Published online Feb 28, 2024. doi: 10.3748/wjg.v30.i8.779
Peer-review started: November 6, 2023
First decision: December 4, 2023
Revised: December 14, 2023
Accepted: January 29, 2024
Article in press: January 29, 2024
Published online: February 28, 2024
Processing time: 112 Days and 23.3 Hours
In this editorial, we comment on the article entitled “Advances and key focus areas in gastric cancer immunotherapy: A comprehensive scientometric and clinical trial review (1999-2023),” which was published in the recent issue of the World Journal of Gastroenterology. We focused on the results of the authors’ bibliometric analysis concerning gastric cancer immunotherapy, which they analyzed in depth by compiling the relevant publications of the last 20 years. Before that, we briefly describe the most recent data concerning the epidemiological parameters of gastric cancer (GC) in different countries, attempting to give an interpretation based on the etiological factors involved in the etiopathogenesis of the neoplasm. We then briefly discuss the conservative treatment (chemotherapy) of the various forms of this malignant neoplasm. We describe the treatment of resectable tumors, locally advanced neoplasms, and unresectable (advanced) cases. Special attention is given to modern therapeutic approaches with emphasis on immunotherapy, which seems to be the future of GC treatment, especially in combination with chemotherapy. There is also a thorough analysis of the results of the study under review in terms of the number of scientific publications, the countries in which the studies were conducted, the authors, and the scientific centers of origin, as well as the clinical studies in progress. Finally, an attempt is made to draw some con-clusions and to point out possible future directions.
Core Tip: Gastric cancer (GC) remains a major cause of morbidity and mortality worldwide. Conservative treatment in the form of chemotherapy has recently made remarkable advances, particularly in the field of immunotherapy. Immunotherapy of GC represents one of the most important fields of research worldwide today, particularly in China, the United States, and Japan, as well as in some Western European countries. Several treatment regimens have been approved and are being implemented with satisfactory results. Also, several treatment regimens are currently under investigation, which are expected to improve the disappointing prognosis of this malignant neoplasm.
- Citation: Triantafillidis JK, Konstadoulakis MM, Papalois AE. Immunotherapy of gastric cancer: Present status and future perspectives. World J Gastroenterol 2024; 30(8): 779-793
- URL: https://www.wjgnet.com/1007-9327/full/v30/i8/779.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i8.779
In this editorial, we comment on the article by Li et al[1] entitled "Advances and key focus areas in gastric cancer immunotherapy: A comprehensive scientometric and clinical trial review (1999-2023)" published in the recent issue of the World Journal of Gastroenterology[1]. Given the very interesting conclusions of the study regarding the number and type of publications, journals, centers, and countries of origin of the papers, the most recent data on the epidemiological parameters of gastric cancer (GC) and chemotherapy of the various types of this malignant neoplasm will be briefly described. Special attention is given to modern therapeutic approaches with particular emphasis on immunotherapy, which seems to be the future of GC treatment, mainly in combination with chemotherapy.
GC is a major cause of morbidity and mortality worldwide particularly in South and Central Asian countries. GC is a complex and heterogeneous disease whose etiopathogenesis includes many risk factors. Despite the recent trend of decreasing incidence, prevalence, and mortality in several countries, GC is one of the most common human malignancies with high mortality. This is particularly true for some Asian countries such as China in which it represents the third most common malignancy. The spatial differences in GC incidence and mortality are very evident, especially in developing regions of the world where the adoption of effective prevention and early diagnosis strategies is evident[2]. Its etiology is multifactorial including environmental, genetic, and infectious factors. The prognosis even today is still disappointing with an overall 5-year survival of less than 5%.
GC remains one of the most important malignant causes of morbidity and mortality worldwide. The prevalence and incidence of the disease change over time. Thus, while in some countries and demographic groups there is a significant decrease, in other regions of the world epidemiological parameters remain stable or even increase such as in Asia. Changes have also been observed in GC incidence rates in the two main sites of occurrence (non-cardia and cardia), in countries where the rates of the disease are increasing[3]. The reasons why a decrease in GC incidence has been observed are primarily related to the introduction of the electric refrigerator through which good food preservation and subsequent inhibition of microbial proliferation, along with other factors such as the reduction in the consumption of smoked, salted, and canned foods, and an increase in the consumption of fresh fruits and vegetables, have reduced the dietary factors that promote gastric carcinogenesis, alongside with the reduction in smoking in Western countries. On the other hand, excessive consumption of antibiotics reduced Helicobacter pylori (H. pylori) infection, a major contributor to gastric carcinogenesis. Nevertheless, GC is not only caused only by H. pylori as only 2%-5% of individuals with H. pylori infection develop GC[4].
Regarding the epidemiological parameters, it is estimated that about one million patients are diagnosed with GC every year worldwide[5]. It is the 7th most common cancer and the 6th most commonly diagnosed cancer in the world[5]. The countries with the highest GC incidence rates (more than 15 new cases/100000 population) are Mongolia, Japan, Korea, Tajikistan, and Kyrgyzstan. Countries with higher than average rates include Eastern European countries, Turkey, Mali, Portugal, Peru, Ecuador, and Colombia. Lower rates (less than 5 new cases/100000 population) are observed in Western countries such as France, United States, Canada, United Kingdom, Norway, Sweden, Norway, Sweden, Australia, and Central African countries, although this could partly be attributed to low diagnostic capacity. The disease mainly affects males. In total, 66% of new GC cases diagnosed in 2020 were male. The highest cumulative GC risk is observed in East Asia (2.64%) and the lowest in Southern Africa (0.42%)[6]. East Asia has the highest incidence of GC, followed by Eastern and Central Europe. Enteric type of GC is more common in Caucasians, while GC located in the gastric cardia is less frequent in Africa and Latin America. Tumor protein p53 (TP53), low-density lipoprotein receptor-related protein 1B, and AT-rich interactive domain-containing protein 1A (ARID1A) are the genes that are most frequently altered in all population groups. Finally, African patients are younger, and the proportion of women is higher than men[7].
In China, 396500 new cases of GC were diagnosed (276300 men, 120200 women) in 2016, corresponding to 1086 newly diagnosed cases every day[8]. In recent years, it has become apparent that both the incidence and mortality of GC in China have shown a downward trend, probably due to the implementation of effective governmental prevention strategies and changes in many aspects of individual life adopted by the Chinese people. As is the case in most countries of the world, H. pylori infection, poor dietary habits, smoking, and family history of GC are the main risk factors for GC in China[9]. The overall crude and age-standardized rates of incidence of GC by the standard Chinese population are 28.7 per 100000 and 17.6 per 100000, respectively. The incidence in males (25.1 per 100000) is 2.5 times higher than that in females (10.3 per 100000 population). The incidence of GC varies in different parts of this huge country as a result of different living conditions and environmental factors. For example, the age-standardized rate of GC is higher in Northwest China, lower in East and Central China, and even lower in South China. Apparently due to environmental factors the incidence of the disease was higher in rural compared to urban areas[10]. It would be of interest to compare the epidemiological parameters of two great Asian countries, namely Japan and China, where half of the world's GC cases are diagnosed. Epidemiological data suggest that there are divergent trends in GC incidence in the two countries, although without obvious or adequate explanation. It appears that the trends in age-standardized incidence rates for GC for both sexes decreased significantly but the decrease was greater in Japan. In both countries, the risk of GC increases with age. The two countries had divergent trends over the study period, with the risk of GC decreasing in Japanese men but increasing among Chinese men[11].
GC is the fourth most common cause of cancer death worldwide, with 783793 deaths in 2020, of which 502788 were in men. Asia has the highest mortality (575206), followed by Europe (96997), Latin America, and the Caribbean (53392 deaths). The annual percentage change in GC mortality decreased between 1980 and 2005 at a rate of 3%-4% in Europe, Korea, Japan, and Australia[12]. In Latin America, the annual percentage change is lower but stable: (Brazil and Chile-1.6%, Argentina and Mexico-2.3%). Currently, the only country showing an increasing trend in male mortality is Thailand (annual percentage change + 3.92). In the United States, the 5-year survival rate for GC is 31% since the majority of patients already have metastases at the time of diagnosis. However, when there are no metastases at the time of diagnosis, the survival rate increases to 67%[13]. Disease prognosis and survival are better in Asian patients compared to Caucasians[14].
It is known that the tumor suppressor gene TP53 is the gene that shows the highest number of mutations in GC cases. Furthermore, significant mutations have been observed in the kirsten rats sarcomaviral oncogene homolog, beta-catenin, and phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha oncogenes as well as in the tumor suppressor genes mothers against decapentaplegic homolog 4, and adenomatous polyposis coli. Somatic copy number alterations activate oncogenes and inactivate tumor suppressor genes in GC patients. Alterations of RTK/RAS/mitogen-activated protein kinase signaling pathways including human epidermal growth factor receptor 2 (HER2), epidermal growth factor receptor (EGFR), mesenchymal epithelial transition, and fibroblast growth factor receptor (FGFR2) şi RAS are observed in 30%-40% of GC cases. Amplification of cell cycle regulatory genes CCND1, cyclin E1, and cyclin-dependent kinase 6 has also been observed[15].
Mutations in the cadherin 1 (CDH1) gene encoding the cell adhesion protein E-cadherin are currently considered to be the cause of hereditary diffuse-type GC (HDGC), through disruption of cell-to-cell adhesion and activation of oncogenic signaling pathways. In carriers of this mutated gene with a positive history of HDGC, prophylactic gastrectomy is recommended, although the use of systematic protocols with multiple biopsies may be an alternative approach to the problem. Through animal models, molecular drivers have been established in the gastric epithelium of experimental animals with loss of E-cadherin. Based on the above, it is possible to adopt new prevention strategies as well as strategies for targeted treatment in patients with HDGC[16]. DNA methylation is another pathogenic factor that can cause genetic alterations. Methylation of CDH1, runt-related transcription factor 3, p16, and human mutL homolog 1 has been described in GC patients[17]. Finally, mutations in the tumor suppressor gene ARID1A, which encodes the SW1/SNF chromatin remodeling complex, have been described. Mutations in interleukin 17 (IL-17) and IL-10 have been described in Asian populations.
Atrophic gastritis and intestinal metaplasia: They are the most important precancerous conditions. Chronic inflammation induces the transcription factor nuclear factor kappa B, one of the most important mediators of inflammation. The inflammatory process promotes oxidative stress and the production of reactive oxygen species and nitrosamines by leukocytes and macrophages[18].
Ménétrier's disease: It is a hypertrophic gastropathy, characterized by the huge growth of mucus cells in the gastric mucosa. The risk of malignant potential exceeds 10%[19].
Gastric remnant: GC in the gastric remnant is defined as GC occurring at least 5 years after partial gastrectomy. The mechanism of carcinogenesis is related to postoperative hypochlorhydria, which results in bacterial overgrowth with the production of nitroso enzymes. Endoscopic monitoring is the best strategy for prevention and early diagnosis[20].
Gastric polyps: Gastric polyps are usually an incidental finding during gastroscopy. Polyps are removed endoscopically and tested histologically to exclude malignancy. Hyperplastic polyps may undergo carcinomatous transformation through the dysplasia/carcinoma sequence. The risk of malignancy is increased in sessile polyps and advanced polyps (polyps larger than 1 cm). It is estimated that 8%-59% of gastric adenomas occur concomitantly with GC[21].
Gastric microbiota: During the evolutionary course of GC, changes in the microbiota of the stomach are observed. Advances in next-generation sequencing and metagenomics have shown that the stomach microbiota is diverse and includes five major phyla. It appears that the positivity of H. pylori influences other bacterial communities in terms of richness and evenness. H. pylori is a fundamental risk factor for GC development, especially strains positive for Cag pathogenicity island and the CagA oncoprotein. Existing data support that there are differences in the microbiota of patients with GC and patients with precancerous conditions with a decrease in microbial diversity and an increase in the presence of microbes that can produce nitrite. Interestingly, the data support that in GC patients there is an increase in the oral microbiota. All of these data suggest that the gastric microbiota in addition to H. pylori plays a role in the latter stages of gastric carcinogenesis[22]. Furthermore, data derived from experiments in transgenic mouse models with insulin-gastrin transplantation and human gastric microbiome support the view linking the gastric microbiota to GC development[19,23]. Finally, it appears that the colonic microbiota favors GC development through various metabolites. Various antimicrobial therapies, probiotics, phages, dietary elements, and fecal transplantation are being studied regarding their role in GC[24].
H. pylori infection: The mechanisms of carcinogenesis associated with H. pylori infection are based, on the one hand, on the existence and maintenance of chronic inflammation and, on the other hand, on the presence of H. pylori-specific infectious agents, that have the potential to damage gastric epithelial cell DNA and promote genomic instability[25]. Neutrophil migration is triggered by H. pylori. Neutrophils induce nitric oxide synthase as well as reactive oxygen species which in turn lead to DNA damage[26]. DNA damage triggers the process of apoptosis, which manifests as gastric atrophy. Eradication of the infection normalizes the apoptosis rate.
Epstein-Barr virus infection: It is well established that a proportion of GC is etiologically related to Epstein-Barr virus (EBV). In a meta-analysis of 220 studies by Hirabayashi et al[27] that included more than 68000 GC cases, it was found that the frequency of virus detection in cancer cells exceeded 7.5% and was higher in males than females in the diffuse compared to the intestinal type and in the proximal compared to the distal region. Furthermore, EBV prevalence reached the level of 75.9% among lymphoepitheliomatous GC and 26.3% among GC cases in gastric remnant. Assuming that a causal relationship between EBV and GC does indeed exist and based on GLOBOCAN 2020 data, the authors estimated that primary prevention, with the development of an effective EBV vaccine, could prevent 81000 EBV-associated GC cases worldwide annually[27]. It has also been confirmed that EBV induces GC through DNA methylation[28]. EBV-associated GC cells are derived from an EBV-infected monocytic clone. In addition, the vast majority of GC patients have a history of H. pylori infection. It appears that H. pylori infection may influence the development of EBV-associated GC, a subtype of GC. Therefore, it remains unclear whether H. pylori infection is a cofactor for EBV-induced gastric carcinogenesis or whether H. pylori and EBV act independently in the development of GC. The possibilities are either that EBV infection participates in H. pylori-induced GC tumorigenesis, or that H. pylori infection accelerates EBV-initiated carcinogenesis[29].
Diet: Excessive salt in the daily diet has a direct carcinogenic effect[30]. There seems to be a synergistic effect of salt consumption and H. pylori infection in GC patients. The decrease in the incidence of GC has been attributed to efficient food preservation over the last 50 years, as the refrigerator replaced salt as the main method of preservation[31]. Nitrates are chemical compounds containing the NO group. These chemical compounds are not only ingested from food and smoking but also from endogenous sources. Processed meats and dairy products are rich in nitrates. After absorption, nitrates react with amines, amides, or amino acids to form N-nitro compounds. High levels of N-Nitro in the stomach have been associated with the presence of advanced precancerous lesions[32]. In 2015, the World Health Organization classified processed meats as a Group 1 carcinogen. It even appears that fried foods are etiopathogenetically associated with GC. In a meta-analysis of 18 studies, Zhang et al[33] compared the effects of fried food intake in GC patients (n = 5739) and healthy adults (n = 70933). They found a significant positive association between GC risk and fried food intake in both non-East Asians and East Asians[33].
Smoking: The association between smoking and GC has been demonstrated in several epidemiological and metanalysis studies. The European EPIC study showed an increased risk of GC, but this risk decreases after 10 years of smoking cessation. It is estimated that 18% of GC cases are associated with smoking[34].
Non-steroidal anti-inflammatory drugs: These have been shown to reduce the risk of GC[35]. The effect is even more favorable in H. pylori-positive patients[36].
One of the most important primary prevention strategies for GC involves early detection and elimination of H. pylori infection through endoscopic and radiological screening of the asymptomatic population in countries with a high incidence of the disease. In countries with a low prevalence of infection, upper digestive endoscopy with biopsies for any evidence of precancerous lesions is recommended[37]. Therefore, H. pylori eradication and endoscopic surveillance are the most important methods aimed at reducing the incidence and prevalence of GC[38].
H. pylori eradication has been shown to actually reduce the risk of GC. A meta-analysis of 27 studies, with 48606 H. pylori-positive patients, of whom 715 developed GC, showed that patients cured of the infection had a lower incidence of GC[39]. Of note, a recent prospective, randomized, placebo-controlled study with 26.5 years of follow-up demonstrated that H. pylori eradication therapy can provide long-term protection against GC in high-risk populations, particularly those without advanced gastric lesions[40]. However, there is still no consensus on population control. Some guidelines recommend that it is beneficial in populations at increased risk for GC.
From the outset, it is emphasized that a multidisciplinary approach is the appropriate way to treat patients with GC to increase the survival rates of patients. Surgery remains the main treatment approach for cases of surgical GC, while chemotherapy serves as the basis of treatment for metastatic GC. Until recently, the two treatment modalities mentioned above along with radiotherapy and targeted therapy were the main available means of treating GC.
For resectable GC, perioperative chemotherapy has become the standard treatment and ongoing investigations are exploring the potential benefits of targeted therapy or immunotherapy in the perioperative or adjuvant setting. However, differences in the extent of standard lymphadenectomy between Eastern and Western countries have led to different standard treatments: Perioperative (neoadjuvant) and adjuvant therapy[41]. Trastuzumab or pembrolizumab should be added to first-line chemotherapy but only in HER2-positive patients. The combination of immunotherapy with therapies targeting HER2 has shown synergistic effects in preclinical models, and clinical trials in locally advanced GC (AGC) and metastatic GC. In addition, disruption of antibody-drug conjugates (ADCs) and other agents targeting HER2 has resulted in numerous clinical trials with promising results[42]. Trastuzumab can be combined either with fluoropyrimidine and a platinum agent or in combination with capecitabine + oxaliplatin (XELOX)/PF[43]. In HER2-negative patients, treatment regimens include nivolumab, cindilimab, and tislelizumab in combination with first-line chemotherapy. Docetaxel, cisplatin, 5-fluorouracil (DCF), modified DCF, and paclitaxel/oxaliplatin/5-fluorouracil/leucovorin also shows satisfactory activity.
In cases of unresectable locally advanced, recurrent, or metastatic GC, combination anti-HER2 therapy with chemotherapy and possibly pembrolizumab in HER2-positive patients is preferred. HER2 is overexpressed in 10%-20% of patients with GC. The implementation of targeted anti-HER2 therapy as part of the standard of care treatment in metastatic disease improved the prognosis of patients. Of note, the addition of pembrolizumab achieves high objective response rates (ORRs). Regardless of whether or not HER2 is positive, nivolumab is recommended as part of systemic treatment regimens.
The choice of second-line regimen depends on the previous treatment and the performance status of the patients. Ramucirumab combined with paclitaxel is the preferred second-line therapy. The drugs docetaxel, paclitaxel, irinotecan, albumin-paclitaxel, pembrolizumab, nivolumab, vedicitumab, and apatinib mesylate have also been used as second-line therapies. In the large class of patients with metastatic or locally AGC, treatment is complex and involves a combination of agents. These combinations include S-1 + oxaliplatin, docetaxel + oxaliplatin + fluorouracil, docetaxel + oxaliplatin + S-1 (DOS), XELOX, and folinic acid/5-fluorouracil/oxaliplatin chemotherapy (FOLFOX). Administration of intilimab in combination with chemotherapy results in a significant degree of pathological complete response with a satisfactory safety profile. Other phase II studies showed that durvalumab combined with DOS as neoadjuvant chemotherapy gave satisfactory results. Equally satisfactory results were produced by the combination of tremelimumab and durvalumab as neoadjuvant therapy in patients with exceptional GC of high microsatellite instability (MSI).
The significant advances made in recent years in the understanding of factors in the cancer microenvironment have resulted in significant progress in immunotherapy of AGC. Immunotherapy induces the generation of immune responses that destroy cancer cells, achieving satisfactory clinical outcomes with tolerable toxicity. Therefore, this novel strategy is becoming increasingly popular[44]. In healthy individuals, to maintain normal T cell functions, the normal immune checkpoint regulates and controls the actions of ligands and receptors. T cell activation results in the expression of many receptors such as programmed cell death protein 1 (PD-1) or cytotoxic T lymphocyte-associated antigen 4 (CTLA-4). Anti-PD-1/anti-programmed death-ligand 1 (PD-L1) agents inhibit immune checkpoints and activate T cells. The results of monotherapy in GC are disappointing. Therefore, combination therapy is a practical treatment option for metastatic GC[45].
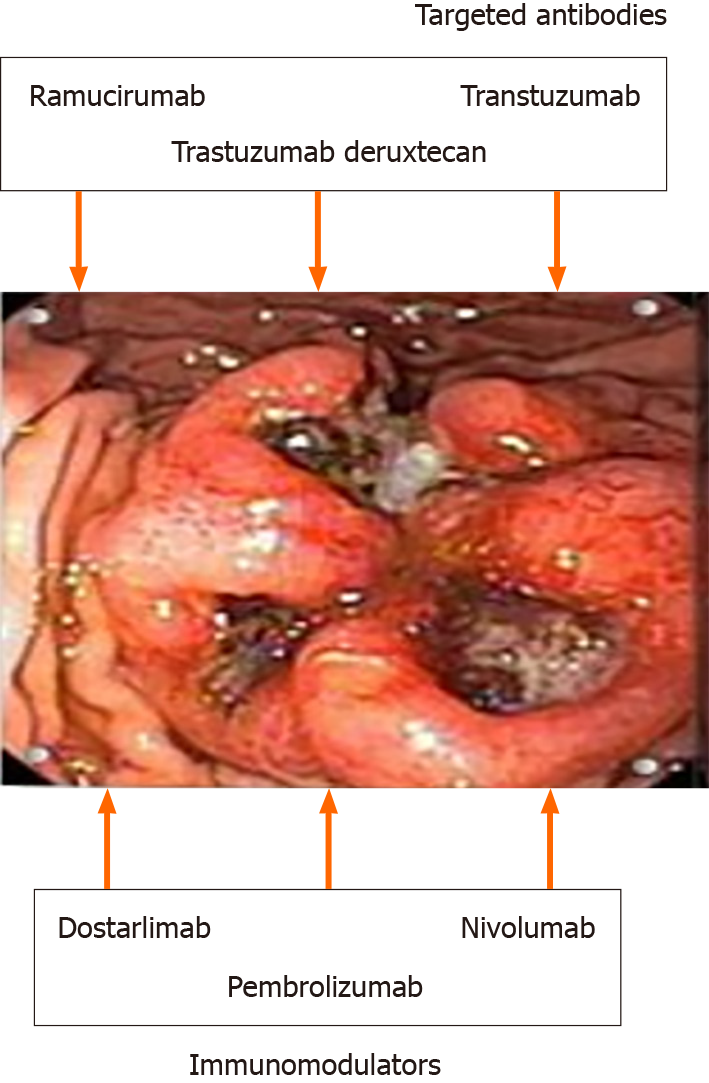
The main classes of immunotherapy are shown in Table 1. Currently, the following strategies are used: immune checkpoint inhibitors (ICIs), tumor vaccines, adoptive immunotherapy, and nonspecific immunomodulators (Figure 1). The increasingly used strategy of administering ICIs is due to their satisfactory efficacy and low toxicity compared to traditional therapies. The combination of immunotherapy and conventional therapy has been shown to achieve more satisfactory results because most GCs are resistant to the administration of a single drug. Nevertheless, many further studies are needed due to the inherent challenges of the complexity of the immune microenvironment and the heterogeneity of immunogenicity.
| Target | Action | Approved for | |
| Targeted antibodies | |||
| Ramucirumab (Cyramza®) | VEGF/VEGFR2 pathway | Inhibits tumor blood vessel growth | Subsets of patients with advanced GC |
| Trastuzumab (Herceptin®) | HER2 pathway | Inhibition | Advanced HER2- + GC |
| Trastuzumab deruxtecan (Enhertu®): Antibody-drug conjugate | HER2 pathway | Inhibition | Subsets of patients with advanced GC |
| Immunomodulators | |||
| Dostarlimab (Jemperli) | PD-1/PD-L1 pathway | Checkpoint inhibitor | Advanced GC that has dMMR |
| Nivolumab (Opdivo®) | PD-1/PD-L1 pathway | Checkpoint inhibitor | Subsets of patients with advanced GC |
| Pembrolizumab (Keytruda®) | PD-1/PD-L1 pathway | Checkpoint inhibitor | Subsets of patients with advanced GC |
The main individual components of immunotherapy are cytokines, immune checkpoints, monoclonal antibodies, bispecific antibodies, ADCs, and chimeric antigen receptors (CARs). Nivolumab is used as a third-line therapy. Ramucirumab and claudiximab are also effective, particularly as long as they are combined with other therapies[46]. Synergistic activity results primarily from the combination of anti-PD-1 and anti-CTLA-4 antibodies, a combination that has been extensively investigated alongside the inclusion of anti-HER2 antibodies. The limitations and challenges of immunotherapy in GC are real and should be addressed shortly. For example, because GC is heterogeneous, the response to immunotherapeutic agents is affected. Therefore, universal targets should be identified such as identifying reliable biomarkers for personalized therapeutic approaches. Existing biomarkers (PD-L1 expression and MSI) do not accurately predict response to therapy. Immunotherapy has moderate efficacy in the advanced stages of GC. Finally, ICIs may cause autoimmune diseases. There is a lack of animal models of GC with all that this implies for the evaluation of therapeutic strategies.
The mode of action of these therapies is of particular interest since the growth of tumor is restricted by restarting the tumor-immune cycle and restoring the normal immune response of the patient. These strategies are discussed in brief below[47].
ICI treatment: The use of ICIs is the most widespread treatment for GC since these inhibitors are particularly effective in solid tumors as well as hematological malignancies with long-term responses without significant toxicity. ICIs such as monoclonal antibodies against PD-1 or PD-L1 can prolong the survival of patients with AGC[48]. It is widely accepted that ICIs by inhibiting excessive activation of the immune response, prevent the occurrence of autoimmunity reactions and damage to autologous tissues. These actions of ICIs are used by cancer cells to inhibit T-cell activation to avoid their destruction. ICIs express their antitumor activity by binding monoclonal antibodies to ICIs on the surface of tumor or immune cells, thereby inhibiting pathological regulation and activating the immune system. ICIs can inhibit the interactions between PD-1 and PD-L1, keep T cells activated, activates natural killer (NK) cells, and prevent cancer cells from evading the destructive influence of immune cells. The most important ICIs are considered to be CTLA-4, PD-1, and PD-L1.
Existing data support that PD-L1 exhibits a significant correlation between its expression level and the benefit expected from ICI application in GC. However, its application is not without drawbacks, such as interobserver variability, the immunohistochemical assay used, and the effect of chemotherapy and radiation[49]. Currently, there are six Food and Drug Administration-approved immunotherapy options for GC (Table 1).
(1) PD-1 inhibitors
Nivolumab: Nivolumab is a humanized monoclonal immunoglobulin G4 (IgG4) PD-1 antibody that, upon binding to the PD-1 receptor, enhances the body's immune response by inhibiting the relation of PD-1 with PD-L1 and PD-L2 and inactivating the relevant pathway. Nivolumab in combination with chemotherapy is used as first-line therapy or nivolumab monotherapy as third- or later-line therapy. In the phase III ATTRACTION-2 trial, nivolumab as a third-line treatment improved overall survival (OS) in patients with AGC[50]. Also the combination of 5-fluorouracil and platinum as a first-line treatment improved OS in patients with HER2-negative AGC (global phase III CheckMate-649 study).
Pembrolizumab: Pembrolizumab is a monoclonal antibody directed against PD-1. It is active in tumors with high MSI or high tumor mutational load. Pembrolizumab acts by blocking the interaction between PD-1 and PD-L1. It activates and proliferates immune system T cells, as well as cytokine production. Pembrolizumab is used in previously treated patients with high-grade satellite instability or patients with tumor mutational burden-high AGC or in combination with trastuzumab and chemotherapy for HER2-positive AGC (United States). Pembrolizumab monotherapy, or combined with chemotherapy, has been used as first-line treatment for AGC. Pembrolizumab-based combination chemotherapy did now show better results.
Dostarlimab: Monoclonal antibody used in previously treated patients with MSI-H AGC (United States).
(2) PD-1 combined with chemotherapy
Treatment modalities that should be applied after ICI combination therapy, such as re-administration of ICI or the use of combination therapy with drugs that have a different mechanism of action, are the subject of intensive research. Several clinical studies are ongoing to apply this therapeutic strategy perioperatively and/or postoperatively in patients with early GC[51]. Satisfactory results are obtained from the combination of ICI and chemotherapy with tolerable toxicity. Thus, the combination of camrelizumab and capecitabine and oxaliplatin (CAPOX) has shown encouraging results in patients with metastatic or AGC in phase II clinical trials.
In the ATTRACTION-4 studies, nivolumab in combination with chemotherapy was shown to have satisfactory anticancer activity with an acceptable level of safety. Patients who received nivolumab in combination with s-1 and oxaliplatin and CapeOX experienced longer remission and objective remission rates[52].
Perioperative administration of ICI combined with chemotherapy in resectable locally AGC has shown encouraging results. However, a significant number of patients have shown resistance to ICIs, highlighting the importance of better patient selection or further combination immunotherapy[53,54]. As the number of available drugs increases, it is important to understand the target biomarkers and drug characteristics and select the optimal therapy for each patient;
(3) PD-L1 inhibitors
PD-L1 is a PD-1 Ligand expressed in various tumors that increases the immune-mediated tumor response by inhibiting the combination of PD-1 and PD-L1.
(4) Avelumab
Avelumab is a fully humanized PD-L1 IgG1 monoclonal antibody which, by binding to PD-L1, reduces the level of immunosuppression by blocking the reaction between PD-L1 and the PD-1 receptor[55]. The drug administered alone or as part of a combination therapy demonstrates satisfactory clinical results possibly in subsequent therapies;
(5) Atezolizumab
Atezolizumab is a monoclonal antibody that by binding to PD-L1 on tumor and immune cells infiltrating the tumor, can destroy cancer cells through the activation of T cells. However, in an earlier clinical trial in a small number of patients who were given the drug as a single treatment, the results were disappointing[56].
(6) Durvalumab
Durvalumab is a humanized monoclonal antibody that binds the PD-L1 protein by inhibiting its binding to the PD-1 protein on the T-cell surface. As a result, cancer cells cannot use the PD-L1/PD-1 pathway to avoid being destroyed by the immune cells. The existing data on its efficacy are largely conflicting[57].
Nonspecific enhancer therapy: This treatment involves various immune modifiers that enhance the immune response when given in combination with other therapies thus enhancing the final effect. These agents include cytokines (CKs), lentinan, Streptococcus preparation (OK-432), and Bacillus Calmette-Guerin (BCG) vaccine.
(1) CKs
CKs are small molecular proteins produced by immune cells that have been stimulated by incubation with a mitogen. They include IL-2, interferon-γ, tumor necrosis factor α, and granulocyte-macrophage colony-stimulating factor, among others. Their actions consist of activation, proliferation and differentiation of lymphocytes[58]. Of these, low-dose IL-2 has been used for the treatment of patients undergoing radical gastrectomy with postoperative adjuvant chemotherapy. The drug achieved an increase in CD4+ T and NK cells in both the periphery and around the tumor[59]. However, CKs have certain side effects and thus could be used as adjuvant molecules in cancer immunotherapy.
(2) Lentinan
Lentinan is quite often used as an immune adjuvant in the treatment of GC. Its actions are indirectly anticancer as it promotes the maturation, differentiation, and proliferation of immune cells[60]. The drug combined with chemotherapy in patients with AGC can significantly prolong the OS of the patients.
(3) BCG vaccine
The BCG vaccine, which is be prepared from attenuated bovine tuberculosis bacillus, enhances macrophage activity and boosts cellular immunity through activation of CD4+ and CD8+ cells[61]. BCG in combination with 5-fluorouracil, adriamycin, mitomycin C prolongs the survival of patients with AGC[62].
Oncolytic virus therapy: The treatment of malignant tumors using natural or genetically modified oncolytic viruses is a new and interesting type of immunotherapy[63]. Oncolytic viruses are divided into inherent oncolytic wild-type strains, which through their affinity with tumor cells, can proliferate and induce their lysis, and viruses in which by modification of the viral genome the virus becomes tumor-selective. Subsequently, through various processes, the virus loses its ability to replicate in normal cells, while its ability to infect cancer cells is enhanced[64].
In a recent study, Yang et al[65] investigated the effect of CF17, a new replication-competent chimeric poxvirus that they administered to intestinal and diffuse GC cell lines. They found that CF17 could infect, and kill cancer cells in dose- and time-dependent manners in vitro. In parallel, in the in vivo experiments, CF17 treatment resulted in a reduction in tumor burden, prevention of ascites formation, and prolonged survival of the experimental animals. These experimental results suggest that CF17 may be used in future studies to treat patients with GC and malignant ascites[65]. In a very recent study, the same group of investigators demonstrated that CF33 oncolytic viruses are capable of delivering functional proteins and demonstrating effective antitumor activity in GCPM models when administered intraperitoneally, suggesting that similar studies in GC patients with peritoneal metastases can be designed and performed in the future[66].
Hori et al[67] investigated the potential activity of adenoviral vectors expressing p53 against peritoneal metastases from diffuse-type GC. They found that the oncolytic adenovirus OBP-702 induced a significantly greater antitumor effect in GC cells compared with Ad-p53 adenovirus, through induction of p53-mediated apoptosis and autophagy and suppression of the tyrosine kinase receptor. The in vivo experiment showed that intraperitoneal administration of the oncolytic adenovirus OBP-702 suppressed peritoneal metastasis of NUGC-4 and GCIY cells compared to Ad-p53, and increased the survival of experimental animals[67]. Currently, the treatment of GC with oncolytic viruses is limited to in vitro research only. There have been no reports from clinical studies.
Tumor vaccine therapy: Treatment based on vaccine-derived elements of the malignant neoplasm relies on the specific immune response induced by the administration of the cancer antigen, which recognizes the cancer antigen on the surface of the antigen-presenting cells[68]. Tumor vaccines primarily involve dendritic cell vaccine (controversial efficacy), nucleic acid vaccine (satisfactory safety, no application in human GC), peptide/protein vaccine (activate the immune system by combining with MHC molecules or T cells of APC), and tumor-associated antigens vaccine. The latter is of particular interest concerning the future treatment of GC. Tumor-associated antigens are proteins produced by cancer cells. A characteristic of these proteins is that they do not show antigenicity under normal conditions. They may, however, elicit immune responses following the appearance of a mutation[69].
Of particular note is the vaccine against H. pylori. This bacterium has been classified as a class I carcinogen by the World Health Organization. Therefore, the development of a vaccine against H. pylori is expected to have a significant impact on the incidence of H. pylori-related GC. Potential antigens of the microbe for vaccine preparation are individual components of the microbe such as bacterial urease, various virulence factors, outer membrane protein, flagella, etc. At present, vaccines based on the microbial elements mentioned above are being tested in experimental models[70].
Νeoantigen-based personalized vaccines: The use of personalized vaccines designed to induce de novo T-cell responses against neoantigens specific to individual patient malignancies has been an area of systematic research in recent years. Results have shown that these vaccines provide potent immunogenicity in many solid cancers. However, the research field contains many elements that need to be answered. A deeper evaluation of the phenotypes, functionality, and long-term memory potential of vaccine-induced CD4+ and CD8+ T-cells is required to achieve optimization of vaccination strategies. Their use is based on the fact that the mutations that occur in cancer cells generate new autoantigen epitopes called neoepitopes or neoantigens. Their advantages are that they are expressed exclusively by the cancer cells causing the generation of specific reactions and that they are derived from somatic mutations causing the generation of short and long-term reactions directed only towards the cancer cells thus preventing the possibility of recurrence of the neoplasm. Of course, this therapeutic method has some disadvantages related to high cost, time delays in vaccine generation, and inconsistency with the most suitable vaccine delivery platform. Results of clinical applications are expected soon[71].
Adoptive cellular immunotherapy: Adoptive immunotherapy consists of selectively isolating sensitized immune cells (T cells and NK cells) from patients or donors and re-administering them to patients to enhance the proliferation of various immune cells. The cells recognize specific tumor antigens and bind to and destroy them[72]. Adoptive immunotherapy includes the following categories: Cytokine-induced killer (CIK) cells, tumor-infiltrating lymphocytes (TILs), NK cell therapy, CAR T cells, and T cell receptor-gene engineered T cells[73].
(1) NK cell therapy
NK cells play an important role in the body's defense processes against various viruses and cancer cells, making a decisive contribution to immune regulation processes[74]. NK cells show satisfactory efficacy against solid tumors while effectively preventing tumor metastasis.
(2) TIL cell therapy
TIL cell therapy is a treatment in which lymphocytes derived from the patient's tumor are cultured and amplified in vitro and then reintroduced into the patient's body[75].
All relevant studies have been conducted in cell lines and there are no clinical data.
CTLA-4 inhibitors: CTLA-4 is a small molecule expressed on the surface of CD4+ and CD8+ T cells. Its inhibition by specific inhibitors results in the restoration of a normal immune response. CTLA-4 inhibitors currently include two monoclonal antibodies ipilimumab and tremelimumab. Ιpilimumab is a fully humanized CTLA-4 antibody inhibitor that will probably find application as part of a combination therapy. Tremelimumab is also a fully humanized IgG2 monoclonal antibody against CTLA-4 which has demonstrated antitumor activity in a variety of solid tumors including gastro-esophageal junction cancer. It appears that this drug has satisfactory safety, which combined with satisfactory efficacy, may increase the survival of patients with AGC.
CIK cells: In recent years, the type of treatment-induced through the so-called “CIK” has been widely discussed. The term “CIK cells” has been increasingly used over the last decade. These cells include features that make them quite a promising therapeutic method for treating cancer due to the antitumor potential of NK cells[76]. Future research in this area will likely focus on the development of more potent antitumor CAR T cells.
CAR T-cell therapy: CAR T-cell therapy is a relatively new approach. CAR T cells, a subset of genetically modified T cells, can recognize specific antigens and their action is independent of major histocompatibility complex (MHC) interactions. Several potential targets for the treatment of GC patients have been identified including claudin 18.2 (CLDN18.2), mesothelin, anthrax toxin receptor 1, and mucin 3A. CAR therapy targets multiple tumor cell targets in GC patients. Among them, intercellular adhesion molecule 1, CAR T cells, and CLDN18.2 CAR T cells have shown good results. However, satisfactory therapeutic responses are not observed in all patients[77].
NK cell therapy: Treatment with NK cells continues to evolve[78]. This treatment is also combined with other forms of immunotherapy to achieve more satisfactory results.
Other biomarker-targeted therapy: Recent studies reporting on new potential molecular targets are investigating the following targeted therapies of AGC: CLDN18.2-targeted therapy, FGFR pathway inhibitors, and EGFR inhibitors. Data on the most important of these, CLDN18.2-targeted therapy, are reported below.
CLDN18.2-targeted therapy: CLDN18.2 is a component of intercellular junctions and is present only in the gastric mucosa. During the processes of carcinogenesis, CLDN18.2 maintains its expression at a rate of 14.1% to 72%, both in gastric adenocarcinoma mucosa and diffuse type cancer[79].
Zolbetuximab is highly promising for the treatment of GC chimeric IgG1 monoclonal antibody that binds to CLDN18.2 thus inducing antibody-dependent cytotoxicity[80]. In several studies, mainly phase II, zolbetuximab showed very satisfactory results. In the MONO study, the drug had an ORR of 9% and a disease control rate of 23% in previously treated patients with GC or EC[81]. In the FAST study, zolbetuximab combined with first-line chemotherapy significantly improved progression-free survival (PFS) and OS in patients with CLDN18.2-positive GC[82]. In the SPOTLIGHT study, zolbetuximab administered with mFOLFOX6 significantly improved median PFS and median OS in patients with CLDN18.2-positive and HER-2-negative AGC[83]. The phase II GLOW study investigated the effect of zolbetuximab with CAPOX as first-line therapy in patients with CLDN18.2-positive, HER2-negative, locally advanced, unresectable, or metastatic GC. The zolbetuximab plus CAPOX combination significantly improved median PFS and median OS[84]. Another promising therapeutic approach targeting CLDN18.2 uses CAR T specific for CLDN18.2. Administration of these cells resulted in partial or complete tumor regression in CLDN18.2-positive patient-derived xenograft models[85]. Therefore, it appears that CLDN18.2 is a novel target for later-line treatment of GC. CLDN18.2 CAR T therapy is expected to become a cornerstone in cellular immunotherapy of solid tumors.
New drugs targeting CLDN18.2 are currently being developed, such as CLDN18.2 bispecific antibodies and ADC analogs. Some of them achieved satisfactory preclinical results representing the field of various clinical studies. The only concern concerning this type of therapy is the kind and severity of adverse effects that may occur since CLDN18.2 is expressed in the normal gastric mucosa.
Tumor microenvironment: The tumor microenvironment differs from that of normal tissues. The main characteristic of this microenvironment is the presence of an inflammatory response ,which favors the accumulation of immune cells and the activation of progenitor cells. The most important cells that promote gastric carcinogenesis through modulation of immune responses and secretion of soluble factors are macrophages. Furthermore, other elements of the stroma such as endothelial cells or blood vessels promote tumor growth through blood flow and secretion of cytokines and chemokines. The fundamental structural and functional changes of cancer-associated fibroblasts and blood vessels are caused by numerous interactions between cancer cells and other stromal components. Nerves and neurotransmitters are also involved in gastric carcinogenesis by acting in the tumor microenvironment[86].
Therefore, it appears that modification of the pro-tumorigenic stroma and creation of an antitumorigenic microenvironment will be promising therapeutic approaches in the future. Many cases of GC exhibit a significant degree of fibrosis due to cancer-associated fibroblasts through the secretion of IL-6, gremlin-1, and other factors. IL-6 activates signal transducer and activator of transcription signaling in cancer cells, inducing tumor growth and metastasis[87]. Therefore, targeting cancer-associated fibroblast-mediated cross-talk would be beneficial in GC. The above leads to the conclusion that a better understanding of all the interactions of the tumor microenvironment is expected to contribute decisively to the discovery of novel therapeutic targets[88].
Bibliometrics is defined as the process of analyzing a large amount of data that aims to identify recent research points and explore cutting-edge trends, revealing the structure of knowledge in a scientific field and listing all data related to scientific productivity and advances achieved in a specific scientific field and a specific period.
In recent years, there has been a boom in bibliometric analysis of articles in various fields. Tools such as CiteSpace and VOSviewer allow visualization of raw data, thus offering comprehensive and intuitive data representation. So-called scientometrics has been applied to analyze literature related to certain fields to identify hotspots and predict future trends[89].
It is an indisputable fact that the scientific medical literature is growing at a rate of 6% per year, a rate that is impossible for even clinicians to keep track of. For example, the scientific articles registered to date in the PubMed database have exceeded 36 million. It is also an undeniable fact that a significant percentage of articles, mainly reviews but also original research articles, repeat the same knowledge published in different journals. The trend toward publication has also resulted in an explosion of biomedical journals that are either listed in PubMed or other databases. This rate of increase in the medical literature is a real challenge for modern doctors, a challenge related both to updating them on the basic data of their specialty and to their ability to review the data of the international bibliography, a necessary element of lifelong updating. Knowledge discovery in databases (KDD) in data analysis consists of a retrospective data analysis[90]. KDD refers to the fluent or automated extraction of knowledge stored in large databases.
CiteSpace II is a system that could be used by clinicians and medical librarians. For the case of clinical research, CiteSpace II proves to be particularly useful for the creation of ontologies, and for the development of evidence-based knowledge bases to support decisions. However, there are several limitations to its use, the most important of which is the learning curve required to define the imaging parameters and the need to provide specialized knowledge. However, despite the existence of some limitations, CiteSpace II is a valuable tool addressed to a variety of users[91].
In this issue of the World Journal of Gastroenterology, the article by Li et al[1], published through bibliometric analysis using the Web of Science Core Collection database, CiteSpace software (6.1.6) and VOSviewer (1.6.18)m assessed the current status and emerging trends regarding the application of immunotherapy in GC patients. Relevant publications from the period 1999 to January 2023 were analyzed. The countries where the studies were carried out, scientific institutions, scientific journals, authors of the articles, bibliographic references, and keywords used were evaluated. The study included 2013 articles by 11730 authors using 726 keywords, from 617 scientific institutions in 71 countries. In addition, 228 clinical trials on immunotherapy, 137 on cell therapy, 274 on ICIs, and 23 using vaccines against GC were evaluated. Finally, the Impact Index Per Article for the top 10 highly cited papers from Reference Citation Analysis was also presented.
The main finding of the bibliometric analysis of the studies was that the immunotherapy for GC has developed significantly during the last few years. China and the United States account for the highest volume of publications. Especially for China, it was found that it accounts for 53.2% of the publications (1070 research articles) with the most authors and scientific institutions. The 10 institutions with the highest number of publications were located in China. Perhaps the huge population of China, smoking habits, increased incidence of H. pylori infection, and increased incidence of GC in the country have led to the large production of related publications, including on immunotherapy. The second place is occupied by the United States with a publication rate of 16.0%. Environmental factors (high-fat diet, obesity, alcohol consumption) are implicated in the etiology of GC in the United States. Finally, Japan, with a publication rate of 11.3%, took third place. In this country, dietary habits (increased consumption of salt and nitrites) combined with genetic factors are probably responsible for the high incidence of the disease. Regarding the annual distribution of scientific articles, an explosive increase in citations was evident, from 22 publications in the year 1999 to 552 in the year 2022. Thirty authors had at least 10 relevant publications while 17 authors collected more than 400 citations. Seven of the ten most frequently cited articles in the international bibliography were published in the last 7 years. Most publications were in journals related to molecular biology, genetics, immunology, nursing, and medicine. As far as clinical trials are concerned, their annual distribution has shown a sharp rise in recent years, especially from the year 2019 onwards. Regarding scientific journals, Frontiers in Oncology took first place with 104 scientific publications, followed by Frontiers in Immunotherapy with 78 and Cancers with 69 publications. The Journal of Clinical Oncology collected the highest number of citations (5044 citations), followed by Cancer Research (3018 citations). The study also showed that the most important points of progress concerned vaccinations, immune checkpoint therapy, and cell therapy. In particular, ICIs, and CAR T, are the most modern options for the treatment of GC. It seems that MSI, tumor microenvironment, mismatch repair deficiency, dendritic cell functions, and adoptive immunotherapy together with ICIs, are the most important future research directions. It also appears that the combined administration of chemotherapy and immunotherapy constitutes the future of GC drug treatment.
Also in a recent study[92], bibliometric analysis was used to investigate the size and trends of scientific research regarding immunotherapy of all types of cancer from 2000 to 2021. In these 20 years, a total of 18778 articles were published with the number of articles skyrocketing. from 366 in 2000 to 3194 in 2021. The United States had the largest number of publications (35.9%). A total of 976 significant topics were identified and further classified into four different groups (immune mechanism, cancer biology, immunotherapy, and clinical trials). The most important search topics included the terms ‘expression,’ ‘chemotherapy,’ ‘dendritic cells,’ ‘pembrolizumab,’ and ‘open-label.’ Liver, breast, lung, and bladder cancer had the greatest number of studies. Of interest is the recent shift from the investigation of pathophysiological mechanisms to the clinical application of immunotherapies, a trend that is predicted to continue in the future.
From these data, it appears that the largest amount of research on immunotherapy of all cancer types is from the United States, although for GC, the data from China are significantly greater in volume compared to the United States counterparts.
Currently, the advances achieved in anti-HER2 and anti-vascular endothelial growth factor receptor therapy are uninterrupted and with a stable outlook. Research efforts include ICIs as monotherapy or in combination with ADCs on which research is increasingly focused. ADCs are potential chemical drugs that selectively target cancer cells by binding specific cell surface receptors with antibodies. Currently, several ADCs are being investigated in GC patient clinical trials targeting receptors such as EGFR, HER-2, HER-3, CLDN18.2, and mucin 1. ADCs combine the specificity of monoclonal antibodies with the potency of cytotoxic agents. They are likely to be the immunotherapy of the future for GC by acting synergistically with chemotherapy.
For future research, the investigation of new prognostic immunotherapy biomarkers to achieve personalized treatment, the further improvement of the combination of immunotherapy with ADC, the application of bispecific immunotherapy antibodies, and the further development of CAR T therapy are suggested. The transmembrane protein CLDN18.2, is, as is well known, the main component of tight junctions, thus contributing to the integrity of the intestinal barrier. The special feature of tumors is the high expression of this protein in the malignant tissue. Its exposure as an extracellular anchor makes it an ideal target for immunotherapy in digestive system cancers[93]. Also, lymphocyte activation gene-3 is a type of immune checkpoint receptor protein. This gene (also known as cluster of differentiation 223), which is mainly expressed in activated immune cells, is highly associated with the appearance and growth of the tumor, thus being a target for the immunotherapy of GC[94].
Current treatment modalities for GC include surgery, chemotherapy, radiotherapy, and molecular-targeted therapy. The trends in clinical trials today are the discovery of new biomarkers and the investigation of more specific treatment options. Immunotherapy has evolved into a very important therapeutic modality for GC, with several possibilities for clinical application such as anti-PD-1 monoclonal antibody being mainly part of combined therapy. The application of immunotherapy against PD-1/PD-L1 in GC also opens up great expectations. However, great caution is required as many issues related to GC immunotherapy are still unresolved. The mechanisms regulating the immune responses are extremely complex and obscure. For example, posttranslational modifications, such as glycosylation, acetylation, and phosphorylation, are involved in the direct regulation of PD-1/PD-L1 protein, as well as cross-talk between PD-1/PD-L1-related signal pathways in GC. The application of ICIs is an important research approach in terms of efficacy and safety. It seems that the combination of several therapeutic agents (immunotherapy and surgery, radiotherapy and chemo-therapy, combination of immunotherapies, and the discovery of new ICIs) will constitute future therapeutic strategies.
In conclusion, there are reasonable hopes for the development of safer and more effective immunotherapies in GC as well as hopes for the establishment of individualized evaluation of combination therapies.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gao W, China; Lin W, China; Miao YD, China S-Editor: Li L L-Editor: Filipodia P-Editor: Yu HG
| 1. | Li YN, Xie B, Zhang Y, He MH, Xing Y, Mu DM, Wang H, Guo R. Advances and key focus areas in gastric cancer immunotherapy: A comprehensive scientometric and clinical trial review (1999-2023). World J Gastroenterol. 2023;29:5593-5617. [PubMed] [DOI] [Full Text] |
| 2. | Yang WJ, Zhao HP, Yu Y, Wang JH, Guo L, Liu JY, Pu J, Lv J. Updates on global epidemiology, risk and prognostic factors of gastric cancer. World J Gastroenterol. 2023;29:2452-2468. [PubMed] [DOI] [Full Text] |
| 3. | Thrift AP, Wenker TN, El-Serag HB. Global burden of gastric cancer: epidemiological trends, risk factors, screening and prevention. Nat Rev Clin Oncol. 2023;20:338-349. [PubMed] [DOI] [Full Text] |
| 4. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [PubMed] [DOI] [Full Text] |
| 5. | Wang S, Zheng R, Li J, Zeng H, Li L, Chen R, Sun K, Han B, Bray F, Wei W, He J. Global, regional, and national lifetime risks of developing and dying from gastrointestinal cancers in 185 countries: a population-based systematic analysis of GLOBOCAN. Lancet Gastroenterol Hepatol. 2024;. [PubMed] [DOI] [Full Text] |
| 6. | Wong MCS, Huang J, Chan PSF, Choi P, Lao XQ, Chan SM, Teoh A, Liang P. Global Incidence and Mortality of Gastric Cancer, 1980-2018. JAMA Netw Open. 2021;4:e2118457. [PubMed] [DOI] [Full Text] |
| 7. | López MJ, Carbajal J, Alfaro AL, Saravia LG, Zanabria D, Araujo JM, Quispe L, Zevallos A, Buleje JL, Cho CE, Sarmiento M, Pinto JA, Fajardo W. Characteristics of gastric cancer around the world. Crit Rev Oncol Hematol. 2023;181:103841. [PubMed] [DOI] [Full Text] |
| 8. | Zheng R, Zhang S, Zeng H, Wang S, Sun K, Chen R, Li L, Wei W, He J. Cancer incidence and mortality in China, 2016. J the National Cancer Center. 2022;2:1-9. [DOI] [Full Text] |
| 9. | Yan X, Lei L, Li H, Cao M, Yang F, He S, Zhang S, Teng Y, Li Q, Xia C, Chen W. Stomach cancer burden in China: Epidemiology and prevention. Chin J Cancer Res. 2023;35:81-91. [PubMed] [DOI] [Full Text] |
| 10. | He J, Wei W. China cancer registry annual report. Beijing: People's Medical Publishing House, 2022. |
| 11. | Li Y, Ren N, Zhang B, Yang C, Li A, Li X, Lei Z, Fei L, Fan S, Zhang J. Gastric cancer incidence trends in China and Japan from 1990 to 2019: Disentangling age-period-cohort patterns. Cancer. 2023;129:98-106. [PubMed] [DOI] [Full Text] |
| 12. | Bertuccio P, Chatenoud L, Levi F, Praud D, Ferlay J, Negri E, Malvezzi M, La Vecchia C. Recent patterns in gastric cancer: a global overview. Int J Cancer. 2009;125:666-673. [PubMed] [DOI] [Full Text] |
| 13. | Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2014. National Cancer Institute; Bethesda, MD: Based on November 2016 SEER data submission, posted to the SEER web site. April 4, 2017. [cited 3 January 2024]. Available from: https://seer.cancer.gov/csr/1975_2014/. |
| 14. | Wang J, Sun Y, Bertagnolli MM. Comparison of gastric cancer survival between Caucasian and Asian patients treated in the United States: results from the Surveillance Epidemiology and End Results (SEER) database. Ann Surg Oncol. 2015;22:2965-2971. [PubMed] [DOI] [Full Text] |
| 15. | Scaltriti M, Eichhorn PJ, Cortés J, Prudkin L, Aura C, Jiménez J, Chandarlapaty S, Serra V, Prat A, Ibrahim YH, Guzmán M, Gili M, Rodríguez O, Rodríguez S, Pérez J, Green SR, Mai S, Rosen N, Hudis C, Baselga J. Cyclin E amplification/overexpression is a mechanism of trastuzumab resistance in HER2+ breast cancer patients. Proc Natl Acad Sci U S A. 2011;108:3761-3766. [PubMed] [DOI] [Full Text] |
| 16. | Gregory SN, Davis JL. CDH1 and hereditary diffuse gastric cancer: a narrative review. Chin Clin Oncol. 2023;12:25. [PubMed] [DOI] [Full Text] |
| 17. | Nakamura J, Tanaka T, Kitajima Y, Noshiro H, Miyazaki K. Methylation-mediated gene silencing as biomarkers of gastric cancer: a review. World J Gastroenterol. 2014;20:11991-12006. [PubMed] [DOI] [Full Text] |
| 18. | Almeida R, Silva E, Santos-Silva F, Silberg DG, Wang J, De Bolós C, David L. Expression of intestine-specific transcription factors, CDX1 and CDX2, in intestinal metaplasia and gastric carcinomas. J Pathol. 2003;199:36-40. [PubMed] [DOI] [Full Text] |
| 19. | Rich A, Toro TZ, Tanksley J, Fiske WH, Lind CD, Ayers GD, Piessevaux H, Washington MK, Coffey RJ. Distinguishing Ménétrier's disease from its mimics. Gut. 2010;59:1617-1624. [PubMed] [DOI] [Full Text] |
| 20. | Tersmette AC, Offerhaus GJ, Tersmette KW, Giardiello FM, Moore GW, Tytgat GN, Vandenbroucke JP. Meta-analysis of the risk of gastric stump cancer: detection of high risk patient subsets for stomach cancer after remote partial gastrectomy for benign conditions. Cancer Res. 1990;50:6486-6489. [PubMed] |
| 21. | Sharaf RN, Shergill AK, Odze RD, Krinsky ML, Fukami N, Jain R, Appalaneni V, Anderson MA, Ben-Menachem T, Chandrasekhara V, Chathadi K, Decker GA, Early D, Evans JA, Fanelli RD, Fisher DA, Fisher LR, Foley KQ, Hwang JH, Jue TL, Ikenberry SO, Khan KM, Lightdale J, Malpas PM, Maple JT, Pasha S, Saltzman J, Dominitz JA, Cash BD; ASGE Standards of Practice Committee. Endoscopic mucosal tissue sampling. Gastrointest Endosc. 2013;78:216-224. [PubMed] [DOI] [Full Text] |
| 22. | Bessède E, Mégraud F. Microbiota and gastric cancer. Semin Cancer Biol. 2022;86:11-17. [PubMed] [DOI] [Full Text] |
| 23. | Liao O, Ye G, Du Q, Ye J. Gastric microbiota in gastric cancer and precancerous stages: Mechanisms of carcinogenesis and clinical value. Helicobacter. 2023;28:e12964. [PubMed] [DOI] [Full Text] |
| 24. | Wang Y, Han W, Wang N, Han M, Ban M, Dai J, Dong Y, Sun T, Xu J. The role of microbiota in the development and treatment of gastric cancer. Front Oncol. 2023;13:1224669. [PubMed] [DOI] [Full Text] |
| 25. | Salvatori S, Marafini I, Laudisi F, Monteleone G, Stolfi C. Helicobacter pylori and Gastric Cancer: Pathogenetic Mechanisms. Int J Mol Sci. 2023;24. [PubMed] [DOI] [Full Text] |
| 26. | Garza-González E, Bosques-Padilla FJ, El-Omar E, Hold G, Tijerina-Menchaca R, Maldonado-Garza HJ, Pérez-Pérez GI. Role of the polymorphic IL-1B, IL-1RN and TNF-A genes in distal gastric cancer in Mexico. Int J Cancer. 2005;114:237-241. [PubMed] [DOI] [Full Text] |
| 27. | Hirabayashi M, Georges D, Clifford GM, de Martel C. Estimating the Global Burden of Epstein-Barr Virus-Associated Gastric Cancer: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol. 2023;21:922-930.e21. [PubMed] [DOI] [Full Text] |
| 28. | Stanland LJ, Luftig MA. The Role of EBV-Induced Hypermethylation in Gastric Cancer Tumorigenesis. Viruses. 2020;12. [PubMed] [DOI] [Full Text] |
| 29. | Iizasa H, Kartika AV, Fekadu S, Okada S, Onomura D, Wadi AFAA, Khatun MM, Moe TM, Nishikawa J, Yoshiyama H. Development of Epstein-Barr virus-associated gastric cancer: Infection, inflammation, and oncogenesis. World J Gastroenterol. 2022;28:6249-6257. [PubMed] [DOI] [Full Text] |
| 30. | Shikata K, Kiyohara Y, Kubo M, Yonemoto K, Ninomiya T, Shirota T, Tanizaki Y, Doi Y, Tanaka K, Oishi Y, Matsumoto T, Iida M. A prospective study of dietary salt intake and gastric cancer incidence in a defined Japanese population: the Hisayama study. Int J Cancer. 2006;119:196-201. [PubMed] [DOI] [Full Text] |
| 31. | Peleteiro B, Lopes C, Figueiredo C, Lunet N. Salt intake and gastric cancer risk according to Helicobacter pylori infection, smoking, tumour site and histological type. Br J Cancer. 2011;104:198-207. [PubMed] [DOI] [Full Text] |
| 32. | Tricker AR. N-nitroso compounds and man: sources of exposure, endogenous formation and occurrence in body fluids. Eur J Cancer Prev. 1997;6:226-268. [PubMed] [DOI] [Full Text] |
| 33. | Zhang T, Song SS, Liu M, Park S. Association of Fried Food Intake with Gastric Cancer Risk: A Systemic Review and Meta-Analysis of Case-Control Studies. Nutrients. 2023;15. [PubMed] [DOI] [Full Text] |
| 34. | Ladeiras-Lopes R, Pereira AK, Nogueira A, Pinheiro-Torres T, Pinto I, Santos-Pereira R, Lunet N. Smoking and gastric cancer: systematic review and meta-analysis of cohort studies. Cancer Causes Control. 2008;19:689-701. [PubMed] [DOI] [Full Text] |
| 35. | Epplein M, Nomura AM, Wilkens LR, Henderson BE, Kolonel LN. Nonsteroidal antiinflammatory drugs and risk of gastric adenocarcinoma: the multiethnic cohort study. Am J Epidemiol. 2009;170:507-514. [PubMed] [DOI] [Full Text] |
| 36. | Wu CY, Wu MS, Kuo KN, Wang CB, Chen YJ, Lin JT. Effective reduction of gastric cancer risk with regular use of nonsteroidal anti-inflammatory drugs in Helicobacter pylori-infected patients. J Clin Oncol. 2010;28:2952-2957. [PubMed] [DOI] [Full Text] |
| 37. | Huang RJ, Laszkowska M, In H, Hwang JH, Epplein M. Controlling Gastric Cancer in a World of Heterogeneous Risk. Gastroenterology. 2023;164:736-751. [PubMed] [DOI] [Full Text] |
| 38. | Grad C, Grad S, Fărcaş RA, Popa S, Dumitraşcu DL. Changing trends in the epidemiology of gastric cancer. Med Pharm Rep. 2023;96:229-234. [PubMed] [DOI] [Full Text] |
| 39. | Liou JM, Lee YC, El-Omar EM, Wu MS. Efficacy and Long-Term Safety of H. pylori Eradication for Gastric Cancer Prevention. Cancers (Basel). 2019;11. [PubMed] [DOI] [Full Text] |
| 40. | Yan L, Chen Y, Chen F, Tao T, Hu Z, Wang J, You J, Wong BCY, Chen J, Ye W. Effect of Helicobacter pylori Eradication on Gastric Cancer Prevention: Updated Report From a Randomized Controlled Trial With 26.5 Years of Follow-up. Gastroenterology. 2022;163:154-162.e3. [PubMed] [DOI] [Full Text] |
| 41. | Guan WL, He Y, Xu RH. Gastric cancer treatment: recent progress and future perspectives. J Hematol Oncol. 2023;16:57. [PubMed] [DOI] [Full Text] |
| 42. | Pous A, Notario L, Hierro C, Layos L, Bugés C. HER2-Positive Gastric Cancer: The Role of Immunotherapy and Novel Therapeutic Strategies. Int J Mol Sci. 2023;24. [PubMed] [DOI] [Full Text] |
| 43. | Triantafyllidi E, Triantafillidis JK. Systematic Review on the Use of Biosimilars of Trastuzumab in HER2+ Breast Cancer. Biomedicines. 2022;10. [PubMed] [DOI] [Full Text] |
| 44. | Jin X, Liu Z, Yang D, Yin K, Chang X. Recent Progress and Future Perspectives of Immunotherapy in Advanced Gastric Cancer. Front Immunol. 2022;13:948647. [PubMed] [DOI] [Full Text] |
| 45. | Fang X, Xu J, Jin K, Qian J. Combining of immunotherapeutic approaches with chemotherapy for treatment of gastric cancer: Achievements and limitations. Int Immunopharmacol. 2023;118:110062. [PubMed] [DOI] [Full Text] |
| 46. | Entezam M, Sanaei MJ, Mirzaei Y, Mer AH, Abdollahpour-Alitappeh M, Azadegan-Dehkordi F, Bagheri N. Current progress and challenges of immunotherapy in gastric cancer: A focus on CAR-T cells therapeutic approach. Life Sci. 2023;318:121459. [PubMed] [DOI] [Full Text] |
| 47. | Zhang Z, Liu N, Sun M. Research Progress of Immunotherapy for Gastric Cancer. Technol Cancer Res Treat. 2023;22:15330338221150555. [PubMed] [DOI] [Full Text] |
| 48. | Liu Y, Hu P, Xu L, Zhang X, Li Z, Li Y, Qiu H. Current Progress on Predictive Biomarkers for Response to Immune Checkpoint Inhibitors in Gastric Cancer: How to Maximize the Immunotherapeutic Benefit? Cancers (Basel). 2023;15. [PubMed] [DOI] [Full Text] |
| 49. | Peixoto RD, Mathias-Machado MC, Jácome A, Gil M, Fogacci J, Sodré B, Passarini T, Chaves A, Diniz PH, Lino F, Palladino A, Souto M, de Castro AC, Garicochea B. PD-L1 testing in advanced gastric cancer-what physicians who treat this disease must know-a literature review. J Gastrointest Oncol. 2023;14:1560-1575. [PubMed] [DOI] [Full Text] |
| 50. | Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, Yeh KH, Yoshikawa T, Oh SC, Bai LY, Tamura T, Lee KW, Hamamoto Y, Kim JG, Chin K, Oh DY, Minashi K, Cho JY, Tsuda M, Chen LT. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461-2471. [PubMed] [DOI] [Full Text] |
| 51. | Yoon J, Kim TY, Oh DY. Recent Progress in Immunotherapy for Gastric Cancer. J Gastric Cancer. 2023;23:207-223. [PubMed] [DOI] [Full Text] |
| 52. | Boku N, Ryu MH, Kato K, Chung HC, Minashi K, Lee KW, Cho H, Kang WK, Komatsu Y, Tsuda M, Yamaguchi K, Hara H, Fumita S, Azuma M, Chen LT, Kang YK. Safety and efficacy of nivolumab in combination with S-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: interim results of a randomized, phase II trial (ATTRACTION-4). Ann Oncol. 2019;30:250-258. [PubMed] [DOI] [Full Text] |
| 53. | Takei S, Kawazoe A, Shitara K. The New Era of Immunotherapy in Gastric Cancer. Cancers (Basel). 2022;14. [PubMed] [DOI] [Full Text] |
| 54. | Li JX, Huang JM, Jiang ZB, Li RZ, Sun A, Lai-Han Leung E, Yan PY. Current Clinical Progress of PD-1/PD-L1 Immunotherapy and Potential Combination Treatment in Non-Small Cell Lung Cancer. Integr Cancer Ther. 2019;18:1534735419890020. [PubMed] [DOI] [Full Text] |
| 55. | Sato Y, Okamoto K, Kida Y, Mitsui Y, Kawano Y, Sogabe M, Miyamoto H, Takayama T. Overview of Chemotherapy for Gastric Cancer. J Clin Med. 2023;12. [PubMed] [DOI] [Full Text] |
| 56. | Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563-567. [PubMed] [DOI] [Full Text] |
| 57. | Bang YJ, Golan T, Dahan L, Fu S, Moreno V, Park K, Geva R, De Braud F, Wainberg ZA, Reck M, Goff L, Laing N, Mi G, Oliveira JM, Wasserstrom H, Lin CC. Ramucirumab and durvalumab for previously treated, advanced non-small-cell lung cancer, gastric/gastro-oesophageal junction adenocarcinoma, or hepatocellular carcinoma: An open-label, phase Ia/b study (JVDJ). Eur J Cancer. 2020;137:272-284. [PubMed] [DOI] [Full Text] |
| 58. | Spitzer JH, Meadows GG. Modulation of perforin, granzyme A, and granzyme B in murine natural killer (NK), IL2 stimulated NK, and lymphokine-activated killer cells by alcohol consumption. Cell Immunol. 1999;194:205-212. [PubMed] [DOI] [Full Text] |
| 59. | Cesana GC, Romano F, Piacentini G, Scotti M, Brenna A, Bovo G, Vaghi M, Aletti G, Caprotti R, Kaufman H, Uggeri F. Low-dose interleukin-2 administered pre-operatively to patients with gastric cancer activates peripheral and peritumoral lymphocytes but does not affect prognosis. Ann Surg Oncol. 2007;14:1295-1304. [PubMed] [DOI] [Full Text] |
| 60. | Nakano H, Namatame K, Nemoto H, Motohashi H, Nishiyama K, Kumada K. A multi-institutional prospective study of lentinan in advanced gastric cancer patients with unresectable and recurrent diseases: effect on prolongation of survival and improvement of quality of life. Kanagawa Lentinan Research Group. Hepatogastroenterology. 1999;46:2662-2668. [PubMed] |
| 61. | Jiang S, Redelman-Sidi G. BCG in Bladder Cancer Immunotherapy. Cancers (Basel). 2022;14. [PubMed] [DOI] [Full Text] |
| 62. | Popiela T, Kulig J, Czupryna A, Szczepanik AM, Zembala M. Efficiency of adjuvant immunochemotherapy following curative resection in patients with locally advanced gastric cancer. Gastric Cancer. 2004;7:240-245. [PubMed] [DOI] [Full Text] |
| 63. | Lawler SE, Speranza MC, Cho CF, Chiocca EA. Oncolytic Viruses in Cancer Treatment: A Review. JAMA Oncol. 2017;3:841-849. [PubMed] [DOI] [Full Text] |
| 64. | Howells A, Marelli G, Lemoine NR, Wang Y. Oncolytic Viruses-Interaction of Virus and Tumor Cells in the Battle to Eliminate Cancer. Front Oncol. 2017;7:195. [PubMed] [DOI] [Full Text] |
| 65. | Yang A, Zhang Z, Chaurasiya S, Park AK, Lu J, Kim SI, Valencia H, Fong Y, Woo Y. Peritoneal-directed chimeric oncolytic virus CF17 prevents malignant ascites and improves survival in gastric cancer peritoneal metastases. Mol Ther Oncolytics. 2023;31:100734. [PubMed] [DOI] [Full Text] |
| 66. | Yang A, Zhang Z, Chaurasiya S, Park AK, Jung A, Lu J, Kim SI, Priceman S, Fong Y, Woo Y. Development of the oncolytic virus, CF33, and its derivatives for peritoneal-directed treatment of gastric cancer peritoneal metastases. J Immunother Cancer. 2023;11. [PubMed] [DOI] [Full Text] |
| 67. | Hori N, Tazawa H, Li Y, Okura T, Kikuchi S, Kuroda S, Ohara T, Noma K, Nishizaki M, Urata Y, Kagawa S, Fujiwara T. Intraperitoneal Administration of p53-armed Oncolytic Adenovirus Inhibits Peritoneal Metastasis of Diffuse-type Gastric Cancer Cells. Anticancer Res. 2023;43:4809-4821. [PubMed] [DOI] [Full Text] |
| 68. | Pan RY, Chung WH, Chu MT, Chen SJ, Chen HC, Zheng L, Hung SI. Recent Development and Clinical Application of Cancer Vaccine: Targeting Neoantigens. J Immunol Res. 2018;2018:4325874. [PubMed] [DOI] [Full Text] |
| 69. | Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69-74. [PubMed] [DOI] [Full Text] |
| 70. | Zhang Y, Li K, Bi Y, Li X, Shan B, Hu D, Zhao L. [Research progress of Helicobacter pylori vaccine]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2023;39:564-570. [PubMed] |
| 71. | Blass E, Ott PA. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat Rev Clin Oncol. 2021;18:215-229. [PubMed] [DOI] [Full Text] |
| 72. | Ramos CA, Heslop HE, Brenner MK. CAR-T Cell Therapy for Lymphoma. Annu Rev Med. 2016;67:165-183. [PubMed] [DOI] [Full Text] |
| 73. | Faghfuri E, Shadbad MA, Faghfouri AH, Soozangar N. Cellular immunotherapy in gastric cancer: adoptive cell therapy and dendritic cell-based vaccination. Immunotherapy. 2022;14:475-488. [PubMed] [DOI] [Full Text] |
| 74. | Sagebiel AF, Steinert F, Lunemann S, Körner C, Schreurs RRCE, Altfeld M, Perez D, Reinshagen K, Bunders MJ. Tissue-resident Eomes(+) NK cells are the major innate lymphoid cell population in human infant intestine. Nat Commun. 2019;10:975. [PubMed] [DOI] [Full Text] |
| 75. | Kumar A, Watkins R, Vilgelm AE. Cell Therapy With TILs: Training and Taming T Cells to Fight Cancer. Front Immunol. 2021;12:690499. [PubMed] [DOI] [Full Text] |
| 76. | Wang X, Tang S, Cui X, Yang J, Geng C, Chen C, Zhou N, Li Y. Cytokine-induced killer cell/dendritic cell-cytokine-induced killer cell immunotherapy for the postoperative treatment of gastric cancer: A systematic review and meta-analysis. Medicine (Baltimore). 2018;97:e12230. [PubMed] [DOI] [Full Text] |
| 77. | 77. Bębnowska D, Grywalska E, Niedźwiedzka-Rystwej P, Sosnowska-Pasiarska B, Smok-Kalwat J, Pasiarski M, Góźdź S, Roliński J, Polkowski W. CAR-T Cell Therapy-An Overview of Targets in Gastric Cancer. J Clin Med.. 2020;9:1894. [PubMed] [DOI] [Full Text] |
| 78. | Mylod E, Lysaght J, Conroy MJ. Natural killer cell therapy: A new frontier for obesity-associated cancer. Cancer Lett. 2022;535:215620. [PubMed] [DOI] [Full Text] |
| 79. | Hong JY, An JY, Lee J, Park SH, Park JO, Park YS, Lim HY, Kim KM, Kang WK, Kim ST. Claudin 18.2 expression in various tumor types and its role as a potential target in advanced gastric cancer. Transl Cancer Res. 2020;9:3367-3374. [PubMed] [DOI] [Full Text] |
| 80. | Singh P, Toom S, Huang Y. Anti-claudin 18.2 antibody as new targeted therapy for advanced gastric cancer. J Hematol Oncol. 2017;10:105. [PubMed] [DOI] [Full Text] |
| 81. | Türeci O, Sahin U, Schulze-Bergkamen H, Zvirbule Z, Lordick F, Koeberle D, Thuss-Patience P, Ettrich T, Arnold D, Bassermann F, Al-Batran SE, Wiechen K, Dhaene K, Maurus D, Gold M, Huber C, Krivoshik A, Arozullah A, Park JW, Schuler M. A multicentre, phase IIa study of zolbetuximab as a single agent in patients with recurrent or refractory advanced adenocarcinoma of the stomach or lower oesophagus: the MONO study. Ann Oncol. 2019;30:1487-1495. [PubMed] [DOI] [Full Text] |
| 82. | Sahin U, Türeci Ö, Manikhas G, Lordick F, Rusyn A, Vynnychenko I, Dudov A, Bazin I, Bondarenko I, Melichar B, Dhaene K, Wiechen K, Huber C, Maurus D, Arozullah A, Park JW, Schuler M, Al-Batran SE. FAST: a randomised phase II study of zolbetuximab (IMAB362) plus EOX vs EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma. Ann Oncol. 2021;32:609-619. [PubMed] [DOI] [Full Text] |
| 83. | Shitara K, Lordick F, Bang YJ, Enzinger PC, Ilson DH, Shah MA, Cutsem EV, Xu RH, Aprile G, Xu J, Chao J, Pazo-Cid R, Kang YK, Yang J, Moran DM, Bhattacharya PP, Arozullah A, Park JW, Ajani JA. Zolbetuximab + mFOLFOX6 as first-line (1L) treatment for patients (pts) withclaudin-18.2+ (CLDN18.2+) / HER2− locally advanced (LA) unresectable or metastatic gastric or gastroesophageal junction (mG/GEJ) adenocarcinoma: primary results from phase 3 SPOTLIGHT study. J Clin Oncol. 2023;41:LBA292. [DOI] [Full Text] |
| 84. | Xu RH, Shitara K, Ajani JA, Bang YJ, Enzinger PC, Ilson DH, Lordick F, Cutsem EV, Plazas JG, Huang J, Shen L, Oh SC, Sunpaweravong P, Hoo HFS, Turk HM, Park JW, Moran DM, Bhattacharya PP, Arozullah A, Shah MA. Zolbetuximab + CAPOX in 1L claudin-18.2+ (CLDN18.2+)/HER2− locally advanced (LA) or metastatic gastric or gastroesophageal junction (mG/GEJ) adenocarcinoma: Primary phase 3 results from GLOW. J Clin Oncol. 2023;41:405736-405736. [DOI] [Full Text] |
| 85. | Jiang H, Shi Z, Wang P, Wang C, Yang L, Du G, Zhang H, Shi B, Jia J, Li Q, Wang H, Li Z. Claudin18.2-Specific Chimeric Antigen Receptor Engineered T Cells for the Treatment of Gastric Cancer. J Natl Cancer Inst. 2019;111:409-418. [PubMed] [DOI] [Full Text] |
| 86. | Hayakawa Y, Sakitani K, Konishi M, Asfaha S, Niikura R, Tomita H, Renz BW, Tailor Y, Macchini M, Middelhoff M, Jiang Z, Tanaka T, Dubeykovskaya ZA, Kim W, Chen X, Urbanska AM, Nagar K, Westphalen CB, Quante M, Lin CS, Gershon MD, Hara A, Zhao CM, Chen D, Worthley DL, Koike K, Wang TC. Nerve Growth Factor Promotes Gastric Tumorigenesis through Aberrant Cholinergic Signaling. Cancer Cell. 2017;31:21-34. [PubMed] [DOI] [Full Text] |
| 87. | Hata M, Kinoshita H, Hayakawa Y, Konishi M, Tsuboi M, Oya Y, Kurokawa K, Hayata Y, Nakagawa H, Tateishi K, Fujiwara H, Hirata Y, Worthley DL, Muranishi Y, Furukawa T, Kon S, Tomita H, Wang TC, Koike K. GPR30-Expressing Gastric Chief Cells Do Not Dedifferentiate But Are Eliminated via PDK-Dependent Cell Competition During Development of Metaplasia. Gastroenterology. 2020;158:1650-1666.e15. [PubMed] [DOI] [Full Text] |
| 88. | Oya Y, Hayakawa Y, Koike K. Tumor microenvironment in gastric cancers. Cancer Sci. 2020;111:2696-2707. [PubMed] [DOI] [Full Text] |
| 89. | van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84:523-538. [PubMed] [DOI] [Full Text] |
| 90. | Babic A. Knowledge discovery for advanced clinical data management and analysis. Stud Health Technol Inform. 1999;68:409-413. [PubMed] |
| 91. | Synnestvedt MB, Chen C, Holmes JH. CiteSpace II: visualization and knowledge discovery in bibliographic databases. AMIA Annu Symp Proc. 2005;2005:724-728. [PubMed] |
| 92. | Yao Z, Lin Z, Wu W. Global research trends on immunotherapy in cancer: A bibliometric analysis. Hum Vaccin Immunother. 2023;19:2219191. [PubMed] [DOI] [Full Text] |
| 93. | Chen J, Xu Z, Hu C, Zhang S, Zi M, Yuan L, Cheng X. Targeting CLDN18.2 in cancers of the gastrointestinal tract: New drugs and new indications. Front Oncol. 2023;13:1132319. [PubMed] [DOI] [Full Text] |
| 94. | Guo Y, Chu HZ, Xu JG. Research progress of immune heckpoint LAG-3 in gastric cancer: a narrative review. Eur Rev Med Pharmacol Sci. 2023;27:248-255. [PubMed] [DOI] [Full Text] |