Published online Mar 7, 2024. doi: 10.3748/wjg.v30.i9.1018
Peer-review started: November 13, 2023
First decision: December 7, 2023
Revised: January 3, 2024
Accepted: January 29, 2024
Article in press: January 29, 2024
Published online: March 7, 2024
Processing time: 113 Days and 21.4 Hours
A consensus meeting of national experts from all major national hepatobiliary centres in the country was held on May 26, 2023, at the Pakistan Kidney and Liver Institute & Research Centre (PKLI & RC) after initial consultations with the experts. The Pakistan Society for the Study of Liver Diseases (PSSLD) and PKLI & RC jointly organised this meeting. This effort was based on a comprehensive literature review to establish national practice guidelines for hilar cholangiocarcinoma (hCCA). The consensus was that hCCA is a complex disease and requires a multidisciplinary team approach to best manage these patients. This coordinated effort can minimise delays and give patients a chance for curative treatment and effective palliation. The diagnostic and staging workup includes high-quality computed tomography, magnetic resonance imaging, and magnetic resonance cholangiopancreatography. Brush cytology or biopsy utilizing endoscopic retrograde cholangiopancreatography is a mainstay for diagnosis. However, histopathologic confirmation is not always required before resection. Endoscopic ultrasound with fine needle aspiration of regional lymph nodes and positron emission tomography scan are valuable adjuncts for staging. The only curative treatment is the surgical resection of the biliary tree based on the Bismuth-Corlette classification. Selected patients with unresectable hCCA can be considered for liver transplantation. Adjuvant chemotherapy should be offered to patients with a high risk of recurrence. The use of preoperative biliary drainage and the need for portal vein embolisation should be based on local multidisciplinary discussions. Patients with acute cholangitis can be drained with endoscopic or percutaneous biliary drainage. Palliative chemotherapy with cisplatin and gemcitabine has shown improved survival in patients with irresectable and recurrent hCCA.
Core Tip: Consensus among national hepatobiliary experts convened at the Pakistan Kidney and Liver Institute & Research Centre emphasized the complexity of hilar cholangiocarcinoma, advocating a multidisciplinary approach for optimal patient management. Diagnostic protocol includes imaging like computed tomography, magnetic resonance imaging, and magnetic resonance cholangiopancreatography, while endoscopic retrograde cholangiopancreatography plays an important role in tissue acquisition. Surgical resection remains the best curative treatment option. For unresectable cases, liver transplantation is considered under strict selection criteria. Preoperative biliary drainage and portal vein embolisation decisions may be needed for selective cases. Adjuvant chemotherapy addresses the risk of recurrence. The role of Immunotherapy is emerging and offers improved survival for irresectable hilar cholangiocarcinoma.
- Citation: Dar FS, Abbas Z, Ahmed I, Atique M, Aujla UI, Azeemuddin M, Aziz Z, Bhatti ABH, Bangash TA, Butt AS, Butt OT, Dogar AW, Farooqi JI, Hanif F, Haider J, Haider S, Hassan SM, Jabbar AA, Khan AN, Khan MS, Khan MY, Latif A, Luck NH, Malik AK, Rashid K, Rashid S, Salih M, Saeed A, Salamat A, Tayyab GUN, Yusuf A, Zia HH, Naveed A. National guidelines for the diagnosis and treatment of hilar cholangiocarcinoma. World J Gastroenterol 2024; 30(9): 1018-1042
- URL: https://www.wjgnet.com/1007-9327/full/v30/i9/1018.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i9.1018
Cholangiocarcinoma (CCA) is the second most common liver-related cancer. It accounts for 10%-20% of mortalities from hepatobiliary malignancies. Hilar CCA (hCCA) is the most frequent type, accounting for 40%-60% of cases. There is currently no national consensus in Pakistan for the appropriate diagnosis and treatment of hCCA. To address this gap, the Pakistan Society for the Study of Liver Diseases (referred to herein as PSSLD) and the Pakistan Kidney and Liver Institute & Research Centre (referred to herein as PKLI & RC) collaborated to conduct a consensus meeting to develop guidelines. These guidelines aim to standardise diagnostic approaches and treatment strategies for patients nationwide.
With no comprehensive national registry and the scarcity of formal hepatobiliary centres, diagnosis and treatment of hCCA have remained suboptimal for patients in Pakistan. However, with the recent development of hepatobiliary centres in major cities, there became a need for national consensus to develop appropriate patient pathways for the diagnosis and treatment of hCCA. The need for such national guidelines was realised and discussions with experts were initiated. Following initial consultations with the collaborative efforts of the PSSLD and the PKLI & RC, a consensus meeting of national experts from all major hepatobiliary centres was arranged on May 26, 2023, at PKLI & RC, Lahore, Pakistan.
These guidelines were developed to standardize diagnostic approaches and treatment strategies nationwide. The basis of guidelines is the literature review of randomised controlled trials (RCTs), meta-analyses, case cohorts and prospective and retrospective studies. These guidelines should not be regarded as the standard of care for all patients. Patients must be managed based on all available clinical data for that case. The guidelines are subject to change, considering future advances in scientific knowledge.
The recommendations are graded according to the Oxford Centre for Evidence-Based Medicine, adapted from the Oxford 2011 Levels of Evidence (Tables 1 and 2).
| Level | Criteria | Simple model for high, intermediate, and low evidence |
| 1 | SR (with homogeneity) of RCT | Further research is unlikely to change our confidence in the estimate of benefit and risk |
| 2 | RCT or observational studies with dramatic effects; SR of lower quality studies (i.e. non-randomised, retrospective) | |
| 3 | Non-randomised controlled cohort/follow-up study/control arm of randomised trial (SR is generally better than an individual study) | Further research (if performed) is likely to have an impact on our confidence in the estimate of benefit and risk and may change the estimate |
| 4 | Case-series, case-control, or historically controlled studies (SR is generally better than an individual study) | |
| 5 | Expert opinion (mechanism-based reasoning) | Any estimate of effect is uncertain |
| Grade | Wording | Criteria |
| Strong | Shall, should, is recommended. Shall not, should not, is not recommended | Evidence, consistency of studies, risk-benefit ratio, patient preferences, ethical obligations, feasibility |
| Weak or open | Can, may, is suggested. May not, is not suggested |
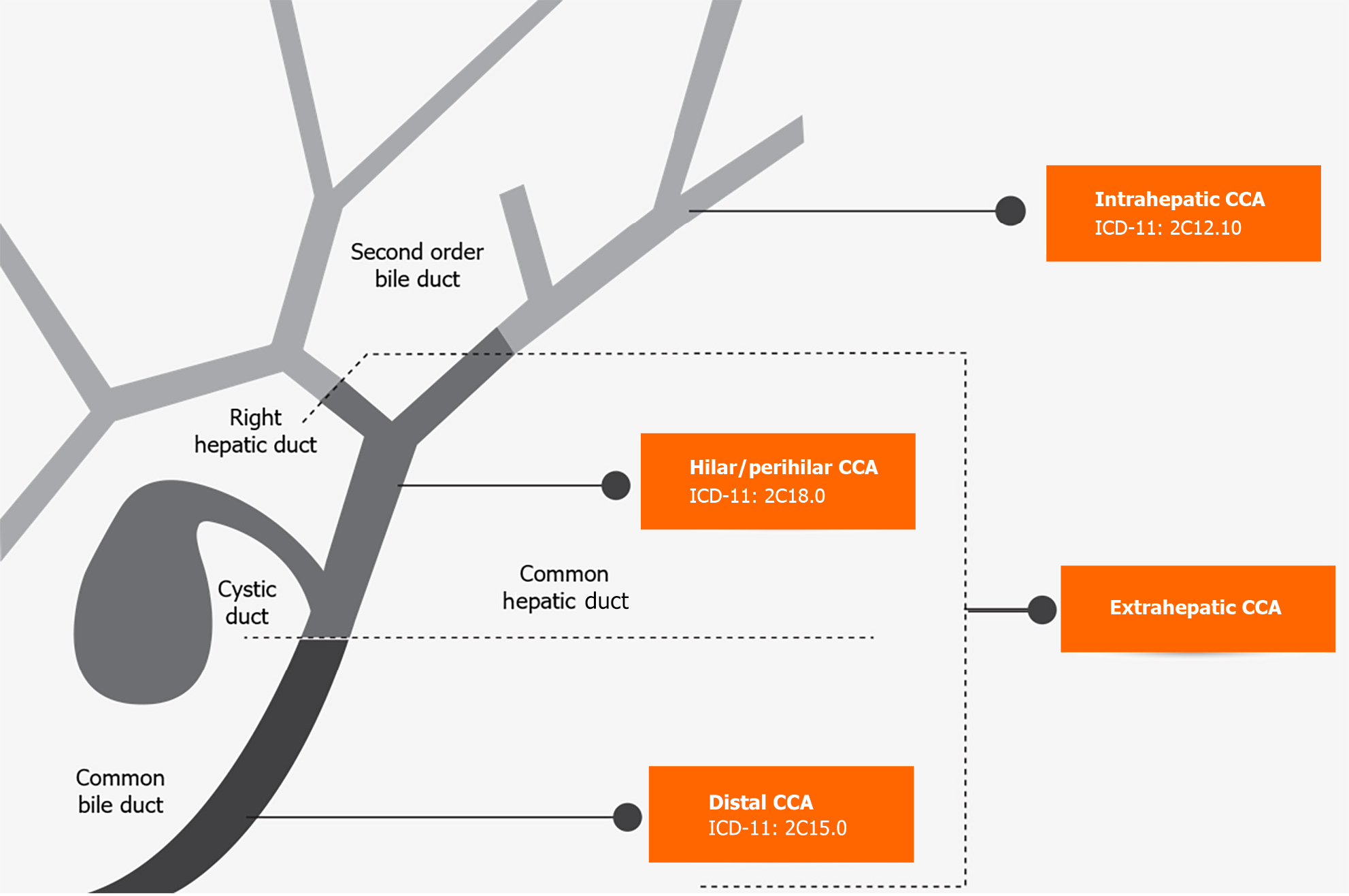
CCA is the second most common liver-related cancer[1]. It accounts for 10%-20% of mortalities from hepatobiliary malignancies[1]. Anatomically, it is classified as intrahepatic and extrahepatic. Extrahepatic CCA (eCCA) is then further classified as hilar/perihilar (hCCA) and distal (dCCA) based on location. Intrahepatic CCA (iCCA) occurs above the second-order bile ducts, while the insertion of the cystic duct distinguishes the hCCA and dCCA types[2] (Figure 1).
Klatskin tumour or hCCA, is the most common type, accounting for 40%-60% of CCA cases, followed by dCCA at 20%-30% and iCCA at 10%-20%[3]. Variances in etiology, risk factors, pathobiology, clinical management, and prognosis are based on anatomical differences. Until 2022, the International Classification of Diseases (commonly known as ICD) did not have a specific code for CCA, resulting in misclassification and difficulty in identifying disease characteristics. The ICD-11 codes were published on January 1, 2022 and now include separate codes for each CCA subtype[4] (Figure 1). The introduction of these new codes will help differentiate the three subtypes of CCA.
CCA typically occurs in individuals over 40, most commonly in the seventh decade of life[5]. Men are more likely to develop CCA than women, with a ratio of 1.0:1.2-1.5[6]. Incidence has been on the rise globally in recent decades, with an increase in mortality for iCCA[7]. It has significant geographical variation and is less common in Western countries compared to some parts of Asia. This difference is mainly attributed to the higher prevalence of established risk factors in some Asian countries. Incidence per 100000 ranges from 85 in northeast Thailand to 0.4 in Canada[8].
Epidemiological data on hCCA is lacking from Pakistan. Only a few local retrospective studies are available on outcomes. Recently, a National Cancer Registry report from Pakistan (2015-2019) showed that liver and intrahepatic bile duct cancers represent 4.43% of all cancers, with a higher prevalence in men compared to women (6.73% vs 2.45%)[9]. In a retrospective analysis of 245 patients with biliary tract malignancy at Aga Khan University, 11.8% were diagnosed with CCA[10]. In another report from Lahore, 34 patients were operated on for CCA over 9 years, and hCCA represented 53% of these cases[11]. In the analysis of 24 patients with hCCA, Dar et al[12] reported a median age at presentation of 49 (23-73) years, with a male to female ratio of 1.4:1.0.
The causes of hCCA remain obscure in many patients. The role of genetic factors needs to be better defined[13,14]. The estimated lifetime incidence of CCA with primary sclerosing cholangitis (PSC) ranges up to 20%[15]. While PSC is a known risk factor for CCA[16], it is attributed to no more than 10% of CCA cases[17]. Hepatobiliary flukes, specifically Opisthorchis viverrini and Clonorchis sinensis have been linked to the development of CCA in Southeast Asia, regardless of site[18]. The presence of hepatitis B virus (commonly known as HBV) and hepatitis C virus (commonly known as HCV) has been linked to an increased risk of developing iCC[3]. Studies do not confirm the association of HBV or HCV with hCCA. Cirrhosis is consistently identified as a risk factor for iCC but not for hCCA[19]. In a meta-analysis by Clements et al[19], choledocholithiasis showed a stronger association with eCCA than iCCA, with odds ratios (ORs) of 18.58 and 10.08, respectively. Choledochal cysts conferred the most significant risk of both iCCA and eCCA with pooled OR of 26.71 [95% confidence interval (95%CI): 15.80-45.16] and 34.94 (95%CI: 24.36-50.12), respectively[19].
Available cohort and case-control studies indicate that high levels of alcohol consumption and tobacco smoking can also increase the likelihood of developing CCA, including hCCA[20]. Conditions such as diabetes, obesity, non-alcoholic fatty liver disease and metabolic syndrome are believed to contribute to the increasing incidence of CCA[3]. However, no significant associations were found between hypertension and obesity[19]. Diabetes has been identified as an important risk factor for both iCCA and eCCA, with ORs of 1.8 (95%CI: 1.5-2.1) and 1.5 (95%CI: 1.3-1.8), respectively[21] (Table 3).
| Established | Less established | Potential (inconclusive data) |
| PSC | IBD likely via PSC | Obesity |
| Choledochal cysts | Cirrhosis | Tobacco smoking |
| Parasitic infections | Hepatitis B and C viruses | Genetic polymorphisms |
| Hepatolithiasis | Diabetes | |
| Choledocholithiasis | Heavy alcohol use | |
| Toxins (Thorotrast contrast agent) | IgG4 related cholangitis | |
| Abnormal junction between the common bile duct and pancreatic duct | ||
| Helicobacter bilis | ||
| Chronic typhoid infection |
Association with other risk factors like IgG4-associated sclerosing cholangitis[22,23], abnormal junction between the bile and pancreatic duct[24], typhoid infection[25,26], and infection with Helicobacter bilis[27,28] need more research before a definitive conclusion can be made.
Recommendation 1: Choledochal cysts, primary sclerosing cholangitis, parasitic infestations, hepatolithiasis and choledocholithiasis should be considered well-established risk factors for hCCA (LoE 2; strong recommendation).
Recommendation 2: Diabetes, alcohol, smoking, and obesity should be considered less well-established risk factors for hCCA (LoE 3; strong recommendation).
CCA can be classified based on anatomy, morphology, and histopathology. Anatomical classification has been discussed earlier in the above section.
CCAs were initially classified as nodular, massive, and diffuse. Rosai called them polypoidal and sclerosing[29]. However, at present, the following classification[30] is being utilised: (1) Mass forming is defined as a small nodule 1-2 cm with bile duct dilatation; (2) intraductal CCA (polypoidal, sessile, or superficially spreading) is along the mucosa. It is confined to the mucosa and does not deeply infiltrate until an advanced stage; and (3) periductal CCA is characterised by annular thickening without mass formation and manifests as complete luminal obstruction.
Most are classified as well to moderately differentiated biliary-type adenocarcinomas[31]. Tubules and glands characterise a typical desmoplastic stroma with a variable inflammatory response. These are further categorised as gastric foveolar, intestinal, and biliary types. Sometimes, papillary groups and sheets are also seen[32]. Perineural and neural invasion is a specific route of invasion, seen in many cases and has prognostic significance. There is also increased invasion of lymphatics[33]. A quantitative grading system based on the percentage of gland formation has been proposed in the College of American Pathologists guidelines and should be followed to standardise reporting[34].
In addition to conventional adenocarcinoma, there are other types, i.e. squamous cell carcinoma, adenosquamous carcinoma, mucinous, signet ring cells, neuroendocrine, clear cells and poorly differentiated. Most of these non-conventional carcinomas have a worse prognosis. Two premalignant conditions have also been identified: high-grade biliary intraepithelial neoplasm and intraductal papillary neoplasm of the biliary tract[35].
Immunohistochemistry can help differentiate metastatic disease by identifying the biliary nature of cells. Conventional markers for adenocarcinomas are CK7, CK20, CK19, P53, MUC5AC, and MUC1. The markers used for squamous cell carcinoma are CK5/6 and for neuroendocrine carcinoma are synaptophysin/chromogranin[36]. Lack of mucin pro
Immunohistochemistry is helpful in the following two scenarios: to differentiate metastatic disease from primary CCA and to distinguish CCA from hepatocellular cancer[37].
It is reported under six categories: (1) Unsatisfactory; (2) negative for malignancy; (3) atypical; (4) benign neoplastic lesions; (5) suspicious for malignancy; and (6) malignant.
Gene sequencing to assess molecular alterations is now emerging to differentiate between benign and malignant strictures[38]. Singhi et al[39] evaluated a 28-gene next-generation sequencing panel using biliary specimens from endoscopic retrograde cholangiopancreatography (ERCP). Next-generation gene sequencing improved sensitivity from 35% to 77% for biliary brushings and 52% to 83% for biliary biopsies.
Recommendation 3: hCCA should be classified as conventional adenocarcinoma or other unconventional tumours based on biliary cytology or biopsy (LoE 2; strong recommendation).
Recommendation 4: College of American Pathologists guidelines should be followed to standardise reporting (LoE 3; strong recommendation).
Recommendation 5: Immunohistochemistry may be done in selected cases to aid diagnosis (LoE 3; weak recommen
Patients generally present with painless jaundice. The alanine aminotransferase/aspartate aminotransferase may be normal or minimally elevated. Alkaline phosphatase levels usually rise in conjunction with bilirubin levels. Biochemical tests of liver function (i.e. albumin, prothrombin time) are normal early in the course of disease. The prothrombin time/international normalized ratio (commonly referred to as INR) may be elevated with prolonged obstruction because of vitamin K malabsorption.
None of the tumour markers are highly sensitive or specific for diagnosis. Carbohydrate antigen 19-9 (CA19-9) is the commonly used tumour marker. The CA19-9 is mainly synthesised by the pancreatic and biliary ductal cells and can be falsely raised in biliary and pancreatic ductal obstruction from benign diseases[40]. It hence cannot be interpreted in the setting of biliary obstruction. Notably, 10% of the patients are non-producers and may have normal CA19-9 levels[41]. The CA19-9 can also be produced by epithelial cells in the stomach, colon, uterus, and salivary glands. Elevated levels can also be seen in urological, pulmonary and gynecological conditions[42].
In patients with PSC, a cut-off value of 129 U/mL had a sensitivity (78.6%), specificity (98.5%), positive predictive value (56.6%), and negative predictive value (99.4%) in predicting CCA[43]. Another study reported a cut-off value of 100 U/mL having a sensitivity (53.0%), and negative predictive value (92.0%) in predicting CCA[41]. In a meta-analysis published in 2015, the overall pooled sensitivity was 0.72 (0.70-0.75) and specificity was 0.84 (0.82-0.85)[44]. The utilization of other tumour markers, i.e. carcinoembryonic antigen and CA-125, in diagnosing hCCA is limited due to their low specificity.
The IgG4 cholangiopathy commonly affects older adults and poses a challenge to diagnosing hCCA, with several reports in the literature[45-48]. With greater recognition of this entity, several guidelines[49-51] now recommend testing for IgG4 cholangiopathy in those with suspected hCCA.
Recommendation 6: CA19-9 is a widely used serum tumour marker for suspected hCCA but does not exhibit high sensitivity and should be carefully interpreted as part of the clinical evaluation (LoE 2; strong recommendation).
Recommendation 7: Testing for IgG4 cholangiopathy should be obtained in suspected cases of hCCA (LoE 4; strong recommendation).
Ultrasound (US) is generally the first imaging modality to evaluate obstructive jaundice. It cannot directly diagnose hCCA but may raise suspicion. Once the diagnosis of hCCA is suspected, the initial radiological examination is often a cross-sectional imaging study, such as computed tomography (CT) or magnetic resonance imaging (MRI) with magnetic resonance cholangiopancreatography (MRCP)[52,53].
The CT is readily available, quick to perform and does not require breath holding, but it carries a risk of radiation exposure and contrast-induced nephropathy. The MRI with MRCP, on the other hand, has no radiation risk. However, it is a longer procedure, requires patient cooperation and may be contraindicated in those with pacemakers and metal implants. While MRI with MRCP is better for soft tissue characterisation and may provide an accurate assessment of longitudinal extension in hCCA, it may overestimate vascular invasion, especially after stenting. In contrast, CT provides better information on vascular invasion[52].
There is no head-to-head comparison of CT vs MRI/MRCP in diagnosing hCCA. In a systematic review article by Zhang et al[54], CT was the most commonly used modality. However, MRI with MRCP is becoming the preferred modality for diagnosing hCCA in the literature[55]. In the Pakistani setting, given the limitations of availability, cost and difficulty in acquiring good-quality images, a CT scan can be used as the preferred diagnostic modality[56].
Positron emission tomography (commonly known as PET)-CT has no clear diagnostic role in helping evaluate issues of local resectability. However, it appears to add some benefit in detecting distant metastatic disease[57]. In one study by Kim et al[58], PET-CT revealed higher accuracy than CT and MRI in the diagnosis of regional lymph node metastases (75.9% vs 60.9%, P = 0.004) and distant metastases (88.3% vs 78.7%, P = 0.004). More studies on the application of PET-CT are needed to determine its utility in staging[54]. Endoscopic US (EUS) with or without fine needle aspiration (FNA)/fine needle biopsy may offer another alternative in staging metastatic lymph nodes[59].
The necessity of establishing a tissue diagnosis before surgery depends upon the clinical situation[60]. It is not critical for planning surgery in patients with characteristic findings of mass-forming malignant biliary obstruction and may not be necessary for planning palliative therapy. Furthermore, tissue sampling with a percutaneous approach with US or CT guidance is not advisable without a visible mass[61]. Detailed knowledge of mimicking diseases and interpretation of biochemical and imaging modalities may lead to a correct diagnosis without the need for a biopsy[61].
Given the complexity of diagnosis and staging, each case of suspected hCCA should be discussed in a multidisciplinary team (MDT) meeting. The MDT should be comprised of radiologists, advanced gastrointestinal endoscopists, hepatobiliary surgeons and oncologists to decide the need for further testing.
Recommendation 8: The initial radiological examination should be a cross-sectional imaging study, such as a CT or MRI and MRCP (LoE 2; strong recommendation).
Recommendation 9: Treatment planning should be done in the presence of resectable hCCA with characteristic imaging features. Tissue diagnosis is not mandatory for such cases (LoE 4; weak recommendation).
Recommendation 10: PET-CT may aid in diagnosing distant metastatic disease and should be considered in surgical planning where added information may change the treatment outcome (LoE 2; weak recommendation).
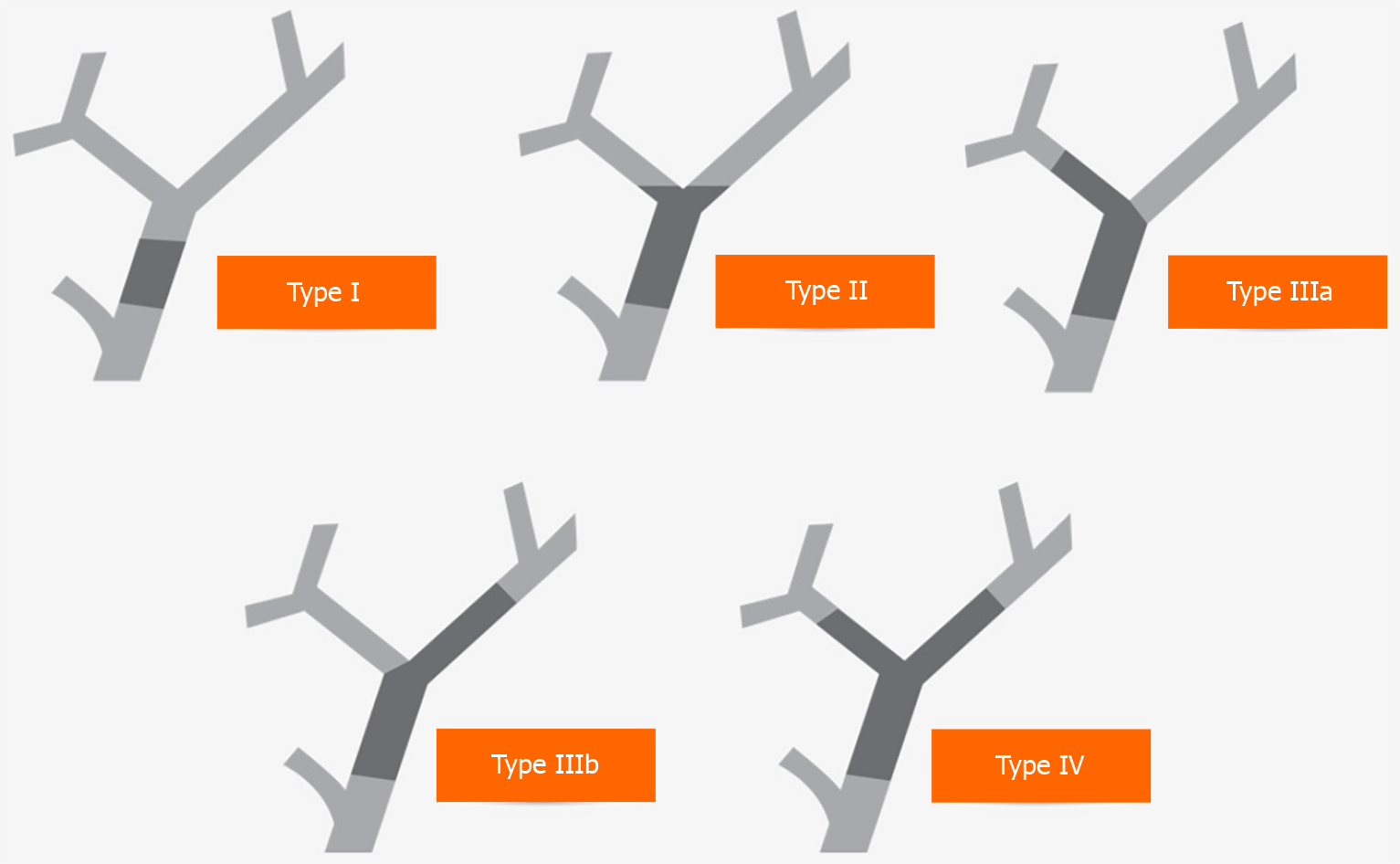
The primary purpose of endobiliary interventions in the diagnostic evaluation of hCCA is to establish histological confirmation and disease staging in the context of Bismuth-Corlette classification (Figure 2) to determine resectability and offer preoperative planning. Biliary strictures remain indeterminate without confirmatory histology, posing a diagnostic dilemma to stratify management decisions. Although in patients with hCCA, preoperative histological confirmation may not be required, around 20% with benign biliary strictures may undergo major surgery for suspected biliary malignancy[62].
The most commonly used modalities for tissue diagnosis in resectable hCCA are ERCP, PTC and intraductal cholangioscopy. Brush cytology, fluoroscopy and cholangioscopy guided forceps biopsy are used to ascertain tissue diagnosis.
The sensitivity of standard brush cytology in the review of 1556 cases has been reported at 41.6% ± 3.2% (99%CI) with a negative predictive value of 58.0% ± 3.2% (99%CI)[63]. In a meta-analysis, Yoon et al[64] revealed pool diagnostic sensitivity of 56.0% (95%CI: 48.8%-63.1%, I2 = 83.0%) with brush cytology alone, 67.0% (95%CI: 60.2%-73.5%, I2 = 72.5%) with biopsy and 70.7% (95%CI: 64.1%-76.8%, I2 = 42.7%) with brushing and biopsy. Supplementary techniques such as fluorescence in situ hybridization (commonly known as FISH) have been suggested to further improve diagnostic sensitivity. Nanda et al[65] reported the diagnostic sensitivity of brush cytology alone, brush cytology with FISH, brush with FISH and biopsy to be 27% vs 77% vs 82%, respectively.
The number of passes also increases the diagnostic sensitivity of brush cytology. In an RCT, Wang et al[66] showed that the sensitivity of brush cytology was 38%, 47%, and 57% in the 10-times, 20-times, and 30-times groups, respectively (P = 0.001). The stricture length of > 1 cm has also been reported as a predictive factor of positive diagnostic yield on brush cytology[67].
Single-operator digital cholangioscopy has emerged as a preferred modality for evaluating indeterminate hilar strictures after inconclusive endobiliary investigations. A systematic review evaluated outcomes of cholangioscopy directed biopsies involving 539 patients and reported a pooled sensitivity of 72% and specificity of 99%[68]. In a meta-analysis, Sun et al[69] studied the performance of single-operator cholangioscopy and revealed the pooled sensitivity and specificity of visual impression (90% and 87%) and spy-bite biopsy (69% and 98%) for the diagnosis of indeterminate biliary strictures.
The role of EUS in hCCA is to stage the disease and sample the hilar mass or locoregional lymph nodes. However, tissue acquisition of hilar mass by EUS carries the risk of seeding metastasis and should be decided in MDT settings[70]. In a meta-analysis, the pooled diagnostic sensitivity and specificity of EUS-FNA for malignant hilar strictures was 76% (95%CI: 66%-85%) and 100% (95%CI: 95%-100%), respectively, with low adverse event rates[71].
Lymph node metastasis is a strong predictor of poor survival in hCCA patients. Malikowski et al[72] reported better regional lymph node detection rates with EUS (89%) than cross-sectional imaging (48%) in hCCA patients. Malignancy was confirmed in 16% of nodes after tissue acquisition via EUS-FNA. Another retrospective multicentre cohort study demonstrated that EUS-FNA detected malignant lymph nodes in 14% of potentially resectable hCCA and avoided surgical exploration[73].
The role of intraductal US in the evaluation of indeterminate biliary strictures is evolving. In a study of 234 indeterminate biliary strictures, the detection rate of malignancy by ERCP/intraductal US was superior to endoscopic trans-papillary biopsy (91% vs 59%, P < 0.0001), EUS (91% vs 74%, P < 0.0001), and CT (91% vs 73%, P < 0.0001)[74].
Recommendation 11: ERCP guided brush cytology and targeted biopsy should be the preferred diagnostic modality to obtain histological confirmation in suspicious or indeterminate biliary strictures (LoE 2; strong recommendation).
Recommendation 12: The number of passes should be increased to enhance the diagnostic sensitivity of brush cytology (LoE 2; strong recommendation).
Recommendation 13: Intraductal cholangioscopy and tissue sampling should be considered in selective cases that remain a diagnostic challenge (LoE 2; strong recommendation).
Recommendation 14: In cases with concern for locoregional metastasis, EUS should be used for staging and tissue sampling (LoE 4; strong recommendation).
Various staging systems have been introduced to define tumour resectability and guide therapy. In 1975, Bismuth and Corlette[75] presented the first staging system. Their classification focused primarily on the level and extension of the tumour along the biliary ductal system. This classification correlated to the procedure required for surgical excision and the establishment of biliary continuity[75,76].
To define resectability, the Memorial Sloan Kettering Cancer Centre staging was introduced in 1998 and was revised in 2001. They incorporated portal vein invasion, the resulting hepatic lobar atrophy, tumour location and extension of bile duct involvement[77]. This staging system provides a framework for defining resectability. However, it does not evaluate the presence of nodal/distant metastasis or arterial involvement.
Mayo Clinic staging was designed for outcome prediction of hCCA patients rather than surgical resection. The Mayo Clinic staging considered the tumour size and multifocality, vascular invasion, lymph node, extra-regional metastasis, and CA19-9 level and performance status to categorize patients into a four-stage system. This staging system reported survival for unresectable hCCA[78,79].
The American Joint Commission on Cancer (commonly referred to as AJCC) staging system, which includes a tumour-node-metastasis (commonly known as TNM) system, is based on pathological findings and is known as pathological staging. It is used postoperatively, has a better prognostic value for resected patients and guides further therapy. The AJCC 8th edition is currently available[80].
To produce a simple and reproducible staging system for hCCA, the International CCA Working Group recently proposed a new classification based on some parameters from the previous systems[2]. The size of the tumour, the extent of the biliary system involvement, hepatic artery and portal vein involvement, lymph node involvement, distant metastases and the volume of the remnant liver after resection are all considered. This system aims to standardise the reporting of hCCA so that resectability and prognosis can be adequately provided.
These staging systems can be supplemented with each other to define resectability, guide the therapy and predict the prognosis in hCCA patients.
Recommendation 15: Bismuth-Corlette classification provides the basis for determining the biliary extent of hCCA and should be used for primary staging and deciding on the surgical technique (LoE 1; strong recommendation).
Recommendation 16: Memorial Sloan Kettering Cancer Centre staging evaluates blood vessel invasion and liver atrophy and should be used for predicting resectability (LoE 3; strong recommendation).
Recommendation 17: American Joint Committee on Cancer TNM staging is based on a comprehensive analysis of postoperative pathological findings. It should be used in predicting the prognosis and postoperative survival of patients (LoE 2; strong recommendation).
The cardinal principle defining resectability is the presence of adequate functional hepatic parenchyma with the achievement of a negative resection margin along with the ability to restore biliary flow in the absence of distant disease[60,81]. Assessment of resectability should be done before any biliary intervention unless the patient is septic. Each case should be discussed in MDT and all hCCA cases should be referred to be managed at high-volume specialist hepatobiliary centres[82,83].
Each patient’s clinical condition and performance status are assessed to ensure they can undergo major hepatic surgery[81]. Cross-sectional images are discussed in MDT meetings[84] for the extent of biliary involvement, the possibility of R0 resection, anatomical variations in hilar structures, quality of hepatic parenchyma and volume of the intended future liver remnant (FLR)[81]. An adequate remnant liver is generally considered as 25% in normal parenchyma[85], while in steatotic and cholestatic livers, the safe limit is 30%-40%[60,81,85]. An inadequate remnant liver may necessitate FLR modulation[12,60,81,86].
Irresectability is defined based on the following parameters: (1) Metastatic spread: once the disease has spread to distant organs, peritoneum and distant lymph nodes[60,81]; (2) patient factors: when the patient is not fit to undergo major liver surgery due to comorbid medical conditions or has a cirrhotic liver with portal hypertension[60,81]; and (3) local factors: there is no consensus regarding local factors determining irresectability[86], hence requiring consideration of individual patient characteristics in MDT discussion[84].
However, the following local criteria make the disease unresectable[60,81]: (1) Bilateral hepatic duct involvement up to secondary biliary radicals; (2) encasement/occlusion of the main portal vein; (3) encasement of portal vein branch with atrophy of contralateral hepatic lobe; and (4) hepatic duct involvement up to secondary biliary radicals with atrophy of the contralateral hepatic lobe.
Several reports[12,81,86-88] recently have shown improved survival in patients with locally advanced disease undergoing major hepatectomies, with portal venous or arterial resection and extended liver resections as right and left trisectionectomies. However, such resections should be performed in highly selected individuals[60]. Portal vein resection is associated with a survival advantage[86,88]. While the clinical benefits of arterial resection for patients with arterial invasion are still unclear[86], this technique results in a higher rate of R0 resection[89].
Recommendation 18: The assessment of disease resectability should be done as a part of hepatobiliary MDT meetings, looking at biliary involvement, lobar atrophy, vascular involvement and FLR (LoE2; strong recommendation).
Most studies have reported that portal vein embolisation (PVE) induces significant hypertrophy of the FLR, thereby increasing the chance of curative resection[90]. The magnitude of FLR hypertrophy varies depending on the extent of liver disease and the technique of PVE[91]. While PVE is generally considered safe, there is a risk of liver failure and other complications, especially in patients with poor liver function or extensive disease. A meta-analysis including 37 publications and 1140 patients undergoing PVE showed liver hypertrophy by an average of 8%-27%, with a complication rate of around 3% and zero mortality[92,93]. Some studies have suggested that PVE may be associated with an increased risk of tumour progression or recurrence[94]. Still, the evidence is conflicting and the exact mechanisms of this effect still need to be fully understood.
The PVE should only be considered in patients who can achieve resectability with liver hypertrophy[95]. The PVE should be performed early enough to allow for adequate FLR hypertrophy but not too early to allow tumour progression[95].
Segment-IV branch PVE can further improve left lateral segment hypertrophy and allow extended resection. However, it comes with a risk of reflux of embolic material to segment II-III FLR portal veins. An alternative would be to perform liver venous deprivation with right and middle hepatic vein embolisation at the same time. Early results from the ongoing HYPER-LIVE01 trial are encouraging[96]. Patients should be closely monitored after PVE for potential complications, including liver failure, portal vein thrombosis and infection. Imaging should be performed to assess the extent of FLR hypertrophy and monitor tumour progression. There is no clear consensus regarding the timing of the scan, but a 4-6-wk window is preferred.
Based on the current evidence, PVE should be considered as a treatment option for patients with hCCA who are not suitable for upfront curative resection but have a chance of achieving resectability with liver hypertrophy. After PVE, if the FLR remains < 20%, liver resection is deemed to be contraindicated.
In the case of biliary dilatation, biliary drainage should be performed before embolisation[97]. Further research is needed to determine the optimal technique of PVE, the predictors of FLR hypertrophy and the effect of PVE on tumour progression and survival outcomes.
Recommendation 19: PVE should only be performed in patients who can achieve resectability with liver hypertrophy (LoE 2; strong recommendation).
Recommendation 20: PVE should be considered in patients whose FLR is less than or equal to 20% of total liver volume (LoE 2; strong recommendation).
Recommendation 21: In patients with biliary dilatation of the FLR, a biliary drainage catheter should be placed before PVE (LoE 2; recommendation strong).
Liver resection for hCCA carries mortality rates between 6.2% and 15.0%, with postoperative morbidity reaching around 60% in Western studies[98-101]. Mortality is linked to postoperative hepatic insufficiency and sepsis, which develops in the compromised liver by previous jaundice, cholangitis and malnutrition[100-102]. The role of preoperative biliary drainage (PBD) in hCCA remains debated. The PBD improves coagulopathy, alleviates renal insufficiency associated with liver failure and provides symptomatic relief[103]. The PBD reduces the risk of cholangitis and postoperative liver failure[104]. However, on the contrary, cholangitis represents the most important complication related to PBD and is an independent prognostic factor for postoperative mortality[98,102,105,106].
While certain centres propose PBD until the serum bilirubin level descends below 2-3 mg/dL, optimal bilirubin levels before surgical resections remain variable across centres[60,107-110]. She et al[111] reported that a cut-off preoperative bilirubin level of > 4.39 mg/dL was associated with more hospital deaths (50.0% vs 8.5%, P = 0.004) and 90-d mortality (50.0% vs 9.8%, P = 0.008).
Biliary drainage of the FLR helps restore metabolic and synthetic liver function and minimises the potential risk of atrophy due to chronic cholestasis. A study involving 287 patients at Memorial Sloan Kettering Cancer Centre and the Academic Medical Centre in Amsterdam also showed improved outcomes after PBD in patients with an FLR < 30%[99]. Major liver resection in 86 jaundiced patients without PBD with a predicted FLR of < 50% was associated with higher morbidity (55% vs 24%, P = 0.04), mortality (23% vs 8%, P = 0.001) and postoperative complications (62% vs 19%, P = 0.003)[112]. A meta-analysis assessing the efficacy of PBD in resectable hCCA involving 2162 patients favored PBD in patients with cholangitis, malnutrition (serum albumin < 3 g/dL), prolonged jaundice and high serum bilirubin levels ≥ 15 mg/dL[113].
ERCP and percutaneous transhepatic biliary drainage (PTBD) are the most used modalities to achieve PBD for hCCA. The selection of drainage modality depends on local expertise, disease complexity, patient fitness and preferences. Giuliante et al[114] showed significantly higher failure rates of PBD at community hospitals than at referral centres (52.7% vs 36.9%, P = 0.002).
Kishi et al[115] reported a higher incidence of major postoperative morbidities (Clavien-Dindo grade ≥ III) in the PTBD (23%) vs non-PTBD (3%) groups (P = 0.01). In a prediction model, Wiggers et al[116] reported that Bismuth-Corlette I and II resectable hCCA could benefit from ERCP as a primary drainage modality. In contrast, Bismuth-Corlette IIIa or IV hCCA and a total bilirubin level above 8.8 mg/dL may be considered for initial PTBD rather than ERCP. European Society of Gastrointestinal Endoscopy (commonly known as ESGE) suggests that an MDT should determine the indication and route for PBD[117].
DRAINAGE, a multicentre RCT, was prematurely terminated because of higher mortality (41% vs 11%, P = 0.03) and cholangitis (59% vs 37%) in PTBD than in endoscopic biliary drainage (EBD) groups[118]. INTERCPT, another RCT, was also prematurely terminated due to higher mortality rates (50% EBD vs 80% PTBD)[119].
Some studies advocate PTBD for its ability to drain specific liver sectors, though advancements in ERCP techniques enable sector-specific drainage in ERCP at experienced centres[120]. The endoscopic approach facilitates enteral drainage, resulting in improved nutritional status[121]. Tumour seeding is another concern requiring meticulous planning for appropriate drainage modality. PTBD was an independent risk factor for seeding metastasis in patients with resectable hCCA than EBD[122,123]. A systematic review showed that EBD was superior to PTBD in the prevention of seeding metastasis (7.8% vs 17.1%, OR: 0.27, 95%CI: 0.13-0.56, P < 0.001)[124].
Endoscopic drainage can be achieved by conventional plastic stents, the inside-stent technique, or endoscopic nasobiliary drainage. The latter is associated with fewer infectious complications but carries a greater risk of catheter dislocation. Data is scarce to recommend the utilization of fully covered self-expandable metal stents for PBD in resectable hCCA[125-130].
The optimal extent of drainage remains controversial and functional liver volume is a better parameter to guide biliary drainage than the placement of unilateral or bilateral stents. Draining more than 50% of the liver volume is an independent factor contributing to improving hyperbilirubinemia with a lower incidence of cholangitis and prolonged survival[131-133]. The preferred drainage side remains the FLR for better peri- and post-operative outcomes[101,134-138].
There is no consensus about the optimal duration between PBD and surgical resection. Cholestasis impairs hepatic regeneration, and restoration of hepatic function may take 4-6 wk after PBD[139]. Multiple factors influence the optimal timing of surgery, including improvement in bilirubin, cholangitis and nutritional status. Time duration varies across centres, ranging from 7 d to 413 d between biliary drainage and surgery[113].
Recommendation 22: PBD of hCCA is not routinely recommended unless indicated in jaundiced patients with any of the following conditions: cholangitis, need for neoadjuvant therapy, preparation for PVE, malnutrition, hepatic or renal insufficiency, predicted FLR volume of ≤ 30% following surgery, and debilitating symptoms such as intense pruritus (LoE 2; strong recommendation).
Recommendation 23: The indication and route for PBD should be decided by an MDT based on patient characteristics, institutional experience, and resource availability (LoE 3; strong recommendation).
Recommendation 24: ERCP over PTBD is recommended for Bismuth-Corlette I and II, while the combination of ERCP and PTBD or PTBD alone is recommended for Bismuth-Corlette III and IV hCCA (LoE 3; weak recommendation).
Recommendation 25: PTBD is recommended in patients with unsuccessful and/or insufficient EBD (LoE 3; strong recommendation).
Surgical resection is the only potentially curative treatment option, with reported 5-year survival from 25%-40% in patients undergoing R0 resection[12,60]. Survival drastically decreases with involved resection margins and lymph node involvement[60,140]. Surgical resection should include complete excision of involved extrahepatic bile ducts with ipsilateral hepatectomy, caudate lobe resection[81,108,141-143], lymphadenectomy[144], hepaticojejunostomy and vascular resection[81,141,144] and reconstruction if required, aiming to obtain negative margins[60,144-146]. Limited resections of bile duct(s) are associated with increased recurrence and poor survival and are not recommended[141]. Hepatectomy can include right and left hepatectomy to right and left trisectionectomies[12,81,141,143,144]. The standard treatment option for Bismuth-Corlette I and II tumour is right hepatectomy, with right-sided resection preferred due to the proximity of the right hepatic artery to the bile duct and the increased length of the extrahepatic portion of the left hepatic duct[147]. Left hepatectomy alone or accompanied by arterial resection and reconstruction of the right hepatic artery is considered in cases of insufficient functional hepatic reserve in case of right hepatectomy[89,148], with large studies showing comparable long-term survival[83]. The choice of resection in Bismuth-Corlette III and selected cases of Bismuth-Corlette IV is dictated by the extent of biliary involvement, lobar atrophy, vascular involvement, side of biliary dominance and hilar anatomical variations, with Bismuth-Corlette IIIa and IV generally requiring right trisectionectomy and Bismuth-Corlette IIIb and IV requiring left trisectionectomy.
Parenchymal sparing hepatectomies may be utilized in highly selected patients[81,141] as they are associated with an increased risk of positive surgical margins and decreased survival[144]. Concomitant pancreaticoduodenectomy may be included to obtain negative resection margins[146,149], as it can be accomplished with demonstrated safety in many reports and is associated with survival benefits[150].
This modality may be employed to exclude metastatic disease and avoid futile laparotomy[81], but the practice remains optional[145].
Regional lymphadenectomy should be performed in all patients undergoing surgical resection[60,86,141,144,151]. The 8th edition of the AJCC TNM staging system recommends the dissection of at least five lymph nodes for accurate staging[151]. The extent of lymphadenectomy remains controversial[151], with Western studies recommending lymphadenectomy of the hepatoduodenal ligament[81] and lymph nodes posterior to the pancreatic head, i.e. No. 12 and No. 13 lymph nodes[151], while Japanese studies recommend inclusion of station 8 lymph nodes along the common hepatic artery[12,140].
Intraoperative frozen section analysis is preferred to obtain negative resection margins if further resection is possible[81,152].
Recommendation 26: Surgical resection should be offered to all potential candidates (LoE 2; strong recommendation).
Recommendation 27: The tumour should be resected along with the involved biliary tree, ipsilateral hemi-liver, and caudate lobe with the aim of achieving a margin-negative resection (LoE 2; strong recommendation).
Recommendation 28: Frozen section assessment of proximal and distal bile duct margins can be considered if further resection is possible (LoE 3; strong recommendation).
Recommendation 29: Hepatectomy with pancreaticoduodenectomy should be considered for positive resection margins (LoE 2; strong recommendation).
Recommendation 30: Hepatectomy with portal vein resection and reconstruction should be considered in case of portal vein involvement (LoE 2; strong recommendation).
Recommendation 31: Hepatectomy with hepatic artery resection and reconstruction can be considered in case of hepatic artery involvement (LoE 2; weak recommendation).
While surgical resection remains the primary treatment for hCCA[81,153], a significant majority present with irresectable disease due to extensive biliary and vascular involvement at the hepatic hilus and underlying parenchymal liver disease such as PSC[81,153]. Earlier attempts to employ orthotopic liver transplantation for such patients resulted in very dismal results[153-156]. This led to the development of combined protocols of neoadjuvant chemoradiation followed by liver transplantation in carefully selected patients[154]. The well-known Mayo Clinic Criteria[157] uses neoadjuvant chemoradiation along with diagnostic, inclusion and exclusion criteria, resulting in improved patient selection[155]. This was subsequently validated in a large multicentre cohort of 214 patients using similar protocols of neoadjuvant treatment, and a 5-year recurrence-free survival (RFS) of 65% was achieved[158,159]. At present, several transplant centres have approved protocols for liver transplantation in hCCA[153,154,159,160]. Patients fulfilling Mayo Clinic Criteria, after completing neoadjuvant chemoradiation, are awarded model for end-stage liver disease exception points by the United Network for Organ Sharing (commonly referred to as UNOS) in the United States[158,159]. On the contrary, this therapeutic option is not utilized in the United Kingdom, Germany, and Japan[159] due to the risk of recurrence under immunosuppression.
As discussed, hCCA diagnosis is challenging. In the setting of liver transplantation, diagnosis requires a dominant stricture of peri -hilar ducts on imaging and one or more of the following: positive endoscopic cytology or biopsy, FISH polysomy, or CA19-9 > 100 U/mL in the absence of obstructive jaundice[155,158].
Liver transplantation with grafts retrieved from both cadaveric and living-related donors has been successfully employed[81,83,153-155,158,159]. Nevertheless, liver transplantation in hCCA is associated with higher rates of arterial and portal venous complications[156,159,161]. The neoadjuvant chemoradiation protocol has been modified by omitting brachytherapy to minimise the risk of hepatic artery thrombosis[159]. Successful liver transplantation may warrant the use of aorto-hepatic conduits[161] and interposition grafts for portal vein reconstruction[155,156,158,159].
The outcomes of upfront liver transplantation for hCCA have been discouraging, with early recurrence and poor long-term survival[155,158,159]. Though established[155,158,159,162], variability is found in components of neoadjuvant chemoradiation protocols[159,163] and the ideal protocol is to be defined[159]. However, a retrospective multicentre report from the European Liver Transplant Registry suggests that in carefully selected patients within the Mayo Clinic Criteria, 5-year survival of 60% could be achieved without neoadjuvant chemoradiation[164], highlighting the significance of strict selection criteria[164]. This merits further exploration in clinical trials[164,165].
Recommendation 32: When considering liver transplantation for hCCA, the diagnosis requires the presence of a dominant stricture of peri-hilar ducts on imaging and one or more of the following: positive endoscopic cytology or biopsy, positive FISH polysomy, and CA19-9 > 100 U/mL in the absence of obstructive jaundice (LoE 2; weak recommendation).
Recommendation 33: For unresectable hCCA within Mayo Clinic Criteria, liver transplantation can be considered after neoadjuvant chemoradiation. The neoadjuvant regimen should include a combination of chemotherapy and radiation (LoE 2; weak recommendation).
Recommendation 34: Upfront liver transplantation can be carefully considered for hCCA, within the Mayo Clinic Criteria, if neoadjuvant treatment is not possible, only in centres with approved protocols (LoE 2; weak recommendation).
Recommendation 35: Given the increased vascular complications, the need for arterial and venous jump grafts (natural or synthetic) should be evaluated in preoperative liver transplant planning (LoE 2; strong recommendation).
After complete surgical resection, almost 60% of patients in the high-risk group (i.e. node-positive and/or margin-positive) develop local recurrence. Unfortunately, there is a dearth of RCTs providing high-quality data on the use of adjuvant treatments. Some studies have shown a lack of benefit of adjuvant treatment in low-risk groups, so these patients may be observed[166]. A retrospective study from MD Anderson Cancer Center showed a 5-year survival of 36% and a locoregional recurrence rate of 38% in patients with positive resection margin or positive lymph nodes who received adjuvant chemoradiotherapy. On the contrary, a 5-year survival of 42% and a locoregional recurrence rate of 37% was seen in patients with negative resection margins and negative lymph nodes with no adjuvant treatment[167]. A Korean study on patients treated with adjuvant radiotherapy showed a 5-year survival of 36%, 35%, and 0% in patients with negative margins, positive margins and gross residual disease, respectively[168]. Intention-to-treat analysis of the BILCAP study[169] showed a median overall survival (OS) of 49.6 mo (95%CI: 35.10-59.10) in the patient group treated with adjuvant capecitabine compared with 36.1 mo (95%CI: 29.70-44.20) in the observation group [adjusted hazard ratio (HR): 0.84; 95%CI: 0.67-1.06]. In the protocol-specified sensitivity analysis, adjusting for minimisation factors, nodal status, grade and sex, the OS hazard ratio was 0.74 (95%CI: 0.59-0.94). The benefit of adjuvant therapy extended more to patients with margin-positive surgery and node-positive disease. A concise summary of the relevant clinical trials is provided in Table 4[169-171].
| Study | Design | Sample size | Treatment | Control | Key findings |
| JCOG1202, ASCOT | Phase 3 | Total: 440; CCA: 180 | S-11 | Observation | 3-yr OS: 77.1% vs 67.6% (95%CI: 61.0%-73.3%); 3-yr RFS: 62.4% vs 50.9% (95%CI: 44.1%-57.2%) |
| BILCAP | Phase 3 | Total: 447; CCA: 284 | Capecitabine, duration: 6 months | Observation | OS (month): 51.1 vs 36.4 (95%CI: 34.6%-59.1%); RFS (month): 24.4 vs 17.5 (95%CI: 18.6%-35.9%) |
| SWOG S0809 | Phase 2 | Total: 79; CCA: 53 | gemcitabine and capecitabine followed by CRRT | None | Median OS: 35 months (R0, 34 months, R1, 35 months) |
Recommendation 36: For patients with resected, margin-negative hCCA with negative regional nodes, the following options are available based on local experience, available expertise and availability of drugs: (1) Fluoropyrimidine (5-fluorouracil or capecitabine) or gemcitabine-based chemotherapy (LoE 2; weak recommendation); (2) Fluoropyrimidine-based chemoradiotherapy (LoE 2; weak recommendation); and (3) Observation (LoE 2; weak recommendation).
Recommendation 37: For patients with positive margins or positive regional lymph nodes, treatment options include: (1) Fluoropyrimidine- or gemcitabine-based chemotherapy (LoE 2; strong recommendation); (2) fluoropyrimidine-based chemoradiotherapy (LoE 2; strong recommendation); and (3) fluoropyrimidine or gemcitabine-based chemotherapy followed by fluoropyrimidine-based chemoradiotherapy (LoE 2; strong recommendation).
Recurrent disease after surgical resection of hCCA is a foremost concern and is associated with poor prognosis. The major determinants of recurrence are resection margin status and lymph node metastasis[172-174]. Lymph nodal positivity and R1/2 resection are associated with early recurrence and poor survival outcomes[167]. The 5-year OS after hCCA resection ranges from 20%-42%[135,172-177]. In a recent systematic review and meta-analysis, Liang et al[174] extrapolated numerous factors that have prognostic value in determining the RFS and OS. The proven independent risk factors of OS are preoperative bilirubin levels (> 3 mg/dL), preoperative CA19-9 levels (> 150 U/mL), tumour size (2-3 cm), major vascular invasion, T-stage of disease (T3/4), lymph node metastasis (N-stage), moderate to poor tumour differentiation (grade 2 and 3), resection margin status, perineural and lymphovascular invasion[172-174]. Adjuvant chemotherapy has a positive impact on OS[174]. In a large retrospective study, Komaya et al[175] found that 5-year OS and RFS were significantly better in R0 resection than in R1 resection groups (48.5% vs 17.7% and 58.5% vs 10.4%, respectively). Further in-depth analysis revealed that 5-year RFS in the R0 resection group worsened as the number of poor prognostic factors increased[175]. Based on these observations, patients may be classified into high risk (R1 resection or R0 with one/more than one poor prognostic factors) and low risk (R0 resection with no risk factor).
Therefore, follow-up visits and postoperative treatment may be formulated based on identifying high-risk and low-risk groups after hCCA resection. As the high-risk group has a high chance of recurrence and poor OS, close surveillance is required[175]. The follow-up visit should include an assessment of clinical parameters, LFTs, tumour markers (CA19-9) and imaging every 2-3 months for the 1st 2 years and then every 6 months for up to 5 years. Imaging should include US at each visit and contrast CT scan of the chest and abdomen or MRI at 6-mo intervals or when clinical parameters mandate. These patients should be discussed in MDT meetings for adjuvant chemoradiotherapy for better outcomes[174]. A low-risk group should be followed every 3 months for the 1st year, 6-mo intervals for the 2nd year and annually for up to 5 years[178,179].
Liver transplantation for unresectable hCCA in a selective cohort after neoadjuvant protocol demonstrates a promising overall outcome[155,158,178,180]. Although a significant body of literature demonstrates superior OS and RFS after liver transplantation for hCCA[155,156,158,162,180,181], recent meta-analysis demonstrates the heterogeneity of these data in terms of patient selection (PSC vs non-PSC hCCA) and inherent limitations in study designs and data analyses resulting in wide variability in results[182]. Nonetheless, 5-year OS and RFS for patients undergoing liver transplantation after neoadjuvant protocol exceeds 50% and 65%, respectively[158,182]. Despite inconsistencies in outcomes, significant factors responsible for disease recurrence and patient survival are a response to neoadjuvant chemoradiation and residual disease in the explanted liver[156,181].
In addition, the main outstanding issues in patients undergoing liver transplantation after neoadjuvant protocol are vascular (late hepatic artery thrombosis: 18.9% and portal vein thrombosis: 37.8%) and biliary complications (anastomotic stricture: 39.2%) as a consequence of irradiated porta hepatis[156,162]. This evidence supports the necessity of robust surveillance protocols. Thus, in addition to usual post-transplant surveillance, a high-risk surveillance strategy for the detection of recurrence should be employed for those who have undergone liver transplantation.
Recommendation 38: The high-risk group (R1 resection or R0 with one or more than one poor prognostic factor) should be followed every 3 months with clinical examination, CA19-9 and US. The CT scan should be done every 6 months for up to 5 years (LoE 2; weak recommendation).
Recommendation 39: The low-risk group (R0 resection with no risk factor) should be followed every 3 months with clinical examination, CA19-9 and US. The CT scan should be done every 6 months for the 1st year and then annually for up to 5 years (LoE 2; weak recommendation).
Recommendation 40: Post-transplant surveillance should follow a high-risk protocol (LoE 2; weak recommendation).
Metastatic CCA carries limited treatment options and has a poor prognosis[183]. Systemic chemotherapy is the mainstay of treatment. Combination chemotherapy with cisplatin and gemcitabine has been the standard of care. It has shown an OS (HR: 0.64, 95%CI: 0.52-0.80, P < 0.001) and median progression-free survival (HR: 0.63, 95%CI: 0.51-0.77, P < 0.001) benefit compared to single agent gemcitabine in ABC-02 trial[184]. In patients with limited renal function, oxaliplatin may be substituted for cisplatin[185]. In the phase III TOPAZ-1 trial[186], the combination of cisplatin and gemcitabine was augmented with the programmed death ligand 1 (PDL1) immune checkpoint inhibitor (ICI) durvalumab resulting in improved response rate, progression-free survival and OS (primary endpoint; HR: 0.76, 95%CI: 0.64-0.91) compared to cisplatin and gemcitabine alone. Another similar study, KEYNOTE-966[187], using pembrolizumab as an immunotherapy partner, came to a similar conclusion. Median OS was 12.7 mo (95%CI: 11.50-13.60) in the pembrolizumab group vs 10.9 mo (95%CI: 9.90-11.60) in the placebo group (HR: 0.83, 95%CI: 0.72-0.95). Hence, this combination with ICI is considered the first-line treatment for advanced biliary tract cancers (BTCs). The availability and cost of ICI are challenging in low-middle-income countries like Pakistan. Therefore, these medicines can be discussed on a case-by-case basis, especially in patients who are PDL1 positive or have high microsatellite instability.
Molecular analysis should be carried out before or during first-line therapy to evaluate options for second and later lines of treatment in advanced disease. Approximately 40% of patients with biliary tract cancers harbor genetic alterations, which are potential targets for precision medicine[188]. Fibroblast growth factor receptor (FGFR) or isocitrate dehydrogenase 1 inhibitors may be incorporated for patients with FGFR or isocitrate dehydrogenase alterations[189,190]. Immunotherapy with ICIs has shown promise in a subset of patients with high microsatellite instability or mismatch repair-deficient tumours[191]. Palliative care should be integrated early in the treatment plan to address symptoms, improve quality of life, and provide psychosocial support. Close monitoring of treatment response and regular reassessment of the treatment strategy is essential, considering the dynamic nature of metastatic CCA and the potential for subsequent treatment modifications or clinical trial enrollment.
Recommendation 41: ICIs are now incorporated in first-line regimens and should be used with gemcitabine and cisplatin in metastatic CCA, depending on availability (LoE 2; strong recommendation).
Recommendation 42: In patients with FGFR alteration, FGFR inhibitors (e.g., pemigatinib) should be considered as second-line therapy (LoE 3; weak recommendation).
Recommendation 43: Early integration of palliative care, focusing on symptom management, quality of life improvement and psychosocial support, is essential in the management of metastatic CCA (LoE 3; weak recommendation).
Approximately 20%-30% of patients with hCCA are diagnosed at a stage when surgical resection can be offered. Furthermore, comorbidities preclude surgical resection in a significant number of patients. The median survival after resection can be up to 4 years; without resection, it is less than 1 year[192].
For patients with a good performance status who have hyperbilirubinemia despite stenting, a non-gemcitabine-based regimen such as leucovorin-modulated fluorouracil (5-FU), or a fluoropyrimidine plus oxaliplatin such as FOLFOX or CAPOX, infusional 5-FU is recommended. Objective response rates for 5-FU alone or 5-FU-based combination therapies range from 0-34%, and the median survival is usually 6 mo[193]. For patients with a borderline performance status or extensive comorbidity, options include leucovorin-modulated 5-FU or single-agent capecitabine[194]. Other locoregional therapies, such as photodynamic therapy, radiofrequency ablation, trans-arterial chemoembolisation, drug-eluting bead trans-arterial chemoembolisation, selective intraarterial radiotherapy with 90-Y microspheres and external beam radiation therapy are available. However, no prospective RCTs have shown a survival benefit with these therapies[195,196].
Supportive care helps patients meet the physical, practical, emotional, and spiritual challenges of cancer. It is essential to cancer care, especially after treatment has ended. The end of cancer treatment may bring mixed emotions. Even though treatment has ended, patients need help for pain, jaundice, loss of appetite, cholangitis, liver abscess and liver failure. The majority of these patients are candidates for palliative treatment[41,197-199]. The main aim of palliative treatment is to improve the quality of life by minimizing the number of hospitalizations. One of the main goals of palliation is to eliminate obstructive jaundice caused by the tumour, which can be achieved by PTBD or endoluminal stent therapy.
With the recent advances in interventional endoscopy and radiology, palliative therapy for patients with advanced hCCA is still suboptimal. Ashat et al[200] reported that draining more than 50% of the liver volume is an important predictor of treatment effectiveness. Given the significant morbidity and mortality related to recurrent cholangitis, meticulous optimization of biliary drainage is critical to improving survival rates in patients with advanced hCCA[201].
The superiority of self-expanding metal stents (SEMS) compared to plastic stents in unresectable hCCA has been observed in several studies[202,203]. In a metanalysis, SEMS had a lower risk of stent occlusion from sludge compared to the plastic stent [relative risk (95%CI): Uncovered SEMS vs plastic stent, 0.09 (0.04-0.18); and covered SEMS vs plastic stent, 0.17 (0.08-0.37)][204] Self-expanding metal stents are hence preferred in patients with life expectancy of > 3 mo[205].
The majority of studies on the natural progression of hCCA without any cancer treatment are retrospective in design and a large number of patients who were treated with only best supportive care had advanced cancer with a poor performance status (performance status 3-4)[192,206,207]. In a Korean study on biliary tract cancers with best supportive care, the OS for intrahepatic, extrahepatic and ampulla of Vater cancer was 4.7 mo, 9.7 mo and 11.2 mo, respectively[208]. In multivariate analysis, variables associated with poor prognosis were metastatic biliary cancer (HR: 2.19, P = 0.001), high baseline carcinoembryonic antigen level, defined as > 4.0 ng/mL (HR: 1.51, P = 0.024) and high baseline CA19-9 > 100 U/mL (HR: 1.93, P = 0.001)[208].
Recommendation 44: Palliative biliary drainage should be attempted at hepatobiliary centres (LoE 3; strong recommendation).
Recommendation 45: Biliary drainage offers significant survival benefits. The goal of drainage should be normalization and not just improvement of bilirubin levels (LoE 4; weak recommendation).
Recommendation 46: SEMS should be preferred for palliative drainage in those with life expectancy > 3 months (LoE 3; strong recommendation).
Recommendation 47: Patients who have advanced hCCA with high bilirubin and poor performance status of 3-4 should be offered supportive care (LoE 2; strong recommendation).
Given the complexity of diagnosis and staging, each case of suspected hCCA should be discussed in a MDT meeting regarding surgical resection, tissue diagnosis, PBD, PVE and palliative drainage. Surgical resection remains the curative option. Immunotherapy is gaining prominence and presents a potential for enhanced survival in cases of unresectable hCCA.
Dr Imran Ali Syed provided assistance during revision of the manuscript. Dr Amjad Zafar, Dr Kaleem ullah and Muhammad Khawar Shahzad attended the consensus meeting and provided valuable input.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Pakistan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Feng YJ, China; Zharikov YO, Russia S-Editor: Chen YL L-Editor: Filipodia P-Editor: Yu HG
| 1. | Gatto M, Bragazzi MC, Semeraro R, Napoli C, Gentile R, Torrice A, Gaudio E, Alvaro D. Cholangiocarcinoma: update and future perspectives. Dig Liver Dis. 2010;42:253-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 133] [Article Influence: 8.9] [Reference Citation Analysis (1)] |
| 2. | Deoliveira ML, Schulick RD, Nimura Y, Rosen C, Gores G, Neuhaus P, Clavien PA. New staging system and a registry for perihilar cholangiocarcinoma. Hepatology. 2011;53:1363-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 208] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 3. | Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, Lind GE, Folseraas T, Forbes SJ, Fouassier L, Geier A, Calvisi DF, Mertens JC, Trauner M, Benedetti A, Maroni L, Vaquero J, Macias RI, Raggi C, Perugorria MJ, Gaudio E, Boberg KM, Marin JJ, Alvaro D. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol. 2016;13:261-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 972] [Article Influence: 108.0] [Reference Citation Analysis (0)] |
| 4. | ICD-11 for Mortality and Morbidity Statistics. 2C18 Malignant neoplasms of perihilar bile duct. 2023. [cited 12 December 2023]. Available from: https://icd.who.int/browse11/L-m/en#/http://id.who.int/icd/entity/1071237724. |
| 5. | Blechacz BR, Gores GJ. Cholangiocarcinoma. Clin Liver Dis. 2008;12:131-150, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 173] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 6. | Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 848] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 7. | Vithayathil M, Khan SA. Current epidemiology of cholangiocarcinoma in Western countries. J Hepatol. 2022;77:1690-1698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 92] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 8. | Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 2019;39 Suppl 1:19-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 500] [Article Influence: 83.3] [Reference Citation Analysis (0)] |
| 9. | Ikram A, Pervez S, Khadim MT, Sohaib M, Uddin H, Badar F, Masood AI, Khattak ZIA, Naz S, Rahat T, Murad N, Memon FN, Abid S, Bashir F, Rafique I, Mustafa MA, Kumar R, Shafiq A. National Cancer Registry of Pakistan: First Comprehensive Report of Cancer Statistics 2015-2019. J Coll Physicians Surg Pak. 2023;33:625-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 10. | Kumar S, Masood N, Shaikh AJ, Valimuhammad AT, Haider G, Lal A, Niamatullah N. Clinical presentation and outcomes of patients with biliary malignancies: the Aga Khan University experience. Asian Pac J Cancer Prev. 2009;10:463-466. [PubMed] |
| 11. | Anwar MuhammadI, Qureshi MI, Shafi M, Durrani KM. Challenges in Surgical Management of Extra-Hepatic Cholangiocarcinoma: A Case Series of 9-Year Experience in Pakistan. Ann King Edw Med Univ. 2019;25:3053. [DOI] [Full Text] |
| 12. | Dar FS, Atiq M, Shahzadi N, Ainy SK, Rana A, Bhatti ABH. Outcomes after Surgical Resection of Hilar Cholangiocarcinoma. J Coll Physicians Surg Pak. 2019;29:874-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Huang WY, Gao YT, Rashid A, Sakoda LC, Deng J, Shen MC, Wang BS, Han TQ, Zhang BH, Chen BE, Rosenberg PS, Chanock SJ, Hsing AW. Selected base excision repair gene polymorphisms and susceptibility to biliary tract cancer and biliary stones: a population-based case-control study in China. Carcinogenesis. 2008;29:100-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Wardell CP, Fujita M, Yamada T, Simbolo M, Fassan M, Karlic R, Polak P, Kim J, Hatanaka Y, Maejima K, Lawlor RT, Nakanishi Y, Mitsuhashi T, Fujimoto A, Furuta M, Ruzzenente A, Conci S, Oosawa A, Sasaki-Oku A, Nakano K, Tanaka H, Yamamoto Y, Michiaki K, Kawakami Y, Aikata H, Ueno M, Hayami S, Gotoh K, Ariizumi SI, Yamamoto M, Yamaue H, Chayama K, Miyano S, Getz G, Scarpa A, Hirano S, Nakamura T, Nakagawa H. Genomic characterization of biliary tract cancers identifies driver genes and predisposing mutations. J Hepatol. 2018;68:959-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 263] [Article Influence: 37.6] [Reference Citation Analysis (1)] |
| 15. | Boonstra K, Weersma RK, van Erpecum KJ, Rauws EA, Spanier BW, Poen AC, van Nieuwkerk KM, Drenth JP, Witteman BJ, Tuynman HA, Naber AH, Kingma PJ, van Buuren HR, van Hoek B, Vleggaar FP, van Geloven N, Beuers U, Ponsioen CY; EpiPSCPBC Study Group. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology. 2013;58:2045-2055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 505] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 16. | Aune D, Sen A, Norat T, Riboli E, Folseraas T. Primary sclerosing cholangitis and the risk of cancer, cardiovascular disease, and all-cause mortality: a systematic review and meta-analysis of cohort studies. Sci Rep. 2021;11:10646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 17. | LaRusso NF, Shneider BL, Black D, Gores GJ, James SP, Doo E, Hoofnagle JH. Primary sclerosing cholangitis: summary of a workshop. Hepatology. 2006;44:746-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 168] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 18. | Brindley PJ, Bachini M, Ilyas SI, Khan SA, Loukas A, Sirica AE, Teh BT, Wongkham S, Gores GJ. Cholangiocarcinoma. Nat Rev Dis Primers. 2021;7:65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 468] [Article Influence: 117.0] [Reference Citation Analysis (0)] |
| 19. | Clements O, Eliahoo J, Kim JU, Taylor-Robinson SD, Khan SA. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: A systematic review and meta-analysis. J Hepatol. 2020;72:95-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 342] [Article Influence: 68.4] [Reference Citation Analysis (1)] |
| 20. | Lee BS, Cha BH, Park EC, Roh J. Risk factors for perihilar cholangiocarcinoma: a hospital-based case-control study. Liver Int. 2015;35:1048-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Welzel TM, Graubard BI, El-Serag HB, Shaib YH, Hsing AW, Davila JA, McGlynn KA. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case-control study. Clin Gastroenterol Hepatol. 2007;5:1221-1228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 386] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 22. | Toyohara T, Nakazawa T, Zakharia K, Shimizu S, Miyabe K, Harada K, Notohara K, Yamada T, Hayashi K, Naitoh I, Kataoka H. IgG4-related Sclerosing Cholangitis Complicated with Cholangiocarcinoma and Detected by Forkhead Box P3 Immunohistochemical Staining. Intern Med. 2021;60:859-866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Kurita Y, Fujita Y, Sekino Y, Watanabe S, Iwasaki A, Kagawa K, Tanida E, Yagi S, Hasegawa S, Sato T, Hosono K, Kato S, Kobayashi N, Ichikawa Y, Endo I, Nakajima A, Kubota K. IgG4-related sclerosing cholangitis may be a risk factor for cancer. J Hepatobiliary Pancreat Sci. 2021;28:524-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Roukounakis NE, Kuhn JA, McCarty TM. Association of an abnormal pancreaticobiliary junction with biliary tract cancers. Proc (Bayl Univ Med Cent). 2000;13:11-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Abdel Wahab M, Mostafa M, Salah T, Fouud A, Kandeel T, Elshobary M, Abd Allah OF, Elghawalby N, Sultan A, Ezzat F. Epidemiology of hilar cholangiocarcinoma in Egypt: single center study. Hepatogastroenterology. 2007;54:1626-1631. [PubMed] |
| 26. | Robbins S, Chuang VP, Hersh T. The development of hepatobiliary cancer in a carrier of Salmonella typhus. Am J Gastroenterol. 1988;83:675-678. [PubMed] |
| 27. | Xiao M, Gao Y, Wang Y. Helicobacter species infection may be associated with cholangiocarcinoma: a meta-analysis. Int J Clin Pract. 2014;68:262-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Segura-López FK, Güitrón-Cantú A, Torres J. Association between Helicobacter spp. infections and hepatobiliary malignancies: a review. World J Gastroenterol. 2015;21:1414-1423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | Rosai J. Ackerman’s Surgical Pathology. 8th edition. Mosby Hardcover, 1996. |
| 30. | Jang JY, Lee JS, Kim H-J, Shim J-J, Kim JH, Kim BH, Kwon CH, Lee SD, Lee HW, Jeong WK, Choi J-Y, Ko HK, Lee DH, Kim H, Kim B, Yoon SM, Yoon WS, Um SH. The General Rules for the Study of Primary Liver Cancer. J Liver Cancer. 2017;17:19-44.. [RCA] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Castellano-Megías VM, Ibarrola-de Andrés C, Colina-Ruizdelgado F. Pathological aspects of so called "hilar cholangiocarcinoma". World J Gastrointest Oncol. 2013;5:159-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Nakanuma Y, Sato Y, Harada K, Sasaki M, Xu J, Ikeda H. Pathological classification of intrahepatic cholangiocarcinoma based on a new concept. World J Hepatol. 2010;2:419-427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 233] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 33. | Ebata T, Kamiya J, Nishio H, Nagasaka T, Nimura Y, Nagino M. The concept of perihilar cholangiocarcinoma is valid. Br J Surg. 2009;96:926-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 34. | Washington MK, Berlin J, Branton PA, Burgart LJ, Carter DK, Compton CC, Fitzgibbons PL, Frankel WL, Jessup JM, Kakar S, Minsky B, Nakhleh RE, Vauthey JN; Members of the Cancer Committee, College of American Pathologists. Protocol for the examination of specimens from patients with carcinoma of the perihilar bile ducts. Arch Pathol Lab Med. 2010;134:e19-e24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2439] [Article Influence: 487.8] [Reference Citation Analysis (3)] |
| 36. | Basturk O, Farris AB, Adsay NV. Immunohistology of the Pancreas, Biliary Tract, and Liver. 2011. [cited 12 December 2023]. Available from: https://www.researchgate.net/publication/285205866_Immunohistology_of_the_Pancreas_Biliary_Tract_and_Liver. |
| 37. | Mocan LP, Rusu I, Melincovici CS, Boșca BA, Mocan T, Crăciun R, Spârchez Z, Iacobescu M, Mihu CM. The Role of Immunohistochemistry in the Differential Diagnosis between Intrahepatic Cholangiocarcinoma, Hepatocellular Carcinoma and Liver Metastasis, as Well as Its Prognostic Value. Diagnostics (Basel). 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 38. | Vij M, Puri Y, Rammohan A, G G, Rajalingam R, Kaliamoorthy I, Rela M. Pathological, molecular, and clinical characteristics of cholangiocarcinoma: A comprehensive review. World J Gastrointest Oncol. 2022;14:607-627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (4)] |
| 39. | Singhi AD, Nikiforova MN, Chennat J, Papachristou GI, Khalid A, Rabinovitz M, Das R, Sarkaria S, Ayasso MS, Wald AI, Monaco SE, Nalesnik M, Ohori NP, Geller D, Tsung A, Zureikat AH, Zeh H, Marsh JW, Hogg M, Lee K, Bartlett DL, Pingpank JF, Humar A, Bahary N, Dasyam AK, Brand R, Fasanella KE, McGrath K, Slivka A. Integrating next-generation sequencing to endoscopic retrograde cholangiopancreatography (ERCP)-obtained biliary specimens improves the detection and management of patients with malignant bile duct strictures. Gut. 2020;69:52-61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 121] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 40. | La'ulu SL, Roberts WL. Performance characteristics of five automated CA 19-9 assays. Am J Clin Pathol. 2007;127:436-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 41. | Patel AH, Harnois DM, Klee GG, LaRusso NF, Gores GJ. The utility of CA 19-9 in the diagnoses of cholangiocarcinoma in patients without primary sclerosing cholangitis. Am J Gastroenterol. 2000;95:204-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 285] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 42. | Lee T, Teng TZJ, Shelat VG. Carbohydrate antigen 19-9 - tumor marker: Past, present, and future. World J Gastrointest Surg. 2020;12:468-490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 159] [Cited by in RCA: 157] [Article Influence: 31.4] [Reference Citation Analysis (4)] |
| 43. | Levy C, Lymp J, Angulo P, Gores GJ, Larusso N, Lindor KD. The value of serum CA 19-9 in predicting cholangiocarcinomas in patients with primary sclerosing cholangitis. Dig Dis Sci. 2005;50:1734-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 226] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 44. | Liang B, Zhong L, He Q, Wang S, Pan Z, Wang T, Zhao Y. Diagnostic Accuracy of Serum CA19-9 in Patients with Cholangiocarcinoma: A Systematic Review and Meta-Analysis. Med Sci Monit. 2015;21:3555-3563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 45. | Nguyen-tat M, Gamstätter T, Marquardt JU, Geißinger E, Schadmand-Fischer S, Lang H, Siegel E, Schuchmann M, Galle PR, Wörns MA. IgG4-related sclerosing cholangitis mimicking cholangiocarcinoma. Z Gastroenterol. 2012;50:1008-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 46. | Bochatay L, Majno P, Giostra E, Frossard JL. Isolated Liver Hilar Infiltration by IgG4 Inflammation Mimicking Cholangiocarcinoma. Case Rep Gastroenterol. 2016;10:512-517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 47. | Erdogan D, Kloek JJ, ten Kate FJ, Rauws EA, Busch OR, Gouma DJ, van Gulik TM. Immunoglobulin G4-related sclerosing cholangitis in patients resected for presumed malignant bile duct strictures. Br J Surg. 2008;95:727-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 48. | Nakazawa T, Naitoh I, Hayashi K, Miyabe K, Simizu S, Joh T. Diagnosis of IgG4-related sclerosing cholangitis. World J Gastroenterol. 2013;19:7661-7670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 78] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 49. | Khan SA, Davidson BR, Goldin RD, Heaton N, Karani J, Pereira SP, Rosenberg WM, Tait P, Taylor-Robinson SD, Thillainayagam AV, Thomas HC, Wasan H; British Society of Gastroenterology. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut. 2012;61:1657-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 593] [Article Influence: 45.6] [Reference Citation Analysis (1)] |
| 50. | Kamisawa T, Nakazawa T, Tazuma S, Zen Y, Tanaka A, Ohara H, Muraki T, Inui K, Inoue D, Nishino T, Naitoh I, Itoi T, Notohara K, Kanno A, Kubota K, Hirano K, Isayama H, Shimizu K, Tsuyuguchi T, Shimosegawa T, Kawa S, Chiba T, Okazaki K, Takikawa H, Kimura W, Unno M, Yoshida M. Clinical practice guidelines for IgG4-related sclerosing cholangitis. J Hepatobiliary Pancreat Sci. 2019;26:9-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 51. | NCCN Guidelines. Acute Lymphoblastic Leukemia. 2023. [cited 12 December 2023]. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1517. |
| 52. | Elbanna KY, Kielar AZ. Computed Tomography Versus Magnetic Resonance Imaging for Hepatic Lesion Characterization/Diagnosis. Clin Liver Dis (Hoboken). 2021;17:159-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 53. | Suthar M, Purohit S, Bhargav V, Goyal P. Role of MRCP in Differentiation of Benign and Malignant Causes of Biliary Obstruction. J Clin Diagn Res. 2015;9:TC08-TC12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 54. | Zhang H, Zhu J, Ke F, Weng M, Wu X, Li M, Quan Z, Liu Y, Zhang Y, Gong W. Radiological Imaging for Assessing the Respectability of Hilar Cholangiocarcinoma: A Systematic Review and Meta-Analysis. Biomed Res Int. 2015;2015:497942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 55. | Masselli G, Gualdi G. Hilar cholangiocarcinoma: MRI/MRCP in staging and treatment planning. Abdom Imaging. 2008;33:444-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 56. | Forsmark CE, Diniz AL, Zhu AX. Consensus conference on hilar cholangiocarcinoma. HPB (Oxford). 2015;17:666-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 57. | Breitenstein S, Apestegui C, Clavien PA. Positron emission tomography (PET) for cholangiocarcinoma. HPB (Oxford). 2008;10:120-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 58. | Kim JY, Kim MH, Lee TY, Hwang CY, Kim JS, Yun SC, Lee SS, Seo DW, Lee SK. Clinical role of 18F-FDG PET-CT in suspected and potentially operable cholangiocarcinoma: a prospective study compared with conventional imaging. Am J Gastroenterol. 2008;103:1145-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 144] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 59. | Strongin A, Singh H, Eloubeidi MA, Siddiqui AA. Role of endoscopic ultrasonography in the evaluation of extrahepatic cholangiocarcinoma. Endosc Ultrasound. 2013;2:71-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 60. | Mansour JC, Aloia TA, Crane CH, Heimbach JK, Nagino M, Vauthey JN. Hilar cholangiocarcinoma: expert consensus statement. HPB (Oxford). 2015;17:691-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 280] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 61. | Buc E, Lesurtel M, Belghiti J. Is preoperative histological diagnosis necessary before referral to major surgery for cholangiocarcinoma? HPB (Oxford). 2008;10:98-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 62. | Singh A, Gelrud A, Agarwal B. Biliary strictures: diagnostic considerations and approach. Gastroenterol Rep (Oxf). 2015;3:22-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 63. | Burnett AS, Calvert TJ, Chokshi RJ. Sensitivity of endoscopic retrograde cholangiopancreatography standard cytology: 10-y review of the literature. J Surg Res. 2013;184:304-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 64. | Yoon SB, Moon SH, Ko SW, Lim H, Kang HS, Kim JH. Brush Cytology, Forceps Biopsy, or Endoscopic Ultrasound-Guided Sampling for Diagnosis of Bile Duct Cancer: A Meta-Analysis. Dig Dis Sci. 2022;67:3284-3297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 65. | Nanda A, Brown JM, Berger SH, Lewis MM, Barr Fritcher EG, Gores GJ, Keilin SA, Woods KE, Cai Q, Willingham FF. Triple modality testing by endoscopic retrograde cholangiopancreatography for the diagnosis of cholangiocarcinoma. Therap Adv Gastroenterol. 2015;8:56-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 66. | Wang J, Xia M, Jin Y, Zheng H, Shen Z, Dai W, Li X, Kang M, Wan R, Lu L, Hu B, Wan X, Cai X. More Endoscopy-Based Brushing Passes Improve the Detection of Malignant Biliary Strictures: A Multicenter Randomized Controlled Trial. Am J Gastroenterol. 2022;117:733-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 67. | Mahmoudi N, Enns R, Amar J, AlAli J, Lam E, Telford J. Biliary brush cytology: factors associated with positive yields on biliary brush cytology. World J Gastroenterol. 2008;14:569-573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 68. | Badshah MB, Vanar V, Kandula M, Kalva N, Badshah MB, Revenur V, Bechtold ML, Forcione DG, Donthireddy K, Puli SR. Peroral cholangioscopy with cholangioscopy-directed biopsies in the diagnosis of biliary malignancies: a systemic review and meta-analysis. Eur J Gastroenterol Hepatol. 2019;31:935-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 69. | Sun X, Zhou Z, Tian J, Wang Z, Huang Q, Fan K, Mao Y, Sun G, Yang Y. Is single-operator peroral cholangioscopy a useful tool for the diagnosis of indeterminate biliary lesion? A systematic review and meta-analysis. Gastrointest Endosc. 2015;82:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 70. | Rauws EA, Kloek JJ, Gouma DJ, Van Gulik TM. Staging of cholangiocarcinoma: the role of endoscopy. HPB (Oxford). 2008;10:110-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 71. | Sadeghi A, Mohamadnejad M, Islami F, Keshtkar A, Biglari M, Malekzadeh R, Eloubeidi MA. Diagnostic yield of EUS-guided FNA for malignant biliary stricture: a systematic review and meta-analysis. Gastrointest Endosc. 2016;83:290-8.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 72. | Malikowski T, Levy MJ, Gleeson FC, Storm AC, Vargas EJ, Topazian MD, Abu Dayyeh BK, Iyer PG, Rajan E, Gores GJ, Roberts LR, Chandrasekhara V. Endoscopic Ultrasound/Fine Needle Aspiration Is Effective for Lymph Node Staging in Patients With Cholangiocarcinoma. Hepatology. 2020;72:940-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 73. | de Jong DM, van de Vondervoort S, Dwarkasing RS, Doukas M, Voermans RP, Verdonk RC, Polak WG, de Jonge J, Koerkamp BG, Bruno MJ, van Driel LMJW. Endoscopic ultrasound in patients with resectable perihilar cholangiocarcinoma: impact on clinical decision-making. Endosc Int Open. 2023;11:E162-E168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 74. | Heinzow HS, Kammerer S, Rammes C, Wessling J, Domagk D, Meister T. Comparative analysis of ERCP, IDUS, EUS and CT in predicting malignant bile duct strictures. World J Gastroenterol. 2014;20:10495-10503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 75. | Bismuth H, Corlette MB. Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg Gynecol Obstet. 1975;140:170-178. [PubMed] |
| 76. | Paul A, Kaiser GM, Molmenti EP, Schroeder T, Vernadakis S, Oezcelik A, Baba HA, Cicinnati VR, Sotiropoulos GC. Klatskin tumors and the accuracy of the Bismuth-Corlette classification. Am Surg. 2011;77:1695-1699. [PubMed] |
| 77. | Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz BS J, Youssef BA M, Klimstra D, Blumgart LH. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507-17; discussion 517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 964] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 78. | Chaiteerakij R, Harmsen WS, Marrero CR, Aboelsoud MM, Ndzengue A, Kaiya J, Therneau TM, Sanchez W, Gores GJ, Roberts LR. A new clinically based staging system for perihilar cholangiocarcinoma. Am J Gastroenterol. 2014;109:1881-1890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 79. | Blechacz BR, Sanchez W, Gores GJ. A conceptual proposal for staging ductal cholangiocarcinoma. Curr Opin Gastroenterol. 2009;25:238-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 80. | Lee AJ, Chun YS. Intrahepatic cholangiocarcinoma: the AJCC/UICC 8th edition updates. Chin Clin Oncol. 2018;7:52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 147] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 81. | Hartog H, Ijzermans JN, van Gulik TM, Groot Koerkamp B. Resection of Perihilar Cholangiocarcinoma. Surg Clin North Am. 2016;96:247-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 82. | van Keulen AM, Franssen S, van der Geest LG, de Boer MT, Coenraad M, van Driel LMJW, Erdmann JI, Haj Mohammad N, Heij L, Klümpen HJ, Tjwa E, Valkenburg-van Iersel L, Verheij J, Groot Koerkamp B, Olthof PB; Dutch Hepatocellular & Cholangiocarcinoma Group (DHCG). Nationwide treatment and outcomes of perihilar cholangiocarcinoma. Liver Int. 2021;41:1945-1953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 83. | Mueller M, Breuer E, Mizuno T, Bartsch F, Ratti F, Benzing C, Ammar-Khodja N, Sugiura T, Takayashiki T, Hessheimer A, Kim HS, Ruzzenente A, Ahn KS, Wong T, Bednarsch J, D'Silva M, Koerkamp BG, Jeddou H, López-López V, de Ponthaud C, Yonkus JA, Ismail W, Nooijen LE, Hidalgo-Salinas C, Kontis E, Wagner KC, Gunasekaran G, Higuchi R, Gleisner A, Shwaartz C, Sapisochin G, Schulick RD, Yamamoto M, Noji T, Hirano S, Schwartz M, Oldhafer KJ, Prachalias A, Fusai GK, Erdmann JI, Line PD, Smoot RL, Soubrane O, Robles-Campos R, Boudjema K, Polak WG, Han HS, Neumann UP, Lo CM, Kang KJ, Guglielmi A, Park JS, Fondevila C, Ohtsuka M, Uesaka K, Adam R, Pratschke J, Aldrighetti L, De Oliveira ML, Gores GJ, Lang H, Nagino M, Clavien PA. Perihilar Cholangiocarcinoma - Novel Benchmark Values for Surgical and Oncological Outcomes From 24 Expert Centers. Ann Surg. 2021;274:780-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 108] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 84. | Coelen RJ, Huiskens J, Olthof PB, Roos E, Wiggers JK, Schoorlemmer A, van Delden OM, Klümpen HJ, Rauws EA, van Gulik TM. Compliance with evidence-based multidisciplinary guidelines on perihilar cholangiocarcinoma. United European Gastroenterol J. 2017;5:519-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 85. | Guglielmi A, Ruzzenente A, Conci S, Valdegamberi A, Iacono C. How much remnant is enough in liver resection? Dig Surg. 2012;29:6-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 247] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 86. | Miyazaki M, Yoshitomi H, Miyakawa S, Uesaka K, Unno M, Endo I, Ota T, Ohtsuka M, Kinoshita H, Shimada K, Shimizu H, Tabata M, Chijiiwa K, Nagino M, Hirano S, Wakai T, Wada K, Isayama H, Okusaka T, Tsuyuguchi T, Fujita N, Furuse J, Yamao K, Murakami K, Yamazaki H, Kijima H, Nakanuma Y, Yoshida M, Takayashiki T, Takada T. Clinical practice guidelines for the management of biliary tract cancers 2015: the 2nd English edition. J Hepatobiliary Pancreat Sci. 2015;22:249-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 166] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 87. | Shimizu H, Kimura F, Yoshidome H, Ohtsuka M, Kato A, Yoshitomi H, Furukawa K, Miyazaki M. Aggressive surgical resection for hilar cholangiocarcinoma of the left-side predominance: radicality and safety of left-sided hepatectomy. Ann Surg. 2010;251:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 88. | Neuhaus P, Jonas S, Bechstein WO, Lohmann R, Radke C, Kling N, Wex C, Lobeck H, Hintze R. Extended resections for hilar cholangiocarcinoma. Ann Surg. 1999;230:808-18; discussion 819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 479] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 89. | Rebelo A, Friedrichs J, Grilli M, Wahbeh N, Partsakhashvili J, Ukkat J, Klose J, Ronellenfitsch U, Kleeff J. Systematic review and meta-analysis of surgery for hilar cholangiocarcinoma with arterial resection. HPB (Oxford). 2022;24:1600-1614. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 90. | Yi F, Zhang W, Feng L. Efficacy and safety of different options for liver regeneration of future liver remnant in patients with liver malignancies: a systematic review and network meta-analysis. World J Surg Oncol. 2022;20:399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 91. | Johnson TN, Tucker GT, Tanner MS, Rostami-Hodjegan A. Changes in liver volume from birth to adulthood: a meta-analysis. Liver Transpl. 2005;11:1481-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 237] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 92. | Abulkhir A, Limongelli P, Healey AJ, Damrah O, Tait P, Jackson J, Habib N, Jiao LR. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg. 2008;247:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 474] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 93. | Palavecino M, Abdalla EK, Madoff DC, Vauthey JN. Portal vein embolization in hilar cholangiocarcinoma. Surg Oncol Clin N Am. 2009;18:257-267, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 94. | de Graaf W, van den Esschert JW, van Lienden KP, van Gulik TM. Induction of tumor growth after preoperative portal vein embolization: is it a real problem? Ann Surg Oncol. 2009;16:423-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 95. | Abdalla EK, Hicks ME, Vauthey JN. Portal vein embolization: rationale, technique and future prospects. Br J Surg. 2001;88:165-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 304] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 96. | Guiu B, Quenet F, Panaro F, Piron L, Cassinotto C, Herrerro A, Souche FR, Hermida M, Pierredon-Foulongne MA, Belgour A, Aho-Glele S, Deshayes E. Liver venous deprivation versus portal vein embolization before major hepatectomy: future liver remnant volumetric and functional changes. Hepatobiliary Surg Nutr. 2020;9:564-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 97. | Kennedy TJ, Yopp A, Qin Y, Zhao B, Guo P, Liu F, Schwartz LH, Allen P, D'Angelica M, Fong Y, DeMatteo RP, Blumgart LH, Jarnagin WR. Role of preoperative biliary drainage of liver remnant prior to extended liver resection for hilar cholangiocarcinoma. HPB (Oxford). 2009;11:445-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 98. | Ratti F, Cipriani F, Ferla F, Catena M, Paganelli M, Aldrighetti LA. Hilar cholangiocarcinoma: preoperative liver optimization with multidisciplinary approach. Toward a better outcome. World J Surg. 2013;37:1388-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 99. | Wiggers JK, Groot Koerkamp B, Cieslak KP, Doussot A, van Klaveren D, Allen PJ, Besselink MG, Busch OR, D'Angelica MI, DeMatteo RP, Gouma DJ, Kingham TP, van Gulik TM, Jarnagin WR. Postoperative Mortality after Liver Resection for Perihilar Cholangiocarcinoma: Development of a Risk Score and Importance of Biliary Drainage of the Future Liver Remnant. J Am Coll Surg. 2016;223:321-331.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 158] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 100. | Giuliante F, Ardito F, Guglielmi A, Aldrighetti L, Ferrero A, Calise F, Giulini SM, Jovine E, Breccia C, De Rose AM, Pinna AD, Nuzzo G. Association of Lymph Node Status With Survival in Patients After Liver Resection for Hilar Cholangiocarcinoma in an Italian Multicenter Analysis. JAMA Surg. 2016;151:916-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 101. | Kimura N, Young AL, Toyoki Y, Wyatt JI, Toogood GJ, Hidalgo E, Prasad KR, Kudo D, Ishido K, Hakamada K, Lodge JPA. Radical operation for hilar cholangiocarcinoma in comparable Eastern and Western centers: Outcome analysis and prognostic factors. Surgery. 2017;162:500-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 102. | Ribero D, Zimmitti G, Aloia TA, Shindoh J, Fabio F, Amisano M, Passot G, Ferrero A, Vauthey JN. Preoperative Cholangitis and Future Liver Remnant Volume Determine the Risk of Liver Failure in Patients Undergoing Resection for Hilar Cholangiocarcinoma. J Am Coll Surg. 2016;223:87-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 103. | Sarmiento JM, Nagorney DM. Hepatic resection in the treatment of perihilar cholangiocarcinoma. Surg Oncol Clin N Am. 2002;11:893-908, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 104. | Liu F, Li Y, Wei Y, Li B. Preoperative biliary drainage before resection for hilar cholangiocarcinoma: whether or not? A systematic review. Dig Dis Sci. 2011;56:663-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 105. | Celotti A, Solaini L, Montori G, Coccolini F, Tognali D, Baiocchi G. Preoperative biliary drainage in hilar cholangiocarcinoma: Systematic review and meta-analysis. Eur J Surg Oncol. 2017;43:1628-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 106. | Wang Y, Fu W, Tang Z, Meng W, Zhou W, Li X. Effect of preoperative cholangitis on prognosis of patients with hilar cholangiocarcinoma: A systematic review and meta-analysis. Medicine (Baltimore). 2018;97:e12025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 107. | Cho MS, Kim SH, Park SW, Lim JH, Choi GH, Park JS, Chung JB, Kim KS. Surgical outcomes and predicting factors of curative resection in patients with hilar cholangiocarcinoma: 10-year single-institution experience. J Gastrointest Surg. 2012;16:1672-1679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 108. | Nimura Y, Hayakawa N, Kamiya J, Kondo S, Shionoya S. Hepatic segmentectomy with caudate lobe resection for bile duct carcinoma of the hepatic hilus. World J Surg. 1990;14:535-43; discussion 544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 320] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 109. | Zhang XF, Beal EW, Merath K, Ethun CG, Salem A, Weber SM, Tran T, Poultsides G, Son AY, Hatzaras I, Jin L, Fields RC, Weiss M, Scoggins C, Martin RCG, Isom CA, Idrees K, Mogal HD, Shen P, Maithel SK, Schmidt CR, Pawlik TM. Oncologic effects of preoperative biliary drainage in resectable hilar cholangiocarcinoma: Percutaneous biliary drainage has no adverse effects on survival. J Surg Oncol. 2018;117:1267-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 110. | Parks RW, Currie EJ, Madhavan KK, Garden OJ. Increased bacterobilia associated with preoperative biliary drainage in patients with hilar cholangiocarcinoma. HPB. 2000;2:375-381. [DOI] [Full Text] |
| 111. | She WH, Cheung TT, Ma KW, Tsang SHY, Dai WC, Chan ACY, Lo CM. Defining the optimal bilirubin level before hepatectomy for hilar cholangiocarcinoma. BMC Cancer. 2020;20:914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 112. | Abdel Wahab M, El Hanafy E, El Nakeeb A, Hamdy E, Atif E, Sultan AM. Postoperative Outcome after Major Liver Resection in Jaundiced Patients with Proximal Bile Duct Cancer without Preoperative Biliary Drainage. Dig Surg. 2015;32:426-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 113. | Mehrabi A, Khajeh E, Ghamarnejad O, Nikdad M, Chang DH, Büchler MW, Hoffmann K. Meta-analysis of the efficacy of preoperative biliary drainage in patients undergoing liver resection for perihilar cholangiocarcinoma. Eur J Radiol. 2020;125:108897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 114. | Giuliante F, Ardito F, Aldrighetti L, Ferrero A, Pinna AD, De Carlis L, Cillo U, Jovine E, Portolani N, Gruttadauria S, Mazzaferro V, Massani M, Rosso E, Ettorre GM, Ratti F, Guglielmi A; Italian Association of HepatoBilioPancreatic Surgeons-AICEP; Italian Association of Hepato-Biliary-Pancreatic Surgeons-AICEP, Cescon M, Colasanti M, Di Sandro S, Gringeri E, Russolillo N, Ruzzenente A, Sposito C, Zanello M, Zimmitti G. Liver resection for perihilar cholangiocarcinoma: Impact of biliary drainage failure on postoperative outcome. Results of an Italian multicenter study. Surgery. 2021;170:383-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 115. | Kishi Y, Shimada K, Nara S, Esaki M, Kosuge T. The type of preoperative biliary drainage predicts short-term outcome after major hepatectomy. Langenbecks Arch Surg. 2016;401:503-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 116. | Wiggers JK, Groot Koerkamp B, Coelen RJ, Rauws EA, Schattner MA, Nio CY, Brown KT, Gonen M, van Dieren S, van Lienden KP, Allen PJ, Besselink MG, Busch OR, D'Angelica MI, DeMatteo RP, Gouma DJ, Kingham TP, Jarnagin WR, van Gulik TM. Preoperative biliary drainage in perihilar cholangiocarcinoma: identifying patients who require percutaneous drainage after failed endoscopic drainage. Endoscopy. 2015;47:1124-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 117. | Dumonceau JM, Tringali A, Papanikolaou IS, Blero D, Mangiavillano B, Schmidt A, Vanbiervliet G, Costamagna G, Devière J, García-Cano J, Gyökeres T, Hassan C, Prat F, Siersema PD, van Hooft JE. Endoscopic biliary stenting: indications, choice of stents, and results: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline - Updated October 2017. Endoscopy. 2018;50:910-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 480] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 118. | Coelen RJS, Roos E, Wiggers JK, Besselink MG, Buis CI, Busch ORC, Dejong CHC, van Delden OM, van Eijck CHJ, Fockens P, Gouma DJ, Koerkamp BG, de Haan MW, van Hooft JE, IJzermans JNM, Kater GM, Koornstra JJ, van Lienden KP, Moelker A, Damink SWMO, Poley JW, Porte RJ, de Ridder RJ, Verheij J, van Woerden V, Rauws EAJ, Dijkgraaf MGW, van Gulik TM. Endoscopic versus percutaneous biliary drainage in patients with resectable perihilar cholangiocarcinoma: a multicentre, randomised controlled trial. Lancet Gastroenterol Hepatol. 2018;3:681-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 128] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 119. | Elmunzer BJ, Smith ZL, Tarnasky P, Wang AY, Yachimski P, Banovac F, Buscaglia JM, Buxbaum J, Chak A, Chong B, Coté GA, Draganov PV, Dua K, Durkalski V, Geller BS, Jamil LH, Keswani RN, Khashab MA, Law R, Lo SK, McCarthy S, Selby JB, Singh VK, Taylor JR, Willingham FF, Spitzer RL, Foster LD; INTERCPT study group and the United States Cooperative for Outcomes Research in Endoscopy (USCORE). An Unsuccessful Randomized Trial of Percutaneous vs Endoscopic Drainage of Suspected Malignant Hilar Obstruction. Clin Gastroenterol Hepatol. 2021;19:1282-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 120. | Kloek JJ, van der Gaag NA, Aziz Y, Rauws EA, van Delden OM, Lameris JS, Busch OR, Gouma DJ, van Gulik TM. Endoscopic and percutaneous preoperative biliary drainage in patients with suspected hilar cholangiocarcinoma. J Gastrointest Surg. 2010;14:119-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 121. | Maguchi H, Takahashi K, Katanuma A, Osanai M, Nakahara K, Matuzaki S, Urata T, Iwano H. Preoperative biliary drainage for hilar cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2007;14:441-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 122. | Komaya K, Ebata T, Yokoyama Y, Igami T, Sugawara G, Mizuno T, Yamaguchi J, Nagino M. Verification of the oncologic inferiority of percutaneous biliary drainage to endoscopic drainage: A propensity score matching analysis of resectable perihilar cholangiocarcinoma. Surgery. 2017;161:394-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 123. | Hirano S, Tanaka E, Tsuchikawa T, Matsumoto J, Kawakami H, Nakamura T, Kurashima Y, Ebihara Y, Shichinohe T. Oncological benefit of preoperative endoscopic biliary drainage in patients with hilar cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2014;21:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 124. | Wang L, Lin N, Xin F, Ke Q, Zeng Y, Liu J. A systematic review of the comparison of the incidence of seeding metastasis between endoscopic biliary drainage and percutaneous transhepatic biliary drainage for resectable malignant biliary obstruction. World J Surg Oncol. 2019;17:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 125. | Nimura Y. Preoperative biliary drainage before resection for cholangiocarcinoma (Pro). HPB (Oxford). 2008;10:130-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 126. | Kawasaki S, Imamura H, Kobayashi A, Noike T, Miwa S, Miyagawa S. Results of surgical resection for patients with hilar bile duct cancer: application of extended hepatectomy after biliary drainage and hemihepatic portal vein embolization. Ann Surg. 2003;238:84-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 227] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 127. | Arakura N, Takayama M, Ozaki Y, Maruyama M, Chou Y, Kodama R, Ochi Y, Hamano H, Nakata T, Kajikawa S, Tanaka E, Kawa S. Efficacy of preoperative endoscopic nasobiliary drainage for hilar cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2009;16:473-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 128. | Takahashi Y, Sasahira N, Sasaki T, Inoue Y, Mise Y, Sato T, Ono Y, Oba A, Saiura A, Ito H. The role of stent placement above the papilla (inside-stent) as a bridging therapy for perihilar biliary malignancy: an initial experience. Surg Today. 2021;51:1795-1804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 129. | Ishiwatari H, Kawabata T, Kawashima H, Nakai Y, Miura S, Kato H, Shiomi H, Fujimori N, Ogura T, Inatomi O, Kubota K, Fujisawa T, Takenaka M, Mori H, Noguchi K, Fujii Y, Sugiura T, Ideno N, Nakafusa T, Masamune A, Isayama H, Sasahira N. Clinical Outcomes of Inside Stents and Conventional Plastic Stents as Bridge-to-Surgery Options for Malignant Hilar Biliary Obstruction. Dig Dis Sci. 2023;68:1139-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 130. | Mori H, Kawashima H, Ohno E, Ishikawa T, Yamao K, Mizutani Y, Iida T, Nakamura M, Ishigami M, Onoe S, Mizuno T, Ebata T, Fujishiro M. Comparison of an Inside Stent and a Fully Covered Self-Expandable Metallic Stent as Preoperative Biliary Drainage for Patients with Resectable Perihilar Cholangiocarcinoma. Can J Gastroenterol Hepatol. 2022;2022:3005210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 131. | Vienne A, Hobeika E, Gouya H, Lapidus N, Fritsch J, Choury AD, Chryssostalis A, Gaudric M, Pelletier G, Buffet C, Chaussade S, Prat F. Prediction of drainage effectiveness during endoscopic stenting of malignant hilar strictures: the role of liver volume assessment. Gastrointest Endosc. 2010;72:728-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 221] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 132. | Takahashi E, Fukasawa M, Sato T, Takano S, Kadokura M, Shindo H, Yokota Y, Enomoto N. Biliary drainage strategy of unresectable malignant hilar strictures by computed tomography volumetry. World J Gastroenterol. 2015;21:4946-4953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 133. | Bulajic M, Panic N, Radunovic M, Scepanovic R, Perunovic R, Stevanovic P, Ille T, Zilli M, Bulajic M. Clinical outcome in patients with hilar malignant strictures type II Bismuth-Corlette treated by minimally invasive unilateral versus bilateral endoscopic biliary drainage. Hepatobiliary Pancreat Dis Int. 2012;11:209-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 134. | Dinant S, Gerhards MF, Rauws EA, Busch OR, Gouma DJ, van Gulik TM. Improved outcome of resection of hilar cholangiocarcinoma (Klatskin tumor). Ann Surg Oncol. 2006;13:872-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 141] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 135. | Hirano S, Kondo S, Tanaka E, Shichinohe T, Tsuchikawa T, Kato K, Matsumoto J, Kawasaki R. Outcome of surgical treatment of hilar cholangiocarcinoma: a special reference to postoperative morbidity and mortality. J Hepatobiliary Pancreat Sci. 2010;17:455-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 136. | van Gulik TM, Kloek JJ, Ruys AT, Busch OR, van Tienhoven GJ, Lameris JS, Rauws EA, Gouma DJ. Multidisciplinary management of hilar cholangiocarcinoma (Klatskin tumor): extended resection is associated with improved survival. Eur J Surg Oncol. 2011;37:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 137. | Seyama Y, Kubota K, Sano K, Noie T, Takayama T, Kosuge T, Makuuchi M. Long-term outcome of extended hemihepatectomy for hilar bile duct cancer with no mortality and high survival rate. Ann Surg. 2003;238:73-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 246] [Article Influence: 11.2] [Reference Citation Analysis (1)] |
| 138. | Rocha FG, Matsuo K, Blumgart LH, Jarnagin WR. Hilar cholangiocarcinoma: the Memorial Sloan-Kettering Cancer Center experience. J Hepatobiliary Pancreat Sci. 2010;17:490-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 139. | Koyama K, Takagi Y, Ito K, Sato T. Experimental and clinical studies on the effect of biliary drainage in obstructive jaundice. Am J Surg. 1981;142:293-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 123] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 140. | Liang L, Li C, Wang MD, Xing H, Diao YK, Jia HD, Lau WY, Pawlik TM, Zhang CW, Shen F, Huang DS, Yang T. The value of lymphadenectomy in surgical resection of perihilar cholangiocarcinoma: a systematic review and meta-analysis. Int J Clin Oncol. 2021;26:1575-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 141. | Nishio H, Nagino M, Nimura Y. Surgical management of hilar cholangiocarcinoma: the Nagoya experience. HPB (Oxford). 2005;7:259-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 95] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 142. | Hu YF, Hu HJ, Lv TR, He ZQ, Dai YS, Li FY. Should more aggressive surgical resection be considered in the treatment for Bismuth types I and II hilar cholangiocarcinoma? A meta-analysis. Asian J Surg. 2023;46:4115-4123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 143. | Kovalenko YA, Zharikov YO, Konchina NA, Gurmikov BN, Marinova LA, Zhao AV. Perihilar cholangiocarcinoma: A different concept for radical resection. Surg Oncol. 2020;33:270-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 144. | Capussotti L, Viganò L. Local Resection Without Hepatectomy. In: Lau W. Hilar Cholangiocarcinoma. Dordrecht: Springer, 2013. [DOI] [Full Text] |
| 145. | Gavriilidis P, Askari A, Roberts KJ, Sutcliffe RP. Appraisal of the current guidelines for management of cholangiocarcinoma-using the Appraisal of Guidelines Research and Evaluation II (AGREE II) Instrument. Hepatobiliary Surg Nutr. 2020;9:126-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 146. | D'Souza MA, Valdimarsson VT, Campagnaro T, Cauchy F, Chatzizacharias NA, D'Hondt M, Dasari B, Ferrero A, Franken LC, Fusai G, Guglielmi A, Hagendoorn J, Hidalgo Salinas C, Hoogwater FJH, Jorba R, Karanjia N, Knoefel WT, Kron P, Lahiri R, Langella S, Le Roy B, Lehwald-Tywuschik N, Lesurtel M, Li J, Lodge JPA, Martinou E, Molenaar IQ, Nikov A, Poves I, Rassam F, Russolillo N, Soubrane O, Stättner S, van Dam RM, van Gulik TM, Serrablo A, Gallagher TM, Sturesson C; E-AHPBA scientific and research committee. Hepatopancreatoduodenectomy -a controversial treatment for bile duct and gallbladder cancer from a European perspective. HPB (Oxford). 2020;22:1339-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 147. | Wu W, Cheng Q, Chen J, Chen D, Feng X, Wu J. Left-side vs. right-side hepatectomy for hilar cholangiocarcinoma: a meta-analysis. World J Surg Oncol. 2021;19:107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 148. | Abbas S, Sandroussi C. Systematic review and meta-analysis of the role of vascular resection in the treatment of hilar cholangiocarcinoma. HPB (Oxford). 2013;15:492-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 149. | Fancellu A, Sanna V, Deiana G, Ninniri C, Turilli D, Perra T, Porcu A. Current role of hepatopancreatoduodenectomy for the management of gallbladder cancer and extrahepatic cholangiocarcinoma: A systematic review. World J Gastrointest Oncol. 2021;13:625-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 150. | Lim JH, Choi GH, Choi SH, Kim KS, Choi JS, Lee WJ. Liver resection for Bismuth type I and Type II hilar cholangiocarcinoma. World J Surg. 2013;37:829-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 151. | Li J, Zhou MH, Ma WJ, Li FY, Deng YL. Extended lymphadenectomy in hilar cholangiocarcinoma: What it will bring? World J Gastroenterol. 2020;26:3318-3325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (36)] |
| 152. | Ribero D, Amisano M, Lo Tesoriere R, Rosso S, Ferrero A, Capussotti L. Additional resection of an intraoperative margin-positive proximal bile duct improves survival in patients with hilar cholangiocarcinoma. Ann Surg. 2011;254:776-81; discussion 781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 153. | Moris D, Kostakis ID, Machairas N, Prodromidou A, Tsilimigras DI, Ravindra KV, Sudan DL, Knechtle SJ, Barbas AS. Comparison between liver transplantation and resection for hilar cholangiocarcinoma: A systematic review and meta-analysis. PLoS One. 2019;14:e0220527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 154. | Sudan D, DeRoover A, Chinnakotla S, Fox I, Shaw B Jr, McCashland T, Sorrell M, Tempero M, Langnas A. Radiochemotherapy and transplantation allow long-term survival for nonresectable hilar cholangiocarcinoma. Am J Transplant. 2002;2:774-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 216] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 155. | Rea DJ, Heimbach JK, Rosen CB, Haddock MG, Alberts SR, Kremers WK, Gores GJ, Nagorney DM. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg. 2005;242:451-8; discussion 458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 428] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 156. | Tan EK, Rosen CB, Heimbach JK, Gores GJ, Zamora-Valdes D, Taner T. Living Donor Liver Transplantation for Perihilar Cholangiocarcinoma: Outcomes and Complications. J Am Coll Surg. 2020;231:98-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 157. | Heimbach JK, Haddock MG, Alberts SR, Nyberg SL, Ishitani MB, Rosen CB, Gores GJ. Transplantation for hilar cholangiocarcinoma. Liver Transpl. 2004;10:S65-S68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 158. | Darwish Murad S, Kim WR, Harnois DM, Douglas DD, Burton J, Kulik LM, Botha JF, Mezrich JD, Chapman WC, Schwartz JJ, Hong JC, Emond JC, Jeon H, Rosen CB, Gores GJ, Heimbach JK. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology. 2012;143:88-98.e3; quiz e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 395] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 159. | Breuer E, Mueller M, Doyle MB, Yang L, Darwish Murad S, Anwar IJ, Merani S, Limkemann A, Jeddou H, Kim SC, López-López V, Nassar A, Hoogwater FJH, Vibert E, De Oliveira ML, Cherqui D, Porte RJ, Magliocca JF, Fischer L, Fondevila C, Zieniewicz K, Ramírez P, Foley DP, Boudjema K, Schenk AD, Langnas AN, Knechtle S, Polak WG, Taner CB, Chapman WC, Rosen CB, Gores GJ, Dutkowski P, Heimbach JK, Clavien PA. Liver Transplantation as a New Standard of Care in Patients With Perihilar Cholangiocarcinoma? Results From an International Benchmark Study. Ann Surg. 2022;276:846-853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 160. | Machairas N, Kostakis ID, Tsilimigras DI, Prodromidou A, Moris D. Liver transplantation for hilar cholangiocarcinoma: A systematic review. Transplant Rev (Orlando). 2020;34:100516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 161. | Bhatti ABH, Dar FS, Qureshi AI, Haider S, Khan NA. Saphenous vein conduits for hepatic arterial reconstruction in living donor liver transplantation. Langenbecks Arch Surg. 2019;404:293-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 162. | Tan EK, Taner T, Heimbach JK, Gores GJ, Rosen CB. Liver Transplantation for Peri-hilar Cholangiocarcinoma. J Gastrointest Surg. 2020;24:2679-2685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 163. | Keltner SJ, Hallemeier C, Wang K, Tao R, Shah S, Heimbach J, Kharofa JR. Neoadjuvant Therapy Regimens for Hilar Cholangiocarcinoma Before Liver Transplant. Am J Clin Oncol. 2023;46:276-278. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 164. | Mantel HT, Westerkamp AC, Adam R, Bennet WF, Seehofer D, Settmacher U, Sánchez-Bueno F, Fabregat Prous J, Boleslawski E, Friman S, Porte RJ; European Liver and Intestine Transplant Association (ELITA). Strict Selection Alone of Patients Undergoing Liver Transplantation for Hilar Cholangiocarcinoma Is Associated with Improved Survival. PLoS One. 2016;11:e0156127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 165. | Schmelzle M, Benzing C, Fischer L, Herden U, Sterneck M, Settmacher U, Bauschke A, Neumann U, Pelzer U, Müller T, Strassburg C, Lang H, Becker T, Königsrainer A, Nadalin S, Quante M, Paul A, Friess H, Klempnauer J, Richter N, Vondran F, Pascher A, Rösch T, Schöning W, Krenzien F, Öllinger R, Seehofer D, Neuhaus P, Pratschke J. Feasibility and Efficacy of Adjuvant Chemotherapy With Gemcitabine After Liver Transplantation for Perihilar Cholangiocarcinoma - A Multi-Center, Randomized, Controlled Trial (pro-duct001). Front Oncol. 2022;12:910871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 166. | Horgan AM, Amir E, Walter T, Knox JJ. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol. 2012;30:1934-1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 560] [Cited by in RCA: 514] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 167. | Borghero Y, Crane CH, Szklaruk J, Oyarzo M, Curley S, Pisters PW, Evans D, Abdalla EK, Thomas MB, Das P, Wistuba II, Krishnan S, Vauthey JN. Extrahepatic bile duct adenocarcinoma: patients at high-risk for local recurrence treated with surgery and adjuvant chemoradiation have an equivalent overall survival to patients with standard-risk treated with surgery alone. Ann Surg Oncol. 2008;15:3147-3156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 168. | Kim S, Kim SW, Bang YJ, Heo DS, Ha SW. Role of postoperative radiotherapy in the management of extrahepatic bile duct cancer. Int J Radiat Oncol Biol Phys. 2002;54:414-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 72] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 169. | Bridgewater J, Fletcher P, Palmer DH, Malik HZ, Prasad R, Mirza D, Anthony A, Corrie P, Falk S, Finch-Jones M, Wasan H, Ross P, Wall L, Wadsley J, Evans TR, Stocken D, Stubbs C, Praseedom R, Ma YT, Davidson B, Neoptolemos J, Iveson T, Cunningham D, Garden OJ, Valle JW, Primrose J; BILCAP study group. Long-Term Outcomes and Exploratory Analyses of the Randomized Phase III BILCAP Study. J Clin Oncol. 2022;40:2048-2057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 115] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 170. | Nakachi K, Ikeda M, Konishi M, Nomura S, Katayama H, Kataoka T, Todaka A, Yanagimoto H, Morinaga S, Kobayashi S, Shimada K, Takahashi Y, Nakagohri T, Gotoh K, Kamata K, Shimizu Y, Ueno M, Ishii H, Okusaka T, Furuse J; Hepatobiliary and Pancreatic Oncology Group of the Japan Clinical Oncology Group (JCOG-HBPOG). Adjuvant S-1 compared with observation in resected biliary tract cancer (JCOG1202, ASCOT): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet. 2023;401:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 133] [Article Influence: 66.5] [Reference Citation Analysis (0)] |
| 171. | Ben-Josef E, Guthrie KA, El-Khoueiry AB, Corless CL, Zalupski MM, Lowy AM, Thomas CR Jr, Alberts SR, Dawson LA, Micetich KC, Thomas MB, Siegel AB, Blanke CD. SWOG S0809: A Phase II Intergroup Trial of Adjuvant Capecitabine and Gemcitabine Followed by Radiotherapy and Concurrent Capecitabine in Extrahepatic Cholangiocarcinoma and Gallbladder Carcinoma. J Clin Oncol. 2015;33:2617-2622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 274] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 172. | Tang Z, Yang Y, Zhao Z, Wei K, Meng W, Li X. The clinicopathological factors associated with prognosis of patients with resectable perihilar cholangiocarcinoma: A systematic review and meta-analysis. Medicine (Baltimore). 2018;97:e11999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 173. | Bird NTE, McKenna A, Dodd J, Poston G, Jones R, Malik H. Meta-analysis of prognostic factors for overall survival in patients with resected hilar cholangiocarcinoma. Br J Surg. 2018;105:1408-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 174. | Liang L, Li C, Jia HD, Diao YK, Xing H, Pawlik TM, Lau WY, Shen F, Huang DS, Zhang CW, Yang T. Prognostic factors of resectable perihilar cholangiocarcinoma: a systematic review and meta-analysis of high-quality studies. Ther Adv Gastrointest Endosc. 2021;14:2631774521993065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 175. | Komaya K, Ebata T, Yokoyama Y, Igami T, Sugawara G, Mizuno T, Yamaguchi J, Nagino M. Recurrence after curative-intent resection of perihilar cholangiocarcinoma: analysis of a large cohort with a close postoperative follow-up approach. Surgery. 2018;163:732-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 176. | Noji T, Tsuchikawa T, Mizota T, Okamura K, Nakamura T, Tamoto E, Shichinohe T, Hirano S. Surgery for recurrent biliary carcinoma: results for 27 recurrent cases. World J Surg Oncol. 2015;13:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 177. | Ruys AT, van Haelst S, Busch OR, Rauws EA, Gouma DJ, van Gulik TM. Long-term survival in hilar cholangiocarcinoma also possible in unresectable patients. World J Surg. 2012;36:2179-2186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 178. | Hu HJ, Mao H, Shrestha A, Tan YQ, Ma WJ, Yang Q, Wang JK, Cheng NS, Li FY. Prognostic factors and long-term outcomes of hilar cholangiocarcinoma: A single-institution experience in China. World J Gastroenterol. 2016;22:2601-2610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 179. | Cai JQ, Cai SW, Cong WM, Chen MS, Chen P, Chen XP, Chen YL, Chen YF, Dai CL, Huang Q, Huang ZY, Jiang B, Jiang KW, Li B, Li ZF, Liang LJ, Liu B, Liu HC, Liu LX, Liu QG, Liu R, Liu YB, Lu JG, Lu SC, Lu Y, Mao YL, Mei B, Niu J, Peng BG, Qin X, Qiu YD, Wang GY, Wang YD, Wang ZM, Wan RH, Wu YF, Xing BC, Xia F, Xu GL, Yang JM, Yu XF, Zeng Y, Zeng YY, Zhang BX, Zhang BH, Zhang QY, Zhang SJ, Zhang WG, Zhang YJ, Zhang ZW, Zhou D, Zhou WP. Diagnosis and treatment of cholangiocarcinoma: a consensus from surgical specialists of China. J Huazhong Univ Sci Technolog Med Sci. 2014;34:469-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 180. | Heimbach JK, Gores GJ, Haddock MG, Alberts SR, Nyberg SL, Ishitani MB, Rosen CB. Liver transplantation for unresectable perihilar cholangiocarcinoma. Semin Liver Dis. 2004;24:201-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 191] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 181. | Lehrke HD, Heimbach JK, Wu TT, Jenkins SM, Gores GJ, Rosen CB, Mounajjed T. Prognostic Significance of the Histologic Response of Perihilar Cholangiocarcinoma to Preoperative Neoadjuvant Chemoradiation in Liver Explants. Am J Surg Pathol. 2016;40:510-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 182. | Cambridge WA, Fairfield C, Powell JJ, Harrison EM, Søreide K, Wigmore SJ, Guest RV. Meta-analysis and Meta-regression of Survival After Liver Transplantation for Unresectable Perihilar Cholangiocarcinoma. Ann Surg. 2021;273:240-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 183. | Casadei-Gardini A, Leone F, Brandi G, Scartozzi M, Silvestris N, Santini D, Faloppi L, Aglietta M, Satolli MA, Rizzo A, Lonardi S, Aprile G, Fornaro L. Survival trends over 20 years in patients with advanced cholangiocarcinoma: Results from a national retrospective analysis of 922 cases in Italy. Front Oncol. 2023;13:1128930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 184. | Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J; ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2617] [Cited by in RCA: 3167] [Article Influence: 211.1] [Reference Citation Analysis (1)] |
| 185. | Sharma A, Kalyan Mohanti B, Pal Chaudhary S, Sreenivas V, Kumar Sahoo R, Kumar Shukla N, Thulkar S, Pal S, Deo SV, Pathy S, Ranjan Dash N, Kumar S, Bhatnagar S, Kumar R, Mishra S, Sahni P, Iyer VK, Raina V. Modified gemcitabine and oxaliplatin or gemcitabine + cisplatin in unresectable gallbladder cancer: Results of a phase III randomised controlled trial. Eur J Cancer. 2019;123:162-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 186. | Bouattour M, Valle JW, Vogel A, Kim JW, Kitano M, Chen J-S, III HAB, Zaucha R, Qin S, Evesque L, Zhen DB, Gupta VG, Park JO, Żotkiewicz M, Rokutanda N, Cohen G, Oh D-Y. Characterization of long-term survivors in the TOPAZ-1 study of durvalumab or placebo plus gemcitabine and cisplatin in advanced biliary tract cancer. J Clin Onco. 2023;41:531-531. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 187. | Kelley RK, Ueno M, Yoo C, Finn RS, Furuse J, Ren Z, Yau T, Klümpen HJ, Chan SL, Ozaka M, Verslype C, Bouattour M, Park JO, Barajas O, Pelzer U, Valle JW, Yu L, Malhotra U, Siegel AB, Edeline J, Vogel A; KEYNOTE-966 Investigators. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023;401:1853-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 473] [Article Influence: 236.5] [Reference Citation Analysis (0)] |
| 188. | Jusakul A, Cutcutache I, Yong CH, Lim JQ, Huang MN, Padmanabhan N, Nellore V, Kongpetch S, Ng AWT, Ng LM, Choo SP, Myint SS, Thanan R, Nagarajan S, Lim WK, Ng CCY, Boot A, Liu M, Ong CK, Rajasegaran V, Lie S, Lim AST, Lim TH, Tan J, Loh JL, McPherson JR, Khuntikeo N, Bhudhisawasdi V, Yongvanit P, Wongkham S, Totoki Y, Nakamura H, Arai Y, Yamasaki S, Chow PK, Chung AYF, Ooi LLPJ, Lim KH, Dima S, Duda DG, Popescu I, Broet P, Hsieh SY, Yu MC, Scarpa A, Lai J, Luo DX, Carvalho AL, Vettore AL, Rhee H, Park YN, Alexandrov LB, Gordân R, Rozen SG, Shibata T, Pairojkul C, Teh BT, Tan P. Whole-Genome and Epigenomic Landscapes of Etiologically Distinct Subtypes of Cholangiocarcinoma. Cancer Discov. 2017;7:1116-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 678] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 189. | Javle M, Lowery M, Shroff RT, Weiss KH, Springfeld C, Borad MJ, Ramanathan RK, Goyal L, Sadeghi S, Macarulla T, El-Khoueiry A, Kelley RK, Borbath I, Choo SP, Oh DY, Philip PA, Chen LT, Reungwetwattana T, Van Cutsem E, Yeh KH, Ciombor K, Finn RS, Patel A, Sen S, Porter D, Isaacs R, Zhu AX, Abou-Alfa GK, Bekaii-Saab T. Phase II Study of BGJ398 in Patients With FGFR-Altered Advanced Cholangiocarcinoma. J Clin Oncol. 2018;36:276-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 518] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 190. | Abou-Alfa GK, Macarulla T, Javle MM, Kelley RK, Lubner SJ, Adeva J, Cleary JM, Catenacci DV, Borad MJ, Bridgewater J, Harris WP, Murphy AG, Oh DY, Whisenant J, Lowery MA, Goyal L, Shroff RT, El-Khoueiry AB, Fan B, Wu B, Chamberlain CX, Jiang L, Gliser C, Pandya SS, Valle JW, Zhu AX. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:796-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 714] [Article Influence: 142.8] [Reference Citation Analysis (0)] |
| 191. | Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, Geva R, Gottfried M, Penel N, Hansen AR, Piha-Paul SA, Doi T, Gao B, Chung HC, Lopez-Martin J, Bang YJ, Frommer RS, Shah M, Ghori R, Joe AK, Pruitt SK, Diaz LA Jr. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol. 2020;38:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2120] [Cited by in RCA: 2019] [Article Influence: 403.8] [Reference Citation Analysis (0)] |
| 192. | Park J, Kim MH, Kim KP, Park DH, Moon SH, Song TJ, Eum J, Lee SS, Seo DW, Lee SK. Natural History and Prognostic Factors of Advanced Cholangiocarcinoma without Surgery, Chemotherapy, or Radiotherapy: A Large-Scale Observational Study. Gut Liver. 2009;3:298-305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 143] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 193. | Ducreux M, Rougier P, Fandi A, Clavero-Fabri MC, Villing AL, Fassone F, Fandi L, Zarba J, Armand JP. Effective treatment of advanced biliary tract carcinoma using 5-fluorouracil continuous infusion with cisplatin. Ann Oncol. 1998;9:653-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 112] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 194. | Fornaro L, Cereda S, Aprile G, Di Girolamo S, Santini D, Silvestris N, Lonardi S, Leone F, Milella M, Vivaldi C, Belli C, Bergamo F, Lutrino SE, Filippi R, Russano M, Vaccaro V, Brunetti AE, Rotella V, Falcone A, Barbera MA, Corbelli J, Fasola G, Aglietta M, Zagonel V, Reni M, Vasile E, Brandi G. Multivariate prognostic factors analysis for second-line chemotherapy in advanced biliary tract cancer. Br J Cancer. 2014;110:2165-2169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 195. | Feisthammel J, Mössner J, Hoffmeister A. Palliative Endoscopic Treatment Options in Malignancies of the Biliopancreatic System. Viszeralmedizin. 2014;30:238-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 196. | Blechacz B. Cholangiocarcinoma: Current Knowledge and New Developments. Gut Liver. 2017;11:13-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 347] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 197. | Ghazale A, Chari ST, Zhang L, Smyrk TC, Takahashi N, Levy MJ, Topazian MD, Clain JE, Pearson RK, Petersen BT, Vege SS, Lindor K, Farnell MB. Immunoglobulin G4-associated cholangitis: clinical profile and response to therapy. Gastroenterology. 2008;134:706-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 667] [Cited by in RCA: 585] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 198. | Vauthey JN, Loyer E, Chokshi P, Lahoti S. Case 57: eosinophilic cholangiopathy. Radiology. 2003;227:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 199. | Aloia TA, Charnsangavej C, Faria S, Ribero D, Abdalla EK, Vauthey JN, Curley SA. High-resolution computed tomography accurately predicts resectability in hilar cholangiocarcinoma. Am J Surg. 2007;193:702-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 200. | Ashat M, Arora S, Klair JS, Childs CA, Murali AR, Johlin FC. Bilateral vs unilateral placement of metal stents for inoperable high-grade hilar biliary strictures: A systemic review and meta-analysis. World J Gastroenterol. 2019;25:5210-5219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 201. | Geller A. Klatskin tumor--palliative therapy: the jury is still out or may be not yet in... Gastrointest Endosc. 2009;69:63-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 202. | Paik WH, Park YS, Hwang JH, Lee SH, Yoon CJ, Kang SG, Lee JK, Ryu JK, Kim YT, Yoon YB. Palliative treatment with self-expandable metallic stents in patients with advanced type III or IV hilar cholangiocarcinoma: a percutaneous versus endoscopic approach. Gastrointest Endosc. 2009;69:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 188] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 203. | Raju RP, Jaganmohan SR, Ross WA, Davila ML, Javle M, Raju GS, Lee JH. Optimum palliation of inoperable hilar cholangiocarcinoma: comparative assessment of the efficacy of plastic and self-expanding metal stents. Dig Dis Sci. 2011;56:1557-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 204. | Park CH, Park SW, Jung JH, Jung ES, Kim JH, Park DH. Comparative Efficacy of Various Stents for Palliation in Patients with Malignant Extrahepatic Biliary Obstruction: A Systematic Review and Network Meta-Analysis. J Pers Med. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 205. | Rerknimitr R, Angsuwatcharakon P, Ratanachu-ek T, Khor CJ, Ponnudurai R, Moon JH, Seo DW, Pantongrag-Brown L, Sangchan A, Pisespongsa P, Akaraviputh T, Reddy ND, Maydeo A, Itoi T, Pausawasdi N, Punamiya S, Attasaranya S, Devereaux B, Ramchandani M, Goh KL; Asia-Pacific Working Group on Hepatobiliary Cancers. Asia-Pacific consensus recommendations for endoscopic and interventional management of hilar cholangiocarcinoma. J Gastroenterol Hepatol. 2013;28:593-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 186] [Article Influence: 15.5] [Reference Citation Analysis (1)] |
| 206. | Sharma A, Dwary AD, Mohanti BK, Deo SV, Pal S, Sreenivas V, Raina V, Shukla NK, Thulkar S, Garg P, Chaudhary SP. Best supportive care compared with chemotherapy for unresectable gall bladder cancer: a randomized controlled study. J Clin Oncol. 2010;28:4581-4586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 212] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 207. | Ji JH, Kim YS, Park I, Lee SI, Kim RB, Park JO, Oh SY, Hwang IG, Jang JS, Song HN, Kang JH. Chemotherapy versus Best Supportive Care in Advanced Biliary Tract Carcinoma: A Multi-institutional Propensity Score Matching Analysis. Cancer Res Treat. 2018;50:791-800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 208. | Ji JH, Song HN, Kim RB, Oh SY, Lim HY, Park JO, Park SH, Kim MJ, Lee SI, Ryou SH, Hwang IG, Jang JS, Kim HJ, Choi JY, Kang JH. Natural history of metastatic biliary tract cancer (BTC) patients with good performance status (PS) who were treated with only best supportive care (BSC). Jpn J Clin Oncol. 2015;45:256-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |