Published online Mar 7, 2024. doi: 10.3748/wjg.v30.i9.1073
Peer-review started: November 4, 2023
First decision: December 27, 2023
Revised: January 2, 2024
Accepted: February 6, 2024
Article in press: February 6, 2024
Published online: March 7, 2024
Processing time: 122 Days and 6.8 Hours
Hepatocrinology explores the intricate relationship between liver function and the endocrine system. Chronic liver diseases such as liver cirrhosis can cause endocrine disorders due to toxin accumulation and protein synthesis disruption. Despite its importance, assessing endocrine issues in cirrhotic patients is frequently neglected. This article provides a comprehensive review of the epidemiology, pathophysiology, diagnosis, and treatment of endocrine disturbances in liver cirrhosis. The review was conducted using the PubMed/Medline, EMBASE, and Scielo databases, encompassing 172 articles. Liver cirrhosis is associated with endocrine disturbances, including diabetes, hypoglycemia, sarcopenia, thyroid dysfunction, hypogonadotropic hypogonadism, bone disease, adrenal insufficiency, growth hormone dysfunction, and secondary hyperaldosteronism. The optimal tools for diagnosing diabetes and detecting hypoglycemia are the oral glucose tolerance test and continuous glucose monitoring system, respectively. Sarcopenia can be assessed through imaging and functional tests, while other endocrine disorders are evaluated using hormonal assays and imaging studies. Treatment options include metformin, glucagon-like peptide-1 analogs, sodium-glucose co-transporter-2 inhibitors, and insulin, which are effective and safe for diabetes control. Established standards are followed for managing hypoglycemia, and hormone replacement therapy is often necessary for other endocrine dysfunctions. Liver transplantation can address some of these problems.
Core Tip: This review explores hepatocrinology, the interplay between liver function and the endocrine system, focusing on endocrine disorders in liver cirrhosis. Cirrhosis triggers endocrine disorders like diabetes, hypoglycemia, sarcopenia, hepatic bone disease, secondary hyperaldosteronism, and thyroid, adrenal and growth hormone dysfunction. Diagnosis includes biochemical and hormonal assays and imaging studies. Treatment involves drugs like metformin, insulin, and hormone replacement therapies, while liver transplantation can alleviate certain conditions. Timely recognition and intervention are crucial for reducing associated morbidity and mortality in cirrhotic patients.
- Citation: Quiroz-Aldave JE, Gamarra-Osorio ER, Durand-Vásquez MDC, Rafael-Robles LDP, Gonzáles-Yovera JG, Quispe-Flores MA, Concepción-Urteaga LA, Román-González A, Paz-Ibarra J, Concepción-Zavaleta MJ. From liver to hormones: The endocrine consequences of cirrhosis. World J Gastroenterol 2024; 30(9): 1073-1095
- URL: https://www.wjgnet.com/1007-9327/full/v30/i9/1073.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i9.1073
Hepatocrinology is the field that investigates the bidirectional interaction between hepatic pathophysiology and the endocrine system[1]. This relationship was established over 70 years ago when cases of gynecomastia and hypogonadism were documented in patients with Laennec’s liver cirrhosis (LC). Pathological findings were also observed in their adrenal glands and pancreas[2]. The liver synthesizes hormones and hormone-transporting proteins[1] and plays a key role in the body’s metabolic detoxification[3]. Individuals with chronic liver diseases (CLD) often exhibit endocrinological disorders[3,4]. Hepatic dysfunction can directly impact endocrine gland function due to toxin accumulation and indirectly affect protein synthesis and transport[3].
LC, a consequence of hepatic inflammation and fibrosis, significantly contributes to morbidity and mortality in patients with CLD[5]. It can lead to the development of hepatocellular carcinoma (HCC) and hepatic decompensation, characterized by manifestations such as ascites, hepatic encephalopathy, and bleeding from gastroesophageal varices[5,6].
Despite its prognostic significance, the evaluation of endocrine pathologies in cirrhotic patients is often overlooked by specialists[3,7,8]. Therefore, this manuscript aims to provide an updated narrative review on the epidemiology, pathophysiology, diagnosis, and treatment of endocrine disturbances in patients with LC.
A narrative review was conducted through a bibliographic search in the PubMed/Medline, EMBASE, and Scielo databases, focusing on the search terms “hepatocrinology”, “liver cirrhosis”, “endocrine disturbances”, “diabetes mellitus”, “hypoglycemia”, “sarcopenia”, “thyroid dysfunction”, “adrenal dysfunction”, “hypogonadism”, “hepatic bone disease”, “secondary hyperaldosteronism”, and “growth disorders”. Systematic reviews, meta-analyses, clinical trials, narrative reviews, retrospective studies, cross-sectional studies, prospective studies, and case reports were included, all closely aligned with the objectives of this manuscript. The search was limited to documents written in Spanish or English, excluding conference abstracts. Articles were not excluded based on their publication date. A total of 172 articles were included and are cited in the References section.
LC ranks 15th in terms of disability-adjusted life years[9]. In 2017, the global prevalence of compensated LC was estimated at 1395 cases per 100000 population, and for decompensated LC, it was 132 cases per 100000 population[6], predominantly affecting males (60%)[10]. LC accounts for 2.4% of global deaths, with approximately two-thirds of these cases occurring in men[10]. Common causes of LC include hepatitis B and C infections, alcoholism, and non-alcoholic fatty liver disease (NAFLD)[5,6]. Alcoholism is responsible for 60% of cirrhosis cases in regions such as Europe, North America, and Latin America[9].
In early 2020, an international expert group introduced the metabolic dysfunction-associated fatty liver disease (MAFLD) definition, which offers a more inclusive approach than NAFLD. MAFLD identifies a broader range of patients with hepatic steatosis and high risks of metabolic disorders, including diverse causes of liver steatosis[11,12]. Additionally, metabolic dysfunction-associated steatohepatitis (MASH) involves liver inflammation in the diagnosis[13]. Upon examination of this updated definition, it is found that the prevalence of MAFLD accounts for approximately one-third of the global population[14].
Around 80% of cirrhotic patients experience impaired glucose tolerance (IGT), and 20%-30% develop diabetes mellitus (DM)[15,16]. Prevalence increases with the severity of hepatic dysfunction[15,17] and is higher in cases secondary to MAFLD (56%), cryptogenic cirrhosis (51%), hepatitis C virus (32%), alcoholic cirrhosis (27%), and hepatitis B virus (22%)[15]. Approximately half of cirrhotic patients develop hypoglycemia[18]. Patients with both DM and LC have 2.7 times more episodes of hypoglycemia than those without LC. Risk factors include advanced age, renal impairment, and the use of insulin, sulfonylureas, beta-blockers, or fibrates[19]. Hypoglycemia is more common during concurrent infections and is associated with high mortality rates[1,20]. Sarcopenia prevalence ranges from 30% to 70%, regardless of LC etiology, increasing with hepatic dysfunction severity and reaching 100% in liver transplant candidates[21,22].
Thyroid dysfunction prevalence varies from 13% to 61% in cirrhotic patients[23]. Osteoporosis affects 3%-43% of cirrhotic patients, particularly in the lumbar spine, femur, and hip[23]. Adrenal insufficiency has been reported in 15%-72% of cirrhotic patients[7]. This wide variability is possibly due to differences in clinical scenarios where these hormonal axes were studied. More than half of cirrhotic patients exhibit hypogonadism[24].
The liver produces insulin-like growth factor 1 (IGF-1) and betatrophin, influencing insulin sensitivity, angiotensinogen, contributing to blood pressure regulation, hepcidin, crucial in iron metabolism, and thrombopoietin, regulating platelet production. It also synthesizes hormone-transporting proteins such as IGF-binding protein (IGFBP), sex hormone-binding globulin (SHBG), thyroid hormone-binding globulin (TBG), transthyretin, corticosteroid-binding globulin, and vitamin D-binding protein[1].
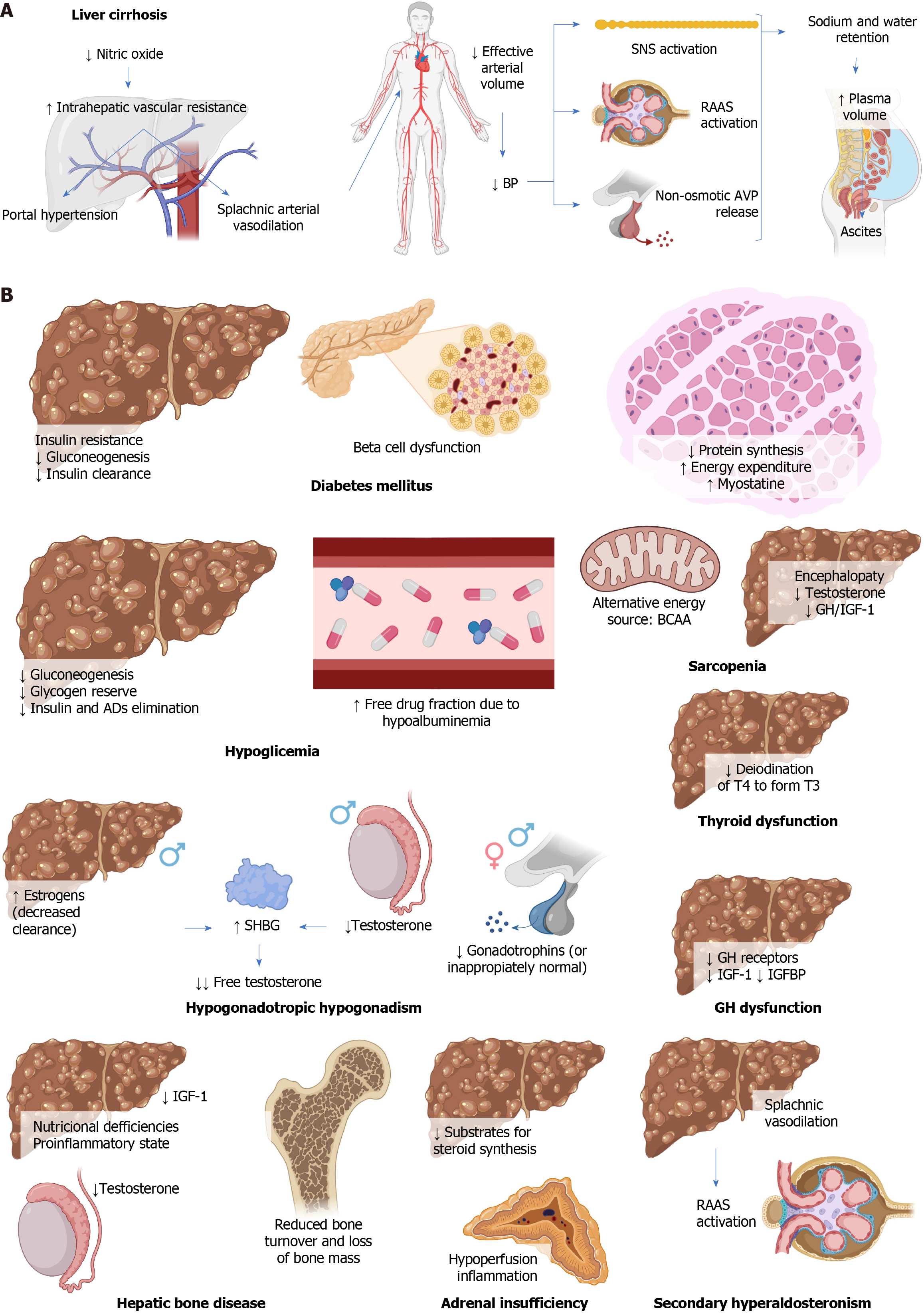
LC, characterized by histological structural alterations, triggers distortion in hepatic angioarchitecture leading to portal hypertension. This results from an imbalance in intrahepatic circulation, favoring vasoconstriction due to increased vasoconstrictors compared to vasodilators, causing rapid changes in portal pressure[5].
In LC, the production of nitric oxide, a vasodilator, decreases in sinusoidal endothelial cells, worsening intrahepatic vascular resistance and contributing to portal hypertension. This increase in intrahepatic vascular resistance elevates portal pressure, causing circulatory disturbances such as splanchnic arterial vasodilation[5]. Splanchnic circulation contributes approximately 25% to systemic vascular resistance, so splanchnic vasodilation leads to a decrease in effective arterial blood volume. This triggers systemic hypotension, inadequate arterial filling, activation of the sympathetic nervous system, the renin-angiotensin-aldosterone system (RAAS), and non-osmotic release of vasopressin, counteracting vasodilation by promoting salt and water retention, increasing plasma volume. Some of this excess plasma volume shifts to the peritoneal space, resulting in ascites[5]. Figure 1A summarizes the pathophysiology of LC.
The described pathophysiological alterations lead to and/or are associated with multiple endocrine disorders, detailed below.
DM plays a significant role in the development of MAFLD and LC. DM associated with LC is known as hepatogenic diabetes[17], and although its pathophysiology is not fully understood, it involves systemic insulin resistance due to alterations in adipokine secretion, proinflammatory cytokines, and free fatty acids[1,25,26]. It also involves a decrease in non-oxidative disposal and oxidative metabolism of glucose, along with LC-induced sarcopenia[27]. Hepatic insulin resistance is observed in cirrhotic patients with DM but not in those without DM[16,17]. Gluconeogenesis is either maintained or decreased[27]. Hyperinsulinemia is observed, more pronounced in hepatogenic diabetes, possibly due to reduced hepatic clearance caused by portosystemic shunting and hepatocyte dysfunction[17]. However, it is unclear whether this hyperinsulinemia is a cause of insulin resistance or an adaptive response to it[27].
Beta-cell dysfunction due to alcohol results from its direct toxic effect, while in hemochromatosis, damage originates from excessive iron accumulation in these cells. In the context of MAFLD, free fatty acids can deposit in pancreatic islets. Regarding hepatitis C virus infection, both direct damage through viral toxicity and indirect damage mediated by autoimmune responses can be observed[27]. Decreased pancreatic beta-cell function is crucial in the transition to overt DM, suggesting that cirrhotic patients might benefit from interventions to protect or enhance the function of these cells, such as thiazolidinediones and incretin-based therapies[17] (Figure 1B).
Underlying mechanisms of hypoglycemia in LC include glycogen depletion, reduced gluconeogenesis, and alterations in insulin and other drug metabolism by the liver[1,20], which is further diminished due to renal dysfunction associated with LC. Hypoalbuminemia and fluid retention increase the free fraction of drugs[28].
Patients with HCC may experience hypoglycemia due to a syndrome known as non-islet cell tumor hypoglycemia. This syndrome is characterized by decreased serum levels of insulin, C-peptide, and beta-hydroxybutyrate, along with increased levels of IGF-2 and the IGF-2/IGF-1 ratio[29-31] (Figure 1B).
Sarcopenia is a syndrome of muscle loss, the definition of which in LC is still debated. Its pathophysiology in this context involves multiple factors, including reduced protein synthesis due to decreased intake, hypercatabolism, and increased energy expenditure. Chronic depletion of hepatic glycogen leads to the search for alternative substrates for energy production, such as fatty acids and benefits of branched-chain amino acid (BCAA), especially during prolonged fasting states like sleep, as these patients experience a state of ‘accelerated fasting’, a deteriorated adaptive response in which gluconeogenesis, fat oxidation, and ketogenesis rates increase[21,32]. Hepatic encephalopathy leads to physical inactivity and, additionally, hyperammonemia and increased production of myostatin, a member of the transforming growth factor β superfamily that inhibits muscle protein synthesis and increases its degradation[21,32]. Alcohol decreases ureagenesis and contributes to hyperammonemia, in addition to its direct toxic effects on muscles[22]. Insulin resistance, common in LC and MAFLD, leads to decreased protein synthesis, increased catabolism, and lipotoxicity[21]. Testosterone and growth hormone (GH)/IGF-1 normally stimulate protein synthesis and suppress myostatin production, but their decrease in LC contributes to sarcopenia[22,32].
Myosteatosis is the pathological accumulation of fat in skeletal muscles and affects half of cirrhotic patients, worsening with age and liver dysfunction progression. In addition to sarcopenia, it holds significant prognostic importance[22] (Figure 1B).
LC can lead to hypogonadotropic hypogonadism. In males, the main clinical manifestation is gynecomastia, although decreased libido, hair loss, sarcopenia, obesity, and fatigue can also occur. In females, common menstrual irregularities include oligomenorrhea or amenorrhea[1,24].
In males, this condition is due to hyperestrogenism caused by reduced hepatic clearance of estrogen due to portal hypertension, decreased testosterone production, increased SHBG levels, and hypothalamic-pituitary dysfunction[1,24]. The increase in SHBG levels is likely due to enhanced hepatic production, hyperstimulated by hyperestrogenism and lacking suppression due to lower testosterone levels. The reduction in free testosterone is even greater since the existing testosterone binds strongly to SHBG[33]. In females, the primary mechanism is hypothalamic-pituitary dysfunction[1,24].
Except for LC caused by alcohol, suppressed or inappropriately normal levels of gonadotropins are observed, which might reverse after liver transplantation[1,24]. Other involved mechanisms include LC-induced hyperprolactinemia, inhibiting gonadotropins, and IGF-1 deficiency, which also contributes to hypogonadism[23] (Figure 1B).
The liver is the primary organ where the conversion of thyroxine to its active form, triiodothyronine, occurs through type 1 deiodinase, making LC commonly associated with thyroid hormone dysfunction[23]. Furthermore, thyroid hormones regulate hepatocyte metabolic rate. Hepatic dysfunction can affect thyroid function and vice versa[34]. A higher fre
Hypothyroidism is associated with dyslipidemia and a higher body mass index (BMI), increasing the risk of MAFLD. Reduced thyroid hormones decrease intrahepatic lipolysis and triglyceride clearance; they also increase tumor necrosis factor α (TNF-α) and leptin while decreasing adiponectin, thus contributing to hepatic inflammation and fibrosis[35].
Thyroid autoimmunity is often found in patients with autoimmune liver diseases such as autoimmune hepatitis, primary biliary cirrhosis, and primary sclerosing cholangitis[1,36].
Hyperthyroidism can cause liver dysfunction due to prolonged exposure to elevated thyroid hormone levels, leading to direct hepatic toxicity and hepatic necrosis due to right-sided heart failure caused by longstanding thyrotoxicosis[35].
Additionally, an increase in serum TBG concentration has been observed in HCC, normalizing after tumor resection[1] (Figure 1B).
LC often leads to disturbances in bone metabolism, such as osteoporosis, and less frequently, osteomalacia or rickets. These effects result from a combination of factors, including nutritional deficiencies, the proinflammatory state, and hypogonadism[23]. Vitamin D levels are inversely associated with the degree of hepatic dysfunction, overall mortality, and infections in cirrhotic patients, as well as the severity and prognosis in HCC[37,38]. Decreased IGF-1 production contributes to reduced bone turnover and bone mass loss[1,39,40] (Figure 1B).
Adrenal insufficiency has been observed in cirrhotic patients, especially in situations of septic shock or hepatic decompensation, known as hepato-adrenal syndrome[41]. This phenomenon has also been described in critically ill cirrhotic patients, with or without sepsis, as well as in liver transplant recipients and even some stable patients[42,43]. The exact mechanism of this phenomenon is not yet fully understood; however, it has been suggested that the reduction in hepatic transport of cholesterol through high-density lipoproteins (HDL), due to decreased levels of apolipoprotein A1 and enzymatic activity of lecithin-cholesterol acyltransferase, could reduce the available substrates for steroid synthesis in the adrenal cortex[1,7,44]. This, in turn, could result in the adrenal glands’ inability to produce adequate amounts of cortisol, especially in stressful situations, leading to adrenal insufficiency[42]. Additionally, other possible mechanisms have been suggested, such as chronic hypoperfusion of the adrenal glands due to splanchnic vasodilation and the impact of proinflammatory cytokines such as interleukin 1 (IL-1), IL-6, and TNF-α, which tend to increase in LC and could suppress both corticotropin-releasing hormone and adrenocorticotropic hormone secretion, as well as reduce peripheral tissues’ sensitivity to glucocorticoids[7,23,42] (Figure 1B).
IGF-1 and IGFBP are mainly synthesized in the liver and play a fundamental role in regulating cellular growth and differentiation[23]. GH stimulates the production of IGF-1 in the liver, essential for growth and development in childhood and the maintenance of cardiovascular health in adults[23]. The actions of IGF-1 are locally and systemically regulated by IGFBP. In LC, GH levels increase due to a heightened response to GH-releasing hormone, but hepatic synthesis capacity of IGF-1 decreases due to reduced hepatic mass, fewer GH receptors in the cirrhotic liver, and blockade exerted by certain IGFBP[1,23]. Consequently, the negative feedback exerted by IGF-1 diminishes, leading to further elevation of GH levels[23].
Linear growth in children with LC is often restricted despite elevated GH levels, due to GH resistance caused by reduced hepatic synthesis of IGF-1 and IGFBP-3[45] (Figure 1B).
Splanchnic vasodilation in LC leads to a decrease in effective arterial blood volume, stimulating the RAAS, the sympathetic nervous system, and arginine-vasopressin, causing retention of sodium and water. Angiotensin II induces systemic arterial vasoconstriction, stimulates vasopressin release, maximizes sodium (and water) reabsorption, and strongly activates the sympathetic nervous system. Besides its effects on sodium retention and blood pressure elevation, aldosterone activates mineralocorticoid receptors in non-epithelial tissues. In the presence of high sodium intake, it causes oxidative stress, endothelial dysfunction, inflammation, fibrosis, and remodeling, leading to cardiovascular and renal injuries. Additionally, it can lead to peripheral insulin resistance and metabolic syndrome[46-48] (Figure 1B).
Within the diagnosis of LC, it is crucial to assess the disease stage, quantify hepatic fibrosis, identify the presence of portal hypertension, and determine underlying causes. Although liver biopsy remains the gold standard, non-invasive tests such as the Fibrosis-4 Index and hepatic elastography can be utilized to evaluate the degree of fibrosis, with elastography being a practical choice[5].
The Child-Pugh classification stands as one of the most widely used methods for evaluating the prognosis in cirrhotic patients. It categorizes patients into three groups with varying survival prognoses. We use the term “compensated cirrhosis” to refer to Child-Pugh class A, encompassing patients with normal liver function and those with mildly impaired liver function (slight elevation in serum bilirubin or prothrombin time, or a history of decompensation such as ascites, jaundice, variceal bleeding, hepatorenal syndrome, or encephalopathy). The term “decompensated cirrhosis” refers to Child-Pugh classes B and C, including patients with moderate or severe impairment in liver function, presence of ascites and/or encephalopathy, and a history of decompensation[49,50].
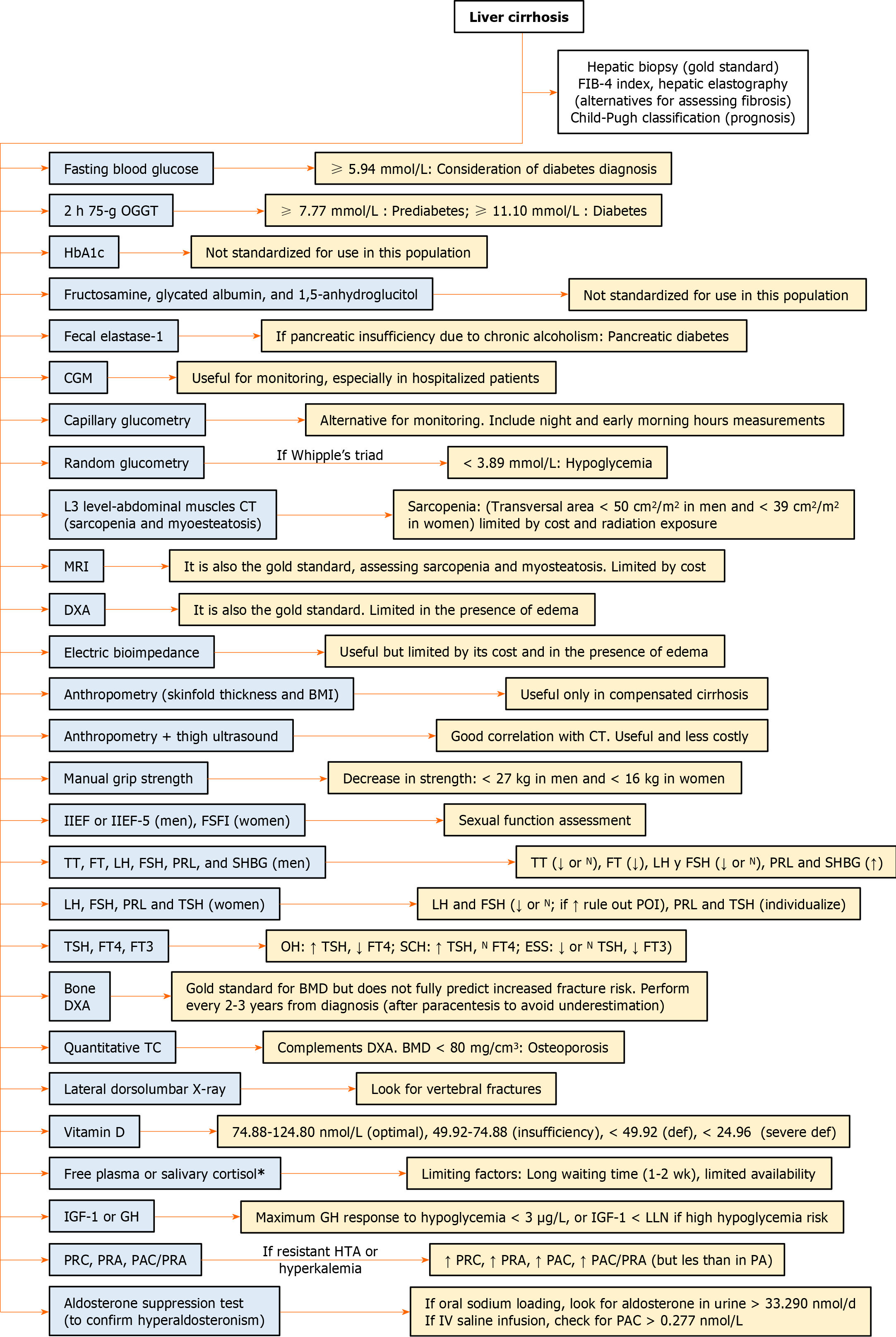
For the study of endocrine disorders associated with LC, relevant biochemical and hormonal tests are required, as explained below, and summarized in Figure 2.
Fasting plasma glucose (FPG) measurement is economical but demands specific conditions for accuracy. The optimal threshold for diagnosing DM in patients with CLD has not been defined, as traditional cutoffs have reduced sensitivity in these patients. For instance, in patients with LC due to hepatitis C, a FPG level of 5.94 mmol/L (107 mg/dL) might be considered diagnostic of DM, although it remains uncertain if this cutoff could apply to LC from other etiologies[51,52]. In patients with LC and DM, FPG levels are often normal or low due to alterations in hepatic gluconeogenesis[25,28,50,51]. The 75 g oral glucose tolerance test (OGTT) can diagnose IGT and identify more cases of DM[52].
Hemoglobin A1c (HbA1c) is commonly used in DM but is inaccurate in cirrhotic patients due to factors such as reduced red blood cell lifespan from hypersplenism, anemia, gastrointestinal bleeding, or vitamin deficiency. In patients with LC and DM, the average HbA1c reported is 39 mmol/mol (5.7%)[25,28,50,51,53].
Hepatogenic diabetes distinguishes itself from type 2 DM by its weaker association with traditional risk factors and complications. FPG levels and HbA1c are normal. Diagnosis requires an abnormal result on the 75-g OGTT[17].
The most suitable method for monitoring blood glucose in cirrhotic patients with DM is controversial. Continuous glucose monitoring (CGM) has proven more accurate than HbA1c measurement in these patients[53]. Glycated albumin and fructosamine reflect glycemic control over the last 2 wk to 3 wk, but their accuracy decreases in LC due to altered protein metabolism. Corrections have been proposed but lack validation[52]. CGM has also been found superior to fructosamine measurement in cirrhotic patients[53]. Serum 1,5-anhydroglucitol is a sensitive glycemic marker in patients with DM, but its accuracy decreases in LC due to altered metabolism and malnutrition[52]. Capillary blood glucose monitoring is a suitable alternative for glycemic monitoring, and it’s recommended to perform it at different times of the day, including nighttime and early morning, as up to 20% of patients with HbA1c below 7% experience nocturnal hypoglycemia[50].
On the other hand, some cirrhotic patients might suffer from pancreatic diabetes, characterized by concurrent exocrine pancreatic insufficiency within the context of chronic pancreatitis triggered by chronic alcoholism, a contributing factor to LC. This condition can be diagnosed through fecal elastase 1 testing[50,54].
The Endocrine Society recommends investigating hypoglycemia when the Whipple triad is present (neuroglycopenic and autonomic symptoms of hypoglycemia, evidence of low blood glucose, and symptom resolution upon glucose elevation). In healthy individuals, symptoms of hypoglycemia manifest when blood glucose is below 3.05 mmol/L (55.00 mg/dL). However, in cases of recurrent hypoglycemia, the thresholds for symptoms can be lower. Hypoglycemia in people without DM is rare and can be caused by critical illnesses such as renal, hepatic, or cardiac failure[55]. For people with DM, the American Diabetes Association defines hypoglycemia as a glucose concentration below 3.89 mmol/L (70.00 mg/dL)[56]. A study in patients with chronic or acute hepatic failure defining hypoglycemia as blood glucose below 2.80 mmol/L (50.40 mg/dL) demonstrated that half of the patients experienced a hypoglycemic episode during their hospitalization, establishing it as an independent risk factor for 90-d mortality (OR = 8.72, P = 0.01)[57]. Another study in hospitalized cirrhotic patients found an association between hypoglycemia and positive blood cultures (P = 0.01), also serving as a predictor for bacteremia and mortality. Seventy-five percent of the patients in this study had compensated LC, suggesting that alterations in hepatic gluconeogenesis could exist and be clinically relevant in early stages of liver disease[58].
Up to 8% of cirrhotic patients have been reported to experience silent hypoglycemia, which could lead to permanent brain damage, highlighting the need for regular glucose monitoring[19]. In high-risk hospitalized patients with DM, including those with hepatic dysfunction, CGM is recommended for the rapid detection of nocturnal hypoglycemia[59].
Muscle mass can be assessed using magnetic resonance imaging or computed tomography (CT), which are currently considered the gold standard, enabling the diagnosis of both sarcopenia and myosteatosis[22]. CT measures the cross-sectional area of abdominal skeletal muscles at the level of the third lumbar vertebra, making it the most used modality with validated specific cutoff points by gender (< 50 cm2/m2 in men and < 39 cm2/m2 in women, generally). However, its cost and repeated exposure to radiation may limit its use[22,60]. Dual-energy X-ray absorptiometry (DXA) is another current gold standard, although its utility is limited in the presence of edema[22]. Electrical bioimpedance is also a useful tool, albeit constrained by its high cost and the presence of edema[21,22]. Less commonly used methods include mid-arm muscle circumference and quadriceps ultrasound[21]. Anthropometric assessments, such as skinfold measurements and BMI, can be helpful in the context of compensated LC but are much less reliable in cases of more severe liver dysfunction. On the other hand, the combination of anthropometry and ultrasound assessment of thigh muscle thickness has shown significant correlation with CT results, offering a useful and less expensive approach[22].
Muscle strength can be evaluated by measuring handgrip strength, which also allows for the detection of reduced strength (defined as handgrip strength < 27 kg in men and < 16 kg in women) in the context of concurrent myosteatosis, where there might be an increase in muscle volume at the expense of fat infiltration[22,60].
In patients with LC, hypogonadism affects reproductive and sexual function, leading to a negative impact on their quality of life[24].
In clinical practice, having objective tools to assess the presence and severity of erectile dysfunction in patients is useful, such as the International Index of Erectile Function (IIEF)[61], and its short version, the IIEF-5, which addresses erectile and orgasmic function, sexual desire, as well as sexual intercourse and overall satisfaction[62]. The Female Sexual Function Index (FSFI) covers domains such as desire, arousal, lubrication, orgasm, satisfaction, and pain[63].
In males, the initial evaluation should include testosterone, luteinizing hormone (LH), follicle-stimulating hormone (FSH), prolactin (PRL), and SHBG. When interpreting results, it is important to consider that in patients with LC, SHBG production is altered, and the reference ranges for testosterone depend on the assay platforms used for androgen measurement[24]. A low total or low-normal testosterone level in the context of symptoms of reproductive dysfunction or sexual dysfunction requires further evaluation with measurement of free or bioavailable testosterone and concurrent LH and FSH values. Testosterone concentrations vary throughout the day and from day to day, so total testosterone concentrations should be measured in fasting samples on different mornings. In cases of SHBG alteration, such as in LC, or when the initial total testosterone concentration is close to the lower limit of the normal range, free testosterone concentrations should be determined. The lower limit of the normal range for total testosterone in young, non-obese, healthy men is 9.2 nmol/L (264 ng/dL). A harmonized reference range for free testosterone has not yet been established, so laboratory-specific lower limits can be utilized. Once hypogonadism is documented, LH and FSH tests should be requested. Elevated values suggest primary hypogonadism, whereas low or inappropriately normal values indicate secondary (hypogonadotropic) hypogonadism[64].
In females, the initial approach should include FSH, LH, PRL, and thyroid-stimulating hormone (TSH). Ideally, FSH and LH should be measured on the second or third day of the menstrual cycle; however, this is often not possible due to the presence of oligomenorrhea or amenorrhea[65]. Elevated PRL levels require further investigation, while abnormal TSH indicates thyroid dysfunction. Elevated LH and FSH concentrations suggest primary ovarian insufficiency, whereas low or normal levels in the presence of irregular menstruation are more consistent with hypothalamic or pituitary pathology (hypogonadotropic hypogonadism)[24,66].
Thyroid dysfunction is associated with abnormalities in liver enzymes and should be ruled out in cases of unknown elevation[34]. Regular thyroid function tests are necessary for patients with cirrhosis, including measurements of TSH, free thyroxine, and free triiodothyronine (FT3)[67].
Hypothyroidism: Clinical manifestations of hypothyroidism are nonspecific and can mimic a state of liver dysfunction with symptoms such as fatigue, myalgias, cramps, dyspnea, and edema. It may rarely present with ascites or mimic hepatic encephalopathy in cirrhotic patients. Hypothyroidism should be suspected and ruled out in patients with LC and seemingly refractory hepatic encephalopathy treatment[68]. Primary hypothyroidism is often associated with a moderate elevation of liver enzymes[69]. These abnormalities usually normalize after hormonal replacement therapy with levothyroxine[34]. Histology is usually normal; however, the relationship between hypothyroidism and liver fibrosis has been evaluated compared to euthyroidism, showing a 2.48 times higher risk[70].
Subclinical and overt hypothyroidism present nonspecific symptoms. Hypothyroidism has been associated with a 61% increased risk of progression to MASH in patients with MAFLD[71].
Euthyroid sick syndrome: This clinical entity is characterized by low FT3 and normal or low TSH concentration. These alterations usually occur in critically ill patients, approximately 70% of those admitted to the intensive care unit and have been associated with increased mortality[72]. This syndrome lacks clinical manifestations or characteristic physical exam findings. However, it should be noted that it can affect patients with preexisting thyroid diseases, and the use of certain drugs in these critically ill patients can alter thyroid hormone secretion[73]. FT3 inclusion in mortality prediction scales for patients with liver failure, such as the FT3-MELD score, performs better in short-term mortality prediction[74].
Hyperthyroidism: The liver is a significant target of excess thyroid hormones, reflected in the fact that 15%-76% of newly diagnosed hyperthyroid patients exhibit abnormal liver function tests[67]. The most common abnormalities in patients with thyrotoxicosis are elevated alkaline phosphatase (64%), gamma-glutamyl transferase (62%), alanine aminotransferase (37%), and aspartate aminotransferase (27%)[67]. Histologically, patients with hyperthyroidism may present with mild liver injury, with centrolobular cholestasis; however, progression of damage is rare and normalizes with treatment[34]. Liver involvement in overt hyperthyroidism is usually self-limiting, although there are reported cases of patients with thyrotoxicosis and fulminant liver failure, especially if coexisting with heart failure or in cases of thyroid storm. Thyrotoxicosis should be considered in the differential diagnosis of causes of acute liver failure, especially in patients with known thyroid dysfunction history[75].
Bone disease is one of the primary complications of LC and can result in fractures spontaneously or following low-intensity traumas, increasing morbidity and decreasing the quality of life. The prevalence of osteoporosis in cirrhotic patients varies according to the cause, being higher in cases secondary to cholestatic disease, ranging between 20%-44%[76,77]. Regarding the distribution of alterations in bone mineral density (BMD), it has been observed that abnormalities are more common in the lumbar spine, with no differences at the hip level[78]. DXA is considered the method to measure BMD and diagnose osteoporosis in cirrhotic patients. However, BMD assessment cannot fully predict or explain the risk of increased fractures, making it necessary to use complementary diagnostic tools such as quantitative CT[78]. A quantitative tomographic analysis with a finding of BMD below 80 mg/cm³ diagnoses osteoporosis[79]. Within the findings in bone microstructure, it has been found that the trabecular bone fraction and trabecular thickness were reduced in advanced cholestatic liver disease[80]. Cirrhotic patients have been found to have lower trabecular bone volume, lower cortical BMD in the tibia, and lower Z scores in the lumbar spine and hip[78].
Due to these findings and the alterations in calcium and vitamin D metabolism that can occur in patients with liver injury, DXA is recommended when the diagnosis of LC is confirmed and then repeated every two or three years[81]. Also, since ascites can cause an underestimation of BMD, increasing values by up to 4.2%-7.0% after paracentesis, measuring BMD immediately after paracentesis is recommended. Additionally, a lateral X-ray of the dorsal and lumbar spine segments or vertebral morphometry by DXA should be performed to look for vertebral fractures[77]. Ideally, BMD measurement should be done with the trabecular bone score, which can help identify cirrhotic patients at high risk of vertebral fractures[82].
In LC, vitamin D requirements might increase, so its levels should be measured, along with serum calcium, serum phosphorus, and urinary calcium[81]. The optimal vitamin D levels are controversial. Most authors suggest that optimal levels are between 74.88 nmol/L and 124.80 nmol/L (30-50 ng/mL), while levels from 49.92 nmol/L to 74.88 nmol/L (20-30 ng/mL) are considered insufficient. Below 49.92 nmol/L (20 ng/mL) is classified as vitamin D deficiency, and below 24.96 nmol/L (10 ng/mL) is considered severe deficiency[38].
Adrenal insufficiency in cirrhotic patients is a common but underdiagnosed problem due to its clinical presentation similarity with stress-induced adrenal insufficiency and the challenge of finding an appropriate diagnostic method to evaluate the hypothalamic-pituitary-adrenal axis in patients with evident decreased synthesis of albumin and corticosteroid-binding globulin. As described in the pathophysiology, this decreased synthesis is the main mechanism causing this disorder[7,83,84].
Ninety percent of cortisol is bound to transport proteins synthesized by the liver, so as hepatic function deteriorates, the prevalence of adrenal insufficiency increases[7,85,86]. The prevalence of adrenal insufficiency in non-hospitalized patients ranges from 26%-49%, and in hospitalized patients, it ranges from 51%-82%. Moreover, in Child-Pugh class A patients, the prevalence of adrenal insufficiency is 0%-44%, in Child-Pugh class B patients, it is 23%-54%, and in Child-Pugh class C patients, it is 57%-68%. Therefore, its evaluation is of vital importance in critically ill patients and in advanced stages of hepatic dysfunction[7,84-87].
Despite the lack of a consensus, the presence of adrenal insufficiency should be evaluated in patients with refractory hypotension, patients with sodium levels less than 125 mmol/L, and patients with HDL levels less than 0.39 mmol/L (15 mg/dL)[85,88-90].
In non-cirrhotic patients, adrenal insufficiency is diagnosed by an inadequate response to the standard 250 μg ACTH stimulation, defined as a maximum total cortisol level of 496.62 nmol/L (18 μg/dL). Additionally, the cortisol delta less than 248.31 nmol/L (9 μg/dL) in critically ill patients or the total cortisol level at 8 a.m. lower than 137.95 nmol/L (5 μg/dL) is routinely used. The decrease in protein synthesis in cirrhotic patients complicates the use of these diagnostic methods. Therefore, only the measurement of free plasma cortisol or salivary cortisol can be used, and these should be measured in parallel with total cortisol to assess probable deficiency, with an average delay of 1 wk to 2 wk and limited availability in emergency services. Based on the above, we suggest measuring these in situations where the patient’s clinical condition raises doubts about the diagnosis[83,87,91].
In patients at high risk of complications such as hypoglycemia, measuring IGF-1 levels below normal limits is often sufficient. However, the standard diagnosis is defined by a maximum GH response to hypoglycemia below 3 μg/L (3 ng/mL)[85,92-96]. However, this test can lead to severe hypoglycemia, need close supervision, and is contraindicated in some patients. The macimorelin stimulation test has become the gold standard for diagnosis of GH deficiency[97], however, there is no data regarding its performance in patients with LC.
Patients may be asymptomatic or present with new or resistant hypertension, fatigue, headache, polyuria, and polydipsia. Hypokalemia, mild hypernatremia, and mild hypermagnesemia are often found[47,48,98].
Plasma renin concentration and plasma renin activity (PRA) levels are part of the initial diagnostic approach and are elevated, unlike in primary hyperaldosteronism. Plasma aldosterone concentration (PAC) is also elevated, as well as the PAC/PRA ratio, although not at the levels observed in primary hyperaldosteronism [PAC > 0.554 nmol/L (20 ng/dL) and PAC/PRA > 30][47,48,98].
Confirmation of the diagnosis of hyperaldosteronism involves aldosterone suppression, often through a 3-d oral sodium load (5000 mg of sodium in the diet or 90 mEq of sodium in tablets) followed by aldosterone measurements in urine. A value above 33.29 nmol/d (12 μg/d) confirms the diagnosis. Intravenous infusion of 2 L of isotonic saline over 4 h is an alternative, requiring a PAC value that exceeds 0.277 nmol/L (10 ng/dL) to confirm the diagnosis, although it has a 30% false negative rate[47,48].
The goals of DM management in cirrhotic patients vary based on the presence of decompensation. For compensated patients, it is recommended to maintain the targets set by the American Diabetes Association: FPG between 4.44 and 7.22 mmol/L (80-130 mg/dL) and postprandial glucose less than 9.99 mmol/L (180 mg/dL). However, in decompensated cirrhotic patients, considering the decreased survival associated with cirrhosis complications, more flexible objectives are proposed: FPG between 5 and 8.32 mmol/L (90-150 mg/dL), postprandial glucose less than 11.1 mmol/L (200 mg/dL), and pre-meal glucose levels between 5.55 and 11.1 mmol/L (100-200 mg/dL)[28,50].
Regarding physical activity, a regimen of 150 to 300 min of intense physical activity per week, divided between aerobic exercises and resistance training, is recommended[28,99]. Alcohol cessation can aid in weight control. Additionally, following a Mediterranean diet pattern with 50%-65% of carbohydrates is advised to prevent hypoglycemia[100].
Metformin, with its short half-life and renal elimination, improves insulin resistance and carries a low risk of hypoglycemia. In patients with compensated LC, it can be used as a first-line medication, given the findings suggesting a reduced risk of HCC[25,28,50]. A study showed that metformin use in diabetic patients was associated with reduced mortality [Hazard ratio (HR): 0.68, 95% confidence interval (95%CI): 0.61-0.75], although not with rates of HCC or hepatic decompensation[101]. Other studies have confirmed reduced mortality[102] and demonstrated a decrease in the risk of decompensation[103] and progression to HCC[104-106]. Metformin is often avoided in patients with liver diseases due to concerns about lactic acidosis. However, metformin is not inherently hepatotoxic, and metformin-associated lactic acidosis is extremely rare, with an incidence of fewer than 10 per 100000 patient-years of exposure in patients without renal insufficiency[52,107,108]. It is recommended to use a maximum of 1500 mg/d of metformin in cirrhotic patients and to avoid it entirely in contexts of hypoxemia, dehydration, sepsis, or decompensated cirrhosis[50,109].
The metabolism and duration of action of sulfonylureas vary. Long-acting ones like glyburide and glimepiride may pose a higher risk of hypoglycemia, especially in patients with renal impairment, compared to other sulfonylureas like glipizide[100]. A study revealed that sulfonylureas were associated with a reduced risk of major cardiovascular events (aHR: 0.69, 95%CI: 0.61-0.80), stroke (aHR: 0.66, 95%CI: 0.53-0.83), ischemic heart disease (aHR: 0.66, 95%CI: 0.53-0.83), and heart failure (aHR: 0.71, 95%CI: 0.55-0.92), with no significant differences in severe hypoglycemia risk (aHR: 0.78, 95%CI: 0.52-1.10)[110]. This study was conducted in compensated cirrhotic patients, suggesting the cautious use of sulfonylureas in these cases but in lower doses than usual to prevent hypoglycemia[28,50,100]. However, they are contraindicated in decompensated LC[109].
Regarding meglitinides, there are no specific guidelines available. However, they might serve as an alternative to sulfonylureas, although repaglinide should be avoided due to its lower clearance and potential hepatotoxicity[28,50]. Nateglinide can be used in Child-Pugh A and B patients, with doses lower than usual to prevent hypoglycemia[50].
Thiazolidinediones are insulin sensitizers useful in the treatment of MAFLD and MASH[52]. A meta-analysis demonstrated that pioglitazone improves advanced fibrosis but with a high prevalence of side effects such as weight gain and lower extremity edema[111]. They have shown normalization of liver enzymes in patients with MASH, along with reduced hepatocellular lesions, parenchymal inflammation, and fibrosis[52]. They can cause dose-dependent weight gain[112,113]. Conversely, a study showed that the rate of major vascular events for thiazolidinedione users was higher, with aHR of 1.81 (1.28-2.55) for stroke, 1.59 (1.03-2.44) for coronary artery disease, and 2.09 (1.22-3.60) for heart failure, with rosiglitazone posing a higher risk than pioglitazone[114]. Hence, careful use is recommended, and they should be avoided in decompensated cirrhotic patients[28,50]. Pioglitazone can be cautiously used in patients with LC, suitable for those classified as Child-Pugh class A, but it should be avoided if liver enzymes exceed three times the upper limit of the normal range and in patients of classes B and C[50,109]. Rosiglitazone is associated with adverse cardiovascular effects in DM and cirrhotic patients[28].
Dipeptidyl peptidase-4 inhibitors (DPP-4i) prevent the inactivation of glucagon-like peptide 1 (GLP-1) primarily and gastric inhibitory polypeptide (GIP), ensuring glycemic control without the risk of hypoglycemia, weight gain, or reduction in muscle mass[28]. Their use has been associated with major episodes of liver decompensation (aHR: 1.35, 95%CI: 1.03-1.77), variceal bleeding (aHR: 1.67, 95%CI: 1.11-2.52), and liver failure (aHR: 1.35, 95%CI: 1.02-1.79), although without a significant increase in mortality[115]. Vildagliptin carries a high risk of hepatotoxicity, while other DPP-4i can be used in Child-Pugh A cirrhotic patients without dose adjustment. Caution is required in Child-Pugh B patients, and they are not recommended for Child-Pugh C patients[50,109]. Except for linagliptin, DPP-4i are eliminated via renal excretion[28], necessitating dose adjustment in renal dysfunction[25].
GLP-1 receptor agonists (GLP-1 RA) offer various beneficial effects in patients with DM, such as improved insulin sensitivity in adipose and hepatic tissues, inhibition of glucagon secretion, increased satiety, delayed gastric emptying, weight loss, and reduced hepatic lipogenesis. They improve glycemia without the risk of hypoglycemia as they stimulate insulin production in a glucose-dependent manner[28]. Studies have shown that GLP-1 RA use is associated with a lower risk of mortality (aHR: 0.47, 95%CI: 0.32-0.69), cardiovascular events (aHR: 0.60, 95%CI: 0.41-0.87), and decompensated LC (aHR: 0.70, 95%CI: 0.49-0.99)[116]. GLP-1 RA has been linked to a lower rate of hepatic decompensations compared to DPP-4i use (105.2 vs 144.0 per 1000 person-years) and a lower rate of ascites and variceal bleeding. Similar results were found when comparing GLP-1 RA with sulfonylureas. No significant differences were observed in the rate of hepatic decompensations when comparing GLP-1 RA with sodium-glucose cotransporter 2 inhibitors (SGLT-2i)[117]. In patients at high risk of sarcopenia, closer monitoring is recommended due to GLP-1 RA-induced weight loss, which may include lean mass loss[118]. Liraglutide and semaglutide have shown effectiveness in improving NASH[119,120]. GLP-1 RA undergoes no hepatic metabolism, enabling their use without dose adjustment in Child-Pugh A cirrhotic patients. Consideration might be given to their cautious use in class B patients. However, due to limited data in patients with liver insufficiency, their use should be avoided in class C patients[50,109]. The dual GLP-1 and GIP agonist (tirzepatide) has been shown to improve MAFLD and MASH[121-123], and the triple GLP-1, GIP, and glucagon agonist (retatrutide) has similar effects in animal models[124,125], although their effects in managing DM in patients with LC remain unknown.
SGLT-2i act in the proximal tubule of the nephron, increasing urinary glucose and sodium excretion[28]. Apart from reducing glycemia and providing cardiovascular and renal benefits[126], they have been reported to improve aminotransferase levels and hepatic steatosis in patients with DM and MAFLD[127,128]. SGLT-2i are comparable in efficacy to GLP-1 RA in reducing hepatic decompensations[117]. A study found that the use of SGLT-2i in cirrhotic patients prevents worsening liver function and hepatic fibrosis[129]. There are also case reports where the addition of SGLT-2i controlled ascites, hydrothorax, and peripheral edema[130]. These effects are akin to loop diuretics[131], but more studies are needed to confirm their safety and efficacy[132]. Inhibition of renal sodium reabsorption could ameliorate the LC-associated RAAS dysfunction, thus reducing renin secretion; however, this hypothesis requires further confirmation in additional studies[133]. SGLT-2 inhibitors are safe in Child-Pugh A patients; however, caution is required when using them in class B patients and they should be avoided in class C due to the risk of dehydration and hypotension associated with their use. Lower doses should be considered when initiating treatment in cirrhotic patients[28,109].
Alpha-glucosidase inhibitors, such as acarbose, interfere with gastrointestinal carbohydrate absorption, reducing postprandial hyperglycemia. This mechanism is relevant in cirrhotic patients due to their impaired splanchnic and peripheral glucose uptake, making them susceptible to postprandial hyperglycemia[28]. Another potential advantage of this drug is its effect on hepatic encephalopathy by decreasing proteolytic flora, which elevates blood ammonia levels, and its associated laxative effect[28,50]. Alpha-glucosidase inhibitors are relatively safe and can be used without dose modification in Child-Pugh class A and B patients, but they are not recommended in class C patients. They are also not recommended in cases of poorly controlled DM due to their insufficient hypoglycemic effectiveness[50,109].
Approximately 40%-50% of endogenously produced insulin is metabolized in the liver. Despite this, insulin therapy remains the most effective treatment for patients with LC, albeit with the caution of an increased risk of hypoglycemia. In cirrhotic patients with compensated DM, insulin therapy is suggested for those unable to use oral medications, those with inadequate glycemic control [FPG above 16.65 mmol/L (300 mg/dL) or HbA1c above 86mmol/mol (10%)], or in conditions such as sepsis, encephalopathy, bleeding, or acute kidney injury. Insulin is the preferred choice for patients with DM and decompensated LC[28]. It’s important to note that during LC decompensations, hepatic insulin metabolism is further reduced, potentially decreasing the need for insulin, necessitating close glucose monitoring[109,134].
Sliding scale-based insulin therapy is not recommended. Ideally, insulin regimens should include a basal insulin to control overnight and fasting glucose. Long-acting insulin analogs such as glargine, detemir, degludec, or neutral protamine lispro (NPL) are preferred[28]. Glargine and degludec have a duration of action of approximately 24 h, while detemir duration is shorter. Glargine and detemir cannot be mixed with fast-acting insulins due to altered kinetics, but degludec can. NPL’s activity is similar to neutral protamine Hagedorn insulin, with slightly higher hypoglycemia risk than the others[28].
Given the frequent postprandial hyperglycemia in cirrhotic patients, fast-acting insulins are often added. Rapid-acting insulin analogs (lispro, aspart, or glulisine) or regular short-acting human insulin can be used to cover additional insulin needs[28]. Gentile and colleagues conducted a 12-wk study involving 50 patients who received comparable doses of insulin lispro and regular insulin before meals, in addition to insulin glargine. The study found that pre-meal blood glucose levels were similar between the lispro and regular insulin groups. However, the increase in blood glucose levels at one and two hours after meals was significantly higher in patients treated with regular insulin, with a difference of 2.22 mmol/L (40 mg/dL) at two hours. Furthermore, patients using regular insulin experienced greater reductions in glucose levels at 3 h and 4 h after meals and during the night. After two weeks, the incidence of hypoglycemia was significantly higher in patients using regular insulin compared to lispro (9.7 vs 1.8, P < 0.001). Consequently, the study recommended insulin lispro due to its superior safety profile in managing postprandial hyperglycemia[135].
In patients with CLD, it is recommended to reduce insulin dosage by 25% and advise patients about the risk of hypoglycemia[28]. Initiation of insulin therapy is advised at a dose of 0.1-0.2 IU/kg/d, potentially increased if blood glucose levels are significantly elevated. Subsequent doses can be adjusted based on glucose levels, with a recommended increase of 2 units every 3 d if glucose remains above the desired range. Additionally, the addition of rapid-acting insulin can be considered if glycemic goals are not met, ideally starting at 4 units or 10% of the basal insulin, increasing by 1-2 units every 3-4 d[28]. Insulin dosing should be titrated as needed, and the use of insulin analogs is preferred to reduce the risk of hypoglycemia[109].
Statins exhibit pleiotropic effects and dose-dependent alterations in hepatic enzymes[136]. Kruppel-like factor 2 (KLF-2), a component of hepatic endothelium, is induced in early stages of LC, serving as a defense mechanism against fibrosis and endothelial dysfunction during disease progression, albeit insufficiently. Statins enhance KLF-2 expression through the Rac1-MEK5-ERK5-MEF2 pathway, augmenting their protective effects. Atorvastatin, simvastatin, rosuvastatin, fluvastatin, and lovastatin have demonstrated benefits in reducing the risk of hepatic decompensation, variceal bleeding, and mortality, especially in compensated cirrhotic patients[136,137]. Statin use is associated with a lower risk of LC progression in chronic hepatitis B patients[138]. Nationwide studies revealed statin use’s correlation with reduced HCC development, regardless of DM or LC presence[139]. Statin therapy is notably advantageous for males, individuals with DM, and those with high fibrosis-4 index at study initiation[140]. In patients with DM, statin use is linked to a reduced likelihood of advanced fibrosis[141].
The management of obesity in liver disease patients is complex due to potential exacerbation of sarcopenia. Therefore, it is recommended to implement moderate caloric restriction (reducing by 500-800 kcal/d) while ensuring a standard or higher protein intake to prevent muscle catabolism, aiming to achieve a weight reduction of 5%-10%. The use of anti-obesity medications is not recommended in these patients, and the evidence for bariatric procedures is insufficient to make recommendations[28].
Regarding the potential remission of DM, liver transplantation is less effective in patients with impaired beta cell function. This raises the need for a reevaluation of the indication and timing of this procedure in these patients[17].
The primary objective in treating hypoglycemia is the swift correction of blood glucose levels to a safe range, minimizing the risk of cerebral damage and alleviating symptoms. In conscious individuals, the recommended approach involves oral administration of 15-20 g of glucose, although any carbohydrate containing glucose can be utilized. Glucose levels should be re-evaluated after 15 min, and if hypoglycemia persists, the treatment should be repeated. Following the increase in blood glucose, a meal should be consumed to prevent recurrence of hypoglycemia. Glucagon is reserved for severe hypoglycemia and is administered parenterally (intramuscular, intravenous, or subcutaneous)[56,142,143]. In most cases, managing hypoglycemia involves an increased caloric intake throughout the day. Additionally, individuals are advised to abstain from alcohol and consider altering or adjusting doses of hypoglycemic drugs such as insulin and sulfonylureas[144].
Managing DM in cirrhotic patients can be complicated by cognitive impairment due to hepatic encephalopathy, affecting their ability to avoid dosing errors, miss meals, and recognize hypoglycemic episodes[28]. Consequently, many patients will require greater support from their social network. The effective management of hypoglycemia in patients with DM and LC necessitates prevention of further episodes. Therefore, patients using oral antidiabetic agents require dose adjustments or changes in the prescribed medication. Similarly, insulin users may need dosage reductions or, in some cases, temporary discontinuation[59].
The management of sarcopenia necessitates a multidimensional approach emphasizing lifestyle modifications, nutrition, exercise, and adjuvant pharmacotherapy. Recommendations include discontinuation of alcohol and tobacco use, optimizing sleep quality, and enhancing psychological well-being. The European Association for the Study of the Liver (EASL) and the European Society for Clinical Nutrition and Metabolism (ESPEN) advocate for a daily caloric intake of 35-40 kcal/kg and a protein intake of 1.2-1.5 g/kg[145,146]. Additionally, considering the potential benefits of BCAA supplementation, EASL recommends its long-term use at a dosage of 0.25 g/kg/d for patients with advanced hepatic dysfunc
Moderate-intensity aerobic and resistance exercises enhance cardiovascular capacity and muscle mass, significantly improving the quality of life, especially in patients with compensated LC and to a lesser extent in decompensated LC patients[21]. Treatments targeting ammonia reduction, hormonal supplements, and myostatin inhibitors are still under investigation[21,22,147].
Addressing sexual dysfunction in cirrhotic patients requires a multidisciplinary approach. In males with LC and low testosterone levels, supplementation has shown additional benefits in total body lean mass and bone mass. Therefore, it could be considered as complementary therapy to enhance frailty in cirrhosis[148]. Regarding the use of phosphodiesterase-5 inhibitors (PDE-5i), four are approved by the Food and Drug Administration: Sildenafil, tadalafil, avanafil, and vardenafil. None of these are recommended for Child-Pugh C patients, and if prescribed, dosage reduction is common due to decreased clearance[24]. Currently, the use of PDE-5i is being evaluated for potential benefits in portal hemodynamics and hepatic encephalopathy[149].
In women, treatment should address reproductive and/or sexual dysfunction. Fundamental intervention strategies include lifestyle modifications and psychological support[24]. Transdermal testosterone supplementation has been assessed for short-term efficacy in improving female sexual dysfunction, with few significant androgenic adverse effects. However, there are no studies on its use in cirrhotic patients[150]. Regarding reproductive dysfunction, women with compensated LC and minimal portal hypertension typically maintain regular menstrual cycles and preserved fertility. If pregnancy is undesirable, contraception should be offered[151]. In women with compensated LC, there are no restrictions on the use of any hormonal contraceptive methods, whereas in those with severe and decompensated LC, risks often outweigh the benefits[152].
Liver transplantation restores physiological levels of testosterone, estradiol, SHBG, gonadotropins, and PRL[45].
Hypothyroidism: Patients with LC and hypothyroidism must be treated immediately according to current guidelines. LC can cause malabsorption, so severely hypothyroid patients with cirrhosis may require higher doses of levothyroxine[67,153]. If the alterations are consistent with subclinical hypothyroidism, other factors such as TSH level, presence of autoantibodies, and patient’s age will be evaluated. Current guidelines for subclinical hypothyroidism do not emphasize the hepatic function status in the treatment decision algorithm[72].
Euthyroid sick syndrome: Isolated low FT3 levels are not recommended to be treated, despite the numerous available studies; the efficacy of hormonal therapy in cases of euthyroid sick syndrome remains unclear[72].
Hyperthyroidism: Like hypothyroidism, immediate treatment should be initiated in cases of overt hyperthyroidism, following current guidelines. Considering the known effect of thiamazole on liver function, there are no controlled studies in Child-Pugh B or C patients. Therefore, it is recommended to start with a dose of 20 mg in Child-Pugh A patients, followed by continuous monitoring of liver function[154-156]. Seventy-seven to eighty-three percent of hyperthyroid patients who initiate treatment early achieve normalization of liver enzymes[157]; however, approximately 1%-2% of patients develop fulminant hepatitis[35]. Propylthiouracil has a higher risk of liver failure and is not considered an ideal therapeutic option for managing hyperthyroidism in patients with LC. Definitive treatments such as radioactive iodine therapy or thyroidectomy can be considered based on the patient’s clinical condition[67]. There is no evidence to suggest that liver transplantation restores thyroid function[45].
The recommendations for osteoporosis treatment in cirrhotic patients do not differ from general guidelines. Lifestyle changes, cessation of tobacco and alcohol use, corticosteroid dose reduction, and increasing physical activity as much as possible to improve spinal mechanics and strength are recommended[77]. Caloric intake recommendations vary between 30-50 kcal/kg/d, with protein intake of 1.0-1.8 g/kg/d, depending on the severity of malnutrition[158]. While treatment is provided to patients with osteoporosis due to the high risk of fractures, given that osteopenia is a risk factor for vertebral fractures, prophylactic treatment is recommended in patients with osteopenia[159].
Regarding supplements, the total calcium intake should be 1000-1500 mg/d, with calcium carbonate being the most consumed supplement. It is recommended to take it with meals[77,158,160]. In patients with LC and proven vitamin D deficiency, efforts should be made to restore vitamin D levels[38]. Although vitamin D deficiency has been linked to higher overall mortality and hepatic decompensation in patients with advanced CLD, there is insufficient evidence to routinely recommend vitamin D supplementation in patients with LC[38,161,162].
Hormonal therapy should be individualized and could be applied in patients with hypogonadism; however, estrogens may increase the risk of cholestasis[77,79].
Regarding treatments that enhance bone formation, intermittent parathyroid hormone administration lacks sufficient evidence in patients with advanced liver disease. For drugs reducing bone resorption, oral bisphosphonates may cause esophagitis or esophageal ulcers; hence, the parenteral use of bisphosphonates such as zoledronic acid is recommended. Due to its annual administration, compliance can be improved[158]. Etidronate and alendronate have been used in patients with primary biliary cirrhosis, and after 1-2 years of treatment, there was improvement in BMD with few adverse effects[160].
Raloxifene has been used as a therapeutic option for osteoporosis, but it has not yet been applied in cirrhotic patients. However, it might have a potential beneficial effect on lumbar spine BMD[77,158].
Unlike diagnosis, there is no controversy in the treatment of critically ill patients. Intravenous hydrocortisone at a dose of 200 mg/d to 300 mg/d divided into 3 to 4 doses is indicated, with subsequent progressive titration based on the patient’s clinical evolution. For non-critically ill patients without symptoms, literature is limited; therefore, oral hydrocortisone at a dose of 15 mg to 20 mg divided into two doses per day is suggested, only in cases of persistent hypotension and hyponatremia[86,87,91,163,164].
In all scenarios, the evidence from research demonstrating a clear benefit for patients replacing GH leans towards its use for periods ranging from 6 months to 12 months. Even in studies with a limited number of patients, the primary benefit observed is the prevention of muscle mass loss. In cases where a deficiency of GH is demonstrated, especially if the patient is a candidate for liver transplantation, replacement therapy is administered via daily injections, independent of body weight and based on age (age below 30 years: 0.4-0.5 mg/d; 30 to 60 years: 0.2-0.3 mg/d; and age over 60 years: 0.1-0.2 mg/d), with subsequent dose titration to maintain IGF-1 within the standard deviation of -2 to 2, always assessing the clinical benefit and side effects. There is no data in LC with long-acting GH preparations. For individuals in whom a decrease in GH is not demonstrated, there is no evidence to adequately justify its use currently. Any apparent beneficial effects do not persist over time, and potential side effects, such as alterations in glucose metabolism and possible neoplasms, have a latency period[92,94,95,165-168].
Although liver transplantation restores physiological levels of GH, IGF-1, and IGFBP-3[45], it achieves only partial restoration of growth, resulting in pubertal delay and reduced adult height[1].
The treatment of LC as an underlying condition is a crucial part of managing secondary hyperaldosteronism. Angio
Managing ascites involves enhancing renal sodium excretion with diuretics and restricting sodium intake. Large-volume paracentesis and transjugular intrahepatic portosystemic shunts are also useful in managing refractory ascites. For cirrhotic patients with refractory ascites, the first-line standard of care is the mineralocorticoid antagonist, spironolactone. However, the natriuretic doses of aldosterone antagonists have not been studied in patients with heart failure[46]. Although loop diuretics have much higher natriuretic potency than distal diuretics, high-dose furosemide (up to 160 mg/d) in cirrhotic patients without renal failure produces satisfactory natriuresis in only 50% of patients. In cases of marked hyperaldosteronism, there is no response to furosemide, necessitating the use of high doses of spironolactone (400 to 600 mg/d). Thus, the International Club of Ascites defined diuretic resistance in patients with ascites as the lack of response to a low-sodium diet and treatment with high-dose diuretics (400 mg/d of spironolactone and 160 mg/d of furosemide)[46]. Adverse effects of spironolactone, given its partial affinity for progesterone and androgen receptors, include painful gynecomastia and hypogonadism in males[169]. On the other hand, it may have beneficial effects in polycystic ovary syndrome[170].
Eplerenone is a more selective mineralocorticoid receptor antagonist with greater specificity for the mineralocorticoid receptor, resulting in fewer side effects related to gynecomastia and hypogonadism compared to spironolactone[171].
Finerenone is a nonsteroidal mineralocorticoid receptor antagonist with high potency and strong selectivity for the mineralocorticoid receptor, overcoming significant barriers seen in previous generations of mineralocorticoid receptor antagonists[171]. Additionally, finerenone prevents aldosterone-induced endothelial cell apoptosis and smooth muscle cell proliferation, thereby reducing vascular remodeling[172] (Table 1).
| Endocrinopathy | Interventions | Comments | Ref. |
| Diabetes mellitus | Therapeutic goals: Compensated cirrhosis: FPG 4.44-7.22 mmol/L, PP glucose < 9.99 mmol/L; decompensated cirrhosis: FPG 5.00-8.32 mmol/L, PP glucose < 11.10 mmol/L, and pre-meal glucose 5.55-11.10 mmol/L | [28,50] | |
| Metformin | Maximum 1500 mg/d. Do not use in hypoxemia, dehydration, sepsis, or Child-Pugh B and C | [50,109] | |
| Sulfonylureas | Can be used in low doses in Child-Pugh A. Contraindicated in Child-Pugh B and C | [28,50,100,109] | |
| Meglitinides | Do not use repaglinide. Nateglinide can be used in Child-Pugh A and B in lower doses | [28,50] | |
| Thiazolidinediones | Do not use rosiglitazone. Pioglitazone can be used in Child-Pugh A | [28,50,109] | |
| DPP-4 inhibitors | Can be used in Child-Pugh A without modifying the dose, except for vildagliptin. Use with caution in Child-Pugh B, but not in Child-Pugh C | [50,109] | |
| GLP-1 RA | Can be used in Child-Pugh A without modifying the dose. Use with caution in Child-Pugh B, but not in Child-Pugh C, due to limited data Unknown for Tirzepatide and Retatrutide | [50,109] | |
| SGLT-2i | Can be used in Child-Pugh A, starting with lower doses. Use with caution in Child-Pugh B but avoid in Child-Pugh C due to the risk of dehydration and hypotension | [28,109] | |
| Alpha-glucosidase inhibitors | Can be used in Child-Pugh A and B but are not recommended in Child-Pugh C | [50,109] | |
| Insulin | Reserve for those unable to use oral medications, fail to achieve adequate glycemic control, or in cases of sepsis, encephalopathy, bleeding, or acute kidney injury. It is the choice in DM and decompensated LC; start with 0.1-0.2 IU/kg/d (higher if blood glucose is very high). Adjust the dose based on blood glucose, varying by 2 units every 3 d if necessary. Add rapid-acting insulin if goals are not achieved, starting with 4 units or 10% of basal insulin and increasing by 1-2 units every 3-4 d as needed. Prefer analog insulins for a lower risk of hypoglycemia | [28,109] | |
| Statins | Generally recommended for males, individuals with diabetes, and those with a high Fibrosis-4 index at the beginning of the study | [140] | |
| Liver transplantation | The worse the beta cell function, the less effective liver transplantation will be in achieving DM remission | [17] | |
| Hypoglycemia | New episodes should be prevented by adjusting the dose or modifying the drugs used | ||
| 15-20 g of oral glucose | For conscious patients. Reassess in 15 min and repeat if necessary | [56,142,143] | |
| Parenteral glucagon | For unconscious patients or those with severe hypoglycemia | [56,142,143] | |
| Sarcopenia | Nutritional support | Caloric intake of 35-40 kcal/kg and protein intake of 1.2-1.5 g/kg. Eat at intervals of 2-3 h and include a bedtime snack with 50 g of carbohydrates and 20 g of protein | [145,146] |
| BCAA | Long-term supplementation at a dose of 0.25 g/kg/d | [146] | |
| Exercise | Recommended moderate-intensity aerobic and resistance exercises | [21] | |
| Others | Treatments to reduce ammonia, hormonal supplements, and myostatin inhibitors are still under study | [21,22,147] | |
| Male hypogonadism | Testosterone supplementation | Considered adjunctive therapy to improve frailty | [148] |
| PDE-5i | Sildenafil, tadalafil, avanafil, and vardenafil are FDA-approved and can be used in Child-Pugh A and B at lower doses | [24] | |
| Liver transplantation | Restores physiological levels of testosterone, estradiol, SHBG, gonadotropins, and prolactin | [45] | |
| Female hypogonadism | Lifestyle modifications and psychological support should be included | [24] | |
| Contraception | In compensated cirrhosis, hormonal contraceptives can be used without restriction, but in decompensated cirrhosis, the risks outweigh the benefits | [152] | |
| Liver transplantation | Restores physiological levels of testosterone, estradiol, SHBG, gonadotropins, and prolactin | [45] | |
| Overt hypothyroidism | Levothyroxine | Immediate treatment per general recommendations, considering higher doses might be necessary due to malabsorption | [67,153] |
| Subclinical hypothyroidism | Levothyroxine | Treatment per general recommendations. Liver function is not part of the treatment decision | [72] |
| Euthyroid sick syndrome | Hormonal treatment is not recommended | [72] | |
| Hyperthyroidism | Thiamazole | Recommended starting dose of 20mg in Child-Pugh A with continuous monitoring of liver function. Not recommended in Child-Pugh B and C | [154-156] |
| Propylthiouracil | Its use is not recommended | [67] | |
| Definitive treatments | Radioactive iodine therapy or thyroidectomy based on patient’s need and clinical condition | [67] | |
| Liver transplantation | Not recommended to restore thyroid function | [45] | |
| Hepatic bone disease | Calcium | 1000-1500 mg/d, taken with food | [77,158,160] |
| 25-OH vitamin D | Supplement only if there is a deficiency of vitamin D | [38] | |
| PTH | There is not enough evidence in advanced cirrhosis | [158] | |
| Bisphosphonates | Parenteral use is recommended | [158] | |
| Raloxifene | There is not enough evidence in cirrhosis | [77,158] | |
| Adrenal insufficiency | Hydrocortisone | Critically ill patients: 200-300 mg/d intravenously, divided into 3 to 4 doses, with progressive titration based on the patient’s clinical evolution; non-critically ill patients without symptoms: 15-20 mg/d orally, divided into 2 doses, only if persistent hypotension and hyponatremia are present | [86,87,91,163,164] |
| Growth hormone dysfunction | GH | If GH deficiency is demonstrated: 0.4-0.5 mg/d (< 30 years), 0.2-0.3 mg/d (30-60 years), 0.1-0.2 mg/d (> 60 years), with dose titration to maintain IGF-1 within the standard deviation of -2 to 2. | [92,94,95,165-168] |
| Secondary hyperaldosteronism | Loop diuretics | High doses of furosemide (up to 160 mg/d) in cases without renal insufficiency, although with limited effectiveness | [46] |
| Mineralocorticoid receptor antagonists | Spironolactone (up to 400-600 mg/d). Eplerenone is more selective with fewer side effects. Finerenone is a potent and selective non-steroidal antagonist | [46,171] | |
LC exerts a significant impact on the endocrine system, although it may go unnoticed despite being associated with increased morbidity and mortality. For this reason, a comprehensive and multidisciplinary approach is necessary, allowing not only the management of hepatic pathology but also timely diagnosis and treatment of associated endocrinological disorders.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Peru
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: He YF, China S-Editor: Chen YL L-Editor: A P-Editor: Zheng XM
| 1. | Kalra S, Bhattacharya S, Rawal P. Hepatocrinology. Med Sci (Basel). 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (1)] |
| 2. | Lloyd CW, Williams RH. Endocrine changes associated with Laennec's cirrhosis of the liver. Am J Med. 1948;4:315-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 104] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Kumar KV, Pawah AK, Manrai M. Occult endocrine dysfunction in patients with cirrhosis of liver. J Family Med Prim Care. 2016;5:576-580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Maheshwari A, Thuluvath PJ. Endocrine diseases and the liver. Clin Liver Dis. 2011;15:55-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet. 2021;398:1359-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 863] [Article Influence: 215.8] [Reference Citation Analysis (1)] |
| 6. | Huang DQ, Terrault NA, Tacke F, Gluud LL, Arrese M, Bugianesi E, Loomba R. Global epidemiology of cirrhosis - aetiology, trends and predictions. Nat Rev Gastroenterol Hepatol. 2023;20:388-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 377] [Article Influence: 188.5] [Reference Citation Analysis (0)] |
| 7. | Wentworth BJ, Siragy HM. Adrenal Insufficiency in Cirrhosis. J Endocr Soc. 2022;6:bvac115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (1)] |
| 8. | Afraz S, Kapila N. Endocrinology for the hepatologist. Clin Liver Dis (Hoboken). 2023;22:1-6. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Devarbhavi H, Asrani SK, Arab JP, Nartey YA, Pose E, Kamath PS. Global burden of liver disease: 2023 update. J Hepatol. 2023;79:516-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 853] [Reference Citation Analysis (4)] |
| 10. | GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:245-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1165] [Cited by in RCA: 1014] [Article Influence: 202.8] [Reference Citation Analysis (4)] |
| 11. | Jeeyavudeen MS, Khan SKA, Fouda S, Pappachan JM. Management of metabolic-associated fatty liver disease: The diabetology perspective. World J Gastroenterol. 2023;29:126-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 12. | Gill MG, Majumdar A. Metabolic associated fatty liver disease: Addressing a new era in liver transplantation. World J Hepatol. 2020;12:1168-1181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 13. | Wang S, Friedman SL. Found in translation-Fibrosis in metabolic dysfunction-associated steatohepatitis (MASH). Sci Transl Med. 2023;15:eadi0759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 63] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 14. | Pipitone RM, Ciccioli C, Infantino G, La Mantia C, Parisi S, Tulone A, Pennisi G, Grimaudo S, Petta S. MAFLD: a multisystem disease. Ther Adv Endocrinol Metab. 2023;14:20420188221145549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 109] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 15. | Lee WG, Wells CI, McCall JL, Murphy R, Plank LD. Prevalence of diabetes in liver cirrhosis: A systematic review and meta-analysis. Diabetes Metab Res Rev. 2019;35:e3157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (1)] |
| 16. | Li H, Sun F, Yang W, Huang M, Pan C, Lin C. Clinical study of abnormal glucose metabolism and insulin resistance in patients with liver cirrhosis. Am J Transl Res. 2021;13:3522-3528. [PubMed] |
| 17. | Grancini V, Trombetta M, Lunati ME, Zimbalatti D, Boselli ML, Gatti S, Donato MF, Resi V, D'Ambrosio R, Aghemo A, Pugliese G, Bonadonna RC, Orsi E. Contribution of β-cell dysfunction and insulin resistance to cirrhosis-associated diabetes: Role of severity of liver disease. J Hepatol. 2015;63:1484-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Majeed A, Arafat M, Ali I. Hypoglycemia in patients presenting with Liver Cirrhosis. PJMHS. 2017;11:1211-1213. [DOI] [Full Text] |
| 19. | Yen FS, Hou MC, Liu JS, Hsu CC, Hwu CM. Severe hypoglycemia in patients with liver cirrhosis and type 2 diabetes. Front Med (Lausanne). 2022;9:962337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Anno T, Kaneto H, Shigemoto R, Kawasaki F, Kawai Y, Urata N, Kawamoto H, Kaku K, Okimoto N. Hypoinsulinemic hypoglycemia triggered by liver injury in elderly subjects with low body weight: case reports. Endocrinol Diabetes Metab Case Rep. 2018;2018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Dhaliwal A, Armstrong MJ. Sarcopenia in cirrhosis: A practical overview. Clin Med (Lond). 2020;20:489-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 22. | Fox R, Stenning K, Slee A, Macnaughtan J, Davies N. Sarcopenia in liver cirrhosis: Prevalence, pathophysiology and therapeutic strategies. Anal Biochem. 2022;647:114581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 23. | Eshraghian A, Taghavi SA. Systematic review: endocrine abnormalities in patients with liver cirrhosis. Arch Iran Med. 2014;17:713-721. [PubMed] |
| 24. | Neong SF, Billington EO, Congly SE. Sexual Dysfunction and Sex Hormone Abnormalities in Patients With Cirrhosis: Review of Pathogenesis and Management. Hepatology. 2019;69:2683-2695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 25. | Kumar R. Hepatogenous Diabetes: An Underestimated Problem of Liver Cirrhosis. Indian J Endocrinol Metab. 2018;22:552-559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Radwan HA, Hamed EH, Saleh OM. Significance of Serum Adiponectin and Insulin Resistance Levels in Diagnosis of Egyptian Patients with Chronic Liver Disease and HCC. Asian Pac J Cancer Prev. 2019;20:1833-1839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Orsi E, Grancini V, Menini S, Aghemo A, Pugliese G. Hepatogenous diabetes: Is it time to separate it from type 2 diabetes? Liver Int. 2017;37:950-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (3)] |
| 28. | Puri P, Kotwal N. An Approach to the Management of Diabetes Mellitus in Cirrhosis: A Primer for the Hepatologist. J Clin Exp Hepatol. 2022;12:560-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Yu B, Douli R, Suarez JA, Gutierrez VP, Aldiabat M, Khan M. Non-islet cell tumor hypoglycemia as an initial presentation of hepatocellular carcinoma coupled with end-stage liver cirrhosis: A case report and review of literature. World J Hepatol. 2020;12:519-524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Garla V, Sonani H, Palabindala V, Gomez-Sanchez C, Subauste J, Lien LF. Non-islet Cell Hypoglycemia: Case Series and Review of the Literature. Front Endocrinol (Lausanne). 2019;10:316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 31. | Regino CA, López-Montoya V, López-Urbano F, Alvarez JC, Roman-Gonzalez A. Paraneoplastic Hypoglycemia in Hepatocarcinoma: Case Report and Literature Review. Cureus. 2020;12:e12013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 32. | Kim Y. Emerging Treatment Options for Sarcopenia in Chronic Liver Disease. Life (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Sinclair M, Grossmann M, Gow PJ, Angus PW. Testosterone in men with advanced liver disease: abnormalities and implications. J Gastroenterol Hepatol. 2015;30:244-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 34. | Burra P. Liver abnormalities and endocrine diseases. Best Pract Res Clin Gastroenterol. 2013;27:553-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 35. | Opoku-Akyeampong NAAS, Agyei-Nkansah A, Yorke E. Liver dysfunction associated with hyperthyroidism: Lessons from 2 Case reports. Clin Case Rep. 2021;9:e04067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Silveira MG, Mendes FD, Diehl NN, Enders FT, Lindor KD. Thyroid dysfunction in primary biliary cirrhosis, primary sclerosing cholangitis and non-alcoholic fatty liver disease. Liver Int. 2009;29:1094-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 37. | Konstantakis C, Tselekouni P, Kalafateli M, Triantos C. Vitamin D deficiency in patients with liver cirrhosis. Ann Gastroenterol. 2016;29:297-306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 38. | Ravaioli F, Pivetti A, Di Marco L, Chrysanthi C, Frassanito G, Pambianco M, Sicuro C, Gualandi N, Guasconi T, Pecchini M, Colecchia A. Role of Vitamin D in Liver Disease and Complications of Advanced Chronic Liver Disease. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 39. | Barbu EC, Chițu-Tișu CE, Lazăr M, Olariu C, Bojincă M, Ionescu RA, Ion DA, Bădărău IA. Hepatic Osteodystrophy: A Global (Re)View of the Problem. Acta Clin Croat. 2017;56:512-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Ehnert S, Aspera-Werz RH, Ruoß M, Dooley S, Hengstler JG, Nadalin S, Relja B, Badke A, Nussler AK. Hepatic Osteodystrophy-Molecular Mechanisms Proposed to Favor Its Development. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 41. | Singh RR, Walia R, Sachdeva N, Bhalla A, Singh A, Singh V. Relative adrenal insufficiency in cirrhotic patients with ascites (hepatoadrenal syndrome). Dig Liver Dis. 2018;50:1232-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 42. | Karagiannis AK, Nakouti T, Pipili C, Cholongitas E. Adrenal insufficiency in patients with decompensated cirrhosis. World J Hepatol. 2015;7:1112-1124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 43. | Wentworth BJ, Schliep M, Novicoff W, Siragy HM, Geng CX, Henry ZH. Relative adrenal insufficiency in the non-critically ill patient with cirrhosis: A systematic review and meta-analysis. Liver Int. 2023;43:660-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 44. | Trifan A, Chiriac S, Stanciu C. Update on adrenal insufficiency in patients with liver cirrhosis. World J Gastroenterol. 2013;19:445-456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 45. | Gariani K, Toso C, Philippe J, Orci LA. Effects of liver transplantation on endocrine function: a systematic review. Liver Int. 2016;36:1401-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 46. | Bansal S, Lindenfeld J, Schrier RW. Sodium retention in heart failure and cirrhosis: potential role of natriuretic doses of mineralocorticoid antagonist? Circ Heart Fail. 2009;2:370-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 47. | Papadopoulou-Marketou N, Vaidya A, Dluhy R, Chrousos GP. Hyperaldosteronism. 2020 Aug 6. In: Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000–. [PubMed] |
| 48. | Dominguez A, Muppidi V, Gupta S. Hyperaldosteronism. 2023 Feb 12. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. [PubMed] |
| 49. | Ruf A, Dirchwolf M, Freeman RB. From Child-Pugh to MELD score and beyond: Taking a walk down memory lane. Ann Hepatol. 2022;27:100535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 50. | Boursier J, Anty R, Carette C, Cariou B, Castera L, Caussy C, Fontaine H, Garioud A, Gourdy P, Guerci B, Guillaume M, Michot N, Minello A, Ouizeman DJ, Serfaty L, Bonnet F, Vergès B, Petit JM; AFEF and SFD. Management of diabetes mellitus in patients with cirrhosis: An overview and joint statement. Diabetes Metab. 2021;47:101272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 51. | Nishida T. Diagnosis and Clinical Implications of Diabetes in Liver Cirrhosis: A Focus on the Oral Glucose Tolerance Test. J Endocr Soc. 2017;1:886-896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 52. | Chung W, Promrat K, Wands J. Clinical implications, diagnosis, and management of diabetes in patients with chronic liver diseases. World J Hepatol. 2020;12:533-557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (11)] |
| 53. | Addepally NS, George N, Martinez-Macias R, Garcia-Saenz-de-Sicilia M, Kim WR, Duarte-Rojo A. Hemoglobin A1c Has Suboptimal Performance to Diagnose and Monitor Diabetes Mellitus in Patients with Cirrhosis. Dig Dis Sci. 2018;63:3498-3508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 54. | ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K, Leon J, Lyons SK, Perry ML, Prahalad P, Pratley RE, Seley JJ, Stanton RC, Gabbay RA; on behalf of the American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46:S19-S40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 1332] [Article Influence: 666.0] [Reference Citation Analysis (70)] |
| 55. | Cryer PE, Axelrod L, Grossman AB, Heller SR, Montori VM, Seaquist ER, Service FJ; Endocrine Society. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2009;94:709-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 712] [Cited by in RCA: 747] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 56. | ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K, Leon J, Lyons SK, Perry ML, Prahalad P, Pratley RE, Seley JJ, Stanton RC, Gabbay RA; on behalf of the American Diabetes Association. 6. Glycemic Targets: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46:S97-S110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 390] [Article Influence: 195.0] [Reference Citation Analysis (0)] |
| 57. | Yang X, Liu X, Wang L, Xu J, Wen J. Hypoglycemia on admission in patients with acute on chronic liver failure: a retrospective cohort analyzing the current situation, risk factors, and associations with prognosis. Ann Palliat Med. 2023;12:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 58. | Saiman Y, Mahmud N. Hypoglycemia is an early, independent predictor of bacteremia and in-hospital death in patients with cirrhosis. Eur J Gastroenterol Hepatol. 2021;33:e693-e699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 59. | McCall AL, Lieb DC, Gianchandani R, MacMaster H, Maynard GA, Murad MH, Seaquist E, Wolfsdorf JI, Wright RF, Wiercioch W. Management of Individuals With Diabetes at High Risk for Hypoglycemia: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2023;108:529-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 48] [Reference Citation Analysis (0)] |
| 60. | Kuchay MS, Martínez-Montoro JI, Llamoza-Torres CJ, Fernández-García JC, Ramos-Molina B. Liver cirrhosis and sarcopenia: a dreadful combination. Hepatobiliary Surg Nutr. 2022;11:729-731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 61. | Rosen RC, Cappelleri JC, Gendrano N 3rd. The International Index of Erectile Function (IIEF): a state-of-the-science review. Int J Impot Res. 2002;14:226-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 731] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 62. | Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Peña BM. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11:319-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1933] [Cited by in RCA: 2175] [Article Influence: 83.7] [Reference Citation Analysis (0)] |
| 63. | Meston CM, Derogatis LR. Validated instruments for assessing female sexual function. J Sex Marital Ther. 2002;28 Suppl 1:155-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 123] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 64. | Bhasin S, Brito JP, Cunningham GR, Hayes FJ, Hodis HN, Matsumoto AM, Snyder PJ, Swerdloff RS, Wu FC, Yialamas MA. Testosterone Therapy in Men With Hypogonadism: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2018;103:1715-1744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 786] [Cited by in RCA: 984] [Article Influence: 140.6] [Reference Citation Analysis (0)] |
| 65. | Klein DA, Paradise SL, Reeder RM. Amenorrhea: A Systematic Approach to Diagnosis and Management. Am Fam Physician. 2019;100:39-48. [PubMed] |
| 66. | Gordon CM, Ackerman KE, Berga SL, Kaplan JR, Mastorakos G, Misra M, Murad MH, Santoro NF, Warren MP. Functional Hypothalamic Amenorrhea: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2017;102:1413-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 273] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 67. | Piantanida E, Ippolito S, Gallo D, Masiello E, Premoli P, Cusini C, Rosetti S, Sabatino J, Segato S, Trimarchi F, Bartalena L, Tanda ML. The interplay between thyroid and liver: implications for clinical practice. J Endocrinol Invest. 2020;43:885-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 68. | Khairy RN, Mullen KD. Hypothyroidism as a mimic of liver failure in a patient with cirrhosis. Ann Intern Med. 2007;146:315-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 69. | Targher G, Montagnana M, Salvagno G, Moghetti P, Zoppini G, Muggeo M, Lippi G. Association between serum TSH, free T4 and serum liver enzyme activities in a large cohort of unselected outpatients. Clin Endocrinol (Oxf). 2008;68:481-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 70. | Bano A, Chaker L, Muka T, Mattace-Raso FUS, Bally L, Franco OH, Peeters RP, Razvi S. Thyroid Function and the Risk of Fibrosis of the Liver, Heart, and Lung in Humans: A Systematic Review and Meta-Analysis. Thyroid. 2020;30:806-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 71. | Kim D, Kim W, Joo SK, Bae JM, Kim JH, Ahmed A. Subclinical Hypothyroidism and Low-Normal Thyroid Function Are Associated With Nonalcoholic Steatohepatitis and Fibrosis. Clin Gastroenterol Hepatol. 2018;16:123-131.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 135] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 72. | Sciacchitano S, Capalbo C, Napoli C, Anibaldi P, Salvati V, De Vitis C, Mancini R, Coluzzi F, Rocco M. Nonthyroidal Illness Syndrome: To Treat or Not to Treat? Have We Answered the Question? A Review of Metanalyses. Front Endocrinol (Lausanne). 2022;13:850328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 73. | Fliers E, Boelen A. An update on non-thyroidal illness syndrome. J Endocrinol Invest. 2021;44:1597-1607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 74. | Feng HL, Li Q, Cao WK, Yang JM. Changes in thyroid function in patients with liver failure and their clinical significance: A clinical study of non-thyroidal illness syndrome in patients with liver failure. Hepatobiliary Pancreat Dis Int. 2020;19:561-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 75. | Hayat MH, Moazzam Z, Ziogas IA, Yousaf A, Hayat M. Thyroid Storm Presenting as Acute Liver Failure in a Patient with Graves' Disease. Cureus. 2020;12:e10333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 76. | Leslie WD, Bernstein CN, Leboff MS; American Gastroenterological Association Clinical Practice Commitee. AGA technical review on osteoporosis in hepatic disorders. Gastroenterology. 2003;125:941-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 160] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 77. | Santos LA, Romeiro FG. Diagnosis and Management of Cirrhosis-Related Osteoporosis. Biomed Res Int. 2016;2016:1423462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 78. | Wakolbinger R, Muschitz C, Scheriau G, Bodlaj G, Kocijan R, Feichtinger X, Schanda JE, Haschka J, Resch H, Pietschmann P. Bone microarchitecture and bone turnover in hepatic cirrhosis. Osteoporos Int. 2019;30:1195-1204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 79. | Ionele CM, Turcu-Stiolica A, Subtirelu MS, Ungureanu BS, Sas TN, Rogoveanu I. Osteoporosis Assessment among Adults with Liver Cirrhosis. J Clin Med. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 80. | Guichelaar MM, Malinchoc M, Sibonga J, Clarke BL, Hay JE. Bone metabolism in advanced cholestatic liver disease: analysis by bone histomorphometry. Hepatology. 2002;36:895-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 81. | Handzlik-Orlik G, Holecki M, Wilczyński K, Duława J. Osteoporosis in liver disease: pathogenesis and management. Ther Adv Endocrinol Metab. 2016;7:128-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 82. | Ogiso Y, Hanai T, Nishimura K, Miwa T, Maeda T, Imai K, Suetsugu A, Takai K, Shimizu M. Usefulness of the Trabecular Bone Score in Assessing the Risk of Vertebral Fractures in Patients with Cirrhosis. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 83. | Ray G, Bhargav PM. A Study of Hormonal Abnormalities in Chronic Liver Disease. J Assoc Physicians India. 2019;67:47-52. [PubMed] |
| 84. | Levick C, Aspinall RJ. Prevalence and clinical significance of relative adrenal insufficiency in decompensated cirrhosis. J R Coll Physicians Edinb. 2019;49:274-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 85. | Piano S, Favaretto E, Tonon M, Antonelli G, Brocca A, Sticca A, Mareso S, Gringeri E, Scaroni C, Plebani M, Russo FP, Burra P, Cillo U, Angeli P. Including Relative Adrenal Insufficiency in Definition and Classification of Acute-on-Chronic Liver Failure. Clin Gastroenterol Hepatol. 2020;18:1188-1196.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 86. | Annane D, Pastores SM, Rochwerg B, Arlt W, Balk RA, Beishuizen A, Briegel J, Carcillo J, Christ-Crain M, Cooper MS, Marik PE, Umberto Meduri G, Olsen KM, Rodgers SC, Russell JA, Van den Berghe G. Guidelines for the Diagnosis and Management of Critical Illness-Related Corticosteroid Insufficiency (CIRCI) in Critically Ill Patients (Part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Crit Care Med. 2017;45:2078-2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 193] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 87. | Jang JY, Kim TY, Sohn JH, Lee TH, Jeong SW, Park EJ, Lee SH, Kim SG, Kim YS, Kim HS, Kim BS. Relative adrenal insufficiency in chronic liver disease: its prevalence and effects on long-term mortality. Aliment Pharmacol Ther. 2014;40:819-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 88. | Javorsky BR, Raff H, Carroll TB, Algeciras-Schimnich A, Singh RJ, Colón-Franco JM, Findling JW. New Cutoffs for the Biochemical Diagnosis of Adrenal Insufficiency after ACTH Stimulation using Specific Cortisol Assays. J Endocr Soc. 2021;5:bvab022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 85] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 89. | Dichtel LE, Schorr M, Loures de Assis C, Rao EM, Sims JK, Corey KE, Kohli P, Sluss PM, McPhaul MJ, Miller KK. Plasma Free Cortisol in States of Normal and Altered Binding Globulins: Implications for Adrenal Insufficiency Diagnosis. J Clin Endocrinol Metab. 2019;104:4827-4836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 90. | Schreier B, Zipprich A, Uhlenhaut H, Gekle M. Mineralocorticoid receptors in non-alcoholic fatty liver disease. Br J Pharmacol. 2022;179:3165-3177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 91. | Téllez L, Guerrero A. Management of Liver Decompensation in Advanced Liver Disease (Renal Impairment, Liver Failure, Adrenal Insufficiency, Cardiopulmonary Complications). Clin Drug Investig. 2022;42:15-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 92. | Doycheva I, Erickson D, Watt KD. Growth hormone deficiency and NAFLD: An overlooked and underrecognized link. Hepatol Commun. 2022;6:2227-2237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 93. | Assaad SN, Cunningham GR, Samaan NA. Abnormal growth hormone dynamics in chronic liver disease do not depend on severe parenchymal disease. Metabolism. 1990;39:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 94. | Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Shalet SM, Vance ML; Endocrine Society's Clinical Guidelines Subcommittee, Stephens PA. Evaluation and treatment of adult growth hormone deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2006;91:1621-1634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 238] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 95. | Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Vance ML; Endocrine Society. Evaluation and treatment of adult growth hormone deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1587-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 519] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 96. | Takahashi Y. The Role of Growth Hormone and Insulin-Like Growth Factor-I in the Liver. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 145] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 97. | Garcia JM, Biller BMK, Korbonits M, Popovic V, Luger A, Strasburger CJ, Chanson P, Medic-Stojanoska M, Schopohl J, Zakrzewska A, Pekic S, Bolanowski M, Swerdloff R, Wang C, Blevins T, Marcelli M, Ammer N, Sachse R, Yuen KCJ. Macimorelin as a Diagnostic Test for Adult GH Deficiency. J Clin Endocrinol Metab. 2018;103:3083-3093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 98. | Mancia G, Kreutz R, Brunström M, Burnier M, Grassi G, Januszewicz A, Muiesan ML, Tsioufis K, Agabiti-Rosei E, Algharably EAE, Azizi M, Benetos A, Borghi C, Hitij JB, Cifkova R, Coca A, Cornelissen V, Cruickshank JK, Cunha PG, Danser AHJ, Pinho RM, Delles C, Dominiczak AF, Dorobantu M, Doumas M, Fernández-Alfonso MS, Halimi JM, Járai Z, Jelaković B, Jordan J, Kuznetsova T, Laurent S, Lovic D, Lurbe E, Mahfoud F, Manolis A, Miglinas M, Narkiewicz K, Niiranen T, Palatini P, Parati G, Pathak A, Persu A, Polonia J, Redon J, Sarafidis P, Schmieder R, Spronck B, Stabouli S, Stergiou G, Taddei S, Thomopoulos C, Tomaszewski M, Van de Borne P, Wanner C, Weber T, Williams B, Zhang ZY, Kjeldsen SE. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J Hypertens. 2023;41:1874-2071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 1355] [Article Influence: 677.5] [Reference Citation Analysis (0)] |
| 99. | Elkrief L, Rautou PE, Sarin S, Valla D, Paradis V, Moreau R. Diabetes mellitus in patients with cirrhosis: clinical implications and management. Liver Int. 2016;36:936-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 148] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 100. | Castera L, Cusi K. Diabetes and cirrhosis: Current concepts on diagnosis and management. Hepatology. 2023;77:2128-2146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 40] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 101. | Kaplan DE, Serper M, John BV, Tessiatore KM, Lerer R, Mehta R, Fox R, Aytaman A, Baytarian M, Hunt K, Albrecht J, Taddei TH; Veterans Outcomes and Cost Associated with Liver disease Study Group. Effects of Metformin Exposure on Survival in a Large National Cohort of Patients With Diabetes and Cirrhosis. Clin Gastroenterol Hepatol. 2021;19:2148-2160.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 102. | Zhang X, Harmsen WS, Mettler TA, Kim WR, Roberts RO, Therneau TM, Roberts LR, Chaiteerakij R. Continuation of metformin use after a diagnosis of cirrhosis significantly improves survival of patients with diabetes. Hepatology. 2014;60:2008-2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 142] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 103. | Ampuero J, Ranchal I, Nuñez D, Díaz-Herrero Mdel M, Maraver M, del Campo JA, Rojas Á, Camacho I, Figueruela B, Bautista JD, Romero-Gómez M. Metformin inhibits glutaminase activity and protects against hepatic encephalopathy. PLoS One. 2012;7:e49279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (2)] |
| 104. | Lai SW, Chen PC, Liao KF, Muo CH, Lin CC, Sung FC. Risk of hepatocellular carcinoma in diabetic patients and risk reduction associated with anti-diabetic therapy: a population-based cohort study. Am J Gastroenterol. 2012;107:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 252] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 105. | Donadon V, Balbi M, Mas MD, Casarin P, Zanette G. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients with chronic liver disease. Liver Int. 2010;30:750-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 223] [Article Influence: 14.9] [Reference Citation Analysis (1)] |
| 106. | Chen HP, Shieh JJ, Chang CC, Chen TT, Lin JT, Wu MS, Lin JH, Wu CY. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies. Gut. 2013;62:606-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 307] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 107. | Richy FF, Sabidó-Espin M, Guedes S, Corvino FA, Gottwald-Hostalek U. Incidence of lactic acidosis in patients with type 2 diabetes with and without renal impairment treated with metformin: a retrospective cohort study. Diabetes Care. 2014;37:2291-2295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 108. | Trinkley KE, Anderson HD, Nair KV, Malone DC, Saseen JJ. Assessing the incidence of acidosis in patients receiving metformin with and without risk factors for lactic acidosis. Ther Adv Chronic Dis. 2018;9:179-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 109. | Gangopadhyay KK, Singh P. Consensus Statement on Dose Modifications of Antidiabetic Agents in Patients with Hepatic Impairment. Indian J Endocrinol Metab. 2017;21:341-354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 110. | Yen FS, Lai JN, Wei JC, Chiu LT, Hwu CM, Hou MC, Hsu CC. Sulfonylureas may be useful for glycemic management in patients with diabetes and liver cirrhosis. PLoS One. 2020;15:e0243783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 111. | Musso G, Cassader M, Paschetta E, Gambino R. Thiazolidinediones and Advanced Liver Fibrosis in Nonalcoholic Steatohepatitis: A Meta-analysis. JAMA Intern Med. 2017;177:633-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 342] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 112. | Filipova E, Uzunova K, Kalinov K, Vekov T. Effects of pioglitazone therapy on blood parameters, weight and BMI: a meta-analysis. Diabetol Metab Syndr. 2017;9:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 113. | Panikar V, Kale NJ, Hoskote SS, Deogaonkar N, Joshi SR. Effect of Low (7.5 mg/day), Standard (15 mg/ day) and High (30 mg/day) Dose Pioglitazone Therapy on Glycemic Control and Weight Gain in Recently-Diagnosed Type 2 Diabetes Patients. J Assoc Physicians India. 2015;63:36-39. [PubMed] |
| 114. | Yen FS, Wei JC, Chiu LT, Hsu CC, Hou MC, Hwu CM. Thiazolidinediones were associated with higher risk of cardiovascular events in patients with type 2 diabetes and cirrhosis. Liver Int. 2021;41:110-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 115. | Yen FS, Wei JC, Yip HT, Hwu CM, Hou MC, Hsu CC. Dipeptidyl peptidase-4 inhibitors may accelerate cirrhosis decompensation in patients with diabetes and liver cirrhosis: a nationwide population-based cohort study in Taiwan. Hepatol Int. 2021;15:179-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 116. | Yen FS, Hou MC, Cheng-Chung Wei J, Shih YH, Hsu CY, Hsu CC, Hwu CM. Glucagon-like Peptide-1 Receptor Agonist Use in Patients With Liver Cirrhosis and Type 2 Diabetes. Clin Gastroenterol Hepatol. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 117. | Simon TG, Patorno E, Schneeweiss S. Glucagon-Like Peptide-1 Receptor Agonists and Hepatic Decompensation Events in Patients With Cirrhosis and Diabetes. Clin Gastroenterol Hepatol. 2022;20:1382-1393.e19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 64] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 118. | Morris SM, Armstrong MJ, Newsome PN. Safety and Efficacy of Glucagon-like Peptide 1 Receptor Agonists in Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2022;20:1220-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 119. | Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, Hazlehurst JM, Guo K; LEAN trial team, Abouda G, Aldersley MA, Stocken D, Gough SC, Tomlinson JW, Brown RM, Hübscher SG, Newsome PN. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1472] [Article Influence: 163.6] [Reference Citation Analysis (1)] |
| 120. | Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, Sanyal AJ, Sejling AS, Harrison SA; NN9931-4296 Investigators. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N Engl J Med. 2021;384:1113-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 1221] [Article Influence: 305.3] [Reference Citation Analysis (0)] |
| 121. | Hartman ML, Sanyal AJ, Loomba R, Wilson JM, Nikooienejad A, Bray R, Karanikas CA, Duffin KL, Robins DA, Haupt A. Effects of Novel Dual GIP and GLP-1 Receptor Agonist Tirzepatide on Biomarkers of Nonalcoholic Steatohepatitis in Patients With Type 2 Diabetes. Diabetes Care. 2020;43:1352-1355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 240] [Article Influence: 48.0] [Reference Citation Analysis (1)] |
| 122. | Valenzuela-Vallejo L, Guatibonza-García V, Mantzoros CS. Recent guidelines for Non-Alcoholic Fatty Liver disease (NAFLD)/ Fatty Liver Disease (FLD): Are they already outdated and in need of supplementation? Metabolism. 2022;136:155248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 123. | Polyzos SA, Goulas A, Papaioannidou P. Tirzepatide for Diabetes and Obesity: A New Window to the Treatment of Non-alcoholic Steatohepatitis. Curr Med Chem. 2023;30:2476-2479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 124. | Choi IY, Kim JK, Lee JS, Park E, Kim YH, Jung SY, Kim SJ. Effect of a Novel Long-Acting GLP-1/GIP/Glucagon Triple Agonist (HM15211) in a NASH and Fibrosis Animal Model. Diabetes. 2018;67 Suppl:1106. [DOI] [Full Text] |
| 125. | Nevola R, Epifani R, Imbriani S, Tortorella G, Aprea C, Galiero R, Rinaldi L, Marfella R, Sasso FC. GLP-1 Receptor Agonists in Non-Alcoholic Fatty Liver Disease: Current Evidence and Future Perspectives. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 129] [Reference Citation Analysis (0)] |
| 126. | Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Sabatine MS. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1634] [Cited by in RCA: 1924] [Article Influence: 320.7] [Reference Citation Analysis (0)] |
| 127. | Sattar N, Fitchett D, Hantel S, George JT, Zinman B. Empagliflozin is associated with improvements in liver enzymes potentially consistent with reductions in liver fat: results from randomised trials including the EMPA-REG OUTCOME® trial. Diabetologia. 2018;61:2155-2163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 130] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 128. | Kuchay MS, Krishan S, Mishra SK, Farooqui KJ, Singh MK, Wasir JS, Bansal B, Kaur P, Jevalikar G, Gill HK, Choudhary NS, Mithal A. Effect of Empagliflozin on Liver Fat in Patients With Type 2 Diabetes and Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial (E-LIFT Trial). Diabetes Care. 2018;41:1801-1808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 432] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 129. | Mascolo A, Di Napoli R, Balzano N, Cappetta D, Urbanek K, De Angelis A, Scisciola L, Di Meo I, Sullo MG, Rafaniello C, Sportiello L. Safety profile of sodium glucose co-transporter 2 (SGLT2) inhibitors: A brief summary. Front Cardiovasc Med. 2022;9:1010693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 130. | Petroni ML, Brodosi L, Marchesini G. The treatment of diabetes in advanced liver disease: change of a paradigm. Ann Hepatol. 2023;28:100772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 131. | Montalvo-Gordon I, Chi-Cervera LA, García-Tsao G. Sodium-Glucose Cotransporter 2 Inhibitors Ameliorate Ascites and Peripheral Edema in Patients With Cirrhosis and Diabetes. Hepatology. 2020;72:1880-1882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 132. | Kumar K, Kulkarni A. Letter to the Editor: Sodium-Glucose Cotransporter-2 Inhibitors Are Not the Magic Pills for Control of Ascites in Cirrhosis and Diabetes. Hepatology. 2021;73:865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 133. | Saffo S, Taddei T. SGLT2 inhibitors and cirrhosis: A unique perspective on the comanagement of diabetes mellitus and ascites. Clin Liver Dis (Hoboken). 2018;11:141-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 134. | Yen FS, Hsu CC, Wei JC, Hou MC, Hwu CM. Selection and Warning of Evidence-Based Antidiabetic Medications for Patients With Chronic Liver Disease. Front Med (Lausanne). 2022;9:839456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Reference Citation Analysis (0)] |
| 135. | Gentile S, Strollo F, Ceriello A. Insulin treatment of people with diabetes mellitus and chronic liver disease. Ann Hepatol. 2016;15:287-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 136. | Vargas JI, Arrese M, Shah VH, Arab JP. Use of Statins in Patients with Chronic Liver Disease and Cirrhosis: Current Views and Prospects. Curr Gastroenterol Rep. 2017;19:43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 137. | Kreidieh M, Hamadi R, Alsheikh M, Al Moussawi H, Deeb L. Statin Use in Patients With Chronic Liver Disease and Cirrhosis: Current Evidence and Future Directions. Gastroenterology Res. 2022;15:1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 138. | Yun B, Ahn SH, Yoon JH, Kim BK. Statin use and risk of progression to liver cirrhosis in chronic hepatitis B independent of conventional risk factors: A nationwide study. Hepatol Commun. 2022;6:2455-2464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 139. | Kim G, Jang SY, Nam CM, Kang ES. Statin use and the risk of hepatocellular carcinoma in patients at high risk: A nationwide nested case-control study. J Hepatol. 2018;68:476-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 140. | Vell MS, Loomba R, Krishnan A, Wangensteen KJ, Trebicka J, Creasy KT, Trautwein C, Scorletti E, Seeling KS, Hehl L, Rendel MD, Zandvakili I, Li T, Chen J, Vujkovic M, Alqahtani S, Rader DJ, Schneider KM, Schneider CV. Association of Statin Use With Risk of Liver Disease, Hepatocellular Carcinoma, and Liver-Related Mortality. JAMA Netw Open. 2023;6:e2320222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 141. | Ciardullo S, Perseghin G. Statin use is associated with lower prevalence of advanced liver fibrosis in patients with type 2 diabetes. Metabolism. 2021;121:154752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 142. | Pontiroli AE, Rizzo M, Tagliabue E. Use of glucagon in severe hypoglycemia is scarce in most countries, and has not been expanded by new ready-to-use glucagons. Diabetol Metab Syndr. 2022;14:193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 143. | Isaacs D, Clements J, Turco N, Hartman R. Glucagon: Its evolving role in the management of hypoglycemia. Pharmacotherapy. 2021;41:623-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 144. | Ahmed FW, Majeed MS, Kirresh O. Non-Diabetic Hypoglycemia. 2023 Jul 17. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. [PubMed] |
| 145. | Bischoff SC, Bernal W, Dasarathy S, Merli M, Plank LD, Schütz T, Plauth M. ESPEN practical guideline: Clinical nutrition in liver disease. Clin Nutr. 2020;39:3533-3562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 197] [Article Influence: 39.4] [Reference Citation Analysis (2)] |
| 146. | European Association for the Study of the Liver. European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70:172-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 675] [Article Influence: 112.5] [Reference Citation Analysis (2)] |
| 147. | Bojko M. Causes of Sarcopenia in Liver Cirrhosis. Clin Liver Dis (Hoboken). 2019;14:167-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 148. | Deng N, Mallepally N, Peng FB, Kanji A, Marcelli M, Hernaez R. Serum testosterone levels and testosterone supplementation in cirrhosis: A systematic review. Liver Int. 2021;41:2358-2370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 149. | Kreisel W, Lazaro A, Trebicka J, Grosse Perdekamp M, Schmitt-Graeff A, Deibert P. Cyclic GMP in Liver Cirrhosis-Role in Pathophysiology of Portal Hypertension and Therapeutic Implications. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 150. | Achilli C, Pundir J, Ramanathan P, Sabatini L, Hamoda H, Panay N. Efficacy and safety of transdermal testosterone in postmenopausal women with hypoactive sexual desire disorder: a systematic review and meta-analysis. Fertil Steril. 2017;107:475-482.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 151. | Allen AM, Hay JE. Review article: the management of cirrhosis in women. Aliment Pharmacol Ther. 2014;40:1146-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 152. | Giard JM, Terrault NA. Women with Cirrhosis: Prevalence, Natural History, and Management. Gastroenterol Clin North Am. 2016;45:345-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 153. | Quiroz-Aldave JE, Concepción-Zavaleta MJ, Durand-Vásquez MDC, Concepción-Urteaga LA, Gamarra-Osorio ER, Suárez-Rojas J, Rafael-Robles LDP, Paz-Ibarra J, Román-González A. Refractory Hypothyroidism: Unraveling the Complexities of Diagnosis and Management. Endocr Pract. 2023;29:1007-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 154. | Shen C, Zhao CY, Liu F, Wang YD, Yu J. Acute-on-chronic liver failure due to thiamazole in a patient with hyperthyroidism and trilogy of Fallot: case report. BMC Gastroenterol. 2010;10:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 155. | Rink C, Klaua M, Mertens E, Schneider G, Nilius R. [Thyrostatic therapy in patients with liver cirrhosis]. Z Gesamte Inn Med. 1985;40:300-302. [PubMed] |
| 156. | . Seven days in medicine: 22-28 Jan 2020. BMJ. 2020;368:m320. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 157. | Yorke E. Hyperthyroidism and Liver Dysfunction: A Review of a Common Comorbidity. Clin Med Insights Endocrinol Diabetes. 2022;15:11795514221074672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 158. | Jeong HM, Kim DJ. Bone Diseases in Patients with Chronic Liver Disease. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 159. | Guañabens N, Cerdá D, Monegal A, Pons F, Caballería L, Peris P, Parés A. Low bone mass and severity of cholestasis affect fracture risk in patients with primary biliary cirrhosis. Gastroenterology. 2010;138:2348-2356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 160. | Patel N, Muñoz SJ. Bone disease in cirrhosis. Clin Liver Dis (Hoboken). 2015;6:96-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 161. | Yoshiji H, Nagoshi S, Akahane T, Asaoka Y, Ueno Y, Ogawa K, Kawaguchi T, Kurosaki M, Sakaida I, Shimizu M, Taniai M, Terai S, Nishikawa H, Hiasa Y, Hidaka H, Miwa H, Chayama K, Enomoto N, Shimosegawa T, Takehara T, Koike K. Evidence-based clinical practice guidelines for Liver Cirrhosis 2020. J Gastroenterol. 2021;56:593-619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 229] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 162. | Grover I, Gunjan D, Singh N, Benjamin J, Ramakrishnan L, Pandey RM, Sati HC, Saraya A. Effect of Vitamin D Supplementation on Vitamin D Level and Bone Mineral Density in Patients With Cirrhosis: A Randomized Clinical Trial. Am J Gastroenterol. 2021;116:2098-2104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 163. | Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, Weiss YG, Benbenishty J, Kalenka A, Forst H, Laterre PF, Reinhart K, Cuthbertson BH, Payen D, Briegel J; CORTICUS Study Group. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358:111-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1696] [Cited by in RCA: 1358] [Article Influence: 79.9] [Reference Citation Analysis (0)] |
| 164. | Solà E, Solé C, Simón-Talero M, Martín-Llahí M, Castellote J, Garcia-Martínez R, Moreira R, Torrens M, Márquez F, Fabrellas N, de Prada G, Huelin P, Lopez Benaiges E, Ventura M, Manríquez M, Nazar A, Ariza X, Suñé P, Graupera I, Pose E, Colmenero J, Pavesi M, Guevara M, Navasa M, Xiol X, Córdoba J, Vargas V, Ginès P. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo-controlled trial. J Hepatol. 2018;69:1250-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 168] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 165. | Wallace JD, Abbott-Johnson WJ, Crawford DH, Barnard R, Potter JM, Cuneo RC. GH treatment in adults with chronic liver disease: a randomized, double-blind, placebo-controlled, cross-over study. J Clin Endocrinol Metab. 2002;87:2751-2759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 166. | Pan CS, Weiss JJ, Fourman LT, Buckless C, Branch KL, Lee H, Torriani M, Misra M, Stanley TL. Effect of recombinant human growth hormone on liver fat content in young adults with nonalcoholic fatty liver disease. Clin Endocrinol (Oxf). 2021;94:183-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 167. | Zhou H, Sun L, Zhang S, Wang Y, Wang G. Effect of long-term growth hormone replacement on glucose metabolism in adults with growth hormone deficiency: a systematic review and meta-analysis. Pituitary. 2021;24:130-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 168. | Shen L, Sun CM, Li XT, Liu CJ, Zhou YX. Growth hormone therapy and risk of recurrence/progression in intracranial tumors: a meta-analysis. Neurol Sci. 2015;36:1859-1867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 169. | Lainscak M, Pelliccia F, Rosano G, Vitale C, Schiariti M, Greco C, Speziale G, Gaudio C. Safety profile of mineralocorticoid receptor antagonists: Spironolactone and eplerenone. Int J Cardiol. 2015;200:25-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 124] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 170. | Witchel SF, Oberfield SE, Peña AS. Polycystic Ovary Syndrome: Pathophysiology, Presentation, and Treatment With Emphasis on Adolescent Girls. J Endocr Soc. 2019;3:1545-1573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 292] [Cited by in RCA: 293] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 171. | Palanisamy S, Funes Hernandez M, Chang TI, Mahaffey KW. Cardiovascular and Renal Outcomes with Finerenone, a Selective Mineralocorticoid Receptor Antagonist. Cardiol Ther. 2022;11:337-354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 172. | Vitale G, Coscia K, Zavatta G, Morelli MC, Vicennati V. Secondary hyperaldosteronism and liver fibrosis in patients with compensated chronic liver disease or portal hypertension. Eur J Intern Med. 2022;99:118-120. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |