Published online Feb 28, 2024. doi: 10.3748/wjg.v30.i8.855
Peer-review started: December 8, 2023
First decision: December 27, 2023
Revised: January 4, 2024
Accepted: January 29, 2024
Article in press: January 29, 2024
Published online: February 28, 2024
Processing time: 80 Days and 9.9 Hours
Reflux esophagitis has an increasing prevalence and complex and diverse symptoms. Identifying its risk factors is crucial to understanding the etiology, prevention, and management of the disease. The occurrence of reflux esophagitis may be associated with food reactions, Helicobacter pylori (H. pylori) infection, and metabolic syndromes.
To investigate the risk factors for reflux esophagitis and analyze the effects of immunoglobulin (Ig) G-mediated food intolerance, H. pylori infection, and metabolic syndrome on reflux esophagitis.
Outpatients attending the Second Medical Center of the PLA General Hospital between 2017 and 2021 were retrospectively enrolled. The patients’ basic information, test results, gastroscopy results, H. pylori test results, and IgG-mediated food intolerance results were collected. Multivariate logistic regression analysis was used to analyze risk factors for reflux esophagitis. Statistical mediation analysis was used to evaluate the effects of IgG-mediated food intolerance and metabolic syndrome on H. pylori infection affecting reflux esophagitis.
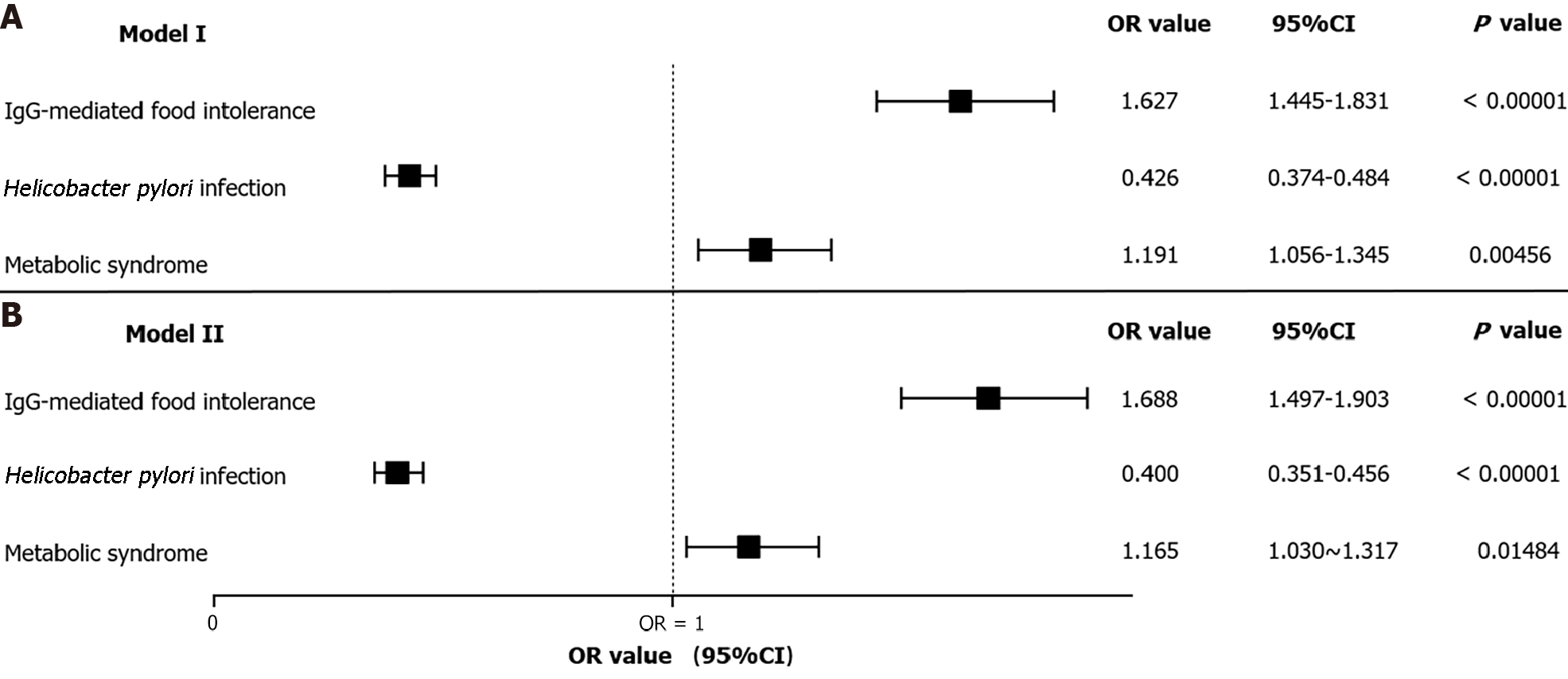
A total of 7954 outpatients were included; the prevalence of reflux esophagitis, IgG-mediated food intolerance, H. pylori infection, and metabolic syndrome were 20.84%, 61.77%, 35.91%, and 60.15%, respectively. Multivariate analysis showed that the independent risk factors for reflux esophagitis included IgG-mediated food intolerance (OR = 1.688, 95%CI: 1.497-1.903, P < 0.00001) and metabolic syndrome (OR = 1.165, 95%CI: 1.030-1.317, P = 0.01484), and the independent protective factor for reflux esophagitis was H. pylori infection (OR = 0.400, 95%CI: 0.351-0.456, P < 0.00001). IgG-mediated food intolerance had a partially positive mediating effect on H. pylori infection as it was associated with reduced occurrence of reflux esophagitis (P = 0.0200). Metabolic syndrome had a partially negative mediating effect on H. pylori infection and reduced the occurrence of reflux esophagitis (P = 0.0220).
Patients with IgG-mediated food intolerance and metabolic syndrome were at higher risk of developing reflux esophagitis, while patients with H. pylori infection were at lower risk. IgG-mediated food intolerance reduced the risk of reflux esophagitis pathogenesis in patients with H. pylori infection; however, metabolic syndrome increased the risk of patients with H. pylori infection developing reflux esophagitis.
Core Tip: This retrospective study investigated the effects of IgG-mediated food intolerance, Helicobacter pylori (H. pylori) infection, and metabolic syndrome on reflux esophagitis. In 7954 outpatients, the prevalence of reflux esophagitis was 20.84%. Patients with IgG-mediated food intolerance and metabolic syndrome are at higher risk of developing reflux esophagitis, while patients with H. pylori infection are at lower risk. IgG-mediated food intolerance reduces the risk of reflux esophagitis pathogenesis in patients with H. pylori infection; however, metabolic syndrome increases the risk of patients with H. pylori infection developing reflux esophagitis.
- Citation: Wang LH, Su BB, Wang SS, Sun GC, Lv KM, Li Y, Shi H, Chen QQ. Immunoglobulin G-mediated food intolerance and metabolic syndrome influence the occurrence of reflux esophagitis in Helicobacter pylori-infected patients. World J Gastroenterol 2024; 30(8): 855-862
- URL: https://www.wjgnet.com/1007-9327/full/v30/i8/855.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i8.855
Gastroesophageal reflux disease (GERD) is a range of diseases arising from the reflux of gastroduodenal content, and its pathogenesis is related to the immuno-inflammatory cascade[1]. Reflux esophagitis (RE), a phenotype of GERD, is defined as a visible mucosal rupture in the distal esophagus[2]. RE accounts for 30% of GERD cases, with an increasing prevalence and complex and diverse symptoms which greatly affect the quality of life; its long-term treatment re-quirements consume significant healthcare resources and create a socioeconomic burden[3]. Identifying the risk factors for RE is crucial to understanding the etiology, prevention, and management of the disease.
Food intolerance (FI) refers to the discomfort caused by a food or food component at a dose that is normally tolerated; this is responsible for most adverse food reactions. FI can be asymptomatic and manifest only as a high immunoglobulin (Ig) G response to stimulation by specific food antigens[4]. Certain foods can cause an imbalance between pro-inflammatory and anti-inflammatory cytokines, which is conducive to the production of an inflammatory environment and activation of the immune system[5]; this phenomenon may affect the development of RE. Metabolic syndrome is a risk factor for RE[6,7]. The effects of Helicobacter pylori (H. pylori) infection on reflux remain controversial[8-10]. This study aimed to explore the risk factors for RE and analyze the effects of IgG-mediated FI, H. pylori infection, and metabolic syndrome on RE.
This was a retrospective cross-sectional study. Outpatients attending the Second Medical Center of PLA General Hospital between 2017 and 2021 were enrolled.
The inclusion criteria were as follows: (1) Successful gastroscopy at our hospital; (2) successful completion of the IgG-mediated FI test; and (3) successful completion of the H. pylori test.
The exclusion criteria were as follows: (1) Missing test results; and (2) severe cardiovascular, cerebrovascular, or consciousness impairment preventing completion of the examination.
The data collected from outpatient records included the following: (1) Basic information: age, sex, systolic blood pressure, diastolic blood pressure, smoking history, and drinking history; (2) test results: white blood cell count, hemoglobin, C-reactive protein, folic acid, homocysteine, brain natriuretic peptide precursor, total cholesterol, fasting blood glucose, creatinine, total protein, and 25-hydroxyvitamin D3; (3) diseases: metabolic syndrome and H. pylori infection; (4) gastroscopy results: presence of RE; and (5) IgG-mediated FI results.
Diagnostic criteria for reflux esophagitis: RE, diagnosed by the presence of mucosal rupture, was detected by gastroscopy and classified according to the current international common Los Angeles grading (LA) standard. LA-A type is categorized by the presence of one or more mucosal breakages ≤ 5 mm in length, LA-B type is categorized by the presence of one or more mucosal breakages > 5 mm in length with no fusion lesion, LA-C type is categorized by the presence of mucosal breakages with fusion lesions < 75% of the esophageal circumference, and LA-D type is categorized by the presence of mucosal breakages with fusion lesions ≥ 75% of the esophageal circumference[3].
Diagnostic criteria for IgG-mediated FI: ELISA was performed to detect delayed allergens, namely, specific IgG antibodies for 21 foods (beef, chicken, cod, corn, crab, egg, mushroom, milk, pork, rice, shrimp, soybean, tomatoes, wheat, brewer's yeast, garlic, ginger, onion, cottage cheese, red pepper, and sesame). All reagents were equilibrated to room temperature (20 °C-28 °C) and a standard curve was drawn. The absorbance value of each microwell was read with a microplate reader and the concentration of IgG antibodies was evaluated based on the absorbance of each well and the standard curve. IgG-positive food was defined as IgG antibody concentrations ≥ 50 U/mL. IgG-mediated FI was diagnosed when there was ≥ 1 IgG-positive food[11].
Diagnostic criteria for metabolic syndrome: Metabolic syndrome was diagnosed by the presence of at least three of the following: (1) Abdominal obesity (namely, central obesity): waist circumference ≥ 90 cm for men and ≥ 85 cm for women; (2) hyperglycemia: fasting blood glucose ≥ 6.1 mmol/L or blood glucose ≥ 7.8 mmol/L obtained 2 h after sugar loading or patients diagnosed with diabetes who were receiving treatment; (3) hypertension: blood pressure ≥ 130/85 mmHg (1 mmHg = 0.133 kPa) or patients diagnosed with hypertension who were receiving treatment; (4) fasting triacylglycerol ≥ 1.70 mmol/L; and (5) fasting high-density lipoprotein cholesterol < l.04 mmol/L[12].
Diagnostic criteria for H.pylori infection: The diagnostic criteria for H. pylori infection included the use of a 13C-urea breath test or the histologic examination of gastric biopsy specimens as diagnostic tools[13].
Normally distributed continuous variables are presented as means ± SD, and independent t-tests were used for between-group comparisons. Categorical variables are presented as numbers or percentages, and χ2 tests were used for between-group comparisons. Multivariate logistic regression analysis was used to analyze risk factors for RE. Mediation effect analysis was used to evaluate the effects of IgG-mediated FI and metabolic syndrome on the occurrence of RE caused by H. pylori infection. In this study, SPSS (version 26.0; IBM Corp., Armonk, NY, United States) was applied to organize and statistically analyze the data; a P-value < 0.05 was considered statistically significant.
A total of 7954 outpatients were included; 1658 (20.84%) had RE. Notably, RE had a high prevalence among older patients, men, and patients with smoking and alcohol consumption histories. Furthermore, comparing the diseases and test results between the non-RE and RE groups, the RE group demonstrated higher positive IgG-mediated FI, metabolic syndrome, white blood cell count, hemoglobin, homocysteine, fasting blood glucose, and creatinine levels and a lower rate of H. pylori infection; all P-values were < 0.05 (Table 1).
| Characteristics | Non-reflux esophagitis (n = 6296) | Reflux esophagitis (n = 1658) | P value |
| Basic information | |||
| Age (yr), mean ± SD | 49.76 ± 8.00 | 50.81 ± 7.83 | < 0.001 |
| Sex (male) [n (%)] | 4177 (66.34) | 1504 (90.71) | < 0.001 |
| Systolic blood pressure (mmHg), mean ± SD | 123.40 ± 137.49 | 126.12 ± 16.78 | 0.421 |
| Diastolic blood pressure (mmHg), mean ± SD | 80.58 ± 11.57 | 82.63 ± 11.06 | < 0.001 |
| Drinking history [n (%)] | < 0.001 | ||
| No | 2567 (40.77) | 387 (23.34) | |
| Yes | 3592 (57.05) | 1231 (74.25) | |
| Abstain | 137 (2.17) | 40 (2.41) | |
| Smoking history [n (%)] | < 0.001 | ||
| No | 4073 (64.69) | 788 (47.53) | |
| Yes | 1777 (28.22) | 720 (43.43) | |
| Abstain | 446 (7.08) | 150 (9.05) | |
| Test results | |||
| White blood cell count (109/L), mean ± SD | 5.87 ± 1.44 | 6.21 ± 1.55 | < 0.001 |
| Hemoglobin (g/L), mean ± SD | 143.31 ± 15.33 | 150.17 ± 12.61 | < 0.001 |
| C-reactive protein (mg/L), mean ± SD | 0.16 ± 0.36 | 0.16 ± 0.31 | 0.999 |
| Folic acid (ng/mL), mean ± SD | 9.50 ± 4.39 | 8.58 ± 4.05 | < 0.001 |
| Homocysteine (μmol/L), mean ± SD | 12.35 ± 9.38 | 13.46 ± 6.96 | < 0.001 |
| Brain natriuretic peptide precursor (pg/mL), mean ± SD | 34.59 ± 41.53 | 30.29 ± 49.06 | < 0.001 |
| Total cholesterol (mmol/L), mean ± SD | 4.76 ± 0.90 | 4.72 ± 0.95 | 0.103 |
| Fasting blood glucose (mmol/L), mean ± SD | 5.50 ± 1.17 | 5.68 ± 1.20 | < 0.001 |
| Creatinine (μmol/L), mean ± SD | 67.92 ± 13.97 | 72.21 ± 12.79 | < 0.001 |
| Total protein (g/L), mean ± SD | 70.14 ± 5.39 | 69.82 ± 5.25 | 0.031 |
| 25-hydroxyvitamin D3 (ng/mL), mean ± SD | 19.79 ± 6.01 | 20.13 ± 6.14 | 0.040 |
| Diseases [n (%)] | |||
| IgG-mediated food intolerance | 3781 (60.05) | 1132 (68.28) | < 0.001 |
| Helicobacter pylori infection | 2478 (39.36) | 378 (22.80) | < 0.001 |
| Metabolic syndrome | 3671 (58.31) | 1113 (67.13) | < 0.001 |
Multivariate analysis showed that the risk factors for RE included IgG-mediated FI and metabolic syndrome and that H. pylori infection was a protective factor for RE (Figure 1).
The x2 test was used to analyze IgG-mediated FI and H. pylori infection. The incidence of IgG-mediated FI was significantly lower in the H. pylori-infected group (1700/2856, 59.52%) than in the H. pylori-non-infected group (3213/5098, 63.02%; P = 0.002). The exposure variable was H. pylori infection, the outcome variable was RE, and IgG-mediated FI was used as the mediating variable in the mediation effect analysis. The results showed that the total effect of H. pylori infection on RE was -0.122064, the direct effect of H. pylori infection on RE was -0.119349, the mediation effect of H. pylori infection on RE through IgG-mediated FI was -0.002715, and the mediation effect accounted for 0.022242 of the total effect; the effects showed statistically significant differences (all P values < 0.05) (Table 2). IgG-mediated FI had a partially positive mediating effect on H. pylori infection in reducing the occurrence of RE.
| Indicators | Effect value | 95%CI | P value |
| Total effect | -0.122064 | -0.138193, -0.104456 | < 0.0001 |
| Average direct effect | -0.119349 | -0.135905, -0.102256 | < 0.0001 |
| Average mediation effect | -0.002715 | -0.004145, -0.000316 | 0.0200 |
| Average mediation effect percentage | 0.022242 | 0.002679, 0.034859 | 0.0200 |
The x2 test was used to analyze metabolic syndrome and H. pylori infection; the incidence of metabolic syndrome was significantly higher in the H. pylori-infected group (1767/2856, 61.87%) than in the H. pylori-non-infected group (3017/5098, 59.18%; P = 0.019). The exposure variable was H. pylori infection, the outcome variable was RE, and metabolic syndrome was used as the mediating variable for the mediation effect analysis. The results showed that the total effect of H. pylori infection on RE was -0.121571, the direct effect of H. pylori infection on RE was -0.122715, the mediation effect of H. pylori infection on RE through metabolic syndrome was 0.001144, and the mediation effect accounted for -0.009413 in the total effect; the effects showed statistically significant differences (all P-values < 0.05) (Table 3). Metabolic syndrome had a partially negative mediating effect on H. pylori infection in reducing the occurrence of RE.
| Indicators | Effect value | 95%CI | P value |
| Total effect | -0.121571 | -0.138064, -0.104781 | < 0.0001 |
| Average direct effect | -0.122715 | -0.139268, -0.105907 | < 0.0001 |
| Average mediation effect | 0.001144 | 0.000182, 0.002307 | 0.0220 |
| Average mediation effect percentage | -0.009413 | -0.018807, -0.001479 | 0.0220 |
RE, also known as erosive esophagitis, is a type of GERD with a complex etiology and an increasing prevalence. Research on its pathogenesis has progressed in recent years, ranging from the invasion of reflux and the destruction of the anti-reflux barrier to immunity, inflammation and biomarkers on a systemic scale[14]. RE demonstrates a high rate of relapse after treatment and is associated with esophageal strictures, bleeding, carcinogenesis, and other complications, which affect the quality of life and long-term prognosis of patients. Therefore, it is critical to explore the risk factors for RE and implement early clinical interventions. The population prevalence of FI is 20%[4], resulting from reactions to food components, non-celiac gluten sensitivity, or defects in enzymes and transport[15]. Furthermore, food may induce inflammatory responses, which are crucial in the development of RE. Metabolic syndrome and its associated components are also risk factors for RE[6], and H. pylori infection plays an essential role in RE[8,10]. Therefore, the occurrence of RE may be associated with food reactions, H. pylori infection, and metabolic syndromes. This study focused on three relatively common clinical diseases, evaluated risk factors associated with the occurrence of RE, and explored the pathogenic effects of IgG-mediated FI, H. pylori infection, and metabolic syndrome on RE to provide a clinical basis for the etiology of the disease; this is crucial knowledge to prevent the occurrence of reflux and improve patients' quality of life.
Our study is the first to investigate the effects of IgG-mediated FI on RE in a large population and confirm that IgG-mediated FI contributes to the development of RE. Studies have suggested that diet may activate the immune system and alter the levels of inflammatory markers in the blood; increased pro-inflammatory cytokines and decreased anti-inflammatory cytokines have been linked to diet-associated immune system activation[16]. Furthermore, the promotion and release of inflammatory cytokines and cells during inflammatory reactions involve continuous interdependent cellular communication, which is crucial in the pathogenesis of GERD[1,17]. When FI occurs, a decrease in anti-inflammatory cytokines and the release of inflammatory cells and factors cause reflux; however, further studies are necessary to better understand this phenomenon. Most patients with FI are asymptomatic, which results in such patients receiving less attention[18]. In this study, the prevalence of IgG-mediated FI, which is crucial in the development of RE, was 61.77%; therefore, it is imperative to critically evaluate IgG-mediated FI. Screening for IgG-mediated FI in patients diagnosed with RE or in high-risk groups and avoiding related foods that induce FI in daily life may be crucial in reducing the occurrence of reflux.
This study suggests that metabolic syndrome is a risk factor for RE, consistent with other studies[6,7]. The pathological commonality between the diseases associated with metabolic syndrome is a chronic low-grade inflammation state, which activates various inflammatory signal cascades and leads to the activation of NF-kB, Jun amino-terminal kinase, and inflammatory processes; this results in the recruitment of immune cells, which further aggravates inflammation[19,20]. The mechanism by which metabolic syndrome promotes the development of RE may be associated with the mechanical effect of increased abdominal pressure caused by a large amount of adipose tissue. Furthermore, substances secreted by adipose tissue, such as TNF-α, IL-6, leptin, and insulin-like growth factor-1, significantly contribute to the occurrence, development, and carcinogenesis of GERD[6]. Therefore, controlling metabolic factors such as blood glucose, blood pressure, lipid levels, and body weight can significantly reduce the occurrence of RE.
In this study, H. pylori infection was considered a protective factor against RE. The possible mechanism has been analyzed: H. pylori infection causes gastric mucosal atrophy and acid production damage, and bacterial ammonia production neutralizes acidic substances and reduces acid reflux. Additionally, it leads to the activation of vagal receptors on the fundus and cardia, consequently increasing serum gastrin secretion, improving lower esophageal sphincter pressure, reducing reflux of gastric contents, and protecting the esophageal mucosa. The effect of H. pylori infection on reflux is controversial; some studies have suggested that H. pylori infection is a risk factor for RE, while others suggested that there is no relationship between them[8-10]. The possible reasons for the controversy between this study and other studies could be that other studies mostly assessed the impact of H. pylori eradication on RE through quality of life and reflux symptoms, and the drugs used to eradicate H. pylori significantly reduced the symptoms of reflux, which affected accuracy and confidence in the results. Therefore, screening for H. pylori infection is not routinely required for the clinical treatment of RE, and the treatment options for H. pylori infection are not indicated as anti-reflux therapy; this is consistent with the recommendations of the 2013 guidelines of the American College of Gastroenterology[21].
This study confirmed the effect of IgG-mediated FI and metabolic syndrome in reducing the occurrence of RE caused by H. pylori infection. A study conducted in southwest China revealed that H. pylori infection was negatively associated with food-specific IgG in eggs, milk, and wheat; this is consistent with the results of this study[22]. H. pylori infection stimulates the production of Foxp3+ regulatory T cells, which have strong immunosuppressive properties, control the degree of self and non-autoantigen reactions, and produce immune tolerance, consequently protecting against H. pylori infection. Immune tolerance suppresses the immune response caused by FI, which reduces the occurrence of RE. In people with FI, increased intestinal permeability allows food substances to enter the circulation; concurrently, the immune system recognizes certain food molecules as harmful substances and initiates an immune response to these substances, resulting in food-specific IgG[23], which further activates the immune effect and enhances the protective effect of H. pylori infection against the occurrence of RE. Therefore, IgG-mediated FI reduces the incidence of RE in patients with H. pylori infection. In addition, the results showed that H. pylori infection increased the incidence of metabolic syndrome. H. pylori infection can upregulate the expression of various inflammatory factors (including C-reactive protein, tumor necrosis factor, and various interleukins), promote insulin resistance, and initiate metabolic syndromes[24]. Metabolic syndrome results in chronic over-nutrition and excess energy, exceeding the metabolic capacity of tissues, which leads to metabolic stress and weakens the protective effect of H. pylori infection against reflux. Therefore, metabolic syndrome increases the incidence of RE in patients with H. pylori infection.
Lifestyle modification, dietary control, and pharmacotherapy are the primary treatment options for RE; however, the complex pathogenesis of RE and the critical role of the immuno-inflammatory cascade in its development may complicate the management of symptoms and increase susceptibility to relapse after treatment. This study evaluated the risk factors for RE and explored the effects of IgG-mediated FI, H. pylori infection, and metabolic syndrome on RE. Patients with IgG-mediated FI and metabolic syndrome were at a higher risk of RE; however, patients with H. pylori infection had a lower risk of RE. IgG-mediated FI reduced the risk of RE in patients with H. pylori infection; however, metabolic syndrome increased the risk of RE in patients with H. pylori infection. Therefore, in the management of RE, preventing the induction of IgG-mediated FI and optimal diagnosis and treatment of metabolic syndrome may reduce the occurrence of RE; this represents a strategy to achieve prevention and early treatment of RE in the future.
This was a single-center retrospective clinical study, and the test results showed certain deviations. Therefore, prospective and multicenter studies should be conducted to evaluate the factors influencing RE. The mechanisms underlying the effects of IgG-mediated FI, H. pylori infection, and metabolic syndrome in RE should be evaluated regarding the molecular perspective and the associated pathways.
Patients with IgG-mediated FI and metabolic syndrome are at a higher risk of RE; however, patients with H. pylori infection have a lower risk of RE. IgG-mediated FI reduces the risk of RE in patients with H. pylori infection; however, metabolic syndrome increases the risk of RE in patients with H. pylori infection.
Reflux esophagitis has an increasing prevalence and complex and diverse symptoms. Identifying its risk factors is crucial to understanding the etiology, prevention, and management of the disease.
The occurrence of reflux esophagitis may be associated with food reactions, Helicobacter pylori (H. pylori) infection, and metabolic syndromes.
To investigate the risk factors for reflux esophagitis and analyze the effects of IgG-mediated food intolerance, H. pylori infection, and metabolic syndrome on reflux esophagitis.
This retrospective study analyzed endoscopic images of outpatients attending the Second Medical Center of PLA General Hospital between 2017 and 2021, classified them into non-RE and RE groups, and further explored the differences in IgG-mediated food intolerance, H. pylori infection, and metabolic syndrome of the different groups.
In 7954 outpatients, the prevalence of reflux esophagitis was 20.84%.
Patients with IgG-mediated food intolerance and metabolic syndrome are at higher risk of developing reflux esophagitis, while those with H. pylori infection are at lower risk. IgG-mediated FI reduces the risk of RE in patients with H. pylori infection, while metabolic syndrome increases the risk of RE in these patients.
In the management of RE, preventing the induction of IgG-mediated FI and optimal diagnosis and treatment of metabolic syndrome may reduce the occurrence of RE.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ushiku T, Japan S-Editor: Gong ZM L-Editor: A P-Editor: Yu HG
| 1. | Huo X, Agoston AT, Dunbar KB, Cipher DJ, Zhang X, Yu C, Cheng E, Zhang Q, Pham TH, Tambar UK, Bruick RK, Wang DH, Odze RD, Spechler SJ, Souza RF. Hypoxia-inducible factor-2α plays a role in mediating oesophagitis in GORD. Gut. 2017;66:1542-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 2. | Fass R, Boeckxstaens GE, El-Serag H, Rosen R, Sifrim D, Vaezi MF. Gastro-oesophageal reflux disease. Nat Rev Dis Primers. 2021;7:55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 103] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 3. | Maret-Ouda J, Markar SR, Lagergren J. Gastroesophageal Reflux Disease: A Review. JAMA. 2020;324:2536-2547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 203] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 4. | Tuck CJ, Biesiekierski JR, Schmid-Grendelmeier P, Pohl D. Food Intolerances. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 5. | Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol. 2006;48:677-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 600] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 6. | Fu S, Xu M, Zhou H, Wang Y, Tan Y, Liu D. Metabolic syndrome is associated with higher rate of gastroesophageal reflux disease: a meta-analysis. Neurogastroenterol Motil. 2022;34:e14234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Niigaki M, Adachi K, Hirakawa K, Furuta K, Kinoshita Y. Association between metabolic syndrome and prevalence of gastroesophageal reflux disease in a health screening facility in Japan. J Gastroenterol. 2013;48:463-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Zhao T, Liu F, Li Y. Effects of Helicobacter pylori eradication on esophageal motility, esophageal acid exposure, and gastroesophageal reflux disease symptoms. Front Cell Infect Microbiol. 2023;13:1082620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Hirata K, Suzuki H, Matsuzaki J, Masaoka T, Saito Y, Nishizawa T, Iwasaki E, Fukuhara S, Okada S, Hibi T. Improvement of reflux symptom related quality of life after Helicobacter pylori eradication therapy. J Clin Biochem Nutr. 2013;52:172-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Yucel O. Interactions between Helicobacter pylori and gastroesophageal reflux disease. Esophagus. 2019;16:52-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Coucke F. Food intolerance in patients with manifest autoimmunity. Observational study. Autoimmun Rev. 2018;17:1078-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr; International Diabetes Federation Task Force on Epidemiology and Prevention; Hational Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8720] [Cited by in RCA: 10540] [Article Influence: 658.8] [Reference Citation Analysis (0)] |
| 13. | Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton J, Graham DY, Hunt R, Moayyedi P, Rokkas T, Rugge M, Selgrad M, Suerbaum S, Sugano K, El-Omar EM; European Helicobacter and Microbiota Study Group and Consensus panel. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2220] [Cited by in RCA: 1980] [Article Influence: 247.5] [Reference Citation Analysis (1)] |
| 14. | Hu JY, Lv M, Zhang KL, Qiao XY, Wang YX, Wang FY. Evaluating the causal relationship between human blood metabolites and gastroesophageal reflux disease. World J Gastrointest Oncol. 2023;15:2169-2184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (10)] |
| 15. | Lomer MC. Review article: the aetiology, diagnosis, mechanisms and clinical evidence for food intolerance. Aliment Pharmacol Ther. 2015;41:262-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 16. | Ohtsuka Y. Food intolerance and mucosal inflammation. Pediatr Int. 2015;57:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Hait EJ, McDonald DR. Impact of Gastroesophageal Reflux Disease on Mucosal Immunity and Atopic Disorders. Clin Rev Allergy Immunol. 2019;57:213-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Turnbull JL, Adams HN, Gorard DA. Review article: the diagnosis and management of food allergy and food intolerances. Aliment Pharmacol Ther. 2015;41:3-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 160] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 19. | Catrysse L, van Loo G. Inflammation and the Metabolic Syndrome: The Tissue-Specific Functions of NF-κB. Trends Cell Biol. 2017;27:417-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 229] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 20. | Grandl G, Wolfrum C. Hemostasis, endothelial stress, inflammation, and the metabolic syndrome. Semin Immunopathol. 2018;40:215-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 211] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 21. | Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108:308-28; quiz 329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1136] [Cited by in RCA: 1117] [Article Influence: 93.1] [Reference Citation Analysis (0)] |
| 22. | Liu Y, Shuai P, Liu YP, Li DY. Association between Helicobacter pylori infection and food-specific immunoglobulin G in Southwest China. World J Clin Cases. 2021;9:9815-9824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 23. | Shakoor Z, AlFaifi A, AlAmro B, AlTawil LN, AlOhaly RY. Prevalence of IgG-mediated food intolerance among patients with allergic symptoms. Ann Saudi Med. 2016;36:386-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Cheng DD, He C, Ai HH, Huang Y, Lu NH. The Possible Role of Helicobacter pylori Infection in Non-alcoholic Fatty Liver Disease. Front Microbiol. 2017;8:743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |