INTRODUCTION
Aging is broadly defined as the time-dependent decline in function that affects most organisms. López-Otín et al[1], in conjunction with recent research findings, has revised the concept of “aging hallmarks” by incorporating an additional 12 aging features, including macroautophagy deficiencies, chronic inflammation, and dysbiosis. Owing to advancements in living standards, economic growth, and healthcare infrastructure, population aging has emerged as the primary demographic phenomenon globally. Currently, the proportion of elderly people over the age of 60 worldwide is about one ninth, with projections indicating that by the year 2050, this ratio will escalate to one-fifth[2]. Human aging is characterized by molecular, structural, and functional alterations in various organ systems, including the liver. Within the intensifying milieu of aging, age-associated liver dysfunction has become a compelling clinical challenge that demands urgent attention and resolution.
Cellular damage, if left unattended or irreparable, can lead to cellular apoptosis or senescence, which is the fundamental cellular process employed by the organism in its defense against cancer. Similarly, following exposure to damage and stress signals, aging can irreversibly arrest the G0/G1 phase of the cell cycle. This phenomenon restricts the proliferative potential of damaged cells[3], ultimately leading to changes in the microenvironment and tissue homeostasis. Upon entering a state of cellular senescence, cells undergo morphological transformations characterized by permanent cell cycle arrest, displaying distinctive traits such as altered secretion profiles, macromolecular damage, and metabolic shifts. These alterations include an upregulated expression of senescence-associated β-galactosidase, acquisition of the senescence-associated secretory phenotype (SASP), elevated levels of P16INK4a (P16) and P21Cip1/Waf1 (P21), and increased levels of reactive oxygen species (ROS)[4]. In particular, the senescence of hepatic cells can potentially contribute to intracellular lipid accumulation, fibrosis, and inflammation, while concurrently secreting age-associated inflammatory mediators.
In 2023, the global hepatology community renamed nonalcoholic fatty liver disease (NAFLD) as metabolic dysfunction-associated steatotic liver disease (MASLD). MASLD is the most common chronic liver disease worldwide. Patients presenting hepatic steatosis and at least one of five cardiometabolic risk factors are diagnosed with MASLD. Generally, the initial hepatic steatosis observed in MASLD is considered reversible. However, persistent metabolic disturbances can lead to aberrant liver cell metabolism, resulting in the accumulation of lipids, such as fatty acids, cholesterol, and other lipid metabolites. This, in turn, induces the onset of MASLD. Excessive lipid accumulation in the liver can lead to lipotoxicity, mitochondrial dysfunction, increased ROS levels, and inflammation. These events signify the transition from MASLD to metabolic dysfunction-associated steatohepatitis (MASH) or the progression to more severe stages[5]. Younossi et al[6] conducted an extensive analysis involving 8515431 samples from 22 countries and demonstrated that the global prevalence of MASLD was 25.24%. The highest rates of prevalence were identified in the Middle East and South America, with Africa exhibiting the lowest prevalence. Notably, MASLD exhibited strong associations with the components of metabolic syndrome, including obesity, diabetes, and dyslipidemia. The median age of the MASLD population in the United States in 2015 was 50 years old. Researchers predict that it will increase to 55 years old by 2030. This indicates that with an increase in population aging, the incidence of MASLD-related liver diseases and mortality rates will increase in the United States[7].
RELATIONSHIP BETWEEN AGING AND MASLD
The aging phenotype exhibits remarkable stability, displaying resistance to mitotic stimuli and apoptosis[8]. In healthy cells, senescence may be triggered primarily by factors such as oxidative stress, mitochondrial imbalance, and chronic inflammation either independently or synergistically. The progressive accumulation of senescent cells can elicit bystander effects, ultimately leading to organ aging and functional impairments[4]. Age dependence is the main risk factor for chronic liver diseases, including MASLD and MASH. The accumulation of aging cells drives liver steatosis, and liver cell aging is closely related to the progression of MASLD[9]. The intricate mechanisms governing the pathogenesis of MASLD are not completely clear. Elevated hepatic lipid accumulation is a significant risk factor for MASLD, with hepatic cell senescence playing a contributory role in the development of hepatic steatosis. This can be best described by the “two-hit” hypothesis. “First hit” denotes the initial occurrence of excessive hepatic lipid accumulation due to alterations in lipid metabolism, which leads to the development of nonalcoholic fatty liver (NAFL). The “second hit” entails the induction of hepatic cell damage, including oxidative stress, inflammatory cytokine release, and mitochondrial dysfunction, building upon the backdrop of hepatic lipotoxicity. This second hit can precipitate the progression of NAFL towards MASH and, in more severe cases, fibrosis, cirrhosis, hepatocellular carcinoma, and even death[10]. In recent years, the “multiple-hit” hypothesis has emerged as a more elaborate concept for elucidating the pathogenesis of MASLD. This theory underscores the pivotal roles played by gut microbiota (GM), insulin resistance, and adipose tissue-derived adipokines. These factors, through their interconnected relationships along the gut–liver axis, interact synergistically and causally, contributing to the progression of MASLD[11].
