Published online Apr 21, 2025. doi: 10.3748/wjg.v31.i15.101695
Revised: January 21, 2025
Accepted: March 21, 2025
Published online: April 21, 2025
Processing time: 206 Days and 17.5 Hours
Gastroparesis may repeatedly induce diabetic ketoacidosis (DKA), and the differential diagnosis of these diseases is challenging because of similar gastrointestinal symptoms. If DKA is accompanied by gastroparesis, patients present with persis
To achieve early detection and diagnosis of DKA + gastroparesis to enable early treatment aimed at relieving gastrointestinal symptoms and preventing re-induction of DKA.
We conducted a case-control study in which 15 patients hospitalized for DKA at the Endocrinology Department of Peking Union Medical College Hospital and diagnosed with DKA and gastroparesis between December 1999 and January 2023 (DKA + gastroparesis group) were included. Then, we selected 60 DKA patients without DKA as a control group (DKA alone group) based on gender, age, disease course, and diabetes subtype in a 1:4 matching ratio. Clinical manifestations and physical and laboratory examination results were statistically compared between the groups.
The DKA + gastroparesis group was composed of nine males and six females, with a mean age of 35 ± 11 years, while the DKA alone group included 34 males and 26 females, with a mean age of 34 ± 17 years. In the DKA + gastroparesis group, urine ketone levels normalized, while gastrointestinal symptoms persisted despite treatment, and the tests indicated lower glycosylated hemoglobin levels (HbA1c; 7.07% vs 11.51%, P < 0.01), largest amplitude of glycemic excursions (5.86 vs 17.41, P < 0.01), standard deviation of blood glucose (SDBG; 2.69 vs 5.83, P < 0.01), and coefficient of blood glucose variation (0.31 vs 0.55, P = 0.014) compared with the DKA alone group. Probable gastroparesis was considered at HbA1c < 8.55%. Besides, the patients in the DKA + gastroparesis group had lower body mass index (19.28 kg/m2vs 23.86 kg/m2, P = 0.02) and higher high density lipoprotein cholesterol level (2.34 mmol/L vs 1.05 mmol/L, P = 0.019) compared to the DKA alone group, but no difference was observed in the remaining lipid profiles between the two groups.
Gastroparesis should be considered in DKA patients who fail to have improved gastrointestinal symptoms after ketone elimination and acidosis correction, particularly when the HbA1c level is < 8.55%.
Core Tip: Persistent gastroparesis can repeatedly trigger diabetic ketoacidosis (DKA) and is difficult to correct. We compared the clinical manifestations and biomarkers between patients with DKA alone and those with DKA + gastroparesis to help promptly detect and diagnose DKA with gastroparesis, relieve gastrointestinal symptoms, and prevent re-induction of DKA. Notably, we found that if patients with DKA present persistent gastrointestinal symptoms without relief, lower glycosylated hemoglobin (HbA1c) levels, lower body mass index, and higher high-density lipoprotein cholesterol levels after ketone elimination and acidosis correction, gastroparesis should be considered in clinical practice, particularly when the HbA1c level is < 8.55%. Furthermore, gastrointestinal examinations should be performed in a timely manner to facilitate diagnosis.
- Citation: Han L, Peng QY, Yu J, Liu YW, Li W, Ping F, Zhang HB, Li YX, Xu LL. Early detection of gastroparesis with diabetic ketoacidosis as initial manifestation: A case-control study. World J Gastroenterol 2025; 31(15): 101695
- URL: https://www.wjgnet.com/1007-9327/full/v31/i15/101695.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i15.101695
Gastroparesis is a gastrointestinal disease with objective evidence of delayed gastric emptying of solid foods after excluding mechanical distal gastric obstruction. Clinical symptoms of gastric retention include nausea, vomiting, early satiety, and abdominal distension[1]. Diabetic gastroparesis is a serious complication in patients with diabetes during disease progression, and a gastroparesis risk of up to 50% exists in patients with types 1 and 2 diabetes and poor blood glucose control[2]. In addition to gastrointestinal symptoms, diabetic gastroparesis may manifest as hypoglycemia, which is induced by frequent glucose variation secondary to delayed postprandial plasma glucose elevation due to slowed gastrointestinal motility[3]; it may also manifest as hyperglycemic symptoms of diabetic ketoacidosis (DKA) or hypertonic hyperglycemia syndrome (HHS). The risk of DKA or HHS was 4-fold higher in patients with diabetic gastroparesis than in those without[4]. In gastroparesis patients, delayed gastric emptying leads to delayed peak blood glucose levels. Unchanged pre-meal insulin timing increases the risk of postprandial early hypoglycemia and pre-meal hyperglycemia. Therefore, delayed gastric emptying complicates blood glucose control in patients with diabetes, especially in those receiving insulin treatment[5]. Gastroparesis is a chronic condition, with an average symptom duration of 26.5 months[6]; however, a sharp rise in blood glucose can alter gastric emptying in the short term by influencing glucose-excited/inhibited neurons in gastric excitation/inhibition neural pathways[5]. For example, a rapid increase in blood glucose in DKA can induce acute gastroparesis, and they may occur simultaneously. DKA patients often present with similar gastrointestinal symptoms similar to gastroparesis patients, including nausea, vomiting, and abdominal pain. Thus, the differential diagnosis of DKA and gastroparesis is challenging based on clinical symptoms, and imaging examinations, such as gastric emptying scintigraphy, wireless motility capsule, and 13C-urea breath test, are not routinely performed for DKA patients, which makes DKA + gastroparesis easily neglected. During the diagnosis and treatment of patients with DKA, DKA is caused by gastroparesis only in a small number of patients. Misdiagnosis by physicians may result in these patients enduring persistent gastrointestinal symptoms, poor blood glucose control, and recurrent DKA in a short period. Therefore, the early diagnosis of DKA + gastroparesis is necessary but challenging for clinicians. However, there is limited research on this topic, and the conclusions are inconsistent. Two case reports reported DKA with gastroparesis[6,7], while others reported that moderate DKA does not affect gastric emptying[8]. The small sample size, severity of DKA, blood glucose levels, and regional factors may help explain these differences. Therefore, the present study statistically analyzed the differences in clinical manifestations and biochemical indices between patients with gastroparesis-induced DKA and those with DKA only, with an aim to provide a reference for clinical diagnosis and treatment of these conditions.
This was a cross-sectional study. A total of 1176 patients who were hospitalized for DKA at the Endocrinology Department of Peking Union Medical College Hospital (PUMCH) between December 1999 and January 2023 were included. To compare the differences between DKA + gastroparesis and DKA alone patients, we reviewed the medical records of 15 patients diagnosed with DKA + gastroparesis between December 1999 and January 2023 (DKA + gastroparesis group). To select a control group, we adopted a matched design, typical of case-control studies. To optimize statistical power, we used a 1:4 matching ratio. Each patient was paired with four controls based on gender, age, disease course, and diabetes subtype in a 1:4 ratio. Finally, 60 DKA alone patients of similar gender, age, disease course, and diabetes subtype were selected as a control group.
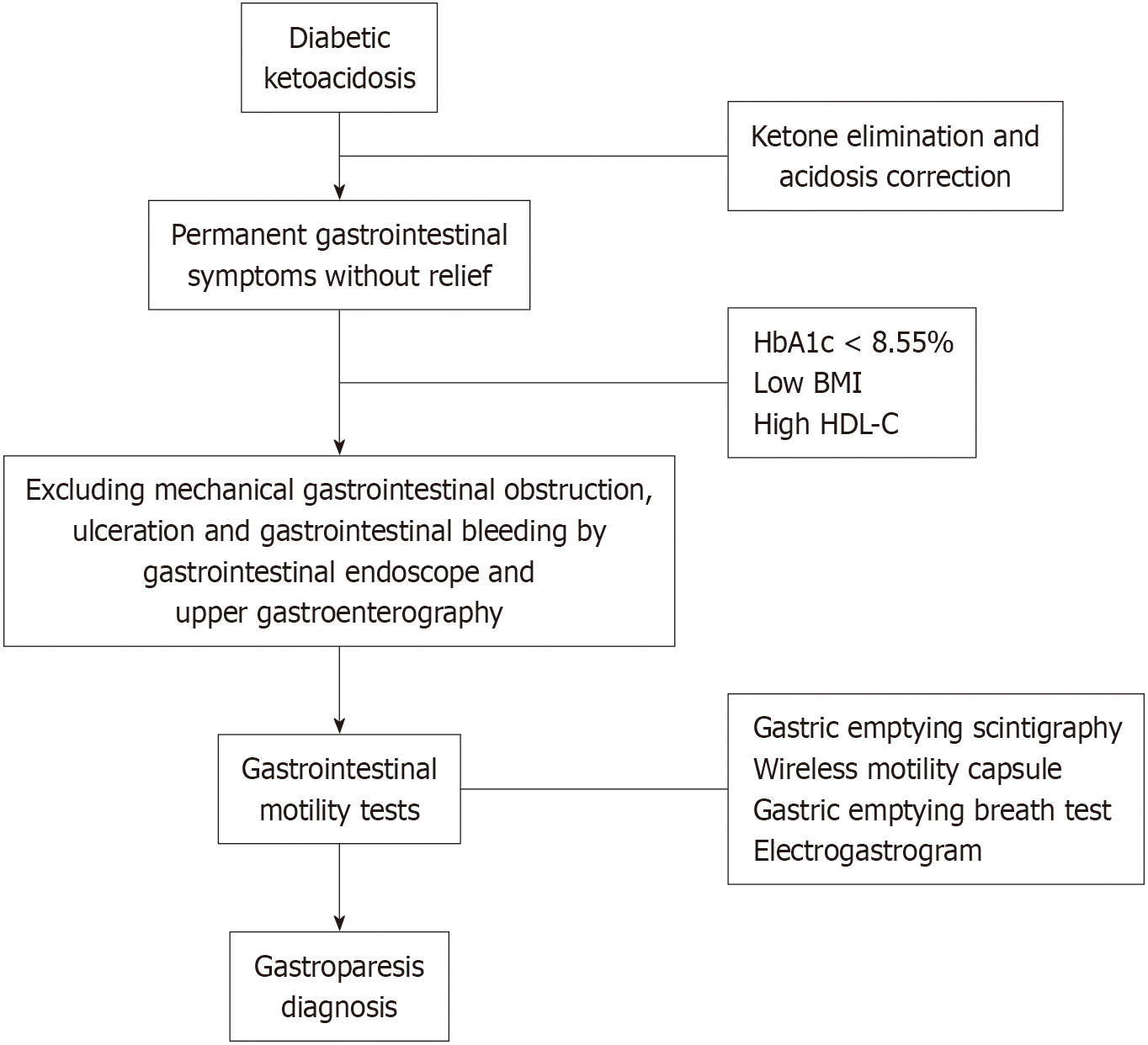
The inclusion criteria for the 15 patients with DKA + gastroparesis were as follows: (1) Patients previously diagnosed as having type 1 or 2 diabetic mellitus; (2) DKA was diagnosed according to the following criteria: Arterial blood gas pH < 7.3 and/or HCO3- < 18 mmol/L, blood ketone > 3.0 mmol/L, or urine ketone body (++) or above, and blood glucose > 13.9 mmol/L; (3) Patients who met the diagnostic criteria for gastroparesis: Presence of clinical manifestations, such as nausea, vomiting, early satiety, abdominal distension, and other gastrointestinal symptoms; 5-hour gastric emptying < 50%; or 48-hour total gastrointestinal emptying < 90% after excluding mechanical gastrointestinal obstruction, ulceration, and gastrointestinal bleeding by gastrointestinal endoscopy, upper gastroenterography, or abdominal X-ray plain scan, or other imaging diagnosis evidence including gastric dysrhythmia and bradygastria confirmed by electrogastrogram; (4) Laboratory tests included routine blood examination, liver and kidney function, inflammatory markers, and routine stool testing to help exclude gastrointestinal infections; and (5) Patients with DKA whose urine ketone levels normalized, while gastrointestinal symptoms persisted despite treatment.
A total of 60 DKA alone patients of similar age, gender, disease course, and diabetes subtype to the DKA + gastroparesis group were retrieved using the PUMCH medical record query and analysis system as controls, and the inclusion criteria were as follows: (1) Diagnosis of type 1 or 2 diabetes mellitus but with no history of gastroparesis; (2) DKA was diagnosed according to the following criteria: Arterial blood gas pH < 7.3 and/or HCO3- < 18 mmol/L, blood ketone > 3.0 mmol/L, or urine ketone body (++) or above, and blood glucose > 13.9 mmol/L; and (3) patients who were hospitalized for DKA and achieved improvement in gastrointestinal symptoms after the treatment of ketone elimination and acidosis correction. The control group patients were regularly followed at our outpatient clinic, and to date, none have presented with unexplained gastrointestinal symptoms or received a diagnosis of gastroparesis upon gastrointestinal examination. The Gastroparesis Cardinal Symptom Index (GCSI), a screening questionnaire, was used to assess the severity of gastroparesis-related symptoms. It comprises nine items and measures important symptoms associated with gastroparesis, including nausea or vomiting, postprandial fullness or early satiety, and abdominal bloating. If no gastrointestinal symptoms exist, a GCSI score < 18 points does not meet the clinical suspicion criteria for gastroparesis[9]. Therefore, gastroparesis was ruled out clinically.
General data, clinical symptoms and manifestations, and laboratory examination data were collected from the medical records of patients in the two groups, and the clinical indices were statistically analyzed between the groups. Particularly, the largest amplitude of glycemic excursions (LAGE), standard deviation of blood glucose (SDBG), mean amplitude of glucose excursions (MAGE), coefficient of blood glucose variation (CV), and pre- and post-prandial blood glucose variations were calculated from daily multipoint blood glucose recordings during hospitalization in both DKA and DKA + gastroparesis patients.
Statistical analyses were performed using SPSS software (version 27.0; IBM Corp., Armonk, NY, United States). The normally distributed quantitative data are presented as the mean ± SD, while the qualitative data with a skewed distribution are expressed as median and quartiles (Q1, Q3). Laboratory examination results were comparatively analyzed between the DKA + gastroparesis and DKA groups. The sample size of the DKA + gastroparesis group was < 20, and the normality test lacked sufficient power. Intergroup comparisons of continuous variables with a skewed distribution, including age, body mass index (BMI), course of disease, total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), glycosylated hemoglobin (HbA1c), LAGE, SDBG, MAGE, CV, and preprandial and postprandial blood glucose variation, were performed using the Mann-Whitney U test. The significance level of α = 0.05 was used, and P < 0.05 for two-tailed tests indicated that the difference between groups was statistically significant. R studio software (version 4.3.1) was employed to plot ROC curves and calculate the area under the curve (AUC). AUC > 0.5 and P < 0.05 indicated appreciated prediction and diagnostic value, with larger AUC values indicating higher diagnostic value. MedCalc software was used for validation and to calculate the cut-offs and corresponding sensitivity and specificity indices.
A total of 75 patients were enrolled in the study, including 15 DKA + gastroparesis patients [9 (60%) males and 6 (40%) females; mean age: 35 ± 11 years; ratio of type 1/type 2/other special types diabetic patients: 7:7:1; mean course of diabetes mellitus: 7 (6, 11 years)] and 60 DKA alone patients [34 (56.7%) males and 26 (43.3%) females; mean age: 34 ± 17 years; ratio of type 1/type 2/unclear type diabetic patients: 19/28/13; mean course of diabetes mellitus: 7 (0, 14) years] (Table 1).
| DKA + gastroparesis (n = 15) | DKA alone (n = 60) | P value1 | |
| Age (mean, ± SD) (years) | 35.47 ± 10.97 | 33.60 ± 17.1 | 0.474 |
| Female patients, n (%) | 6.0 (40.0) | 26.0 (43.3) | 0.818 |
| Type 2 DM, n (%) | 7.0 (46.7) | 28.0 (46.7) | 0.495 |
| Course of disease (years) | 9.0 (5.0-13.0) | 7.0 (0-14.0) | 0.265 |
| BMI (kg/m2) | 19.28 ± 3.16b | 23.86 ± 5.90b | 0.007 |
| TC (mmol/L) | 6.09 ± 2.64 | 5.26 ± 2.14 | 0.263 |
| TG (mmol/L) | 3.82 ± 4.39 | 2.85 ± 5.41 | 0.553 |
| HDL-C (mmol/L) | 2.34 ± 1.60a | 1.05 ± 0.36a | 0.019 |
| LDL-C (mmol/L) | 4.44 ± 2.61 | 3.08 ± 1.31 | 0.09 |
| HbA1c (%) | 7.07 ± 1.04a | 11.51 ± 2.75a | 0.01 |
| SDBG (mmol/L) | 2.69 ± 0.56a | 5.83 ± 2.07a | 0.01 |
| LAGE (mmol/L) | 5.86 ± 2.87a | 17.41 ± 7.22a | 0.01 |
| MAGE (mmol/L) | 4.50 ± 1.62 | 6.36 ± 3.41 | 0.125 |
| CV (%) | 0.31 ± 0.11a | 0.55 ± 0.25a | 0.014 |
| Blood glucose variation before and after meals (mmol/L) | 2.85 ± 1.79 | 3.44 ± 4.19 | 0.473 |
| Fasting C-peptide (nmol/L) | 0.44 ± 0.33 | 0.75 ± 0.75 | 0.353 |
| Fasting insulin (μU/mL) | 23.40 ± 27.86 | 20.40 ± 32.71 | 0.9 |
| ACR (mg/g) | 245.22 ± 320.06 | 125.12 ± 416.71 | 0.536 |
Clinical symptoms of polyuria, polydipsia, dehydration, respiratory compensation, and changes in cognitive state before therapy quickly improved after ketone elimination and acidosis correction in both groups. Patients in the DKA + gastroparesis group exhibited several types of gastrointestinal discomfort. Apart from nausea and vomiting, abdominal distension was particularly prominent and was characterized by early satiety, postprandial fullness, and prolonged duration. This sensation of fullness significantly affected their ability to eat. Other symptoms included reflux, heartburn, abdominal pain, and constipation. The gastrointestinal symptoms persisted even after the correction of ketonuria and acidosis. Prompt diagnosis and treatment using prokinetic agents led to significant improvement in gastrointestinal symptoms, with abdominal distension exhibiting the most remarkable improvement. In contrast, patients in the DKA alone group mainly experienced nausea and vomiting, with other gastrointestinal symptoms being less pronounced. Gastrointestinal symptoms rapidly disappeared after the resolution of ketonuria.
Lower HbA1c levels, LAGE, SDBG, and CV were observed in the DKA + gastroparesis group (7.07% vs 11.51%, P < 0.01; 5.86 vs 17.41, P < 0.01; 2.69 vs 5.83, P < 0.01; 0.31 vs 0.55, P = 0.014, respectively), but no significant difference was observed in MAGE between the two groups. BMI was lower and HDL-C level was higher in the DKA + gastroparesis group than in the DKA group (19.28 vs 23.86 kg/m2, P = 0.02; 2.34 vs 1.05 mmol/L, P = 0.019), and no significant differences were observed in TC, TG, or LDL-C between the two groups. Similarly, no remarkable differences were observed in the fasting C-peptide level, fasting insulin level, and urinary albumin creatinine ratio between the groups (Table 1).
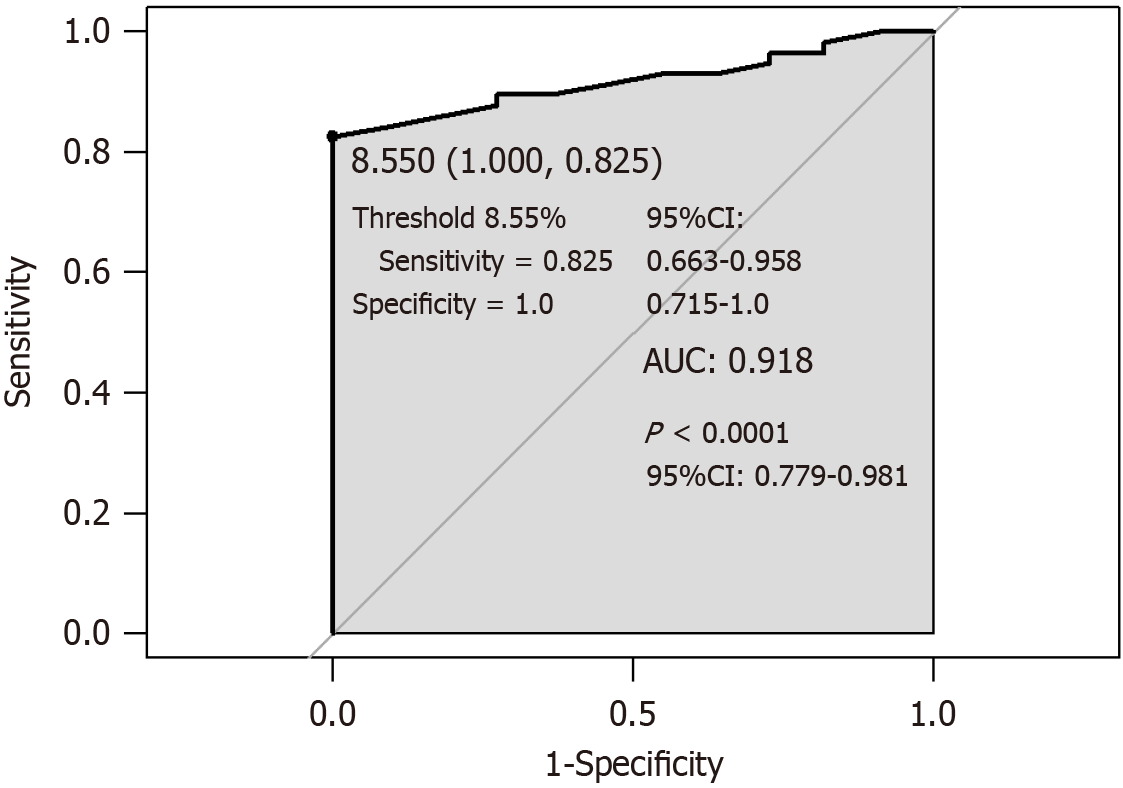
For lower HbA1c levels in the DKA + gastroparesis group, we further investigated whether HbA1c could be used to predict gastroparesis in DKA patients. The AUC (Figure 1) was 0.918 (P < 0.0001, 95% confidence interval [CI]: 0.779-0.981), cutoff was 8.55% (sensitivity = 0.825, 95%CI: 0.663-0.958; specificity = 1, 95%CI: 0.715-1.0), and HbA1c < 8.55% indicated probable DKA + gastroparesis.
This study summarized the clinical manifestations and biochemical indices of DKA + gastroparesis, and the study results suggested that if patients with DKA have no improvement in gastrointestinal symptoms after urine ketone normalization and acidosis correction, gastroparesis should be considered. The patients with DKA as onset symptom who were finally diagnosed with gastroparesis had significant differences in blood glucose variation and lipid from DKA patients, specifically lower BMI and HbA1c level, higher HDL-C level, and smaller random blood glucose variation; HbA1c < 8.55% indicates a greater probability of gastroparesis in DKA patients.
Serum HbA1c level is not only an important factor influencing the gastric emptying time in patients with type 2 diabetes[10,11], but its increase is also a risk factor for gastrointestinal symptoms in these patients, as reported in a cross-sectional epidemiological study[12]. Poor blood glucose control and high blood glucose and HbA1c levels are regarded as early predictors of gastroparesis in type 2 diabetes mellitus and risk factors for its progression[13,14]. Notably, this study demonstrated that patients diagnosed with DKA + gastroparesis had lower HbA1c levels, suggesting good blood glucose control in the short term; at HbA1c < 8.55%, probable gastroparesis was considered for DKA patients, which may be inconsistent with previous research findings. This can be attributed to several factors. Due to varying disease durations among patients, hyperglycemia is indeed a key factor in the development of gastroparesis, with significantly elevated HbA1c levels observed in early and mid-stage cases. However, in our study, in patients with gastroparesis, the condition persisted for a long time before admission, leading to reduced food intake, early satiety, weight loss, lower blood glucose levels, and smaller random blood glucose variations. However, because hyperglycemia is a known risk factor for gastroparesis, clinicians may easily overlook the diagnosis of gastroparesis when the HbA1c level is only mildly elevated. Currently, there is a lack of relevant foundational and clinical research, so the other potential pathophysiological mechanisms remain unclear. Further prospective studies are needed to gain a deeper understanding of these findings. Our study results proved that for DKA patients with HbA1c < 8.55%, close attention must be paid to changes in gastrointestinal symptoms, and if these symptoms persist after urine ketone levels normalize, relevant examinations should be performed to identify gastroparesis.
None of previous studies have indicated that lipid dysmetabolism is associated with gastroparesis[15], but the later can delay the arrival time of nutrients to the small intestine and thus result in continuous lipid absorption and elevation of blood lipids, especially TG, and the affected patients have a poor response to conventional lipid-lowering treatment[16]. In a rat experiment of diabetic gastroparesis, postprandial hypertriglyceridemia was observed and significantly improved after treatment with gastro-kinetic agents[17]. In this study, both the DKA alone and DKA + gastroparesis groups exhibited lipid abnormalities based on the average levels of each lipid component. Lipid abnormalities may not be recognized as diagnostic factors. However, patients with DKA + gastroparesis had a lower BMI but higher HDL-C levels though there were no significant differences in TC, TG, or LDL-C levels between the groups, which is not consistent with results from previous studies. The underlying causes and pathophysiological mechanisms may be considered as follows: (1) In gastroparesis patients with a chronic and prolonged disease, an underlying gastrointestinal neuropathy exists[18], which may be re-induced or worsened by acute hyperglycemia in DKA, and previous long-term gastrointestinal discomfort can lead to a decrease in food intake, body weight, and BMI. Weight loss can also increase HDL-C levels. For every 1 kg of weight loss, HDL-C levels can reportedly rise by 0.35-0.46 mg/dL[19]; and (2) HDL-C is primarily syn
Based on previous case reports, patients with DKA + gastroparesis presented with persistent vomiting without relief, nausea, abdominal pain, and other gastrointestinal symptoms, which did not improve after simple hydration and insulin treatment; the imaging examination indicated gastric retention, and the aforementioned symptoms were relieved after gastrointestinal decompression and drainage using a nasogastric tube[6]. Meanwhile, a case study showed that gastroparesis could repeatedly induce DKA; thus, it was regarded as one of the factors that aggravates or induces DKA[7]. If DKA patients present with persistent gastrointestinal symptoms without relief, lower HbA1c levels and BMI, and higher HDL-C levels after ketone elimination and acidosis correction, gastroparesis should be considered, and gastrointestinal examinations such as gastric emptying scintigraphy, wireless motility capsule, and 13C-urea breath test should be performed promptly[22]. Moreover, decompression and gastrointestinal prokinetic therapy, such as metoclopramide, erythromycin, domperidone, and mosapride, should be initiated as soon as possible to prevent the re-induction of DKA.
This study has several strengths. First, few previous studies on DKA + gastroparesis exist; specifically, only two case reports have reported it. Limited literature exists on the incidence and clinical characteristics of DKA + gastroparesis patients. This is the first clinical study to retrospectively analyze the differences in clinical manifestations and biochemical indices between DKA + gastroparesis patients and those with DKA alone. Although only 15 patients with DKA + gastroparesis were included, the sample size was relatively large, which has considerable reference value for clinical applications. Second, many indices, besides HbA1c level, were used in this study to evaluate daily blood glucose variations, such as CV, SDBG, LAGE, and MAGE. The findings of lower CV, LAGE, and SDBG levels in the DKA + gastroparesis group reflected a smaller daily blood glucose variation, which assessed blood glucose control and variation from a different perspective.
This study also has some limitations. First, this was a single-center study with a small sample size, and the generalization of the statistical analysis results is limited. Advancements in primary-tier diabetes management have made the early detection of gastroparesis and DKA in community hospitals possible, enabling timely intervention without hospitalization. In contrast, some patients with DKA complicated by gastroparesis may be initially discharged after resolution of ketoacidosis, with the diagnosis of coexisting gastroparesis becoming evident only after multiple DKA-related hospital admissions. Consequently, the number of hospitalized patients with DKA and coexisting gastroparesis remains limited. Although our study involved a relatively small sample size, it offers certain advantages over previous studies, particularly in terms of sample size. Future studies with larger sample sizes and multicenter design are necessary to validate our conclusions. Second, inherent limitations associated with retrospective studies exist: (1) Our study may have been subject to unmeasured or unaccounted-for biases and confounding factors. To address this, we employed matched controls in the selection of the control group to minimize selection bias and mitigate the influence of confounders. However, owing to the nature of retrospective studies, eliminating these potential biases and confounders remains challenging; and (2) Clinical data were incomplete and difficult to trace. For example, when calculating the daily blood glucose variation, the blood glucose data at seven time points within a day were required[23] to compute the CV, but only the blood glucose data at five time points were collected by reviewing the medical records of the patients in this study, resulting in a bias in the computed blood glucose variation value. Future large-scale prospective studies are needed to validate these findings and explore the underlying causal relationships and potential mechanisms.
In summary, gastroparesis should be considered in clinical practice if patients with DKA experience persistent gas
Continuous gastroparesis can repeatedly trigger DKA, which is challenging to address. The risk of recurrent DKA can be fundamentally reduced by promptly identifying and treating triggers. Prospective studies with larger sample sizes and multicenter design are required to enhance the depth of the research and provide more valuable insights for clinical practice.
We express our sincere gratitude to Ling-Ling Xu, who made great contributions to manuscript revision.
| 1. | Shin AS, Camilleri M. Diagnostic assessment of diabetic gastroparesis. Diabetes. 2013;62:2667-2673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Aswath GS, Foris LA, Ashwath AK, Patel K. Diabetic Gastroparesis. 2023 Mar 27. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. [PubMed] |
| 3. | Sang MM, Wu TZ, Sun ZL. [Advances in the pathogenesis and clinical management of diabetes-related gastrointestinal symptoms]. Zhonghua Tangniaobing Zazhi. 2021;13:513-516. [DOI] [Full Text] |
| 4. | Butalia S, Johnson JA, Ghali WA, Rabi DM. Clinical and socio-demographic factors associated with diabetic ketoacidosis hospitalization in adults with Type 1 diabetes. Diabet Med. 2013;30:567-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Goyal RK. Gastric Emptying Abnormalities in Diabetes Mellitus. N Engl J Med. 2021;384:1742-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 6. | Sharayah AM, Hajjaj N, Osman R, Livornese D. Gastroparesis in a patient with diabetic ketoacidosis. Cleve Clin J Med. 2019;86:238-239. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Pape A, Nguyen HV, Flack JR. Recurrent diabetic ketoalkalosis in a patient with Type 1 diabetes mellitus and severe gastroparesis. Diabet Med. 2010;27:607-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Jalleh RJ, Phillips L, Umapathysivam MM, Jones KL, Marathe CS, Watson LE, Bound M, Rayner CK, Horowitz M. Gastric emptying during and following resolution of moderate diabetic ketoacidosis in type 1 diabetes: a case series. BMJ Open Diabetes Res Care. 2024;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 9. | Brown LK, Xu J, Freedman BI, Hsu FC, Bowden DW, Koch KL. Symptoms Suggestive of Gastroparesis in a Community-Based Cohort of European Americans and African Americans with Type 2 Diabetes Mellitus. Dig Dis Sci. 2020;65:2321-2330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Javadi H, Bayani H, Mogharrabi M, Pashazadeh AM, Semnani S, Semnani S, Nabipour I, Assadi M. Relation between clinical features and gastric emptying time in diabetic patients. Nucl Med Rev Cent East Eur. 2015;18:3-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Izzy M, Lee M, Johns-Keating K, Kargoli F, Beckoff S, Chun K, Tokayer A. Glycosylated hemoglobin level may predict the severity of gastroparesis in diabetic patients. Diabetes Res Clin Pract. 2018;135:45-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Bytzer P, Talley NJ, Hammer J, Young LJ, Jones MP, Horowitz M. GI symptoms in diabetes mellitus are associated with both poor glycemic control and diabetic complications. Am J Gastroenterol. 2002;97:604-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 139] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Asghar S, Asghar S, Shahid S, Sajjad H, Abdul Nasir J, Usman M. Gastroparesis-Related Symptoms in Patients With Type 2 Diabetes Mellitus: Early Detection, Risk Factors, and Prevalence. Cureus. 2023;15:e35787. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Bharucha AE, Kudva Y, Basu A, Camilleri M, Low PA, Vella A, Zinsmeister AR. Relationship between glycemic control and gastric emptying in poorly controlled type 2 diabetes. Clin Gastroenterol Hepatol. 2015;13:466-476.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Bharucha AE, Kudva YC, Prichard DO. Diabetic Gastroparesis. Endocr Rev. 2019;40:1318-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 134] [Article Influence: 22.3] [Reference Citation Analysis (3)] |
| 16. | Adar T, Lysy J. Pseudodyslipidemia: are we over-treating dyslipidemia in diabetic patients with undiagnosed gastroparesis? Endocrine. 2014;45:26-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Xie W, Xing D, Zhao Y, Su H, Meng Z, Chen Y, Du L. A new tactic to treat postprandial hyperlipidemia in diabetic rats with gastroparesis by improving gastrointestinal transit. Eur J Pharmacol. 2005;510:113-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Tseng PH, Chao CC, Cheng YY, Chen CC, Yang PH, Yang WK, Wu SW, Wu YW, Cheng MF, Yang WS, Wu MS, Hsieh ST. Diabetic visceral neuropathy of gastroparesis: Gastric mucosal innervation and clinical significance. Eur J Neurol. 2022;29:2097-2108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Reference Citation Analysis (0)] |
| 19. | Bays HE, Kirkpatrick CF, Maki KC, Toth PP, Morgan RT, Tondt J, Christensen SM, Dixon DL, Jacobson TA. Obesity, dyslipidemia, and cardiovascular disease: A joint expert review from the Obesity Medicine Association and the National Lipid Association 2024. J Clin Lipidol. 2024;18:e320-e350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 20. | Endo Y, Fujita M, Ikewaki K. HDL Functions-Current Status and Future Perspectives. Biomolecules. 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 21. | Nunes VS, Leança CC, Panzoldo NB, Parra E, Cazita PM, Nakandakare ER, de Faria EC, Quintão EC. HDL-C concentration is related to markers of absorption and of cholesterol synthesis: Study in subjects with low vs. high HDL-C. Clin Chim Acta. 2011;412:176-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Camilleri M, Kuo B, Nguyen L, Vaughn VM, Petrey J, Greer K, Yadlapati R, Abell TL. ACG Clinical Guideline: Gastroparesis. Am J Gastroenterol. 2022;117:1197-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 145] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 23. | Sieber J, Flacke F, Link M, Haug C, Freckmann G. Improved Glycemic Control in a Patient Group Performing 7-Point Profile Self-Monitoring of Blood Glucose and Intensive Data Documentation: An Open-Label, Multicenter, Observational Study. Diabetes Ther. 2017;8:1079-1085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |