Published online Apr 21, 2025. doi: 10.3748/wjg.v31.i15.103512
Revised: March 4, 2025
Accepted: April 2, 2025
Published online: April 21, 2025
Processing time: 148 Days and 1.4 Hours
Transjugular intrahepatic portosystemic shunts (TIPSs) are generally used for the management of complications of portal hypertension in patients with decom
Core Tip: Hepatic encephalopathy (HE) is the main complication following transjugular intrahepatic portosystemic shunt (TIPS), significantly impairs patients’ quality of life. Preoperative evaluation is crucial to mitigate the risk of post-TIPS HE. The shunt diameter and embolization of spontaneous portosystemic shunts should be considered according to the portal pressure gradient. Rifaximin is effective for preventing post-TIPS HE. Endovascular shunt reduction is a potential in
- Citation: Li Y, Wu YT, Wu H. Management of hepatic encephalopathy following transjugular intrahepatic portosystemic shunts: Current strategies and future directions. World J Gastroenterol 2025; 31(15): 103512
- URL: https://www.wjgnet.com/1007-9327/full/v31/i15/103512.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i15.103512
Transjugular intrahepatic portosystemic shunt (TIPS) placement is a standard procedure for treating portal hypertension-related complications, which mainly include recurrent ascites and variceal rebleeding. The major drawbacks of TIPS have been shunt dysfunction and the development of hepatic encephalopathy (HE)[1]. With technical progress and the use of polytetrafluoroethylene-covered stents, the rate of shunt dysfunction has dramatically decreased[2]. However, HE, which is induced and exacerbated by TIPS placement, continues to pose a significant clinical challenge in patients with decompensated cirrhosis[3]. The incidence of post-TIPS HE ranges from 35% to 50%, seriously affects the quality of life of patients and their families, increases readmission and is a major cause of health care expenses[4-7].
The diagnosis of overt HE (OHE) is usually straightforward in clinical practice once other known brain diseases are excluded. However, minimal HE (MHE) is characterized by subtle signs and symptoms that can be detected only through specialized psychometric tests. HE can be classified into three types according to its pathogenesis: Type A is found in patients with acute liver failure, type B is found in those with portosystemic shunts, and type C is found in those with cirrhosis[8]. This review focuses mainly on patients with type C HE. HE is classified into two main categories based on severity: Covert HE, including MHE and West Haven grades 0-1; and OHE, which includes grades 2-4. Furthermore, in terms of its temporal evolution, OHE can be classified as episodic, recurrent (more than one episode within a 6-month period) or persistent (no return to normal or baseline neuropsychiatric function between episodes)[8].
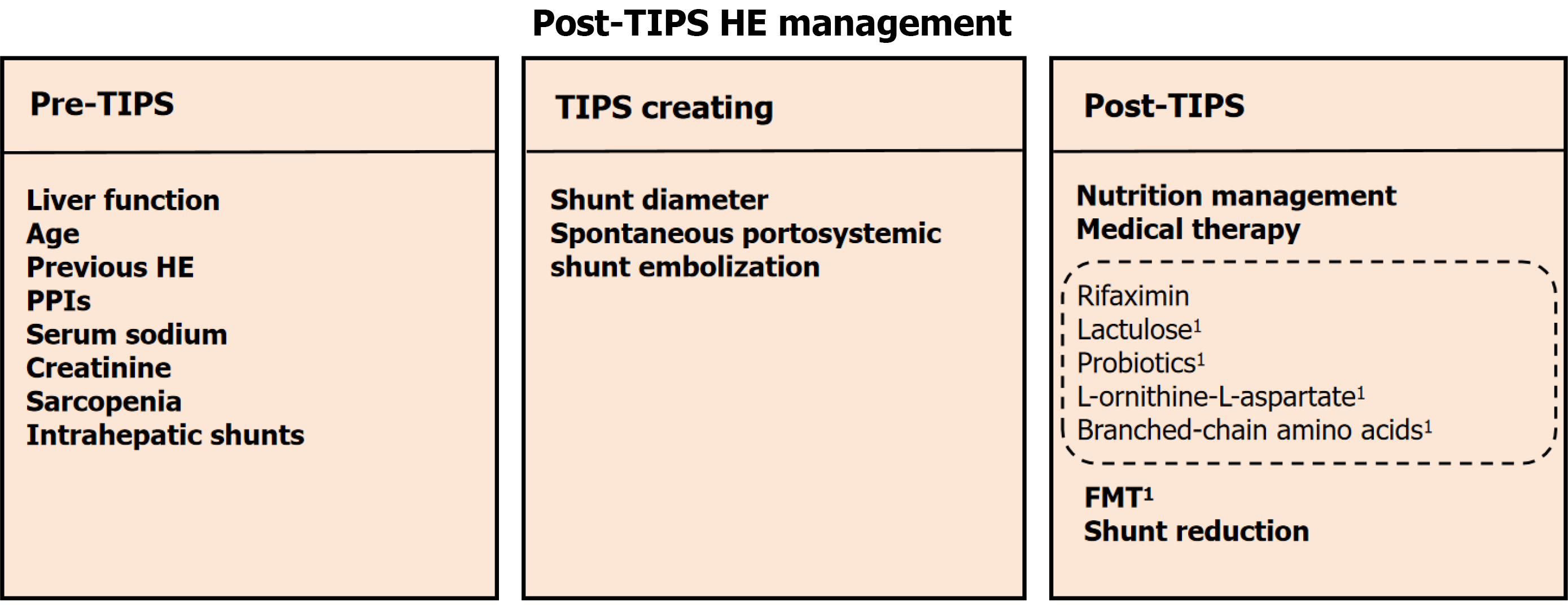
Post-TIPS HE is closely associated with patient baseline characteristics, portosystemic shunt diameter, status of spontaneous portosystemic shunt (SPSS) embolization, and postoperative management. It is imperative for clinicians to understand the pathophysiological mechanisms of post-TIPS HE to effectively prevent and manage this complication. Therefore, this review outlines comprehensive strategies for the prevention and treatment of post-TIPS HE based on its underlying mechanisms, encompassing preoperative, intraoperative, and postoperative approaches (Figure 1). We aim to offer practical recommendations for minimizing HE occurrence and improving patient outcomes after TIPS.
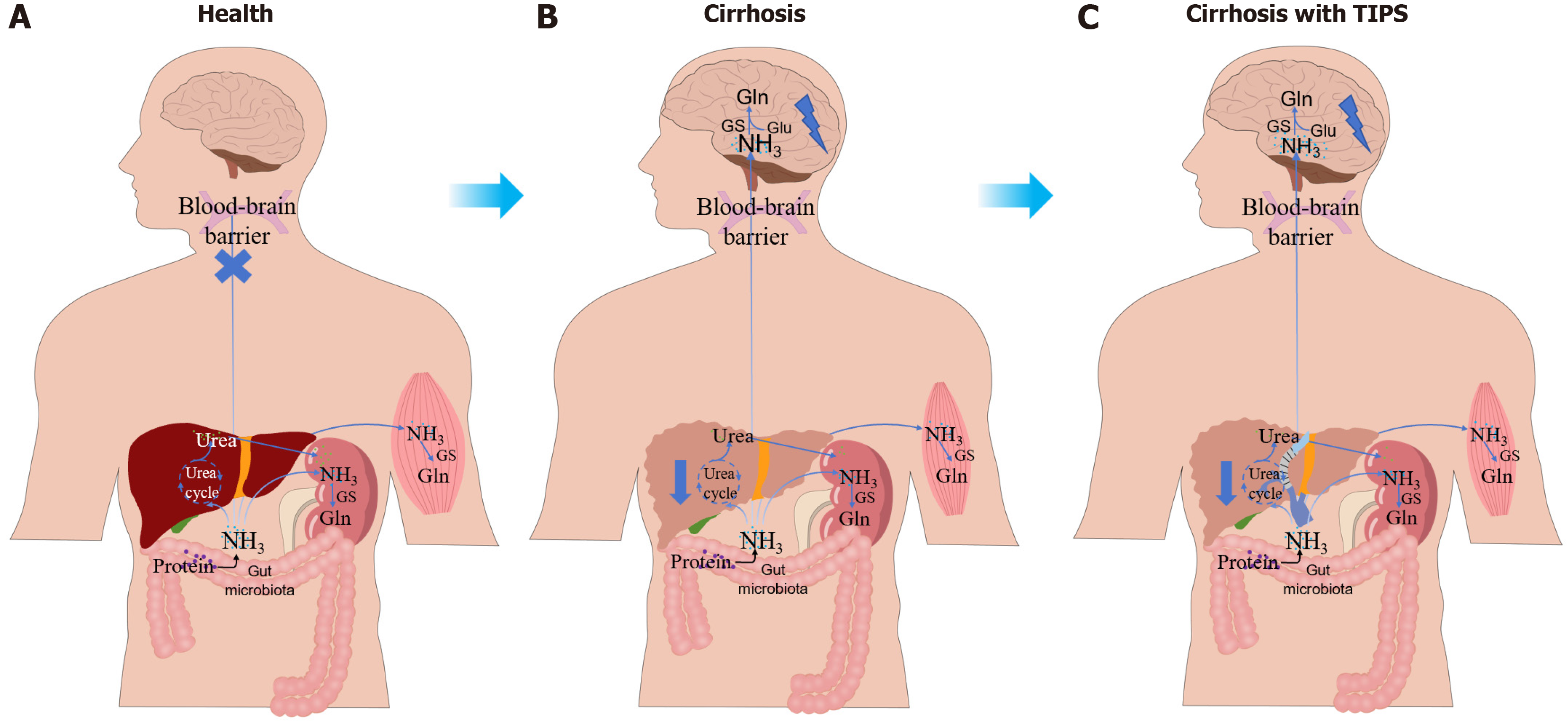
The pathogenesis of HE in cirrhotic patients primarily involves hyperammonemia, systemic and neuroinflammation, oxidative stress, and dysfunction of the gut-liver-brain axis[9]. Additionally, genetic and epigenetic factors have been implicated in the development of HE[10]. Among these, hyperammonemia is regarded as the central driver of HE[11].
In healthy individuals, ammonia production primarily occurs in the colon due to the activity of the gut microbiota, which converts glutamine into ammonia via enterocytic glutaminase[12]. Ammonia from the gut undergoes the urea cycle within hepatocytes to form urea, which is then excreted by the kidneys. Indeed, muscles also provide alternative pa
Systemic inflammation plays a pivotal role in the pathogenesis of HE in patients with cirrhosis, contributing to increased BBB permeability and eliciting a neuroinflammatory response via the activation of microglia and brain endothelial cells[21-23]. The abrupt hemodynamic changes induced by TIPS may facilitate the passage of inflammatory cytokines across the dysfunctional BBB, triggering neuroinflammation and increasing the risk of post-TIPS HE. Li et al[24] reported a significant association between preoperative serum interleukin-6 (IL-6) levels and the development of post-TIPS OHE in a prospective cohort study of 125 cirrhosis patients who underwent TIPS placement. Furthermore, toxic substances such as ammonia can activate microglia and cause the secretion of pro-inflammatory cytokines like IL-β and tumor necrosis factor-alpha (TNF-α), which promote the development of HE[25]. Further research is required to explore the role of inflammation in post-TIPS HE and to determine whether preoperative anti-inflammatory strategies can mitigate its occurrence.
Oxidative stress, frequently observed in cirrhosis, compromises BBB permeability through the reactivity of reactive oxygen and nitrogen species with lipids, proteins, and DNA, playing a crucial role in the pathogenesis of HE[26]. It induces the release of reactive oxygen species and mitochondrial dysfunction, leading to an increase in GABAergic tone in the central nervous system, thereby contributing to the development of HE[27]. Additionally, elevated ammonia and inflammation further direct oxidative stress in neurons, resulting in reactive oxygen species production and the development of HE[28]. Elevated blood ammonia levels following TIPS may promote the development of HE through this mechanism, and further investigations are needed in future studies to elucidate this relationship. Recent research suggests that antioxidant properties of vitamin D may alleviate oxidative stress in HE, but the role of vitamin D in patients undergoing TIPS procedures should be further explored[29].
The functional integrity of the gut barrier and optimal hepatic function are essential for maintaining the homeostasis of the gut-liver-brain axis in a healthy state, effectively preventing the translocation of toxic metabolic products into the brain[30]. However, in patients with liver cirrhosis, the integrity of the gut barrier is compromised, and gut permeability is increased as a result of systemic and local inflammation, as well as bacterial translocation. This disruption facilitates the harmful metabolites into the bloodstream[31]. Compromised hepatic function leads to inadequate detoxification of gut-derived metabolites, facilitating their passage across the BBB and promoting the development of HE. Following TIPS, increased intestinal absorption facilitates the systemic circulation of toxic metabolites, which bypass hepatic detoxification and directly enter the brain, thereby increased the risk of HE. Additionally, in a prospective study of 106 TIPS-treated cirrhotic patients, the post-TIPS HE group presented increased autochthonous taxa and decreased pathogenic taxa relative to the non-HE group[32]. He et al[33] also identified that high baseline phenylethylamine levels, which are derived mainly from Ruminococcus gnavus, are correlated with a sevenfold higher risk of post-TIPS HE. A prospective study revealed no genus-level gut microbiota differences in hepatitis B-related portal hypertension patients with or without pre-TIPS HE, but post-TIPS HE was associated with increased Morganella, a urease-producing bacterium that elevates blood ammonia[34-36]. These findings indicate that post-TIPS HE may be closely associated with gut microbiota dysbiosis, highlighting its potential as a therapeutic and preventive target for HE following TIPS placement.
HE is intricately linked to genetic and epigenetic mechanisms involving ammonia metabolism, neuroinflammation, and neurotransmitter regulatory pathways. Astrocytic glutamine synthetase (GS) converts glutamate and ammonia into glutamine, contributing to astrocyte swelling[37,38]. Activation of the intestinal GS gene has been linked to an increased risk of HE in cirrhotic patients[39]. Furthermore, the presence of two long alleles in the GS microsatellite promoter region was predominantly observed in HE patients[40]. Myocyte enhancer factor 2C, a key transcription factor in neuronal development and plasticity, is regulated by lnc240 through interaction with miR-1264-5p. In cirrhotic HE mice, lnc240 downregulation enhances the binding of miR-1264-5p binding to myocyte enhancer factor 2C, leading to neuronal dysfunction[41]. Gut barrier integrity is vital for gut-liver-brain axis homeostasis. In decompensated states, defensin and mucin sulfation genes were downregulated, whereas IL-1, IL-6, and TNF-related genes are upregulated in intestinal epithelial cells[42]. RNA sequencing analysis in a HE mouse model revealed that astrocytes upregulated corticoid receptor and oxidative stress signaling, while microglia displayed transcriptome changes associated with immune cell recruitment, highlighting the inflammation and hypoxia pathways in HE pathogenesis[43]. These findings provide critical insights into the pathophysiological mechanisms of HE and offer novel perspectives for precision medicine and targeted therapies. TIPS placement may further activate pathways associated with the development of HE on the aforementioned genetic basis, thereby increasing the incidence of HE, which warrants further validation in future studies.
Specific biomarkers are crucial for pre-TIPS patient selection, significantly reducing the incidence of post-TIPS HE. Beyond traditional hepatic biochemical markers such as albumin, creatinine, and total bilirubin, an increasing number of biomarkers have been identified as associated with post-TIPS HE[44,45]. Elevated preoperative serum IL-6 levels are strongly linked to post-TIPS HE, indicating a higher risk of severe HE in cirrhotic patients[24]. In contrast, Tiede et al[46] reported no associations between systemic inflammatory markers (IL-6, TNF-α, and IL-β) and post-TIPS OHE in a two-year prospective study of 62 patients. Variations in preoperative liver function and patient characteristics likely con
Artificial intelligence (AI) algorithms are integral to fields such as biomics and healthcare analytics. In HE management, AI-based analysis of multidimensional data has revolutionized early detection, risk assessment, and tailored therapeutic interventions[49]. AI-based models, such as machine learning and deep learning algorithms, analyze biomarkers and imaging data to identify subtle patterns predictive of HE onset and progression. Yang et al[50] compared various classification methods for HE prediction in cirrhosis, demonstrating that the weighted random forest model is suitable for developing a risk assessment system for HE. Bajaj et al[51] established a 20-microbial species stool signature using machine learning, which was validated in a multicenter cohort, to distinguish HE from non-HE cases and correct misdiagnoses, thereby improving diagnostic precision and risk stratification for HE. Radiomics enhances these approaches by extracting high-dimensional quantitative features from medical imaging, such as magnetic resonance imaging and computed tomography, to identify biomarkers linked to disease severity and neurological outcomes. Chen et al[52] constructed a model (area under the curve = 0.719) to predict post-TIPS OHE via 3D liver and spleen evaluation, optimizing the TIPS candidacy assessment. Models utilizing clinical and hepatic vascular characteristics from computed tomography images (area under the curve = 0.814) showed robust performance in predicting OHE risk, with good calibration[53]. Collectively, these technologies enable more precise diagnosis, enhanced prediction of HE episodes, and personalized therapeutic strategies, ultimately improving patient outcomes. Future research should focus on refining the accuracy of post-TIPS HE prediction.
Pre-TIPS liver function is significantly correlated with postoperative prognosis, and a Child-Pugh score > 13 or a model for end-stage liver disease score > 19 has been advocated as a contraindication for TIPS[54]. Common factors that affect HE after TIPS include age, a history of HE, the use of proton pump inhibitors (PPIs), serum sodium and creatinine levels, and the presence of sarcopenia[55,56]. Advanced age is a significant risk factor for post-TIPS HE. A meta-analysis revealed that patients over 70 years of age had a significantly greater incidence of HE after TIPS and a marked increase in readmission rates within 30 days[57,58]. This finding may be related to increased BBB permeability and decreased function of neural cells in elderly patients[59]. Moreover, a history of HE increases the incidence of HE following TIPS, and recurrent or persistent OHE has been advocated as a contraindication for TIPS[60]. PPIs are widely used in gastrointestinal diseases such as reflux esophagitis and peptic ulcers. A retrospective study revealed that the duration of preoperative PPI use was positively correlated with the incidence of postoperative HE, and the incidence of HE decreased after the discontinuation of PPIs[61]. This could be associated with PPIs modifying the pH of the gastrointestinal tract, which results in alterations in the gut microbiota that subsequently impact blood ammonia metabolism[62]. However, a study including 397 patients with liver cirrhosis who received TIPS revealed that 59.1% of patients treated with PPIs did not have a clear indication for PPI use[63]. Therefore, it is essential to adhere strictly to appropriate prescribing in
The selection of the TIPS stent diameter is crucial for the occurrence of HE after TIPS. A stent diameter that is too small can result in insufficient shunting and ineffective reduction of portal pressure, whereas a stent diameter that is too large increases the incidence of postoperative HE. Currently, a uniform recommendation for the selection of the stent diameter is lacking. The use of the smallest possible stent that can effectively control the complications of portal hypertension is generally advised. The 2023 American Association for the Study of Liver Diseases guidelines recommend a stent diameter of 8 mm[70]. The European guidelines do not specify a particular stent diameter[71]. According to the Baveno VII guidelines, a hepatic venous pressure gradient of < 12 mmHg or a reduction in the portal pressure gradient (PPG) of more than 50% from baseline after stent placement can effectively reduce the risk of recurrent variceal bleeding[72].
Several studies have compared the impact of placing stents with different diameters on portal hypertension complications and the incidence of HE. Initially, patients who underwent TIPS were randomly assigned to either the 8-mm diameter stent group or the 10-mm diameter stent group by Riggio et al[73]. They reported that the incidence of portal hypertension complications was greater in the 8-mm diameter stent group, whereas there was no significant difference in HE between the two groups. However, a meta-analysis including 5 studies and 489 patients indicated that, compared with a 10-mm stent, placing an 8-mm diameter stent did not significantly affect variceal rebleeding but did reduce the incidence of HE[74]. A randomized controlled trial (RCT) revealed that, compared with a 10-mm diameter stent, an 8-mm diameter stent not only reduced liver impairment but also halved the risk of HE, with no significant difference in rebleeding rates[75]. Furthermore, Trebicka et al[76] reported that covered stents with an 8 mm diameter may improve survival compared with 10 mm stents. The above studies suggest that an 8-mm diameter stent may be recommended for use in patients to prevent variceal bleeding because it does not increase the risk of rebleeding or liver impairment. However, the most critical factor in selecting the stent diameter is the patient’s baseline characteristics and treatment goals rather than the specific size of the stent. A retrospective study revealed that a 6-mm shunt significantly reduced the incidence of OHE, protected perioperative liver function, and did not affect the rebleeding rate in patients with a small liver volume[77]. For patients with cirrhosis and refractory ascites who are at high risk for postoperative HE, a stent diameter smaller than 8 mm may be more appropriate[18].
Adjustable stents provide a balanced approach for managing post-TIPS HE and complications related to portal hypertension. Monnin-Bares et al[78] introduced a technique using a balloon-expandable stent-graft and a lasso catheter for adjustable shunt diameter reduction, permitting reversible titration via stent-graft dilation or lasso tightening. The use of adjustable stents requires further validation in large-scale studies to confirm their efficacy and safety.
SPSS was identified in 71% of patients with medically refractory HE[79]. These shunts develop as a compensatory mechanism to alleviate portal hypertension, allowing blood to flow from the portal vein directly into the systemic circulation via collateral vessels[33]. Spontaneous splenorenal shunts and spontaneous gastrorenal shunts are relatively common types of SPSSs in patients with cirrhosis[80]. The presence of spontaneous shunts allows some of the portal venous blood to bypass the liver and directly enter the systemic circulation, including gut-derived toxins such as ammonia. These toxins can cross the BBB and interfere with the function of neuroglial cells[37]. Once formed, SPSSs tend to persist and do not easily regress. One-third of SPSSs remain even after TIPS normalizes portal pressure, which may be one of the reasons for persistent HE after TIPS[81]. A multicenter study revealed that the total area of the SPSS can independently predict the incidence of HE and mortality in patients with cirrhosis[82]. An RCT revealed that in patients with variceal bleeding, the combination of TIPS and embolization of a large SPSS decreased the incidence of post-TIPS HE without significantly increasing the risk of other complications[83]. Additionally, a meta-analysis conducted by Yang et al[84] involving a total of 4 studies and 1243 patients demonstrated that the prevalence of SPSS is associated with an increased risk of OHE after TIPS and that embolization of the SPSS during TIPS placement reduces the risk of OHE. The North American practice guidelines recommend considering embolization for SPSSs > 6 mm to reduce the risk of HE after TIPS[85]. Commonly used methods for SPSS embolization include balloon-occluded retrograde transvenous obliteration, plug-assisted retrograde transvenous obliteration, and coil-assisted retrograde transvenous obliteration[86]. However, the above studies have certain limitations, such as small sample sizes and the inclusion of patients with cirrhosis, primarily due to hepatitis B virus infection.
Nutrition management: Malnutrition is a prevalent issue in patients with liver cirrhosis, affects 20%-50% of this patient population, and poses a risk for the development of post-TIPS HE[48,87]. Although restricted protein intake was previously recommended for patients with HE, more recent research has suggested that protein restriction in patients with HE should be based on the severity of HE and the patient’s current nutritional status[88,89]. The European Association for the Study of the Liver (EASL) clinical practice guidelines also recommend that daily protein and caloric intake for patients with HE should not fall below the general recommendations for cirrhotic patients, with a minimum daily caloric intake of 35 kcal/kg and a minimum daily protein intake of 1.2-1.5 g/kg[90]. The use of vegetables and dairy protein over animal protein is encouraged[91]. An RCT of 120 cirrhotic patients demonstrated that nutritional therapy (30-35 kcal/kg and 1.0-1.5 g of vegetable protein/kg of ideal body weight/day) effectively treats MHE and improves health-related quality of life[92]. Kato et al[93] also reported that 8 weeks of nutritional support improved MHE in 68.4% of patients. A recent retrospective cohort study revealed that nutritional counseling effectively improved mortality and prevented OHE in patients with alcohol-associated liver disease[94].
However, to date, protein intake management has focused primarily on the prevention and treatment of HE in patients with liver cirrhosis. Only one retrospective prospective study reported that early dietary intervention can reduce the incidence of HE after TIPS in patients with cirrhosis[95]. Therefore, guidelines that recommend dietary management for the prevention of HE after TIPS placement are currently lacking. In clinical practice, both nutritional interventions and dietary counseling are essential and cost-effective measures. However, their efficacy is often constrained by patient compliance and variations in dietary habits.
Medical therapy: Lactulose, a nonabsorbable disaccharide metabolized by the colonic microbiota into short-chain organic acids, creates an acidic environment that inhibits NH3-producing bacteria, promotes beneficial microorganisms, and converts NH3 to nonabsorbable NH4+ to reduce ammonia levels[96]. Wang et al[97] demonstrated that lactulose is a safe and effective treatment for MHE because it decreases the abundance of Pediococcus, Clostridium, and Pseudomonas and increases the abundance of Anaerosinus and Akkermansia. The EASL guidelines recommend lactulose as the first-line treatment for HE, and it is effective for treating covert HE, preventing HE, and providing secondary prophylaxis after the first episode of OHE[90]. A recent clinical trial demonstrated that lactulose plus rifaximin decreased ammonia production in patients with cirrhosis, as quantified by the constant ammonia infusion technique, which measures whole-body ammonia metabolism[98]. In addition, lactulose promotes the elimination of accumulated blood in the gastrointestinal tract of patients with concurrent esophageal variceal bleeding and suppresses the production and absorption of ammonia to prevent HE. However, some studies have also shown that lactulose can lead to complications such as bloating, flatulence, and severe and unpredictable diarrhea, which may result in dehydration[99]. Current studies on the use of lactulose for the treatment and prevention of HE after TIPS are limited. An RCT from 2005 by Riggio et al[100] suggested that lactulose is not effective in the prophylaxis of HE after TIPS compared with no treatment. However, this study only compared the incidence of HE within 1 month after TIPS, so factors including insufficient medication duration cannot be completely excluded. Furthermore, Seifert et al[101] reported that the combination of lactulose and rifaximin is more effective in patients with HE prior to TIPS than in those with no history of HE. Lactulose is effective in the treatment and prevention of HE in patients with cirrhosis who have not received a TIPS. However, most current treatments and preventive measures for HE after TIPS are not significantly different from those used in the control group. This lack of efficacy may be due to insufficient medication time for patients and the impact of preoperative HE episodes. Alternatively, the preventive effect of lactulose through the aforementioned mechanisms may be insufficient to prevent HE in patients with a high risk of HE compared with patients who have not received a TIPS. Further large-scale RCTs are needed to explore the effects of lactulose alone on HE in patients who have undergone TIPS.
Rifaximin, a nonsystemic antibiotic based on rifamycin, has low gastrointestinal absorption and broad-spectrum antibacterial action without ototoxicity or nephrotoxicity[102]. Owing to its minimal absorption, it has high bioavailability within the gastrointestinal tract[99]. Rifaximin can be used to treat HE by improving bacterial translocation and systemic endotoxemia, reducing systemic inflammation, and repairing the intestinal barrier[103,104]. Rifaximin selectively in
Probiotics: HE is linked to an imbalance of in intestinal homeostasis[111]. Probiotics primarily affect the intestine, treating HE by regulating intestinal flora dysbiosis, enhancing intestinal mucosal barrier function, and modulating intestinal immunity and inflammation-related factors[112]. Various probiotics contain different combinations of bacterial species and play different roles in the treatment and prevention of HE. Bajaj et al[113] demonstrated that Lactobacillus GG is associated with reduced endotoxemia and microecological imbalance, whereas Stadlbauer et al[114] reported that Lactobacillus casei Shirota restored neutrophil phagocytic capacity. Yang et al[115] found that ProBiotic-4 (Bifidobacterium lactis, Lactobacillus casei, Bifidobacterium bifidum, and Lactobacillus acidophilus) alleviated cognitive impairment in aged mice via the suppression of Toll-like receptor 4- and RIG-I-induced nuclear factor-kappa B signaling and inflammation. Most current studies on probiotic treatment for HE include Bifidobacterium longum, Lactobacillus bulgaricus, and Streptococcus thermophilus which are key components of their probiotic formulations[116,117]. Probiotics are as effective as lactulose and rifaximin in reversing MHE and preventing OHE, with the added advantage of having fewer side effects[118]. Xia et al[119] demonstrated the efficacy of probiotics in treating MHE in patients with hepatitis B virus-induced cirrhosis by comparing changes in cognitive function, the gut microbiota, and venous blood ammonia levels after three months of probiotic treatment vs no treatment. Another RCT reported that taking probiotics for three months is effective in preventing the occurrence of HE in patients with liver cirrhosis[120]. Dhiman et al[121] conducted a double-blind RCT and revealed that the oral administration of probiotics for six months can prevent the recurrence of HE and reduce hospitalization rates. Notably, while the aforementioned studies indicate the effectiveness of probiotics in the treatment or prevention of HE, these studies have included populations from different regions, with inconsistent probiotic administration times and varying types of probiotics. Therefore, the optimal duration for probiotic treatment and the generalizability of these probiotics in HE patients are unclear.
However, the role of probiotics in the prevention and treatment of post-TIPS HE remains uncertain. Probiotics reduce ammonia production by modulating the composition of the intestinal flora, thereby reducing the amount of ammonia that enters the BBB and lowering the incidence of HE. Theoretically, although the use of portal-systemic shunts is increased after TIPS, the incidence of post-TIPS HE is reduced if less ammonia is absorbed from the intestine into the blood. This has been demonstrated in our preliminary study on the prophylaxis of HE after TIPS with probiotics (ChiCTR2200062996). Multicenter prospective RCTs are needed to confirm the role of probiotics in the treatment and prevention of HE following TIPS.
L-ornithine-L-aspartate: The main metabolic pathway for ammonia is the synthesis of urea in the liver through the urea cycle, and ornithine and aspartate are essential substrates in the urea cycle. Moreover, in extrahepatic tissues such as the brain and muscles, ammonia is converted into glutamine, a nontoxic transport form, through the action of GS, which is activated by ornithine and aspartate[122]. L-ornithine-L-aspartate (LOLA) is a combination of ornithine and aspartate that plays an important role in reducing blood ammonia levels both within and outside the liver[123].
A double-blind RCT conducted by Jain et al[124], which focused primarily on patients with grade 3 to 4 OHE, revealed that the continuous intravenous infusion of LOLA for 5 days, in addition to lactulose and rifaximin treatment, was more effective in improving HE grades and reducing the time to recovery from HE than lactulose and rifaximin alone were and decreased 28-day mortality. Other studies have shown that, compared with lactulose and probiotics, LOLA has an equivalent therapeutic effect on HE[125]. Whether the effect of LOLA treatment on HE is associated with the different types of HE (acute HE, chronic HE, or MHE) and the route of LOLA administration (oral or intravenous) remains controversial a review of the treatment and prevention of HE in patients with liver cirrhosis indicated that the efficacy of LOLA is not related to different types of HE or the route of administration[126]. Jiang et al[127] reported that the LOLA is effective in the treatment of acute HE and chronic HE but ineffective in the treatment of MHE. A meta-analysis revealed that oral administration of LOLA is more effective than intravenous infusion in the treatment of MHE[128]. Bai et al[113] compared the venous blood ammonia levels of patients who did and did not receive a LOLA infusion and reported a decrease in venous blood ammonia levels in the group that received a LOLA infusion 7 days post-TIPS. Furthermore, the levels of transaminases and bilirubin were significantly lower in the LOLA-treated group than in the control group, indicating that LOLA can improve liver function after TIPS[113]. However, Seifert et al[101] found no significant dif
Branched-chain amino acids: The occurrence of HE is related to an imbalance in the ratio of branched-chain amino acids (BCAAs) (valine, leucine, and isoleucine) to aromatic amino acids (AAAs) (phenylalanine, tyrosine, and tryptophan) in the blood[130]. In healthy individuals, BCAAs and AAAs provide energy for the body and participate in protein syn
Supplementation with BCAAs can increase the ratio of BCAAs in the blood, restoring amino acid balance and thereby inhibiting the production and release of false neurotransmitters, thus treating HE[131]. In addition, BCAAs decrease the occurrence of HE by regulating the gut microbiota and improving gut barrier function[132]. An RCT revealed that patients with alcoholic cirrhosis and HE who received a combination of antibiotics and BCAAs had faster and more complete recoveries than did those who received antibiotics alone[133]. Additionally, Vidot et al[134] found that the combination of BCAAs and prebiotics was more effective in treating HE than prebiotics alone. However, in an RCT that included 116 patients with cirrhosis and a previous episode of HE, supplementation with BCAAs in the diet did not reduce the recurrence rate of HE but did improve MHE and skeletal muscle mass[135]. Furthermore, an early study suggested that BCAAs reduce the concentration of AAAs in the blood of HE patients but do not improve brain function[136]. Therefore, the EASL clinical practice guidelines state that while BCAAs are beneficial for HE patients, their role in preventing HE recurrence still needs to be further validated by high-quality RCTs[71]. Regarding the treatment and prevention of HE after TIPS placement, further research on the role of BCAAs is still needed.
Fecal microbiota transplantation: The intestinal microbiome, which consists of various bacteria, fungi, viruses, and other organisms, has proinflammatory or anti-inflammatory effects[137]. In healthy individuals, the intestinal microbiota remains in a balanced state. In patients with liver cirrhosis combined with HE, the reduced production of bile acids disrupts this balance, leading to an increase in harmful bacteria and a decrease in beneficial bacteria[111]. The reduced production of short-chain fatty acids leads to an increase in the intestinal pH, allowing urease-producing bacteria to continue to grow and produce ammonia and endotoxins, which results in hyperammonamia, systemic inflammation, and impaired intestinal barrier function[138].
Fecal microbiota transplantation (FMT) involves transferring fecal materials from healthy donors to patients with dysregulated intestinal environments to restore the gut microbiota balance. Administration methods include colo
Although changes in the gut microbiota are associated with the occurrence of HE after TIPS, no studies have yet been conducted on the use of FMT for the treatment of HE following TIPS placement has not yet been studied. Li et al[32] conducted a prospective longitudinal study of 106 patients with cirrhosis who had undergone TIPS and reported that gut microbiota dysbiosis was associated with the occurrence of post-TIPS HE. These findings suggest that the gut microbiome is a target for the treatment of post-TIPS HE. Additionally, in the open-label study by Bloom et al[141] mentioned above, four patients (40%) had received a TIPS prior to FMT, and TIPS did not significantly affect the treatment of HE with FMT.
Post-TIPS HE is correlated with gut microbiota shifts. In a study by Li et al[146], two cirrhotic patients with recurrent grade 2-3 HE experienced improved liver function and a significant decline in HE attacks following three FMT treatments. Bloom et al[147] reported in their open-label study that 40% of patients (n = 4) had a history of TIPS before receiving FMT, and TIPS placement did not significantly impact the therapeutic outcomes of FMT for HE. These findings suggest that the gut microbiome could be a potential therapeutic target for post-TIPS HE and that the role of FMT needs further validation through RCTs.
Endovascular techniques for shunt reduction: An episode of OHE occurs in 35% to 50% of patients after TIPS, whereas refractory HE, including recurrent or persistent HE that cannot be controlled by dietary and pharmacological in
Notably, while the abovementioned studies offer insights into managing post-TIPS refractory HE, their small sample sizes and absence of control groups indicate that the observed improvements in HE could be due to factors unrelated to stent reduction. Moreover, comparing the differences in symptoms related to portal hypertension, liver function, and patient survival rates between those who have undergone stent reduction and those who have not is essential to assess the efficacy of stent reduction precisely for patients with refractory HE following TIPS placement.
First, early detection and identification of MHE following TIPS is crucial for the timely removal of precipitating factors, thereby preventing the occurrence of OHE. The creation of an efficient predictive model for MHE based on serum biochemical indicators, cognitive assessment systems, and radiomics warrants further investigation. Second, when sarcopenia is targeted as a therapeutic endpoint, comprehensive preoperative and postoperative nutritional assessments for patients who receive a TIPS, along with dietary guidance provided by nutritionists, may represent a nonpharmacological approach to prevent HE after TIPS placement. Third, the optimal size of the shunt channel that should be embolized to increase the prevention efficiency of HE after TIPS creation needs further investigation. Fourth, future research should focus on developing adjustable stents based on PPG and post-TIPS HE. Finally, the efficacy and safety of FMT as a treatment for HE following TIPS placement need to be investigated in the future.
This review is based on physiopathology and provides a detailed exposition about the assessment of patients before TIPS placement, the determination of stent diameter during the procedure, the combination of SPSS embolization, and patient diet, medication, FMT and stent reduction management after TIPS placement. Although TIPS increases the risk of HE in patients, most cases of HE can be effectively controlled by dietary intervention and drug management, and only a small number of recurrent or persistent HE cases require stent reduction. An increasing number of management strategies have been shown to be effective in the treatment of post-TIPS HE. A recent study confirmed that post-TIPS HE does not affect patient survival rates[154]. TIPS also decreases the further decompensation rates of complications other than HE[155]. Therefore, we believe that HE should not be a major barrier to the performance of TIPS. Multicenter RCTs are needed to further validate the effectiveness of the aforementioned HE management strategies after TIPS and to explore new therapeutic options in the future.
| 1. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1777] [Cited by in RCA: 1818] [Article Influence: 259.7] [Reference Citation Analysis (2)] |
| 2. | Bureau C, Garcia-Pagan JC, Otal P, Pomier-Layrargues G, Chabbert V, Cortez C, Perreault P, Péron JM, Abraldes JG, Bouchard L, Bilbao JI, Bosch J, Rousseau H, Vinel JP. Improved clinical outcome using polytetrafluoroethylene-coated stents for TIPS: results of a randomized study. Gastroenterology. 2004;126:469-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 338] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 3. | Fonio P, Discalzi A, Calandri M, Doriguzzi Breatta A, Bergamasco L, Martini S, Ottobrelli A, Righi D, Gandini G. Incidence of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt (TIPS) according to its severity and temporal grading classification. Radiol Med. 2017;122:713-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 4. | Bai M, Qi XS, Yang ZP, Yang M, Fan DM, Han GH. TIPS improves liver transplantation-free survival in cirrhotic patients with refractory ascites: an updated meta-analysis. World J Gastroenterol. 2014;20:2704-2714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 127] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (2)] |
| 5. | Hudson M, Schuchmann M. Long-term management of hepatic encephalopathy with lactulose and/or rifaximin: a review of the evidence. Eur J Gastroenterol Hepatol. 2019;31:434-450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 6. | Montagnese S, Bajaj JS. Impact of Hepatic Encephalopathy in Cirrhosis on Quality-of-Life Issues. Drugs. 2019;79:11-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 7. | Zuo L, Lv Y, Wang Q, Yin Z, Wang Z, He C, Guo W, Niu J, Bai W, Li K, Yu T, Yuan X, Chen H, Liu H, Xia D, Wang E, Luo B, Li X, Yuan J, Han N, Nie Y, Fan D, Han G. Early-Recurrent Overt Hepatic Encephalopathy Is Associated with Reduced Survival in Cirrhotic Patients after Transjugular Intrahepatic Portosystemic Shunt Creation. J Vasc Interv Radiol. 2019;30:148-153.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 8. | American Association for the Study of Liver Diseases; European Association for the Study of the Liver. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases. J Hepatol. 2014;61:642-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 332] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 9. | Arjunan A, Sah DK, Jung YD, Song J. Hepatic Encephalopathy and Melatonin. Antioxidants (Basel). 2022;11:837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Warskulat U, Kreuels S, Müller HW, Häussinger D. Identification of osmosensitive and ammonia-regulated genes in rat astrocytes by Northern blotting and differential display reverse transcriptase-polymerase chain reaction. J Hepatol. 2001;35:358-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Jaffe A, Lim JK, Jakab SS. Pathophysiology of Hepatic Encephalopathy. Clin Liver Dis. 2020;24:175-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Frieg B, Görg B, Gohlke H, Häussinger D. Glutamine synthetase as a central element in hepatic glutamine and ammonia metabolism: novel aspects. Biol Chem. 2021;402:1063-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 13. | Sheasgreen C, Lu L, Patel A. Pathophysiology, diagnosis, and management of hepatic encephalopathy. Inflammopharmacology. 2014;22:319-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Butterworth RF, Giguère JF, Michaud J, Lavoie J, Layrargues GP. Ammonia: key factor in the pathogenesis of hepatic encephalopathy. Neurochem Pathol. 1987;6:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 289] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 15. | Wijdicks EF. Hepatic Encephalopathy. N Engl J Med. 2016;375:1660-1670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 293] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 16. | Zhang LJ, Zhong J, Lu GM. Multimodality MR imaging findings of low-grade brain edema in hepatic encephalopathy. AJNR Am J Neuroradiol. 2013;34:707-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Jindal A, Jagdish RK. Sarcopenia: Ammonia metabolism and hepatic encephalopathy. Clin Mol Hepatol. 2019;25:270-279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 18. | Deltenre P, Zanetto A, Saltini D, Moreno C, Schepis F. The role of transjugular intrahepatic portosystemic shunt in patients with cirrhosis and ascites: Recent evolution and open questions. Hepatology. 2023;77:640-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 45] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 19. | Liu J, Ma J, Yang C, Chen M, Shi Q, Zhou C, Huang S, Chen Y, Wang Y, Li T, Xiong B. Sarcopenia in Patients with Cirrhosis after Transjugular Intrahepatic Portosystemic Shunt Placement. Radiology. 2022;303:711-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 64] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 20. | Bajaj JS, Pyrsopoulos NT, Rahimi RS, Heimanson Z, Allen C, Rockey DC. Serum Ammonia Levels Do Not Correlate With Overt Hepatic Encephalopathy Severity in Hospitalized Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2024;22:1950-1952.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Rose CF, Amodio P, Bajaj JS, Dhiman RK, Montagnese S, Taylor-Robinson SD, Vilstrup H, Jalan R. Hepatic encephalopathy: Novel insights into classification, pathophysiology and therapy. J Hepatol. 2020;73:1526-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 274] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 22. | Maharshi S, Sharma BC. Prophylaxis of hepatic encephalopathy: current and future drug targets. Hepatol Int. 2024;18:1096-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 23. | Jayakumar AR, Rama Rao KV, Norenberg MD. Neuroinflammation in hepatic encephalopathy: mechanistic aspects. J Clin Exp Hepatol. 2015;5:S21-S28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 24. | Li J, Liu Y, Li M, Rong X, Yuan Z, Ren C, Liu S, Li L, Zhao C, Gao L, Feng D. Association of preoperative IL-6 levels with overt HE in patients with cirrhosis after TIPS. Hepatol Commun. 2023;7:e0128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 25. | Cui W, Sun CM, Liu P. Alterations of blood-brain barrier and associated factors in acute liver failure. Gastroenterol Res Pract. 2013;2013:841707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6483] [Cited by in RCA: 6354] [Article Influence: 276.3] [Reference Citation Analysis (0)] |
| 27. | Bai Y, Li K, Li X, Chen X, Zheng J, Wu F, Chen J, Li Z, Zhang S, Wu K, Chen Y, Wang Y, Yang Y. Effects of oxidative stress on hepatic encephalopathy pathogenesis in mice. Nat Commun. 2023;14:4456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 28. | Bobermin LD, Wartchow KM, Flores MP, Leite MC, Quincozes-Santos A, Gonçalves CA. Ammonia-induced oxidative damage in neurons is prevented by resveratrol and lipoic acid with participation of heme oxygenase 1. Neurotoxicology. 2015;49:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Johnson CD, Stevens CM, Bennett MR, Litch AB, Rodrigue EM, Quintanilla MD, Wallace E, Allahyari M. The Role of Vitamin D Deficiency in Hepatic Encephalopathy: A Review of Pathophysiology, Clinical Outcomes, and Therapeutic Potential. Nutrients. 2024;16:4007. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 30. | Mancini A, Campagna F, Amodio P, Tuohy KM. Gut : liver : brain axis: the microbial challenge in the hepatic encephalopathy. Food Funct. 2018;9:1373-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 31. | Aguirre Valadez JM, Rivera-Espinosa L, Méndez-Guerrero O, Chávez-Pacheco JL, García Juárez I, Torre A. Intestinal permeability in a patient with liver cirrhosis. Ther Clin Risk Manag. 2016;12:1729-1748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Li M, Li K, Tang S, Lv Y, Wang Q, Wang Z, Luo B, Niu J, Zhu Y, Guo W, Bai W, Wang E, Xia D, Wang Z, Li X, Yuan J, Yin Z, Trebicka J, Han G. Restoration of the gut microbiota is associated with a decreased risk of hepatic encephalopathy after TIPS. JHEP Rep. 2022;4:100448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 33. | He X, Hu M, Xu Y, Xia F, Tan Y, Wang Y, Xiang H, Wu H, Ji T, Xu Q, Wang L, Huang Z, Sun M, Wan Y, Cui P, Liang S, Pan Y, Xiao S, He Y, Song R, Yan J, Quan X, Wei Y, Hong C, Liao W, Li F, El-Omar E, Chen J, Qi X, Gao J, Zhou H. The gut-brain axis underlying hepatic encephalopathy in liver cirrhosis. Nat Med. 2025;31:627-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 34. | Zhao HW, Zhang JL, Liu FQ, Yue ZD, Wang L, Zhang Y, Dong CB, Wang ZC. Alterations in the gut microbiome after transjugular intrahepatic portosystemic shunt in patients with hepatitis B virus-related portal hypertension. World J Gastroenterol. 2024;30:3668-3679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 35. | Minnullina L, Pudova D, Shagimardanova E, Shigapova L, Sharipova M, Mardanova A. Comparative Genome Analysis of Uropathogenic Morganella morganii Strains. Front Cell Infect Microbiol. 2019;9:167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 36. | Wang Q, Wang B, Saxena V, Miles L, Tiao J, Mortensen JE, Nathan JD. The gut-liver axis: impact of a mouse model of small-bowel bacterial overgrowth. J Surg Res. 2018;221:246-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Häussinger D, Kircheis G, Fischer R, Schliess F, vom Dahl S. Hepatic encephalopathy in chronic liver disease: a clinical manifestation of astrocyte swelling and low-grade cerebral edema? J Hepatol. 2000;32:1035-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 305] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 38. | Olde Damink SW, Jalan R, Dejong CH. Interorgan ammonia trafficking in liver disease. Metab Brain Dis. 2009;24:169-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 39. | Romero-Gómez M, Jover M, Del Campo JA, Royo JL, Hoyas E, Galán JJ, Montoliu C, Baccaro E, Guevara M, Córdoba J, Soriano G, Navarro JM, Martínez-Sierra C, Grande L, Galindo A, Mira E, Mañes S, Ruiz A. Variations in the promoter region of the glutaminase gene and the development of hepatic encephalopathy in patients with cirrhosis: a cohort study. Ann Intern Med. 2010;153:281-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 40. | Mayer LB, Krawczyk M, Grünhage F, Lammert F, Stokes CS. A genetic variant in the promoter of phosphate-activated glutaminase is associated with hepatic encephalopathy. J Intern Med. 2015;278:313-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 41. | Zhang H, Yu G, Li J, Tu C, Hui Y, Liu D, Chen M, Zhang J, Gong X, Guo G. Overexpressing lnc240 Rescues Learning and Memory Dysfunction in Hepatic Encephalopathy Through miR-1264-5p/MEF2C Axis. Mol Neurobiol. 2023;60:2277-2294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 42. | Jiang X, Xu Y, Fagan A, Patel B, Zhou H, Bajaj JS. Single nuclear RNA sequencing of terminal ileum in patients with cirrhosis demonstrates multi-faceted alterations in the intestinal barrier. Cell Biosci. 2024;14:25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 43. | Claeys W, Van Hoecke L, Lernout H, De Nolf C, Van Imschoot G, Van Wonterghem E, Verhaege D, Castelein J, Geerts A, Van Steenkiste C, Vandenbroucke RE. Experimental hepatic encephalopathy causes early but sustained glial transcriptional changes. J Neuroinflammation. 2023;20:130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 44. | Yao C, Huang L, Wang M, Mao D, Wang M, Zheng J, Long F, Huang J, Liu X, Zhang R, Xie J, Cheng C, Yao F, Huang G. Establishment and validation of a nomogram model for riskprediction of hepatic encephalopathy: a retrospective analysis. Sci Rep. 2023;13:19544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 45. | Peng Y, Wei Q, Liu Y, Wu Z, Zhang H, Wu H, Chai J. Prediction and Risk Factors for Prognosis of Cirrhotic Patients with Hepatic Encephalopathy. Gastroenterol Res Pract. 2021;2021:5623601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 46. | Tiede A, Stockhoff L, Ehrenbauer AF, Rieland H, Cornberg M, Meyer BC, Gabriel MM, Wedemeyer H, Hinrichs JB, Weissenborn K, Falk CS, Maasoumy B. Value of systemic inflammation markers for the detection of minimal and prediction of overt hepatic encephalopathy after TIPS insertion. Metab Brain Dis. 2024;40:58. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 47. | Dantas Machado AC, Ramos SF, Gauglitz JM, Fassler AM, Petras D, Aksenov AA, Kim UB, Lazarowicz M, Barnard Giustini A, Aryafar H, Vodkin I, Warren C, Dorrestein PC, Zarrinpar A, Zarrinpar A. Portosystemic shunt placement reveals blood signatures for the development of hepatic encephalopathy through mass spectrometry. Nat Commun. 2023;14:5303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 48. | Nardelli S, Lattanzi B, Torrisi S, Greco F, Farcomeni A, Gioia S, Merli M, Riggio O. Sarcopenia Is Risk Factor for Development of Hepatic Encephalopathy After Transjugular Intrahepatic Portosystemic Shunt Placement. Clin Gastroenterol Hepatol. 2017;15:934-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 151] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 49. | Xu X, Yang Y, Tan X, Zhang Z, Wang B, Yang X, Weng C, Yu R, Zhao Q, Quan S. Hepatic encephalopathy post-TIPS: Current status and prospects in predictive assessment. Comput Struct Biotechnol J. 2024;24:493-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 50. | Yang H, Li X, Cao H, Cui Y, Luo Y, Liu J, Zhang Y. Using machine learning methods to predict hepatic encephalopathy in cirrhotic patients with unbalanced data. Comput Methods Programs Biomed. 2021;211:106420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 51. | Bajaj JS, O'Leary JG, Jakab SS, Fagan A, Sikaroodi M, Gillevet PM. Gut microbiome profiles to exclude the diagnosis of hepatic encephalopathy in patients with cirrhosis. Gut Microbes. 2024;16:2392880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 52. | Chen X, Wang T, Ji Z, Luo J, Lv W, Wang H, Zhao Y, Duan C, Yu X, Li Q, Zhang J, Chen J, Zhang X, Huang M, Zhou S, Lu L, Huang M, Fu S. 3D automatic liver and spleen assessment in predicting overt hepatic encephalopathy before TIPS: a multi-center study. Hepatol Int. 2023;17:1545-1556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 53. | Chen X, Huang M, Yu X, Chen J, Xu C, Jiang Y, Li Y, Zhao Y, Duan C, Luo Y, Zhang J, Lv W, Li Q, Luo J, Dong D, An T, Lu L, Fu S. Hepatic-associated vascular morphological assessment to predict overt hepatic encephalopathy before TIPS: a multicenter study. Hepatol Int. 2024;18:1238-1248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (1)] |
| 54. | García-Pagán JC, Saffo S, Mandorfer M, Garcia-Tsao G. Where does TIPS fit in the management of patients with cirrhosis? JHEP Rep. 2020;2:100122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 55. | Ehrenbauer AF, Schneider H, Stockhoff L, Tiede A, Lorenz C, Dirks M, Witt J, Gabriel MM, Wedemeyer H, Hinrichs JB, Weissenborn K, Maasoumy B. Predicting overt hepatic encephalopathy after TIPS: Value of three minimal hepatic encephalopathy tests. JHEP Rep. 2023;5:100829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 56. | Fagiuoli S, Bruno R, Debernardi Venon W, Schepis F, Vizzutti F, Toniutto P, Senzolo M, Caraceni P, Salerno F, Angeli P, Cioni R, Vitale A, Grosso M, De Gasperi A, D'Amico G, Marzano A; AISF TIPS Special Conference. Consensus conference on TIPS management: Techniques, indications, contraindications. Dig Liver Dis. 2017;49:121-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 57. | Ahmed Z, Farooq U, Faiza Arif S, Aziz M, Iqbal U, Nawaz A, Lee-Smith W, Badal J, Mahmood A, Kobeissy A, Nawras A, Hassan M, Saab S. Transjugular Intrahepatic Portosystemic Shunt Outcomes in the Elderly Population: A Systematic Review and Meta-Analysis. Gastroenterology Res. 2022;15:325-333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 58. | Pan JJ, Chen C, Caridi JG, Geller B, Firpi R, Machicao VI, Hawkins IF Jr, Soldevila-Pico C, Nelson DR, Morelli G. Factors predicting survival after transjugular intrahepatic portosystemic shunt creation: 15 years' experience from a single tertiary medical center. J Vasc Interv Radiol. 2008;19:1576-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 59. | Hassoun Z, Deschênes M, Lafortune M, Dufresne MP, Perreault P, Lepanto L, Gianfelice D, Bui B, Pomier-Layrargues G. Relationship between pre-TIPS liver perfusion by the portal vein and the incidence of post-TIPS chronic hepatic encephalopathy. Am J Gastroenterol. 2001;96:1205-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 60. | Bai M, Qi X, Yang Z, Yin Z, Nie Y, Yuan S, Wu K, Han G, Fan D. Predictors of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt in cirrhotic patients: a systematic review. J Gastroenterol Hepatol. 2011;26:943-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 143] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 61. | Dai R, Sag AA, Martin JG, Befera NT, Pabon-Ramos WM, Suhocki PV, Smith TP, Kim CY, Muir AJ, Ronald J. Proton pump inhibitor use is associated with increased rates of post-TIPS hepatic encephalopathy: Replication in an independent patient cohort. Clin Imaging. 2021;77:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 62. | Tsai CF, Chen MH, Wang YP, Chu CJ, Huang YH, Lin HC, Hou MC, Lee FY, Su TP, Lu CL. Proton Pump Inhibitors Increase Risk for Hepatic Encephalopathy in Patients With Cirrhosis in A Population Study. Gastroenterology. 2017;152:134-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 134] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 63. | Sturm L, Bettinger D, Giesler M, Boettler T, Schmidt A, Buettner N, Thimme R, Schultheiss M. Treatment with proton pump inhibitors increases the risk for development of hepatic encephalopathy after implantation of transjugular intrahepatic portosystemic shunt (TIPS). United European Gastroenterol J. 2018;6:1380-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 64. | Merola J, Chaudhary N, Qian M, Jow A, Barboza K, Charles H, Teperman L, Sigal S. Hyponatremia: A Risk Factor for Early Overt Encephalopathy after Transjugular Intrahepatic Portosystemic Shunt Creation. J Clin Med. 2014;3:359-372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 65. | Guevara M, Baccaro ME, Torre A, Gómez-Ansón B, Ríos J, Torres F, Rami L, Monté-Rubio GC, Martín-Llahí M, Arroyo V, Ginès P. Hyponatremia is a risk factor of hepatic encephalopathy in patients with cirrhosis: a prospective study with time-dependent analysis. Am J Gastroenterol. 2009;104:1382-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 159] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 66. | Liao Y, Zhang L, Wang JT, Yue ZD, Fan ZH, Wu YF, Zhang Y, Dong CB, Wang XQ, Cui T, Meng MM, Bao L, Chen SB, Liu FQ, Wang L. A novel nomogram predicting overt hepatic encephalopathy after transjugular intrahepatic portosystemic shunt in portal hypertension patients. Sci Rep. 2023;13:15244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 67. | Liu J, Yang C, Yao J, Bai Y, Li T, Wang Y, Shi Q, Wu X, Ma J, Zhou C, Huang S, Xiong B. Improvement of sarcopenia is beneficial for prognosis in cirrhotic patients after TIPS placement. Dig Liver Dis. 2023;55:918-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 68. | Shi W, Yin H, Yu Z, Li Y, Bai X, Fu S, Duan C, Xu W, Yang Y. Myosteatosis is an independent risk factor for overt hepatic encephalopathy after transjugular intrahepatic portosystemic shunting. Eur J Gastroenterol Hepatol. 2024;36:897-903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 69. | Li J, Feng D, Pang N, Zhao C, Gao L, Liu S, Li L. Controlling nutritional status score as a new indicator of overt hepatic encephalopathy in cirrhotic patients following transjugular intrahepatic portosystemic shunt. Clin Nutr. 2022;41:560-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 70. | Lee EW, Eghtesad B, Garcia-Tsao G, Haskal ZJ, Hernandez-Gea V, Jalaeian H, Kalva SP, Mohanty A, Thabut D, Abraldes JG. AASLD Practice Guidance on the use of TIPS, variceal embolization, and retrograde transvenous obliteration in the management of variceal hemorrhage. Hepatology. 2024;79:224-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 59] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 71. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on the management of hepatic encephalopathy. J Hepatol. 2022;77:807-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 222] [Article Influence: 74.0] [Reference Citation Analysis (1)] |
| 72. | de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1537] [Cited by in RCA: 1499] [Article Influence: 499.7] [Reference Citation Analysis (2)] |
| 73. | Riggio O, Ridola L, Angeloni S, Cerini F, Pasquale C, Attili AF, Fanelli F, Merli M, Salvatori FM. Clinical efficacy of transjugular intrahepatic portosystemic shunt created with covered stents with different diameters: results of a randomized controlled trial. J Hepatol. 2010;53:267-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 74. | Huang Z, Yao Q, Zhu J, He Y, Chen Y, Wu F, Hua T. Efficacy and safety of transjugular intrahepatic portosystemic shunt (TIPS) created using covered stents of different diameters: A systematic review and meta-analysis. Diagn Interv Imaging. 2021;102:279-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 75. | Wang Q, Lv Y, Bai M, Wang Z, Liu H, He C, Niu J, Guo W, Luo B, Yin Z, Bai W, Chen H, Wang E, Xia D, Li X, Yuan J, Han N, Cai H, Li T, Xie H, Xia J, Wang J, Zhang H, Wu K, Fan D, Han G. Eight millimetre covered TIPS does not compromise shunt function but reduces hepatic encephalopathy in preventing variceal rebleeding. J Hepatol. 2017;67:508-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 149] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 76. | Trebicka J, Bastgen D, Byrtus J, Praktiknjo M, Terstiegen S, Meyer C, Thomas D, Fimmers R, Treitl M, Euringer W, Sauerbruch T, Rössle M. Smaller-Diameter Covered Transjugular Intrahepatic Portosystemic Shunt Stents Are Associated With Increased Survival. Clin Gastroenterol Hepatol. 2019;17:2793-2799.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 77. | Yan H, Xiang Z, Zhao C, Luo S, Liu H, Li M, Huang M. 6-mm shunt transjugular intrahepatic portosystemic shunt in patients with severe liver atrophy and variceal bleeding. Eur Radiol. 2024;34:4697-4707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 78. | Monnin-Bares V, Thony F, Sengel C, Bricault I, Leroy V, Ferretti G. Stent-graft narrowed with a lasso catheter: an adjustable TIPS reduction technique. J Vasc Interv Radiol. 2010;21:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 79. | Riggio O, Efrati C, Catalano C, Pediconi F, Mecarelli O, Accornero N, Nicolao F, Angeloni S, Masini A, Ridola L, Attili AF, Merli M. High prevalence of spontaneous portal-systemic shunts in persistent hepatic encephalopathy: a case-control study. Hepatology. 2005;42:1158-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 132] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 80. | Nardelli S, Riggio O, Gioia S, Puzzono M, Pelle G, Ridola L. Spontaneous porto-systemic shunts in liver cirrhosis: Clinical and therapeutical aspects. World J Gastroenterol. 2020;26:1726-1732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 63] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (3)] |
| 81. | Vidal-González J, Simón-Talero M, Genescà J. Should prophylactic embolization of spontaneous portosystemic shunts be routinely performed during transjugular intrahepatic portosystemic shunt placement? Dig Liver Dis. 2018;50:1324-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 82. | Praktiknjo M, Simón-Talero M, Römer J, Roccarina D, Martínez J, Lampichler K, Baiges A, Low G, Llop E, Maurer MH, Zipprich A, Triolo M, Maleux G, Fialla AD, Dam C, Vidal-González J, Majumdar A, Picón C, Toth D, Darnell A, Abraldes JG, López M, Jansen C, Chang J, Schierwagen R, Uschner F, Kukuk G, Meyer C, Thomas D, Wolter K, Strassburg CP, Laleman W, La Mura V, Ripoll C, Berzigotti A, Calleja JL, Tandon P, Hernandez-Gea V, Reiberger T, Albillos A, Tsochatzis EA, Krag A, Genescà J, Trebicka J; Baveno VI-SPSS group of the Baveno Cooperation. Total area of spontaneous portosystemic shunts independently predicts hepatic encephalopathy and mortality in liver cirrhosis. J Hepatol. 2020;72:1140-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 115] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 83. | Lv Y, Chen H, Luo B, Bai W, Li K, Wang Z, Xia D, Guo W, Wang Q, Li X, Yuan J, Cai H, Xia J, Yin Z, Fan D, Han G. Concurrent large spontaneous portosystemic shunt embolization for the prevention of overt hepatic encephalopathy after TIPS: A randomized controlled trial. Hepatology. 2022;76:676-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 60] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 84. | Yang M, Qiu Y, Wang W. Concurrent spontaneous portosystemic shunt embolization for the prevention of overt hepatic encephalopathy after TIPS: A systematic review and meta-analysis. Dig Liver Dis. 2024;56:978-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 85. | Boike JR, Thornburg BG, Asrani SK, Fallon MB, Fortune BE, Izzy MJ, Verna EC, Abraldes JG, Allegretti AS, Bajaj JS, Biggins SW, Darcy MD, Farr MA, Farsad K, Garcia-Tsao G, Hall SA, Jadlowiec CC, Krowka MJ, Laberge J, Lee EW, Mulligan DC, Nadim MK, Northup PG, Salem R, Shatzel JJ, Shaw CJ, Simonetto DA, Susman J, Kolli KP, VanWagner LB; Advancing Liver Therapeutic Approaches (ALTA) Consortium. North American Practice-Based Recommendations for Transjugular Intrahepatic Portosystemic Shunts in Portal Hypertension. Clin Gastroenterol Hepatol. 2022;20:1636-1662.e36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 144] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 86. | Lee EW, Lee AE, Saab S, Kee ST. Retrograde Transvenous Obliteration (RTO): A New Treatment Option for Hepatic Encephalopathy. Dig Dis Sci. 2020;65:2483-2491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 87. | Nutritional status in cirrhosis. Italian Multicentre Cooperative Project on Nutrition in Liver Cirrhosis. J Hepatol. 1994;21:317-325. [PubMed] |
| 88. | Nguyen DL, Morgan T. Protein restriction in hepatic encephalopathy is appropriate for selected patients: a point of view. Hepatol Int. 2014;8:447-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 89. | Daftari G, Tehrani AN, Pashayee-Khamene F, Karimi S, Ahmadzadeh S, Hekmatdoost A, Salehpour A, Saber-Firoozi M, Hatami B, Yari Z. Dietary protein intake and mortality among survivors of liver cirrhosis: a prospective cohort study. BMC Gastroenterol. 2023;23:227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 90. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70:172-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 674] [Article Influence: 112.3] [Reference Citation Analysis (2)] |
| 91. | Iqbal U, Jadeja RN, Khara HS, Khurana S. A Comprehensive Review Evaluating the Impact of Protein Source (Vegetarian vs. Meat Based) in Hepatic Encephalopathy. Nutrients. 2021;13:370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 92. | Maharshi S, Sharma BC, Sachdeva S, Srivastava S, Sharma P. Efficacy of Nutritional Therapy for Patients With Cirrhosis and Minimal Hepatic Encephalopathy in a Randomized Trial. Clin Gastroenterol Hepatol. 2016;14:454-460.e3; quiz e33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 93. | Kato A, Tanaka H, Kawaguchi T, Kanazawa H, Iwasa M, Sakaida I, Moriwaki H, Murawaki Y, Suzuki K, Okita K. Nutritional management contributes to improvement in minimal hepatic encephalopathy and quality of life in patients with liver cirrhosis: A preliminary, prospective, open-label study. Hepatol Res. 2013;43:452-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 94. | Hanai T, Nishimura K, Unome S, Miwa T, Nakahata Y, Imai K, Suetsugu A, Takai K, Shimizu M. Nutritional counseling improves mortality and prevents hepatic encephalopathy in patients with alcohol-associated liver disease. Hepatol Res. 2024;54:1089-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 95. | Luo L, Fu S, Zhang Y, Wang J. Early diet intervention to reduce the incidence of hepatic encephalopathy in cirrhosis patients: post-Transjugular Intrahepatic Portosystemic Shunt (TIPS) findings. Asia Pac J Clin Nutr. 2016;25:497-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 96. | Gerber T, Schomerus H. Hepatic encephalopathy in liver cirrhosis: pathogenesis, diagnosis and management. Drugs. 2000;60:1353-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 63] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 97. | Wang MW, Ma WJ, Wang Y, Ma XH, Xue YF, Guan J, Chen X. Comparison of the effects of probiotics, rifaximin, and lactulose in the treatment of minimal hepatic encephalopathy and gut microbiota. Front Microbiol. 2023;14:1091167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 98. | Eriksen PL, Djernes L, Vilstrup H, Ott P. Clearance and production of ammonia quantified in humans by constant ammonia infusion - the effects of cirrhosis and ammonia-targeting treatments. J Hepatol. 2023;79:340-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 99. | Bass NM, Mullen KD, Sanyal A, Poordad F, Neff G, Leevy CB, Sigal S, Sheikh MY, Beavers K, Frederick T, Teperman L, Hillebrand D, Huang S, Merchant K, Shaw A, Bortey E, Forbes WP. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 868] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 100. | Riggio O, Masini A, Efrati C, Nicolao F, Angeloni S, Salvatori FM, Bezzi M, Attili AF, Merli M. Pharmacological prophylaxis of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt: a randomized controlled study. J Hepatol. 2005;42:674-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 165] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 101. | Seifert LL, Schindler P, Schoster M, Weller JF, Wilms C, Schmidt HH, Maschmeier M, Masthoff M, Köhler M, Heinzow H, Wildgruber M. Recurrence of Hepatic Encephalopathy after TIPS: Effective Prophylaxis with Combination of Lactulose and Rifaximin. J Clin Med. 2021;10:4763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 102. | Scarpignato C, Pelosini I. Rifaximin, a poorly absorbed antibiotic: pharmacology and clinical potential. Chemotherapy. 2005;51 Suppl 1:36-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 174] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 103. | Patel VC, Lee S, McPhail MJW, Da Silva K, Guilly S, Zamalloa A, Witherden E, Støy S, Manakkat Vijay GK, Pons N, Galleron N, Huang X, Gencer S, Coen M, Tranah TH, Wendon JA, Bruce KD, Le Chatelier E, Ehrlich SD, Edwards LA, Shoaie S, Shawcross DL. Rifaximin-α reduces gut-derived inflammation and mucin degradation in cirrhosis and encephalopathy: RIFSYS randomised controlled trial. J Hepatol. 2022;76:332-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 132] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 104. | Bajaj JS, Heuman DM, Sanyal AJ, Hylemon PB, Sterling RK, Stravitz RT, Fuchs M, Ridlon JM, Daita K, Monteith P, Noble NA, White MB, Fisher A, Sikaroodi M, Rangwala H, Gillevet PM. Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PLoS One. 2013;8:e60042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 254] [Cited by in RCA: 339] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 105. | Kimer N, Pedersen JS, Tavenier J, Christensen JE, Busk TM, Hobolth L, Krag A, Al-Soud WA, Mortensen MS, Sørensen SJ, Møller S, Bendtsen F; members of the CoRif study group. Rifaximin has minor effects on bacterial composition, inflammation, and bacterial translocation in cirrhosis: A randomized trial. J Gastroenterol Hepatol. 2018;33:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 106. | de Wit K, Beuers U, Mukha A, Stigter ECA, Gulersonmez MC, Ramos Pittol JM, Middendorp S, Takkenberg RB, van Mil SWC. Rifaximin stimulates nitrogen detoxification by PXR-independent mechanisms in human small intestinal organoids. Liver Int. 2023;43:649-659. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 107. | de Wit K, Schaapman JJ, Nevens F, Verbeek J, Coenen S, Cuperus FJC, Kramer M, Tjwa ETTL, Mostafavi N, Dijkgraaf MGW, van Delden OM, Beuers UHW, Coenraad MJ, Takkenberg RB. Prevention of hepatic encephalopathy by administration of rifaximin and lactulose in patients with liver cirrhosis undergoing placement of a transjugular intrahepatic portosystemic shunt (TIPS): a multicentre randomised, double blind, placebo controlled trial (PEARL trial). BMJ Open Gastroenterol. 2020;7:e000531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 108. | Bureau C, Thabut D, Jezequel C, Archambeaud I, D'Alteroche L, Dharancy S, Borentain P, Oberti F, Plessier A, De Ledinghen V, Ganne-Carrié N, Carbonell N, Rousseau V, Sommet A, Péron JM, Vinel JP. The Use of Rifaximin in the Prevention of Overt Hepatic Encephalopathy After Transjugular Intrahepatic Portosystemic Shunt : A Randomized Controlled Trial. Ann Intern Med. 2021;174:633-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 110] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 109. | Bouza E, Alcalá L, Marín M, Valerio M, Reigadas E, Muñoz P, González-Del Vecchio M, de Egea V. An outbreak of Clostridium difficile PCR ribotype 027 in Spain: risk factors for recurrence and a novel treatment strategy. Eur J Clin Microbiol Infect Dis. 2017;36:1777-1786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 110. | Zullo A, Hassan C, Ridola L, Lorenzetti R, Campo SM, Riggio O. Rifaximin therapy and hepatic encephalopathy: Pros and cons. World J Gastrointest Pharmacol Ther. 2012;3:62-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 111. | Bajaj JS. The role of microbiota in hepatic encephalopathy. Gut Microbes. 2014;5:397-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 130] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 112. | Patel R, DuPont HL. New approaches for bacteriotherapy: prebiotics, new-generation probiotics, and synbiotics. Clin Infect Dis. 2015;60 Suppl 2:S108-S121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 172] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 113. | Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, Puri P, Sterling RK, Luketic V, Stravitz RT, Siddiqui MS, Fuchs M, Thacker LR, Wade JB, Daita K, Sistrun S, White MB, Noble NA, Thorpe C, Kakiyama G, Pandak WM, Sikaroodi M, Gillevet PM. Randomised clinical trial: Lactobacillus GG modulates gut microbiome, metabolome and endotoxemia in patients with cirrhosis. Aliment Pharmacol Ther. 2014;39:1113-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 219] [Article Influence: 19.9] [Reference Citation Analysis (1)] |
| 114. | Stadlbauer V, Mookerjee RP, Hodges S, Wright GA, Davies NA, Jalan R. Effect of probiotic treatment on deranged neutrophil function and cytokine responses in patients with compensated alcoholic cirrhosis. J Hepatol. 2008;48:945-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 234] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 115. | Yang X, Yu D, Xue L, Li H, Du J. Probiotics modulate the microbiota-gut-brain axis and improve memory deficits in aged SAMP8 mice. Acta Pharm Sin B. 2020;10:475-487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 261] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 116. | Pratap Mouli V, Benjamin J, Bhushan Singh M, Mani K, Garg SK, Saraya A, Joshi YK. Effect of probiotic VSL#3 in the treatment of minimal hepatic encephalopathy: A non-inferiority randomized controlled trial. Hepatol Res. 2015;45:880-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 117. | Pereg D, Kotliroff A, Gadoth N, Hadary R, Lishner M, Kitay-Cohen Y. Probiotics for patients with compensated liver cirrhosis: a double-blind placebo-controlled study. Nutrition. 2011;27:177-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 118. | Viramontes Hörner D, Avery A, Stow R. The Effects of Probiotics and Symbiotics on Risk Factors for Hepatic Encephalopathy: A Systematic Review. J Clin Gastroenterol. 2017;51:312-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 119. | Xia X, Chen J, Xia J, Wang B, Liu H, Yang L, Wang Y, Ling Z. Role of probiotics in the treatment of minimal hepatic encephalopathy in patients with HBV-induced liver cirrhosis. J Int Med Res. 2018;46:3596-3604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 120. | Lunia MK, Sharma BC, Sharma P, Sachdeva S, Srivastava S. Probiotics prevent hepatic encephalopathy in patients with cirrhosis: a randomized controlled trial. Clin Gastroenterol Hepatol. 2014;12:1003-8.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 154] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 121. | Dhiman RK, Rana B, Agrawal S, Garg A, Chopra M, Thumburu KK, Khattri A, Malhotra S, Duseja A, Chawla YK. Probiotic VSL#3 reduces liver disease severity and hospitalization in patients with cirrhosis: a randomized, controlled trial. Gastroenterology. 2014;147:1327-37.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 255] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 122. | Abraldes JG, Caraceni P, Ghabril M, Garcia-Tsao G. Update in the Treatment of the Complications of Cirrhosis. Clin Gastroenterol Hepatol. 2023;21:2100-2109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 33] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 123. | Stauch S, Kircheis G, Adler G, Beckh K, Ditschuneit H, Görtelmeyer R, Hendricks R, Heuser A, Karoff C, Malfertheiner P, Mayer D, Rösch W, Steffens J. Oral L-ornithine-L-aspartate therapy of chronic hepatic encephalopathy: results of a placebo-controlled double-blind study. J Hepatol. 1998;28:856-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 101] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 124. | Jain A, Sharma BC, Mahajan B, Srivastava S, Kumar A, Sachdeva S, Sonika U, Dalal A. L-ornithine L-aspartate in acute treatment of severe hepatic encephalopathy: A double-blind randomized controlled trial. Hepatology. 2022;75:1194-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 125. | Bai M, Yang Z, Qi X, Fan D, Han G. l-ornithine-l-aspartate for hepatic encephalopathy in patients with cirrhosis: a meta-analysis of randomized controlled trials. J Gastroenterol Hepatol. 2013;28:783-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 126. | Goh ET, Stokes CS, Sidhu SS, Vilstrup H, Gluud LL, Morgan MY. L-ornithine L-aspartate for prevention and treatment of hepatic encephalopathy in people with cirrhosis. Cochrane Database Syst Rev. 2018;5:CD012410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 127. | Jiang Q, Jiang XH, Zheng MH, Chen YP. L-Ornithine-l-aspartate in the management of hepatic encephalopathy: a meta-analysis. J Gastroenterol Hepatol. 2009;24:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 128. | Butterworth RF, McPhail MJW. L-Ornithine L-Aspartate (LOLA) for Hepatic Encephalopathy in Cirrhosis: Results of Randomized Controlled Trials and Meta-Analyses. Drugs. 2019;79:31-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 129. | Rees CJ, Oppong K, Al Mardini H, Hudson M, Record CO. Effect of L-ornithine-L-aspartate on patients with and without TIPS undergoing glutamine challenge: a double blind, placebo controlled trial. Gut. 2000;47:571-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 130. | Gluud LL, Dam G, Les I, Marchesini G, Borre M, Aagaard NK, Vilstrup H. Branched-chain amino acids for people with hepatic encephalopathy. Cochrane Database Syst Rev. 2017;5:CD001939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 131. | Soeters PB, Fischer JE. Insulin, glucagon, aminoacid imbalance, and hepatic encephalopathy. Lancet. 1976;2:880-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 163] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 132. | Badal BD, Fagan A, Tate V, Mousel T, Gallagher ML, Puri P, Davis B, Miller J, Sikaroodi M, Gillevet P, Gedguadas R, Kupcinkas J, Thacker L, Bajaj JS. Substitution of One Meat-Based Meal With Vegetarian and Vegan Alternatives Generates Lower Ammonia and Alters Metabolites in Cirrhosis: A Randomized Clinical Trial. Clin Transl Gastroenterol. 2024;15:e1. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 133. | Cerra FB, Cheung NK, Fischer JE, Kaplowitz N, Schiff ER, Dienstag JL, Bower RH, Mabry CD, Leevy CM, Kiernan T. Disease-specific amino acid infusion (F080) in hepatic encephalopathy: a prospective, randomized, double-blind, controlled trial. JPEN J Parenter Enteral Nutr. 1985;9:288-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 117] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 134. | Vidot H, Cvejic E, Finegan LJ, Shores EA, Bowen DG, Strasser SI, McCaughan GW, Carey S, Allman-Farinelli M, Shackel NA. Supplementation with Synbiotics and/or Branched Chain Amino Acids in Hepatic Encephalopathy: A Pilot Randomised Placebo-Controlled Clinical Study. Nutrients. 2019;11:1810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 135. | Les I, Doval E, García-Martínez R, Planas M, Cárdenas G, Gómez P, Flavià M, Jacas C, Mínguez B, Vergara M, Soriano G, Vila C, Esteban R, Córdoba J. Effects of branched-chain amino acids supplementation in patients with cirrhosis and a previous episode of hepatic encephalopathy: a randomized study. Am J Gastroenterol. 2011;106:1081-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 136. | Wahren J, Denis J, Desurmont P, Eriksson LS, Escoffier JM, Gauthier AP, Hagenfeldt L, Michel H, Opolon P, Paris JC, Veyrac M. Is intravenous administration of branched chain amino acids effective in the treatment of hepatic encephalopathy? A multicenter study. Hepatology. 1983;3:475-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 108] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 137. | Basson AR, Zhou Y, Seo B, Rodriguez-Palacios A, Cominelli F. Autologous fecal microbiota transplantation for the treatment of inflammatory bowel disease. Transl Res. 2020;226:1-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 138. | Cong J, Zhou P, Zhang R. Intestinal Microbiota-Derived Short Chain Fatty Acids in Host Health and Disease. Nutrients. 2022;14:1977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 115] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 139. | Ramai D, Zakhia K, Ofosu A, Ofori E, Reddy M. Fecal microbiota transplantation: donor relation, fresh or frozen, delivery methods, cost-effectiveness. Ann Gastroenterol. 2019;32:30-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 140. | Youngster I, Mahabamunuge J, Systrom HK, Sauk J, Khalili H, Levin J, Kaplan JL, Hohmann EL. Oral, frozen fecal microbiota transplant (FMT) capsules for recurrent Clostridium difficile infection. BMC Med. 2016;14:134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 137] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 141. | Bloom PP, Donlan J, Torres Soto M, Daidone M, Hohmann E, Chung RT. Fecal microbiota transplant improves cognition in hepatic encephalopathy and its effect varies by donor and recipient. Hepatol Commun. 2022;6:2079-2089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 142. | Li Y, Lv L, Ye J, Fang D, Shi D, Wu W, Wang Q, Wu J, Yang L, Bian X, Jiang X, Jiang H, Yan R, Peng C, Li L. Bifidobacterium adolescentis CGMCC 15058 alleviates liver injury, enhances the intestinal barrier and modifies the gut microbiota in D-galactosamine-treated rats. Appl Microbiol Biotechnol. 2019;103:375-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 143. | Bajaj JS, Kassam Z, Fagan A, Gavis EA, Liu E, Cox IJ, Kheradman R, Heuman D, Wang J, Gurry T, Williams R, Sikaroodi M, Fuchs M, Alm E, John B, Thacker LR, Riva A, Smith M, Taylor-Robinson SD, Gillevet PM. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: A randomized clinical trial. Hepatology. 2017;66:1727-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 454] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 144. | Bajaj JS, Shamsaddini A, Acharya C, Fagan A, Sikaroodi M, Gavis E, McGeorge S, Khoruts A, Fuchs M, Sterling RK, Lee H, Gillevet PM. Multiple bacterial virulence factors focused on adherence and biofilm formation associate with outcomes in cirrhosis. Gut Microbes. 2021;13:1993584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 145. | Gao J, Nie R, Chang H, Yang W, Ren Q. A meta-analysis of microbiome therapies for hepatic encephalopathy. Eur J Gastroenterol Hepatol. 2023;35:927-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Reference Citation Analysis (1)] |
| 146. | Li J, Wang D, Sun J. Application of fecal microbial transplantation in hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. Medicine (Baltimore). 2022;101:e28584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 147. | Bloom PP, Tapper EB, Young VB, Lok AS. Microbiome therapeutics for hepatic encephalopathy. J Hepatol. 2021;75:1452-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 148. | Riggio O, Angeloni S, Salvatori FM, De Santis A, Cerini F, Farcomeni A, Attili AF, Merli M. Incidence, natural history, and risk factors of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt with polytetrafluoroethylene-covered stent grafts. Am J Gastroenterol. 2008;103:2738-2746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 205] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 149. | Maleux G, Verslype C, Heye S, Wilms G, Marchal G, Nevens F. Endovascular shunt reduction in the management of transjugular portosystemic shunt-induced hepatic encephalopathy: preliminary experience with reduction stents and stent-grafts. AJR Am J Roentgenol. 2007;188:659-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 150. | Fanelli F, Salvatori FM, Rabuffi P, Boatta E, Riggio O, Lucatelli P, Passariello R. Management of refractory hepatic encephalopathy after insertion of TIPS: long-term results of shunt reduction with hourglass-shaped balloon-expandable stent-graft. AJR Am J Roentgenol. 2009;193:1696-1702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 151. | Rowley MW, Choi M, Chen S, Hirsch K, Seetharam AB. Refractory Hepatic Encephalopathy After Elective Transjugular Intrahepatic Portosystemic Shunt: Risk Factors and Outcomes with Revision. Cardiovasc Intervent Radiol. 2018;41:1765-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 152. | Shah RJ, Alqadi MM, Duvvuri M, Kim YJ, Tyagi R, Lokken RP, Gaba RC. Clinical Outcomes and Patency after Transjugular Intrahepatic Portosystemic Shunt Reduction for Overshunting Adverse Events. J Vasc Interv Radiol. 2022;33:1507-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 153. | Li X, Partovi S, Coronado WM, Gadani S, Martin C, Thompson D, Levitin A, Kapoor B. Hepatic Encephalopathy After TIPS Placement: Predictive Factors, Prevention Strategies, and Management. Cardiovasc Intervent Radiol. 2022;45:570-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 154. | Nardelli S, Riggio O, Marra F, Gioia S, Saltini D, Bellafante D, Adotti V, Guasconi T, Ridola L, Rosi M, Caporali C, Fanelli F, Roccarina D, Bianchini M, Indulti F, Spagnoli A, Merli M, Vizzutti F, Schepis F. Episodic overt hepatic encephalopathy after transjugular intrahepatic portosystemic shunt does not increase mortality in patients with cirrhosis. J Hepatol. 2024;80:596-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 155. | Larrue H, D'Amico G, Olivas P, Lv Y, Bucsics T, Rudler M, Sauerbruch T, Hernandez-Gea V, Han G, Reiberger T, Thabut D, Vinel JP, Péron JM, García-Pagán JC, Bureau C. TIPS prevents further decompensation and improves survival in patients with cirrhosis and portal hypertension in an individual patient data meta-analysis. J Hepatol. 2023;79:692-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 52] [Article Influence: 26.0] [Reference Citation Analysis (1)] |