Published online May 14, 2025. doi: 10.3748/wjg.v31.i18.106670
- This article has been corrected.
- See: World J Gastroenterol. Jul 7, 2025; 31(25): 109912
Revised: April 7, 2025
Accepted: April 18, 2025
Published online: May 14, 2025
Processing time: 70 Days and 13.3 Hours
Given the growing burden of colorectal cancer (CRC) as a global health challenge, it becomes imperative to focus on strategies that can mitigate its impact. Post-treatment surveillance has emerged as essential for early detection of recurrence, significantly improving patient outcomes. However, intensive surveillance stra
Core Tip: Given the increasing incidence of colorectal cancer, especially among younger populations, effective post-treatment surveillance is essential for early detection of recurrence and improved outcomes. Intensive surveillance has variable efficacy, underscoring the need for personalized, risk-based protocols. Suboptimal adherence to guidelines further highlights the need for efficient, individualized approaches. Circulating tumor DNA shows promise as a biomarker, offering high specificity and diagnostic accuracy. Additionally, artificial intelligence models utilizing patient and tumor data have the potential to refine surveillance, with predictive accuracy ranging from 0.581 to 0.979. Nonetheless, cost, accessibility, and validation remain significant barriers to widespread clinical implementation.
- Citation: Negoi I. Personalized surveillance in colorectal cancer: Integrating circulating tumor DNA and artificial intelligence into post-treatment follow-up. World J Gastroenterol 2025; 31(18): 106670
- URL: https://www.wjgnet.com/1007-9327/full/v31/i18/106670.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i18.106670
Given the growing burden of colorectal cancer (CRC) as a global health challenge, with its incidence and mortality doubling over the last 30 years and increasing in patients younger than 50 years, it becomes imperative to focus on strategies that can mitigate its impact[1,2]. There is a continuous preoccupation for healthcare professionals to increase post-treatment healthy life years with reintegration in the society of patients with CRC, with 43.54% of young and middle-aged patients returning to work within 100 days[3]. Among these, post-treatment surveillance has emerged as a crucial element for early detection of recurrence, thereby significantly improving patient outcomes.
Patients with CRC should undergo post-treatment surveillance to enable early detection of recurrence, typically following guidelines that recommend regular follow-up clinic visits, periodic imaging, serial carcinoembryonic antigen measurements, and colonoscopy[4]. However, despite recommendations, adherence to surveillance protocols is often suboptimal internationally, with studies indicating that only a minority of patients fully comply with all guideline-directed surveillance follow-up investigations[5]. An analysis of the Netherlands Cancer Registry showed that 18.3%, 41.4%, and 56.1% of the patients had fewer carcinoembryonic antigen (CEA), imaging, and colonoscopy tests than recommended[6].
There is a 21.4% [95% confidence interval (CI): 19.5%-23.3%] 5-year cumulative incidence for tumor recurrence for radically resected stage II-III CRCs[7]. A total of 87.3% of recurrences were detected in the first 3 years, with a median time to detection of 1.1 years. There was a significant difference in overall survival (OS) between patients with recurrence retreated with curative intent (5-year OS of 58.6%) and those who received palliative treatment (5-year OS of 7.7%)[7].
Considering that the number of cancer survivors is increasing globally, risk-adapted follow-up strategies should be implemented, tailoring the frequency and modality of surveillance to patient-specific risk factors (e.g., tumor stage, molecular characteristics, or response to therapy) to focus resources on those most likely to benefit, an approach that remains underutilized in current practice[8,9]. International guidelines lack consensus regarding the integration of emerging technologies.
This editorial outlines the marked heterogeneity in post-treatment surveillance protocols for CRC across different healthcare systems and discusses related challenges and evidence gaps. We highlight how these variations impact patients and healthcare resources and contextualize recent advances [including circulating tumor DNA (ctDNA) and artificial intelligence (AI)-based innovations] that promise to optimize surveillance.
The aim of this article is to detail the heterogeneity of surveillance protocols after radical resection of CRC worldwide and the significant challenges and variability of evidence related to this important topic in terms of its impact on patients and healthcare systems.
Given the significant impact of CRC globally, understanding the effectiveness of surveillance strategies, as explored in a study by Sala-Miquel et al[10], is of paramount importance.
Sala-Miquel et al[10] analyzed the accuracy of postoperative colonoscopy, computed tomography (CT), and tumor markers used for surveillance in a Spanish cohort of patients with resected non-metastatic CRC. This article is interesting considering its analysis of factors correlated with compliance to guidelines in a cohort of patients who underwent radical colorectal resection between 2010 and 2015 and were surveyed for 5 years. Spanish guidelines recommend CEA and carbohydrate antigen 19-9 evaluation every 6 months for the first 5 years, with thoracic and abdominopelvic CT each year and colonoscopy in years 1 and 4. The authors revealed that adherence to surveillance [odds ratio (OR) = 0.30, 95%CI: 0.20 to 0.46], age at diagnosis (OR = 0.93, 0.91 to 0.95), and early stages (OR = 0.38, 95%CI: 0.24 to 0.61) were independently associated with better 5-year OS. The cohort included 574 patients, 160 (27.9%) of whom had rectal cancer. There were 25 (16.3%) and 128 (83.7%) cases of local and systemic recurrences, respectively.
Patient adherence to the surveillance protocol decreased over time, with 74% and 36.50% of CT scans performed on an appropriate date in the first and fifth year, respectively. Colonoscopy was performed at an appropriate date in 68.1% of patients in the first year and in 15% of patients in the fourth year.
There are significant variations and controversies throughout the international guidelines regarding the postoperative surveillance of patients with CRC, stages I-III, and even more for patients with resected stage IV CRC[11]. Although professional societies usually recommend an intensive approach, a less intensive approach seems to be non-inferior in terms of OS[12]; thus, a personalized surveillance protocol that balances patient, disease, and healthcare system factors is required[11].
A randomized controlled trial (RCT) including 2509 patients from 24 centers in Sweden, Denmark, and Uruguay compared high-frequency (chest, abdomen, pelvis CT, and CEA at 6, 12, 18, 24, and 36 months after resection) with low-frequency (months 12 and 36) surveillance in patients with stages II-III CRC[13]. The 5-year overall and cancer-specific mortality rates were 13.0% vs 14.1% (P = 0.43) and 10.6% vs 11.4% (P = 0.52), respectively, in high- and low-frequency surveillance[13].
Zhao et al[14] meta-analyzed the results of 17 RCTs (8039 patients) that compared intensive and less-intensive surveillance. Intensive surveillance [hazard ratio (HR) = 0.85, 95%CI: 0.74 to 0.97, I2 = 30%] as well as subgroups with intensive frequency (HR = 0.82, 95%CI: 0.69 to 0.97) and intensive test (HR = 0.75, 95%CI: 0.64 to 0.87) surveillance protocols were associated with improved OS[14]. However, there was significant clinical heterogeneity (I2 = 30%) of interventions in this study, and the protocols of the included studies were developed several decades ago[14].
A Cochrane meta-analysis of 19 studies with 13216 patients published in 2019 showed that surveillance of patients with non-metastatic CRC did not improve OS (high-quality evidence), CRC-specific survival (moderate-quality evidence), or relapse-free survival (high-quality evidence)[15].
However, intensive surveillance is associated with a higher rate of surgical re-resections[16,17]. In the Follow-up After Colorectal Surgery (FACS) trial, the rate of curative surgery for recurrence was 2.3% in the minimum surveillance group, compared with 6.7%, 8%, and 6.6% in the CEA monitoring, CT, and CEA + CT groups, respectively[17]. The authors concluded that if present for any strategy, the survival benefit was small. Long-term surveillance of the FACS study revealed no survival difference between the arms (P = 0.45) and no difference in the quality of life of patients[16]. Although intensive imaging or CEA monitoring was associated with an increased rate of curative resections of recurrences, the observed lack of survival benefits may be partially explained by the size of the trial, which had only 31% power to detect a 5% effect on survival[17].
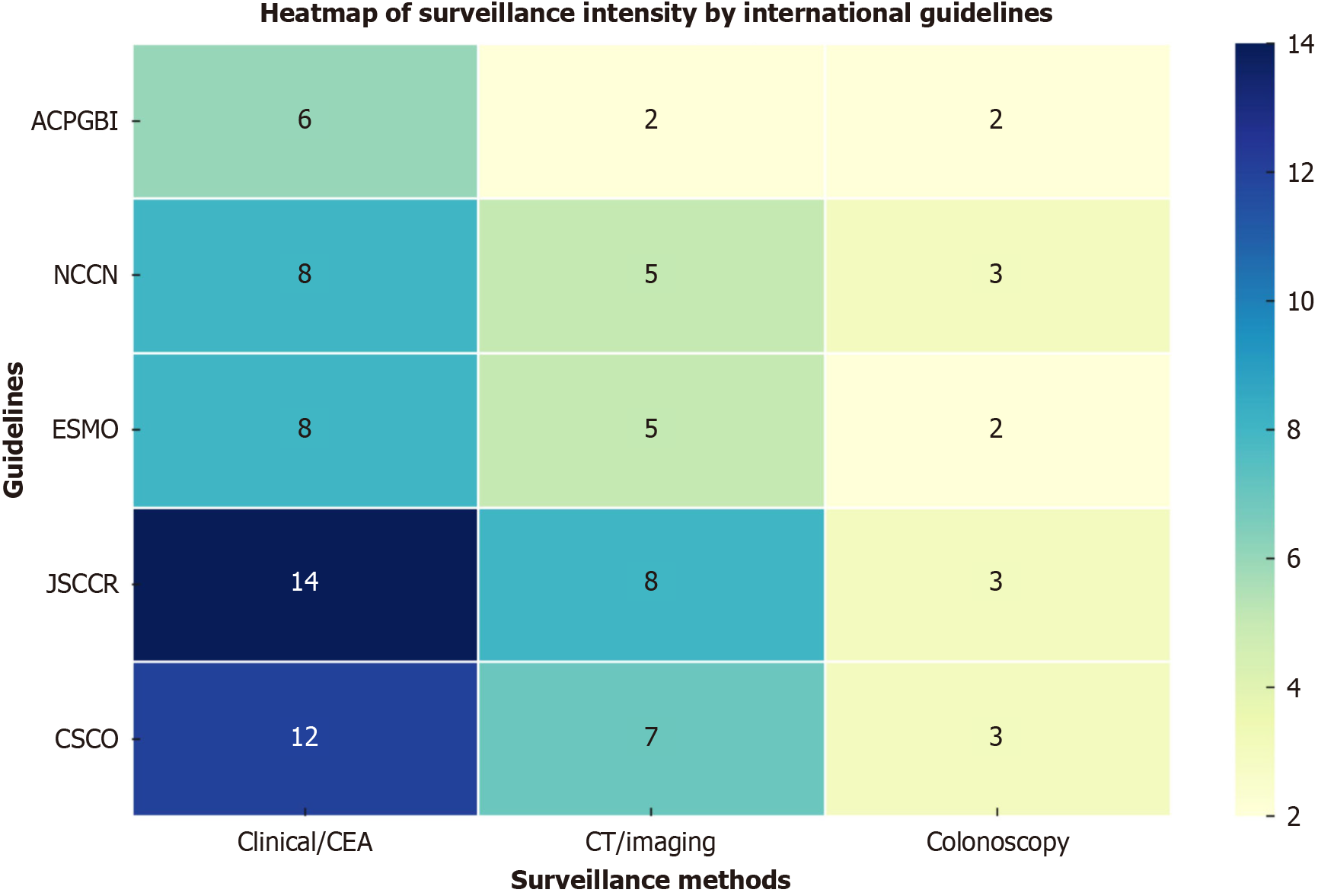
However, analyses of international guidelines revealed significant heterogeneity in surveillance recommendations among different countries (Figure 1 and Table 1).
| Surveillance method | ACPGBI (United Kingdom/Ireland)[17] | NCCN (United States)[18,19] | ESMO (Europe)[20,21] | JSCCR (Japan)[22] | CSCO (China)[23] |
| Clinical exam & CEA | Every 6 months (for 3 years) | Every 3-6 months (years 1-2), then every 6 months (up to 5 years) | Every 3-6 months (years 1-3), 6-12 months (years 4-5) | Every 3 months (years 1-3), every 6 months (years 4-5) | Stage I: Every 6 months; stage II-III: Every 3 months (years 1-3), then every 6 months (years 4-5) |
| CT | Every 6 months (minimum two evaluations) | Every 6-12 months (up to 5 years), Stage IV: Every 3-6 months (first 2 years) | Every 6-12 months (years 1-3), annually (years 4-5) | Every 6 months (years 1-3), twice per year stage III, annually stages I-II (years 4-5) | Stage III/IV: Every 6-12 months (years 1-5), ultrasound or CT for lower stages |
| Colonoscopy | Year 1, then every 5 years | Year 1; if adenoma present annually, if no adenoma, repeat at year 3, then every 5 years | Starting year 1, then every 3-5 years | Colon cancer: Years 1 & 3; Rectal cancer: Years 1, 2 & 3 | Year 1, then year 3, afterwards every 5 years |
| Additional imaging | Not routinely recommended | Not routinely recommended | Not routinely recommended | Not routinely recommended | Liver ultrasound recommended for stage II |
The Association of Coloproctology of Great Britain and Ireland guidelines recommend: (1) A minimum of two CT evaluations of the thorax, abdomen, and pelvis, every 6 months; (2) CEA monitoring for the first 3 years; and (3) A surve
The National Comprehensive Cancer Network (NCCN) 2025 colon and rectum cancer guidelines recommend the following for stages II-III disease: (1) Clinical examination and CEA monitoring every 3-6 months for 2 years, and then every 6 months up to 5 years; (2) CT of the thorax, abdomen, and pelvis at 6-12 months up to 5 years; and (3) Colono
The European Society for Medical Oncology colon and rectal guidelines recommend for the first 3 years: (1) Clinical and CEA evaluation every 3-6 months; (2) Chest, abdominal, and pelvic CT every 6-12 months; and (3) Colonoscopy starting from year 1 after resection, every 3-5 years. For years 4 and 5 after resection, clinical examination and CEA are recommended at 6-12 months, and CT every 12 months[21,22].
The Japanese Society for Cancer of the Colon and Rectum guidelines recommend the following for the first 3 years: (1) Clinical examination and tumor marker every 3 months; (2) Chest, abdominal, and pelvic CT every 6 months; and (3) Colonoscopy in years 1 and 3 for colon cancers; and in years 1, 2, and 3 for rectal cancers. For years 4 and 5: (1) Clinical examination and tumor markers every 6 months; and (2) CT twice per year for stage III, and once per year for stages I-II[23].
The Chinese Society of Clinical Oncology guidelines recommend an interval of 6 months for stage I, and 3 months for stage II-III up to 3 years, and 6 months for the next 2 years, with the following: (1) Clinical examination; (2) CEA; (3) Liver ultrasound for stage II; (4) Chest, abdominal, and pelvic CT for stage III or abdominal ultrasonography or CEA; and (5) Colonoscopy within 1 year after surgery, then in year 3, and every 5 years[23]. For patients with completely resected or ablated stage IV CRC, the evaluation should be done every 3 months for 3 years, and every 6 months for the next 2 years. CT should be done each 6-12 months[24].
According to all guidelines, colonoscopy should be performed within 3-6 months after surgery, if not performed before surgery.
There is an increasing trend for total neoadjuvant therapy (TNT) in patients with rectal cancer, especially in microsatellite stable locally advanced tumors[25]. This strategy improved the pathological complete response in patients with resected cancer or the clinical complete response (cCR) in patients managed nonoperatively (NOM) to 38% compared to 21% in patients managed with traditional chemoradiation[26,27]. The initial evaluation after TNT completion to determine the cCR status and patients’ possibility of choosing NOM should be done at 1-3 months[25,28]. Patients with rectal cancer with cCR opting for NOM should follow an additional surveillance protocol to timely detect tumor regrowth in order to have timely salvage surgery that offers similar disease-free survival compared to the initial resection[29].
In the Organ Preservation in Patients with Rectal Adenocarcinoma Treated with Neoadjuvant Therapy trial (OPRA trial NCT04270760), tumor regrowth was observed in 81 of 324 patients with stages II-III rectal cancer, 94% of cases within the first 2 years, and 99% of cases within the first 3 years[29–31].
The surveillance strategy used in patients managed NOM, complementary to the standard protocol, usually includes: (1) Digital rectal examination and flexible rectoscopy each 3 months for the first 2 years, and each 6 months up to 5 years; and (2) Pelvic MRI twice per year for the first 3 years, and each year up to 5 years[20,25]. Endoscopic biopsy has a high false-negative rate for assessing adenocarcinoma persistence and is not mandatory[32].
Transitioning from traditional surveillance methods, emerging technologies such as ctDNA offer a more nuanced approach to monitor tumor burden in patients. ctDNA or liquid biopsy is used to identify and monitor the tumor burden in a specific patient, which is important for diagnosis, prognosis, and evaluation of the therapeutic response. ctDNA is a biomarker of minimal residual disease and is strongly correlated with the prognosis of patients with CRC[33].
Benešová et al[34] showed that ctDNA was identified in 22 of 22 recurrences (100%) compared to 73% by imaging and 68% by tumor markers.
Li et al[35] analyzed ctDNA in 394 plasma samples from 60 patients with curative resection of CRC liver metastases surveyed for a median of 31.3 months. Longitudinal ctDNA monitoring had a sensitivity of 100% to identify tumor recurrence; imagistic tumor recurrence was identified in 75% of patients within 5 months after the first ctDNA positive test. 61% of patients had liver-limited recurrences, 72.0% had repeated local therapies, and 60.0% had no evidence of relapse after re-resection/ablation[35]. ctDNA was present before surgery, after surgery, and at the end of multimodal therapy in 96.7%, 58.3%, and 39.7% of patients, respectively[36].
Ultradeep sequencing analysis of plasma cell-free DNA in a cohort of 125 Danish patients with stages I-III CRC revealed that ctDNA was associated with recurrence before and after adjuvant chemotherapy and longitudinal surveillance[37]. ctDNA was detectable preoperatively in 88.5% of the patients, and after radical treatment, long-term ctDNA monitoring identified 14 of 16 (87.5%) recurrences. Moreover, the 30-day postoperative ctDNA positivity was associated with a seven-time higher risk of tumor relapse (HR = 7.2, 95%CI: 2.7 to 190; P < 0.001). ctDNA positivity during surveillance was associated with a 40 times higher risk of recurrence (HR = 43.5, 95%CI: 9.8 to 193.5; P < 0.001). Serial surveillance showed that ctDNA analyses indicated recurrence within a mean of 8.7 months (range 0.8–16.5 months) before standard imagistics[37].
By contrast, in a study by Tie et al[38], ctDNA was found in only 20 of 96 (21%) postsurgical samples and was inde
The GALAXY and CIRCULATE-Japan studies investigated ctDNA measurements before and after surgery in 1039 patients with stages II-IV CRC[39]. ctDNA was detected preoperatively in 94.5%, 97.2%, and 84.2% of patients with stages II, III, and IV disease, respectively. Positive ctDNA at 4 weeks after surgery was associated with a higher recurrence risk (HR = 10; P < 0.0001) and was the most significant prognostic factor associated with relapse in patients with stages II-III disease (HR = 10.82; P < 0.001)[39].
However, ctDNA analysis is variable in terms of quantification efficiency, sensitivity, and reproducibility[40]. Assay sensitivity is a major challenge, considering that less than 1% of cell-free DNA in a specific patient has origins in the tumoral tissue[41]. Stetson et al[42] compared four plasma next-generation sequencing tests and found a range of sensitivity from 38% to 89% and positive predictive value from 36% to 80%. The majority of discordances was observed when variant allele frequency was less than 1%[42]. There is variability between ctDNA and tissue profiling, with a reported concordance for CRC of 75%[43]. From a cohort of 923 patients with CRC 565 (61.2%) had mutations that could be targeted according to the NCCN guidelines. 5.1% of CRC patients had at least one actionable variant detected uniquely by ctDNA, and 19.8% of at least unique actionable variants detected uniquely by tissue profiling[43].
A real-world analysis of the utilization and performance of ctDNA monitoring revealed in early stages I-III CRC a preoperative detection rate of 54.3%[44]. In metastatic stage IV, the pretreatment detection rate was 87.7%[44]. ctDNA could vary with the organ where the cancer started (more sensitive in the liver than in the stomach, for example, in the study by Nguyen Hoang et al[44]), but also with the variant allele frequency, presenting a higher detection rate in metastatic cancers than in early-stage cancers.
Another challenge related to ctDNA is cost-effectiveness. Although initial costs are higher, ctDNA testing in stage II colon cancer to guide chemotherapy reduces costs by $221.684 and $116.720 for commercial and Medicare Advantage payers, respectively[45]. A European analysis revealed that ctDNA would be cost-effective if the testing would cost less than 1500 dollars, or will predict treatment response, or its performance will improve substantially[46].
In recent years, there has been massive development of AI, machine learning (ML), and other models in oncology, showing good discrimination and clinical utility[47,48]. Recent studies have highlighted the expanding role of AI in tailoring the post-resection surveillance of CRC. ML models, including patient and tumor features, can predict the recurrence risk more accurately than conventional staging. Osman et al[49] developed a ML algorithm for predicting survival in patients with CRC, including data from 364316 patients from the Surveillance, Epidemiology, and End Results (SEER) database and 1572 from a Korean database. The most influential parameters of the ML model were age, the number of examined lymph nodes, and tumor size. This model had a better prediction of 5-year survival than tumor-node-metastasis staging (area under the receiver operating characteristic curve = 0.80 vs 0.73; P < 0.001)[49].
The utility of deep learning (DL) algorithms based on imaging to predict tumor recurrence was shown by Zhao et al[50], who included data from 207 consecutive patients and 13248 ultrasound images of CRC liver metastases. Early recurrence after thermal ablation was observed in 49% of cases, and DL combined with a clinical algorithm (including T stage, lymph node metastases of the primary CRC, and preoperative chemotherapy) presented an area under the curve (AUC) of 0.78, which was higher than that of DL (AUC = 0.76) and the clinical model (AUC = 0.67) (P < 0.001)[50].
AI may help to identify patients who require adjuvant therapy or intensive monitoring. Such AI-driven risk stratification enables personalized surveillance, concentrating intensive surveillance on high-risk patients and potentially de-intensifying monitoring for low-risk patients[51]. Although attractive, van der Reijd et al[52] investigated CT radiomics models to predict tumor progression of CRCLM after thermal ablation and revealed that the clinical and radiomics models were not reproducible when reassessed in internal and external cohorts.
Xiao et al[53] developed and validated a DL approach based on histology to predict 5-year recurrence risk. The developed model had AUC of 0.833 and 0.715 for the validation and external cohort, respectively. There was a significant difference in relapse-free survival (RFS) between the high and low DL model factors (45.7% vs 82.5%, HR = 3.89; P < 0.001). The use of this prognostic model revealed that adjuvant chemotherapy was associated with better RFS in stage II disease with high scores (HR = 0.15; P < 0.001)[53].
The workflow of CEA measurement may be optimized by the ML algorithm using clinical, pathological, and serial CEA measurements[54]. The AUC was 0.71 for the five postoperative CEA measurements[54]. A systematic review of 52 studies showed that the threshold to continue investigations for CEA should be 10 µg/L, and that the frequency of testing should be increased to monthly for the first 3 months, and then every 2 months up to 1 year[55]. After the second CEA test, the decision to further analyze should be based on the trend of CEA values, which should have a decreased threshold for investigation[55].
AI enhances the quality of surveillance of colonoscopy and CT. AI significantly increased the colonoscopy adenoma detection rate (29.1% vs 20.3%; P < 0.001) and the number of detected polyps (0.53 vs 0.31; P < 0.001)[56]. A prediction model was developed to help prioritize patients at high risk for CRC when the surveillance colonoscopy capacity is exceeded[57]. This model performed better than the guideline-based surveillance, with an AUC of 0.71-0.73 (57%-70% of detected cancers had the first three deciles of the score) vs 0.52-0.52[56]. AI-assisted imaging based on the DL system achieved an accuracy of 97.2% compared to 86.0% of radiologists for CRC detection on CT without bowel preparation[58].
On the other hand, external validation of a ML model is of major importance before clinical implementation, but is rarely reported in studies[59]. There are limitations to the external validation of AI models[60], with Van Calster et al[61] affirming that there is no validated prediction model, considering that populations, measurements, and patient characteristic evolution vary over time[62].
Effective surveillance of CRC treatment is undergoing a transformative shift, moving away from standardized regimens towards personalized, patient-specific strategies. Current international disparities in surveillance guidelines highlight the urgent need for harmonization through evidence-based adaptive frameworks. Beyond traditional clinical tools, the emerging roles of ctDNA and AI offer powerful precision approaches but also introduce practical challenges, such as assay sensitivity limitations, cost-effectiveness considerations, and the generalizability of AI models in diverse clinical settings.
To fully realize the potential of personalized CRC surveillance, several research priorities have been proposed: (1) Conducting multicenter randomized trials that rigorously evaluate the clinical utility and cost-effectiveness of ctDNA-guided surveillance in comparison to conventional approaches; (2) Establishing robust external validation standards for AI-driven predictive models, ensuring reproducibility and real-world applicability across diverse populations; and (3) Developing integrated surveillance protocols that combine ctDNA and AI technologies with patient-centered surveillance models that address patient compliance, quality of life, and healthcare resource allocation simultaneously.
Future surveillance strategies should also prioritize the incorporation of patient-reported outcomes to align clinical efficacy with patient preferences and quality of life.
Ultimately, transitioning to individualized surveillance protocols informed by innovative biomarkers and intelligent analytics represents a critical step toward optimizing patient outcomes and healthcare efficiency in CRC survivorship.
| 1. | Pinheiro M, Moreira DN, Ghidini M. Colon and rectal cancer: An emergent public health problem. World J Gastroenterol. 2024;30:644-651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 18] [Article Influence: 18.0] [Reference Citation Analysis (4)] |
| 2. | GBD 2019 Colorectal Cancer Collaborators. Global, regional, and national burden of colorectal cancer and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol Hepatol. 2022;7:627-647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 327] [Cited by in RCA: 374] [Article Influence: 124.7] [Reference Citation Analysis (0)] |
| 3. | Hu D, Li Y, Zhang H, Wang LL, Liu WW, Yang X, Xiao MZ, Zhang HL, Li J. Return to work in young and middle-aged colorectal cancer survivors: Factors influencing self-efficacy, fear, resilience, and financial toxicity. World J Gastroenterol. 2025;31:100357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (3)] |
| 4. | Kupfer SS, Lubner S, Coronel E, Pickhardt PJ, Tipping M, Graffy P, Keenan E, Ross E, Li T, Weinberg DS. Adherence to postresection colorectal cancer surveillance at National Cancer Institute-designated Comprehensive Cancer Centers. Cancer Med. 2018;7:5351-5358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Ford ME, Sterba KR, Armeson K, Malek AM, Knight KD, Zapka J. Factors Influencing Adherence to Recommended Colorectal Cancer Surveillance: Experiences and Behaviors of Colorectal Cancer Survivors. J Cancer Educ. 2019;34:938-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Boute TC, van Eekelen R, Elferink MAG, Birgit LW 1st, de Wilt JHW, Vink GR, Greuter MJE, Coupé VMH. Follow-Up Adherence After Treatment With Curative Intent for Stage II and III Colorectal Cancer Patients. Cancer Med. 2025;14:e70667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Hansdotter P, Scherman P, Petersen SH, Mikalonis M, Holmberg E, Rizell M, Naredi P, Syk I. Patterns and resectability of colorectal cancer recurrences: outcome study within the COLOFOL trial. BJS Open. 2021;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 8. | Mollica MA, Mayer DK, Oeffinger KC, Kim Y, Buckenmaier SS, Sivaram S, Muha C, Taib NA, Andritsch E, Asuzu CC, Bochis OV, Diaz S, Trill MD, Garcia PJ, Grassi L, Uchitomi Y, Shaikh AJ, Jefford M, Lee HJ, Johansen C, Luyirika E, Maher EJ, Mallillin MMB, Maniragaba T, Mehnert-Theuerkauf A, Pramesh CS, Siesling S, Spira O, Sussman J, Tang L, Hai NV, Yalcin S, Jacobsen PB. Follow-Up Care for Breast and Colorectal Cancer Across the Globe: Survey Findings From 27 Countries. JCO Glob Oncol. 2020;6:1394-1411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Hang D, Knudsen MD, Song M. Moving Toward Personalized Colorectal Cancer Follow-Up Care. JAMA Oncol. 2024;10:29-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Sala-Miquel N, Carrasco-Muñoz J, Bernabeu-Mira S, Mangas-Sanjuan C, Baile-Maxía S, Madero-Velázquez L, Ausina V, Yuste A, Gómez-González L, Romero Simó M, Zapater P, Jover R. Diagnostic yield of follow-up in patients undergoing surgery for non-metastatic colorectal cancer. World J Gastroenterol. 2025;31:100155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (5)] |
| 11. | Liu SL, Cheung WY. Role of surveillance imaging and endoscopy in colorectal cancer follow-up: Quality over quantity? World J Gastroenterol. 2019;25:59-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Mokhles S, Macbeth F, Farewell V, Fiorentino F, Williams NR, Younes RN, Takkenberg JJ, Treasure T. Meta-analysis of colorectal cancer follow-up after potentially curative resection. Br J Surg. 2016;103:1259-1268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 13. | Wille-Jørgensen P, Syk I, Smedh K, Laurberg S, Nielsen DT, Petersen SH, Renehan AG, Horváth-Puhó E, Påhlman L, Sørensen HT; COLOFOL Study Group. Effect of More vs Less Frequent Follow-up Testing on Overall and Colorectal Cancer-Specific Mortality in Patients With Stage II or III Colorectal Cancer: The COLOFOL Randomized Clinical Trial. JAMA. 2018;319:2095-2103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 165] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 14. | Zhao Y, Yi C, Zhang Y, Fang F, Faramand A. Intensive follow-up strategies after radical surgery for nonmetastatic colorectal cancer: A systematic review and meta-analysis of randomized controlled trials. PLoS One. 2019;14:e0220533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Jeffery M, Hickey BE, Hider PN. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev. 2019;9:CD002200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 16. | Mant D, Gray A, Pugh S, Campbell H, George S, Fuller A, Shinkins B, Corkhill A, Mellor J, Dixon E, Little L, Perera-Salazar R, Primrose J. A randomised controlled trial to assess the cost-effectiveness of intensive versus no scheduled follow-up in patients who have undergone resection for colorectal cancer with curative intent. Health Technol Assess. 2017;21:1-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 17. | Primrose JN, Perera R, Gray A, Rose P, Fuller A, Corkhill A, George S, Mant D; FACS Trial Investigators. Effect of 3 to 5 years of scheduled CEA and CT follow-up to detect recurrence of colorectal cancer: the FACS randomized clinical trial. JAMA. 2014;311:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 346] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 18. | Leong K, Hartley J, Karandikar S. Association of Coloproctology of Great Britain & Ireland (ACPGBI): Guidelines for the Management of Cancer of the Colon, Rectum and Anus (2017) - Follow Up, Lifestyle and Survivorship. Colorectal Dis. 2017;19 Suppl 1:67-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Benson AB, Venook AP, Adam M, Chang G, Chen YJ, Ciombor KK, Cohen SA, Cooper HS, Deming D, Garrido-Laguna I, Grem JL, Haste P, Hecht JR, Hoffe S, Hunt S, Hussan H, Johung KL, Joseph N, Kirilcuk N, Krishnamurthi S, Malla M, Maratt JK, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Shogan B, Skibber JM, Sofocleous CT, Tavakkoli A, Willett CG, Wu C, Gurski LA, Snedeker J, Jones F. Colon Cancer, Version 3.2024, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2024;22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 64] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 20. | Benson AB, Venook AP, Adam M, Chang G, Chen YJ, Ciombor KK, Cohen SA, Cooper HS, Deming D, Garrido-Laguna I, Grem JL, Haste P, Hecht JR, Hoffe S, Hunt S, Hussan H, Johung KL, Joseph N, Kirilcuk N, Krishnamurthi S, Malla M, Maratt JK, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Shogan B, Skibber JM, Sofocleous CT, Tavakkoli A, Willett CG, Wu C, Jones F, Gurski L. NCCN Guidelines® Insights: Rectal Cancer, Version 3.2024. J Natl Compr Canc Netw. 2024;22:366-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 60] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 21. | Argilés G, Tabernero J, Labianca R, Hochhauser D, Salazar R, Iveson T, Laurent-Puig P, Quirke P, Yoshino T, Taieb J, Martinelli E, Arnold D; ESMO Guidelines Committee. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:1291-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 807] [Article Influence: 161.4] [Reference Citation Analysis (0)] |
| 22. | Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, Arnold D; ESMO Guidelines Committee. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv22-iv40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1112] [Cited by in RCA: 1199] [Article Influence: 149.9] [Reference Citation Analysis (0)] |
| 23. | Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, Hasegawa K, Hotta K, Ishida H, Ishiguro M, Ishihara S, Kanemitsu Y, Kinugasa Y, Murofushi K, Nakajima TE, Oka S, Tanaka T, Taniguchi H, Tsuji A, Uehara K, Ueno H, Yamanaka T, Yamazaki K, Yoshida M, Yoshino T, Itabashi M, Sakamaki K, Sano K, Shimada Y, Tanaka S, Uetake H, Yamaguchi S, Yamaguchi N, Kobayashi H, Matsuda K, Kotake K, Sugihara K; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25:1-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1024] [Cited by in RCA: 1311] [Article Influence: 262.2] [Reference Citation Analysis (1)] |
| 24. | Diagnosis And Treatment Guidelines For Colorectal Cancer Working Group CSOCOC. Chinese Society of Clinical Oncology (CSCO) diagnosis and treatment guidelines for colorectal cancer 2018 (English version). Chin J Cancer Res. 2019;31:117-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 135] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 25. | Scott AJ, Kennedy EB, Berlin J, Brown G, Chalabi M, Cho MT, Cusnir M, Dorth J, George M, Kachnic LA, Kennecke HF, Loree JM, Morris VK, Perez RO, Smith JJ, Strickland MR, Gholami S. Management of Locally Advanced Rectal Cancer: ASCO Guideline. J Clin Oncol. 2024;42:3355-3375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 40] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 26. | Garcia-Aguilar J, Chow OS, Smith DD, Marcet JE, Cataldo PA, Varma MG, Kumar AS, Oommen S, Coutsoftides T, Hunt SR, Stamos MJ, Ternent CA, Herzig DO, Fichera A, Polite BN, Dietz DW, Patil S, Avila K; Timing of Rectal Cancer Response to Chemoradiation Consortium. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol. 2015;16:957-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 516] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 27. | Cercek A, Roxburgh CSD, Strombom P, Smith JJ, Temple LKF, Nash GM, Guillem JG, Paty PB, Yaeger R, Stadler ZK, Seier K, Gonen M, Segal NH, Reidy DL, Varghese A, Shia J, Vakiani E, Wu AJ, Crane CH, Gollub MJ, Garcia-Aguilar J, Saltz LB, Weiser MR. Adoption of Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer. JAMA Oncol. 2018;4:e180071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 445] [Article Influence: 63.6] [Reference Citation Analysis (1)] |
| 28. | Verheij FS, Omer DMR, Williams H, Buckley JT, Lin ST, Qin L, Thompson HM, Yuval JB, Gollub MJ, Wu AJ, Saltz LB, Garcia-aguilar J; on behalf of the OPRA Consortium. Sustained organ preservation in patients with rectal cancer treated with total neoadjuvant therapy: Long-term results of the OPRA trial. J Clin Oncol. 2023;41:3520-3520. [DOI] [Full Text] |
| 29. | Verheij FS, Omer DM, Williams H, Lin ST, Qin LX, Buckley JT, Thompson HM, Yuval JB, Kim JK, Dunne RF, Marcet J, Cataldo P, Polite B, Herzig DO, Liska D, Oommen S, Friel CM, Ternent C, Coveler AL, Hunt S, Gregory A, Varma MG, Bello BL, Carmichael JC, Krauss J, Gleisner A, Guillem JG, Temple L, Goodman KA, Segal NH, Cercek A, Yaeger R, Nash GM, Widmar M, Wei IH, Pappou EP, Weiser MR, Paty PB, Smith JJ, Wu AJ, Gollub MJ, Saltz LB, Garcia-Aguilar J. Long-Term Results of Organ Preservation in Patients With Rectal Adenocarcinoma Treated With Total Neoadjuvant Therapy: The Randomized Phase II OPRA Trial. J Clin Oncol. 2024;42:500-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 137] [Article Influence: 137.0] [Reference Citation Analysis (0)] |
| 30. | Thompson H, Kim JK, Yuval JB, Verheij F, Patil S, Gollub MJ, Wu AJ, Lee M, Hezel AF, Marcet J, Cataldo P, Polite BN, Herzig D, Liska D, Oommen S, Friel C, Ternent CA, Coveler AL, Hunt SR, Garcia-aguilar J. Survival and organ preservation according to clinical response after total neoadjuvant therapy in locally advanced rectal cancer patients: A secondary analysis from the organ preservation in rectal adenocarcinoma (OPRA) trial. J Clin Oncol. 2021;39:3509-3509. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Garcia-Aguilar J, Patil S, Gollub MJ, Kim JK, Yuval JB, Thompson HM, Verheij FS, Omer DM, Lee M, Dunne RF, Marcet J, Cataldo P, Polite B, Herzig DO, Liska D, Oommen S, Friel CM, Ternent C, Coveler AL, Hunt S, Gregory A, Varma MG, Bello BL, Carmichael JC, Krauss J, Gleisner A, Paty PB, Weiser MR, Nash GM, Pappou E, Guillem JG, Temple L, Wei IH, Widmar M, Lin S, Segal NH, Cercek A, Yaeger R, Smith JJ, Goodman KA, Wu AJ, Saltz LB. Organ Preservation in Patients With Rectal Adenocarcinoma Treated With Total Neoadjuvant Therapy. J Clin Oncol. 2022;40:2546-2556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 521] [Article Influence: 173.7] [Reference Citation Analysis (0)] |
| 32. | Langenfeld SJ, Davis BR, Vogel JD, Davids JS, Temple LKF, Cologne KG, Hendren S, Hunt S, Garcia Aguilar J, Feingold DL, Lightner AL, Paquette IM; Clinical Practice Guidelines Committee of the American Society of Colon and Rectal Surgeons. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Rectal Cancer 2023 Supplement. Dis Colon Rectum. 2024;67:18-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 33. | Dawood ZS, Alaimo L, Lima HA, Moazzam Z, Shaikh C, Ahmed AS, Munir MM, Endo Y, Pawlik TM. Circulating Tumor DNA, Imaging, and Carcinoembryonic Antigen: Comparison of Surveillance Strategies Among Patients Who Underwent Resection of Colorectal Cancer-A Systematic Review and Meta-analysis. Ann Surg Oncol. 2023;30:259-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 34. | Benešová L, Hálková T, Ptáčková R, Semyakina A, Menclová K, Pudil J, Ryska M, Levý M, Šimša J, Pazdírek F, Hoch J, Blaha M, Minárik M. Significance of postoperative follow-up of patients with metastatic colorectal cancer using circulating tumor DNA. World J Gastroenterol. 2019;25:6939-6948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 35. | Li Y, Xu J, Hu X, Chen Y, Liu F, Chen Y, Ma X, Dong Q, Sun L, Mo S, Zhang L, He X, Tong S, Wu H, Li W, Cai S, Zhu S, Pan Q, Peng J. Personalized circulating tumor DNA monitoring improves recurrence surveillance and management after curative resection of colorectal liver metastases: a prospective cohort study. Int J Surg. 2024;110:2776-2787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 36. | Li Y, Xu J, Chen Y, Ma X, Hu X, Chen Y, Sun L, Wu H, Xiu Z, Dong Q, Chen L, Liu J, Cai S, Peng J. Effect of dynamic circulating tumor DNA minimal residual disease detection on recurrence surveillance and second resection rate after curative intent resection of colorectal liver metastasis: A prospective cohort study. J Clin Oncol. 2023;41:242-242. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 37. | Reinert T, Henriksen TV, Christensen E, Sharma S, Salari R, Sethi H, Knudsen M, Nordentoft I, Wu HT, Tin AS, Heilskov Rasmussen M, Vang S, Shchegrova S, Frydendahl Boll Johansen A, Srinivasan R, Assaf Z, Balcioglu M, Olson A, Dashner S, Hafez D, Navarro S, Goel S, Rabinowitz M, Billings P, Sigurjonsson S, Dyrskjøt L, Swenerton R, Aleshin A, Laurberg S, Husted Madsen A, Kannerup AS, Stribolt K, Palmelund Krag S, Iversen LH, Gotschalck Sunesen K, Lin CJ, Zimmermann BG, Lindbjerg Andersen C. Analysis of Plasma Cell-Free DNA by Ultradeep Sequencing in Patients With Stages I to III Colorectal Cancer. JAMA Oncol. 2019;5:1124-1131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 307] [Cited by in RCA: 645] [Article Influence: 107.5] [Reference Citation Analysis (0)] |
| 38. | Tie J, Cohen JD, Wang Y, Christie M, Simons K, Lee M, Wong R, Kosmider S, Ananda S, McKendrick J, Lee B, Cho JH, Faragher I, Jones IT, Ptak J, Schaeffer MJ, Silliman N, Dobbyn L, Li L, Tomasetti C, Papadopoulos N, Kinzler KW, Vogelstein B, Gibbs P. Circulating Tumor DNA Analyses as Markers of Recurrence Risk and Benefit of Adjuvant Therapy for Stage III Colon Cancer. JAMA Oncol. 2019;5:1710-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 452] [Article Influence: 113.0] [Reference Citation Analysis (0)] |
| 39. | Kotani D, Oki E, Nakamura Y, Yukami H, Mishima S, Bando H, Shirasu H, Yamazaki K, Watanabe J, Kotaka M, Hirata K, Akazawa N, Kataoka K, Sharma S, Aushev VN, Aleshin A, Misumi T, Taniguchi H, Takemasa I, Kato T, Mori M, Yoshino T. Molecular residual disease and efficacy of adjuvant chemotherapy in patients with colorectal cancer. Nat Med. 2023;29:127-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 277] [Article Influence: 138.5] [Reference Citation Analysis (0)] |
| 40. | Li W, Huang X, Patel R, Schleifman E, Fu S, Shames DS, Zhang J. Analytical evaluation of circulating tumor DNA sequencing assays. Sci Rep. 2024;14:4973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 41. | Diehl F, Schmidt K, Choti MA, Romans K, Goodman S, Li M, Thornton K, Agrawal N, Sokoll L, Szabo SA, Kinzler KW, Vogelstein B, Diaz LA Jr. Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008;14:985-990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2240] [Cited by in RCA: 2112] [Article Influence: 124.2] [Reference Citation Analysis (0)] |
| 42. | Stetson D, Ahmed A, Xu X, Nuttall BRB, Lubinski TJ, Johnson JH, Barrett JC, Dougherty BA. Orthogonal Comparison of Four Plasma NGS Tests With Tumor Suggests Technical Factors are a Major Source of Assay Discordance. JCO Precis Oncol. 2019;3:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 43. | Iams WT, Mackay M, Ben-Shachar R, Drews J, Manghnani K, Hockenberry AJ, Cristofanilli M, Nimeiri H, Guinney J, Benson AB 3rd. Concurrent Tissue and Circulating Tumor DNA Molecular Profiling to Detect Guideline-Based Targeted Mutations in a Multicancer Cohort. JAMA Netw Open. 2024;7:e2351700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 35] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 44. | Nguyen Hoang V, Nguyen N, Le DN, Nguyen VS, Nguyen Thi HG, Vu HT, Ho HH, Le HM, Nguyen TD, Vo HN, Le Thi TS, Ha VT, Nguyen HD, Nguyen Thi MP, Tran Thi KP, Vi TD, Hoang MT, Vu HG, Nguyen T, Phan VT, Tam Phuc NH, Nguyen DS, Nguyen H, Tu LN. Real-World Utilization and Performance of Circulating Tumor DNA Monitoring to Predict Recurrence in Solid Tumors. JCO Oncol Adv. 2025;2. [DOI] [Full Text] |
| 45. | Li Y, Heer AK, Sloane HS, Edelstein DL, Tie J, Gibbs P, Barzi A. Budget Impact Analysis of Circulating Tumor DNA Testing for Colon Cancer in Commercial Health and Medicare Advantage Plans. JAMA Health Forum. 2024;5:e241270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 46. | Kramer A, Greuter MJE, Schraa SJ, Vink GR, Phallen J, Velculescu VE, Meijer GA, van den Broek D, Koopman M, Roodhart JML, Fijneman RJA, Retèl VP, Coupé VMH. Early evaluation of the effectiveness and cost-effectiveness of ctDNA-guided selection for adjuvant chemotherapy in stage II colon cancer. Ther Adv Med Oncol. 2024;16:17588359241266164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 47. | Cai LQ, Yang DQ, Wang RJ, Huang H, Shi YX. Establishing and clinically validating a machine learning model for predicting unplanned reoperation risk in colorectal cancer. World J Gastroenterol. 2024;30:2991-3004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (2)] |
| 48. | Rompianesi G, Pegoraro F, Ceresa CD, Montalti R, Troisi RI. Artificial intelligence in the diagnosis and management of colorectal cancer liver metastases. World J Gastroenterol. 2022;28:108-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 71] [Cited by in RCA: 72] [Article Influence: 24.0] [Reference Citation Analysis (3)] |
| 49. | Osman MH, Mohamed RH, Sarhan HM, Park EJ, Baik SH, Lee KY, Kang J. Machine Learning Model for Predicting Postoperative Survival of Patients with Colorectal Cancer. Cancer Res Treat. 2022;54:517-524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 50. | Zhao QX, He XL, Wang K, Cheng ZG, Han ZY, Liu FY, Yu XL, Hui Z, Yu J, Chao A, Liang P. Deep learning model based on contrast-enhanced ultrasound for predicting early recurrence after thermal ablation of colorectal cancer liver metastasis. Eur Radiol. 2023;33:1895-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 51. | Staal FCR, Taghavi M, van der Reijd DJ, Gomez FM, Imani F, Klompenhouwer EG, Meek D, Roberti S, de Boer M, Lambregts DMJ, Beets-Tan RGH, Maas M. Predicting local tumour progression after ablation for colorectal liver metastases: CT-based radiomics of the ablation zone. Eur J Radiol. 2021;141:109773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 52. | van der Reijd DJ, Guerendel C, Staal FCR, Busard MP, De Oliveira Taveira M, Klompenhouwer EG, Kuhlmann KFD, Moelker A, Verhoef C, Starmans MPA, Lambregts DMJ, Beets-Tan RGH, Benson S, Maas M. Independent validation of CT radiomics models in colorectal liver metastases: predicting local tumour progression after ablation. Eur Radiol. 2024;34:3635-3643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 53. | Xiao H, Weng Z, Sun K, Shen J, Lin J, Chen S, Li B, Shi Y, Kuang M, Song X, Weng W, Peng S. Predicting 5-year recurrence risk in colorectal cancer: development and validation of a histology-based deep learning approach. Br J Cancer. 2024;130:951-960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Reference Citation Analysis (0)] |
| 54. | Mohammadian Rad N, Sosef O, Seegers J, Koolen LJER, Hoofwijk JJWA, Woodruff HC, Hoofwijk TAGM, Sosef M, Lambin P. Prognostic models for colorectal cancer recurrence using carcinoembryonic antigen measurements. Front Oncol. 2024;14:1368120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 55. | Shinkins B, Nicholson BD, James T, Pathiraja I, Pugh S, Perera R, Primrose J, Mant D. What carcinoembryonic antigen level should trigger further investigation during colorectal cancer follow-up? A systematic review and secondary analysis of a randomised controlled trial. Health Technol Assess. 2017;21:1-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 56. | Wang P, Berzin TM, Glissen Brown JR, Bharadwaj S, Becq A, Xiao X, Liu P, Li L, Song Y, Zhang D, Li Y, Xu G, Tu M, Liu X. Real-time automatic detection system increases colonoscopic polyp and adenoma detection rates: a prospective randomised controlled study. Gut. 2019;68:1813-1819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 398] [Cited by in RCA: 547] [Article Influence: 91.2] [Reference Citation Analysis (0)] |
| 57. | Levin TR, Jensen CD, Marks AR, Schlessinger D, Liu V, Udaltsova N, Badalov J, Layefsky E, Corley DA, Nugent JR, Lee JK. Development and External Validation of a Prediction Model for Colorectal Cancer Among Patients Awaiting Surveillance Colonoscopy Following Polypectomy. Gastro Hep Adv. 2024;3:671-683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 58. | Yao L, Li S, Tao Q, Mao Y, Dong J, Lu C, Han C, Qiu B, Huang Y, Huang X, Liang Y, Lin H, Guo Y, Liang Y, Chen Y, Lin J, Chen E, Jia Y, Chen Z, Zheng B, Ling T, Liu S, Tong T, Cao W, Zhang R, Chen X, Liu Z. Deep learning for colorectal cancer detection in contrast-enhanced CT without bowel preparation: a retrospective, multicentre study. EBioMedicine. 2024;104:105183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 59. | Chalkidis G, McPherson JP, Beck A, Newman MG, Guo JW, Sloss EA, Staes CJ. External Validation of a Machine Learning Model to Predict 6-Month Mortality for Patients With Advanced Solid Tumors. JAMA Netw Open. 2023;6:e2327193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 60. | Youssef A, Pencina M, Thakur A, Zhu T, Clifton D, Shah NH. External validation of AI models in health should be replaced with recurring local validation. Nat Med. 2023;29:2686-2687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 66] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 61. | Van Calster B, Steyerberg EW, Wynants L, van Smeden M. There is no such thing as a validated prediction model. BMC Med. 2023;21:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 118] [Article Influence: 59.0] [Reference Citation Analysis (0)] |