Published online May 21, 2025. doi: 10.3748/wjg.v31.i19.105617
Revised: March 15, 2025
Accepted: April 1, 2025
Published online: May 21, 2025
Processing time: 111 Days and 13.9 Hours
The “obesity paradox” in hepatocellular carcinoma (HCC) suggests patients with obesity may experience better treatment outcomes compared to patients without obesity. Wang et al highlighted this paradox in HCC immunotherapy, demon
Core Tip: The “obesity paradox” in hepatocellular carcinoma suggests better outcomes in patients with obesity undergoing immunotherapy, potentially due to leptin-driven immune modulation and enhanced nutritional reserves. While promising, these findings have only been demonstrated with lenvatinib and camrelizumab, and have not yet been observed with more commonly used immunotherapy treatments for hepatocellular carcinoma, such as nivolumab, pembrolizumab, or beva
- Citation: Sierra L, Abu-Hammour MN, Chatterjee A, Simons-Linares CR. Obesity paradox role in the immunosuppressive treatment of hepatocellular carcinoma. World J Gastroenterol 2025; 31(19): 105617
- URL: https://www.wjgnet.com/1007-9327/full/v31/i19/105617.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i19.105617
The “obesity paradox” in liver disease refers to the observation that, contrary to the general expectation that obesity worsens health outcomes, patients with obesity with certain liver diseases may have better survival rates and treatment responses compared to patients without obesity. The study by Wang et al[1] provides further insight into this underexplored issue, examining the impact of body mass index (BMI) on outcomes of lenvatinib plus camrelizumab treatment in advanced hepatocellular carcinoma (HCC). This study highlights the “obesity paradox” in immunotherapy for HCC and suggests its potential applicability to other liver diseases in various contexts. The purpose of our brief communication is to expand the understanding of immunosuppressant treatment strategies for individuals with obesity and HCC, providing insights that may guide clinical decisions. Additionally, it will be pivotal for further research studying the role of obesity in other immunotherapies, as well as their role in other liver diseases.
Wang et al[1] demonstrated that overweight patients and those with obesity (BMI ≥ 25 kg/m²) achieved superior progression-free survival (8.53 months vs 6.30 months, P < 0.001) and overall survival (OS, 15.30 months vs 11.90 months, P = 0.001) compared to their non-overweight counterparts. Subgroup analyses further revealed that patients with obesity (BMI ≥ 30 kg/m²) had the best progression-free survival (10.00 months, hazard ratio 0.13, 95% confidence interval: 0.06-0.31) and OS (16.60 months, hazard ratio 0.28; 95% confidence interval: 0.14-0.57) compared to patients without obesity[1]. This paradoxical relationship suggests that the metabolic and immunological changes associated with obesity may enhance the efficacy of certain immune checkpoint inhibitors. Notably, the study was conducted in a subset of the population with hepatitis B virus-related HCC and did not include other causes of HCC, such as hepatitis C virus, metabolic-associated fatty liver disease, or alcohol-related liver disease. Additionally, the study focused solely on treatment with lenvatinib plus camrelizumab, excluding other commonly used anti-programmed death-1 (PD-1) therapies for HCC, such as nivolumab or pembrolizumab. This limitation makes it challenging to extrapolate the findings to routine clinical practice.
Multiple studies have shown that the presence of obesity may improve inpatient mortality in cirrhotic patients[2-4]. For instance, a study by Karagozian et al[2] found that cirrhotic patients with obesity had a lower crude mortality rate (2.7% vs 3.5%, P = 0.02) compared to cirrhotic patients without obesity, despite having longer hospital stays and higher healthcare costs[2]. One key factor is the enhanced nutritional reserve in patients with obesity, which may provide a buffer against the catabolic stress of acute illness. The degradation of fatty acids in critically ill patients releases 3-hydroxybutyrate, which significantly decreases phenylalanine-to-tyrosine degradation and reduces net forearm phenylalanine release, leading to a reduction in protein breakdown and enhanced muscle regeneration post-hospitalization[5,6]. In HCC, studies have demonstrated that lean patients with metabolic-associated fatty liver disease-associated HCC have poorer long-term surgical outcomes compared to overweight and obese patients, suggesting an obesity paradox in this subgroup. Specifically, in one study, lean patients exhibited a 5-year OS of 55.4% and recurrence-free survival of 35.1%, whereas overweight patients had a 5-year OS of 71.3% and recurrence-free survival of 55.6%[7]. Another study using data from the Korean Central Cancer Registry indicated that overweight males with HCC had better OS compared to normal-weight males, particularly after transarterial chemoembolization[8].
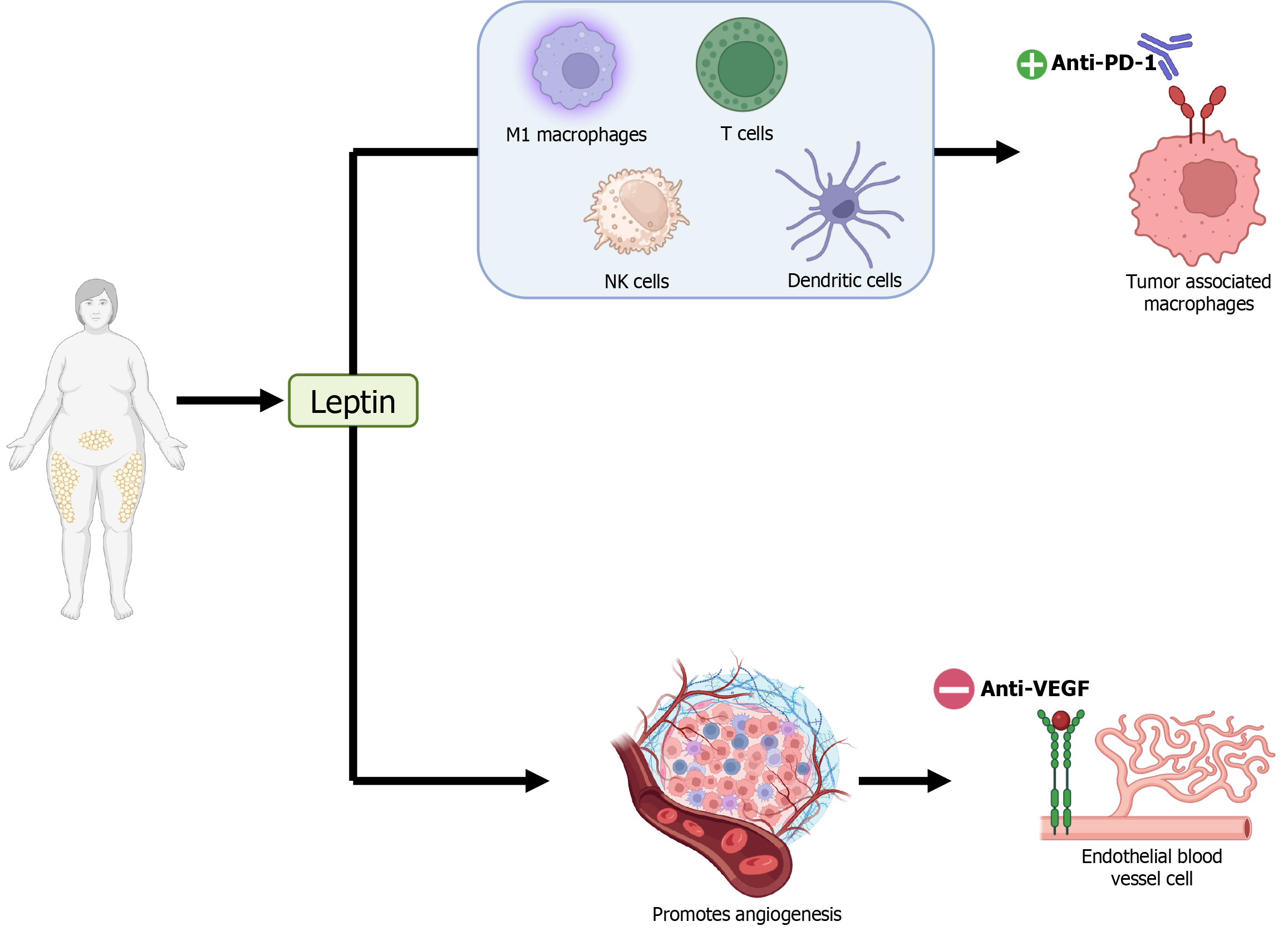
Adipose cells cause an increase in leptin, which is a proinflammatory adipokine believed to be the primary factor responsible for differences in the effectiveness of immunotherapy for HCC. Leptin enhances the efficacy of anti-PD-1 checkpoint therapy through several mechanisms. It repolarizes tumor-associated macrophages to an inflammatory type 1 macrophages-like phenotype with antitumor activity. Leptin also downregulates the immunosuppressive function of regulatory T-cells, boosting CD8+ T-cell activity. Additionally, it activates natural killer cells and enhances the migratory and stimulatory capacity of dendritic cells, further promoting antitumor immunity (Figure 1). However, while these mechanisms benefit anti-PD-1 therapy, obesity may reduce the effectiveness of other treatments. For instance, 5-fluorouracil may be less effective in HCC patients with obesity due to reduced dihydropyrimidine dehydrogenase activity, which exacerbates toxicity. Similarly, anti-vascular endothelial growth factor (VEGF) treatments like bevacizumab may be less effective in patients with obesity. Obesity increases the production of alternative proangiogenic factors, such as interleukin-6 and fibroblast growth factor 2, which promote angiogenesis independently of VEGF, reducing the efficacy of anti-VEGF therapies (Figure 1).
The impact of immunosuppressants on HCC patients with obesity remains largely unexplored. In fact, the article by Wang et al[1] is the first to study this intervention in a real-world population. However, the treatment analyzed is not one of the most commonly used immunotherapies, leaving an opportunity for further research on how obesity influences the effectiveness of treatments such as nivolumab, pembrolizumab, or bevacizumab for HCC. Additionally, it is unclear whether the benefits diminish once a patient loses weight through interventions such as lifestyle modification, glucagon-like peptide-1 therapy, or bariatric surgery. Further research on this subject is crucial to guide clinicians in tailoring treatments based on individual patient characteristics.
| 1. | Wang YQ, Pan D, Yao ZY, Li YQ, Qu PF, Wang RB, Gu QH, Jiang J, Han ZX, Liu HN. Impact of baseline body mass index on the long-term prognosis of advanced hepatocellular carcinoma treated with immunotherapy. World J Gastroenterol. 2024;30:4132-4148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 2. | Karagozian R, Bhardwaj G, Wakefield DB, Baffy G. Obesity paradox in advanced liver disease: obesity is associated with lower mortality in hospitalized patients with cirrhosis. Liver Int. 2016;36:1450-1456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Jiang H, Li M, Yu H, Huang Y, Yang B, Wu B, Yang Y. Body mass index and waist-to-height ratio effect on mortality in non-alcoholic fatty liver: revisiting the obesity paradox. Front Endocrinol (Lausanne). 2024;15:1419715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 4. | Endo K, Kakisaka K, Abe T, Yusa K, Nakaya I, Watanabe T, Suzuki A, Yoshida Y, Oikawa T, Miyasaka A, Kuroda H, Matsumoto T. Positive impact of obesity on the prognosis of liver cirrhosis. J Gastroenterol Hepatol. 2024;39:1663-1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Goossens C, Weckx R, Derde S, Dufour T, Vander Perre S, Pauwels L, Thiessen SE, Van Veldhoven PP, Van den Berghe G, Langouche L. Adipose tissue protects against sepsis-induced muscle weakness in mice: from lipolysis to ketones. Crit Care. 2019;23:236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 6. | Swierczynski J, Sledzinski T, Slominska E, Smolenski R, Sledzinski Z. Serum phenylalanine concentration as a marker of liver function in obese patients before and after bariatric surgery. Obes Surg. 2009;19:883-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Zhang W, Li MY, Li ZQ, Diao YK, Liu XK, Guo HW, Wu XC, Wang H, Wang SY, Zhou YH, Lu J, Lin KY, Gu WM, Chen TH, Li J, Liang YJ, Yao LQ, Wang MD, Li C, Yin DX, Pawlik TM, Lau WY, Shen F, Chen Z, Yang T. Long-term outcomes following hepatectomy in patients with lean non-alcoholic fatty liver disease-associated hepatocellular carcinoma versus overweight and obese counterparts: A multicenter analysis. Asian J Surg. 2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Cha B, Yu JH, Jin YJ, Suh YJ, Lee JW. Survival Outcomes According to Body Mass Index in Hepatocellular Carcinoma Patient: Analysis of Nationwide Cancer Registry Database. Sci Rep. 2020;10:8347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |