Published online Feb 28, 2024. doi: 10.3748/wjg.v30.i8.806
Peer-review started: December 10, 2023
First decision: December 27, 2023
Revised: December 27, 2023
Accepted: January 31, 2024
Article in press: January 31, 2024
Published online: February 28, 2024
Processing time: 77 Days and 21.6 Hours
Approximately 50%-70% of patients with hepatocellular carcinoma experience recurrence within five years after curative hepatic resection or ablation. As a result, many patients receive adjuvant therapy after curative resection or ablation in order to prolong recurrence-free survival. The therapy recommended by national guidelines can differ, and guidelines do not specify when to initiate adjuvant therapy or how long to continue it. These and other unanswered questions around adjuvant therapies make it difficult to optimize them and determine which may be more appropriate for a given type of patient. These questions need to be addressed by clinicians and researchers.
Core Tip: Several questions need to be addressed by clinical researchers about the use of adjuvant therapy to prolong recurrence-free survival of patients with hepatocellular carcinoma following potentially curative treatment.
- Citation: Zhong JH. Adjuvant therapy for hepatocellular carcinoma: Dilemmas at the start of a new era. World J Gastroenterol 2024; 30(8): 806-810
- URL: https://www.wjgnet.com/1007-9327/full/v30/i8/806.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i8.806
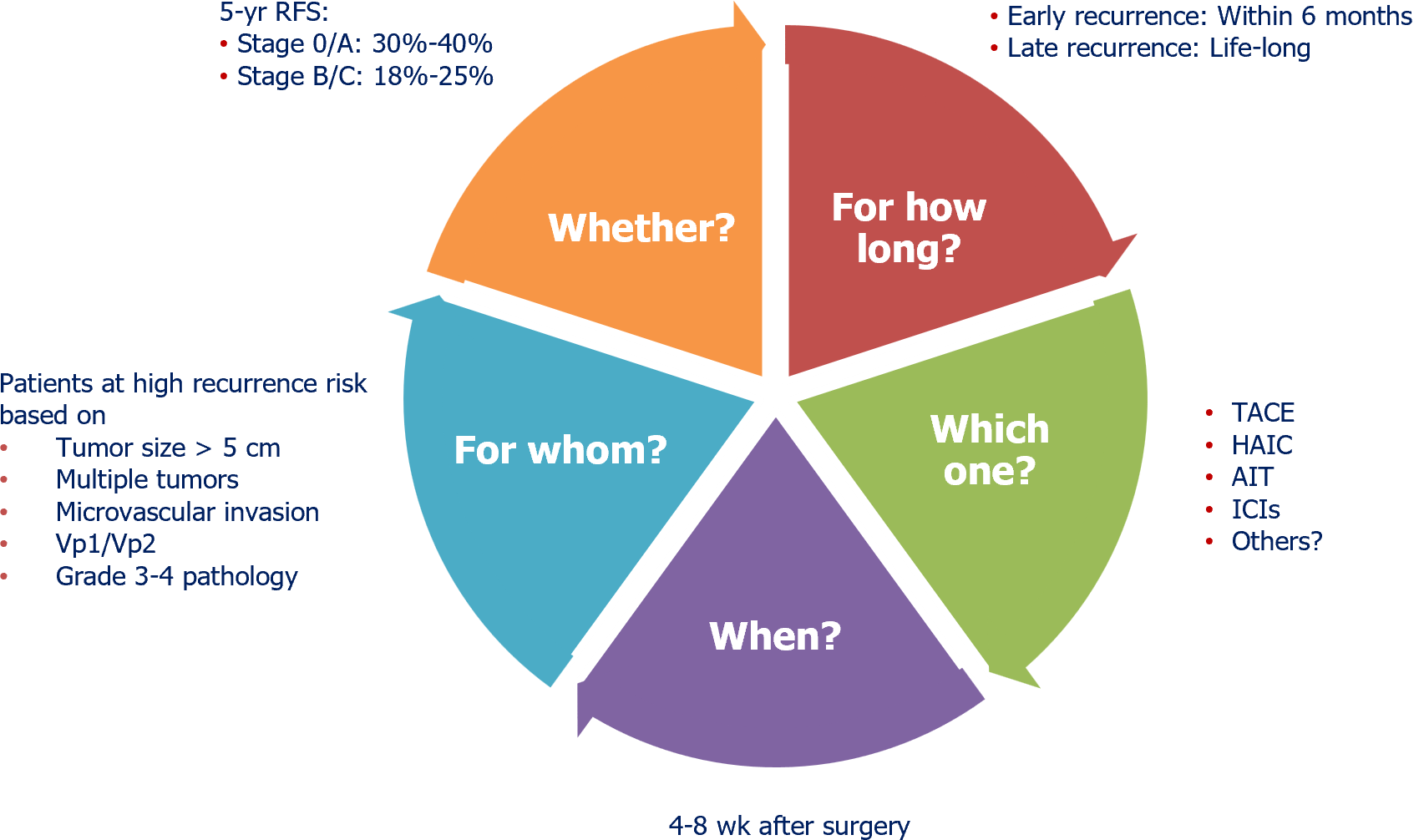
Primary or recurrent hepatocellular carcinoma (HCC) in certain patients can be treated through potentially curative hepatic resection or local ablation[1,2], which is typically defined as complete resection of the tumor, return of alpha fetoprotein levels to normal, and no sign of recurrence 4-8 wk later on contrast-enhanced computed tomography or magnetic resonance imaging[3]. Unfortunately, 50%-70% of patients experience intra- or extrahepatic metastases within five years after such procedures, and these metastases are the most frequent cause of HCC-related death[1,2]. For example, patients with primary HCC in the “very early” or “early” stages according to the Barcelona Clinic Liver Cancer staging system show 5-year recurrence rates of 40.7% after hepatectomy and 29.3% after local ablation[4], and the rate after hepatectomy falls to 18%-25% if the HCC is “intermediate” or “advanced”[5].
Therefore many patients are given adjuvant therapy after curative resection or ablation in order to prolong recurrence-free survival. However, international consensus is lacking about many aspects of adjuvant therapy, including which is the best type for a given type of patient, when it should be performed, and how long it should last. The question has even been raised whether adjuvant therapy is effective at all in certain contexts. These are important questions that need to be addressed through well-designed research and informed discussion.
Adjuvant therapy increases treatment costs and risks of adverse events, so it should not be administered routinely to all patients whose tumors have been completely removed by resection or ablation. Instead, national guidelines recommend it for certain types of patients. The Chinese Liver Cancer staging system[3] and the American Association for the Study of Liver Diseases[1] recommend it for patients with factors associated with high risk of recurrence, such as tumor size > 5 cm, presence of > 3 tumors, micro- or macrovascular invasion, or poor tumor differentiation.
Whether these guidelines are optimal is questionable, in light of evidence identifying additional potential risk factors, such as the absence of a tumor capsule, tumor rupture, narrow resection margin (≤ 2 cm) and alpha fetoprotein ≥ 400 ng/mL[1,3]. In addition, the risk factors in guidelines have been associated primarily with recurrence within 6 months after curative treatment, meaning that the guidelines neglect liver cirrhosis and chronic hepatitis, which have been linked primarily to late recurrence[6,7]. The evidence base for all these risk factors should be expanded to the point that they can be taken into account in future versions of guidelines. Another question that should be addressed is whether adjuvant therapy is effective for all etiologies of HCC: For example, immune checkpoint inhibitors may offer limited benefit to patients with HCC linked to non-alcoholic steatohepatitis[8].
Based on extensive evidence from randomized controlled trials, Chinese Liver Cancer guidelines mention several adjuvant therapies as effective: Transarterial chemoembolization, hepatic arterial infusion chemotherapy, molecular targeted drugs, and adoptive immunotherapy[3]. In contrast, guidelines from South Korea[9] and the United States[1] do not recommend adjuvant transarterial chemoembolization or hepatic arterial infusion chemotherapy, although the South Korean guidelines do recommend adoptive immunotherapy based on strong evidence, while the United States guidelines mention immune checkpoint inhibition for the first time in the latest revision. Guidelines from the United States and China, but not South Korea, recommend adjuvant antiviral therapy with tenofovir or entecavir for patients with HCC related to chronic infection with hepatitis B virus[1,3].
The evidence base for the efficacy of some adjuvant therapies remains to be solidified. Only one randomized controlled trial has explored adjuvant use of the tyrosine kinase inhibitor sorafenib[10], reporting no significant benefit on recurrence-free or overall survival relative to placebo, and randomized trials of other molecular targeted drugs are ongoing. For example, an evaluation of the adjuvant combination of atezolizumab and bevacizumab has yet to reach the endpoint of median recurrence-free survival[11], although one study suggested that the two therapeutic antibodies may synergize to inhibit tumor angiogenesis, regulatory T proliferation and myeloid cell inflammation[12]. One study has suggested that molecular targeted drugs can potentiate adjuvant immune checkpoint blockade[11]. The current landscape of clinical evidence does not provide multiple, clearly effective treatments based on molecular targeted drugs, which makes it difficult to identify which ones may be optimal for given types of patient. Several network meta-analyses have examined the landscape but failed to converge on clear recommendations for clinical practice because of heterogeneity among patient populations and treatment protocols.
This is a key consideration given the inevitable side effects of adjuvant therapy, yet no major guidelines recommend a particular start time. Most randomized controlled trials initiate it 4-8 wk after curative resection. This question should be explored in clinical trials, which should consider that the optimal timing of initiation likely depends on perioperative complications, wound healing, residual liver function, and patient characteristics such as performance status and comorbidities.
The evidence base around immune checkpoint blockade and molecular targeted drugs does not clearly indicate minimal or maximal duration of adjuvant treatment. In one trial, sorafenib therapy was scheduled for 48 months, but it lasted closer to 12-13 months because of the lack of efficacy and high frequency of adverse events[10]. In another trial, the combination of atezolizumab and bevacizumab was scheduled for 12 months, and it lasted a median of 11 months[11]. This duration may be too long, at least for certain types of patients: Immune checkpoint inhibitor therapy for 6 months was sufficient to prolong recurrence-free survival in one prospective study[13], and median progression-free survival was shorter than 12 months among patients with unresectable HCC who were treated with immune checkpoint inhibitors alone or together with molecular targeted drugs[14,15].
These observations suggest that 12 months of immune checkpoint inhibition may be excessive and, in any case, that the duration of adjuvant therapy will need to be determined based on its mechanism(s) of action. The indications for transarterial therapy, molecular targeted drugs, adoptive immunotherapy and immune checkpoint inhibition were originally formulated for patients with unresectable HCC, so they may not be optimal for patients whose disease is in an early, resectable stage and who are likely to survive long enough for late recurrence to be a concern. For example, patients with resectable disease who are chronically infected with hepatitis B virus should probably continue antiviral therapy for the long term, perhaps even the rest of their lives[16-18].
The costs and adverse effects of adjuvant therapy dictate that clinical researchers better define what therapies should be administered to which patients when and for how long (Figure 1), and that the best evidence be integrated into the next versions of consensus guidelines. This task becomes more urgent as more medical centers administer molecular targeted drugs and immune checkpoint inhibitors to HCC patients[19,20]. Eventually guidelines will also need to take stock of the growing use of neoadjuvant and “conversion” therapies, which promise to make potentially curative treatment accessible to patients with traditionally unresectable HCC.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jackson T, United States S-Editor: Wang JJ L-Editor: A P-Editor: Chen YX
| 1. | Singal AG, Llovet JM, Yarchoan M, Mehta N, Heimbach JK, Dawson LA, Jou JH, Kulik LM, Agopian VG, Marrero JA, Mendiratta-Lala M, Brown DB, Rilling WS, Goyal L, Wei AC, Taddei TH. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology. 2023;78:1922-1965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 736] [Article Influence: 368.0] [Reference Citation Analysis (23)] |
| 2. | Federica C, Gianluca F, Margherita R, Federica P, Federica I, Andrea CG, Francesco De C, Massimo C, Luca A. Surgery for hepatocellular carcinoma and intrahepatic cholangiocarcinoma: milestone changes in the last two decades potentially affecting current guidelines. Hepatoma Res. 2023;9:13. [DOI] [Full Text] |
| 3. | Zhou J, Sun H, Wang Z, Cong W, Zeng M, Zhou W, Bie P, Liu L, Wen T, Kuang M, Han G, Yan Z, Wang M, Liu R, Lu L, Ren Z, Zeng Z, Liang P, Liang C, Chen M, Yan F, Wang W, Hou J, Ji Y, Yun J, Bai X, Cai D, Chen W, Chen Y, Cheng W, Cheng S, Dai C, Guo W, Guo Y, Hua B, Huang X, Jia W, Li Q, Li T, Li X, Li Y, Liang J, Ling C, Liu T, Liu X, Lu S, Lv G, Mao Y, Meng Z, Peng T, Ren W, Shi H, Shi G, Shi M, Song T, Tao K, Wang J, Wang K, Wang L, Wang X, Xiang B, Xing B, Xu J, Yang J, Yang Y, Ye S, Yin Z, Zeng Y, Zhang B, Zhang L, Zhang S, Zhang T, Zhang Y, Zhao M, Zhao Y, Zheng H, Zhou L, Zhu J, Zhu K, Shi Y, Xiao Y, Yang C, Wu Z, Dai Z, Cai J, Cai X, Shen F, Qin S, Teng G, Dong J, Fan J. Guidelines for the Diagnosis and Treatment of Primary Liver Cancer (2022 Edition). Liver Cancer. 2023;12:405-444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 194] [Reference Citation Analysis (0)] |
| 4. | Wang JH, Wang CC, Hung CH, Chen CL, Lu SN. Survival comparison between surgical resection and radiofrequency ablation for patients in BCLC very early/early stage hepatocellular carcinoma. J Hepatol. 2012;56:412-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 272] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 5. | Zhong JH, Ke Y, Wang YY, Li LQ. Liver resection for patients with hepatocellular carcinoma and macrovascular invasion, multiple tumours, or portal hypertension. Gut. 2015;64:520-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Li Z, Tan C, Liu X, Feng Z, Li K. Early and late recurrence after hepatectomy in patients with low-level HBV-DNA hepatocellular carcinoma under antiviral therapy. Infect Agent Cancer. 2022;17:56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 7. | Nevola R, Ruocco R, Criscuolo L, Villani A, Alfano M, Beccia D, Imbriani S, Claar E, Cozzolino D, Sasso FC, Marrone A, Adinolfi LE, Rinaldi L. Predictors of early and late hepatocellular carcinoma recurrence. World J Gastroenterol. 2023;29:1243-1260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 103] [Cited by in RCA: 115] [Article Influence: 57.5] [Reference Citation Analysis (3)] |
| 8. | Kara W, Anna Mae D, Cynthia AM. Challenges and barriers in hepatocellular carcinoma (HCC) surveillance for patients with non-alcoholic fatty liver disease (NAFLD). Hepatoma Res. 2023;9:11. [DOI] [Full Text] |
| 9. | Korean Liver Cancer Association (KLCA) and National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea Practice Guidelines for the Management of Hepatocellular Carcinoma. Korean J Radiol. 2022;23:1126-1240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 85] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 10. | Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, Cai J, Poon RT, Han KH, Tak WY, Lee HC, Song T, Roayaie S, Bolondi L, Lee KS, Makuuchi M, Souza F, Berre MA, Meinhardt G, Llovet JM; STORM investigators. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16:1344-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 787] [Article Influence: 78.7] [Reference Citation Analysis (0)] |
| 11. | Qin S, Chen M, Cheng AL, Kaseb AO, Kudo M, Lee HC, Yopp AC, Zhou J, Wang L, Wen X, Heo J, Tak WY, Nakamura S, Numata K, Uguen T, Hsiehchen D, Cha E, Hack SP, Lian Q, Ma N, Spahn JH, Wang Y, Wu C, Chow PKH; IMbrave050 investigators. Atezolizumab plus bevacizumab versus active surveillance in patients with resected or ablated high-risk hepatocellular carcinoma (IMbrave050): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2023;402:1835-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 222] [Article Influence: 111.0] [Reference Citation Analysis (0)] |
| 12. | Zhu AX, Abbas AR, de Galarreta MR, Guan Y, Lu S, Koeppen H, Zhang W, Hsu CH, He AR, Ryoo BY, Yau T, Kaseb AO, Burgoyne AM, Dayyani F, Spahn J, Verret W, Finn RS, Toh HC, Lujambio A, Wang Y. Molecular correlates of clinical response and resistance to atezolizumab in combination with bevacizumab in advanced hepatocellular carcinoma. Nat Med. 2022;28:1599-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 339] [Article Influence: 113.0] [Reference Citation Analysis (0)] |
| 13. | Li L, Wu PS, Liang XM, Chen K, Zhang GL, Su QB, Huo RR, Xie RW, Huang S, Ma L, Zhong JH. Adjuvant immune checkpoint inhibitors associated with higher recurrence-free survival in postoperative hepatocellular carcinoma (PREVENT): a prospective, multicentric cohort study. J Gastroenterol. 2023;58:1043-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 14. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 4682] [Article Influence: 936.4] [Reference Citation Analysis (2)] |
| 15. | Kelley RK, Rimassa L, Cheng AL, Kaseb A, Qin S, Zhu AX, Chan SL, Melkadze T, Sukeepaisarnjaroen W, Breder V, Verset G, Gane E, Borbath I, Rangel JDG, Ryoo BY, Makharadze T, Merle P, Benzaghou F, Banerjee K, Hazra S, Fawcett J, Yau T. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23:995-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 349] [Article Influence: 116.3] [Reference Citation Analysis (1)] |
| 16. | Massimo F, Mariacristina P, Michele M, Francesco Rosario Paolo I, Marianna C, Bruno C, Teresa Antonia S. Risk of hepatocellular carcinoma development in long-term nucles(t)ide analog suppressed patients with chronic hepatitis B. Hepatoma Res. 2023;9:3. [DOI] [Full Text] |
| 17. | Zhou J, Wang FD, Li LQ, Li YJ, Wang SY, Chen EQ. Antiviral Therapy Favors a Lower Risk of Liver Cirrhosis in HBeAg-negative Chronic Hepatitis B with Normal Alanine Transaminase and HBV DNA Positivity. J Clin Transl Hepatol. 2023;11:1465-1475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Huang DQ, Hoang JK, Kamal R, Tsai PC, Toyoda H, Yeh ML, Yasuda S, Leong J, Maeda M, Huang CF, Won Jun D, Ishigami M, Tanaka Y, Uojima H, Ogawa E, Abe H, Hsu YC, Tseng CH, Alsudaney M, Yang JD, Yoshimaru Y, Suzuki T, Liu JK, Landis C, Dai CY, Huang JF, Chuang WL, Schwartz M, Dan YY, Esquivel C, Bonham A, Yu ML, Nguyen MH. Antiviral Therapy Utilization and 10-Year Outcomes in Resected Hepatitis B Virus- and Hepatitis C Virus-Related Hepatocellular Carcinoma. J Clin Oncol. 2024;JCO2300757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Reference Citation Analysis (0)] |
| 19. | Mandlik DS, Mandlik SK, Choudhary HB. Immunotherapy for hepatocellular carcinoma: Current status and future perspectives. World J Gastroenterol. 2023;29:1054-1075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 67] [Article Influence: 33.5] [Reference Citation Analysis (3)] |
| 20. | Llovet JM, Kudo M, Merle P, Meyer T, Qin S, Ikeda M, Xu R, Edeline J, Ryoo BY, Ren Z, Masi G, Kwiatkowski M, Lim HY, Kim JH, Breder V, Kumada H, Cheng AL, Galle PR, Kaneko S, Wang A, Mody K, Dutcus C, Dubrovsky L, Siegel AB, Finn RS; LEAP-002 Investigators. Lenvatinib plus pembrolizumab versus lenvatinib plus placebo for advanced hepatocellular carcinoma (LEAP-002): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2023;24:1399-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 193] [Article Influence: 96.5] [Reference Citation Analysis (0)] |