Published online May 7, 2025. doi: 10.3748/wjg.v31.i17.106508
Revised: March 27, 2025
Accepted: April 22, 2025
Published online: May 7, 2025
Processing time: 60 Days and 8 Hours
Colorectal cancer (CRC) during pregnancy poses significant risks to both maternal and fetal health; however, this topic remains under researched globally.
To investigate the impacts of clinical features, pathology type, treatment strate
To address this research gap, we analyzed the clinical and pathological characteristics of pCRC by collecting and evaluating clinicopathological data from 43 patients treated at the National Cancer Center/Cancer Hospital, Chinese Acade
Treatment for pCRC was initiated with surgery and/or chemotherapy. Among 43 patients, 37 underwent surgery, including 21 radical resections (5 prenatal and 16 postpartum resections) and 16 palliative surgeries. Chemotherapy (with regimens such as CapeOx or FOLFOX4) was administered to 37 patients. Six advanced-stage patients received chemotherapy alone. The gestational outcomes among the patients varied. Specifically, 5 patients who were diagnosed in early pregnancy chose abortion. Additionally, in mid-pregnancy, 3 patients underwent abortion, 1 required induced labor, and 2 underwent cesarean delivery with healthy neonates. Among the 3 late-pregnancy diagnoses, 1 patient underwent induced abortion, 1 delivered via cesarean section with a healthy fetus, and 1 underwent stillbirth management. The 5-year survival rate was 59.8%, with a rate of 100% for stage I/II patients, 75% for stage III patients, and 21.1% for stage IV patients.
Patients with poorly differentiated tumors exhibited worse outcomes than those with moderately and highly differentiated tumors. Early-stage diagnosis and timely treatment significantly improved maternal survival and fetal outcomes in pregnant patients with CRC. Advanced tumor stages and delayed diagnosis were observed to be associated with poorer maternal prognoses and may require interventions that compromise fetal survival. Fetal outcomes depend on the pathological stage of the mother’s cancer, the gestational age at diagnosis, and treatment strategies.
Core Tip: Pregnancy-associated colorectal cancer (pCRC) is a rare disease with a poor prognosis. Research on the prognosis of pCRC, particularly regarding factors influencing maternal and fetal outcomes, remains limited in Asia. This study encompassed all pCRC patients from Chinese cancer centers over a 24-year period and employed rigorous retrospective research methods. The findings highlight that while tumor location and advanced maternal age did not significantly impact survival, tailored strategies balancing maternal and fetal health are crucial, especially in cases of poorly differentiated tumors and advanced-stage disease.
- Citation: Fan LW, Shang C, Lin Q, Tian YT, Xu DK. Clinicopathological characteristics and prognostic outcomes of pregnancy-associated colorectal cancer: A 24-year experience. World J Gastroenterol 2025; 31(17): 106508
- URL: https://www.wjgnet.com/1007-9327/full/v31/i17/106508.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i17.106508
According to global cancer statistics for 2020, colorectal cancer (CRC) is the second deadliest cancer worldwide, and the mortality rate of CRC in female patients has surpassed that of cervical cancer, thus indicating that CRC has become the most lethal cancer after breast cancer[1]. As a very specific condition of CRC, pregnancy-associated CRC (pCRC) is defined as CRC diagnosed within 9 months before obstetric delivery and 12 months after childbirth[2]. Due to inadequate knowledge of pCRC, its manifestations are easily neglected by surgeons and obstetricians, thus putting patients at risk of pregnancy-specific complications[3]. This lack of awareness is extremely harmful to the fetus and mother; however, few studies have been conducted on this disease worldwide, and most of these studies are sporadic case reports that lack systematic research. Therefore, we collected and analyzed the clinical data of 43 patients with pCRC who were admitted to the Cancer Hospital of the Chinese Academy of Medical Sciences/National Cancer Center to investigate their clinical features, pathological types, and prognoses.
A retrospective study was conducted on 43 patients with pCRC at the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences, from 1999 to 2023. Clinical data, including pathological characteristics, treatment stra
All of the statistical analyses were performed using Sangerbox[4], SPSS (version 26.0) and GraphPad Prism 9. Qualitative data are presented as numbers with percentages, whereas quantitative data are presented as the mean ± SD. Continuous variables were compared via the Student’s t test, and categorical variables were compared using the χ2 and Spearman tests. All of the tests were two-sided, and a level of 0.05 was used to indicate statistical significance. Kaplan-Meier survival curves were used for analysis and compared via a log-rank test.
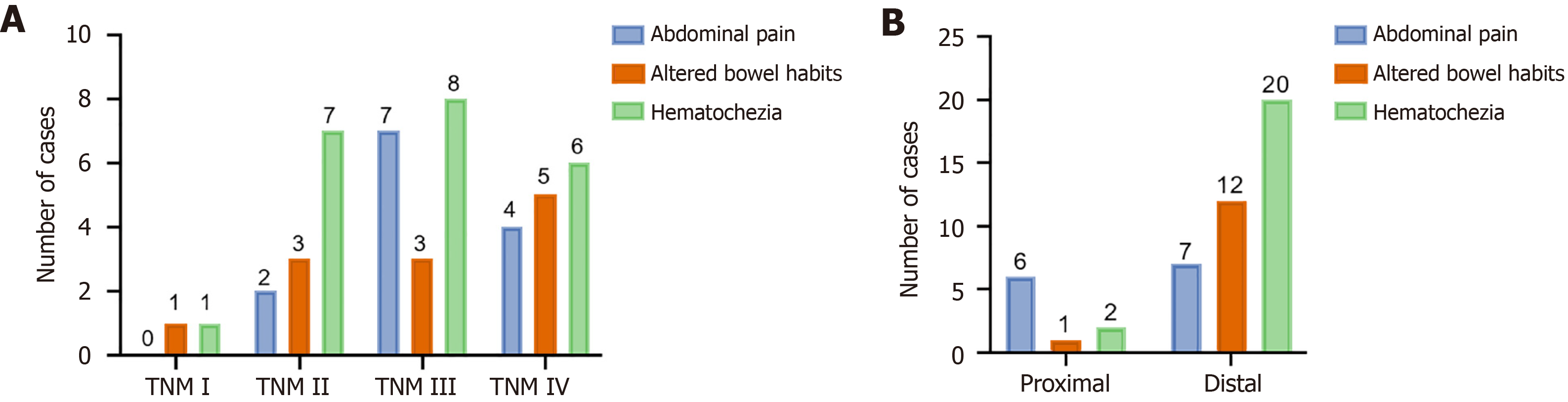
Among the 43 analyzed patients, the age ranged from 27-41 years, with a mean age of 31.9 ± 4 years. Fourteen patients were diagnosed during pregnancy, and 29 patients were diagnosed within 12 months after delivery. The most common initial symptoms of CRC during pregnancy included changes in bowel habits (n = 28), abdominal distension and pain (n = 13), and anal discomfort (n = 2).
All 43 patients were biopsied by electronic colonoscopy and pathologically confirmed. The cohort included 25 patients with poorly differentiated adenocarcinoma, 16 patients with moderately differentiated adenocarcinoma, and 2 patients with well-differentiated adenocarcinoma. Among these patients, 40 had adenocarcinoma, 2 had signet-ring cell carcinoma (SRCC), and 1 had mucinous carcinoma. According to the eighth Edition of the American Joint Committee on Cancer staging criteria[5], 2 patients were classified as stage I, 10 patients as stage II, 18 patients as stage III, and 13 patients as stage IV. Using the splenic flexure as the dividing point, 33 patients had tumors located in the distal region of the splenic flexure, whereas 10 patients had tumors in the proximal region of the splenic flexure. In this study, 13 patients had metastases, including 9 liver metastases, 7 ovarian metastases, 9 peritoneal and omental metastases, 3 mesenteric metastases, 10 retroperitoneal lymph node metastases, and 1 supraclavicular lymph node metastasis (Table 1).
| Characteristic | All pCRC, n | pCRC diagnosed < 35-year | pCRC diagnosed ≥ 35-year | P value |
| Total | 43 | 32 (74.4) | 11 (25.6) | < 0.001 |
| Location of CRC | 0.715 | |||
| Proximal | 10 | 7 (70) | 3 (30) | |
| Distal | 33 | 25 (75.8) | 8 (24.2) | |
| Pathological stage | 0.871 | |||
| TNM I | 2 | 1 (50) | 1 (50) | |
| TNM II | 10 | 7 (70) | 3 (30) | |
| TNM III | 18 | 15 (83.3) | 3 (16.7) | |
| TNM IV | 13 | 9 (69.2) | 4 (30.8) | |
| Survival (months) | 0.676 | |||
| mean | 52.3 | 52.6 | 51.5 | |
| Range | 1-208 | 1-208 |
If pCRC is diagnosed during pregnancy, treatment for these patients is entirely different from that for postpartum patients. When choosing a treatment plan, it is advisable to consider the statuses of both the mother and the fetus, as well as the order and timing of treatment[6,7].
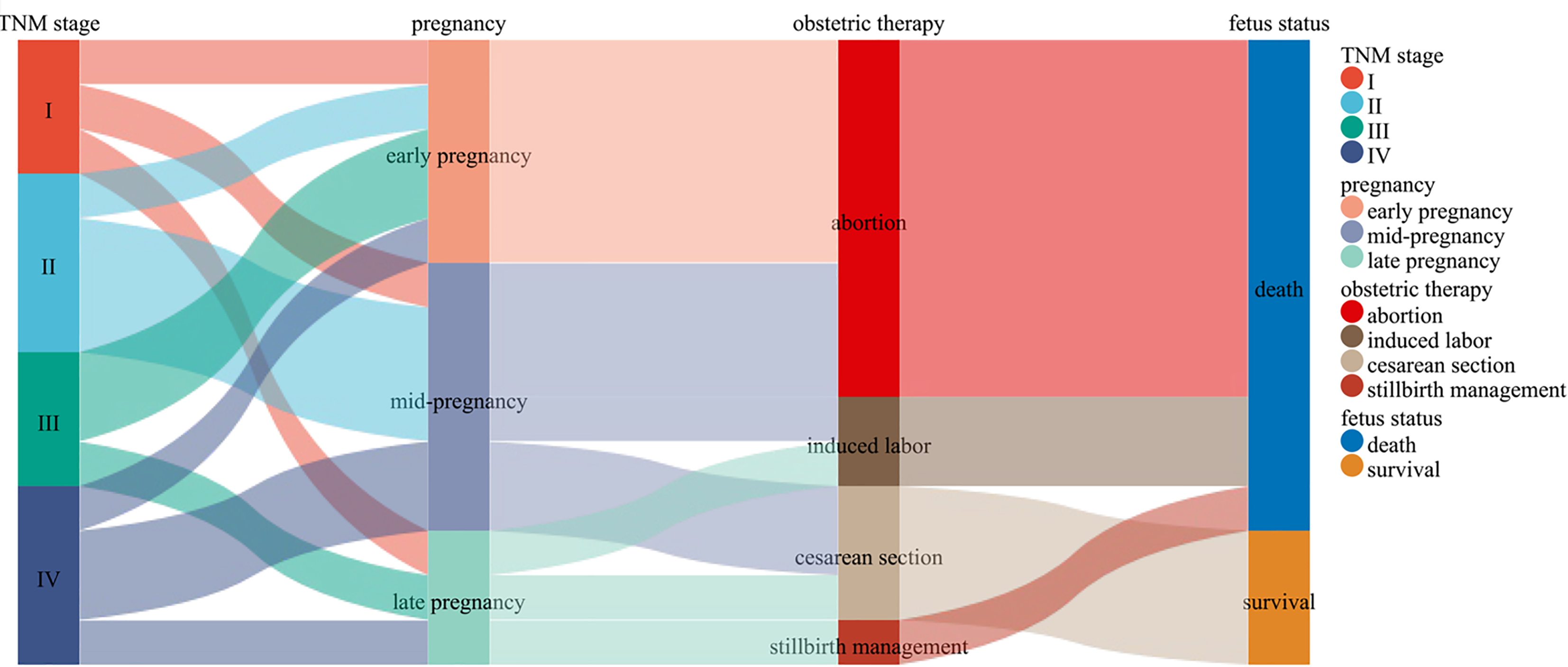
Obstetric treatment: Among the 43 patients, 14 were diagnosed during pregnancy: 5 in the early stage (within 12 weeks), all of whom opted for abortion; 6 in the mid-pregnancy stage (13-27 weeks), 3 of them made the decision to abort, 1 had an induced abortion, and 2 had a cesarean section; and 3 in the late-pregnancy stage (beyond 28 weeks). Among the latter group of patients, one patient underwent an induced abortion, one delivered via cesarean section, and one received stillbirth management. The fetus that was delivered via cesarean section was viable and in good health. Additionally, 29 patients were diagnosed within one year postpartum, including 12 normal deliveries and 17 cesarean deliveries, and all of the fetuses were healthy and alive (Figure 1).
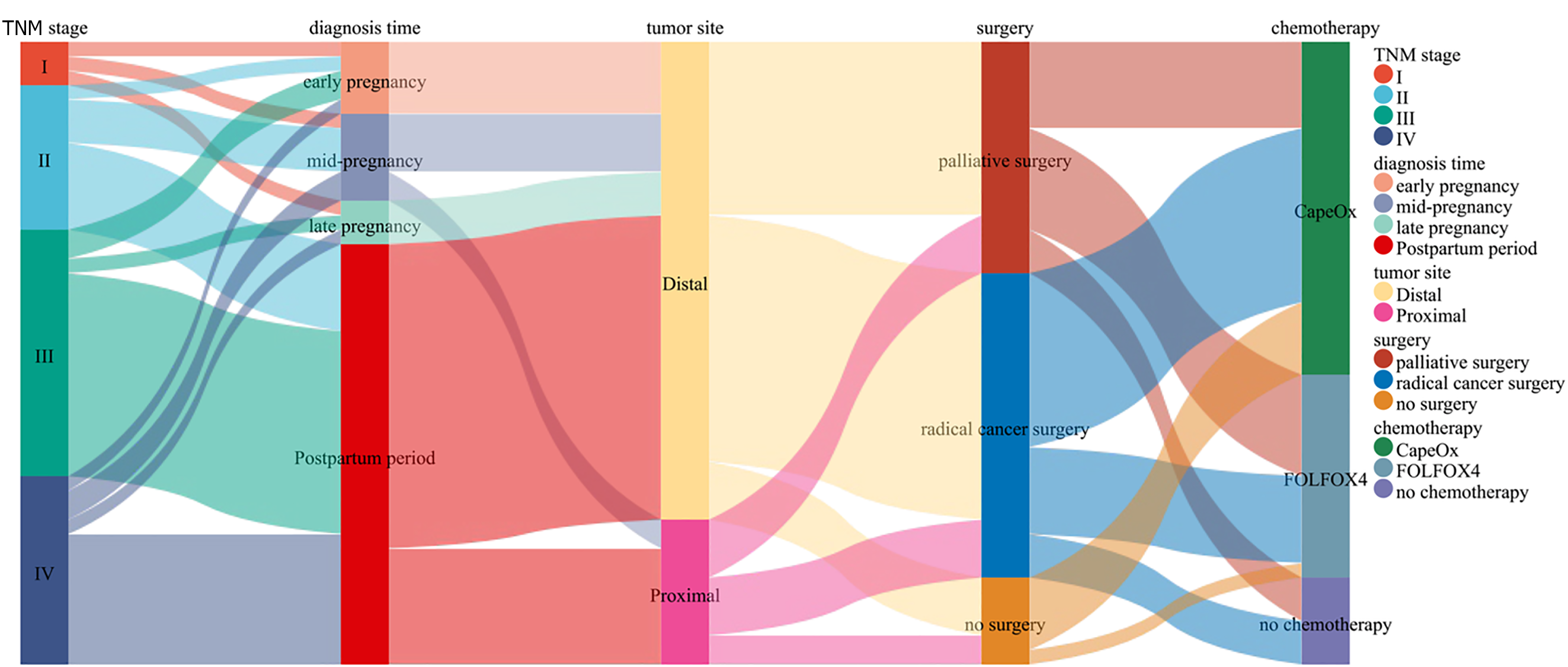
Tumor treatment: Among the 43 patients, 37 underwent surgical treatment, with the surgeries including 21 radical surgeries and 16 palliative surgeries. Among the 21 patients who underwent radical surgery, 5 were pregnant, of whom 1 patient in early pregnancy (within 12 weeks) underwent radical surgery after medical abortion according to obstetric evaluation; 2 patients in mid-pregnancy (13-27 weeks) experienced abortion or induced labor and underwent radical resection of sigmoid colon cancer; and 2 patients in late pregnancy (beyond 28 weeks) underwent either induced labor or cesarean section. The fetus delivered via cesarean section was healthy and survived, and both patients underwent radical rectal cancer surgery after delivery. Among these 5 cases of radical surgery during pregnancy, only 1 fetus was delivered via cesarean section after obstetric evaluation, and the fetus was alive and healthy because of the diagnosis occurring in late pregnancy. In the remaining 4 cases, the fetuses died. Among these 43 patients, 37 patients received chemotherapy, of whom 6 did not undergo surgical treatment due to the late pathology stages and received only chemotherapy with CapeOx or FOLFOX4. The remaining 31 patients received surgical combination chemotherapy, of whom 18 underwent adjuvant chemotherapy with CapeOx or FOLFOX4 after radical surgery, and the remaining 13 underwent chemotherapy after palliative surgery, with 5 patients being treated with FOLFOX4 and 8 patients being treated with CapeOx (Table 2, Figure 2).
| Treatment strategy | Pregnancy | Postpartum (within 12 months) | Total (n = 43) | ||
| Early pregnancy (within 12 weeks) | Mid-pregnancy (13-27 weeks) | Late pregnancy (beyond 28 weeks) | |||
| Type of surgery | |||||
| Palliative surgery | 3 | 4 | 1 | 8 | 16 (37.2) |
| Radical surgery | 1 | 2 | 2 | 16 | 21 (48.8) |
| No surgery | 1 | 0 | 0 | 5 | 6 (14.0) |
| Chemotherapy | |||||
| CapOx | 2 | 4 | 2 | 15 | 23 (53.5) |
| FLOX4 | 3 | 1 | 1 | 9 | 14 (32.5) |
| No chemotherapy | 0 | 1 | 0 | 5 | 6 (14.0) |
| Obstetric treatment | |||||
| Abortion | 5 | 3 | 0 | \ | 8 (18.6) |
| Induced labor | 0 | 1 | 1 | \ | 2 (4.7) |
| Stillbirth | 0 | 0 | 1 | \ | 1 (2.3) |
| Cesarean section | 0 | 2 | 1 | 17 | 20 (46.5) |
| Normal labor | 0 | 0 | 0 | 12 | 12 (27.9) |
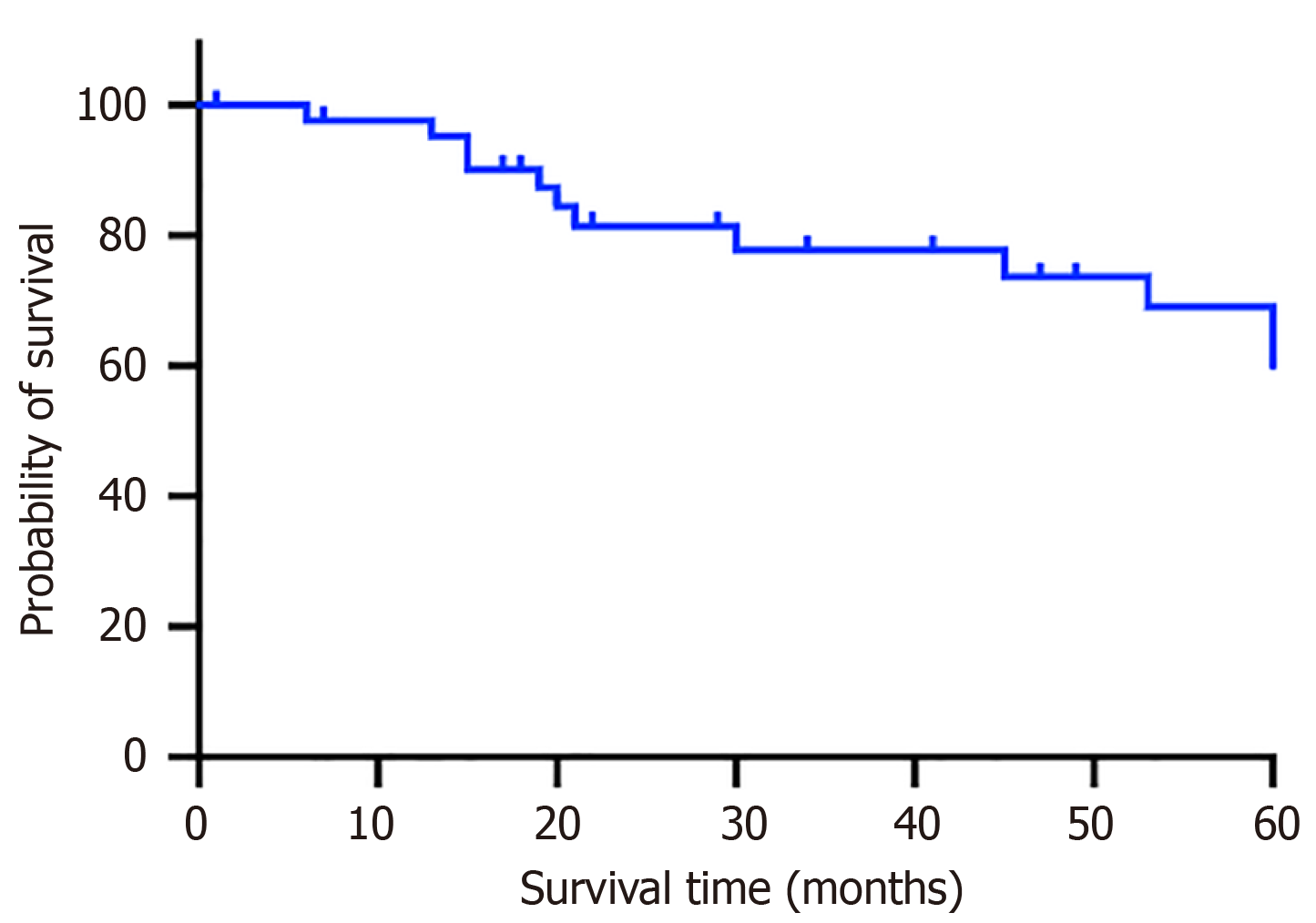
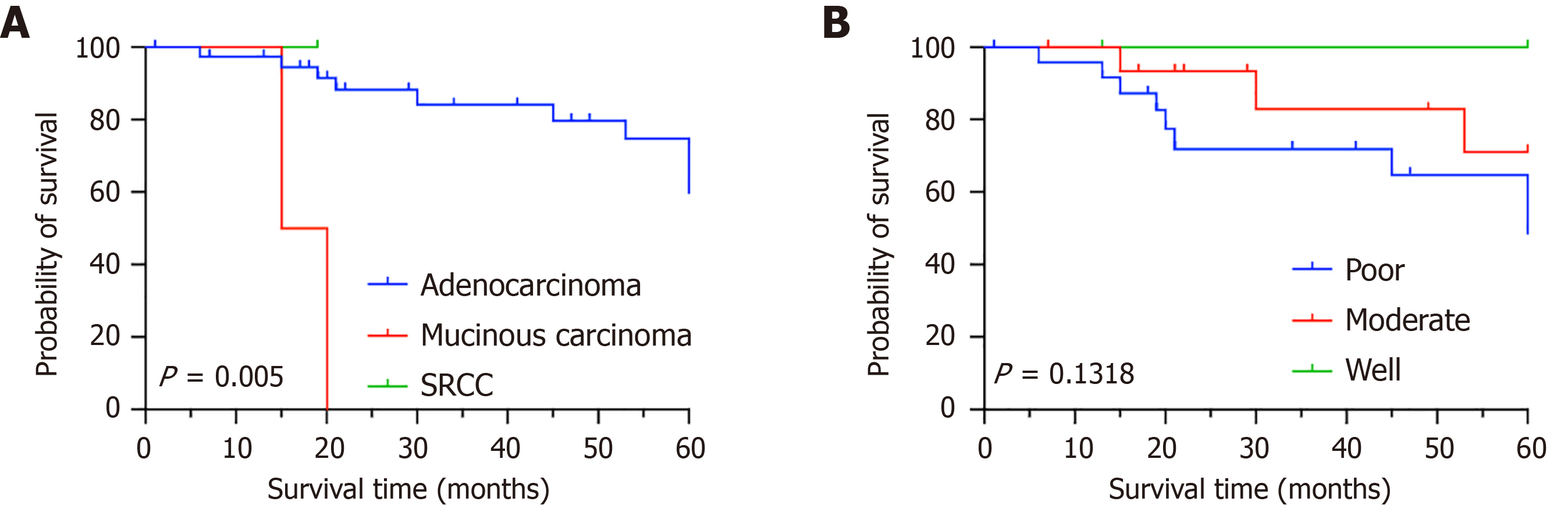
The 5-year OS rate of the 43 pCRC patients was 65.12% (Figure 3). The pathologic categories among the 43 pCRC patients included mucinous carcinoma (n = 1, 2.3%), SRCC (n = 2, 4.7%), and adenocarcinomas (n = 40, 93%) (P = 0.0005). The 5-year OS rate of patients with adenocarcinomas was 75% (Figure 4A). For poorly differentiated tumors (n = 25, 58.1%), moderately differentiated tumors (n = 16, 37.2%), and highly differentiated tumors (n = 2, 4.7%), the 5-year OS rates were 64%, 81.25%, and 100%, respectively (P = 0.1318) (Figure 4B).
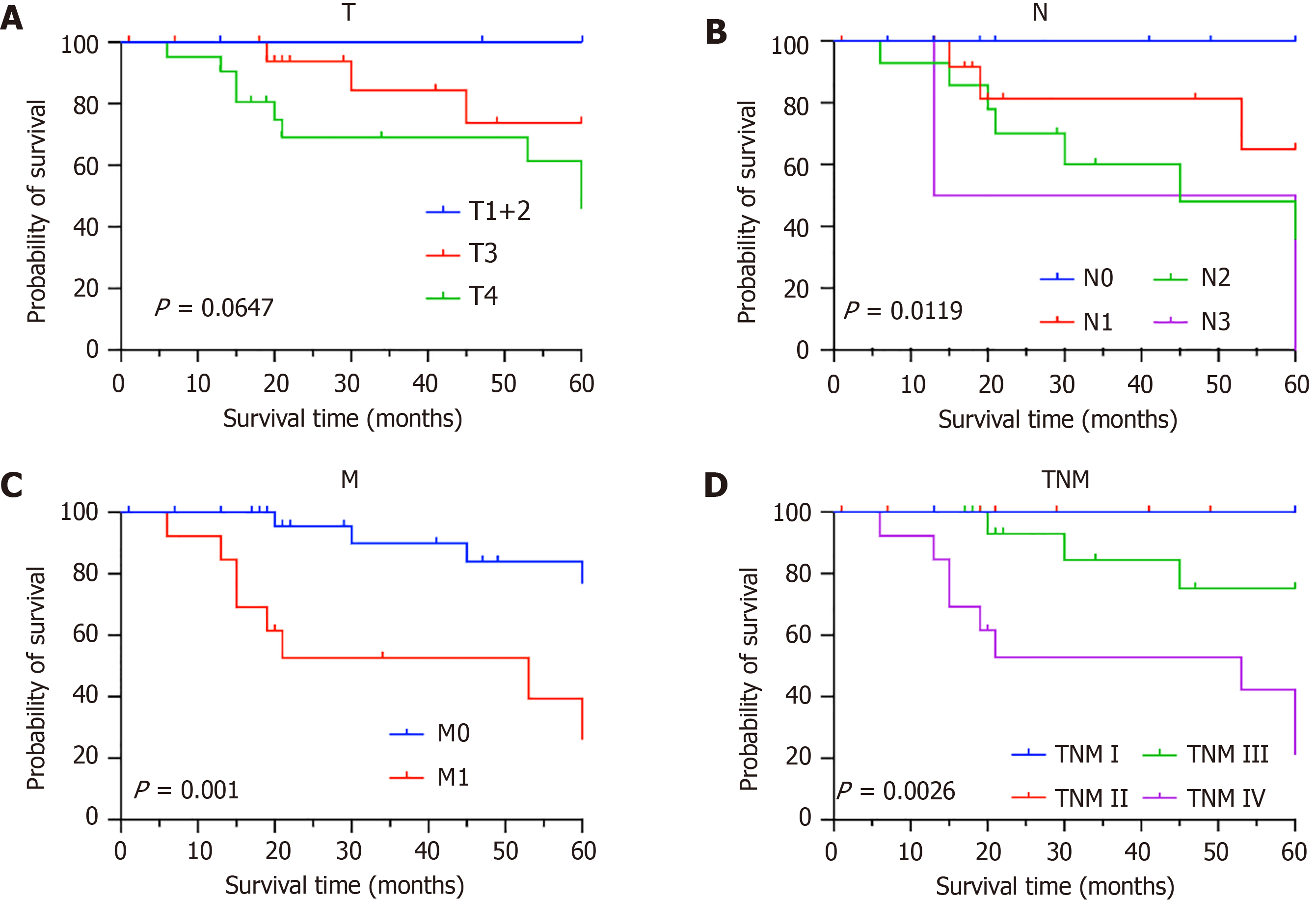
The patients were divided into three groups according to the extent of tumor invasion (T), including the T1 + T2, T3, and T4 groups; moreover, the 5-year OS rates of each group were compared (P = 0.0647) (Figure 5A). According to the degree of lymph node (N) metastasis, the three groups were categorized into N0, N1, and N2-N3 groups, and the 5-year OS rates for these groups were 100%, 78.57% and 43.75%, respectively (P = 0.0119) (Figure 5B). The following two groups were categorized according to the status of distant metastasis (M): M0 and M1 groups. The 5-year OS rates of the two groups were compared, and a statistically significant difference was observed (P = 0.001) (Figure 5C). Among patients without distant metastases, the 5-year OS rate was 86.67%. However, among those with distant metastases, the 5-year OS rate was 38.46%.
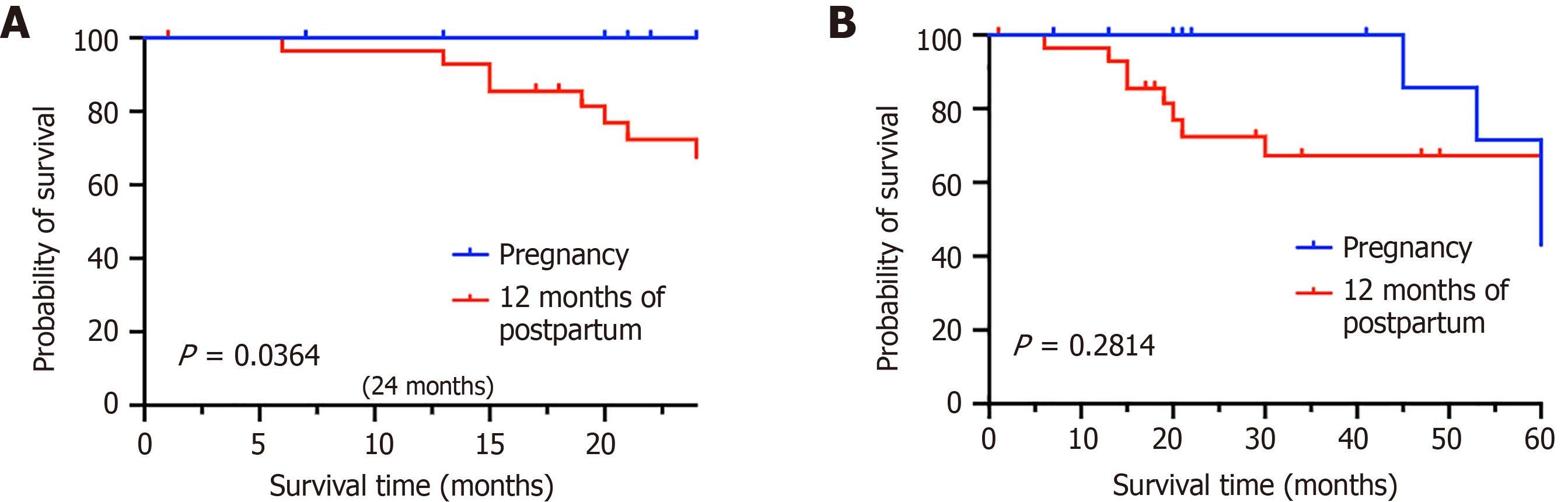
After treatment, patients with tumor-node-metastasis stages I and II disease (n = 12, 27.9%) had a 5-year OS rate of 100%. Patients with stage III disease (n = 18, 41.9%) had a 5-year OS rate of 75%, and patients with stage IV disease (n = 13, 30.2%) had a 5-year OS rate of 21.1%. Therefore, it was demonstrated that tumor-node-metastasis staging is strongly associated with the 5-year OS rate (P = 0.0026) (Figure 5D). Fourteen patients (32.6%) were diagnosed during pregnancy, and 29 patients (67.4%) were diagnosed one year postpartum. The 2-year survival rates for these two groups are 100% and 72.41%, respectively (P = 0.0364) (Figure 6A), and the 5-year OS rates for these two groups are 71.43% and 72.41%, respectively (P = 0.2814) (Figure 6B).
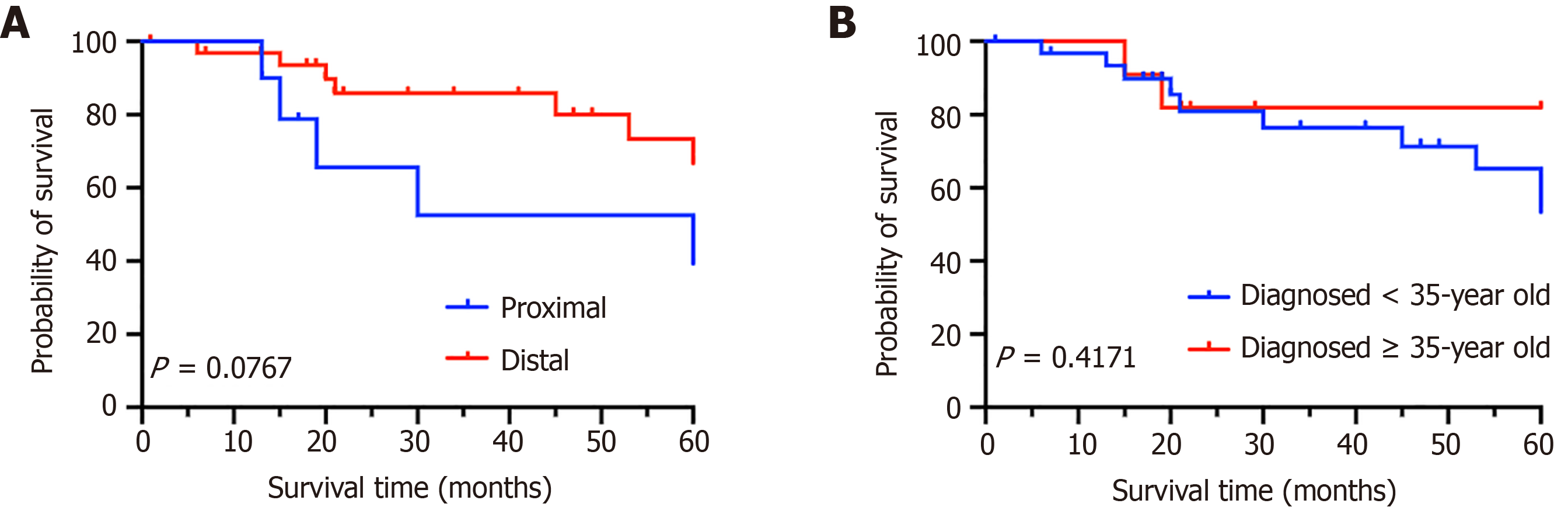
There were 10 patients (23.3%) with tumors located proximal to the splenic flexure and 33 patients (76.7%) with tumors located distal to the splenic flexure; additionally, the 5-year OS rates for the two groups were 50% and 78.79%, respectively (P = 0.0767) (Figure 7A). Among the 32 patients < 35-years-old (74.4%) and the other 11 patients 35-years-old (25.6%), the 5-year OS rates were 68.75% and 81.82%, respectively (P = 0.4171) (Figure 7B). The study found that tumor location and whether the patient is an advanced maternal age woman do not affect the 5-year OS rate.
According to global cancer statistics for 2020, new cases of CRC in Chinese women account for 48.6% of the 9.2 million global cases, with mortality due to CRC representing 55.5% of the 4.4 million global cancer deaths. CRC is the second most common cancer occurring in women, accounting for 9.4% of all cancer types; moreover, CRC is the third leading cause of cancer-related deaths, accounting for 9.5% of all cancer fatalities[1]. Additionally, statistical research by the China Cancer Center has shown that CRC has become the fourth most common cancer in Chinese women[8]. Every year, the incidence of CRC in pregnant women has increased. Obstetricians, gynecologists, and surgeons encounter significant challenges in the diagnosis and treatment of pCRC, as there is limited research and literature on this topic.
The clinical manifestations of pCRC are often masked by the symptoms and signs of pregnancy, and most surgeons, obstetricians, and gynecologists lack sufficient awareness of this phenomenon, which makes it easy for pCRC to be misdiagnosed and underdiagnosed[2]. In this study, most of the patients were diagnosed with advanced-stage tumors. Specifically, 41.9% of patients were diagnosed with stage III disease, and 30.2% of patients were diagnosed with stage IV disease (Figure 8).
Studies have shown that if a pCRC patient is diagnosed at an earlier point in time, a better prognosis can be achieved[7]. However, during pregnancy, the displacement of abdominal tissues and organs and increased pressure in the abdomen increase the difficulty of performing specialized examinations, and most colorectal examinations, such as digital rectal examination (DRE), endoscopy, and computed tomography (CT), demonstrate potential risks that can induce miscarriage, preterm delivery, and fetal distress[6,9]. Therefore, only 6 (13.95%) patients in this study terminated their pregnancies and underwent tests such as e-colonoscopy, and more patients elected to cancel or postpone their tests during pregnancy.
DRE is useful for the early detection of rectal cancer during pregnancy because of its simplicity and minimal influence on expectant mothers and fetuses. In this study, 6 patients (3 in early pregnancy, 2 in mid-pregnancy, and 1 in late pregnancy) underwent DRE, 8 patients (4 in early pregnancy, 2 in mid-pregnancy, and 2 in late pregnancy) underwent colonoscopy, and all the patients who were diagnosed within one year after delivery underwent colonoscopy.
In nonpregnant patients, abdominal CT is frequently utilized to identify local invasion or metastases. However, abdominal CT should not be performed during pregnancy (especially in the early stages) due to the teratogenicity of radiation. The pCRC stage can be accessed via magnetic resonance imaging (MRI) rather than CT. Although it is more expensive, the MRI approach is comparable to CT-resolved (N and M) staging[10,11]. Nonemergency MRI examinations should be avoided, especially in the early stages of pregnancy, even though studies assessing the safety of MRI during pregnancy have not revealed any negative consequences[6]. None of the patients in this study underwent CT or MRI procedures during pregnancy.
Even if hormone levels change during pregnancy, carcinoembryonic antigen (CEA) values either do not increase or only marginally increase in pregnant women without cancer[12]. The ratio of pre- to postoperative CEA levels has been linked to survival, according to current researches[13]. Elevated preoperative serum CEA levels are a poor prognostic indicator; specifically, a higher serum CEA level indicates a greater likelihood that the tumor will exhibit extensive growth and a greater likelihood of recurrence after surgery. The serum CEA level decreases to a normal level after surgery. If the serum CEA level does not return to a normal level after surgery, the surgery may be incomplete[13,14]. Unfortunately, there was considerable variation in the clinical collection time of the CEA level in this retrospective study, which created a more challenging scenario in examining the precise relationship between the CEA level and pCRC prognosis. Thus, CEA must be tested both before and after diagnosis and therapy, with all of the measurements being conducted within a consistent time frame. In summary, to increase the rate of early diagnosis, it is necessary to improve multidisciplinary collaboration and increase clinicians’ awareness of pCRC in pregnant females with gastrointestinal symptoms such as hematochezia and altered bowel habits.
Pregnant CRC patients demonstrate diverse clinical outcomes that are influenced by gestational timing and tumor progression. A favorable maternal tumor prognosis was associated with early-stage diagnosis and comprehensive treatment combining surgery and chemotherapy. In contrast, advanced tumor stage was correlated with poorer maternal survival. Moreover, fetal outcomes were generally favorable, with all of the full-term neonates being healthy (regardless of maternal treatment), thereby highlighting the idea that timely diagnosis and appropriate management can achieve a balance between maternal tumor control and fetal survival.
Surgical operation: Pregnancy at less than 20 weeks increases the bodily burden on the patient and induces immunosuppression, which promotes the development and metastasis of CRC. If therapy is postponed because of a missed diagnosis, misdiagnosis, or initiation of tumor treatment after delivery, the mother’s life may be in danger, and the condition may worsen. Radical surgery, which can significantly improve the prognosis of patients, is crucial for individuals with early-stage CRC. Therefore, to begin oncologic treatment as early as possible, induced abortion is often advised. In our study, after the patients’ tumor sites and stages were assessed, 8 of 11 (72.7%) patients in the early to middle stages of pregnancy at the time of diagnosis received oncologic treatment following abortion, with a 5-year survival rate of 40% being observed.
For pCRC patients diagnosed in late pregnancy, it is possible to monitor fetal development and evaluate the risk of tumor advancement before the initiation of oncological treatment, depending on the patient’s willingness to deliver the fetus. If the fetus’s lungs have matured and the number of pregnancy weeks is greater than 32 weeks, the mother may undergo a cesarean delivery before tumor treatment[6]. Among the 3 late pregnancy cases observed in our cohort, only 1 patient underwent cesarean delivery followed by anterior resection of the rectum. Of the remaining 2 cases, 1 patient experienced intrauterine fetal death and underwent systemic chemotherapy after the induction of labor, and the other patient underwent radical CRC surgery after the induction of labor.
In this study, three patients who were diagnosed during pregnancy underwent cesarean section, and no compressive tumor bleeding occurred. The strengthening of supportive treatment and the performance of cesarean section alongside radical tumor resection as early as possible are recommended. Although no complications were observed in this study, emergency surgery should be promptly performed if life-threatening complications (such as intestinal obstruction or perforation) occur.
Chemotherapy and radiotherapy: The risk of fetal teratogenicity associated with radiation and chemotherapy must be carefully considered, thus indicating that the induction of labor may be a recommended option following a diagnosis of pCRC.
In general, radiation therapy can be considered in the treatment of cancer that is located at a far distance from the fetus. However, due to the proximity of the rectum to the uterus, the fetus cannot be adequately protected from pelvic radiation. The radiation doses that are required for CRC treatment can cause serious teratogenic or fatal effects on the fetus. Therefore, it is recommended that this treatment be provided after delivery or the termination of pregnancy[15,16]. In this study, 2 patients in early pregnancy received radiotherapy-synchronized chemotherapy (CapeOX) after abortion. Notably, recent studies have suggested that pelvic radiotherapy for rectal cancer may lead to premature ovarian failure[5,14]. For pCRC patients with reproductive considerations, the selection of the radiotherapy dose and regimen must be carefully evaluated to balance therapeutic efficacy with potential impacts on fertility and fetal health.
Chemotherapeutic drugs may affect the development of early embryos, which significantly increases the risks of stillbirth and intrauterine growth limitations[17]. FOLFOX4 and CapeOx are often used to treat CRC, as recommended by the National Comprehensive Cancer Network Colorectal Cancer Treatment Guidelines[17,18]. Regarding these two commonly used regimens, 5-fluorouracil may cause fetal growth restrictions when used during pregnancy, and there are very limited safety data available on the use of oxaliplatin, capecitabine, and bevacizumab during this time period[19,20]. Although FOLFOX is considered to be the typical chemotherapy regimen for the treatment of nonpregnant patients with advanced CRC, it should be avoided as much as possible during pregnancy[21,22]. Similarly, breastfeeding is contraindicated for mothers who have received chemotherapy postpartum. In this study, all of the patients were managed with adjuvant or systemic chemotherapy following the termination of pregnancy, and none of the infants were breastfed.
This study analyzed 43 patients with pCRC, with a reported 5-year OS rate of 65.12%. In comparison, a cohort study by Mayo, which included 31 pCRC patients, demonstrated a 5-year OS rate of 62%. In the Mayo cohort, the distributions of pathological stages at diagnosis were 19.4% for stage I, 12.9% for stage II, 25.8% for stage III, and 41.9% for stage IV[23]. In our study, the pathological stage distributions at diagnosis were as follows: 4.7% for stage I, 23.2% for stage II, 41.9% for stage III, and 30.2% for stage IV. Notably, the 5-year OS rates in our study were 100% for stages I and II, 75% for stage III, and 21.1% for stage IV, thus underscoring the critical role of pathological stage as a key prognostic factor.
Histological analysis revealed that the majority of pCRC cases were adenocarcinomas, which are associated with a more favorable prognosis. The pathological subtypes included adenocarcinoma (93%), SRCC (4.7%), and mucinous carcinoma (2.3%), with a statistically significant difference in outcomes being observed among these subtypes (P = 0.0005). These findings highlight the impact of histological type on patient prognosis. Interestingly, no statistically significant correlation was observed between tumor differentiation and prognosis in this study, which is a result that warrants further investigation to clarify its clinical implications. In addition to pathological data, we evaluated the influence of patient age and tumor location on the prognosis of pCRC patients. Subgroup analysis revealed that neither the anatomical site of the tumor nor the patient’s age at the time of pregnancy significantly affected the overall prognosis.
Fetal prognoses depend on the pathologic stage of the mother’s cancer, the gestational week at diagnosis, and treatment strategies[24]. Maternal homeostasis and nutrition are essential for normal fetal development. Severe maternal frailty and advanced cancer are strongly linked to fetal malnutrition and a lower likelihood of fetal survival. However, there have been no reports of CRC metastases in utero. This result is primarily due to the protective effect of the placental barrier and the fetal immune system[5]. The associations between pCRC and adverse pregnancy or neonatal outcomes remain poorly understood. Due to the limited follow-up duration, the long-term effects of pCRC on fetal growth remain unclear.
In this study, among 14 patients who were diagnosed during pregnancy, those in early pregnancy opted for ter
Early-stage diagnosis and treatment lead to better maternal survival and favorable fetal outcomes. Advanced tumor stage and delayed diagnosis worsen maternal prognosis and may require interventions that reduce fetal survival. Patient age and tumor site do not affect prognosis, and early detection and optimized treatment strategies are crucial for improving these outcomes.
The authors would like to thank all of the patients and their families for their cooperation and participation. In addition, the authors are thankful to all of the research staff and coinvestigators who were involved in this investigation.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64681] [Article Influence: 16170.3] [Reference Citation Analysis (177)] |
| 2. | Samadder NJ, Smith KR, Wong J, Burt RW, Curtin K. Colorectal cancer in the setting of pregnancy and familial risk. Int J Colorectal Dis. 2020;35:1559-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13300] [Cited by in RCA: 15475] [Article Influence: 2579.2] [Reference Citation Analysis (2)] |
| 4. | Shen W, Song Z, Zhong X, Huang M, Shen D, Gao P, Qian X, Wang M, He X, Wang T, Li S, Song X. Sangerbox: A comprehensive, interaction-friendly clinical bioinformatics analysis platform. Imeta. 2022;1:e36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 491] [Cited by in RCA: 685] [Article Influence: 228.3] [Reference Citation Analysis (0)] |
| 5. | Weiser MR. AJCC 8th Edition: Colorectal Cancer. Ann Surg Oncol. 2018;25:1454-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 651] [Article Influence: 93.0] [Reference Citation Analysis (0)] |
| 6. | Bukhari Y, Hogan NM, Pomeroy M, O'Leary M, Joyce MR. Surgical management of rectal cancer in pregnancy. Int J Colorectal Dis. 2013;28:883-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Neal RD, Tharmanathan P, France B, Din NU, Cotton S, Fallon-Ferguson J, Hamilton W, Hendry A, Hendry M, Lewis R, Macleod U, Mitchell ED, Pickett M, Rai T, Shaw K, Stuart N, Tørring ML, Wilkinson C, Williams B, Williams N, Emery J. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. 2015;112 Suppl 1:S92-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 576] [Cited by in RCA: 725] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 8. | Zheng R, Zhang S, Zeng H, Wang S, Sun K, Chen R, Li L, Wei W, He J. Cancer incidence and mortality in China, 2016. J Natl Cancer Cent. 2022;2:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 852] [Cited by in RCA: 951] [Article Influence: 317.0] [Reference Citation Analysis (1)] |
| 9. | Pellino G, Simillis C, Kontovounisios C, Baird DL, Nikolaou S, Warren O, Tekkis PP, Rasheed S. Colorectal cancer diagnosed during pregnancy: systematic review and treatment pathways. Eur J Gastroenterol Hepatol. 2017;29:743-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Wong EM, Leung JL, Cheng CS, Lee JC, Li MK, Chung CC. Effect of endorectal coils on staging of rectal cancers by magnetic resonance imaging. Hong Kong Med J. 2010;16:421-426. [PubMed] |
| 11. | Kim SH, Lee JM, Lee MW, Kim GH, Han JK, Choi BI. Diagnostic accuracy of 3.0-Tesla rectal magnetic resonance imaging in preoperative local staging of primary rectal cancer. Invest Radiol. 2008;43:587-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Al-Ibrahim A, Parrish J, Dunn E, Swallow C, Maxwell C. Pregnancy and maternal outcomes in women with prior or current gastrointestinal malignancies. J Obstet Gynaecol Can. 2014;36:34-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Egenvall M, Martling A, Veres K, Horváth-Puhó E, Wille-Jørgensen P, Høirup Petersen S, Laurberg S, Sørensen HT, Syk I; COLOFOL Study Group. No benefit of more intense follow-up after surgery for colorectal cancer in the risk group with elevated CEA levels - An analysis within the COLOFOL randomized clinical trial. Eur J Surg Oncol. 2021;47:2053-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Cappell MS. Colon cancer during pregnancy. Gastroenterol Clin North Am. 2003;32:341-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | McCormick A, Peterson E. Cancer in Pregnancy. Obstet Gynecol Clin North Am. 2018;45:187-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Makoshi Z, Perrott C, Al-Khatani K, Al-Mohaisen F. Chemotherapeutic treatment of colorectal cancer in pregnancy: case report. J Med Case Rep. 2015;9:140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Applegate KE. The International Commission on Radiological Protection: Working Toward Keeping Recommendations Fit for Purpose. J Am Coll Radiol. 2023;20:721-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Robson DE, Lewin J, Cheng AW, O'Rourke NA, Cavallucci DJ. Synchronous colorectal liver metastases in pregnancy and post-partum. ANZ J Surg. 2017;87:800-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Farkas L, Garrido-Laguna I, Grem JL, Gunn A, Hecht JR, Hoffe S, Hubbard J, Hunt S, Johung KL, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Gregory KM, Gurski LA. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:329-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1054] [Cited by in RCA: 959] [Article Influence: 239.8] [Reference Citation Analysis (16)] |
| 20. | Levine O, Zbuk K. Colorectal cancer in adolescents and young adults: Defining a growing threat. Pediatr Blood Cancer. 2019;66:e27941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Dotters-Katz S, McNeil M, Limmer J, Kuller J. Cancer and pregnancy: the clinician's perspective. Obstet Gynecol Surv. 2014;69:277-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Leowattana W, Leowattana P, Leowattana T. Systemic treatment for metastatic colorectal cancer. World J Gastroenterol. 2023;29:1569-1588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 49] [Article Influence: 24.5] [Reference Citation Analysis (1)] |
| 23. | Grass F, Spindler BA, Naik ND, Thiels CA, Dozois EJ, Larson DW, Mathis KL. Oncological outcome of peripartum colorectal carcinoma-a single-center experience. Int J Colorectal Dis. 2019;34:899-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Dahling MT, Xing G, Cress R, Danielsen B, Smith LH. Pregnancy-associated colon and rectal cancer: perinatal and cancer outcomes. J Matern Fetal Neonatal Med. 2009;22:204-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |