INTRODUCTION
Acute liver failure (ALF), also referred to as fulminant hepatic failure, is a rare yet serious condition characterized by the rapid deterioration of liver function, often occurring within a span of days to weeks. It can be caused by various factors, including drug overdose, viral infections, and autoimmune diseases[1]. This rapid deterioration of liver function can lead to severe complications, including hepatic encephalopathy, coagulopathy (bleeding disorders), and multi-organ failure[2]. According to a report, patients with ALF had 40.02% mortality[3]. ALF accounts for 6% of all liver disease-related deaths in the United States, and 3000 cases are reported annually. It is more common in Americans than other racial groups and more common in women than men. The peak incidence of ALF is at a relatively young age of 35 years in women and 45 years in men[4]. Drug-induced liver injury is the most common cause of ALF in developed countries, accounting for over 50% of cases. It can be caused by various medications, including acetaminophen (paracetamol) overdose, certain antibiotics, anti-epileptic drugs, and herbal remedies[5]. Recent studies have proposed several cell death pathways, including ferroptosis, pyroptosis, necroptosis, and apoptosis, that are important in the development of ALF[6,7]. Among them, the two distinguishing programmed cell death processes that are distinct from autophagy, apoptosis, and necrosis are ferroptosis and pyroptosis. Both of these processes are vital for the development of ALF as they each modulate distinct inflammatory and immunological responses[8].
The main mechanism behind ferroptosis is reactive oxygen species (ROS)-dependent regulated cell death, with lipid peroxidation, intracellular iron overload, and decreased glutathione peroxidase 4 (GPX4) activity serving as key indicators. The Fenton process results in the production of ROS, which changes hydrogen peroxide into hydroxyl radicals and subsequently causes lipid peroxidation[9]. ROS growth is regulated by the inhibitory activity of GPX4, an enzyme that uses hydrogen ions to neutralize lipid peroxides and hydrogen peroxide. Reduced glutathione (GSH) is transformed into glutathione disulfide (GSSG) by GPX4[10]. In addition to GPX4’s basic function of preserving ROS levels, numerous other pathways are necessary to sustain GPX’s antioxidant activity. System Xc- is a heterodimeric antiport system comprising the subunits SLC3A2 and SLC7A11 that import cystine and output glutamate. The imported cystine is converted into cysteine and GSH to maintain redox equilibrium and shield the cells from ferroptosis[11]. The primary mechanism that sustains the effectiveness of GPX antioxidant action is System Xc-, which inhibits a sequence of processes that lead to a decrease in GSH levels, lipid peroxidation, and, ultimately, ferroptosis[12].
Due to p53’s transcriptional inhibition of SLC7A11, cells are more susceptible to ferroptosis and absorb cystine less efficiently. The cellular response to various triggers of stress, such as hypoxia, starvation, DNA damage, and oncogene activation, is greatly influenced by p53. Furthermore, by inhibiting cystine metabolism and ROS activity, p53 promotes ferroptosis[13].
Pyroptosis is an acute inflammatory form of programmed cell death that is mostly provoked by either canonical or noncanonical inflammasomes. It is characterized by the morphological enlargement of cells followed by lysis, ultimately releasing intracellular material[14]. The liver is constantly exposed to various gut-derived microbial particles known as pathogen-associated molecular patterns (PAMPs) due to the tight connection between portal circulation and the intestines. PAMPs excite local immune cells[15]. When risk signals are detected, intracellular multiprotein complexes known as canonical inflammasomes are formed. These inflammasomes activate caspase-1, which leads to the maturation of interleukin-1 beta (IL-1β), IL-18, and IL-37, ultimately causing pyroptosis. Caspase-11 is activated by noncanonical inflammasomes, causing pyroptosis[16]. Gasdermin D (GSDMD) is a pore-forming protein that incites pro-inflammatory cytokine release and causes pyroptosis[17]. PAMPs stimulate distinct inflammasomes to initiate caspase-1, which subsequently cleaves GSDMD, the pyroptosis executor, into its active N terminal and inactive C terminal. The C-terminal regulatory domain (GSDMD-C) and the pyroptotic N-terminal domain (GSDMD-N) of GSDMD are joined by a linker domain. Inflammatory caspases (caspase-1, caspase-4, and caspase-5) break the linker domain of GSDMD, releasing GSDMD-N from its autoinhibitory GSDMD-C. GSDMD-N demonstrates a remarkable resemblance to membrane lipids, where it oligomerizes to produce pores with a diameter of around 20 nm, causing swelling and cell lysis, while GSDMD-C stays in the cytoplasm[18].
Sirtuin 1 (SIRT1), a nicotinamide adenine dinucleotide (NAD+)-dependent histone deacetylase, is believed to protect cells from oxidative stress injury by mediating Nrf2 production and its downstream targets[19]. SIRT1 plays a complex function in stress responses, energy metabolism, inflammation, and redox balance through the acetylation and deacetylation of certain transcription factors and proteins, including p53[20]. Zhou et al[21] discussed the method by which SIRT1 reduces ALF and how it is linked to hepatocyte death that occurs widely and involves ferroptosis and pyroptosis[21].
ROLE OF p53/GPX4/GSDMD AXIS IN ACUTE LIVER FAILURE
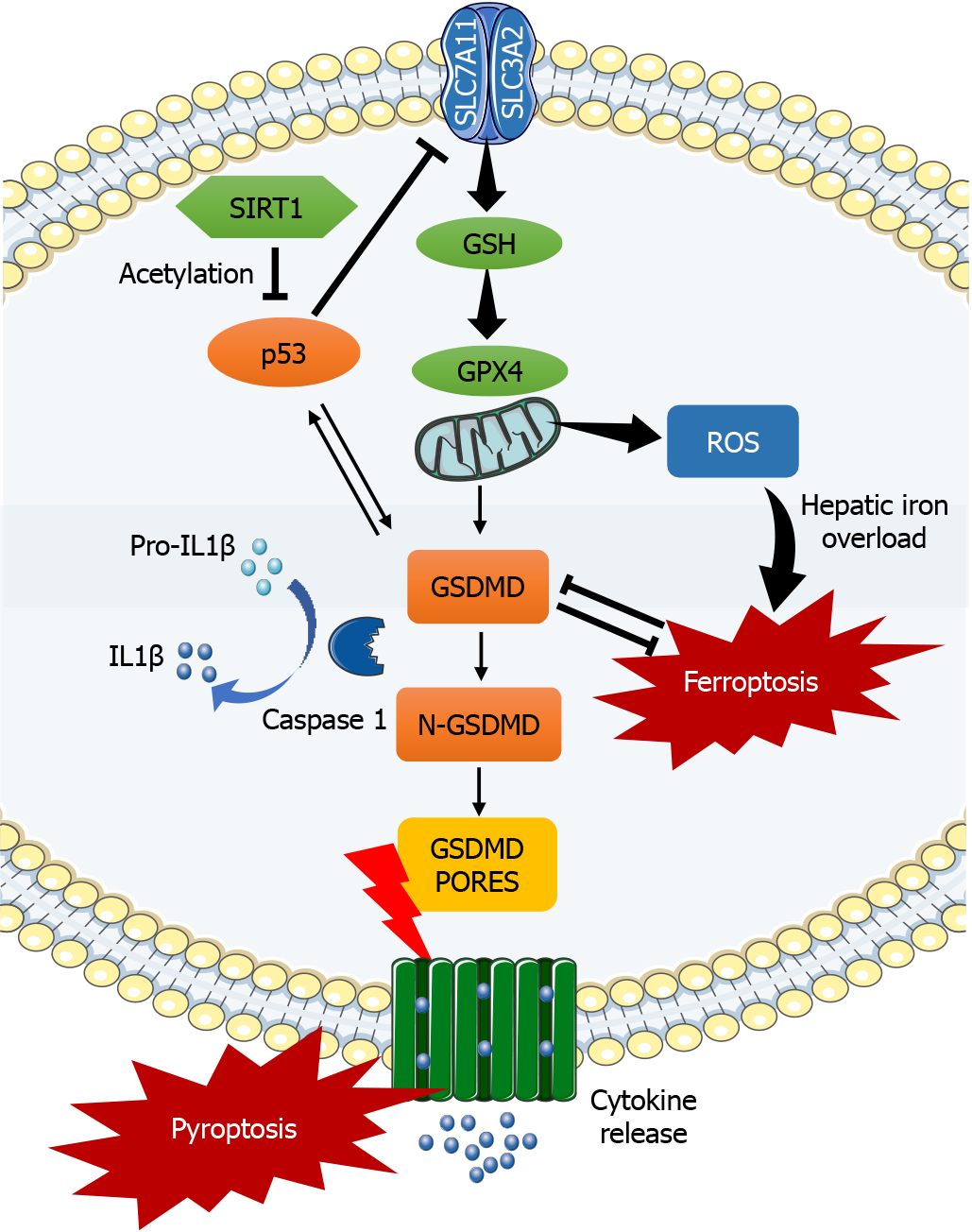
Zhou et al[21] have carefully examined the process by which p53/GPX4/GSDMD signaling pathway blockade caused by SIRT1 activation decreases ferroptosis and pyroptosis in ALF (Figure 1). The increased activity of the enzyme biomarkers alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in human ALF is linked to elevated levels of inflammatory factors, such as tumor necrosis factor-α (TNFα), IL-1β, and IL-6. Furthermore, ferroptosis-related antioxidant protein levels of GPX4 and SLC7A11 drop in ALF, worsening iron deposition and leading to increased expression of GSDMD[21]. It is unclear how ferroptosis and pyroptosis are related to fulminant hepatitis in humans, despite the fact that the etiology of LPS/D-GalN-induced ALF in mice is quite similar to that in humans.
Figure 1 The activation of sirtuin 1 suppresses ferroptosis and pyroptosis in acute liver failure by inhibiting p53, glutathione peroxidase 4, and gasdermin D.
The silent information regulator sirtuin 1 blocks p53 deacetylation and inhibits the glutathione peroxidase 4 (GPX4)/gasdermin D (GSDMD) signaling pathway, which in turn reduces hepatic iron overload and the inflammatory response. Ferroptosis and pyroptosis in acute liver failure are reduced by blocking the p53/GPX4/GSDMD signaling pathway. SIRT1: Silent information regulator sirtuin 1; SLC7A11: Solute carrier family 7a member 11; SLC3A2: Solute carrier family 3a member 2; GSH: Glutathione; GPX4: Glutathione peroxidase 4; GSDMD: Gasdermin D; IL1β: Interleukin-1 beta; ROS: Reactive oxygen species.
GSDMD and GPX4 are essential for ferroptosis and pyroptosis to occur. Conditions leading to GPX4 instability or suppression make cells more susceptible to ferroptosis or even lead to ferroptotic cell death, whereas sufficient GPX4 activity and GSH synthesis are necessary to prevent ferroptosis[22]. A recent study in a murine model found that ferroptosis was significantly involved in liver failure produced by acetaminophen. The results showed higher levels of TNFα, interleukins, and PTGS2, a well-known marker for ferroptosis. This process was considerably suppressed by ferrostatin-1, an inhibitor that is unique to ferroptosis. Ferroptosis may be a viable therapeutic target for liver failure, according to these findings[23]. Increased ROS levels, decreased System Xc-activity, and suppressed GPX4 levels are the main inducers of ferroptosis[24]. The LPS/D-GalN-induced liver failure model was reported to have decreased levels of SLC7A11, HO-1, and GPX4, along with increased ROS production, indicating System Xc- involvement in ferroptosis[25]. The inflammatory action is further prolonged by GSDMD-mediated hepatocyte pyroptosis, which stimulates macrophages by escalating the levels of monocyte chemotactic protein 1/CC chemokine receptor-2[18]. A study reported that pre-administration of oyster-derived Tyr-Ala (YA) peptide improves the elevated levels of GSDMD, along with its regulatory factors like caspase-1, IL-1β and TNFα in the LPS/D-GalN induced ALF model[26]. Many researchers reported that GSDMD gene knockout ameliorates pyroptosis and ferroptosis with reduced inflammatory reactions and hepatocyte loss, which improves ALF[27,28].
The non-classical process of pyroptotic cell death is mediated by caspase-11, which is implicated in myeloid cell knockout[29]. This process was seen in mice lacking the GPX4 gene that were susceptible to a deadly infection and had septic myeloid cells. GPX4 suppressed the caspase-1-dependent NLRP3 inflammasome, suggesting a comprehensive role of GPX4 against pyroptosis[30]. According to a different study, 3,4-dihydroxyphenylethyl alcohol glycoside (DAG) prevents hepatocyte ferroptosis and pyroptosis, which lowers ALF in mice caused by acetaminophen. DAG further decreased the levels of proinflammatory cytokines, histological changes, hepatic neutrophil infiltration, and serum ALT and AST. It also suppressed the levels of MDA adducts and the depletion of GSH, CAT, and SOD enzymes. In vitro, in mouse AML12 hepatocytes exposed to acetaminophen, DAG demonstrated a dose-dependent suppression of proinflammatory factors (IL-1β and IL18), ROS, and the reduction of GSH depletion. Interestingly, in liver tissues and AML12 hepatocytes, DAG increased the expression of GPX4 and decreased that of HO-1, NLRP3, caspase-1, and GSDMD[31].
The p53 tumor suppressor protein is a critical cell cycle and apoptosis regulator. p53 regulates the progression of liver injury and regeneration after acetaminophen overdose[32]. p53 knockout mice (p53KO) showed prolonged steatosis, lower mitochondrial DNA content, and reduced expression of mitochondrial transcription factor A and mitochondrial complexes in acetaminophen-induced liver injury. It also altered metabolic homeostasis and activated proinflammatory and proliferative signaling. Prolonged steatosis in the p53KO group was also linked with p53 targets related to fatty acid balance, SREBP2 protein, and GAMT mRNA expression[32]. Zhou et al’s study[21] demonstrated that the inhibition of p53 and increased GPX4 in the ALF mouse model reduced the inflammatory responses, AST and ALT levels, and ferroptotic events (depletion of GPX4, GSH, and SLC7A11, as well as iron buildup). A cross-talk between ferroptosis and pyroptosis was observed, as evidenced by the reduction of p53 expression and the elevation of GPX4 following GSDMD knockout. Furthermore, ALT and AST levels, ferroptosis markers, and GSDMD were markedly enhanced in response to GPX4 knockdown[21]. The findings suggest the possibility of a positive feedback loop and the alleviation of ferroptosis and pyroptosis in ALF caused by disrupting the p53/GPX4/GSDMD signaling pathway. Another study showed that altering GPX4 protein expression did not influence p53 levels; however, it did operate indirectly by controlling GSDMD, indicating that GPX4 is a downstream regulator of p53. p53-driven ferroptosis is produced in a GPX4-independent manner, and p53 levels remain unaffected by the deletion of ACSL4 and GPX4. However, p53 transcription decreases with GPX4 augmentation, indicating the correlation between GPX4, GSDMD, and p53 in ferroptosis and pyroptosis[33].
SIRT1, a NAD-dependent deacetylase, mediates the function of p53 by direct deacetylation. According to one study, negative regulation of SIRT1 exacerbated the acute hepatic proinflammatory response and induced pyroptosis[34]. However, SIRT1 overexpression eliminated p53 acetylation levels and decreased the release of hepatic enzymes, hepatic oxidative stress, and inflammation in acetaminophen-induced liver injury[35]. The immediate target gene of p53 is microRNA-34a (miR-34a), which concurrently activates p53 via SIRT1. The miR-34a/SIRT1/p53 signaling pathway, crucial for cell division and death, creates a positive feedback loop in which p53 stimulates miR-34a, and miR-34a promotes p53 by blocking SIRT1. Human patients or animal models with several liver disorders, including liver fibrosis, have been reported with higher expression levels of miR-34a[36]. SIRT1 overexpression attenuates liver fibrosis by decreasing p53 acetylation and caspase activation in apoptosis[37]. In the case of myocardial ischemia-reperfusion injury, SIRT1 activation was found to reduce ferroptosis-induced cardiac cell death by overexpression of SLC7A11 and inhibition of p53, indicating the close link of the SIRT1/p53/SLC7A11 axis[38]. Mice treated with resveratrol, a small-molecule SIRT1 activator, did not suffer hepatic ischemia-reperfusion injury[39]. Overall, all the findings establish a cross-talk between ferroptosis and pyroptosis with their primary upstream and downstream regulatory mechanisms. The activation of SIRT1 inhibits p53 and GSDMD activity while stimulating GPX4 action, overall blocking the p53/GPX4/GSDMD axis and protecting the cell from ferroptosis and pyroptosis.
CLINICAL IMPLICATIONS OF ACUTE LIVER FAILURE
ALF can have significant clinical implications, ranging from mild symptoms to life-threatening complications. Some common implications related to ALF are hepatic dysfunction, coagulopathy, multi-organ dysfunction, hepatic encephalopathy, infection susceptibility, and various long-term complications[2]. Early recognition, prompt diagnosis, and appropriate management are essential to improve outcomes and reduce morbidity and mortality associated with ALF[3]. Certain naturally occurring substances, such as resveratrol in red wine, have been recognized as SIRT1 agonists. Resveratrol has drawn interest because of its potential health benefits and capacity to activate SIRT1 and replicate the effects of caloric restriction, which have been linked to longer lifespans in various organisms[39]. Synthetic SIRT1 agonist development has also been the focus of researchers for possible medicinal uses. Compared to natural substances, these synthesized molecules may provide a more robust and tailored activation of SIRT1. Researchers are looking at the potential of SIRT1 agonists in cancer, neurological diseases, and metabolic disorders, among other areas. The clinical advantages and safety of SIRT1 agonists in humans are still being assessed through ongoing research and clinical trials, even though SIRT1 activation shows promising results in preclinical studies. It is important to remember that SIRT1 is a multifaceted molecule whose function in human health is currently being studied. This work demonstrated a connection between the upstream regulatory processes and ferroptosis and pyroptosis. More investigations are required to ascertain the possible therapeutic advantages of targeting SIRT1 to treat various metabolic disorders.
CONCLUSION
To sum up, this editorial examines the data supporting the notion that ferroptosis and pyroptosis are essential hepatocyte death mechanisms in ALF, and that the interplay between these cell death mechanisms promotes the development of ALF. The findings suggest that SIRT1 and p53 can regulate each other in a feedback loop. While SIRT1 has been shown to deacetylate and inhibit p53 activity, thereby promoting cell survival and inhibiting apoptosis, p53 activation can lead to increased expression of SIRT1, which may have various downstream effects on cellular processes. Considering the reported shreds of evidence, we can attenuate ALF by blocking the p53/GPX4/GSDMD signaling pathway and activating SIRT1, which results in reduced ferroptosis and pyroptosis. This signaling pathway, especially SIRT1, might be a promising therapeutic target for liver failure.
ACKNOWLEDGEMENTS
The authors are thankful to the Director, CSIR-Institute of Himalayan Bioresource Technology, Palampur, India, for his continuous support (Communication no. 5627).
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: India
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Shahid N S-Editor: Li L L-Editor: Webster JR P-Editor: Yuan YY