Published online Sep 28, 2024. doi: 10.3748/wjg.v30.i36.4057
Revised: August 22, 2024
Accepted: September 5, 2024
Published online: September 28, 2024
Processing time: 67 Days and 14 Hours
Pancreatic cancer is one of the most lethal malignancies, characterized by poor prognosis and low survival rates. Traditional prognostic factors for pancreatic cancer offer inadequate predictive accuracy, often failing to capture the com
To develop and validate a prognostic model for predicting outcomes in patients with pancreatic cancer using key hypoxia-related molecules.
This pancreatic cancer prognostic model was developed based on the expression levels of the hypoxia-associated genes CAPN2, PLAU, and CCNA2. The results were validated in an independent dataset. This study also examined the corre
The prognostic model demonstrated significant predictive value, with the risk score showing a strong correlation with clinical features: It was significantly associated with tumor grade (G) (bP < 0.01), moderately associated with tumor stage (T) (aP < 0.05), and signi
The prognostic model based on hypoxia-related genes effectively predicts pancreatic cancer outcomes with improved accuracy over traditional factors and can guide treatment selection based on risk assessment.
Core Tip: In this study, a prognostic model based on the expression levels of the hypoxia-related genes CAPN2, PLAU, and CCNA2 was developed for pancreatic cancer. Compared with traditional methods, the model demonstrated superior predictive accuracy, and the model risk score was strongly correlated with clinical features such as cancer stage and tumor size. The risk score was also significantly associated with chemotherapy drug sensitivity and metabolic pathway activity. These findings highlight the model's potential to enhance personalized treatment selection and improve prognosis.
- Citation: Yang F, Jiang N, Li XY, Qi XS, Tian ZB, Guo YJ. Construction and validation of a pancreatic cancer prognostic model based on genes related to the hypoxic tumor microenvironment. World J Gastroenterol 2024; 30(36): 4057-4070
- URL: https://www.wjgnet.com/1007-9327/full/v30/i36/4057.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i36.4057
Among cancers, pancreatic cancer has a highly unsatisfactory prognosis worldwide and has the third-highest mortality rate in the United States; it has a very high degree of malignancy[1]. According to statistics, the 5-year survival rate of patients with pancreatic cancer is less than 5%[2]. It is very aggressive and characterized by dense interstitial tissue, severe hypoxia, and an immunosuppressive microenvironment[3], and its rapid progression, late onset of symptoms, and lack of specific diagnostic methods are the main reasons for its poor prognosis[4]. Past studies have investigated some of the regulatory mechanisms and signalling pathways involved in pancreatic cancer. In recent years, diagnostic and the
The tumor microenvironment, which encompasses the cellular milieu in which tumors subsist, proliferate, and infiltrate, has garnered increasing amounts of scholarly interest due to the expanding recognition of its pivotal role in facilitating intricate interactions between tumors and adjacent microenvironments[6]. Within the context of pancreatic cancer, the microenvironment comprises a minor subset of malignant cells, while fibroblasts, extracellular matrix, endothelial cells, and haematopoietic cells are highly abundant; these components collectively form the intricate pan
The interaction between tumors and the immune system plays a crucial role in the initiation, progression, and treat
Hypoxia and the immune microenvironment play important roles in the tumor microenvironment of pancreatic cancer and in the progression of pancreatic cancer. Through bioinformatics analysis, we conducted screening to identify genes associated with hypoxia in pancreatic cancer and constructed a prognostic model linked to hypoxia, aiming to elucidate the influence of the prognostic model risk score on the immune microenvironment. This study provides new insights into the prognosis and treatment of pancreatic cancer.
The human pancreatic cancer cell line (PANC-1) human pancreatic cancer cell line and the human telomerase reverse transcriptase-immortalized human pancreatic nestin-expressing (hTERT-HPNE) normal pancreatic ductal cell line were stored in the repository of the Department of Hepatobiliary and Pancreatic Medicine at the Affiliated Hospital of Qingdao University (originally obtained from Procell Life Science & Technology). PANC-1 and hTERT-HPNE cells were cultured in DMEM supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin (P/S) (Meilunbio) in a humidified incubator at 37 °C with 5% carbon dioxide. To induce a hypoxic environment, PANC-1 cells were subjected to 24 hours of culture in an anoxic setting using the Ruskinn Invivo 2400 Hypoxia Workstation.
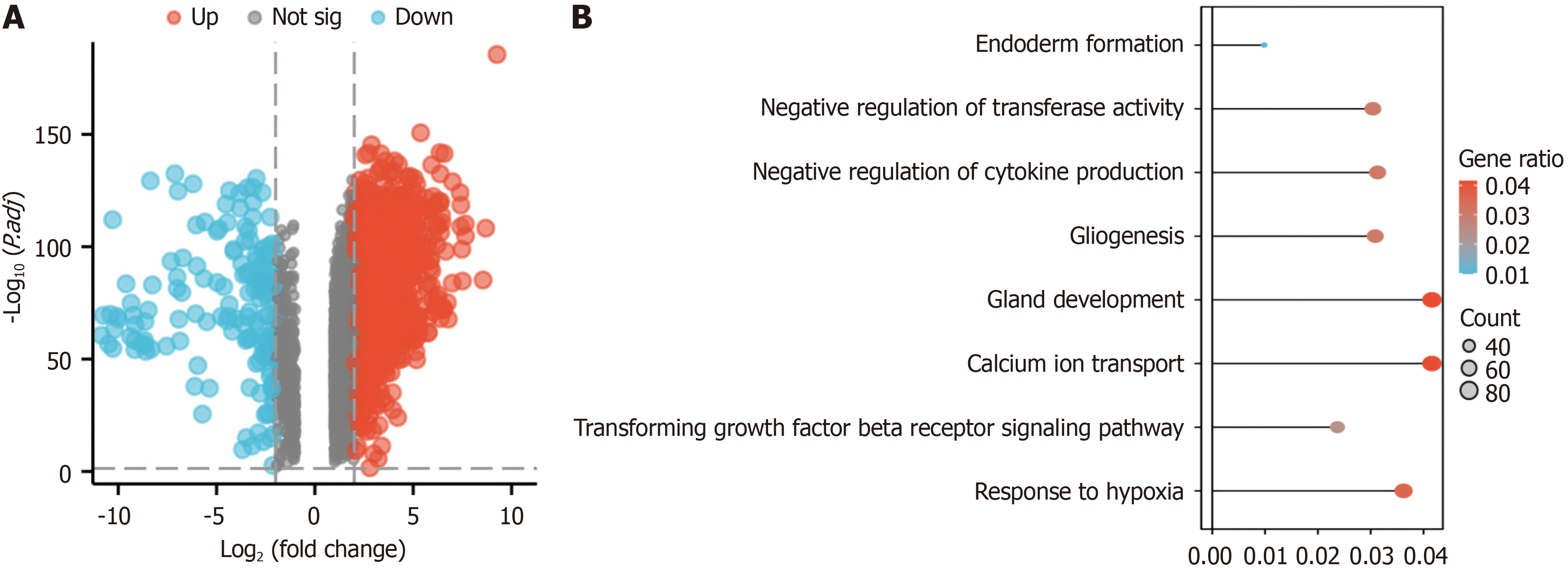
Using gene expression profiling interactive analysis 2 (GEPIA2)[13], we systematically explored the unique gene expression patterns in pancreatic cancer utilizing the log2FoldChange (logFC) and P value as criteria for screening differentially expressed genes (DEGs). Genes with P < 0.05 were considered to be differentially expressed, with log2 fold change (log2FC) > 2 denoting upregulation and log2FC < -2 indicating downregulation. Next, the top ranked genes were visualized using volcano plots.
We used the clusterProfiler package (for enrichment analysis), the msigdbr package (reference gene set sources), and molecular signatures database (MSigDB) gene sets for Gene Ontology (GO)/Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis of DEGs. The genes enriched in pathways and gene sets related to hypoxia were further investigated.
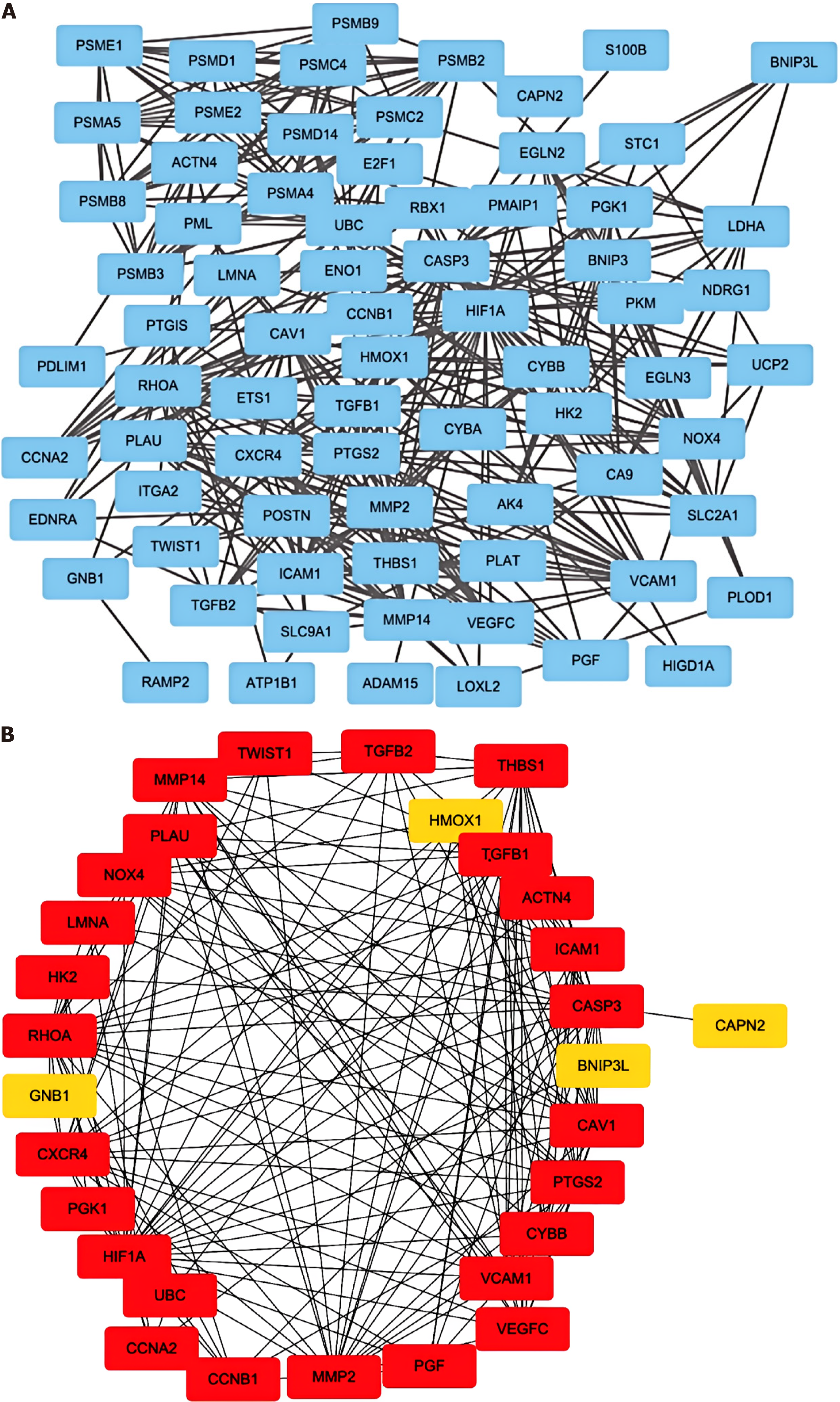
We constructed a protein-protein interaction (PPI) network composed of genes related to the above hypoxia pathways via search tool for the retrieval of interacting genes/proteins (STRING)[14].
We used the “betweenness” algorithm to calculate the scores of hypoxia-related genes in the PPI network. Using the "survival" package, we performed univariate Cox regression analysis of the top 30 genes.
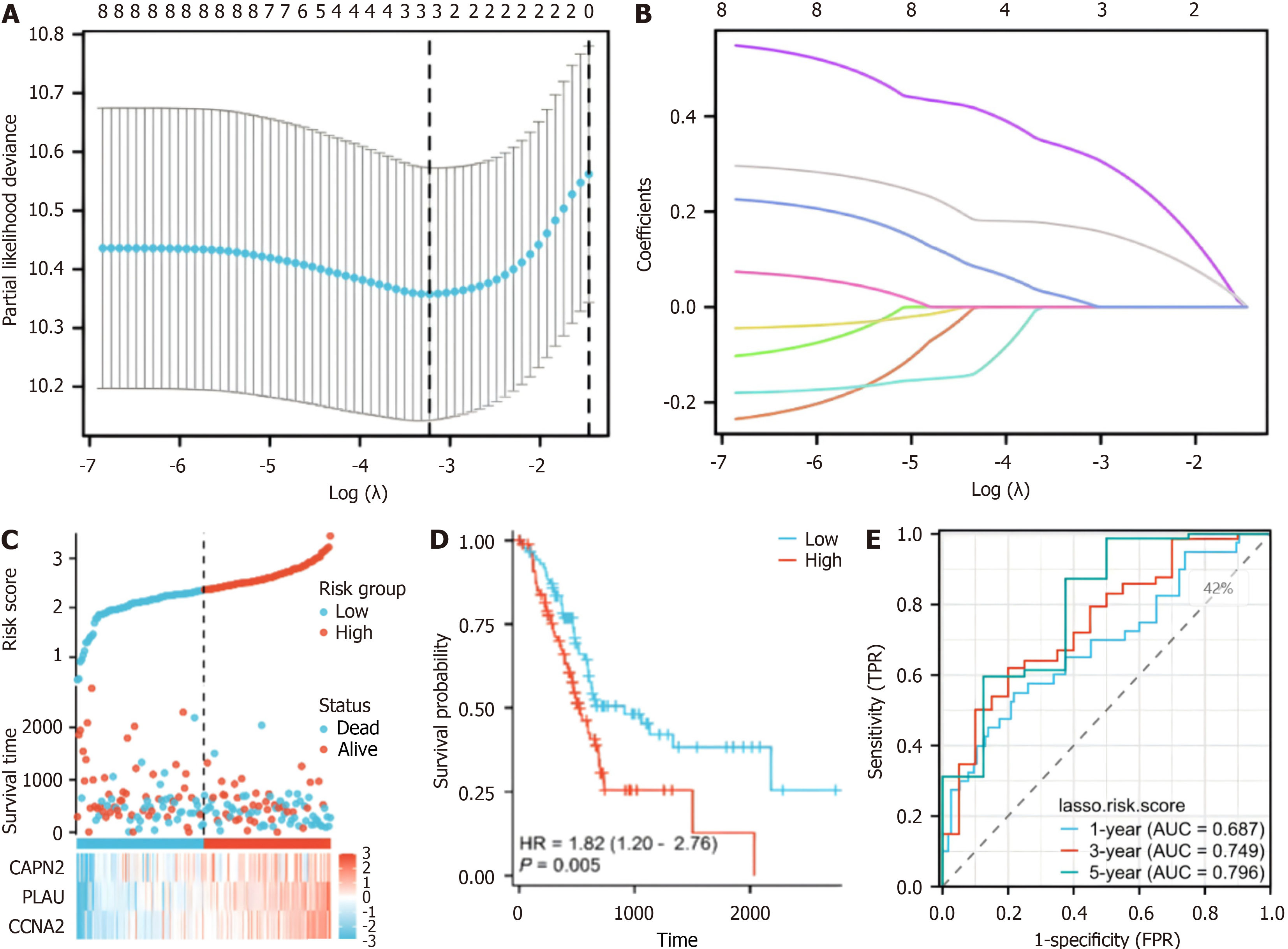
We used the "betweenness" algorithm to assess the betweenness among "30 genes related to hypoxia and performed univariate Cox regression analysis to assess the betweenness among prognostic genes with P < 0.05. We then used prognostic least absolute shrinkage and selection operator (LASSO) coefficients to screen these hypoxia- and prognosis-related genes to construct a prognostic model. Specifically, we constructed a LASSO prognostic model, calculated the lambda value of the model, calculated the coefficient of the variable according to the lambda value, and identified variables with coefficients of 0 by screening and excluded them. In the prognostic model, a coefficient of 0 indicates that there is no correlation between the variables; therefore, these variables have no real significance in the prognostic model.
Relevant RNAseq data and clinical information for pancreatic adenocarcinoma (PAAD) patients were obtained from the cancer genome atlas (TCGA) database (https://portal.gdc.cancer.gov). Employing the "glmnet" package, we inte
To validate our model, we conducted cross-validation using the GSE62452 dataset (Supplementary Table 2). Simultaneously, we employed the "rms" and "survival" packages to compare the model's predictions with against actual outcomes, graphically representing the model's actual and predicted probabilities under diverse conditions. To assess the prognostic superiority of the model over conventional clinical scoring systems, ROC curves were generated for risk scores and relevant clinical variables using the "pROC" software package. Univariate Cox analyses were also conducted for cli
In addition, we analysed the correlation between the risk score derived from our prognostic model and the clinical characteristics of TCGA-PAAD patients. Patients were stratified based on clinical features, and the associations between the risk score and these features in the TCGA-PAAD cohort were thoroughly examined.
Using the Single-sample Gene Set Enrichment Analysis algorithm from the "Gene Set Variation Analysis (GSVA)" package[15], we evaluated the association of infiltrating immune cells with the risk score based on the prognostic model by employing the 24 immune cell markers detailed in the Immunity article[16]. Moreover, the expression levels of im
Using the TCGA-PAAD matrix, the linear ridge function of the ridge package was used for ridge regression analysis to predict the sensitivity of patients to specific drugs. When risk group was considered, the groups displayed differences in drug sensitivity.
The "limma" package was used to analyse the difference between the high- and low-risk groups for hypoxia. After the analysis, the DEGs between risk groups were extracted for gene set enrichment analysis (GSEA). The "KEGG representational state transfer (KEGGREST)" package was used to extract metabolism-related pathways and their related genes from the KEGG database. The enrichment levels of metabolism-related pathways in different hypoxia risk groups were analysed via GSVA.
Total RNA was extracted from PANC-1, hypoxic-treated PANC-1, and hTERT-HPNE cells via incubation in Tri-Reagent (TRIzol) (Invitrogen); reverse transcription was performed using the PrimeScript RT-PCR Kit (Takara, Japan), and RT-qPCR was conducted on an Agilent AriaMX (Agilent) employing SYBR Green (SYBR) (Takara, Japan). Supplementary Table 3 contains the sequences of primers used in this study.
A paired t test or unpaired t test was used to compare experimental data between groups, and one-way analysis of variance was used to compare multiple groups. The figures were generated using GraphPad Prism 7 (GraphPad Soft
Through gene expression analysis, a comprehensive screening process identified 9221 DEGs, consisting of 2457 upre
We identified 30 genes involved in the hypoxia pathway via screening with the STRING database (Figure 2B). Genes with P < 0.05 were identified by screening via univariate Cox regression analysis of key hypoxia-related genes (Supple
A prognostic model was developed for all cancer samples in the training set, and the risk score was calculated as follows: Risk score = 0.00701516690012852 × CAPN2 + 0.163284368708758 × PLAU + 0.317555399984732 × CCNA2 (Figure 3A and B). To assess the ability of our model to predict pancreatic cancer prognosis, 178 patients were categorized into high-risk
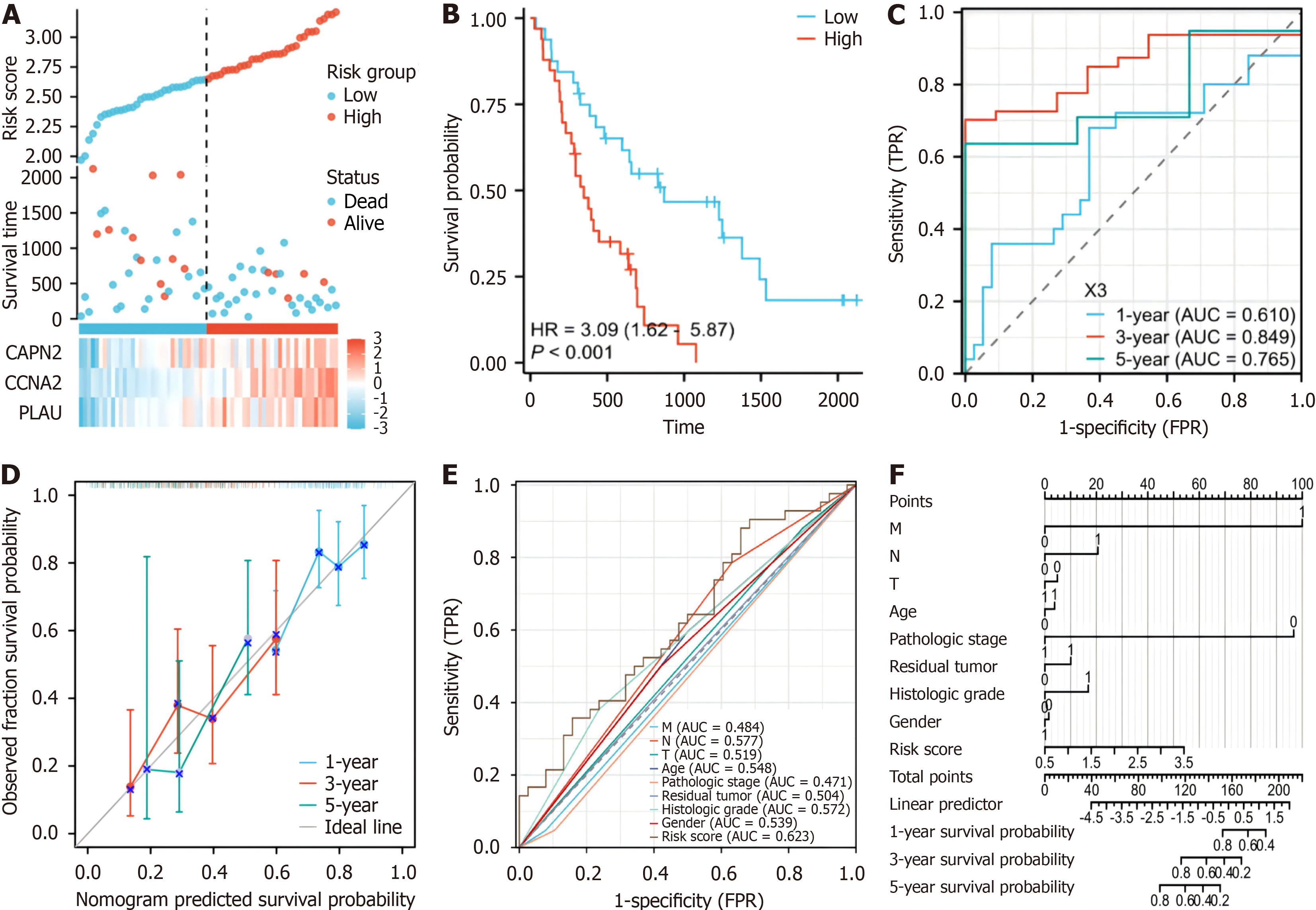
To assess the precision of the established prognostic model, an additional validation dataset on 65 patients with pan
Furthermore, calibration curves were generated, revealing the congruence of our model with the observed survival patterns in patients with PAAD (Figure 4D). The results of the formulation of a nomogram incorporating risk scores and conventional prognostic factors (Figure 4F) revealed that our model displayed an increased AUC compared to that of traditional clinical parameters (Figure 4F). This finding emphasizes the heightened predictive accuracy of our model, underscoring its potential as a robust tool for clinical prognostication.
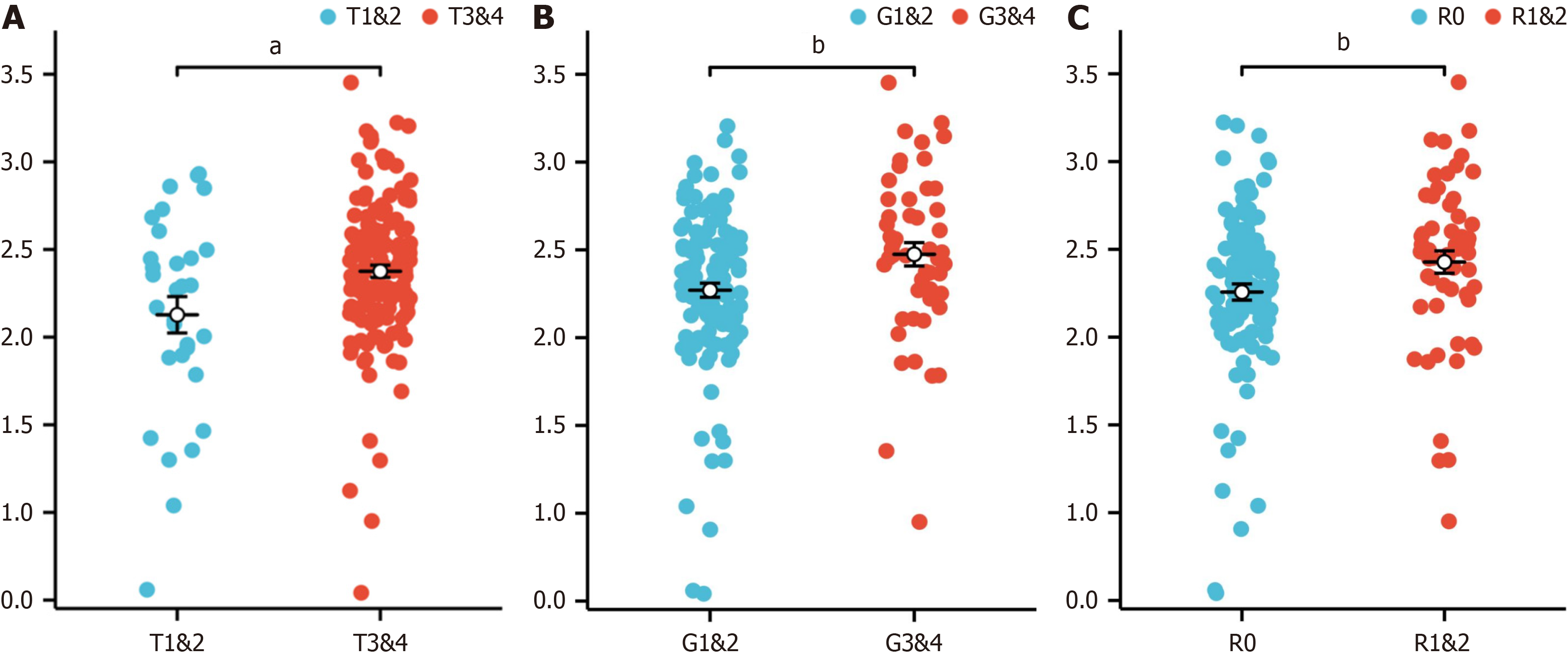
When the correlations between the risk score associated with hypoxia and the clinicopathological characteristics of TCGA-PAAD patients were analysed, the risk score was found to be closely associated with tumor stage (T) (Figure 5A), tumor grade (G) (Figure 5B), and residual tumor status (R) (Figure 5C). These results underscore the potential of our prognostic model to discern and stratify risk across patients with various clinical features, providing valuable insights into the heterogeneity of PAAD.
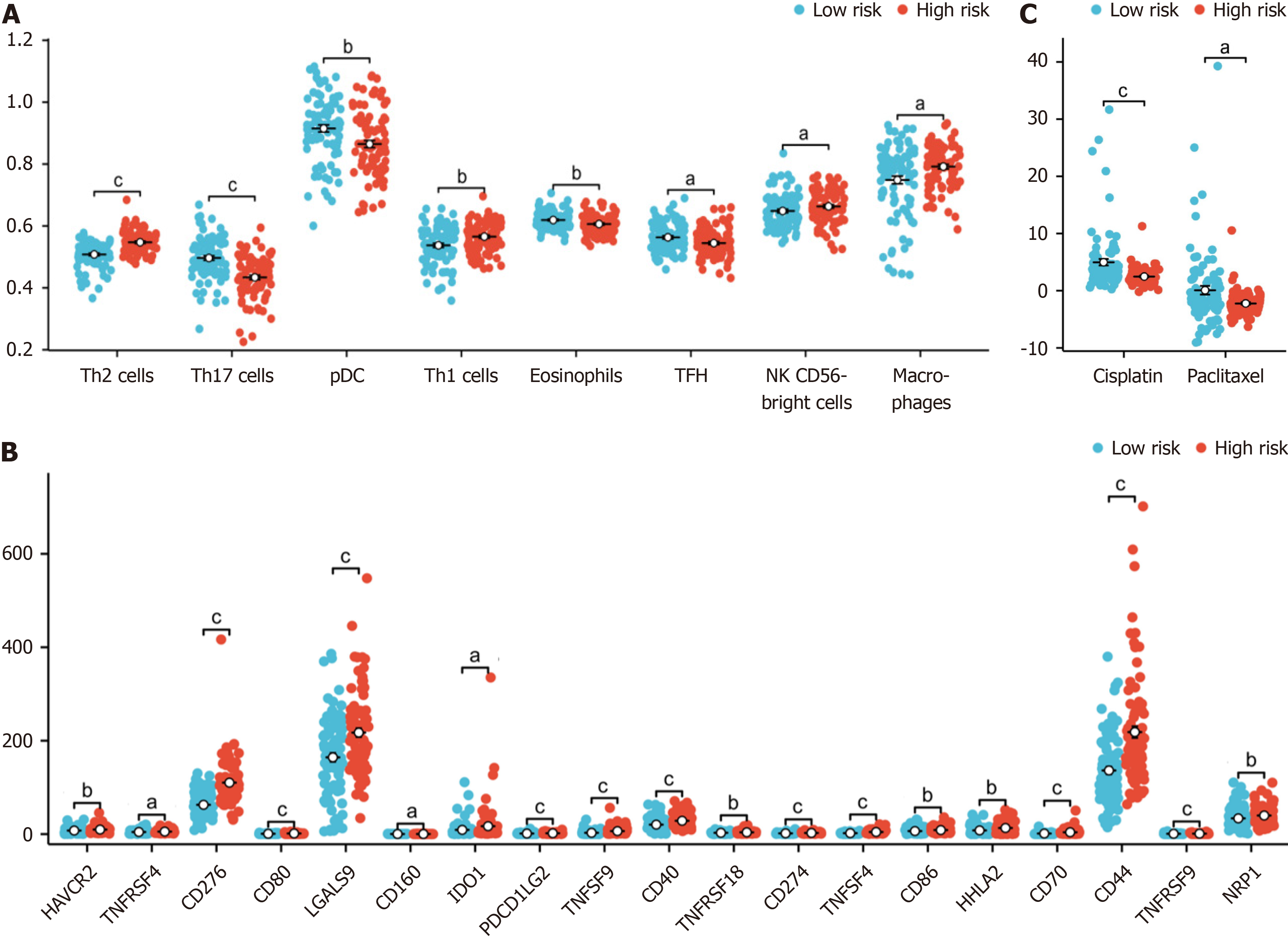
We found that the proportions of macrophages, T helper 1 cells (Th1) , activated dendritic cells (aDCs), “natural killer CD56bright cells (NK CD56bright cells) and T helper 2 cells (Th2) were higher in the high-risk group, while those of T helper 17 cells (Th17), plasmacytoid dendritic cells (pDCs), eosinophils, t follicular helper (TFH), mast cells, CD8 T cells, immature dendritic cells, T cells, B cells, cytotoxic cells and Tem cells were lower in the high-risk group (Figure 6A).
We also found that cluster of CD276, TNFSF4, CD70, TNFSF9, CD44, CD80, CD274, CD40, TNFRSF9, PDCD1 LG2, LGALS9, CD86, HHLA2, HAVCR2, NRP1, TNFRSF18, TNFRSF4, IDO1 and CD160 were differentially expressed between high-risk and low-risk patients grouped according to our model (Figure 6B).
We also explored the relationship between the hypoxia-related risk score and the effect of chemotherapy on PAAD treatment. We found that the half-maximal inhibitory concentrations (IC50) of the chemotherapy drugs cisplatin and paclitaxel were lower in the high-risk group (Figure 6C).
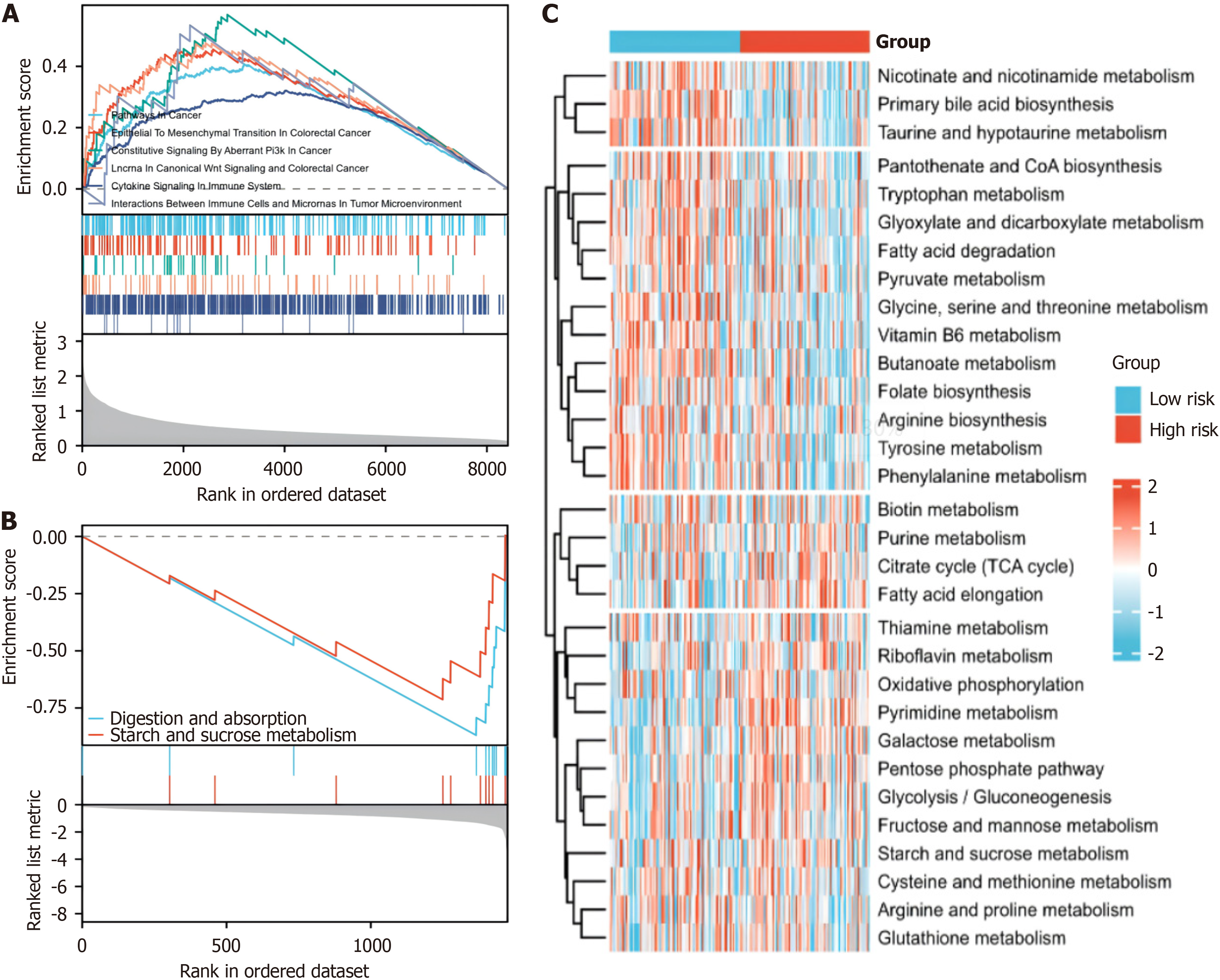
GSEA revealed enrichment of pathways related to tumor progression and immunity in the high-risk group (Figure 7A), whereas pathways associated with tumor progression and immunity were less enriched in the low-risk group (Figure 7B). Our GSVA further revealed substantial alterations in metabolic pathways among the distinct hypoxia risk groups (Figure 7C). This comprehensive examination sheds light on the intricate interplay between hypoxia, the immune res
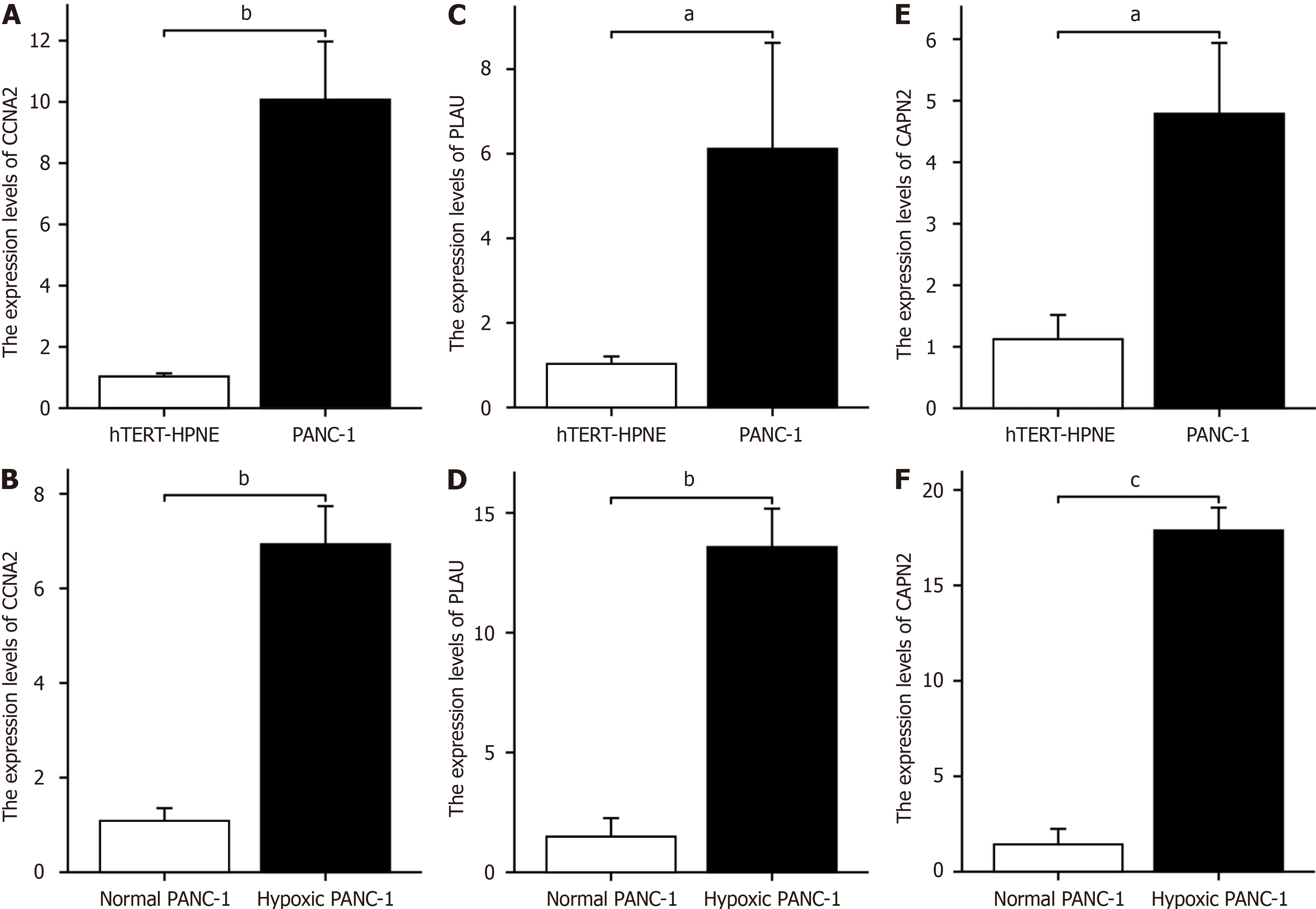
The RT-PCR results revealed that CCNA2 was highly expressed in PANC-1 cells and hTERT-HPNE cells (Figure 8A). The expression levels of CCNA2 in PANC-1 cells subjected to hypoxia was greater than those in untreated cells (Figure 8B). Similarly, PLAU was highly expressed in PANC-1 cells and hTERT-HPNE cells (Figure 8C), the expression levels of CCNA2 in PANC-1 cells subjected to hypoxia was greater than those in untreated cells (Figure 8D). Additionally, CAPN2 was highly expressed in both PANC-1 cells and hTERT-HPNE cells (Figure 8E), the expression levels of CCNA2 in PANC-1 cells subjected to hypoxia was greater than those in untreated cells (Figure 8F).
Pancreatic cancer mainly originates from the pancreatic ductal epithelium; its symptoms are not obvious, and it is difficult to diagnose this disease early[17]. It has a very poor prognosis and a high mortality rate worldwide[18]. Its onset is insidious, and there are no specific symptoms at the beginning of the disease; once there are obvious symptoms, most tumors have reached the advanced stage, which is one of the reasons for the poor prognosis of pancreatic cancer[19]. In this context, identifying new high-efficiency prognostic models that contribute to clinical diagnosis, treatment, and prognosis evaluation is critical. Overall, we conducted a comprehensive analysis of pancreatic cancer using gene ex
Hypoxia is one of the main factors leading to the malignant progression of pancreatic cancer[20]. Hypoxia occurs when the oxygen supply is insufficient to meet the metabolic needs of the tissues[21]. A hypoxic environment can promote the survival and proliferation of cancer cells and induce the expression of genes related to angiogenesis and metastasis, leading to the formation of abnormal and inefficient blood vessels and further leading to a hypoxic environment[22]. Recent studies have shown that HIF-1 plays an important role in this process. Hypoxia-induced HIF-1a overexpression can decrease caspase-3 and caspase-9 expression and inhibit tumor cell apoptosis[23].
Previous studies have highlighted the critical role of hypoxia in pancreatic cancer progression and prognosis. Lin et al[24] identified that hypoxia-induced exosomal circPDK1 promotes pancreatic cancer glycolysis via c-myc activation. Liu et al[25] revealed that m6A methylation regulates hypoxia-induced pancreatic cancer glycolytic metabolism through an ALKBH5-HDAC4-HIF1α positive feedback loop. Chen et al[26] showed that in the hypoxic environment of pancreatic cancer, exosomal miR-30b-5p promotes tumor angiogenesis by inhibiting GJA1 expression. These studies collectively demonstrate the complex interplay between hypoxia, exosomes, epigenetic modifications, and cellular metabolism in pancreatic cancer, providing insights into potential therapeutic targets and diagnostic markers. Our study builds upon these findings by focusing on a more refined set of genes and their specific roles in the hypoxic pancreatic cancer micro
In recent years, an increasing focus has been placed on biomarkers, prognostic markers, and prognostic models in the context of pancreatic ductal adenocarcinoma[27]. We performed enrichment analysis of DEGs between risk grounds in the PAAD dataset via GEPIA2 and constructed a PPI network, and these analyses revealed genes associated with hypoxia. Through univariate Cox analysis, we further screened hypoxia- and prognosis-related genes (CAPN2, PLAU, and CCNA2) from among the above genes and established a prognostic model via LASSO analysis. According to rigorous validation and analysis, our model demonstrated robust reliability in prognosis prediction for pancreatic cancer patients, exhibiting superior performance compared to conventional clinical prognostic factors, as indicated by the area under the ROC curve. The close correlation of our prognostic model with key clinical features, including tumor grade, T stage, and residual tumor status, emphasizes its potential utility for clinicians. Consequently, our model offers a valuable tool for improving the prognosis of pancreatic cancer patients, as it facilitates more effective and timely treatment and reveals potentially effective intervention options.
Our analysis identified three key hypoxia- and prognosis-related genes: PLAU, CCNA2, and CAPN2. PLAU is crucial for extracellular matrix degradation and angiogenesis[28]. CCNA2 regulates cancer cell growth and influences the tumor immune microenvironment[29]. CAPN2 promotes epithelial-mesenchymal transition in pancreatic cancer[30]. These genes likely play significant roles in hypoxia-mediated pancreatic cancer progression, highlighting their potential as pro
The microenvironment of pancreatic cancer has a crucial and complex impact on the biological behaviour of cancer cells[31]. As the tumor evolves, its tissue structure and function dynamically change, forming an immunosuppressive environment that facilitates tumor cell evasion of immune surveillance[32]. Patients with rapidly progressing PAAD typically exhibit insufficient infiltration of immune cells in the tumor immune microenvironment[33]. Research has shown that the NLRP3 signalling pathway and Dectin-1 signalling pathway mediate the predominant effects of immu
The immune checkpoint programmed death-ligand 1 (PD-L1) (CD274) is an important immune regulatory molecule that primarily inhibits the activity of immune cells by binding to programmed death-1 (PD-1) on the surface of immune cells to prevent excessive immune responses[38]. Overexpression of PD-L1 has been observed in various cancers and is associated with tumor progression, metastasis, and treatment resistance[39]. In our study, the immune checkpoint pro
There is a strong link between hypoxia and metabolism, especially at the cellular and tissue levels[41]. In hypoxic environments, cells often choose to produce energy through lactic acid fermentation rather than through oxidative phosphorylation[42]. This choice stems from the fact that lactic acid fermentation is a more rapid way to produce ATP in the absence of oxygen, helping to meet the energy needs of cells[43]. Hypoxia may lead to disruption of the reduction-oxidation (REDOX) balance in cells, which in turn affects multiple metabolic pathways[44]. In our study, GSVA revealed that, compared with those in the low-risk group, the expression of genes in multiple metabolic pathways, including those involved in the REDOX pathway and tricarboxylic acid cycle, tended to increase or decrease, respectively. Dysregulation of these pathways may be a key factor affecting the prognosis of pancreatic cancer. Moreover, through GSEA, we ob
Through RT-qPCR analysis, our investigation revealed notable differences in the expression levels of critical hypoxia-related genes (CAPN2, PLAU, and CCNA2) in pancreatic cancer cells subjected to a hypoxic environment compared to those in untreated pancreatic cancer cells. The substantial upregulation of these genes implies their potential pivotal role in promoting the progression of pancreatic cancer.
While our study provides insights into the role of hypoxia-related genes in the prognosis of patients with pancreatic cancer, there are some limitations related to its retrospective nature, so prospective clinical validation studies are needed. Future research should focus on validating our model's predictive power through prospective trials, exploring the complex interactions between hypoxia and other tumor microenvironment factors, and elucidating the underlying mole
In this study, we established a novel hypoxia-related prognostic model for pancreatic cancer, which demonstrated robust power in predicting patient outcomes. By elucidating the relationships among hypoxia, gene expression, and the immune landscape, we developed a tool for guiding personalized treatment. This integration of hypoxia-related biomarkers may lead to more targeted treatment strategies, potentially improving outcomes in patients with pancreatic cancer.
| 1. | Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395:2008-2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1674] [Article Influence: 334.8] [Reference Citation Analysis (1)] |
| 2. | Padoan A, Plebani M, Basso D. Inflammation and Pancreatic Cancer: Focus on Metabolism, Cytokines, and Immunity. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 253] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 3. | Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607-620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2129] [Cited by in RCA: 2115] [Article Influence: 151.1] [Reference Citation Analysis (3)] |
| 4. | Wolpin BM. Pancreatic Cancer. Hematol Oncol Clin North Am. 2015;29:xiii-xxiv. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Dreyer SB, Chang DK, Bailey P, Biankin AV. Pancreatic Cancer Genomes: Implications for Clinical Management and Therapeutic Development. Clin Cancer Res. 2017;23:1638-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 125] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 6. | Ren B, Cui M, Yang G, Wang H, Feng M, You L, Zhao Y. Tumor microenvironment participates in metastasis of pancreatic cancer. Mol Cancer. 2018;17:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 421] [Article Influence: 60.1] [Reference Citation Analysis (0)] |
| 7. | Chen D, Huang H, Zang L, Gao W, Zhu H, Yu X. Development and Verification of the Hypoxia- and Immune-Associated Prognostic Signature for Pancreatic Ductal Adenocarcinoma. Front Immunol. 2021;12:728062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 8. | Garcea G, Doucas H, Steward WP, Dennison AR, Berry DP. Hypoxia and angiogenesis in pancreatic cancer. ANZ J Surg. 2006;76:830-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Tan Z, Xu J, Zhang B, Shi S, Yu X, Liang C. Hypoxia: a barricade to conquer the pancreatic cancer. Cell Mol Life Sci. 2020;77:3077-3083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 10. | Ru B, Wong CN, Tong Y, Zhong JY, Zhong SSW, Wu WC, Chu KC, Wong CY, Lau CY, Chen I, Chan NW, Zhang J. TISIDB: an integrated repository portal for tumor-immune system interactions. Bioinformatics. 2019;35:4200-4202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 1437] [Article Influence: 287.4] [Reference Citation Analysis (0)] |
| 11. | Li Y, Yang S, Yue H, Yuan D, Li L, Zhao J, Zhao L. Unraveling LGALS1 as a Potential Immune Checkpoint and a Predictor of the Response to Anti-PD1 Therapy in Clear Cell Renal Carcinoma. Pathol Oncol Res. 2020;26:1451-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Lei X, Lei Y, Li JK, Du WX, Li RG, Yang J, Li J, Li F, Tan HB. Immune cells within the tumor microenvironment: Biological functions and roles in cancer immunotherapy. Cancer Lett. 2020;470:126-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 942] [Article Influence: 157.0] [Reference Citation Analysis (0)] |
| 13. | Tang Z, Kang B, Li C, Chen T, Zhang Z. GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019;47:W556-W560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1991] [Cited by in RCA: 3082] [Article Influence: 513.7] [Reference Citation Analysis (0)] |
| 14. | Szklarczyk D, Kirsch R, Koutrouli M, Nastou K, Mehryary F, Hachilif R, Gable AL, Fang T, Doncheva NT, Pyysalo S, Bork P, Jensen LJ, von Mering C. The STRING database in 2023: protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023;51:D638-D646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1815] [Cited by in RCA: 3498] [Article Influence: 1749.0] [Reference Citation Analysis (0)] |
| 15. | Yoshihara K, Shahmoradgoli M, Martínez E, Vegesna R, Kim H, Torres-Garcia W, Treviño V, Shen H, Laird PW, Levine DA, Carter SL, Getz G, Stemke-Hale K, Mills GB, Verhaak RG. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat Commun. 2013;4:2612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3056] [Cited by in RCA: 6455] [Article Influence: 586.8] [Reference Citation Analysis (0)] |
| 16. | Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, Angell H, Fredriksen T, Lafontaine L, Berger A, Bruneval P, Fridman WH, Becker C, Pagès F, Speicher MR, Trajanoski Z, Galon J. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39:782-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1792] [Cited by in RCA: 2957] [Article Influence: 246.4] [Reference Citation Analysis (0)] |
| 17. | McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24:4846-4861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1338] [Cited by in RCA: 1262] [Article Influence: 180.3] [Reference Citation Analysis (39)] |
| 18. | Chiorean EG, Coveler AL. Pancreatic cancer: optimizing treatment options, new, and emerging targeted therapies. Drug Des Devel Ther. 2015;9:3529-3545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 135] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 19. | Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:1039-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1500] [Cited by in RCA: 1711] [Article Influence: 155.5] [Reference Citation Analysis (0)] |
| 20. | Tao J, Yang G, Zhou W, Qiu J, Chen G, Luo W, Zhao F, You L, Zheng L, Zhang T, Zhao Y. Targeting hypoxic tumor microenvironment in pancreatic cancer. J Hematol Oncol. 2021;14:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 255] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 21. | Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18:4266-4276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 841] [Cited by in RCA: 1030] [Article Influence: 85.8] [Reference Citation Analysis (0)] |
| 22. | Shah VM, Sheppard BC, Sears RC, Alani AW. Hypoxia: Friend or Foe for drug delivery in Pancreatic Cancer. Cancer Lett. 2020;492:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 23. | Semenza GL. HIF-1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol. 2001;13:167-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 811] [Cited by in RCA: 919] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 24. | Lin J, Wang X, Zhai S, Shi M, Peng C, Deng X, Fu D, Wang J, Shen B. Hypoxia-induced exosomal circPDK1 promotes pancreatic cancer glycolysis via c-myc activation by modulating miR-628-3p/BPTF axis and degrading BIN1. J Hematol Oncol. 2022;15:128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 102] [Reference Citation Analysis (0)] |
| 25. | Liu X, Feng M, Hao X, Gao Z, Wu Z, Wang Y, Du L, Wang C. m6A methylation regulates hypoxia-induced pancreatic cancer glycolytic metabolism through ALKBH5-HDAC4-HIF1α positive feedback loop. Oncogene. 2023;42:2047-2060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 26. | Chen K, Wang Q, Liu X, Wang F, Yang Y, Tian X. Hypoxic pancreatic cancer derived exosomal miR-30b-5p promotes tumor angiogenesis by inhibiting GJA1 expression. Int J Biol Sci. 2022;18:1220-1237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 95] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 27. | Zhou Z, Cheng Y, Jiang Y, Liu S, Zhang M, Liu J, Zhao Q. Ten hub genes associated with progression and prognosis of pancreatic carcinoma identified by co-expression analysis. Int J Biol Sci. 2018;14:124-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 113] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 28. | Hosen SMZ, Uddin MN, Xu Z, Buckley BJ, Perera C, Pang TCY, Mekapogu AR, Moni MA, Notta F, Gallinger S, Pirola R, Wilson J, Ranson M, Goldstein D, Apte M. Metastatic phenotype and immunosuppressive tumour microenvironment in pancreatic ductal adenocarcinoma: Key role of the urokinase plasminogen activator (PLAU). Front Immunol. 2022;13:1060957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 29. | Chen Q, Shen P, Ge WL, Yang TY, Wang WJ, Meng LD, Huang XM, Zhang YH, Cao SJ, Miao Y, Jiang KR, Zhang JJ. Roundabout homolog 1 inhibits proliferation via the YY1-ROBO1-CCNA2-CDK2 axis in human pancreatic cancer. Oncogene. 2021;40:2772-2784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | Li P, Miao C, Liang C, Shao P, Wang Z, Li J. Silencing CAPN2 Expression Inhibited Castration-Resistant Prostate Cancer Cells Proliferation and Invasion via AKT/mTOR Signal Pathway. Biomed Res Int. 2017;2017:2593674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 31. | Daley D, Mani VR, Mohan N, Akkad N, Ochi A, Heindel DW, Lee KB, Zambirinis CP, Pandian GSB, Savadkar S, Torres-Hernandez A, Nayak S, Wang D, Hundeyin M, Diskin B, Aykut B, Werba G, Barilla RM, Rodriguez R, Chang S, Gardner L, Mahal LK, Ueberheide B, Miller G. Dectin 1 activation on macrophages by galectin 9 promotes pancreatic carcinoma and peritumoral immune tolerance. Nat Med. 2017;23:556-567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 287] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 32. | Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 1360] [Article Influence: 151.1] [Reference Citation Analysis (0)] |
| 33. | Abdel Mouti M, Pauklin S. TGFB1/INHBA Homodimer/Nodal-SMAD2/3 Signaling Network: A Pivotal Molecular Target in PDAC Treatment. Mol Ther. 2021;29:920-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 34. | Sendler M, van den Brandt C, Glaubitz J, Wilden A, Golchert J, Weiss FU, Homuth G, De Freitas Chama LL, Mishra N, Mahajan UM, Bossaller L, Völker U, Bröker BM, Mayerle J, Lerch MM. NLRP3 Inflammasome Regulates Development of Systemic Inflammatory Response and Compensatory Anti-Inflammatory Response Syndromes in Mice With Acute Pancreatitis. Gastroenterology. 2020;158:253-269.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 218] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 35. | Ghosh D, Lippert D, Krokhin O, Cortens JP, Wilkins JA. Defining the membrane proteome of NK cells. J Mass Spectrom. 2010;45:1-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Euchner J, Sprissler J, Cathomen T, Fürst D, Schrezenmeier H, Debatin KM, Schwarz K, Felgentreff K. Natural Killer Cells Generated From Human Induced Pluripotent Stem Cells Mature to CD56(bright)CD16(+)NKp80(+/-)In-Vitro and Express KIR2DL2/DL3 and KIR3DL1. Front Immunol. 2021;12:640672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 37. | Veneziani I, Alicata C, Pelosi A, Landolina N, Ricci B, D'Oria V, Fagotti A, Scambia G, Moretta L, Maggi E. Toll-like receptor 8 agonists improve NK-cell function primarily targeting CD56(bright)CD16(-) subset. J Immunother Cancer. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 38. | Feng M, Xiong G, Cao Z, Yang G, Zheng S, Song X, You L, Zheng L, Zhang T, Zhao Y. PD-1/PD-L1 and immunotherapy for pancreatic cancer. Cancer Lett. 2017;407:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 249] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 39. | Soliman H, Khalil F, Antonia S. PD-L1 expression is increased in a subset of basal type breast cancer cells. PLoS One. 2014;9:e88557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 193] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 40. | Hashimoto S, Furukawa S, Hashimoto A, Tsutaho A, Fukao A, Sakamura Y, Parajuli G, Onodera Y, Otsuka Y, Handa H, Oikawa T, Hata S, Nishikawa Y, Mizukami Y, Kodama Y, Murakami M, Fujiwara T, Hirano S, Sabe H. ARF6 and AMAP1 are major targets of KRAS and TP53 mutations to promote invasion, PD-L1 dynamics, and immune evasion of pancreatic cancer. Proc Natl Acad Sci U S A. 2019;116:17450-17459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 41. | Parks SK, Cormerais Y, Pouysségur J. Hypoxia and cellular metabolism in tumour pathophysiology. J Physiol. 2017;595:2439-2450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 108] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 42. | Wang Q, Wang P, Qin Z, Yang X, Pan B, Nie F, Bi H. Altered glucose metabolism and cell function in keloid fibroblasts under hypoxia. Redox Biol. 2021;38:101815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 43. | Wu Z, Zuo M, Zeng L, Cui K, Liu B, Yan C, Chen L, Dong J, Shangguan F, Hu W, He H, Lu B, Song Z. OMA1 reprograms metabolism under hypoxia to promote colorectal cancer development. EMBO Rep. 2021;22:e50827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 44. | Infantino V, Santarsiero A, Convertini P, Todisco S, Iacobazzi V. Cancer Cell Metabolism in Hypoxia: Role of HIF-1 as Key Regulator and Therapeutic Target. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 283] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 45. | Suthen S, Lim CJ, Nguyen PHD, Dutertre CA, Lai HLH, Wasser M, Chua C, Lim TKH, Leow WQ, Loh TJ, Wan WK, Pang YH, Soon G, Cheow PC, Kam JH, Iyer S, Kow A, Tam WL, Shuen TWH, Toh HC, Dan YY, Bonney GK, Chan CY, Chung A, Goh BKP, Zhai W, Ginhoux F, Chow PKH, Albani S, Chew V. Hypoxia-driven immunosuppression by Treg and type-2 conventional dendritic cells in HCC. Hepatology. 2022;76:1329-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 108] [Article Influence: 36.0] [Reference Citation Analysis (0)] |