Oxidative stress
In response to oxidative stress, organisms generate substances, including ROS or free radicals, that can inflict damage on cellular constituents such as the cell membrane, proteins, and DNA. This damage can ultimately lead to cellular senescence or even apoptosis. The acceleration of oxidative stress due to hepatocyte aging reportedly augments lipid accumulation in the liver, suggesting the pivotal role of oxidative stress in the etiology and progression of MASLD. Oxidative stress initiates DNA damage, as well as instigates autophagy and the secretion of SASP. These processes are concomitant with the activation of the p53-p21 and p16-Rb pathways, leading to premature cellular senescence. Concurrently, oxidative stress exacerbates disruptions in lipid metabolism, promotes inflammatory responses, and induces hepatocyte damage[4]. During the normal aging process of the liver, hepatic stellate cells help drive macrophage differentiation towards the M1 phenotype, exerting anti-tumor effects. Conversely, a study has found that p53-knockout hepatic stellate cells promote macrophage differentiation towards the pro-tumor M2 phenotype, thereby inducing the progression of liver fibrosis, cirrhosis, and even HCC[12].
MASLD is characterized by increased levels of senescent cells compared with the control group. Additionally, patients with MASLD demonstrate significantly higher telomere shortening, along with increased cell cycle arrest, which coincides with an increased expression of p21[13]. Reportedly, Krüppel-like factor 16 (KLF16) can bind to the promoter of peroxisome proliferator-activated receptor alpha (PPARα), thereby activating it. Upregulation of KLF16 expression expedites fatty acid β-oxidation and mitigates oxidative stress responses in db/db and high-fat diet (HFD) mice. This, in turn, results in a reduction in hepatic lipid accumulation and an improvement in MASLD[14]. Oxidative stress induced cellular aging can alter the liver microenvironment, leading to disease progression from simple steatosis to inflammation and fibrosis, as well as hepatocellular carcinoma.
Autophagy
In hepatic cells, autophagy is subject to modulation by both individual and organ-level aging processes, with pronounced attenuation of hepatic autophagy evident in the aging state[15]. The process of aging decreases the quantity and efficiency of autophagosomes, subsequently causing a buildup of lipid droplets and resultant liver damage. Autophagy inhibition is posited as a potential risk factor in the progression of age-related MASLD. An animal study has revealed that the administration of plasma from younger mice restores autophagic activity in aged mice, effectively mitigating liver aging, lipid accumulation, and fibrosis[16]. Using Atg7-deficient mice, researchers have substantiated that the autophagic impairment in liver sinusoidal endothelial cells not only accelerates liver inflammation and fibrosis in the early phases of MASLD but also exacerbates hepatic inflammation and fibrosis during the advanced stages[17].
Mice deficient in Omi/HtrA2 exhibit premature aging symptoms and age-related autophagy inhibition, which results in hepatic dysfunction[18]. Therefore, augmenting autophagy may mitigate aging and hepatic steatosis, thus alleviating MASLD. Mice overexpressing Omi/HtrA2 have demonstrated enhanced autophagic activity, diminished hepatic steatosis, and elevated hepatic fatty acid β-oxidation, which ameliorated HFD-induced MASLD along with hepatic inflammation[19]. P62/sequestosome 1 facilitates the phosphorylation of unc-51 Like autophagy activating kinase 1, thereby promoting autophagy activation and triggering NFE2L2/NRF2 activation. This protects mouse liver cells against lipotoxic damage[20]. AMPK activation is a regulator of autophagy and aging-related changes in AMPK activation may impact autophagic processes, which results in decreased formation of autophagosomes and further hastening of the aging process[21]. In hepatic cells, PPARδ activates the autophagy-lysosome pathway via the AMPK/mammalian target of rapamycin (mTOR) signaling to induce fatty acid β-oxidation, which reduces hepatic lipid levels[22]. Autophagy changes may be an important target for the treatment of age-related MASLD.
Mitochondrial homeostasis
In mouse liver, increased mitochondrial ROS levels are linked with the aging process. Oxidative stress triggers mutations in the mitochondrial DNA, leading to the accumulation of mitochondrial DNA fragments within the cell nucleus. This accumulation subsequently contributes to mitochondrial dysfunction[23]. It has been shown that the absence of the gene encoding the nonhomologous end-joining enzyme known as DNA ligase IV (DNL4) exacerbates linear mitochondrial DNA (mtDNA) aggregation in the nucleus. Cheng et al[24], proposed that linear nuclear mtDNA fragments accelerate aging in yme1-1 mutant cells by affecting nuclear DNA replication, recombination, repair, and transcription. In addition, mice deficient in the antioxidant enzyme superoxide dismutase 1 (SOD1) demonstrate premature aging, along with hepatic damage. Investigations have unveiled that SOD1-deficient (SOD1-/-) mice display shifts in the composition of their GM, including alterations in the ratio of Firmicutes and Bacteroidetes, a significant reduction in lactobacilli, increased hepatic metabolites, and manifestation of a systemic aging phenotype[25]. Moreover, cellular senescence results in mitochondrial dysfunction, which induces respiratory chain disturbances, membrane potential anomalies, and concomitant ROS generation, all of which further induce the development of MASLD[26].
Free fatty acids in the liver are primarily metabolized via two pathways: mitochondrial β-oxidation and esterification into triglycerides. The preservation of mitochondrial homeostasis is a pivotal factor in hepatic lipid metabolism. However, excessive free fatty acids burden the process of mitochondrial β-oxidation, subsequently leading to an imbalance in mitochondrial homeostasis. This imbalance further exacerbates the accumulation of lipids within the hepatic cells, ultimately contributing to the development of MASLD[27]. Omi/HtrA2 is a mitochondrial serine protease and a pro-apoptotic factor that plays a pivotal role in maintaining mitochondrial homeostasis[28]. Within the liver, Omi/HtrA2 mediates mitochondrial stability and autophagy, thus contributing to the amelioration of MASLD. Reportedly, the overexpression of HtrA2/Omi in the mouse liver enhances the expression of genes related to mitochondrial fatty acid β-oxidation, reduces hepatic lipid accumulation, and regulates glucose homeostasis[19]. KLF16 tightly links hepatic lipid metabolism to mitochondrial homeostasis by regulating the transcriptional activity of PPARα. Knockdown of hepatic KLF16 also leads to increased mitochondrial stress and promotes the development of hepatic steatosis and insulin resistance in mice, whereas hepatic-specific PPARα overexpression effectively ameliorates hepatic steatosis induced by KLF16 deficiency and improves mitochondrial imbalance and insulin resistance[14]. The mitochondrial homeostasis is closely related associated with hepatic lipid metabolism and exacerbates the development of MASLD.
Bile acid-mediated metabolism homeostasis
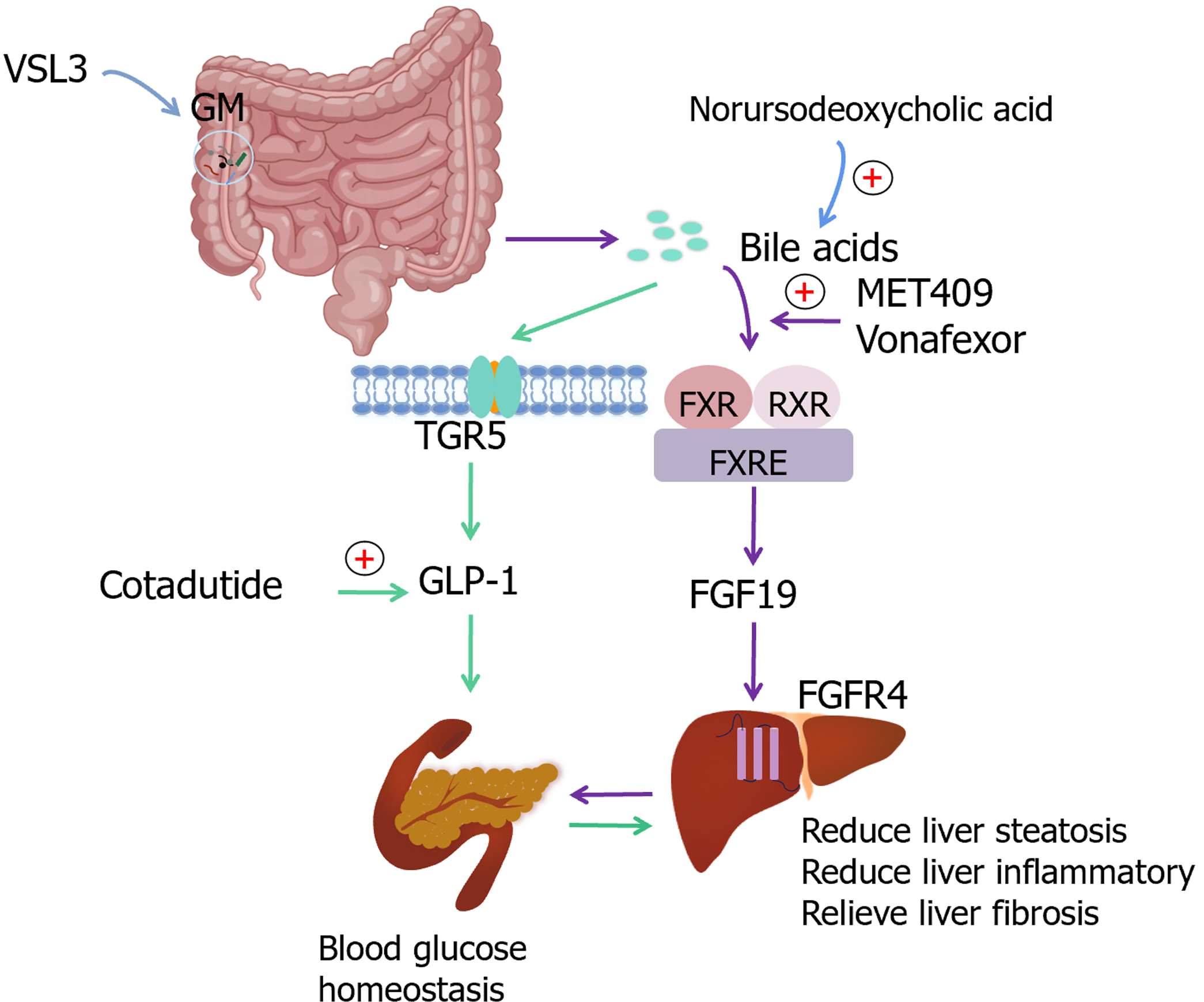
Metabolic irregularities serve as both inducers and outcomes of the aging process and are intricately intertwined in the development of various diseases[29]. Hepatic metabolism declines with aging and is accompanied by a reduction in enzymatic activity, owing to which the elderly are more susceptible to lipid accumulation. Bile acids (Bas), in conjunction with their homologous receptors such as the farnesoid X receptor (FXR) and Takeda G-protein-coupled receptor 5, play integral roles in numerous signaling pathways that are closely linked with MASLD. These pathways encompass BA self-regulation, glucose and lipid metabolism, energy modulation, cell proliferation, detoxification, and immune regulation[30]. BA analogs targeting FXR, TGR5, or both have been shown to effectively mitigate the progression of MASH[31].
BAs regulate metabolism by interacting with nuclear receptors and tightly modulating the diversity and relative abundance of the GM. The GM, along with their metabolic byproducts, may mediate MASLD by inducing endotoxemia, triggering insulin resistance, increasing short-chain fatty acids, elevating endogenous ethanol production, altering choline and BA metabolism, and impacting the host’s immune response[32]. Therefore, the disruption of intracellular BA homeostasis may be a pivotal factor influencing the development of MASLD.
Dysbiosis
Aging results in the restructuring of the GM and is characterized by a decreased Firmicutes-to-Bacteroidetes ratio and reduced overall microbial diversity[33]. The lipopolysaccharide (LPS)/toll-like receptor 4 (TLR4) signaling pathway plays a crucial role in mediating the pathological mechanisms of MASLD. Patients with MASLD demonstrate an overgrowth of intestinal bacteria, which disrupts the intestinal barrier function. This altered gut barrier permeability leads to the translocation of LPS that triggers the activation of the LPS/TLR4/nuclear factor-κB (NF-κB) signaling pathway. That mediates the progression of MASLD to MASH[34]. A study has demonstrated that TLR4-deficient mice showed amelioration in insulin resistance and hepatic steatosis induced by HFD[35]. However, another study revealed that TLR5-deficient mice exhibited characteristics of metabolic syndrome, such as obesity, insulin resistance, and hepatic steatosis. Furthermore, transplanting the GM from TLR5-deficient mice into healthy mice exhibited the performance of metabolic syndrome in healthy mice[36].
Reportedly, GM additionally stimulates the generation of endogenous ethanol[37]. Dysbiosis within the gut microbiome can lead to the overgrowth of ethanol-producing bacteria, resulting in an increase in endogenous ethanol levels and subsequent induction of MASH. This has been demonstrated by Yuan et al[38], who isolated a high-alcohol-producing strain of Klebsiella pneumoniae (HiAlc Kpn) from the fecal samples of patients with Auto-brewery syndrome(ABS)/MASH and found that orally administering the strain to healthy sterile mice induced hepatic steatosis. Their results revealed that HiAlc-Kpn induced mouse MASLD model, the high-alcohol-producing strain of K. pneumoniae, upon colonization in the mouse gut, induced endogenous ethanol production that subsequently impaired the intestinal mucosal barrier. This resulted in heightened intestinal permeability in mice, which exacerbated inflammation in MASLD. Additionally, the transplantation of GM from younger mice to older mice could reverse age-related changes in the gut, eyes, and brain. Aged mice receiving young donor microbiota had reduced cortical and callosal microglia, reduced expression of inflammatory complement protein C3 in the retina, and reduced circulating concentrations of lipopolysaccharide (LPS)-binding protein (LBP), to levels comparable to young mice[39]. Furthermore, Hoyles et al[40] transplanted GM from patients with MASLD into mice maintained on a normal diet and found that the mice developed hepatic steatosis and their gut microbial characteristics realigned to those observed in MASLD.
POTENTIAL THERAPEUTIC TARGETS FOR MASLD
Regarding MASH treatment, this article predominantly focuses on clinical trials assessing agents targeting metabolic pathways, cellular stress responses, and interactions with GM.
Drugs targeting metabolism
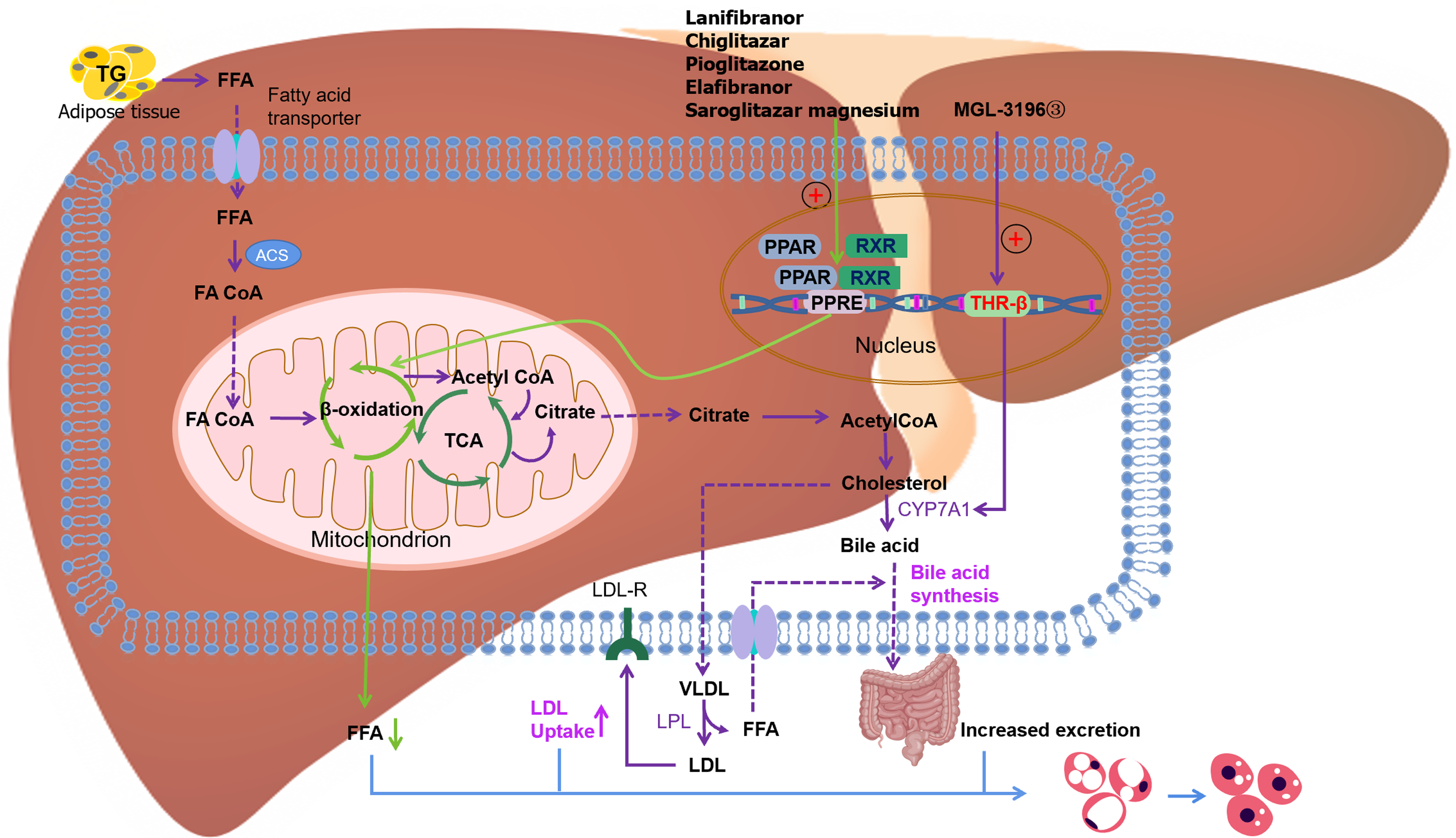
FXR agonists: FXR plays a pivotal role in regulating lipid metabolism, BA homeostasis, and glucose equilibrium. The dysregulation of FXR function has been implicated in the pathogenesis of MASLD, cholestasis, and chronic inflammatory disorders that affect the liver and gastrointestinal tract. Despite a multitude of clinical trials targeting FXR for the management of MASLD/MASH, to date, only obeticholic acid has received approval for the treatment of primary biliary cholangitis. MET409[41] is another agent that has a unique non-BA composition and sustained pharmacokinetic/pharmacodynamic properties. A study has assessed the efficacy of oral MET409 administered once daily over 12 wk in patients with MASH. By the end of the 12-week treatment cycle, MET409 remarkably reduced hepatic fat levels, with an average reduction of 55% (80 mg) and 38% (50 mg) compared with the 6% reduction observed with placebo (P < 0.001). Vonafexor (EYP001a) is a second-generation synthetic, non steroidal, non-bile salt, orally active carboxylic acid FXR agonist currently in development. In patients with MASH and suspected fibrosis, vonafexor has demonstrated efficacy in reducing hepatic fat content and improving liver enzymes. Specifically, 50.0% and 39.3% of patients treated with VONA-100 mg and VONA-200 mg once daily, respectively, demonstrated a reduction in hepatic fat levels of > 30%, whereas only 12.5% of patients treated with placebo demonstrated this effect[42].
PPARs agonists: PPARs represent a subgroup of nuclear transcription factors activated by ligands and are categorically classified within the nuclear receptor superfamily. Post activation, PPARs form heterodimers with retinoid X receptors (RXRs). The resultant PPARγ–RXR heterodimer binds to peroxisome proliferator response elements that are situated upstream of target gene promoters, consequently modulating the transcription of these specific target genes. The PPARs comprise PPARα, PPARβ/δ, and PPARγ that function as sensitive receptors for fatty acids and their derivatives, exerting crucial roles in lipid metabolism[43]. Notably, they can effectively mitigate hepatic steatosis, inflammation, and fibrosis in preclinical models of MASLD, thus underscoring their potential as promising targets for MASLD treatment. Selective agonists targeting PPARα and PPARγ have already demonstrated clinical efficacy, while clinical trials are currently assessing PPARγ agonists such as pioglitazone; dual PPARα/δ agonists such as chiglitazar, saroglitazar magnesium, and elafibranor; and pan-PPAR agonists such as lanifibranor. The activation of intestinal PPARα signaling plays a role in upregulating the expression of fatty acid-binding protein 1, thus facilitating intestinal fatty acid uptake and potentially contributing to the progression of MASH[44]. Elafibranor can improve steatosis, mitigate inflammation, and attenuate fibrosis in rodent models of MASLD/MASH[45]. Moreover, human liver in vitro models have unveiled that PPAR agonists can effectively diminish the increase in lipid levels, quell the secretion of inflammatory chemokines, and modulate the expression of pro-fibrotic genes via diverse mechanisms[46].
Glucagon-like peptide-1 agonists: The discovery of glucagon-like peptide-1 (GLP-1) represents a pivotal milestone in the field of biology, as the molecule significantly affects the regulation of blood glucose levels and the management of body weight. Cotadutide, an agonist of GLP-1R/GcgR, improves MASH and liver fibrosis by regulating mitochondrial function and lipid biosynthesis. Notably, in C6BL29/J mice exposed to an Amylin liver MASH diet for 57 wk, cotadutide displayed greater efficacy in treating MASH than cotadutide combined with obeticholic acid[47]. Reportedly, the dual agonist GLP-1-Fc-FGF21 D1 exhibits notable and sustained hypoglycemic effects in diabetic mouse models. Moreover, in an HFD-induced ob/ob mouse model, GLP-1-Fc-FGF21 D1 has demonstrated robust anti-MASH properties via significant enhancements in liver function, alterations in serum and hepatic lipid profiles, and reduction in the MASLD activity score. Remarkably, its therapeutic efficacy surpasses that of singular FGF21 or GLP-1 analogs[48].
Thyroid hormone receptor-beta agonist: Activation of hepatic thyroid hormone receptor-beta (THR-β) reduces systemic lipid levels, enhances BA synthesis, and promotes lipid oxidation. Resmetirom (MGL-3196) is a liver-targeted and selective THR-β agonist. Studies have demonstrated that administering resmetirom (MGL-3196) to mice with diet-induced fibrotic DIO-MASH can lead to substantial reductions in liver weight, hepatic steatosis, plasma ALT levels, and hepatic and plasma cholesterol levels, as well as a decrease in blood glucose levels. Moreover, the treatment remarkably improved the MASLD activity score, with no discernible impact on body weight[49]. Compared with the placebo group, the resmetirom group was more effective in mitigating hepatic steatosis and ameliorating liver enzyme levels as well as inflammatory markers, resulting in a pronounced improvement in MASH as evidenced by liver biopsy assessments[50]. Furthermore, both 80 and 100 mg resmetirom result in favorable tolerability profiles, devoid of any severe or serious adverse events. The Phase 3 MASH clinical trial (NCT03900429) is now underway and is poised to enroll 900 volunteers with liver biopsy MASH with fibrosis grades I-III in a multinational, double-blind, randomized, placebo-controlled study to address the progression of MASH, cirrhosis, and/or hepatic decompensation (Figure 1).
Figure 1 Signaling pathways of proliferator-activated receptor alpha and thyroid hormone receptor-beta and drugs targeting these pathways.
PPAR: Proliferator-activated receptor; TG: Triglyceride; FFA: Free fatty acid; ACS: Acyl coenzyme A synthetase; FA CoA: Fatty acyl coenzyme A; TCA: Tricarboxylic acid cycle; RXR: Retinoid X receptor; PPRE: PPAR reaction element; THR: Thyroid hormone receptor-beta; LDL: Low-density lipoprotein; LDL-R: Low-density lipoprotein receptor; VLDL: Very low-density lipoprotein.
Other agents: Sodium-dependent glucose co-transporter 2 (SGLT2) inhibitors are a novel class of antidiabetic drugs that inhibit glucose reabsorption in the proximal renal tubules, and have been shown to be effective in reducing hepatic fat content and AST/ALT levels and ameliorating hepatic fibrosis in several studies. Specifically, empagliflozin prevents MASLD progression in ApoE (-/-) mice by inducing autophagy via increased AMPK phosphorylation, reduced mTOR activity, and elevated LC3B expression. Furthermore, it mitigates endoplasmic reticulum stress and inhibits hepatocyte apoptosis[51]. Dapagliflozin, another SGLT2 inhibitor, has been found to activate AMPK and reduce mTOR phosphorylation in Zucker diabetic fatty rats. This effect has additionally been replicated in LO2 cells and HepG2 cells stimulated with palmitic acid. Consequently, the activation of AMPK promotes fatty acid oxidation and induces autophagy, ultimately improving hepatic steatosis[52]. Reportedly, fructose is catalyzed by ketohexokinase to produce fructose-1-phosphate, a metabolite that is primarily metabolized within the liver. Inhibition of fructose metabolism using ketohexokinase inhibitors can mitigate hepatic injury and fibrosis in both murine models and human subjects[53]. High fructose intake induces de novo lipid biosynthesis in the liver. This process does not depend on ATP citrate lyase (ACLY) but rather on the intestinal microflora which metabolizes fructose to acetate and converts the latter to acetyl coenzyme A (acetyl-CoA). Altered intestinal permeability, gut dysbiosis, and increased fructose intake exacerbate hepatic lipid accumulation and contribute to the development of MASLD in elderly patients[54].
Cell stress and cellular senescence
ROS inhibition: Excessive oxidative stress culminates in hepatocyte senescence, thereby instigating the accrual of hepatic fat[55]. Furthermore, hepatic lipotoxicity triggers oxidative stress and the release of inflammatory cytokines, thereby fostering the progression of MASH and, in more severe cases, fibrosis. Senolytics are a class of selective drugs that kill aging cells and can be used for the targeted intervention of cellular senescence. Quercetin, as an example, can elevate hepatic levels of SOD, catalase, and glutathione, concurrently decreasing interleukin (IL)-1β, IL-6, tumor necrosis factor-α, and hepatic lipid accumulation in db/db mice. Furthermore, it can activate the FXR1/TGR5 signaling pathway, thereby contributing to the amelioration of MASLD[56]. In addition, quercetin demonstrates the ability to regulate GM dysbiosis and attenuate endotoxemia-induced upregulation of the TLR-4 pathway. This results in the inhibition of inflammasome activation and stress pathway activation, which reinstates the equilibrium in lipid metabolism gene expression[57]. An ongoing clinical trial (NCT05506488) assessing a combination of dasatinib and quercetin for the clearance of senescent cells offers a potential avenue for addressing MASLD-associated fibrosis. N-acetylcysteine (NAC), an antioxidant with the capacity to reduce ROS levels and induce cellular apoptosis, can significantly reduce obesity, dyslipidemia, hepatic dysfunction, and GM dysbiosis induced by HFD in murine models. However, it is noteworthy that in these mice, using antibiotics for GM depletion resulted in a resurgence of hepatic steatosis and liver injury[58]. Another study has found that supplementing NAC to mice with diet-induced obesity and non-alcoholic steatohepatitis increases the CD4+ T cell population within liver tumor cells. Additionally, it elevates the levels of immunotherapeutic agents M30 and aOX40, thus effectively inhibiting the progression of liver tumors[59].
Myeloperoxidase inhibitor: Recent studies have revealed that patients with MASLD have elevated levels of plasma myeloperoxidase (MPO) and increased hepatic MPO expression compared with healthy controls. Notably, this elevation is more pronounced in patients with MASH. Studies have demonstrated that treatment with the MPO inhibitor AZM198 results in significantly reduced MASH-induced liver damage and fibrosis as well as decreased serum ALT levels and amelioration of hepatic steatosis[60]. Specifically in the elderly population, MASLD was associated with notable alterations in GM abundance, which resulted in compromised gut barrier function and heightened susceptibility to intestinal inflammation and subsequent systemic inflammatory responses. The administration of AZM198 improved the Firmicutes-to-Bacteroidetes ratio and regulated GM composition. Reportedly, TXNIP-deficient mice demonstrated decreased expression of inflammatory factors, reduced LPS levels, improved liver health, and restored intestinal barrier function. Notably, TXNIP is significantly upregulated in the intestinal mucosa of MASH mice. Moreover, studies have demonstrated that inhibiting the activation of the TXNIP-NLRP3 axis can effectively reduce MPO activity and oxidative stress, leading to the restoration of intestinal barrier function in the context of MASH. MPO Inhibitors reduce liver lipid accumulation, inflammation, and fibrosis and ameliorate the development of MASH.
PTUPB inhibitor: PTUPB, a dual inhibitor of soluble epoxide hydrolase and cyclooxygenase-2, exerts its effects by suppressing the PI3K/AKT/mTOR pathway via the modulation of Sirt3[61]. This leads to increased autophagy and decreased hepatocyte senescence, thus inducing the progression of MASLD. Reportedly, PTUPB can mitigate liver injury in HFD-induced MASLD murine models by inducing collagen deposition and lipid accrual; reducing hepatic triglyceride levels; and suppressing the expression of liver aging-associated molecules, such as p16, p53, and p21. Aspirin, an oral irreversible inhibitor of the cyclooxygenases COX-1 and COX-2, prevents the development of MASLD and MASH-related HCC[62].
Other agents: Lubiprostone functions by selectively stimulating type 2 chloride channels located on the apical cell membrane of gastrointestinal epithelial cells, leading to increased intestinal permeability. Research findings have indicated that in patients with constipation and MASLD, lubiprostone reduces liver enzyme levels and exhibits favorable tolerability[63]. Heat shock protein (HSP) 47 is a collagen-specific molecular chaperone residing within the endoplasmic reticulum and plays a pivotal role in ensuring the correct folding, assembly, and extracellular secretion of collagen proteins within the extracellular matrix (ECM). The anomalous accumulation of collagen proteins within the ECM disrupts its structural integrity, thereby precipitating fibrotic processes[64]. These findings suggest that targeting HSP47 is of paramount importance for the treatment of liver fibrosis[65].
Drugs targeting the gut–liver axis
Probiotics: The GM assumes a crucial role in MASLD. Dysbiosis of the GM, attributed to oxidative stress, lifestyle choices, and excessive antibiotic use, leads to impaired intestinal permeability. An increased permeability facilitates the induction of pro-inflammatory cytokines and interferon-mediated factors through the activation of pattern recognition receptors by microbiota, bacterial byproducts, and LPS, thereby significantly contributing to the pathogenesis of MASLD[66]. Aging is concomitant with the deterioration of multiple physiological functions and the exacerbation of inflammatory processes. Age-related alterations in the gastrointestinal tract contribute to an elevated incidence of gastrointestinal inflammatory disorders. Aging is associated with increased production of ROS, which leads to lipid accumulation, DNA damage, and concomitant cellular functional impairments. Furthermore, modifications in GM composition result in the endogenous production of ethanol, which consequently compromises the integrity of the intestinal barrier and incites ROS accumulation in hepatic stellate cells and Kupffer cells[37]. Studies have shown that probiotics can restore homeostasis in the GM and mitigate oxidative stress[67]. Specifically, members of the Lactobacillus genus have demonstrated the ability to modulate the expression of inflammatory cytokines, including but not limited to IL-6, IL-1β, IL-1α, IL-12, and interferon-γ, both in serum and colonic tissues. This immunomodulatory effect is attributed to their capacity to inhibit NF-κB activation via the G protein-coupled receptor 109A pathway, thereby fostering improvements in immune function among aging mice[68]. In animal models of chronic liver injury, probiotics have demonstrated a protective role against hepatic steatosis and liver inflammation by modulating and potentially restoring the GM. Studies evaluating patients with MASH/MASLD have revealed that the probiotic formulation VSL3 displays inherent anti-inflammatory properties and insulin-sensitizing effects, indicating its potential for the treatment of liver fibrosis[69]. Evidence suggests that GM can ameliorate an array of biomarkers associated with inflammation, blood glucose regulation, insulin resistance, lipid anomalies, obesity, and hepatic impairment, including reductions in liver enzymes, hepatic steatosis, and fibrosis. Probiotics exert their influence on the immune system, potentially improving MASLD outcomes, either by fortifying the intestinal barrier or preventing the formation of hepatotoxic metabolites[70] (Figure 2).
Figure 2 Targets related to lipids, bile acids, glucose homeostasis and intestinal microbiota in age-related metabolic dysfunction-associated steatotic liver disease.
GM: Gut microbiota; FXR: Farnesoid X receptor; RXR: Retinoid X receptor; FXRE: FXR reaction element; FGF19: Fibroblast growth factor 19; FGFR4: Fibroblast growth factor receptor 4; TGR5: Takeda G protein–coupled receptor 5; GLP-1: Glucagon-like peptide-1.
Weight loss surgery: Invasive weight loss surgeries may be associated with reduced tolerance among patients but demonstrate notable efficacy, particularly in severely obese individuals. A study assessing patients with severe obesity and MASH who underwent bariatric surgery revealed that 5 years post-surgery, 84% of the patients experienced resolution of inflammation, 70% demonstrated improvements in fibrosis, and 56% exhibited regression of liver fibrosis[71]. Bariatric surgery further modifies the ileal milieu, thereby inducing alterations in the GM or its metabolic products, ultimately ameliorating MASLD.