Published online May 14, 2025. doi: 10.3748/wjg.v31.i18.105443
Revised: March 27, 2025
Accepted: April 24, 2025
Published online: May 14, 2025
Processing time: 110 Days and 18.3 Hours
Retroperitoneal fibrosis is a rare fibro-inflammatory condition which can be classified into idiopathic (accounting for over 75%) and secondary types (due to malignancies, infections, medications, radiotherapy or other conditions). Idio
An abdominal mass was discovered in a 52-year-old man during a routine physi
IRPF is a rare condition that presents considerable diagnostic challenges when lesions arise from the peritoneal space. In cases where imaging findings are atypical, a further puncture biopsy may be necessary to confirm the diagnosis.
Core Tip: This study reports an unusual case of idiopathic retroperitoneal fibrosis that was incidentally discovered on magnetic resonance imaging and 18F-fluorodeoxyglucose positron emission tomography imaging. The mass was primarily situated in the peritoneal cavity rather than the retroperitoneal space, encroaching upon adjacent organs such as the pancreatic head, the hepatic flexure of the colon, and part of the small intestine. The imaging diagnosis can be particularly difficult when the lesion originates in the peritoneal space, and a biopsy may be required prior to surgery.
- Citation: Dong ZY, Zhu HB. Idiopathic retroperitoneal fibrosis arising from peritoneal space: A case report and review of literature. World J Gastroenterol 2025; 31(18): 105443
- URL: https://www.wjgnet.com/1007-9327/full/v31/i18/105443.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i18.105443
Idiopathic retroperitoneal fibrosis (IRPF) is a rare condition characterized by the proliferation of fibro-inflammatory tissue in the retroperitoneal space, often encasing the abdominal aorta, inferior vena cava, and ureters[1-3]. The disease was first described by Ormond in 1948 and therefore is occasionally called Ormond’s disease[1]. The etiology remains unclear, although it is believed to be an autoimmune disease associated with the immunoglobulin G4 infiltration, potentially triggered by factors such as malignancies, infections, medications, radiotherapy or abdominal surgery[4-7]. Although there have been some reports of IRPF, most cases have been located at the retroperitoneal space[8-10]. We report a rare case originating from peritoneal space, which presents significant diagnostic challenges for radiologists due to the uncommon origin and atypical manifestations. This final diagnosis can be achieved through fine-needle aspiration biopsy, thereby effectively preventing unnecessary surgical procedures.
A 52-year-old male patient was admitted to our hospital with the complaint of an abdominal mass incidentally discovered during a routine physical examination.
The patient did not experience recent weight loss or symptoms such as abdominal pain, vomiting, nausea, or diarrhea.
The patient had no relevant past illness.
The patient had a one-year history of hypertension, with a maximum recorded blood pressure of 150/90 mmHg. The patient’s high blood pressure was effectively managed with oral Valsartan daily, which maintained the blood pressure at 120/80 mmHg.
The physical examination showed no abnormalities. No abdominal tenderness or masses were observed. The spleen, liver and enlarged lymph nodes were not palpable.
All laboratory investigations, including hematological tests, liver and kidney function tests, and tumor markers, such as carcinoembryonic antigen, carbohydrate antigen 19-9, carbohydrate antigen 72-4 and carbohydrate antigen 125, were within normal limits (Table 1)[11-18].
| Ref. | Number of cases | Male to female ratio | Age | Main clinical manifestations | Elevated CRP (%) | Elevated ESR (%) | Elevated serum IgG4 (%) | Diagnostic modality |
| Present study | 1 | 1:0 | 52 | No complaints | 0 | 0 | 0 | CT/MRI/PET-CT |
| Zhao et al[11] | 155 | 2.4:1 | 58.1 ± 12.0 | Low back pain | 63 | 86 | 38 | CT/MRI |
| Liao et al[12] | 142 | 5.4:1 | 54.3 ± 11.8 | Weight loss | 59 | 63 | 49 | CT/MRI |
| Hu et al[13] | 70 | 1.1:1 | 52.7 ± 12.9 | 61 | 54 | 44 | CT/MRI | |
| Zampeli et al[14] | 67 | 2.7:1 | 56.0 ± 9.2 | Low back pain | 78 | 69 | 36 | CT/MRI |
| Raffiotta et al[15] | 50 | 1.4:1 | 58.7 (50.9-64.0) | Low back pain | 66 | 93 | 17 | CT/MRI |
| Yachoui et al[16] | 26 | 3.3:1 | 58 (53.2-64.5) | Low back pain | 56 | 77 | 17 | CT |
| Moroni et al[17] | 22 | 1.2:1 | (38-76) | 72 | 68 | PET-CT | ||
| Choi et al[18] | 18 | 3.5:1 | 62 (54-65) | Flank pain | CT/MRI |
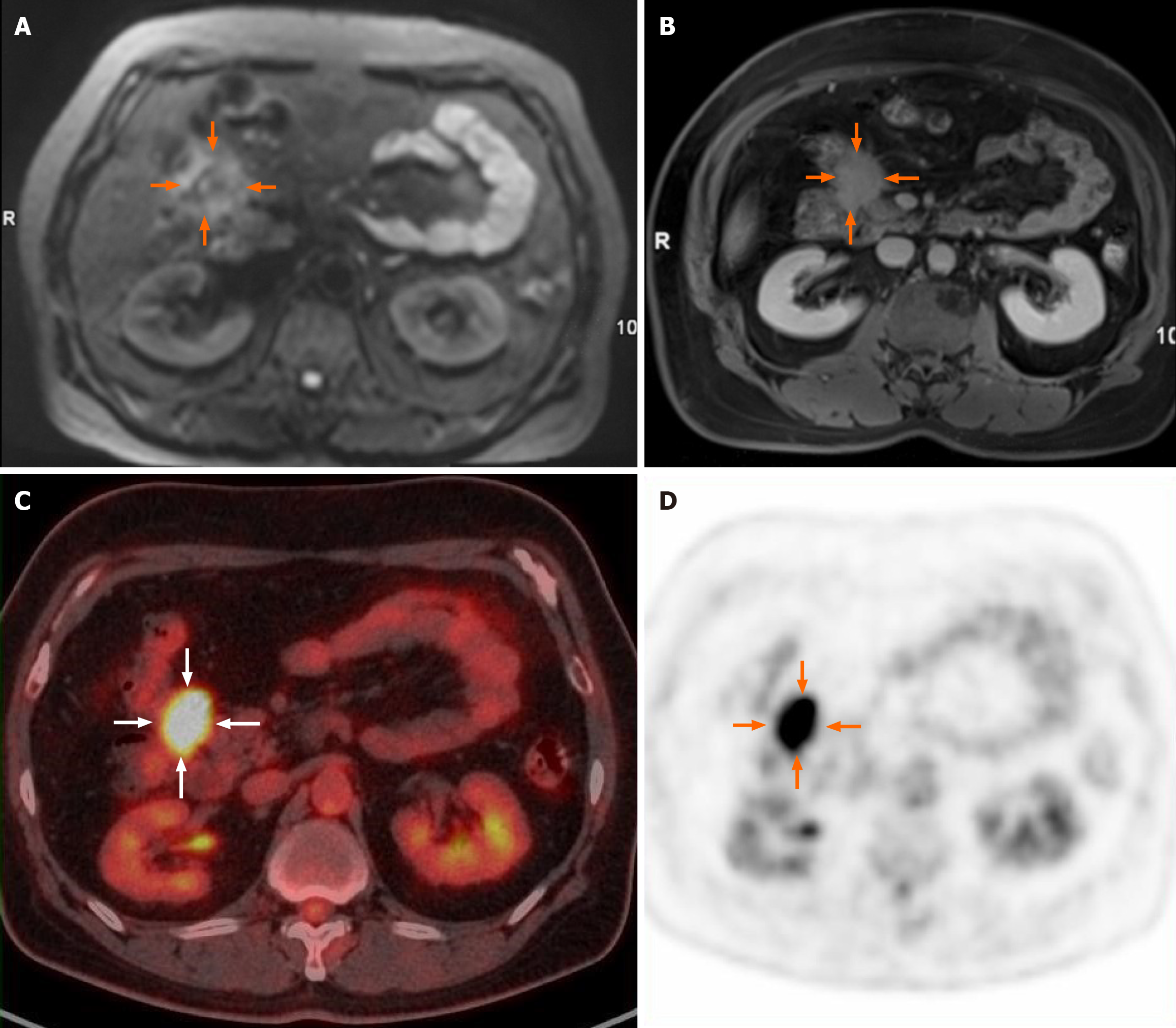
Abdominal magnetic resonance imaging (MRI) revealed an irregular soft tissue mass exhibiting slightly high signal intensity on both T1-weighted and T2-weighted images. The tumor displayed infiltrative changes with poorly defined margins and extended into adjacent structures, including the head of the pancreas and the hepatic flexure of the colon. Notably, it encircled the anterior superior pancreaticoduodenal artery. On diffusion-weighted imaging (DWI) with a β value of 800 mm²/second, the lesion showed slightly to moderately high signal intensity (Figure 1A), with the corresponding apparent diffusion coefficient (ADC) average value of 1.27 × 10-3 mm²/second for the maximum slice. Dynamic contrast-enhanced images indicated uneven enhancement during the arterial phase, with significant enhancement observed in the delayed phase (Figure 1B). 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) revealed increased uptake in the tumor (Figure 1C and D), with a maximum standardized uptake value (SUVmax) of 10.3. No abnormal lymph nodes were detected on PET/computed tomography (CT) images. Given the infiltrative characteristics of the tumor, a malignant neoplasm was suspected.
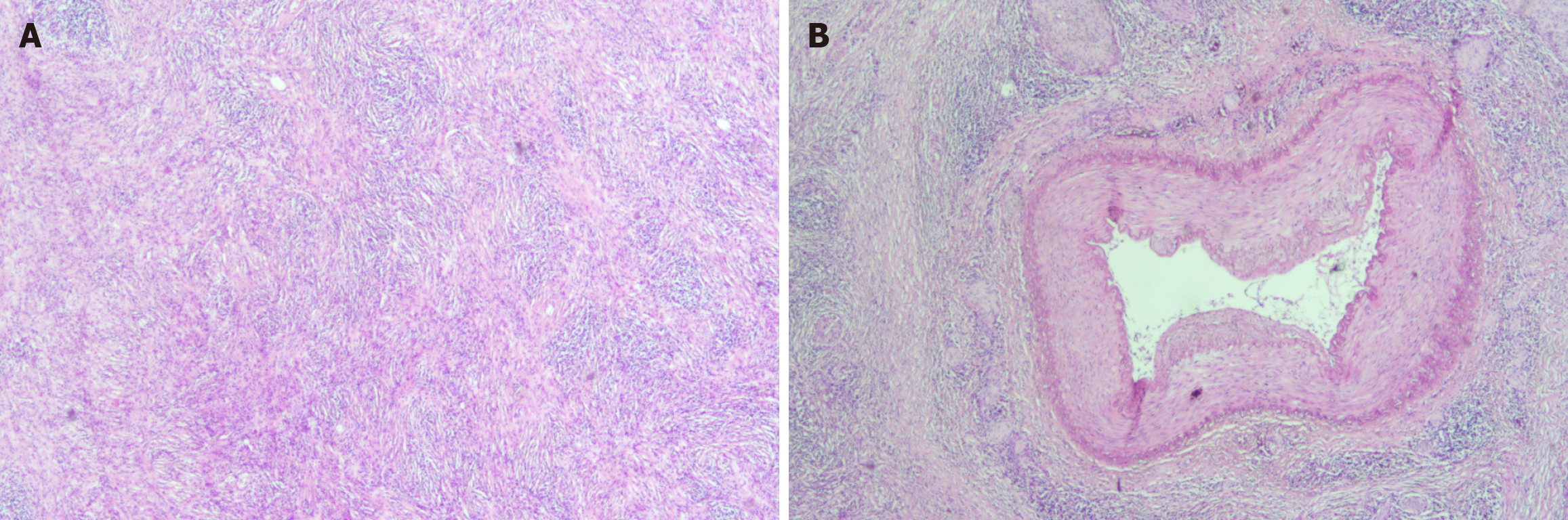
The patient was diagnosed with IRPF (Figure 2A and B) that invaded the muscularis propria of the colon following surgery.
During the surgical procedure, a hard mass was palpated, revealing significant adhesions to the mesenteric root of the colon, a portion of the small intestine and the pancreatic head, which complicated the dissection. In light of these findings, the patient underwent radical resection of the pancreatic head and duodenum, along with a right hemicolectomy and lymph node dissection.
The patient underwent routine follow-up for one year, which confirmed no recurrence.
Retroperitoneal fibrosis (RPF), also known as Ormond’s disease, has an estimated incidence of 0.1 to 1.4 cases per 100000 individuals[19]. The average age at diagnosis typically falls between 40 and 60 years, with a notable male predominance, reflected in a male-to-female ratio of approximately 2:1 to 5:1[20]. This fibroinflammatory disorder is primarily characterized by fibrotic masses in the retroperitoneal space, particularly surrounding the abdominal aorta and iliac arteries. Most cases (up to 70%) of RPF are classified as idiopathic (IRPF), with only a few resulting from specific causes, such as neoplasms, radiotherapy, infections, surgery, and medications[4].
The primary complaint associated with IRPF is often abdominal or back pain, accompanied by fatigue, anorexia, and weight loss. This pain is typically not exacerbated by eating or physical activity. When the lesion encases the ureter and renal blood vessels, it can lead to ureteral dilation, hydronephrosis, and renal hypertension[21]. Ureteral obstruction can even lead to the development of acute or chronic renal failure. In addition, up to one-third of patients may experience newly developed hypertension or a worsening of existing hypertension at diagnosis[22]. The symptoms are relatively non-specific and may not be helpful in differential diagnosis.
The clinical and laboratory manifestations in the present case and previous studies were summarized in Table 1. Laboratory findings in IRPF may show elevated levels of inflammatory markers such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), as well as reduced hemoglobin, indicating an active disease state[23]. However, the laboratory findings tend to be nonspecific and inadequate for establishing a definitive diagnosis.
Imaging examinations are essential for diagnosing and monitoring the treatment response in diseases. CT is the most frequently used imaging modality, employed to assess the location, extent, and potential causes of the condition. CT images typically reveal a homogeneous, well-defined soft tissue mass surrounding the retroperitoneal vessels[24,25]. MRI can provide valuable additionally information in monitoring the disease stage. For example, hyperintense signal intensity on T2-weighted and DWI can be detected due to edematous and vascular tissue with a large number of mononuclear cells during the active stage of the disease. Moreover, Kamper et al[26] noted that DWI can quantitatively differentiate between inactive and active disease as ADC values are higher for inactive IRPF compared to active cases. DWI is also useful for distinguishing inactive IRPF from malignant neoplasms arising in the retroperitoneal space[27]. Furthermore, the enhancement characteristics of IRPF vary across different stages of the disease[28]. In active phases, significant enhancement is observed while gradual enhancement can be commonly detected at the inactive stage.
Recently, the potential of 18F-FDG PET/CT in evaluating IRPF has emerged. For instance, Moroni et al[17] discovered that PET/CT can effectively distinguish between active and inactive IRPF, achieving a sensitivity, specificity, and accuracy of 95.5%, 90.9%, and 93.9%, respectively, when using SUVmax values. Additionally, a significant correlation was observed between SUVmax and various inflammatory markers, including CRP and ESR levels[29]. Moreover, PET/CT has been utilized to predict the early response to treatment. For example, Jansen et al[30] found that the visual PET score decreased after treatment with tamoxifen[30].
The differential diagnosis includes sarcoidosis and inflammatory myofibroblastic tumor. Peritoneal sarcoidosis typically presents as multiple enlarged lymph nodes along the hepatic hilar and mesentery on CT images. Notably, these enlarged lymph nodes usually demonstrate strong FDG uptake on PET images, which can assist in differentiating sarcoidosis from IRPF[31]. Peritoneal inflammatory myofibroblastic tumor can manifest as single or multiple heterogeneous lesions of larger size, often exhibiting central necrosis and early peripheral enhancement on contrast-enhanced images[32]. Despite these distinctions, the similarities in presentation among these conditions can lead to misdiagnosis. Therefore, biopsy may be required to confirm the diagnosis and rule out potential malignancy.
We present a rare case of IRPF originating from the peritoneal space and invading adjacent organs. This condition poses great challenge for clinicians due to its uncommon origin and atypical manifestations. Imaging is crucial for early detection and staging of the disease, and the definitive diagnosis can be confirmed by fine-needle aspiration biopsy, helping to avoid unnecessary surgical procedures.
| 1. | Ormond JK. Bilateral ureteral obstruction due to envelopment and compression by an inflammatory retroperitoneal process. J Urol. 1948;59:1072-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 416] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 2. | Vaglio A, Salvarani C, Buzio C. Retroperitoneal fibrosis. Lancet. 2006;367:241-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 472] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 3. | Li KP, Zhu J, Zhang JL, Huang F. Idiopathic retroperitoneal fibrosis (RPF): clinical features of 61 cases and literature review. Clin Rheumatol. 2011;30:601-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Urban ML, Palmisano A, Nicastro M, Corradi D, Buzio C, Vaglio A. Idiopathic and secondary forms of retroperitoneal fibrosis: a diagnostic approach. Rev Med Interne. 2015;36:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Rossi GM, Rocco R, Accorsi Buttini E, Marvisi C, Vaglio A. Idiopathic retroperitoneal fibrosis and its overlap with IgG4-related disease. Intern Emerg Med. 2017;12:287-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Koo BS, Koh YW, Hong S, Kim YJ, Kim YG, Lee CK, Yoo B. Clinicopathologic characteristics of IgG4-related retroperitoneal fibrosis among patients initially diagnosed as having idiopathic retroperitoneal fibrosis. Mod Rheumatol. 2015;25:194-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Lian L, Wang C, Tian JL. IgG4-related retroperitoneal fibrosis: a newly characterized disease. Int J Rheum Dis. 2016;19:1049-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Petelytska L, Nikitina A, Tarasenko O, Chechotenko I, Mykhailov D, Kravchenko V, Iaremenko O. IgG4-related retroperitoneal fibrosis with acute kidney injury: a case report and literature review. Rheumatol Int. 2023;43:2141-2153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Raval DM, Rathod VM, Dave M, Patel NS, Dobariya R. Idiopathic Retroperitoneal Fibrosis Presented As Urinary Tract Obstruction. Cureus. 2022;14:e29582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 10. | Yan T, Wang Y, Liu Z, Zhang X, Wu Q, Xi M. Idiopathic retroperitoneal fibrosis causing unilateral ureteral and sigmoid colon obstruction: A case report. Medicine (Baltimore). 2017;96:e6105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Zhao J, Li J, Zhang Z. Long-term outcomes and predictors of a large cohort of idiopathic retroperitoneal fibrosis patients: a retrospective study. Scand J Rheumatol. 2019;48:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Liao S, Wang Y, Li K, Zhu J, Zhang J, Huang F. Idiopathic retroperitoneal fibrosis: a cross-sectional study of 142 Chinese patients. Scand J Rheumatol. 2018;47:198-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Hu JQ, Jin ZY, Yu YY, Min DM, Cai Q, Gao J. Clinical characteristics of IgG4-related retroperitoneal fibrosis in a cohort of 117 patients with idiopathic retroperitoneal fibrosis: a retrospective study. Clin Rheumatol. 2025;44:757-766. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Zampeli E, Venetsanopoulou AI, Christaki S, Argyropoulou OD, Boki KA, Manoussakis MN, Skopouli FN, Tzioufas AG, Moutsopoulos HM. Idiopathic retroperitoneal fibrosis: clinical features, treatment modalities, relapse rate in Greek patients and a review of the literature. Clin Exp Rheumatol. 2022;40:1642-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Raffiotta F, da Silva Escoli R, Quaglini S, Rognoni C, Sacchi L, Binda V, Messa P, Moroni G. Idiopathic Retroperitoneal Fibrosis: Long-term Risk and Predictors of Relapse. Am J Kidney Dis. 2019;74:742-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Yachoui R, Sehgal R, Carmichael B. Idiopathic retroperitoneal fibrosis: clinicopathologic features and outcome analysis. Clin Rheumatol. 2016;35:401-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Moroni G, Castellani M, Balzani A, Dore R, Bonelli N, Longhi S, Martinelli I, Messa P, Gerundini P. The value of (18)F-FDG PET/CT in the assessment of active idiopathic retroperitoneal fibrosis. Eur J Nucl Med Mol Imaging. 2012;39:1635-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Choi SJ, Oh JS, Hong S, Lee CK, Yoo B, Hong B, Kim YG. Treatment Response to Idiopathic Retroperitoneal Fibrosis-associated Hydronephrosis With a Focus on IgG4/IgG3 Serum Concentration Ratio. J Rheum Dis. 2021;28:38-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Uibu T, Oksa P, Auvinen A, Honkanen E, Metsärinne K, Saha H, Uitti J, Roto P. Asbestos exposure as a risk factor for retroperitoneal fibrosis. Lancet. 2004;363:1422-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 111] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Kermani TA, Crowson CS, Achenbach SJ, Luthra HS. Idiopathic retroperitoneal fibrosis: a retrospective review of clinical presentation, treatment, and outcomes. Mayo Clin Proc. 2011;86:297-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 21. | Łoń I, Lewandowski J, Wieliczko M, Małyszko J. Retroperitoneal fibrosis, a rare entity with urorenal and vascular subtypes - preliminary data. Ren Fail. 2022;44:688-692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 22. | van Bommel EF. Retroperitoneal fibrosis. Neth J Med. 2002;60:231-242. [PubMed] |
| 23. | Tzou M, Gazeley DJ, Mason PJ. Retroperitoneal fibrosis. Vasc Med. 2014;19:407-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Si M, Zhang K, Li J, He H, Yao Y, Han J, Qiao J. Idiopathic retroperitoneal fibrosis with endometrial cancer: a case report and literature review. BMC Womens Health. 2022;22:399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Peisen F, Thaiss WM, Ekert K, Horger M, Amend B, Bedke J, Nikolaou K, Kaufmann S. Retroperitoneal Fibrosis and its Differential Diagnoses: The Role of Radiological Imaging. Rofo. 2020;192:929-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Kamper L, Brandt AS, Ekamp H, Abanador-Kamper N, Piroth W, Roth S, Haage P. Diffusion-weighted MRI findings of treated and untreated retroperitoneal fibrosis. Diagn Interv Radiol. 2014;20:459-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Bakir B, Yilmaz F, Turkay R, Ozel S, Bilgiç B, Velioglu A, Saka B, Salmaslioglu A. Role of diffusion-weighted MR imaging in the differentiation of benign retroperitoneal fibrosis from malignant neoplasm: preliminary study. Radiology. 2014;272:438-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Kamper L, Brandt AS, Scharwächter C, Kukuk S, Roth S, Haage P, Piroth W. MR evaluation of retroperitoneal fibrosis. Rofo. 2011;183:721-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Ruhlmann V, Poeppel TD, Brandt AS, Grüneisen J, Ruhlmann M, Theysohn JM, Forsting M, Bockisch A, Umutlu L. (18)F-FDG PET/MRI evaluation of retroperitoneal fibrosis: a simultaneous multiparametric approach for diagnosing active disease. Eur J Nucl Med Mol Imaging. 2016;43:1646-1652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Jansen I, Hendriksz TR, Han SH, Huiskes AW, van Bommel EF. (18)F-fluorodeoxyglucose position emission tomography (FDG-PET) for monitoring disease activity and treatment response in idiopathic retroperitoneal fibrosis. Eur J Intern Med. 2010;21:216-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Fu H, Guo X, Chen Z, Wu H, Chen H. Uncommon Imaging Findings of Inflammatory Myofibroblastic Tumor: Report of a Rare Case With Both Omentum and Mesentery Involvement in the Abdominal Cavity. Clin Nucl Med. 2018;43:e407-e409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Liang W, Lin S, Chen Z. Imaging findings of inflammatory myofibroblastic tumor from the greater omentum: One case report. Medicine (Baltimore). 2017;96:e8297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |