng SC, Ghosh S, Chen MH. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:667-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 741] [Cited by in RCA: 754] [Article Influence: 150.8] [Reference Citation Analysis (0)] |
| 23. | Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, Dequanter D, Blecic S, El Afia F, Distinguin L, Chekkoury-Idrissi Y, Hans S, Delgado IL, Calvo-Henriquez C, Lavigne P, Falanga C, Barillari MR, Cammaroto G, Khalife M, Leich P, Souchay C, Rossi C, Journe F, Hsieh J, Edjlali M, Carlier R, Ris L, Lovato A, De Filippis C, Coppee F, Fakhry N, Ayad T, Saussez S. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277:2251-2261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1729] [Cited by in RCA: 1738] [Article Influence: 347.6] [Reference Citation Analysis (0)] |
| 24. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14869] [Cited by in RCA: 12973] [Article Influence: 2594.6] [Reference Citation Analysis (1)] |
| 25. | Buscarini E, Benedetti A, Monica F, Pasquale L, Buttitta F, Cameletti M, Ferrari C, Ricciardiello L; FISMAD: the FISMAD-ALERT Survey Group. Changes in digestive cancer diagnosis during the SARS-CoV-2 pandemic in Italy: A nationwide survey. Dig Liver Dis. 2021;53:682-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 26. | De Soto J, Hakim S, Boyd F. The Pathophysiology of Virulence of the COVID-19 Virus. 2020 Preprint. Available from: Preprints:2020040077. [DOI] [Full Text] |
| 27. | Schiller JT, Lowy DR. An Introduction to Virus Infections and Human Cancer. Recent Results Cancer Res. 2021;217:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 28. | Jafarzadeh A, Gosain R, Mortazavi SMJ, Nemati M, Jafarzadeh S, Ghaderi A. SARS-CoV-2 Infection: A Possible Risk Factor for Incidence and Recurrence of Cancers. Int J Hematol Oncol Stem Cell Res. 2022;16:117-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 29. | Marshall M. The lasting misery of coronavirus long-haulers. Nature. 2020;585:339-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 190] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 30. | Costanzo M, De Giglio MAR, Roviello GN. Deciphering the Relationship between SARS-CoV-2 and Cancer. Int J Mol Sci. 2023;24:7803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 31. | da Mata Kanzaki ECG, Kanzaki I. Viral Genome Integration into the Host Cell Genome: A Double Edged-Sword. Discov Med. 2021;32:141-148. [PubMed] |
| 32. | Akram N, Imran M, Noreen M, Ahmed F, Atif M, Fatima Z, Bilal Waqar A. Oncogenic Role of Tumor Viruses in Humans. Viral Immunol. 2017;30:20-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 33. | Pietropaolo V, Prezioso C, Moens U. Role of Virus-Induced Host Cell Epigenetic Changes in Cancer. Int J Mol Sci. 2021;22:8346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 34. | Zhang L, Richards A, Barrasa MI, Hughes SH, Young RA, Jaenisch R. Reverse-transcribed SARS-CoV-2 RNA can integrate into the genome of cultured human cells and can be expressed in patient-derived tissues. Proc Natl Acad Sci U S A. 2021;118:e2105968118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 158] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 35. | Grandi N, Tramontano E, Berkhout B. Integration of SARS-CoV-2 RNA in infected human cells by retrotransposons: an unlikely hypothesis and old viral relationships. Retrovirology. 2021;18:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Yuan B, Liu HQ, Yang ZR, Chen YX, Liu ZY, Zhang K, Wang C, Li WX, An YW, Wang JC, Song S. Recurrence of positive SARS-CoV-2 viral RNA in recovered COVID-19 patients during medical isolation observation. Sci Rep. 2020;10:11887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 37. | Yahav D, Yelin D, Eckerle I, Eberhardt CS, Wang J, Cao B, Kaiser L. Definitions for coronavirus disease 2019 reinfection, relapse and PCR re-positivity. Clin Microbiol Infect. 2021;27:315-318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 138] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 38. | Wu X, Wang Z, He Z, Li Y, Wu Y, Wang H, Liu Y, Hao F, Tian H. A follow-up study shows that recovered patients with re-positive PCR test in Wuhan may not be infectious. BMC Med. 2021;19:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 39. | Kazazian HH Jr, Moran JV. Mobile DNA in Health and Disease. N Engl J Med. 2017;377:361-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 304] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 40. | Zhang L, Bisht P, Flamier A, Barrasa MI, Friesen M, Richards A, Hughes SH, Jaenisch R. LINE1-Mediated Reverse Transcription and Genomic Integration of SARS-CoV-2 mRNA Detected in Virus-Infected but Not in Viral mRNA-Transfected Cells. Viruses. 2023;15:629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 41. | Al-Eitan L, Mihyar A. The controversy of SARS-CoV-2 integration into the human genome. Rev Med Virol. 2024;34:e2511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 42. | Briggs E, Ward W, Rey S, Law D, Nelson K, Bois M, Ostrov N, Lee HH, Laurent JM, Mita P. Assessment of potential SARS-CoV-2 virus integration into human genome reveals no significant impact on RT-qPCR COVID-19 testing. Proc Natl Acad Sci U S A. 2021;118:e2113065118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 43. | Kanwal R, Gupta S. Epigenetic modifications in cancer. Clin Genet. 2012;81:303-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 350] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 44. | Seligson DB, Horvath S, Shi T, Yu H, Tze S, Grunstein M, Kurdistani SK. Global histone modification patterns predict risk of prostate cancer recurrence. Nature. 2005;435:1262-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 796] [Cited by in RCA: 773] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 45. | Ben-Porath I, Cedar H. Epigenetic crosstalk. Mol Cell. 2001;8:933-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 46. | Chlamydas S, Papavassiliou AG, Piperi C. Epigenetic mechanisms regulating COVID-19 infection. Epigenetics. 2021;16:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 94] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 47. | AbdelHamid SG, Refaat AA, Benjamin AM, Elmawardy LA, Elgendy LA, Manolly MM, Elmaksoud NA, Sherif N, Hamdy NM. Deciphering epigenetic(s) role in modulating susceptibility to and severity of COVID-19 infection and/or outcome: a systematic rapid review. Environ Sci Pollut Res Int. 2021;28:54209-54221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 48. | Bhat S, Rishi P, Chadha VD. Understanding the epigenetic mechanisms in SARS CoV-2 infection and potential therapeutic approaches. Virus Res. 2022;318:198853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 49. | Pinto BGG, Oliveira AER, Singh Y, Jimenez L, Gonçalves ANA, Ogava RLT, Creighton R, Schatzmann Peron JP, Nakaya HI. ACE2 Expression Is Increased in the Lungs of Patients With Comorbidities Associated With Severe COVID-19. J Infect Dis. 2020;222:556-563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 262] [Cited by in RCA: 268] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 50. | Behura A, Naik L, Patel S, Das M, Kumar A, Mishra A, Nayak DK, Manna D, Mishra A, Dhiman R. Involvement of epigenetics in affecting host immunity during SARS-CoV-2 infection. Biochim Biophys Acta Mol Basis Dis. 2023;1869:166634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 51. | Khan MA, Sany MRU, Islam MS, Islam ABMMK. Epigenetic Regulator miRNA Pattern Differences Among SARS-CoV, SARS-CoV-2, and SARS-CoV-2 World-Wide Isolates Delineated the Mystery Behind the Epic Pathogenicity and Distinct Clinical Characteristics of Pandemic COVID-19. Front Genet. 2020;11:765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 132] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 52. | Sosa MS, Bragado P, Aguirre-Ghiso JA. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer. 2014;14:611-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 743] [Cited by in RCA: 854] [Article Influence: 77.6] [Reference Citation Analysis (0)] |
| 53. | Anderson NM, Simon MC. The tumor microenvironment. Curr Biol. 2020;30:R921-R925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 1528] [Article Influence: 382.0] [Reference Citation Analysis (0)] |
| 54. | Barnes BJ, Adrover JM, Baxter-Stoltzfus A, Borczuk A, Cools-Lartigue J, Crawford JM, Daßler-Plenker J, Guerci P, Huynh C, Knight JS, Loda M, Looney MR, McAllister F, Rayes R, Renaud S, Rousseau S, Salvatore S, Schwartz RE, Spicer JD, Yost CC, Weber A, Zuo Y, Egeblad M. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J Exp Med. 2020;217:e20200652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1054] [Cited by in RCA: 1104] [Article Influence: 220.8] [Reference Citation Analysis (0)] |
| 55. | Grivennikov SI, Karin M. Inflammation and oncogenesis: a vicious connection. Curr Opin Genet Dev. 2010;20:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 344] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 56. | Grivennikov SI, Karin M. Inflammatory cytokines in cancer: tumour necrosis factor and interleukin 6 take the stage. Ann Rheum Dis. 2011;70 Suppl 1:i104-i108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 433] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 57. | Aggarwal BB, Vijayalekshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res. 2009;15:425-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 560] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 58. | Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE Jr. Stat3 as an oncogene. Cell. 1999;98:295-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2236] [Cited by in RCA: 2346] [Article Influence: 90.2] [Reference Citation Analysis (0)] |
| 59. | Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883-899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8437] [Cited by in RCA: 8176] [Article Influence: 545.1] [Reference Citation Analysis (0)] |
| 60. | Alahdal M, Elkord E. Exhaustion and over-activation of immune cells in COVID-19: Challenges and therapeutic opportunities. Clin Immunol. 2022;245:109177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 61. | Ahmad A, Fawaz MAM, Aisha A. A comparative overview of SARS-CoV-2 and its variants of concern. Infez Med. 2022;30:328-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 62. | Campbell F, Archer B, Laurenson-Schafer H, Jinnai Y, Konings F, Batra N, Pavlin B, Vandemaele K, Van Kerkhove MD, Jombart T, Morgan O, le Polain de Waroux O. Increased transmissibility and global spread of SARS-CoV-2 variants of concern as at June 2021. Euro Surveill. 2021;26:2100509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 479] [Cited by in RCA: 561] [Article Influence: 140.3] [Reference Citation Analysis (0)] |
| 63. | Davies NG, Abbott S, Barnard RC, Jarvis CI, Kucharski AJ, Munday JD, Pearson CAB, Russell TW, Tully DC, Washburne AD, Wenseleers T, Gimma A, Waites W, Wong KLM, van Zandvoort K, Silverman JD; CMMID COVID-19 Working Group; COVID-19 Genomics UK (COG-UK) Consortium, Diaz-Ordaz K, Keogh R, Eggo RM, Funk S, Jit M, Atkins KE, Edmunds WJ. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021;372:eabg3055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1994] [Cited by in RCA: 1668] [Article Influence: 417.0] [Reference Citation Analysis (0)] |
| 64. | Harvey WT, Carabelli AM, Jackson B, Gupta RK, Thomson EC, Harrison EM, Ludden C, Reeve R, Rambaut A; COVID-19 Genomics UK (COG-UK) Consortium, Peacock SJ, Robertson DL. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19:409-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2349] [Cited by in RCA: 2390] [Article Influence: 597.5] [Reference Citation Analysis (0)] |
| 65. | Willett BJ, Grove J, MacLean OA, Wilkie C, De Lorenzo G, Furnon W, Cantoni D, Scott S, Logan N, Ashraf S, Manali M, Szemiel A, Cowton V, Vink E, Harvey WT, Davis C, Asamaphan P, Smollett K, Tong L, Orton R, Hughes J, Holland P, Silva V, Pascall DJ, Puxty K, da Silva Filipe A, Yebra G, Shaaban S, Holden MTG, Pinto RM, Gunson R, Templeton K, Murcia PR, Patel AH, Klenerman P, Dunachie S; PITCH Consortium; COVID-19 Genomics UK (COG-UK) Consortium, Haughney J, Robertson DL, Palmarini M, Ray S, Thomson EC. SARS-CoV-2 Omicron is an immune escape variant with an altered cell entry pathway. Nat Microbiol. 2022;7:1161-1179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 423] [Cited by in RCA: 429] [Article Influence: 143.0] [Reference Citation Analysis (0)] |
| 66. | Dolton G, Rius C, Hasan MS, Wall A, Szomolay B, Behiry E, Whalley T, Southgate J, Fuller A; COVID-19 Genomics UK (COG-UK) consortium, Morin T, Topley K, Tan LR, Goulder PJR, Spiller OB, Rizkallah PJ, Jones LC, Connor TR, Sewell AK. Emergence of immune escape at dominant SARS-CoV-2 killer T cell epitope. Cell. 2022;185:2936-2951.e19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 74] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 67. | Mlcochova P, Kemp SA, Dhar MS, Papa G, Meng B, Ferreira IATM, Datir R, Collier DA, Albecka A, Singh S, Pandey R, Brown J, Zhou J, Goonawardane N, Mishra S, Whittaker C, Mellan T, Marwal R, Datta M, Sengupta S, Ponnusamy K, Radhakrishnan VS, Abdullahi A, Charles O, Chattopadhyay P, Devi P, Caputo D, Peacock T, Wattal C, Goel N, Satwik A, Vaishya R, Agarwal M; Indian SARS-CoV-2 Genomics Consortium (INSACOG); Genotype to Phenotype Japan (G2P-Japan) Consortium; CITIID-NIHR BioResource COVID-19 Collaboration, Mavousian A, Lee JH, Bassi J, Silacci-Fegni C, Saliba C, Pinto D, Irie T, Yoshida I, Hamilton WL, Sato K, Bhatt S, Flaxman S, James LC, Corti D, Piccoli L, Barclay WS, Rakshit P, Agrawal A, Gupta RK. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature. 2021;599:114-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 952] [Cited by in RCA: 929] [Article Influence: 232.3] [Reference Citation Analysis (0)] |
| 68. | Davis C, Logan N, Tyson G, Orton R, Harvey WT, Perkins JS, Mollett G, Blacow RM; COVID-19 Genomics UK (COG-UK) Consortium, Peacock TP, Barclay WS, Cherepanov P, Palmarini M, Murcia PR, Patel AH, Robertson DL, Haughney J, Thomson EC, Willett BJ; COVID-19 DeplOyed VaccinE (DOVE) Cohort Study investigators. Reduced neutralisation of the Delta (B.1.617.2) SARS-CoV-2 variant of concern following vaccination. PLoS Pathog. 2021;17:e1010022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 116] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 69. | Carabelli AM, Peacock TP, Thorne LG, Harvey WT, Hughes J; COVID-19 Genomics UK Consortium, Peacock SJ, Barclay WS, de Silva TI, Towers GJ, Robertson DL. SARS-CoV-2 variant biology: immune escape, transmission and fitness. Nat Rev Microbiol. 2023;21:162-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 286] [Cited by in RCA: 443] [Article Influence: 221.5] [Reference Citation Analysis (0)] |
| 70. | Choi B, Choudhary MC, Regan J, Sparks JA, Padera RF, Qiu X, Solomon IH, Kuo HH, Boucau J, Bowman K, Adhikari UD, Winkler ML, Mueller AA, Hsu TY, Desjardins M, Baden LR, Chan BT, Walker BD, Lichterfeld M, Brigl M, Kwon DS, Kanjilal S, Richardson ET, Jonsson AH, Alter G, Barczak AK, Hanage WP, Yu XG, Gaiha GD, Seaman MS, Cernadas M, Li JZ. Persistence and Evolution of SARS-CoV-2 in an Immunocompromised Host. N Engl J Med. 2020;383:2291-2293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 860] [Cited by in RCA: 935] [Article Influence: 187.0] [Reference Citation Analysis (0)] |
| 71. | Clark SA, Clark LE, Pan J, Coscia A, McKay LGA, Shankar S, Johnson RI, Brusic V, Choudhary MC, Regan J, Li JZ, Griffiths A, Abraham J. SARS-CoV-2 evolution in an immunocompromised host reveals shared neutralization escape mechanisms. Cell. 2021;184:2605-2617.e18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 141] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 72. | Msomi N, Lessells R, Mlisana K, de Oliveira T. Africa: tackle HIV and COVID-19 together. Nature. 2021;600:33-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 73. | Wilkinson SAJ, Richter A, Casey A, Osman H, Mirza JD, Stockton J, Quick J, Ratcliffe L, Sparks N, Cumley N, Poplawski R, Nicholls SN, Kele B, Harris K, Peacock TP, Loman NJ. Recurrent SARS-CoV-2 mutations in immunodeficient patients. Virus Evol. 2022;8:veac050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 74. | Ghafari M, Liu Q, Dhillon A, Katzourakis A, Weissman DB. Investigating the evolutionary origins of the first three SARS-CoV-2 variants of concern. Front Virol. 2022;2:942555. [DOI] [Full Text] |
| 75. | Lucas M, Karrer U, Lucas A, Klenerman P. Viral escape mechanisms--escapology taught by viruses. Int J Exp Pathol. 2001;82:269-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 76. | Hie B, Zhong ED, Berger B, Bryson B. Learning the language of viral evolution and escape. Science. 2021;371:284-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 171] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 77. | Gil Del Alcazar CR, Alečković M, Polyak K. Immune Escape during Breast Tumor Progression. Cancer Immunol Res. 2020;8:422-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 78. | Chakraborty C, Sharma AR, Bhattacharya M, Lee SS. A Detailed Overview of Immune Escape, Antibody Escape, Partial Vaccine Escape of SARS-CoV-2 and Their Emerging Variants With Escape Mutations. Front Immunol. 2022;13:801522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 100] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 79. | Saini G, Aneja R. Cancer as a prospective sequela of long COVID-19. Bioessays. 2021;43:e2000331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 80. | Jaiswal A, Shrivastav S, Kushwaha HR, Chaturvedi R, Singh RP. Oncogenic potential of SARS-CoV-2-targeting hallmarks of cancer pathways. Cell Commun Signal. 2024;22:447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 81. | Nakov R, Segal JP, Settanni CR, Bibbò S, Gasbarrini A, Cammarota G, Ianiro G. Microbiome: what intensivists should know. Minerva Anestesiol. 2020;86:777-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 82. | Kaźmierczak-Siedlecka K, Vitale E, Makarewicz W. COVID-19 - gastrointestinal and gut microbiota-related aspects. Eur Rev Med Pharmacol Sci. 2020;24:10853-10859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 83. | Xu K, Cai H, Shen Y, Ni Q, Chen Y, Hu S, Li J, Wang H, Yu L, Huang H, Qiu Y, Wei G, Fang Q, Zhou J, Sheng J, Liang T, Li L. [Management of COVID-19: the Zhejiang experience]. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49:147-157 Chinese. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 184] [Reference Citation Analysis (0)] |
| 84. | Ianiro G, Mullish BH, Kelly CR, Kassam Z, Kuijper EJ, Ng SC, Iqbal TH, Allegretti JR, Bibbò S, Sokol H, Zhang F, Fischer M, Costello SP, Keller JJ, Masucci L, van Prehn J, Quaranta G, Quraishi MN, Segal J, Kao D, Satokari R, Sanguinetti M, Tilg H, Gasbarrini A, Cammarota G. Reorganisation of faecal microbiota transplant services during the COVID-19 pandemic. Gut. 2020;69:1555-1563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 117] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 85. | Angelakis E, Million M, Kankoe S, Lagier JC, Armougom F, Giorgi R, Raoult D. Abnormal weight gain and gut microbiota modifications are side effects of long-term doxycycline and hydroxychloroquine treatment. Antimicrob Agents Chemother. 2014;58:3342-3347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 86. | Howell MC, Green R, McGill AR, Dutta R, Mohapatra S, Mohapatra SS. SARS-CoV-2-Induced Gut Microbiome Dysbiosis: Implications for Colorectal Cancer. Cancers (Basel). 2021;13:2676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 87. | Odun-Ayo F, Reddy L. Gastrointestinal Microbiota Dysbiosis Associated with SARS-CoV-2 Infection in Colorectal Cancer: The Implication of Probiotics. Gastroenterol Insights. 2022;13:35-59. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |