Published online Jan 21, 2024. doi: 10.3748/wjg.v30.i3.225
Peer-review started: September 11, 2023
First decision: November 3, 2023
Revised: November 16, 2023
Accepted: December 26, 2023
Article in press: December 26, 2023
Published online: January 21, 2024
Processing time: 128 Days and 19.9 Hours
This comprehensive review elucidates the complex interplay between gut microbiota and constipation in Parkinson’s disease (PD), a prevalent non-motor symptom contributing significantly to patients’ morbidity. A marked alteration in the gut microbiota, predominantly an increase in the abundance of Proteobacteria and Bacteroidetes, is observed in PD-related constipation. Conventional treatments, although safe, have failed to effectively alleviate symptoms, thereby necessitating the development of novel therapeutic strategies. Microbiological interventions such as prebiotics, probiotics, and fecal microbiota transplantation (FMT) hold therapeutic potential. While prebiotics improve bowel movements, probiotics are effective in enhancing stool consistency and alleviating abdominal discomfort. FMT shows potential for significantly alleviating constipation symptoms by restoring gut microbiota balance in patients with PD. Despite promising developments, the causal relationship between changes in gut microbiota and PD-related constipation remains elusive, highlighting the need for further research in this expanding field.
Core Tip: This comprehensive review explores the intricate relationship between gut microbiota and constipation, a prevalent non-motor symptom observed in Parkinson’s disease (PD). Notably, we discuss the significant alterations in gut microbiota, particularly the increase in the abundance of Proteobacteria and Bacteroidetes, associated with PD-related constipation. Although currently available treatments are safe, their effectiveness in providing symptom relief remains suboptimal, necessitating the development of innovative therapeutic approaches. This review delves into the potential of therapies based on microbiological interventions such as prebiotics, probiotics, and fecal microbiota transplantation, in alleviating these symptoms.
- Citation: Yuan XY, Chen YS, Liu Z. Relationship among Parkinson’s disease, constipation, microbes, and microbiological therapy. World J Gastroenterol 2024; 30(3): 225-237
- URL: https://www.wjgnet.com/1007-9327/full/v30/i3/225.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i3.225
Parkinson’s disease (PD) is a neurodegenerative disorder with an increasing incidence worldwide[1]. The doubling of PD cases between 1990 and 2016 is expected to result in more than 12 million patients globally by the year 2050[2,3]. PD is characterized by both motor symptoms (e.g., bradykinesia, resting tremor, and rigidity) and non-motor symptoms (e.g., constipation, depression, impaired olfaction, and rapid eye movement sleep behavior disorder)[4]. Constipation is considered one of the most common precursor symptoms of PD and persists throughout the clinical stages of the disease, with its prevalence increasing as the disease progresses[5,6]. For patients with PD, constipation significantly reduces their ability to carry out daily activities and their overall quality of life[7]. Hence, effective therapeutic approaches to control PD-related constipation are urgently required. The pathological mechanisms of PD-related constipation remain unknown, but they may be associated with recto-anal dysfunction or smooth muscle dystonia in the gastrointestinal tract[8,9]. The role of intestinal microorganisms has attracted increasing research attention in recent years. Accumulating evidence reveals a relationship between gut microbiota and PD-related constipation[10-12]. Consequently, traditional treatment options are shifting toward microecological interventions[13-16]. This review summarizes currently available evidence supporting the roles of gut microbiota in the pathogenesis and treatment of PD-related constipation.
The role of intestinal microbes in the central nervous system (CNS) has garnered increasing interest recently. The gut microbiota is a complex ecological community comprising hundreds of millions of microbes that live in the gut and regulates both normal physiology and disease susceptibility through its collective metabolic activities and host interactions[17]. A growing body of research linking PD to the microbiota-gut-brain axis suggests that gut microbiota and microbial metabolites have an important role in PD pathogenesis by influencing neuroinflammation, barrier function, and neurotransmitter activity[18,19]. The microbiota-gut-brain axis includes the autonomic nervous system, the enteric nervous system (ENS), the hypothalamic-pituitary-adrenal axis, and the intestinal microbes[18]. The gut microbiota and the brain can communicate directly through various signaling molecules or indirectly through the gut-brain axis; similarly, the brain can influence the microbes directly or indirectly through alterations to the gut microbiota envir
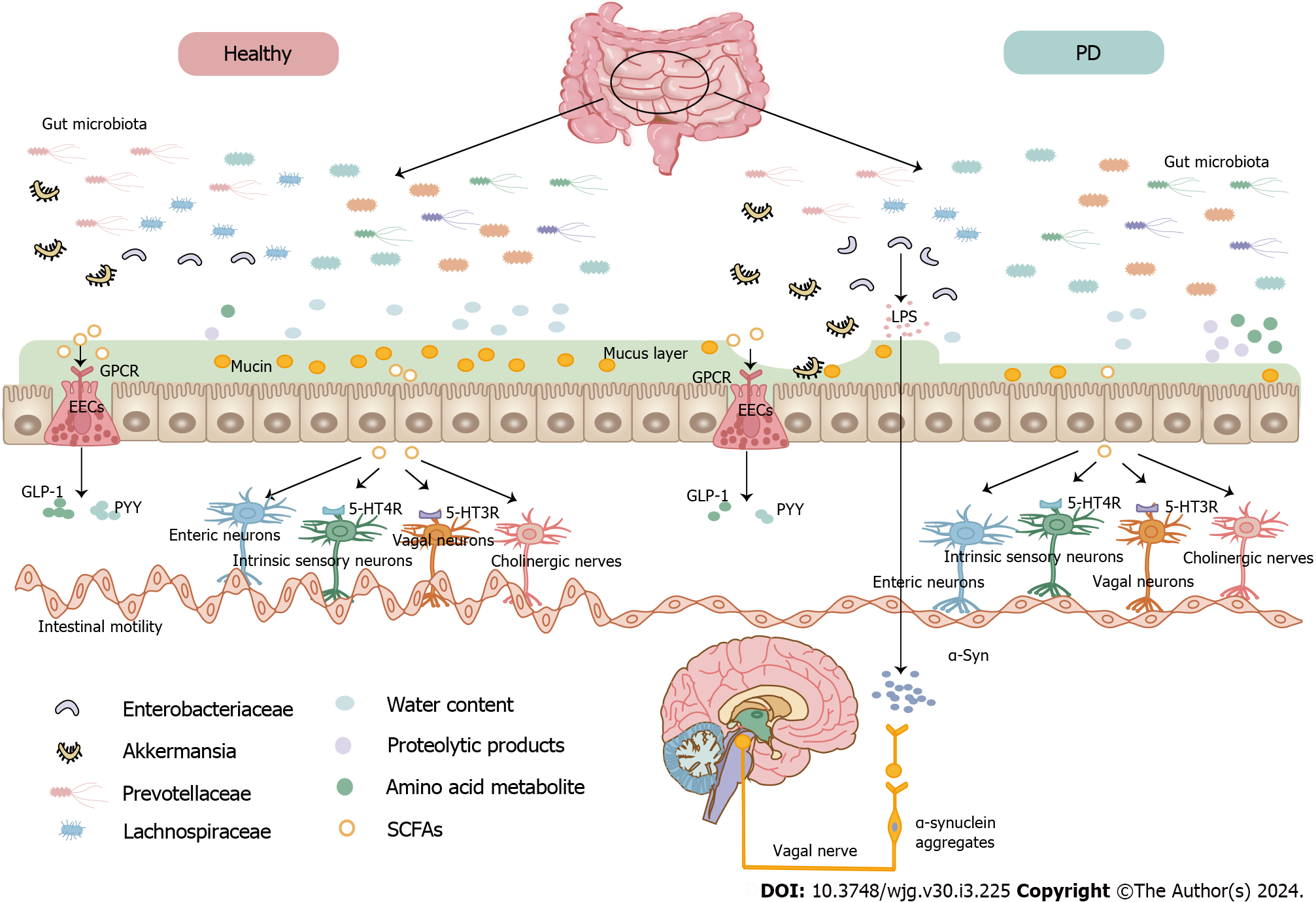
The pathological hallmarks of PD are loss of dopaminergic neurons together with abnormal accumulation of α-synuclein (α-syn) in the substantia nigra and the striatum[21]. Braak et al[22] and Hawkes et al[23] noticed α-synuclein-containing inclusion bodies in the intestines of patients with sporadic PD and hypothesized that the pathology of Lewy body in PD might begin in the gastrointestinal tract and then spread to the brain through the vagal nerve. Human α-syn fibrils were injected into the gut tissue of healthy rodents and transported through the vagus nerve to the dorsal motor nucleus of the vagal nucleus in the brainstem. These results provide the first direct experimental proof that α-syn can propagate from the gut to the brain[24]. Vagotomy has protective effects on the subsequent development of PD, as it can attenuate the pathological spread of α-syn, dopaminergic neuronal degeneration, and motor dysfunction. The vagus nerve is an important route for the transmission of pathological α-syn into the CNS[25-28]. These findings demonstrate that a-syn detection in the ENS could provide an opportunity to identify early PD neuropathology before the disease spreads to other regions and motor symptoms become evident. Shannon et al[29] reported a-syn detection in the neurites of the colonic submucosa in colonic biopsies collected 2-5 years before motor symptom onset in patients with PD[29]. This evidence suggests that a-syn detection in colonic mucosal biopsy samples could serve as a presymptomatic biomarker for PD. Additional evidence revealing a-syn accumulation in colonic biopsies for up to 8 years before motor symptom manifestation further supports the potential of enteric a-syn as a diagnostic biomarker for PD[30]. Pouclet et al[31] performed a comparative analysis of a-syn deposition using biopsy samples collected from the rectum, descending colon, and ascending colon of 26 patients with PD and 9 control subjects. The authors discovered that 23%, 42%, and 65% of patients with PD had a-syn deposition in the rectum, descending colon, and ascending colon, respectively, while control subjects had no a-syn deposition. These findings indicate that enteric α-syn detection has the potential to be used as a sensitive, PD-specific, and clinically useful biomarker for early PD detection.
Constipation, a prevalent non-motor symptom of PD, has been observed in as many as 90% of patients and is a notable early manifestation and risk factor for PD[32-34]. It is nearly three times more prevalent in patients with PD than in healthy individuals[8,35]. Research indicates that the severity of PD-related constipation helps diagnose the PD stage, with 67% sensitivity and 90% specificity[36]. A Taiwanese study revealed that constipation severity correlates with the probability of PD development[37]. A meta-analysis supported this finding, indicating a 2.27-times higher risk of PD in individuals with constipation[33]. Constipation has a significant 76.56% effect on PD and is mediated by gut microbial changes, as a result of altered gut conditions caused by constipation[12,38]. These changes may result in intestinal inflammation and PD symptoms[38]. Causes of PD-related constipation include delayed colon transit and outlet obstruction[8,39]. The clinical course of PD worsens with constipation, resulting in evident severe motor and non-motor symptoms[7,40]. The severity and frequency of constipation also increase as PD advances[41,42]. A unique correlation between gut health and cognitive function has been documented in patients with PD[43]. Studies from Spain suggest a link between constipation and cognitive decline in PD[44]. The presence and severity of constipation are associated with rapidly progressive dementia and reduced subcutaneous fat [45,46].
Evidence suggests an association between gastrointestinal dysfunction and PD medication[47]. Compared to patients with PD who have a normal colonic transit, those with a slow colonic transit require a considerably higher levodopa equivalent daily dose[48]. This indicates that slow colonic transit may delay peak plasma concentration and cause a reduction in the clinical efficacy of levodopa. Long-term PD-related constipation can lead to an abnormal overgrowth of bacterial decarboxylases in the gut[49]. Du et al[11] reported a significant increase in the abundance of the order Lactobacillales in the intestines of patients with PD-related constipation. Levodopa plasma availability has a negative association with Lactobacillus abundance[50], particularly as several bacterial species of the genus Lactobacillus contain genes encoding tyrosine decarboxylase[51]. This enzyme can convert levodopa, a common drug used for PD treatment, into dopamine, affecting blood dopamine levels and potentially causing motor fluctuations. This may necessitate more frequent administration of levodopa and decarboxylase inhibitor treatments[51]. Complex interactions occur between anti-PD medications and gastrointestinal symptoms[52]. Healthy rats treated with PD medication for 14 days exhibited significantly reduced gut motility and altered microbiota composition, including increased abundance of Bifidobacterium and Lactobacillus and decreased abundance of the families Prevotellaceae and Lachnospiraceae[50]. Alterations in microbiota composition may lead to microbial metabolite changes, leading to constipation. A comprehensive meta-analysis demonstrated that pramipexole administration increased constipation risk relative to placebo[53]. Evidence suggests that constipation marginally increased after 1 year in patients with PD on dopaminergic medication, particularly levodopa[54]. Another randomized, double-blind trial showed that pramipexole extended release led to a higher constipation likelihood versus placebo in patients with early PD[55]. A high levodopa equivalent dose increases constipation risk, which nearly doubles with the combination of levodopa and a dopamine agonist[56].
Approximately 80% of patients with PD exhibit a slow colon transit, often twice as long as that recorded in healthy control subjects[39,57,58]. This delayed motility is a sign of impaired peristalsis, which depends on the ENS, a network of two plexuses (myenteric and submucosal) within the gut walls[59]. A significant number of these plexus neurons express vasoactive intestinal peptide (VIP) and nitric oxide synthase — both being crucial for muscle relaxation and vasodilation[60]. PD-associated Lewy bodies are present in VIPergic neurons of the ENS, implying that a slower intestinal transit could primarily result from impaired reflex relaxation caused by the loss of inhibitory motor neurons[61]. Evidence indicates Lewy body-containing neurons in the sympathetic ganglia are immunoreactive to tyrosine hydroxylase, implying that the slow transit could be directly linked to the involvement of colonic myenteric plexus in the PD course[62]. Additionally, the loss of dopaminergic neurons in the ENS likely contributes to slow-transit constipation. Studies have found that dopamine inhibits the release of acetylcholine and slows intestinal motility through presynaptic D2 receptors[63]. Age-related loss of excitatory cholinergic neurons in the colon may also be a factor for the slow colonic transit in PD[64,65]. The type of constipation influences the risk of PD development, and people with slow-transit constipation have a very high likelihood of developing PD[66]. Therefore, individuals aged over 65 years with newly diagnosed slow-transit constipation should be considered for PD screening[66].
More than 60% of patients with PD experience pelvic floor dyssynergia, an uncoordinated action of defecation muscles leading to outlet obstruction[67]. Normal defecation requires the relaxation of pelvic floor and sphincter muscles and a swift return of muscle activity post-defecation. The increase in intra-abdominal pressure, aided by the contraction of glottic, diaphragmatic, and abdominal wall muscles, acts synergistically with the inhibition of pelvic floor and external anal sphincter muscles[68]. In patients with PD, constipation often correlates with a paradoxical contraction of the puborectalis muscle. This abnormal muscle behavior results in defecation obstruction, a decrease in the anorectal angle, and paradoxical perineum ascent[39,69]. PD-related constipation is indicative of significantly weaker gastrointestinal tract function, with slow transit suggesting colonic ENS involvement and outlet obstruction (dystonia) suggesting direct muscle involvement in PD[39]. The severity and duration of PD are closely associated with the degree of constipation[70].
In the context of gut microbiota and PD, functional gut changes in a PD mouse model appear well before the onset of motor symptoms, suggesting a potential gut origin for PD[71]. Alteration in gut function could influence PD progression by modifying gut microbiota composition[72]. Several studies have proposed that gut microbiota alteration could trigger PD development[73,74] and incite immunological activation[75]. Persistent immune responses in the gut can increase intestinal permeability, allowing microbial products and inflammatory mediators to escape from the gut, thereby stimulating systemic immune responses[76]. This proinflammatory immune activity and related conditions can elevate levels of α-synuclein (α-syn) in the gut[77]. Pathologic levels of α-syn can propagate in a prion-like manner from the gut to the brain through the vagus nerve[27,78,79]. One study suggested that oral administration of Proteus mirabilis stimulates α-synuclein aggregation in the brain and colon, resulting in PD symptoms[80]. Another research indicated that the abundance of specific bacterial families could identify patients with PD[36].
Current evidence suggests a delayed colon transit and outlet obstruction, both linked to alpha-synuclein-related neurodegeneration in the ENS, are primary factors for PD-related constipation[36,81]. However, emerging research points out to the imbalance in gut flora as a significant player in the development and progression of PD-related constipation[82]. Studies have found that excessive pre-synaptic α-synuclein production in the colonic myenteric ganglia could cause early defecation impairment[83]. This finding is supported by the fact that transgenic mice overexpressing α-synuclein show impaired colonic transit[84,85]. Moreover, α-synuclein overexpression in the CNS can alter gut function[86,87]. Notably, transplantation of PD microbiota into humanized mice worsened motor symptoms and intestinal dysfunction, implying that α-synuclein overexpression and microbiota imbalance both contribute to disease progression[72]. Research also suggests that gut microbiota may significantly influence gut motor function[88,89]. This finding was confirmed in a study in which aryl hydrocarbon receptor expression induced by the gut microbiota in enteric neurons affected gut motility[90]. In a mouse model of PD induced by rotenone, gut microbiota was seen to influence gastrointestinal dysfunction, indicating its possible role in PD[91]. Distinct differences in gut microbiome between patients with PD and individuals without PD have been identified[92]. A study of 197 patients with PD demonstrated that higher microbial diversity in the gut correlated positively with stool firmness, implying a link between higher microbial diversity and constipation[93]. Furthermore, most PD studies have reported a decrease in the abundance of the families Prevotellaceae and Lachnospiraceae, accompanied by an increase in the abundance of the family Verrucomicrobiaceae (including the genus Akkermansia)[94-97]. This suggests a complex interplay between gut microbiota and PD-related constipation.
Studies reveal that gut microbiota dysbiosis may reduce stool water content, and Prevotella enterotypes increases the stool water content[98,99]. Indeed, patients with Prevotella-enriched enterotypes showed less severe constipation[100]. Hydrogen sulfide secreted by Prevotella, known for protecting dopaminergic neurons, may decrease in concentration in patients with PD who have reduced Prevotella enterotypes, leading to constipation because of increased hydrogen sulfide absorption[101]. Hydrogen sulfide can inhibit colonic contractility by affecting cholinergic and tachykinergic excitatory pathways mediated by neurons[102]. Prevotellaceae and Lachnospiraceae, which produce short-chain fatty acids (SCFAs), can promote gastrointestinal peristalsis[103]. A correlation was found between the genus Akkermansia, particularly Akkermansia muciniphila, and colon transit time[104]. Uncontrolled growth of Akkermansia muciniphila may degrade the mucus layer, leading to drier or harder stools[105,106]. A study on 52 patients with PD found that Enterobacteriaceae, abundant in the colon of patients with PD, negatively correlated with stool frequency[107]. Enterobacteriaceae produce Curli, an amyloid protein that can promote the aggregation of α-syn in the intestine and brain[80,108]. Gut-restricted amyloid inhibitor treatment in mice alleviated motor and constipation-like symptoms[108]. Both commensal and pathogenic bacterial metabolites can influence gut functions[93,109] (Figure 1). SCFAs, glucagon-like peptide 1 (GLP-1), and peptide tyrosine tyrosine (PYY) can modulate gut sympathetic activity and gastrointestinal motility, highlighting the link between gut microbiota and neuronal function[110]. Additionally, SCFAs activate G-protein-coupled receptors on enteroendocrine cells, mediating GLP-1 and PYY secretion[111]. In vitro studies showed that SCFAs stimulate colonic contractions through an enteric reflex involving local sensory and cholinergic nerves[112] and regulate colonic motility through enteric neurons[113]. Changes in the cholinergic phenotype caused by butyrate have a prokinetic effect on colonic motility[99,113]. Alterations in dopamine, 5-HT4 receptors, and β3-adrenoceptors likely lead to colonic dysmotility and constipation in patients with PD[114]. The β3-adrenoceptor in colonic interstitial cells of Cajal inhibits colonic motility by inhibiting pacemaker potential[115]. Dopamine inhibits gastrointestinal motility by activating D1 receptors[116,117], while 5-HT promotes gut motility primarily through the 5-HT4 and 5-HT3 receptors[118,119]. SCFAs can activate 5-HT4 receptors of intrinsic sensory neurons, triggering a peristaltic colonic reflex[120]. Butyrate, which modulates gastrointestinal motility by stimulating 5-HT3 receptors of the vagal sensory fibers[121,122], negatively correlates with constipation severity[123] and increases mucin secretion[124]. Mucin acts as a lubricant, protecting the mucosa and aiding stool excretion[125]. Acetic acid is positively associated with defecation frequency in patients with PD[126].
A study identified higher levels of the harmful amino acid metabolite p-cresol sulfate in the cerebrospinal fluid of patients with PD[127]. The protein degradation byproducts p-cresol and phenylacetylglutamine are also found elevated in the serum of patients with PD, with strong associations with stool consistency and constipation[93]. Glycerolipids, sphingolipids, and sterol lipids are positively associated with constipation in patients with PD[123]. Additionally, constipation positively correlated with pantothenic acid, D-ribose, L-lactic acid, D-alanine, and xanthine in the Luxembourg Parkinson’s Study[128]. In summary, the altered microbiota composition in PD-related constipation might lead to changes in microbial metabolites, especially SCFAs, suggesting the potential for manipulating SCFAs as a novel therapeutic strategy in PD-related constipation. Correlations between PD-related constipation, microorganisms, and their metabolites are summarized in Table 1.
| Positive | Negative | Ref. | |
| Microbial diversity | Alpha diversity | [93,100] | |
| Gut microbiota | Dorea, Oscillospira, Ruminococcus, Lactobacillus plantarum subgroup, Bifidobacterium, Verrucomicrobiaceae, Bradyrhizobiaceae | Faecalibacterium, Roseburia, Enterobacteriaceae cluster, Atopobium cluster | [36,93,100,107,128] |
| Metabolites | p-cresol and its sulfated form, phenylacetylglutamine, xanthine, D-alanine, L-lactic acid, D-ribose, pantothenic acid. glycerolipids, sphingolipids, sterol lipids | Butyrate, acetic acid | [93,128,123,126] |
| Enterotype | Firmicutes | Prevotella | [100] |
Research indicates that the primary microorganisms in patients with PD-related constipation are those belonging to Proteobacteria and Bacteroidetes[14]. According to a study, the most prevalent bacteria in the fecal microbiota of patients with PD-related constipation were from the phylum Bacteroidetes, genus Bacteroides, order Bacteroidales, class Bacteroidia, and family Bacteroidaceae. The study also noted a significantly higher abundance of Bacteroides and a considerably lower abundance of Faecalibacterium in patients with PD-related constipation than in healthy controls[129]. Additionally, Du et al[11] reported that Bifidobacteriales, Lactobacillales, Bacillales, Peptostreptococcales Tissierellales, Desulfovibrionales, and Coriobacteriales were the most abundant microorganisms in the gut of patients with PD-related constipation. These patients also exhibited significantly higher levels of Bacillus, Alistipes, Bifidobacterium, Romboutsia, Adlercreutzia, Desulfovibrio, Butyricicoccus, Bilophila, Intestinibacter, Holdemania,UCG_002 Actinomyces, Lachnospiraceae_UCG_008, Gordonibacter, Raoultibacter, Odoribacter, Oscillibacter, Eubacterium_nodatum_group, and uncultured species than healthy individuals[11]. Interestingly, the gut microbiota of patients with chronic constipation is predominantly characterized by reduced abundance of bifidobacteria and lactobacilli and increased abundance of Bacteroidetes[130-133].
The current treatments for PD-related constipation mainly include prokinetics and laxatives. While these traditional therapies can be safe and effective, they are often limited in fully relieving clinical symptoms, indicating a need for more effective treatments[134,135]. Recent insights into the association between gut microflora and PD-related constipation have led to research exploring how altering gut microflora through prebiotics, probiotics, and fecal microbiota transplantation (FMT) might provide a cure. These interventions could supplement traditional treatments for PD-related constipation.
Prebiotics are selectively utilized substrates that confer health benefits to host microorganisms[136]. Reports suggest that prebiotic fibers can alleviate constipation and improve bowel movements[137]. In particular, diets rich in insoluble fiber improved constipation in patients with PD[138], and a study reported that psyllium is useful in treating constipation in patients with PD, noting that it increased stool frequency and weight, with, on average, three bowel movements per week[139].
Probiotics are live microorganisms that confer health benefits to the host when administered in sufficient amounts and are thought to be another potential treatment for PD-related constipation. They can strengthen the gut barrier and restore normal intestinal microbiota[140], suggesting its potential as a novel treatment strategy for PD-related constipation[141,142]. Initial studies have shown promising results; For instance, patients with PD who took Lactobacillus casei Shirota for 5 weeks showed improved stool consistency[16], and those who took probiotics containing Lactobacillus acidophilus and Bifidobacterium infantis for 3 months experienced reduced abdominal pain and bloating[10]. Further research showed an increase in the number of complete bowel movements in patients with PD-related constipation after drinking fermented milk containing multiple probiotic strains and prebiotic fiber for 4 weeks[143]. A subsequent study reported that taking a multi-strain probiotic combined with prebiotic fiber for 8 weeks improved whole-gut transit time and the frequency of bowel opening in patients with PD-related constipation[144]. Additionally, a randomized controlled trial of 72 patients with PD-related constipation showed that multi-strain probiotics significantly improved weekly spontaneous bowel movements frequency and quality of life scores associated with constipation[15]. Du et al[11] reported that multi-strain probiotics effectively improved constipation symptoms and stool consistency in patients with PD, even altering the composition of their gut microbiota.
FMT is a novel treatment approach that alleviates constipation by restoring the intestinal microenvironment. This method is based on the premise that alterations in the microbiome may affect gut motility through the production of different microbial-derived metabolites, and correcting these disruptions might improve the clinical symptoms[145]. FMT has shown promising results in treating PD-related constipation, as evidenced by increased abundance of Firmicutes and decreased abundance of Proteobacteria and Bacteroidetes in treated patients, leading to effective relief of constipation and tremors[14]. More recent studies support the beneficial role of FMT in improving PD-related constipation symptoms[13]. One study highlighted that FMT significantly reduced Bacteroidetes and increased Prevotella and Blautia in patients with PD-related constipation. Surprisingly, after FMT, the abundance of several other bacterial groups also increased at different times, accompanied by significant decreases in the patients’ Wexner constipation scores and resolution of their constipation symptoms[129]. Such findings underline the therapeutic potential of FMT in rebuilding the gut microbiota of patients with PD-related constipation. Microbial alterations in PD-Related constipation after microbial treatments are summarized in Table 2.
| Microbial treatments | Study design | Participant | Duration | Microbial alterations | Results | Ref. | |
| Increased | Decreased | ||||||
| Probiotics | Randomized controlled clinical trial | 46 | 12 wk | g_Christensenella_sp._Marseille-P2437 | g_Eubacterium_oxidoreducens_group, g_Eubacterium_hallii_group, s_Odoribacter_sp._N54.MGS-14 and Prevotellaceae | The probiotics group increased the average number of complete bowel movements per week as compared to the control group. The improvement rate of constipation in the probiotics group was significantly higher than that in the control group | [18] |
| FMT | Case report | 1 | 3 d | Firmicutes | Proteobacteria, Bacteroidetes | After FMT, patients successfully defecated within 5 min and maintained daily unobstructed defecation until the end of follow-up | [14] |
| FMT | A prospective, single-center study | 11 | 1 d | Blautia, Prevotella | Bacteroidetes | The PAC-QOL and Wexner constipation scores both decreased significantly | [129] |
In prodromal PD, abnormalities related to α-syn can be detected in the colon. Subsequently, α-syn spreads from the gut to the brain through the vagus nerve, which may lead to the development of PD. Constipation is considered one of the precursor symptoms of PD, potentially stemming from α-syn pathology in the ENS. The exact mechanisms driving PD-related constipation are still largely unknown, with potential causes ranging from outlet obstruction to delayed colon transit. Current evidence shows a correlation between PD-related constipation and changes in gut microbiota, suggesting a complex interplay between the gut microbiome and PD-related constipation. However, whether the onset of PD-related constipation precedes intestinal dysbiosis or vice versa is still unknown. Despite the unclear cause-effect relationship, studies indicate that gut microbiota dysbiosis can exacerbate constipation and that restoring the gut microbiota can mitigate these symptoms, suggesting gut microbiota as a potential therapeutic target for PD-related constipation. Microbiological intervention treatments for PD-related constipation, including prebiotics, probiotics, and FMT, can prove beneficial and possibly more effective than traditional treatments.
This review covered longitudinal studies on gut dysbiosis in PD-related constipation. However, it has a few weaknesses. The limited number of studies may not have accurately captured the full longitudinal changes in the microbiota associated with PD-related constipation. Furthermore, there is a scarcity of clinical studies examining intestinal flora specifically in PD-related constipation, making it difficult to infer the particular microbial taxa linked to this condition. In addition, as most studies have been conducted at the phylum and genus levels, further research at the species and strain levels could provide greater mechanistic insights. Therefore, future studies should focus on identifying specific bacterial species that promote PD-related constipation development. Finally, pinpointing the causative microbes could enable targeted microbial therapies for PD-related constipation in the future. However, more rigorous clinical studies are needed to elucidate the precise microbiota compositional and functional changes underlying PD-related constipation before such therapeutic approaches can be applied. However, this is a nascent field of research with various limitations and challenges and hence requires future extensive research.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Govindarajan KK, India; Mortazavian AM, Iran S-Editor: Gong ZM L-Editor: A P-Editor: Yuan YY
| 1. | Okunoye O, Marston L, Walters K, Schrag A. Change in the incidence of Parkinson's disease in a large UK primary care database. NPJ Parkinsons Dis. 2022;8:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 2. | GBD 2016 Parkinson's Disease Collaborators. Global, regional, and national burden of Parkinson's disease, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:939-953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1820] [Cited by in RCA: 1624] [Article Influence: 232.0] [Reference Citation Analysis (0)] |
| 3. | Rocca WA. The burden of Parkinson's disease: a worldwide perspective. Lancet Neurol. 2018;17:928-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 167] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 4. | Kalia LV, Lang AE. Parkinson's disease. Lancet. 2015;386:896-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3143] [Cited by in RCA: 4080] [Article Influence: 408.0] [Reference Citation Analysis (39)] |
| 5. | Safarpour D, Sharzehi K, Pfeiffer RF. Gastrointestinal Dysfunction in Parkinson's Disease. Drugs. 2022;82:169-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Schapira AHV, Chaudhuri KR, Jenner P. Non-motor features of Parkinson disease. Nat Rev Neurosci. 2017;18:435-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 688] [Cited by in RCA: 1209] [Article Influence: 151.1] [Reference Citation Analysis (0)] |
| 7. | Yu QJ, Yu SY, Zuo LJ, Lian TH, Hu Y, Wang RD, Piao YS, Guo P, Liu L, Jin Z, Li LX, Chan P, Chen SD, Wang XM, Zhang W. Parkinson disease with constipation: clinical features and relevant factors. Sci Rep. 2018;8:567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 8. | De Pablo-Fernández E, Passananti V, Zárate-López N, Emmanuel A, Warner T. Colonic transit, high-resolution anorectal manometry and MRI defecography study of constipation in Parkinson's disease. Parkinsonism Relat Disord. 2019;66:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Knudsen K, Krogh K, Østergaard K, Borghammer P. Constipation in parkinson's disease: Subjective symptoms, objective markers, and new perspectives. Mov Disord. 2017;32:94-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 121] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 10. | Georgescu D, Ancusa OE, Georgescu LA, Ionita I, Reisz D. Nonmotor gastrointestinal disorders in older patients with Parkinson's disease: is there hope? Clin Interv Aging. 2016;11:1601-1608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 11. | Morris MJ, Sartor O, de Bono JS, Fizazi K, Tagawa ST. Reply to Timothée Olivier, Kerrington Powell, Vinay Prasad. Lutetium-177-PSMA-617 in Metastatic Castration-resistant Prostate Cancer: Limitations of the VISION Trial. Eur Urol. In press. https://doi.org/10.1016/j.eururo.2022.08.022. Eur Urol. 2023;84:7-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 12. | Fu SC, Shih LC, Wu PH, Hsieh YC, Lee CH, Lin SH, Wang H. Exploring the Causal Effect of Constipation on Parkinson's Disease Through Mediation Analysis of Microbial Data. Front Cell Infect Microbiol. 2022;12:871710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Segal A, Zlotnik Y, Moyal-Atias K, Abuhasira R, Ifergane G. Fecal microbiota transplant as a potential treatment for Parkinson's disease - A case series. Clin Neurol Neurosurg. 2021;207:106791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 77] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 14. | Huang H, Xu H, Luo Q, He J, Li M, Chen H, Tang W, Nie Y, Zhou Y. Fecal microbiota transplantation to treat Parkinson's disease with constipation: A case report. Medicine (Baltimore). 2019;98:e16163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 125] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 15. | Tan AH, Lim SY, Chong KK, A Manap MAA, Hor JW, Lim JL, Low SC, Chong CW, Mahadeva S, Lang AE. Probiotics for Constipation in Parkinson Disease: A Randomized Placebo-Controlled Study. Neurology. 2021;96:e772-e782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 16. | Cassani E, Privitera G, Pezzoli G, Pusani C, Madio C, Iorio L, Barichella M. Use of probiotics for the treatment of constipation in Parkinson's disease patients. Minerva Gastroenterol Dietol. 2011;57:117-121. [PubMed] |
| 17. | Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3051] [Cited by in RCA: 3651] [Article Influence: 280.8] [Reference Citation Analysis (0)] |
| 18. | Wang Q, Luo Y, Ray Chaudhuri K, Reynolds R, Tan EK, Pettersson S. The role of gut dysbiosis in Parkinson's disease: mechanistic insights and therapeutic options. Brain. 2021;144:2571-2593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 154] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 19. | Kaur G, Behl T, Bungau S, Kumar A, Uddin MS, Mehta V, Zengin G, Mathew B, Shah MA, Arora S. Dysregulation of the Gut-Brain Axis, Dysbiosis and Influence of Numerous Factors on Gut Microbiota Associated Parkinson's Disease. Curr Neuropharmacol. 2021;19:233-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 20. | Mayer EA, Nance K, Chen S. The Gut-Brain Axis. Annu Rev Med. 2022;73:439-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 394] [Article Influence: 98.5] [Reference Citation Analysis (0)] |
| 21. | Jankovic J, Tan EK. Parkinson's disease: etiopathogenesis and treatment. J Neurol Neurosurg Psychiatry. 2020;91:795-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 629] [Article Influence: 125.8] [Reference Citation Analysis (0)] |
| 22. | Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner's and Auerbach's plexuses in cases staged for Parkinson's disease-related brain pathology. Neurosci Lett. 2006;396:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 873] [Cited by in RCA: 1009] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 23. | Hawkes CH, Del Tredici K, Braak H. Parkinson's disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol. 2007;33:599-614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 690] [Cited by in RCA: 714] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 24. | Holmqvist S, Chutna O, Bousset L, Aldrin-Kirk P, Li W, Björklund T, Wang ZY, Roybon L, Melki R, Li JY. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014;128:805-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 665] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 25. | Pan-Montojo F, Schwarz M, Winkler C, Arnhold M, O'Sullivan GA, Pal A, Said J, Marsico G, Verbavatz JM, Rodrigo-Angulo M, Gille G, Funk RH, Reichmann H. Environmental toxins trigger PD-like progression via increased alpha-synuclein release from enteric neurons in mice. Sci Rep. 2012;2:898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 300] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 26. | Anselmi L, Bove C, Coleman FH, Le K, Subramanian MP, Venkiteswaran K, Subramanian T, Travagli RA. Ingestion of subthreshold doses of environmental toxins induces ascending Parkinsonism in the rat. NPJ Parkinsons Dis. 2018;4:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 27. | Kim S, Kwon SH, Kam TI, Panicker N, Karuppagounder SS, Lee S, Lee JH, Kim WR, Kook M, Foss CA, Shen C, Lee H, Kulkarni S, Pasricha PJ, Lee G, Pomper MG, Dawson VL, Dawson TM, Ko HS. Transneuronal Propagation of Pathologic α-Synuclein from the Gut to the Brain Models Parkinson's Disease. Neuron 2019; 103: 627-641. e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 949] [Article Influence: 158.2] [Reference Citation Analysis (0)] |
| 28. | Svensson E, Horváth-Puhó E, Thomsen RW, Djurhuus JC, Pedersen L, Borghammer P, Sørensen HT. Vagotomy and subsequent risk of Parkinson's disease. Ann Neurol. 2015;78:522-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 589] [Article Influence: 58.9] [Reference Citation Analysis (0)] |
| 29. | Shannon KM, Keshavarzian A, Dodiya HB, Jakate S, Kordower JH. Is alpha-synuclein in the colon a biomarker for premotor Parkinson's disease? Mov Disord. 2012;27:716-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 344] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 30. | Hilton D, Stephens M, Kirk L, Edwards P, Potter R, Zajicek J, Broughton E, Hagan H, Carroll C. Accumulation of α-synuclein in the bowel of patients in the pre-clinical phase of Parkinson's disease. Acta Neuropathol. 2014;127:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 255] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 31. | Pouclet H, Lebouvier T, Coron E, des Varannes SB, Rouaud T, Roy M, Neunlist M, Derkinderen P. A comparison between rectal and colonic biopsies to detect Lewy pathology in Parkinson's disease. Neurobiol Dis. 2012;45:305-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 32. | Noyce AJ, Bestwick JP, Silveira-Moriyama L, Hawkes CH, Giovannoni G, Lees AJ, Schrag A. Meta-analysis of early nonmotor features and risk factors for Parkinson disease. Ann Neurol. 2012;72:893-901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 616] [Cited by in RCA: 526] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 33. | Adams-Carr KL, Bestwick JP, Shribman S, Lees A, Schrag A, Noyce AJ. Constipation preceding Parkinson's disease: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2016;87:710-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 148] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 34. | Fasano A, Visanji NP, Liu LW, Lang AE, Pfeiffer RF. Gastrointestinal dysfunction in Parkinson's disease. Lancet Neurol. 2015;14:625-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 596] [Article Influence: 59.6] [Reference Citation Analysis (0)] |
| 35. | Kaye J, Gage H, Kimber A, Storey L, Trend P. Excess burden of constipation in Parkinson's disease: a pilot study. Mov Disord. 2006;21:1270-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 36. | Scheperjans F, Aho V, Pereira PA, Koskinen K, Paulin L, Pekkonen E, Haapaniemi E, Kaakkola S, Eerola-Rautio J, Pohja M, Kinnunen E, Murros K, Auvinen P. Gut microbiota are related to Parkinson's disease and clinical phenotype. Mov Disord. 2015;30:350-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1044] [Cited by in RCA: 1068] [Article Influence: 97.1] [Reference Citation Analysis (0)] |
| 37. | Lin CH, Lin JW, Liu YC, Chang CH, Wu RM. Risk of Parkinson's disease following severe constipation: a nationwide population-based cohort study. Parkinsonism Relat Disord. 2014;20:1371-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 38. | Flint HJ, Duncan SH, Scott KP, Louis P. Interactions and competition within the microbial community of the human colon: links between diet and health. Environ Microbiol. 2007;9:1101-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 419] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 39. | Edwards LL, Quigley EM, Harned RK, Hofman R, Pfeiffer RF. Characterization of swallowing and defecation in Parkinson's disease. Am J Gastroenterol. 1994;89:15-25. [PubMed] |
| 40. | Grillo P, Sancesario GM, Mascioli D, Geusa L, Zenuni H, Giannella E, Della Morte D, Mercuri NB, Schirinzi T. Constipation distinguishes different clinical-biochemical patterns in de novo Parkinson's disease. Parkinsonism Relat Disord. 2022;102:64-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 41. | Edwards L, Quigley EM, Hofman R, Pfeiffer RF. Gastrointestinal symptoms in Parkinson disease: 18-month follow-up study. Mov Disord. 1993;8:83-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 93] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 42. | Chen H, Zhao EJ, Zhang W, Lu Y, Liu R, Huang X, Ciesielski-Jones AJ, Justice MA, Cousins DS, Peddada S. Meta-analyses on prevalence of selected Parkinson's nonmotor symptoms before and after diagnosis. Transl Neurodegener. 2015;4:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 133] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 43. | Jones JD, Rahmani E, Garcia E, Jacobs JP. Gastrointestinal symptoms are predictive of trajectories of cognitive functioning in de novo Parkinson's disease. Parkinsonism Relat Disord. 2020;72:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 44. | Santos García D, García Roca L, de Deus Fonticoba T, Cores Bartolomé C, Naya Ríos L, Canfield H, Paz González JM, Martínez Miró C, Jesús S, Aguilar M, Pastor P, Planellas L, Cosgaya M, García Caldentey J, Caballol N, Legarda I, Hernández Vara J, Cabo I, López Manzanares L, González Aramburu I, Ávila Rivera MA, Gómez Mayordomo V, Nogueira V, Puente V, Dotor García-Soto J, Borrué C, Solano Vila B, Álvarez Sauco M, Vela L, Escalante S, Cubo E, Carrillo Padilla F, Martínez Castrillo JC, Sánchez Alonso P, Alonso Losada MG, López Ariztegui N, Gastón I, Kulisevsky J, Blázquez Estrada M, Seijo M, Rúiz Martínez J, Valero C, Kurtis M, de Fábregues O, González Ardura J, Alonso Redondo R, Ordás C, López Díaz L LM, McAfee D, Martinez-Martin P, Mir P; COPPADIS Study Group. Constipation Predicts Cognitive Decline in Parkinson's Disease: Results from the COPPADIS Cohort at 2-Year Follow-up and Comparison with a Control Group. J Parkinsons Dis. 2022;12:315-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 45. | Camacho M, Macleod AD, Maple-Grødem J, Evans JR, Breen DP, Cummins G, Wijeyekoon RS, Greenland JC, Alves G, Tysnes OB, Lawson RA, Barker RA, Williams-Gray CH. Early constipation predicts faster dementia onset in Parkinson's disease. NPJ Parkinsons Dis. 2021;7:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 46. | Yong VW, Tan YJ, Ng YD, Choo XY, Sugumaran K, Chinna K, Md Shah MN, Raja Aman RRA, Moy FM, Mohd Ramli N, Grossmann M, Lim SY, Tan AH. Progressive and accelerated weight and body fat loss in Parkinson's disease: A three-year prospective longitudinal study. Parkinsonism Relat Disord. 2020;77:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (1)] |
| 47. | Kenna JE, Bakeberg MC, Gorecki AM, Chin Yen Tay A, Winter S, Mastaglia FL, Anderton RS. Characterization of Gastrointestinal Symptom Type and Severity in Parkinson's Disease: A Case-Control Study in an Australian Cohort. Mov Disord Clin Pract. 2021;8:245-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 48. | Khoshbin K, Hassan A, Camilleri M. Cohort Study in Parkinsonism: Delayed Transit, Accelerated Gastric Emptying, and Prodromal Dysmotility. Neurol Clin Pract. 2021;11:e407-e413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 49. | Maini Rekdal V, Bess EN, Bisanz JE, Turnbaugh PJ, Balskus EP. Discovery and inhibition of an interspecies gut bacterial pathway for Levodopa metabolism. Science. 2019;364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 264] [Cited by in RCA: 440] [Article Influence: 73.3] [Reference Citation Analysis (0)] |
| 50. | van Kessel SP, Bullock A, van Dijk G, El Aidy S. Parkinson's Disease Medication Alters Small Intestinal Motility and Microbiota Composition in Healthy Rats. mSystems. 2022;7:e0119121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 51. | van Kessel SP, Frye AK, El-Gendy AO, Castejon M, Keshavarzian A, van Dijk G, El Aidy S. Gut bacterial tyrosine decarboxylases restrict levels of levodopa in the treatment of Parkinson's disease. Nat Commun. 2019;10:310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 342] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 52. | van Kessel SP, Auvinen P, Scheperjans F, El Aidy S. Gut bacterial tyrosine decarboxylase associates with clinical variables in a longitudinal cohort study of Parkinsons disease. NPJ Parkinsons Dis. 2021;7:115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 53. | Kulisevsky J, Pagonabarraga J. Tolerability and safety of ropinirole versus other dopamine agonists and levodopa in the treatment of Parkinson's disease: meta-analysis of randomized controlled trials. Drug Saf. 2010;33:147-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 54. | Müller B, Assmus J, Larsen JP, Haugarvoll K, Skeie GO, Tysnes OB; ParkWest study group. Autonomic symptoms and dopaminergic treatment in de novo Parkinson's disease. Acta Neurol Scand. 2013;127:290-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 55. | Hauser RA, Schapira AH, Rascol O, Barone P, Mizuno Y, Salin L, Haaksma M, Juhel N, Poewe W. Randomized, double-blind, multicenter evaluation of pramipexole extended release once daily in early Parkinson's disease. Mov Disord. 2010;25:2542-2549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 56. | Pagano G, Tan EE, Haider JM, Bautista A, Tagliati M. Constipation is reduced by beta-blockers and increased by dopaminergic medications in Parkinson's disease. Parkinsonism Relat Disord. 2015;21:120-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 57. | Jost WH, Schimrigk K. Constipation in Parkinson's disease. Klin Wochenschr. 1991;69:906-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 58. | Knudsen K, Fedorova TD, Bekker AC, Iversen P, Østergaard K, Krogh K, Borghammer P. Objective Colonic Dysfunction is Far more Prevalent than Subjective Constipation in Parkinson's Disease: A Colon Transit and Volume Study. J Parkinsons Dis. 2017;7:359-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 59. | Stirpe P, Hoffman M, Badiali D, Colosimo C. Constipation: an emerging risk factor for Parkinson's disease? Eur J Neurol. 2016;23:1606-1613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 60. | Cersosimo MG, Benarroch EE. Pathological correlates of gastrointestinal dysfunction in Parkinson's disease. Neurobiol Dis. 2012;46:559-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 204] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 61. | Stocchi F, Torti M. Constipation in Parkinson's Disease. Int Rev Neurobiol. 2017;134:811-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 62. | Singaram C, Ashraf W, Gaumnitz EA, Torbey C, Sengupta A, Pfeiffer R, Quigley EM. Dopaminergic defect of enteric nervous system in Parkinson's disease patients with chronic constipation. Lancet. 1995;346:861-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 249] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 63. | Li ZS, Schmauss C, Cuenca A, Ratcliffe E, Gershon MD. Physiological modulation of intestinal motility by enteric dopaminergic neurons and the D2 receptor: analysis of dopamine receptor expression, location, development, and function in wild-type and knock-out mice. J Neurosci. 2006;26:2798-2807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 218] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 64. | Phillips RJ, Pairitz JC, Powley TL. Age-related neuronal loss in the submucosal plexus of the colon of Fischer 344 rats. Neurobiol Aging. 2007;28:1124-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 65. | Phillips RJ, Powley TL. Innervation of the gastrointestinal tract: patterns of aging. Auton Neurosci. 2007;136:1-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 199] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 66. | Song EM, Lee HJ, Jung KW, Kim MJ, Hwang SW, Park SH, Yang DH, Ye BD, Byeon JS, Choe J, Yang SK, Rao SSC, Myung SJ. Long-Term Risks of Parkinson's Disease, Surgery, and Colorectal Cancer in Patients With Slow-Transit Constipation. Clin Gastroenterol Hepatol 2021; 19: 2577-2586. e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 67. | Bassotti G, Maggio D, Battaglia E, Giulietti O, Spinozzi F, Reboldi G, Serra AM, Emanuelli G, Chiarioni G. Manometric investigation of anorectal function in early and late stage Parkinson's disease. J Neurol Neurosurg Psychiatry. 2000;68:768-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 68. | Mathers SE, Kempster PA, Swash M, Lees AJ. Constipation and paradoxical puborectalis contraction in anismus and Parkinson's disease: a dystonic phenomenon? J Neurol Neurosurg Psychiatry. 1988;51:1503-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 101] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 69. | Mathers SE, Kempster PA, Law PJ, Frankel JP, Bartram CI, Lees AJ, Stern GM, Swash M. Anal sphincter dysfunction in Parkinson's disease. Arch Neurol. 1989;46:1061-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 92] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 70. | Edwards LL, Pfeiffer RF, Quigley EM, Hofman R, Balluff M. Gastrointestinal symptoms in Parkinson's disease. Mov Disord. 1991;6:151-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 242] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 71. | Gries M, Christmann A, Schulte S, Weyland M, Rommel S, Martin M, Baller M, Röth R, Schmitteckert S, Unger M, Liu Y, Sommer F, Mühlhaus T, Schroda M, Timmermans JP, Pintelon I, Rappold GA, Britschgi M, Lashuel H, Menger MD, Laschke MW, Niesler B, Schäfer KH. Parkinson mice show functional and molecular changes in the gut long before motoric disease onset. Mol Neurodegener. 2021;16:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (2)] |
| 72. | Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S, Gradinaru V, Chesselet MF, Keshavarzian A, Shannon KM, Krajmalnik-Brown R, Wittung-Stafshede P, Knight R, Mazmanian SK. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson's Disease. Cell 2016; 167: 1469-1480. e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2094] [Cited by in RCA: 2415] [Article Influence: 268.3] [Reference Citation Analysis (0)] |
| 73. | Matheoud D, Cannon T, Voisin A, Penttinen AM, Ramet L, Fahmy AM, Ducrot C, Laplante A, Bourque MJ, Zhu L, Cayrol R, Le Campion A, McBride HM, Gruenheid S, Trudeau LE, Desjardins M. Intestinal infection triggers Parkinson's disease-like symptoms in Pink1(-/-) mice. Nature. 2019;571:565-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 352] [Article Influence: 58.7] [Reference Citation Analysis (1)] |
| 74. | Seguella L, Sarnelli G, Esposito G. Leaky gut, dysbiosis, and enteric glia activation: the trilogy behind the intestinal origin of Parkinson's disease. Neural Regen Res. 2020;15:1037-1038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 75. | Keshavarzian A, Green SJ, Engen PA, Voigt RM, Naqib A, Forsyth CB, Mutlu E, Shannon KM. Colonic bacterial composition in Parkinson's disease. Mov Disord. 2015;30:1351-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 680] [Cited by in RCA: 898] [Article Influence: 89.8] [Reference Citation Analysis (0)] |
| 76. | Houser MC, Tansey MG. The gut-brain axis: is intestinal inflammation a silent driver of Parkinson's disease pathogenesis? NPJ Parkinsons Dis. 2017;3:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 382] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 77. | Kelly LP, Carvey PM, Keshavarzian A, Shannon KM, Shaikh M, Bakay RA, Kordower JH. Progression of intestinal permeability changes and alpha-synuclein expression in a mouse model of Parkinson's disease. Mov Disord. 2014;29:999-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 208] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 78. | Ayers JI, Brooks MM, Rutherford NJ, Howard JK, Sorrentino ZA, Riffe CJ, Giasson BI. Robust Central Nervous System Pathology in Transgenic Mice following Peripheral Injection of α-Synuclein Fibrils. J Virol. 2017;91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 79. | Luk KC, Kehm V, Carroll J, Zhang B, O'Brien P, Trojanowski JQ, Lee VM. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338:949-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1619] [Cited by in RCA: 1935] [Article Influence: 148.8] [Reference Citation Analysis (0)] |
| 80. | Choi JG, Kim N, Ju IG, Eo H, Lim SM, Jang SE, Kim DH, Oh MS. Oral administration of Proteus mirabilis damages dopaminergic neurons and motor functions in mice. Sci Rep. 2018;8:1275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 117] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 81. | Pfeiffer RF. Gastrointestinal Dysfunction in Parkinson's Disease. Curr Treat Options Neurol. 2018;20:54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 82. | Camilleri M, Subramanian T, Pagan F, Isaacson S, Gil R, Hauser RA, Feldman M, Goldstein M, Kumar R, Truong D, Chhabria N, Walter BL, Eskenazi J, Riesenberg R, Burdick D, Tse W, Molho E, Robottom B, Bhatia P, Kadimi S, Klos K, Shprecher D, Marquez-Mendoza O, Hidalgo G, Grill S, Li G, Mandell H, Hughes M, Stephenson S, Vandersluis J, Pfeffer M, Duker A, Shivkumar V, Kinney W, MacDougall J, Zasloff M, Barbut D. Oral ENT-01 Targets Enteric Neurons to Treat Constipation in Parkinson Disease : A Randomized Controlled Trial. Ann Intern Med. 2022;175:1666-1674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 83. | Wang L, Magen I, Yuan PQ, Subramaniam SR, Richter F, Chesselet MF, Taché Y. Mice overexpressing wild-type human alpha-synuclein display alterations in colonic myenteric ganglia and defecation. Neurogastroenterol Motil. 2012;24:e425-e436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 84. | Rota L, Pellegrini C, Benvenuti L, Antonioli L, Fornai M, Blandizzi C, Cattaneo A, Colla E. Constipation, deficit in colon contractions and alpha-synuclein inclusions within the colon precede motor abnormalities and neurodegeneration in the central nervous system in a mouse model of alpha-synucleinopathy. Transl Neurodegener. 2019;8:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 85. | Kuo YM, Nwankwo EI, Nussbaum RL, Rogers J, Maccecchini ML. Translational inhibition of α-synuclein by Posiphen normalizes distal colon motility in transgenic Parkinson mice. Am J Neurodegener Dis. 2019;8:1-15. [PubMed] |
| 86. | O'Donovan SM, Crowley EK, Brown JR, O'Sullivan O, O'Leary OF, Timmons S, Nolan YM, Clarke DJ, Hyland NP, Joyce SA, Sullivan AM, O'Neill C. Nigral overexpression of α-synuclein in a rat Parkinson's disease model indicates alterations in the enteric nervous system and the gut microbiome. Neurogastroenterol Motil. 2020;32:e13726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 87. | Pellegrini C, Fornai M, Colucci R, Tirotta E, Blandini F, Levandis G, Cerri S, Segnani C, Ippolito C, Bernardini N, Cseri K, Blandizzi C, Haskó G, Antonioli L. Alteration of colonic excitatory tachykininergic motility and enteric inflammation following dopaminergic nigrostriatal neurodegeneration. J Neuroinflammation. 2016;13:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 88. | Barbara G, Stanghellini V, Brandi G, Cremon C, Di Nardo G, De Giorgio R, Corinaldesi R. Interactions between commensal bacteria and gut sensorimotor function in health and disease. Am J Gastroenterol. 2005;100:2560-2568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 218] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 89. | Wichmann A, Allahyar A, Greiner TU, Plovier H, Lundén GÖ, Larsson T, Drucker DJ, Delzenne NM, Cani PD, Bäckhed F. Microbial modulation of energy availability in the colon regulates intestinal transit. Cell Host Microbe. 2013;14:582-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 301] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 90. | Obata Y, Castaño Á, Boeing S, Bon-Frauches AC, Fung C, Fallesen T, de Agüero MG, Yilmaz B, Lopes R, Huseynova A, Horswell S, Maradana MR, Boesmans W, Vanden Berghe P, Murray AJ, Stockinger B, Macpherson AJ, Pachnis V. Neuronal programming by microbiota regulates intestinal physiology. Nature. 2020;578:284-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 237] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 91. | Bhattarai Y, Si J, Pu M, Ross OA, McLean PJ, Till L, Moor W, Grover M, Kandimalla KK, Margolis KG, Farrugia G, Kashyap PC. Role of gut microbiota in regulating gastrointestinal dysfunction and motor symptoms in a mouse model of Parkinson's disease. Gut Microbes. 2021;13:1866974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 92. | Tan AH, Lim SY, Lang AE. The microbiome-gut-brain axis in Parkinson disease - from basic research to the clinic. Nat Rev Neurol. 2022;18:476-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 189] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 93. | Cirstea MS, Yu AC, Golz E, Sundvick K, Kliger D, Radisavljevic N, Foulger LH, Mackenzie M, Huan T, Finlay BB, Appel-Cresswell S. Microbiota Composition and Metabolism Are Associated With Gut Function in Parkinson's Disease. Mov Disord. 2020;35:1208-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 197] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 94. | Barichella M, Severgnini M, Cilia R, Cassani E, Bolliri C, Caronni S, Ferri V, Cancello R, Ceccarani C, Faierman S, Pinelli G, De Bellis G, Zecca L, Cereda E, Consolandi C, Pezzoli G. Unraveling gut microbiota in Parkinson's disease and atypical parkinsonism. Mov Disord. 2019;34:396-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 262] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 95. | Hill-Burns EM, Debelius JW, Morton JT, Wissemann WT, Lewis MR, Wallen ZD, Peddada SD, Factor SA, Molho E, Zabetian CP, Knight R, Payami H. Parkinson's disease and Parkinson's disease medications have distinct signatures of the gut microbiome. Mov Disord. 2017;32:739-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 633] [Article Influence: 79.1] [Reference Citation Analysis (0)] |
| 96. | Aho VTE, Pereira PAB, Voutilainen S, Paulin L, Pekkonen E, Auvinen P, Scheperjans F. Gut microbiota in Parkinson's disease: Temporal stability and relations to disease progression. EBioMedicine. 2019;44:691-707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 243] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 97. | Bedarf JR, Hildebrand F, Coelho LP, Sunagawa S, Bahram M, Goeser F, Bork P, Wüllner U. Functional implications of microbial and viral gut metagenome changes in early stage L-DOPA-naïve Parkinson's disease patients. Genome Med. 2017;9:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 411] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 98. | Cao H, Liu X, An Y, Zhou G, Liu Y, Xu M, Dong W, Wang S, Yan F, Jiang K, Wang B. Dysbiosis contributes to chronic constipation development via regulation of serotonin transporter in the intestine. Sci Rep. 2017;7:10322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 183] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 99. | Soret R, Chevalier J, De Coppet P, Poupeau G, Derkinderen P, Segain JP, Neunlist M. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology. 2010;138:1772-1782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 373] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 100. | Heinzel S, Aho VTE, Suenkel U, von Thaler AK, Schulte C, Deuschle C, Paulin L, Hantunen S, Brockmann K, Eschweiler GW, Maetzler W, Berg D, Auvinen P, Scheperjans F. Gut Microbiome Signatures of Risk and Prodromal Markers of Parkinson Disease. Ann Neurol. 2021;90:E1-E12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 101. | Cakmak YO. Provotella-derived hydrogen sulfide, constipation, and neuroprotection in Parkinson's disease. Mov Disord. 2015;30:1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 102. | Martinez-Cutillas M, Gil V, Mañé N, Clavé P, Gallego D, Martin MT, Jimenez M. Potential role of the gaseous mediator hydrogen sulphide (H2S) in inhibition of human colonic contractility. Pharmacol Res. 2015;93:52-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 103. | Chen ZJ, Liang CY, Yang LQ, Ren SM, Xia YM, Cui L, Li XF, Gao BL. Association of Parkinson's Disease With Microbes and Microbiological Therapy. Front Cell Infect Microbiol. 2021;11:619354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 104. | Vandeputte D, Falony G, Vieira-Silva S, Tito RY, Joossens M, Raes J. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut. 2016;65:57-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 575] [Cited by in RCA: 708] [Article Influence: 78.7] [Reference Citation Analysis (0)] |
| 105. | Ceresola ER, Ferrarese R, Preti A, Canducci F. Targeting patients' microbiota with probiotics and natural fibers in adults and children with constipation. Eur Rev Med Pharmacol Sci. 2018;22:7045-7057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 106. | Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, Kayser BD, Levenez F, Chilloux J, Hoyles L; MICRO-Obes Consortium, Dumas ME, Rizkalla SW, Doré J, Cani PD, Clément K. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65:426-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1101] [Cited by in RCA: 1317] [Article Influence: 146.3] [Reference Citation Analysis (0)] |
| 107. | Hasegawa S, Goto S, Tsuji H, Okuno T, Asahara T, Nomoto K, Shibata A, Fujisawa Y, Minato T, Okamoto A, Ohno K, Hirayama M. Intestinal Dysbiosis and Lowered Serum Lipopolysaccharide-Binding Protein in Parkinson's Disease. PLoS One. 2015;10:e0142164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 356] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 108. | Sampson TR, Challis C, Jain N, Moiseyenko A, Ladinsky MS, Shastri GG, Thron T, Needham BD, Horvath I, Debelius JW, Janssen S, Knight R, Wittung-Stafshede P, Gradinaru V, Chapman M, Mazmanian SK. A gut bacterial amyloid promotes α-synuclein aggregation and motor impairment in mice. Elife. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 279] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 109. | Unger MM, Spiegel J, Dillmann KU, Grundmann D, Philippeit H, Bürmann J, Faßbender K, Schwiertz A, Schäfer KH. Short chain fatty acids and gut microbiota differ between patients with Parkinson's disease and age-matched controls. Parkinsonism Relat Disord. 2016;32:66-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 815] [Article Influence: 90.6] [Reference Citation Analysis (0)] |
| 110. | Muller PA, Schneeberger M, Matheis F, Wang P, Kerner Z, Ilanges A, Pellegrino K, Del Mármol J, Castro TBR, Furuichi M, Perkins M, Han W, Rao A, Pickard AJ, Cross JR, Honda K, de Araujo I, Mucida D. Microbiota modulate sympathetic neurons via a gut-brain circuit. Nature. 2020;583:441-446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 279] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 111. | Martin-Gallausiaux C, Marinelli L, Blottière HM, Larraufie P, Lapaque N. SCFA: mechanisms and functional importance in the gut. Proc Nutr Soc. 2021;80:37-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 812] [Article Influence: 162.4] [Reference Citation Analysis (0)] |
| 112. | Yajima T. Contractile effect of short-chain fatty acids on the isolated colon of the rat. J Physiol. 1985;368:667-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 157] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 113. | Cherbut C, Ferrier L, Rozé C, Anini Y, Blottière H, Lecannu G, Galmiche JP. Short-chain fatty acids modify colonic motility through nerves and polypeptide YY release in the rat. Am J Physiol. 1998;275:G1415-G1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 125] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 114. | Zhang X, Li Y, Liu C, Fan R, Wang P, Zheng L, Hong F, Feng X, Zhang Y, Li L, Zhu J. Alteration of enteric monoamines with monoamine receptors and colonic dysmotility in 6-hydroxydopamine-induced Parkinson's disease rats. Transl Res. 2015;166:152-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 115. | Wu MJ, Shin DH, Kim MY, Park CG, Kim YD, Lee J, Park IK, Choi S, So I, Park JS, Jun JY. Functional effects of β3-adrenoceptor on pacemaker activity in interstitial cells of Cajal from the mouse colon. Eur J Pharmacol. 2015;754:32-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 116. | Zizzo MG, Mulè F, Mastropaolo M, Serio R. D1 receptors play a major role in the dopamine modulation of mouse ileum contractility. Pharmacol Res. 2010;61:371-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 117. | Zhang X, Guo H, Xu J, Li Y, Li L, Zhang X, Li X, Fan R, Zhang Y, Duan Z, Zhu J. Dopamine receptor D1 mediates the inhibition of dopamine on the distal colonic motility. Transl Res. 2012;159:407-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 118. | Hoffman JM, Tyler K, MacEachern SJ, Balemba OB, Johnson AC, Brooks EM, Zhao H, Swain GM, Moses PL, Galligan JJ, Sharkey KA, Greenwood-Van Meerveld B, Mawe GM. Activation of colonic mucosal 5-HT(4) receptors accelerates propulsive motility and inhibits visceral hypersensitivity. Gastroenterology 2012; 142: 844-854. e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 219] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 119. | Morita H, Mochiki E, Takahashi N, Kawamura K, Watanabe A, Sutou T, Ogawa A, Yanai M, Ogata K, Fujii T, Ohno T, Tsutsumi S, Asao T, Kuwano H. Effects of 5-HT2B, 5-HT3 and 5-HT4 receptor antagonists on gastrointestinal motor activity in dogs. World J Gastroenterol. 2013;19:6604-6612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 120. | Grider JR, Piland BE. The peristaltic reflex induced by short-chain fatty acids is mediated by sequential release of 5-HT and neuronal CGRP but not BDNF. Am J Physiol Gastrointest Liver Physiol. 2007;292:G429-G437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 120] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 121. | Vincent AD, Wang XY, Parsons SP, Khan WI, Huizinga JD. Abnormal absorptive colonic motor activity in germ-free mice is rectified by butyrate, an effect possibly mediated by mucosal serotonin. Am J Physiol Gastrointest Liver Physiol. 2018;315:G896-G907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 122. | Fukumoto S, Tatewaki M, Yamada T, Fujimiya M, Mantyh C, Voss M, Eubanks S, Harris M, Pappas TN, Takahashi T. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1269-R1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 322] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 123. | Tan AH, Chong CW, Lim SY, Yap IKS, Teh CSJ, Loke MF, Song SL, Tan JY, Ang BH, Tan YQ, Kho MT, Bowman J, Mahadeva S, Yong HS, Lang AE. Gut Microbial Ecosystem in Parkinson Disease: New Clinicobiological Insights from Multi-Omics. Ann Neurol. 2021;89:546-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 131] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 124. | Gaudier E, Jarry A, Blottière HM, de Coppet P, Buisine MP, Aubert JP, Laboisse C, Cherbut C, Hoebler C. Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1168-G1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 251] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 125. | Matsuo K, Ota H, Akamatsu T, Sugiyama A, Katsuyama T. Histochemistry of the surface mucous gel layer of the human colon. Gut. 1997;40:782-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 185] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 126. | Aho VTE, Houser MC, Pereira PAB, Chang J, Rudi K, Paulin L, Hertzberg V, Auvinen P, Tansey MG, Scheperjans F. Relationships of gut microbiota, short-chain fatty acids, inflammation, and the gut barrier in Parkinson's disease. Mol Neurodegener. 2021;16:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 287] [Article Influence: 71.8] [Reference Citation Analysis (1)] |
| 127. | Willkommen D, Lucio M, Moritz F, Forcisi S, Kanawati B, Smirnov KS, Schroeter M, Sigaroudi A, Schmitt-Kopplin P, Michalke B. Metabolomic investigations in cerebrospinal fluid of Parkinson's disease. PLoS One. 2018;13:e0208752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 128. | Baldini F, Hertel J, Sandt E, Thinnes CC, Neuberger-Castillo L, Pavelka L, Betsou F, Krüger R, Thiele I; NCER-PD Consortium. Parkinson's disease-associated alterations of the gut microbiome predict disease-relevant changes in metabolic functions. BMC Biol. 2020;18:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 140] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 129. | Kuai XY, Yao XH, Xu LJ, Zhou YQ, Zhang LP, Liu Y, Pei SF, Zhou CL. Evaluation of fecal microbiota transplantation in Parkinson's disease patients with constipation. Microb Cell Fact. 2021;20:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 110] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 130. | Khalif IL, Quigley EM, Konovitch EA, Maximova ID. Alterations in the colonic flora and intestinal permeability and evidence of immune activation in chronic constipation. Dig Liver Dis. 2005;37:838-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 272] [Article Influence: 13.6] [Reference Citation Analysis (2)] |
| 131. | Kim SE, Choi SC, Park KS, Park MI, Shin JE, Lee TH, Jung KW, Koo HS, Myung SJ; Constipation Research group of Korean Society of Neurogastroenterology and Motility. Change of Fecal Flora and Effectiveness of the Short-term VSL#3 Probiotic Treatment in Patients With Functional Constipation. J Neurogastroenterol Motil. 2015;21:111-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 136] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 132. | Chassard C, Dapoigny M, Scott KP, Crouzet L, Del'homme C, Marquet P, Martin JC, Pickering G, Ardid D, Eschalier A, Dubray C, Flint HJ, Bernalier-Donadille A. Functional dysbiosis within the gut microbiota of patients with constipated-irritable bowel syndrome. Aliment Pharmacol Ther. 2012;35:828-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 261] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 133. | Ohkusa T, Koido S, Nishikawa Y, Sato N. Gut Microbiota and Chronic Constipation: A Review and Update. Front Med (Lausanne). 2019;6:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 222] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 134. | Poirier AA, Aubé B, Côté M, Morin N, Di Paolo T, Soulet D. Gastrointestinal Dysfunctions in Parkinson's Disease: Symptoms and Treatments. Parkinsons Dis. 2016;2016:6762528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 135. | Barboza JL, Okun MS, Moshiree B. The treatment of gastroparesis, constipation and small intestinal bacterial overgrowth syndrome in patients with Parkinson's disease. Expert Opin Pharmacother. 2015;16:2449-2464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 136. | Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD, Verbeke K, Reid G. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14:491-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2256] [Cited by in RCA: 3197] [Article Influence: 399.6] [Reference Citation Analysis (0)] |
| 137. | Meksawan K, Chaotrakul C, Leeaphorn N, Gonlchanvit S, Eiam-Ong S, Kanjanabuch T. Effects of Fructo-Oligosaccharide Supplementation on Constipation in Elderly Continuous Ambulatory Peritoneal Dialysis Patients. Perit Dial Int. 2016;36:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 138. | Astarloa R, Mena MA, Sánchez V, de la Vega L, de Yébenes JG. Clinical and pharmacokinetic effects of a diet rich in insoluble fiber on Parkinson disease. Clin Neuropharmacol. 1992;15:375-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 139. | Ashraf W, Pfeiffer RF, Park F, Lof J, Quigley EM. Constipation in Parkinson's disease: objective assessment and response to psyllium. Mov Disord. 1997;12:946-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 137] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 140. | Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4055] [Cited by in RCA: 5558] [Article Influence: 505.3] [Reference Citation Analysis (2)] |
| 141. | Yin S, Zhu F. Probiotics for constipation in Parkinson's: A systematic review and meta-analysis of randomized controlled trials. Front Cell Infect Microbiol. 2022;12:1038928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 142. | Yan T, Shi J, Li X. Clinical efficacy of probiotics in the treatment of constipation in Parkinson's patients. Minerva Gastroenterol (Torino). 2022;68:369-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 143. | Barichella M, Pacchetti C, Bolliri C, Cassani E, Iorio L, Pusani C, Pinelli G, Privitera G, Cesari I, Faierman SA, Caccialanza R, Pezzoli G, Cereda E. Probiotics and prebiotic fiber for constipation associated with Parkinson disease: An RCT. Neurology. 2016;87:1274-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 261] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 144. | Ibrahim A, Ali RAR, Manaf MRA, Ahmad N, Tajurruddin FW, Qin WZ, Desa SHM, Ibrahim NM. Multi-strain probiotics (Hexbio) containing MCP BCMC strains improved constipation and gut motility in Parkinson's disease: A randomised controlled trial. PLoS One. 2020;15:e0244680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 145. | Ge X, Zhao W, Ding C, Tian H, Xu L, Wang H, Ni L, Jiang J, Gong J, Zhu W, Zhu M, Li N. Potential role of fecal microbiota from patients with slow transit constipation in the regulation of gastrointestinal motility. Sci Rep. 2017;7:441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 145] [Article Influence: 18.1] [Reference Citation Analysis (0)] |