Published online Dec 14, 2024. doi: 10.3748/wjg.v30.i46.4969
Revised: October 3, 2024
Accepted: October 29, 2024
Published online: December 14, 2024
Processing time: 82 Days and 9.5 Hours
In this letter, we commented on the article by Wu et al. We examined the inter
Core Tip: The interplay between gut microbiota, mesenteric adipose tissue, and creeping fat is crucial in Crohn’s disease management. Dysbiosis is characterized by decreased beneficial bacteria and increased pathogenic bacteria and exacerbates inflammation and disease progression. Targeting microbial imbalances and the inflammatory roles of mesenteric adipose tissue and creeping fat may lead to novel therapeutic approaches, including fecal microbiota transplantation, which shows promise in restoring microbial balance and achieving sustained remission in patients with Crohn’s disease. Ongoing research should focus on identifying specific microbial communities and their roles in different phenotypes to optimize personalized treatment strategies and improve clinical outcomes.
- Citation: Hasnaoui A, Trigui R, Giuffrida M. Gut microbiota and mesenteric adipose tissue interactions in shaping phenotypes and treatment strategies for Crohn’s disease. World J Gastroenterol 2024; 30(46): 4969-4976
- URL: https://www.wjgnet.com/1007-9327/full/v30/i46/4969.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i46.4969
Crohn’s disease (CD), which is a form of inflammatory bowel disease, is characterized by chronic inflammation of the gastrointestinal tract. CD was first identified as a distinct condition in 1932. Its incidence has surged dramatically, and it now affects approximately 7000 individuals each year in Europe[1]. This rapid increase highlights CD as a significant global public health challenge. The exact causes of CD remain unidentified; however, genetic predisposition, environmental influences, and gut microbiota are believed to have a complex interplay in its pathogenesis[2]. Dysbiosis in gut microbiota has been consistently observed in patients with CD, yet its precise impact on disease progression remains unclear[3]. Consequently, research aimed at elucidating the role of the gut microbiota in CD as well as studies targeting this pathogenetic mechanism could offer critical insights into the underlying processes of the disease[4]. Such investigations may pave the way for novel therapeutic approaches that enhance both short-term and long-term clinical outcomes[4].
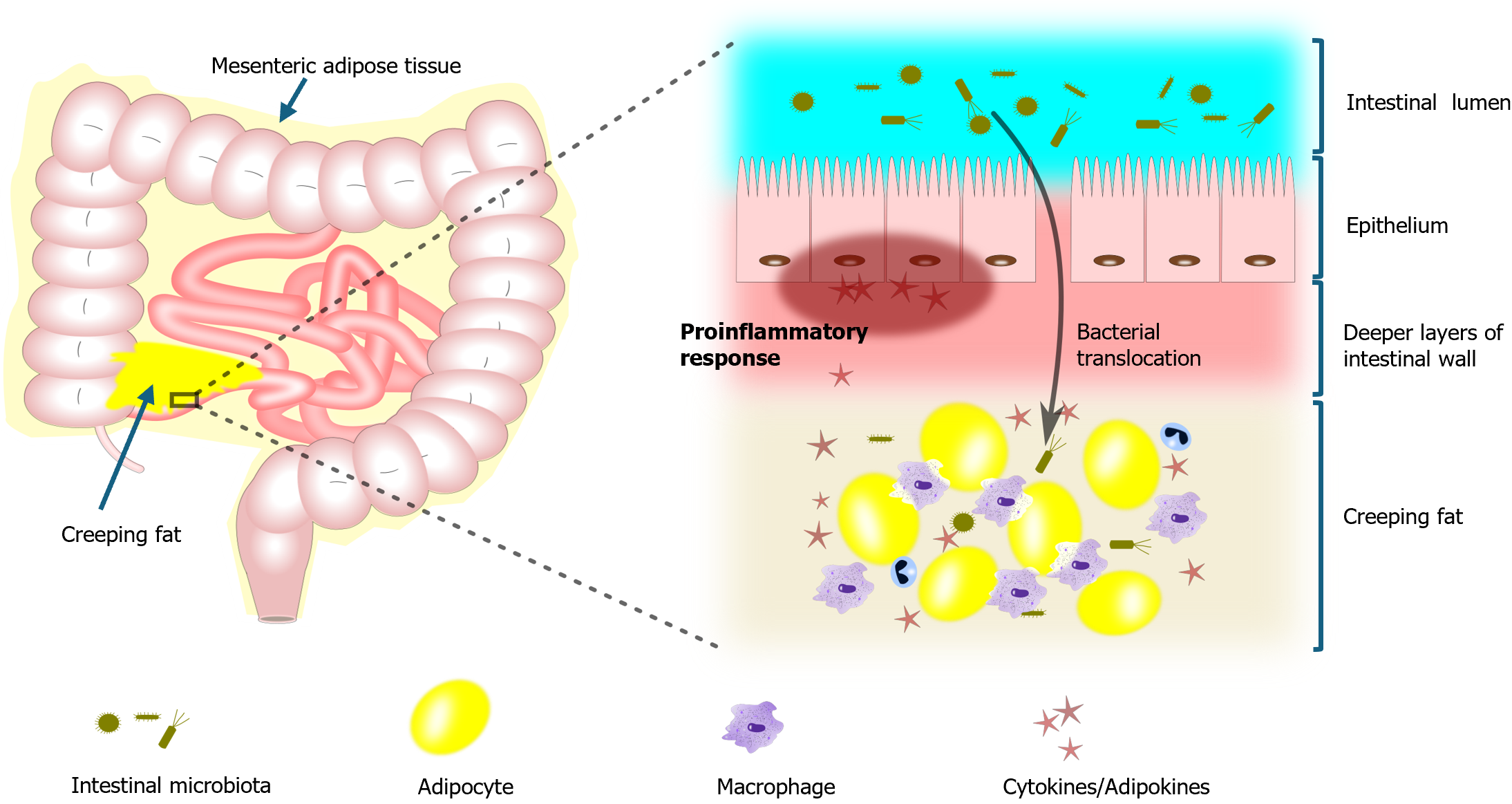
Mesenteric adipose tissue (MAT) and creeping fat (CF) play a crucial role in the pathogenesis of CD through their inflammatory contributions and interactions with disease progression[5]. In patients with CD, these fat tissues exhibit a distinctive profile characterized by the production of elevated levels of inflammatory cytokines and adipokines, which promote the inflammatory response within the digestive tract[6]. Simultaneously, they inhibit the production of beneficial adipokines, making the disease more difficult to control[6]. MAT and CF are not merely passive tissues; they are actively involved in the disease process[7]. The selective enlargement of MAT and the histopathological changes leading to the accumulation of CF around affected bowel regions suggest a direct association between these tissues and the progression of CD[7]. Moreover, CF may also contribute to bacterial translocation in CD, further complicating the progression of the disease[8].
During CD, ongoing changes in the gut microbiota lead to dysbiosis, which ultimately results in damage and dis
In the early phases of CD, the interaction between CF and the altered microbiota may be beneficial, as CF helps contain the bacteria, preventing them from spreading to other areas[10]. However, as the disease advances and the amount of CF increases, this adipose tissue becomes saturated with altered bacteria that continue to proliferate and migrate towards lesion sites, worsening the condition over time[10]. Some researchers suggest that the bacteria that infiltrate the mesentery following intestinal barrier disruption may be the initial trigger for the formation of CF[11]. Although the intricate interactions among MAT, CF, and the intestinal microbiota are not yet fully understood, a study, published in the recent issue of the World Journal of Gastroenterology, has suggested that the complex influence of intestinal microbiota on MAT and CF in shaping CD phenotypes could pave the way for novel therapeutic approaches[12].
A central element in chronic inflammation during CD is dysbiosis, which is characterized by an imbalance in the composition of gut microbial communities[13]. A consistent finding in CD patients is the notable decrease in anti-inflammatory bacteria. For instance, Faecalibacterium prausnitzii (F. prausnitzii) levels are significantly lower compared to healthy individuals[14]. This bacterium, which is known for its anti-inflammatory properties, is particularly important as its reduction correlates with an elevated risk of relapse in patients with ileal CD[15]. Additionally, other beneficial bacteria such as Blautia faecis, Roseburia inulinivorans, Ruminococcus torques, and Clostridium lavalense also show reduced abundance in CD patients[16]. The loss of these microbes, which play crucial roles in maintaining intestinal homeostasis and reducing inflammation, may exacerbate the disease course[17].
Conversely, there is a notable increase in pathogenic bacteria in the gut microbiota of CD patients. Particularly, an expansion in Proteobacteria, including adhesion-invasive Escherichia coli (E. coli), is observed[18]. According to a clinical study published in 2004, adhesion-invasive E. coli was present in approximately 38% of CD patients compared to just 6% in healthy controls, highlighting its significant association with the disease[19]. Additionally, sulfate-reducing bacteria such as Desulfovibrio are more prevalent in patients with CD, leading to the production of hydrogen sulfide, which is a compound known to cause damage to intestinal epithelial cells and contribute to mucosal injury and inflammation[20]. These pathogenic bacteria can adhere to the intestinal mucosa, disrupting gut barrier integrity and promoting inflammatory responses through a cascade of immune responses[21].
Moreover, this inflammatory process is not limited to the intestinal mucosa; it actually extends to the surrounding MAT and CF[22]. Both MAT and CF are sites of active immune responses in CD, contributing to the chronic inflammation that defines the disease[22]. MAT and CF take on an active role in CD by responding to the inflammatory cues generated by the gut microbiota. Inflammatory signals from the microbiota induce MAT and CF to produce additional proinflammatory cytokines, including interleukin-6 (IL-6), IL-1 beta, and tumor necrosis factor-alpha[10]. These cytokines are crucial in sustaining the inflammatory state that characterizes the intestinal environment in CD[10]. The interplay between dysbiotic microbiota and these adipose tissues exacerbates the inflammatory state, leading to persistent and often severe inflammation that can affect the entire gastrointestinal tract[23] (Figure 1).
Obesity contributes to worsening inflammation in CD by increasing visceral adiposity, which is associated with elevated levels of circulating inflammatory markers such as IL-6 and C-reactive protein[24]. This amplifies systemic inflammation, while obesity-related alterations in the gut microbiota further disrupt intestinal barrier function. This allows bacterial translocation into mesenteric fat, leading to adipocyte hyperplasia and activating local immune responses[25,26]. Moreover, research has revealed that the gene expression profile of CF in CD is similar to that of visceral fat in obesity, suggesting that obesity could intensify inflammatory and fibrotic activities in the mesenteric fat seen in CD[27]. Thus, obesity may boost the immune activity of CF, potentially leading to more severe disease outcomes and posing challenges in the management of CD.
In addition to its role in driving inflammation, the gut microbiota also contributes to the development of fibrosis, which is a common and serious complication in CD[12]. Fibrosis in CD is characterized by the excessive deposition of extracellular matrix components, such as collagen, which leads to the thickening and stiffening of the intestinal wall[12]. This fibrotic phenotype is closely linked to the activities of certain bacterial species within a dysbiotic microbiota[28]. When translocating into MAT, pathogenic bacteria from the gut microbiota can activate immune cells, particularly macrophages[29]. This activation triggers the release of fibrotic markers, including alpha-smooth muscle actin and vimentin[29]. These markers are critical in the transformation of fibroblasts into myofibroblasts, which are the cells responsible for producing the extracellular matrix that contributes to fibrosis[28].
As the disease advances, the continuous interaction between the dysbiotic microbiota, MAT, and CF perpetuates the cycle of inflammation leading to fibrosis[30]. This process transforms the intestinal environment into one that is prone to strictures and obstructions, both of which are complications associated with the fibrotic phenotype in CD[30]. Moreover, the fibrosis driven by these interactions is not just a local phenomenon, it has systemic implications as well[31]. The altered microbiota in patients with CD has been shown to influence not only the local intestinal environment but also the overall immune response, further complicating the disease and making it more resistant to conventional treatments[32]. Therefore, understanding the role of gut microbiota in the fibrotic phenotype of CD is crucial for developing targeted therapies that can effectively manage or even reverse this debilitating aspect of the disease.
Through a study published in 2016, the microbiota in ileal and colonic CD were found to be distinct, with specific bacterial species showing different abundances in each phenotype[33]. In ileal CD, severe dysbiosis is consistently observed, characterized by a notable reduction in beneficial bacteria such as F. prausnitzii and an increase in pathogenic taxa like Fusobacterium, which is often associated with heightened disease activity[33]. In contrast, colonic CD tends to display a microbiota profile closer to that of healthy individuals, with less pronounced dysbiosis[33]. These differences suggest that while both forms of CD share inflammatory processes, the microbial ecosystems in the ileum and colon behave differently[34]. The differences in microbial composition between ileal and colonic CD highlight the potential role of genetic factors in driving these distinct phenotypes[35].
Among the most studied ones are genes involved in autophagy, a crucial process for maintaining cellular homeostasis and immune defense[35]. Extensive research has identified more than 30 genomic loci associated with genetic susceptibility to CD, implicating eight key autophagy-related genes such as ATG16 L1, NOD2, IRGM, and LRRK2[36]. These genes play a crucial role in regulating the immune response and microbial homeostasis, particularly in the ileum. Variations in these genes are believed to contribute to the differential inflammatory patterns observed in CD by altering the host’s ability to manage gut bacteria, thus providing insights into disease mechanisms and potential therapeutic targets[36].
In addition to genetic influences, mucosal immune receptors are pivotal in shaping the intestinal environment and influencing inflammatory responses[37]. These mucosal receptors interact with the gut microbiota and contribute to the differential immune profiles seen in ileal vs colonic CD[37]. Understanding the variations in receptor expression and function between these phenotypes can provide deeper insights into the pathophysiology of CD.
CF is a hallmark of CD and plays a central role in its pathogenesis, extending beyond its interaction with gut microbiota and the secretion of proinflammatory cytokines and adipokines[22]. Accurately quantifying CF is crucial for evaluating the severity and extent of CD[23]. Furthermore, its active involvement in disease progression serves as a potential biomarker for monitoring disease activity, providing valuable insights for optimizing patient management and therapeutic strategies[23]. While direct measurement from surgical specimens remains the gold standard, non-invasive imaging techniques such as magnetic resonance enterography (MRE) and computed tomography enterography offer promising alternatives for accurate assessment[38]. These imaging techniques are valuable for monitoring disease progression and predicting complications in CD patients.
MRE has shown utility in visualizing CF, with studies indicating that it persists even during endoscopic remission, suggesting ongoing tissue injury rather than active inflammation[39]. Importantly, the presence of CF on MRE has been linked to more severe disease phenotypes and poorer therapeutic responses, highlighting its potential as a prognostic marker[40]. Computed tomography enterography has also advanced in assessing CF, particularly through the deve
Fecal microbiota transplantation (FMT) involves transferring intestinal microbiota from a healthy donor with the objective of re-establishing a balanced microbial community in the recipient’s gut[41]. In recent years, this approach has gained attention driven by the concept that a disrupted microbiota can be restored by introducing the colonic microbiota from a healthy individual[41].
In 2012, FMT was introduced through randomized controlled trials as a preventive measure against pouchitis, ulcerative colitis, and E. coli infections in patients with hemorrhagic rectocolitis[42]. It was not until 2016 that FMT was recognized as a second-line treatment for preventing recurrent infections of Clostridium difficile, demonstrating an efficacy of up to 80%[43]. Since then, numerous case series and studies with low to moderate levels of evidence have been published regarding the role of FMT in CD. The first randomized controlled trial investigating the use of FMT for this condition was published in 2020 by Sokol et al[44]. This single-blinded pilot study revealed that, although none of the patients reached the primary endpoint of successful donor microbiota engraftment, FMT still showed considerable promise in maintaining remission. The FMT group had a higher steroid-free clinical remission rate (87.5% at 10 weeks) compared to the sham group (44.4%), though the difference was not statistically significant (P = 0.23). Additionally, FMT led to a significant reduction in disease severity (P = 0.03) and prevented an increase in inflammatory markers.
A more recent double-blind, randomized, placebo-controlled pilot study published in 2024 by Zhang et al[45] introduced several novel insights into the use of FMT for treating CD. It was observed that greater residual microbial diversity following antibiotic treatment was significantly correlated with successful donor engraftment (P < 0.05). Furthermore, increased levels of donor engraftment were strongly associated with improved clinical response over time
All these findings highlight the critical role of microbial diversity and specific bacterial taxa in predicting the success of FMT, potentially guiding future strategies to optimize therapeutic outcomes in CD patients.
Despite considerable progress in medical management, including the use of biologic agents and immunosuppressants, a significant proportion of patients with CD still require surgery[46]. It is estimated that 75% of patients with CD will undergo at least one surgical procedure in their lifetime[47]. However, surgery is not curative, and postoperative recurrence remains a significant challenge[46]. Endoscopic recurrence occurs in approximately 60% of patients within the first 6 months after surgery, with nearly 50% of these patients exhibiting clinical recurrence in the first 5 years in the postoperative course[47]. In the ongoing quest to optimize surgical outcomes in CD, two surgical approaches have gained attention: The extended mesenterectomy and the Kono-S anastomosis. Both approaches aim to minimize postoperative recurrence, but they address different aspects of CD pathology[48].
The extended mesenterectomy involves a comprehensive removal of mesenteric tissue, targeting inflammatory mechanisms directly and potentially reducing recurrence by addressing the role of the mesentery in disease progression[49]. However, The SPICY Trial, which is an international multicenter randomized controlled study, found no significant difference in endoscopic recurrence of CD between extended mesenteric resection and conventional resection[50].
The Kono-S anastomosis has gained attention as a promising technique for reducing recurrence in CD, though existing studies show mixed results[51]. A systematic review and meta-analysis, published in 2024[51], compared the outcomes of Kono-S anastomosis with conventional methods in patients undergoing bowel resection for CD. The analysis significantly revealed lower recurrence rates in the Kono-S group, with an endoscopic recurrence rate of 41% compared to 48% in the conventional group and a surgical recurrence rate of 2.7% vs 21%[51].
Monitoring microbial changes: Regular assessment of gut microbiota composition, especially beneficial strains such as F. prausnitzii, can inform us about disease activity and relapse risk, thereby aiding clinical management decisions.
Therapeutic targeting of adipose tissue: The inflammatory roles of MAT and CF indicate potential therapeutic strategies that focus on these tissues to reduce inflammation and improve outcomes in CD patients.
Efficacy of FMT in clinical practice: The promising results of FMT suggest its potential as a second-line treatment for CD, particularly for individuals exhibiting dysbiosis in their gut microbiota.
Microbial diversity as a prognostic indicator: The correlation between enhanced microbial diversity and successful donor microbiota engraftment following FMT highlights the importance of evaluating microbial diversity to guide treatment approaches and improve patient outcomes.
Long-term benefits of microbiota restoration: Achieving successful microbiota restoration through FMT may result in sustained remission and decreased inflammatory markers, underscoring the need for further investigation into optimizing FMT protocols for CD management.
Personalized therapeutic approaches: The identification of unique microbial profiles in different CD phenotypes suggests the potential for personalized treatment strategies tailored to individual microbiota characteristics, enhancing therapeutic effectiveness and patient care.
CD represents a complex interplay of genetic, environmental, and microbial factors that contribute to its pathogenesis and progression[2]. The dysbiosis observed in patients with CD highlights the crucial role of gut microbiota in maintaining intestinal homeostasis and mitigating inflammation[14]. Additionally, the involvement of MAT and CF in the inflammatory processes associated with CD offers a new perspective on potential treatment avenues[10]. These adipose tissues not only contribute to the inflammatory milieu but also interact dynamically with the altered gut microbiota[22]. Consequently, therapeutic strategies that focus on modulating the activity of MAT and CF may provide significant benefits in managing inflammation and preventing disease complications[29].
Furthermore, FMT has emerged as a promising therapeutic modality for CD, demonstrating potential in re-establishing a balanced gut microbiota and achieving sustained remission in some patients[42]. The correlation between microbial diversity and successful treatment outcomes further emphasizes the importance of tailoring FMT protocols to enhance efficacy[45]. Future research should focus on elucidating the specific microbial communities involved in CD and their functional roles in the disease process[33]. This knowledge will not only enhance our understanding of CD but also inform the development of personalized treatment strategies that address the unique microbial profiles associated with different CD phenotypes[33].
The authors wish to express their sincere appreciation to Dorra Maalej, Assistant Professor of English, for her invaluable contribution in the linguistic revision of this manuscript.
| 1. | Crocetti E, Bergamaschi W, Russo AG. Population-based incidence and prevalence of inflammatory bowel diseases in Milan (Northern Italy), and estimates for Italy. Eur J Gastroenterol Hepatol. 2021;33:e383-e389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Li J, Simmons AJ, Hawkins CV, Chiron S, Ramirez-Solano MA, Tasneem N, Kaur H, Xu Y, Revetta F, Vega PN, Bao S, Cui C, Tyree RN, Raber LW, Conner AN, Pilat JM, Jacobse J, McNamara KM, Allaman MM, Raffa GA, Gobert AP, Asim M, Goettel JA, Choksi YA, Beaulieu DB, Dalal RL, Horst SN, Pabla BS, Huo Y, Landman BA, Roland JT, Scoville EA, Schwartz DA, Washington MK, Shyr Y, Wilson KT, Coburn LA, Lau KS, Liu Q. Identification and multimodal characterization of a specialized epithelial cell type associated with Crohn's disease. Nat Commun. 2024;15:7204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 3. | Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, Essers J, Mitrovic M, Ning K, Cleynen I, Theatre E, Spain SL, Raychaudhuri S, Goyette P, Wei Z, Abraham C, Achkar JP, Ahmad T, Amininejad L, Ananthakrishnan AN, Andersen V, Andrews JM, Baidoo L, Balschun T, Bampton PA, Bitton A, Boucher G, Brand S, Büning C, Cohain A, Cichon S, D'Amato M, De Jong D, Devaney KL, Dubinsky M, Edwards C, Ellinghaus D, Ferguson LR, Franchimont D, Fransen K, Gearry R, Georges M, Gieger C, Glas J, Haritunians T, Hart A, Hawkey C, Hedl M, Hu X, Karlsen TH, Kupcinskas L, Kugathasan S, Latiano A, Laukens D, Lawrance IC, Lees CW, Louis E, Mahy G, Mansfield J, Morgan AR, Mowat C, Newman W, Palmieri O, Ponsioen CY, Potocnik U, Prescott NJ, Regueiro M, Rotter JI, Russell RK, Sanderson JD, Sans M, Satsangi J, Schreiber S, Simms LA, Sventoraityte J, Targan SR, Taylor KD, Tremelling M, Verspaget HW, De Vos M, Wijmenga C, Wilson DC, Winkelmann J, Xavier RJ, Zeissig S, Zhang B, Zhang CK, Zhao H; International IBD Genetics Consortium (IIBDGC), Silverberg MS, Annese V, Hakonarson H, Brant SR, Radford-Smith G, Mathew CG, Rioux JD, Schadt EE, Daly MJ, Franke A, Parkes M, Vermeire S, Barrett JC, Cho JH. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3979] [Cited by in RCA: 3603] [Article Influence: 277.2] [Reference Citation Analysis (0)] |
| 4. | Younis N, Zarif R, Mahfouz R. Inflammatory bowel disease: between genetics and microbiota. Mol Biol Rep. 2020;47:3053-3063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 5. | Yang L, Zhang Y, Yao B, Wu Q, Peng L, Yuan L. Timing of first abdominal operation in Crohn's disease based on a diagnostic model. Sci Rep. 2024;14:6099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 6. | Karaskova E, Velganova-Veghova M, Geryk M, Foltenova H, Kucerova V, Karasek D. Role of Adipose Tissue in Inflammatory Bowel Disease. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (1)] |
| 7. | Rowan CR, McManus J, Boland K, O'Toole A. Visceral adiposity and inflammatory bowel disease. Int J Colorectal Dis. 2021;36:2305-2319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 8. | Peyrin-Biroulet L, Gonzalez F, Dubuquoy L, Rousseaux C, Dubuquoy C, Decourcelle C, Saudemont A, Tachon M, Béclin E, Odou MF, Neut C, Colombel JF, Desreumaux P. Mesenteric fat as a source of C reactive protein and as a target for bacterial translocation in Crohn's disease. Gut. 2012;61:78-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 201] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 9. | Goode EC, Watson AJ. Mesenteric fat as a source of CRP and target for bacterial translocation in Crohn's disease. Gastroenterology. 2012;143:496-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Bilski J, Mazur-Bialy A, Wojcik D, Surmiak M, Magierowski M, Sliwowski Z, Pajdo R, Kwiecien S, Danielak A, Ptak-Belowska A, Brzozowski T. Role of Obesity, Mesenteric Adipose Tissue, and Adipokines in Inflammatory Bowel Diseases. Biomolecules. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 11. | Johnson TO, Akinsanmi AO, Ejembi SA, Adeyemi OE, Oche JR, Johnson GI, Adegboyega AE. Modern drug discovery for inflammatory bowel disease: The role of computational methods. World J Gastroenterol. 2023;29:310-331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Wu Q, Yuan LW, Yang LC, Zhang YW, Yao HC, Peng LX, Yao BJ, Jiang ZX. Role of gut microbiota in Crohn's disease pathogenesis: Insights from fecal microbiota transplantation in mouse model. World J Gastroenterol. 2024;30:3689-3704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (2)] |
| 13. | Zuo T, Kamm MA, Colombel JF, Ng SC. Urbanization and the gut microbiota in health and inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2018;15:440-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 188] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 14. | Fujimoto T, Imaeda H, Takahashi K, Kasumi E, Bamba S, Fujiyama Y, Andoh A. Decreased abundance of Faecalibacterium prausnitzii in the gut microbiota of Crohn's disease. J Gastroenterol Hepatol. 2013;28:613-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 220] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 15. | Varela E, Manichanh C, Gallart M, Torrejón A, Borruel N, Casellas F, Guarner F, Antolin M. Colonisation by Faecalibacterium prausnitzii and maintenance of clinical remission in patients with ulcerative colitis. Aliment Pharmacol Ther. 2013;38:151-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 187] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 16. | Takahashi K, Nishida A, Fujimoto T, Fujii M, Shioya M, Imaeda H, Inatomi O, Bamba S, Sugimoto M, Andoh A. Reduced Abundance of Butyrate-Producing Bacteria Species in the Fecal Microbial Community in Crohn's Disease. Digestion. 2016;93:59-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 517] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 17. | Yu CG, Huang Q. Recent progress on the role of gut microbiota in the pathogenesis of inflammatory bowel disease. J Dig Dis. 2013;14:513-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Iaquinto G, Aufiero VR, Mazzarella G, Lucariello A, Panico L, Melina R, Iaquinto S, De Luca A, Sellitto C. Pathogens in Crohn's Disease: The Role of Adherent Invasive Escherichia coli. Crit Rev Eukaryot Gene Expr. 2024;34:83-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Darfeuille-Michaud A, Boudeau J, Bulois P, Neut C, Glasser AL, Barnich N, Bringer MA, Swidsinski A, Beaugerie L, Colombel JF. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn's disease. Gastroenterology. 2004;127:412-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1075] [Cited by in RCA: 1123] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 20. | Loubinoux J, Bronowicki JP, Pereira IA, Mougenel JL, Faou AE. Sulfate-reducing bacteria in human feces and their association with inflammatory bowel diseases. FEMS Microbiol Ecol. 2002;40:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 280] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 21. | Nishida A, Inoue R, Inatomi O, Bamba S, Naito Y, Andoh A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol. 2018;11:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 905] [Article Influence: 113.1] [Reference Citation Analysis (3)] |
| 22. | Hwang N, Kang D, Shin SJ, Yoon BK, Chun J, Kim JW, Fang S. Creeping fat exhibits distinct Inflammation-specific adipogenic preadipocytes in Crohn's disease. Front Immunol. 2023;14:1198905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Seifarth C, Hering NA, Arndt M, Lehmann KS, Stroux A, Weixler B, Kreis ME. Increased proinflammatory cytokines in mesenteric fat in major surgery and Crohn's disease. Surgery. 2021;169:1328-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Colombel JF, Solem CA, Sandborn WJ, Booya F, Loftus EV Jr, Harmsen WS, Zinsmeister AR, Bodily KD, Fletcher JG. Quantitative measurement and visual assessment of ileal Crohn's disease activity by computed tomography enterography: correlation with endoscopic severity and C reactive protein. Gut. 2006;55:1561-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 169] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 25. | Kim A. Dysbiosis: A Review Highlighting Obesity and Inflammatory Bowel Disease. J Clin Gastroenterol. 2015;49 Suppl 1:S20-S24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Boutagy NE, McMillan RP, Frisard MI, Hulver MW. Metabolic endotoxemia with obesity: Is it real and is it relevant? Biochimie. 2016;124:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 286] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 27. | Zulian A, Cancello R, Micheletto G, Gentilini D, Gilardini L, Danelli P, Invitti C. Visceral adipocytes: old actors in obesity and new protagonists in Crohn's disease? Gut. 2012;61:86-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 28. | Laudadio I, Carissimi C, Scafa N, Bastianelli A, Fulci V, Renzini A, Russo G, Oliva S, Vitali R, Palone F, Cucchiara S, Stronati L. Characterization of patient-derived intestinal organoids for modelling fibrosis in Inflammatory Bowel Disease. Inflamm Res. 2024;73:1359-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 29. | Park JM, Kim J, Lee YJ, Bae SU, Lee HW. Inflammatory bowel disease-associated intestinal fibrosis. J Pathol Transl Med. 2023;57:60-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 30. | Wu X, Lin X, Tan J, Liu Z, He J, Hu F, Wang Y, Chen M, Liu F, Mao R. Cellular and Molecular Mechanisms of Intestinal Fibrosis. Gut Liver. 2023;17:360-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 31. | Qian W, Xu Y, Wen W, Huang L, Guo Z, Zhu W, Li Y. Exosomal miR-103a-3p from Crohn's Creeping Fat-Derived Adipose-Derived Stem Cells Contributes to Intestinal Fibrosis by Targeting TGFBR3 and Activating Fibroblasts. J Crohns Colitis. 2023;17:1291-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 32. | Wang J, Yang B, Chandra J, Ivanov A, Brown JM, Rieder F. Preventing fibrosis in IBD: update on immune pathways and clinical strategies. Expert Rev Clin Immunol. 2024;20:727-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 33. | Naftali T, Reshef L, Kovacs A, Porat R, Amir I, Konikoff FM, Gophna U. Distinct Microbiotas are Associated with Ileum-Restricted and Colon-Involving Crohn's Disease. Inflamm Bowel Dis. 2016;22:293-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 34. | Pittayanon R, Lau JT, Leontiadis GI, Tse F, Yuan Y, Surette M, Moayyedi P. Differences in Gut Microbiota in Patients With vs Without Inflammatory Bowel Diseases: A Systematic Review. Gastroenterology. 2020;158:930-946.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 403] [Article Influence: 80.6] [Reference Citation Analysis (0)] |
| 35. | Subramanian A, Jahabardeen A, Thamaraikani T, Vellapandian C. More on the interplay between gut microbiota, autophagy, and inflammatory bowel disease is needed. World J Gastroenterol. 2024;30:3356-3360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 36. | Henderson P, Stevens C. The role of autophagy in Crohn's disease. Cells. 2012;1:492-519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Lu Q, Yang MF, Liang YJ, Xu J, Xu HM, Nie YQ, Wang LS, Yao J, Li DF. Immunology of Inflammatory Bowel Disease: Molecular Mechanisms and Therapeutics. J Inflamm Res. 2022;15:1825-1844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 102] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 38. | Li XH, Feng ST, Cao QH, Coffey JC, Baker ME, Huang L, Fang ZN, Qiu Y, Lu BL, Chen ZH, Li Y, Bettenworth D, Iacucci M, Sun CH, Ghosh S, Rieder F, Chen MH, Li ZP, Mao R. Degree of Creeping Fat Assessed by Computed Tomography Enterography is Associated with Intestinal Fibrotic Stricture in Patients with Crohn's Disease: A Potentially Novel Mesenteric Creeping Fat Index. J Crohns Colitis. 2021;15:1161-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 39. | Rimola J, Alfaro I, Fernández-Clotet A, Castro-Poceiro J, Vas D, Rodríguez S, Masamunt MC, Ordás I, Ricart E, Panés J. Persistent damage on magnetic resonance enterography in patients with Crohn's disease in endoscopic remission. Aliment Pharmacol Ther. 2018;48:1232-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 40. | Althoff P, Schmiegel W, Lang G, Nicolas V, Brechmann T. Creeping Fat Assessed by Small Bowel MRI Is Linked to Bowel Damage and Abdominal Surgery in Crohn's Disease. Dig Dis Sci. 2019;64:204-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 41. | Imdad A, Pandit NG, Zaman M, Minkoff NZ, Tanner-Smith EE, Gomez-Duarte OG, Acra S, Nicholson MR. Fecal transplantation for treatment of inflammatory bowel disease. Cochrane Database Syst Rev. 2023;4:CD012774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 26] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 42. | Guarner F, Khan AG, Garisch J, Eliakim R, Gangl A, Thomson A, Krabshuis J, Lemair T, Kaufmann P, de Paula JA, Fedorak R, Shanahan F, Sanders ME, Szajewska H, Ramakrishna BS, Karakan T, Kim N; World Gastroenterology Organization. World Gastroenterology Organisation Global Guidelines: probiotics and prebiotics October 2011. J Clin Gastroenterol. 2012;46:468-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 245] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 43. | Sokol H, Galperine T, Kapel N, Bourlioux P, Seksik P, Barbut F, Scanzi J, Chast F, Batista R, Joly F, Joly AC, Collignon A, Guery B, Beaugerie L; French Group of Faecal microbiota Transplantation (FGFT). Faecal microbiota transplantation in recurrent Clostridium difficile infection: Recommendations from the French Group of Faecal microbiota Transplantation. Dig Liver Dis. 2016;48:242-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 44. | Sokol H, Landman C, Seksik P, Berard L, Montil M, Nion-Larmurier I, Bourrier A, Le Gall G, Lalande V, De Rougemont A, Kirchgesner J, Daguenel A, Cachanado M, Rousseau A, Drouet É, Rosenzwajg M, Hagege H, Dray X, Klatzman D, Marteau P; Saint-Antoine IBD Network, Beaugerie L, Simon T. Fecal microbiota transplantation to maintain remission in Crohn's disease: a pilot randomized controlled study. Microbiome. 2020;8:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 248] [Article Influence: 49.6] [Reference Citation Analysis (1)] |
| 45. | Zhang YJ, Bousvaros A, Docktor M, Kaplan AL, Rufo PA, Leier M, Weatherly M, Zimmerman L, Nguyen LTT, Barton B, Russell G, Alm EJ, Kahn SA. Higher alpha diversity and Lactobacillus blooms are associated with better engraftment after fecal microbiota transplant in inflammatory bowel disease. Sci Rep. 2024;14:18188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 46. | Chen R, Zheng J, Li C, Chen Q, Zeng Z, Li L, Chen M, Zhang S. Prognostic models for predicting postoperative recurrence in Crohn's disease: a systematic review and critical appraisal. Front Immunol. 2023;14:1215116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 47. | Kelm M, Benatzky C, Buck V, Widder A, Schoettker K, Rosenfeldt M, Brand M, Schlegel N, Germer CT, Meining A, Nusrat A, Flemming S. Positive resection margins in Crohn's disease are a relevant risk factor for postoperative disease recurrence. Sci Rep. 2024;14:10823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 48. | Li Y, Mohan H, Lan N, Wu X, Zhou W, Gong J, Shen B, Stocchi L, Coffey JC, Zhu W. Mesenteric excision surgery or conservative limited resection in Crohn's disease: study protocol for an international, multicenter, randomized controlled trial. Trials. 2020;21:210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 49. | Coffey CJ, Kiernan MG, Sahebally SM, Jarrar A, Burke JP, Kiely PA, Shen B, Waldron D, Peirce C, Moloney M, Skelly M, Tibbitts P, Hidayat H, Faul PN, Healy V, O'Leary PD, Walsh LG, Dockery P, O'Connell RP, Martin ST, Shanahan F, Fiocchi C, Dunne CP. Inclusion of the Mesentery in Ileocolic Resection for Crohn's Disease is Associated With Reduced Surgical Recurrence. J Crohns Colitis. 2018;12:1139-1150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 240] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 50. | van der Does de Willebois EML, Bellato V, Duijvestein M, van der Bilt JDW, van Dongen K, Spinelli A, D'Haens GR, Mundt MW, Furfaro F, Danese S, Vignali A, Bemelman WA, Buskens CJ; SPICY collaborator group. Effect of mesenteric sparing or extended resection in primary ileocolic resection for Crohn's disease on postoperative endoscopic recurrence (SPICY): an international, randomised controlled trial. Lancet Gastroenterol Hepatol. 2024;9:793-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 51. | Cathomas M, Saad B, Taha-Mehlitz S, Vankayalapati DK, Ghazal NE, Mourad MM, Ortlieb N, Than CA, Burri E, Glaser C, Heigl A, Neumann K, Honaker MD, Taha A, Rosenberg R. Safety and effectivity of Kono-S anastomosis in Crohn's patients: a systematic review and Meta-analysis. Langenbecks Arch Surg. 2024;409:227. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |