Published online Mar 7, 2024. doi: 10.3748/wjg.v30.i9.1257
Peer-review started: December 18, 2023
First decision: January 15, 2024
Revised: January 17, 2024
Accepted: February 18, 2024
Article in press: February 18, 2024
Published online: March 7, 2024
Processing time: 78 Days and 23.4 Hours
The increasing popularity of endoscopic submucosal dissection (ESD) as a treatment for early gastric cancer has highlighted the importance of quality assessment in achieving curative resections. This article emphasizes the significance of evaluating ESD quality, not only for curative cases but also for non-curative ones. Postoperative assessment relies on the endoscopic curability (eCura) classification, but management strategies for eCuraC-1 tumour with a positive horizontal margin are unclear. Current research primarily focuses on comparing additional surgical procedures in high-risk patients, while studies specifically targeting eCuraC-1 patients are limited. Exploring management strategies and follow-up outcomes for such cases could provide valuable insights. Furthermore, the application of molecular imaging using near-infrared fluorescent tracers holds promise for precise tumour diagnosis and navigation, potentially impacting the management of early-stage gastric cancer patients. Advancing research in these areas is essential for improving the overall efficacy of endoscopic techniques and refining treatment indications.
Core Tip: The quality control of endoscopic submucosal dissection (ESD) has gained increasing attention, and concurrently, the management of patients with non-curative ESD outcomes is equally crucial. Existing guidelines offer unclear recommendations for the management of patients classified as endoscopic curability C-1 after the procedure, warranting the need for further clinical research to refine treatment strategies for this patient population.
- Citation: Zhu YN, Yuan XL, Liu W, Zhang YH, Mou Y, Hu B, Ye LS. Exploring non-curative endoscopic submucosal dissection: Current treatment optimization and future indication expansion. World J Gastroenterol 2024; 30(9): 1257-1260
- URL: https://www.wjgnet.com/1007-9327/full/v30/i9/1257.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i9.1257
A recent publication by Kim et al[1] highlighted the importance of quality assessment in endoscopic submucosal dissection (ESD) for the treatment of early gastric cancer (EGC). With the widespread utilization of endoscopy, endoscopic treatment, specifically ESD, has become increasingly favoured for the management of EGC, especially when the risk of lymph node metastasis is deemed negligible. Acknowledging this prevailing trend, the authors emphasize that with the increasing popularity of endoscopic therapies, the number of physicians performing ESD has been on the rise each year. Consequently, there has been a corresponding increase in the rate of non-curative resections.
Compared to patients undergoing open surgery, patients undergoing ESD undoubtedly experience less surgical trauma and improved quality of life[2]. Considering the epidemiology of gastric cancer, conducting research to enhance the quality of ESD is imperative, and an increasing number of clinical studies are currently focusing on assessing ESD quality. However, it is essential to note that curative ESD is not the sole focal point; the discourse on non-curative ESD is equally significant.
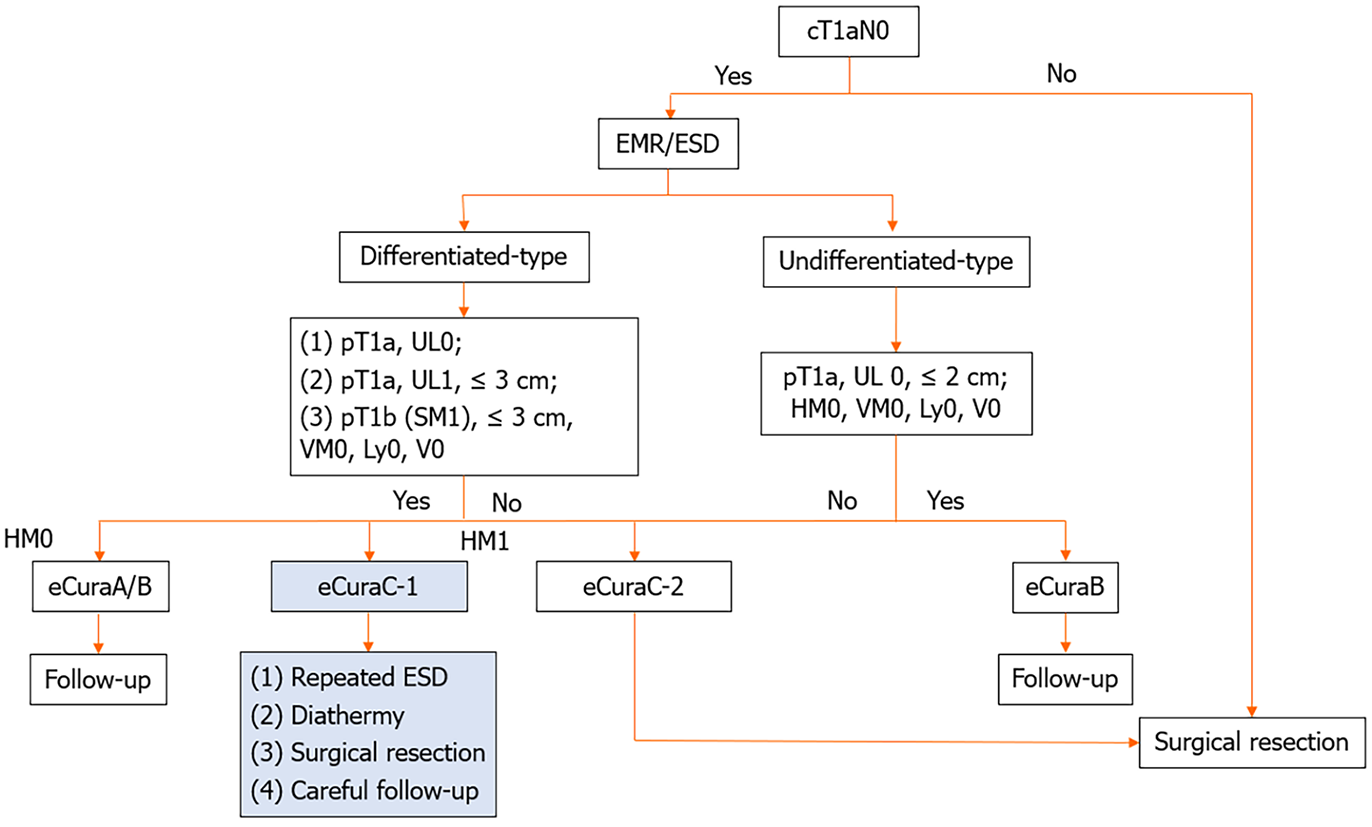
Many studies have focused on the preoperative evaluation of patients. When assessing the risk of non-curative ESD, the size of the tumour and the depth of infiltration are the two most crucial indicators[3] since pathology can be acquired directly through biopsy. However, due to the challenges in evaluating the extent of gastric cancer, especially in assessing infiltration depth, even though endoscopic ultrasound techniques are advanced and several studies have explored predictive models for risk resection[4], difficulties still persist. In regard to postoperative assessment, the commonly utilized classification in Asia for determining the completeness of ESD is the endoscopic curability (eCura) from the Japan Gastroenterological Endoscopy Society, which involves depth of invasion, ulceration, and pathology[5]. In accordance with guidelines[6], the management strategies for eCuraA, eCuraB, and eCuraC-2 are clear and well defined. However, regarding eCuraC-1 tumours, where segmental resection or a positive horizontal margin (HM) serve as the sole noncurative factor, the appropriate approach remains ambiguous. Surgical intervention is not the exclusive choice, and alternative options include repeat ESD, surgery, close observation, and endoscopic coagulation (Figure 1).
Current clinical research on noncurative patients predominantly focuses on directly comparing the efficacy of additional surgical procedures in high-risk patients[7]. Several studies have explored the effectiveness of conservative treatments in elderly patients[8], however, none of these studies categorized outcomes based on postoperative pathology of HM. Globally, large-scale clinical studies specifically targeting eCuraC-1 patients are scarce. The management strategies and corresponding follow-up outcomes for eCuraC-1 patients may represent valuable avenues for future research. We believe that the progressive conduct of such studies plays a crucial role in delineating both the absolute and relative indications for endoscopic treatment and contributes significantly to the overall advancement of endoscopic techniques.
In addition, with the advancement of molecular diagnostic technologies, “molecular imaging” has emerged as a new frontier in tumour diagnosis. Near-infrared fluorescent tracers have been employed for tumour navigation in breast cancer, offering real-time and precise information about the tumour for medical professionals[9]. This technology has been successfully deployed in the field of gastrointestinal cancer, specifically in colorectal tumours, through the utili
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rodrigo L, Spain S-Editor: Chen YL L-Editor: A P-Editor: Yu HG
| 1. | Kim GH. Endoscopic submucosal dissection for early gastric cancer: It is time to consider the quality of its outcomes. World J Gastroenterol. 2023;29:5800-5803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Abe S, Oda I, Suzuki H, Nonaka S, Yoshinaga S, Nakajima T, Sekiguchi M, Mori G, Taniguchi H, Sekine S, Katai H, Saito Y. Long-term surveillance and treatment outcomes of metachronous gastric cancer occurring after curative endoscopic submucosal dissection. Endoscopy. 2015;47:1113-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 3. | Pimingstorfer P, Biebl M, Gregus M, Kurz F, Schoefl R, Shamiyeh A, Spaun GO, Ziachehabi A, Fuegger R. Endoscopic Submucosal Dissection in the Upper Gastrointestinal Tract and the Need for Rescue Surgery-A Multicenter Analysis. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Kim EH, Park JC, Song IJ, Kim YJ, Joh DH, Hahn KY, Lee YK, Kim HY, Chung H, Shin SK, Lee SK, Lee YC. Prediction model for non-curative resection of endoscopic submucosal dissection in patients with early gastric cancer. Gastrointest Endosc. 2017;85:976-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 5. | Ono H, Yao K, Fujishiro M, Oda I, Uedo N, Nimura S, Yahagi N, Iishi H, Oka M, Ajioka Y, Fujimoto K. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer (second edition). Dig Endosc. 2021;33:4-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 308] [Article Influence: 77.0] [Reference Citation Analysis (0)] |
| 6. | Chinese Society of Clinical Oncology (CSCO) Guideline Working Committee. Chinese Society of Clinical Oncology (CSCO) Guidelines for the Diagnosis and Treatment of Gastric Cancer. Zhonghua Yixue Zazhi. 2023;103:27. [RCA] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 7. | Li S, Tian X, Wei J, Shi Y, Zhang H, Huang Y. Long-term outcomes of additional surgery versus non-gastrectomy treatment for early gastric cancer after non-curative endoscopic submucosal dissection: a meta-analysis. Chin Med J (Engl). 2023;136:528-535. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Kishida Y, Takizawa K, Kakushima N, Kawata N, Yoshida M, Yabuuchi Y, Yamamoto Y, Ito S, Imai K, Hotta K, Ishiwatari H, Matsubayashi H, Bando E, Terashima M, Ono H. Endoscopic submucosal dissection versus surgery in elderly patients with early gastric cancer of relative indication for endoscopic resection. Dig Endosc. 2022;34:497-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Lamberts LE, Koch M, de Jong JS, Adams ALL, Glatz J, Kranendonk MEG, Terwisscha van Scheltinga AGT, Jansen L, de Vries J, Lub-de Hooge MN, Schröder CP, Jorritsma-Smit A, Linssen MD, de Boer E, van der Vegt B, Nagengast WB, Elias SG, Oliveira S, Witkamp AJ, Mali WPTM, Van der Wall E, van Diest PJ, de Vries EGE, Ntziachristos V, van Dam GM. Tumor-Specific Uptake of Fluorescent Bevacizumab-IRDye800CW Microdosing in Patients with Primary Breast Cancer: A Phase I Feasibility Study. Clin Cancer Res. 2017;23:2730-2741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 204] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 10. | Boekestijn I, van Oosterom MN, Dell'Oglio P, van Velden FHP, Pool M, Maurer T, Rietbergen DDD, Buckle T, van Leeuwen FWB. The current status and future prospects for molecular imaging-guided precision surgery. Cancer Imaging. 2022;22:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |