INTRODUCTION
Esophageal intramural pseudodiverticulosis (EIPD) is a disease in which the excretory ducts of esophageal submucosal glands show systemic, cystic dilatation. On endoscopic examination, the orifices of the dilated ducts appear as multiple, small mucosal depressions resembling diverticula[1-4]. Chronic inflammation is a frequent complication, and prolonged inflammation results in esophageal stricture with impaired peristaltic movements, which leads to difficulty swallowing or impaction of food[2].
Although EIPD is a rare disease, it can have serious complications, such as esophageal perforation, mediastinitis, and lung abscess, if not adequately treated[5-8]. In this article, I review the epidemiology, clinical manifestations, characteristic endoscopic findings, radiological findings, complications, and medical treatment of EIPD. I also discuss some hypotheses concerning its etiology and pathogenesis, based on my own experience and a review of the literature.
EPIDEMIOLOGY
Since the first clinicopathological report by Mendl et al[9], approximately 320 cases of EIPD have been recorded to date, mainly in Europe and the United States. EIPD affects men more commonly than women and occurs in all age groups, the peak incidence being between 50 and 70 years of age[10-12]. About 20 cases have also been reported in the pediatric population, the youngest patient being diagnosed at 37 d after birth[13]. In a survey of the literature, excluding conference proceeding abstracts, less than 60 cases of EIPD have been reported in Japan.
Many asymptomatic patients are incidentally found to have EIPD by endoscopic examination, and, even if detected, some patients do not wish to undergo treatment or observation because the subjective symptoms are usually mild or, in some cases, absent.
CLINICAL MANIFESTATIONS
The main symptom of EIPD is dysphagia of solid food, which is intermittent and unremarkable in the incipient stages. In many cases, dysphagia progresses slowly, and patients occasionally present for emergency endoscopy due to impaction of food or consult for a thorough endoscopic examination seeking the cause of their weight loss[14-18]. Other symptoms such as a feeling of congestion in the esophagus, chest pain, and hematemesis have been reported[5,19-21]. EIPD is usually diagnosed in individuals of middle age or older, and many patients have a history of heavy drinking or smoking[7,22-25]. Some disorders, such as diabetes mellitus, gastroesophageal reflux disease (GERD), chronic lung diseases, alcoholic liver cirrhosis, esophageal cancer, and collagen-vascular diseases, have been reported as conditions predisposing patients to EIPD[1,23,26].
In pediatric cases, EIPD may manifest several years after surgery for esophageal atresia or tracheoesophageal fistula[27,28]. There are also some reports of cases with underlying asthmatic bronchitis[29] and pediatric patients with siblings suffering from the same disease[30]. In some cases, EIPD presents with vomiting soon after birth and is followed by dysphagia of solid food and growth retardation. The symptoms are aggravated by disturbance of esophageal peristalsis or GERD in the period from approximately 5 years of age to adolescence[31-35]. In some pediatric patients, EIPD becomes apparent after repeated surgical interventions for esophageal hiatal hernia or GERD[36]. Cases of young patients diagnosed with EIPD after ingestion of corrosive substances have also been reported[28,37].
CLINICAL EXAMINATIONS
Endoscopic examination
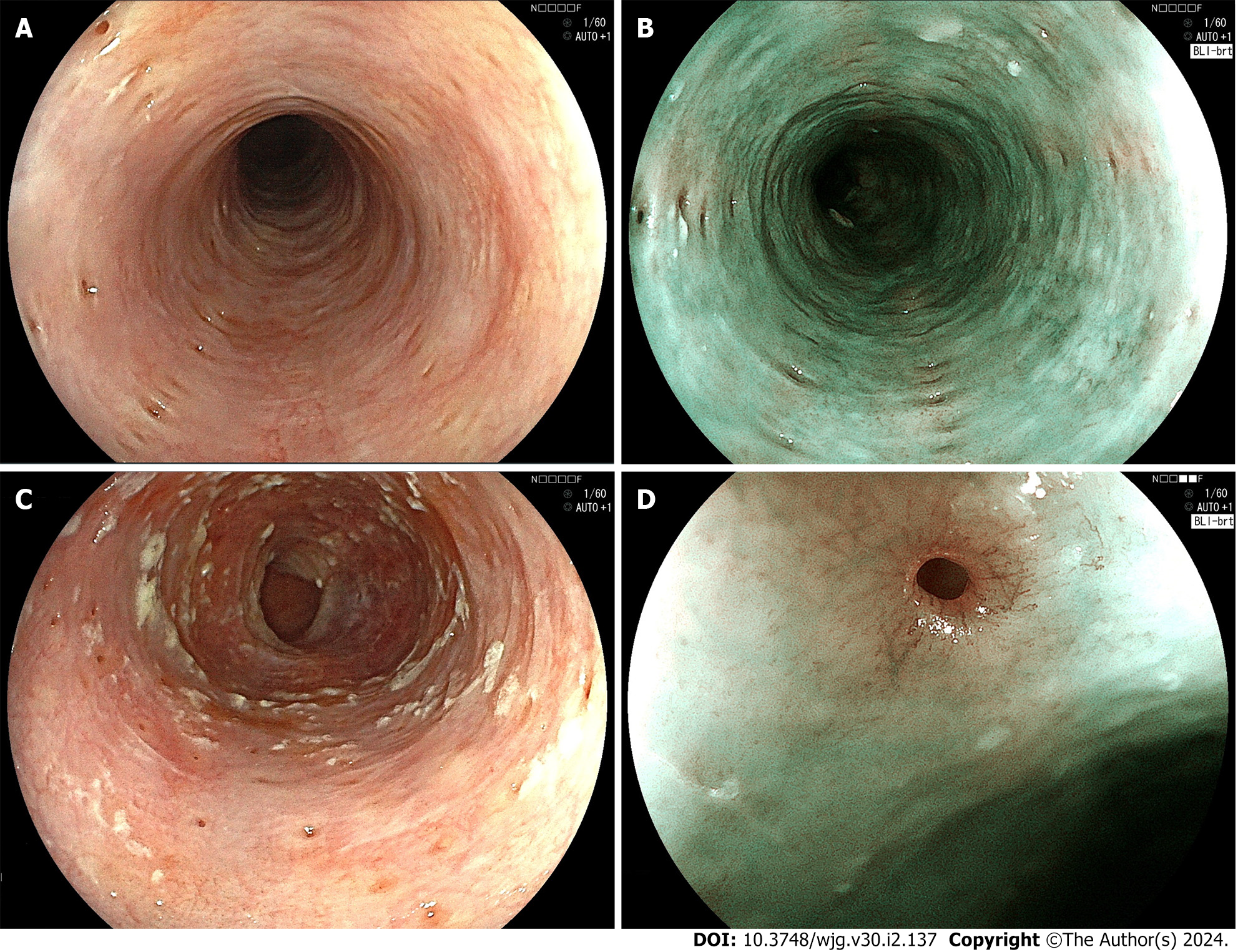
Endoscopy of the esophagus has markedly advanced in recent years, and nowadays most cases of EIPD are diagnosed by endoscopic examination[23]. On endoscopy, EIPD is usually observed as multiple, diverticulum-like, small, localized mucosal depressions measuring approximately 1 mm to 4 mm in diameter (Figure 1A). Narrow band imaging (NBI) or blue laser imaging (BLI) shows multiple small, light-brown spots that tend to be arranged longitudinally on the background mucosa, which is green (Figure 1B). Although the orifices of EIPD are often covered by inflammatory exudate produced by infection with Candida spp., the orifices become more clearly visible after washing away the adherent exudate (Figure 1C). In magnified images, the orifices of the dilated ducts of esophageal glands can be seen in the center of small depressions as pinhole-like, minute spots surrounded by an intraepithelial papillary loop of capillaries that resemble eyelashes[38] (Figure 1D).
Figure 1 Endoscopic examination.
A: White-light endoscopic image. Multiple small diverticulum-like depressions of the mucosa with a diameter of 1-4 mm are seen. Fujifilm EG-L600ZW; B: Blue laser imaging (BLI)-bright image. The light-brown depressions are longitudinally aligned on the green-colored mucosa. Fujifilm EG-L600ZW; C: White-light endoscopic image. The orifices of pseudodiverticula are covered with whitish, plaque-like material. Fujifilm EG-L600ZW; D: BLI-bright, medium magnifying image. The orifice of the pseudodiverticulum is surrounded by blood vessels arranged in an eyelash-like pattern. Fujifilm EG-L600ZW.
Among the cases of EIPD, those in which many small “pseudodiverticula” are distributed along the entire length of the esophagus are called “diffuse EIPD”. In contrast, when the occurrence of multiple “pseudodiverticula” is limited to only a part of the esophagus, it is called “segmental EIPD”. Segmental EIPD can be easily overlooked, especially when it is not associated with esophageal stricture and the subjective symptoms are mild, but endoscopy with NBI or BLI improves the detection rate of the orifices of dilated ducts due to differences in the colors of the mucosa[23].
In 2011, the present author and colleagues reported for the first time that the mucosal orifices of dilated ducts in EIPD periodically open and close asynchronously with respiratory or peristaltic movements[19]. These movements were observed at intervals of approximately 2 min, and clear or white-colored mucus was discharged from the orifices onto the mucosal surface[19,38]. The opening and closing of the mucosal orifices typically starts a few minutes after insertion of the endoscope or after stimulation with air or water.
In EIPD patients complicated with severe Candida esophagitis, EIPD may be overlooked because the orifices of dilated ducts are covered by white plaques or inspissated mucus[11]. There are a few reports of EIPD complicated with severe candidiasis in which cystically dilated ducts coalesced to form a “sinus tract”-like structure in the submucosa[25,39,40]. In many cases of EIPD, inflammation involving the esophageal wall is prolonged, the mucosa is thickened by edema, and the mucosal vascular pattern becomes hardly discernible. Web-like constriction of the esophagus due to inflammatory changes may be occasionally observed[23]. Further progression of inflammation leads to esophageal stricture or disorders of peristaltic movements[10]. In one of our own cases, in which the patient suffered from GERD with Barrett esophagus, the lower portion of the esophagus was constricted[38]. However, according to many previously reported cases of EIPD, stenosis is mostly noted in the upper portion of the esophagus[1,23,41].
Esophagography
A barium esophagogram shows characteristic, numerous, tiny, flask-shaped outpouching within the esophageal wall, when contrast medium flows into the “pseudodiverticula”[19,42]. These outpouchings correspond to cystic dilatation of the ducts of esophageal glands. When the esophagus is regionally narrowed, these outpouchings are often seen in the vicinity of the narrowed portion. Since the quality of endoscopic images was suboptimal in the past and magnifying endoscopes were not widely used until about 10 years ago, EIPD was mainly diagnosed by esophagography. However, infusion of contrast medium into the “pseudodiverticula” is difficult when the dilated ducts are filled with inflammatory exudates, desquamated epithelial cells, or inspissated mucus, or when the orifices of EIPD are very small[28,32,43]. EIPD that was not detected by esophagography is occasionally found at autopsy[3]. It takes time and sufficient knowledge of EIPD to obtain clear esophagographic images of the disease[21].
Computed tomography and esophageal manometry
When the inflammatory process complicating EIPD is prolonged, computed tomography (CT) shows thickening of the esophageal wall due to inflammatory exudation and fibrosis. Intramural air images reflecting dilatation of ducts are also observed[25,40,44,45]. Esophageal manometry may demonstrate decreased peristaltic movements, abnormal motility, and abnormal intraluminal pressure of the esophagus[46,47]. There are several reports of EIPD co-existing with motility disorders, such as achalasia[48,49] or “jackhammer esophagus”[50,51]. To select an appropriate treatment for EIPD, high-resolution manometry is useful for evaluating esophageal peristalsis and sphincter function at the proximal and distal ends of the esophagus[46,47].
PATHOLOGICAL FINDINGS
Since EIPD is a disorder that is rarely treated surgically, pathological findings of the esophagus are often obtained only for autopsied cases or for patients who underwent surgical treatment due to complications or co-existing disorders, such as esophageal cancer. There are very few reports dealing with pathological findings of the esophagus in EIPD, especially in recent years[52].
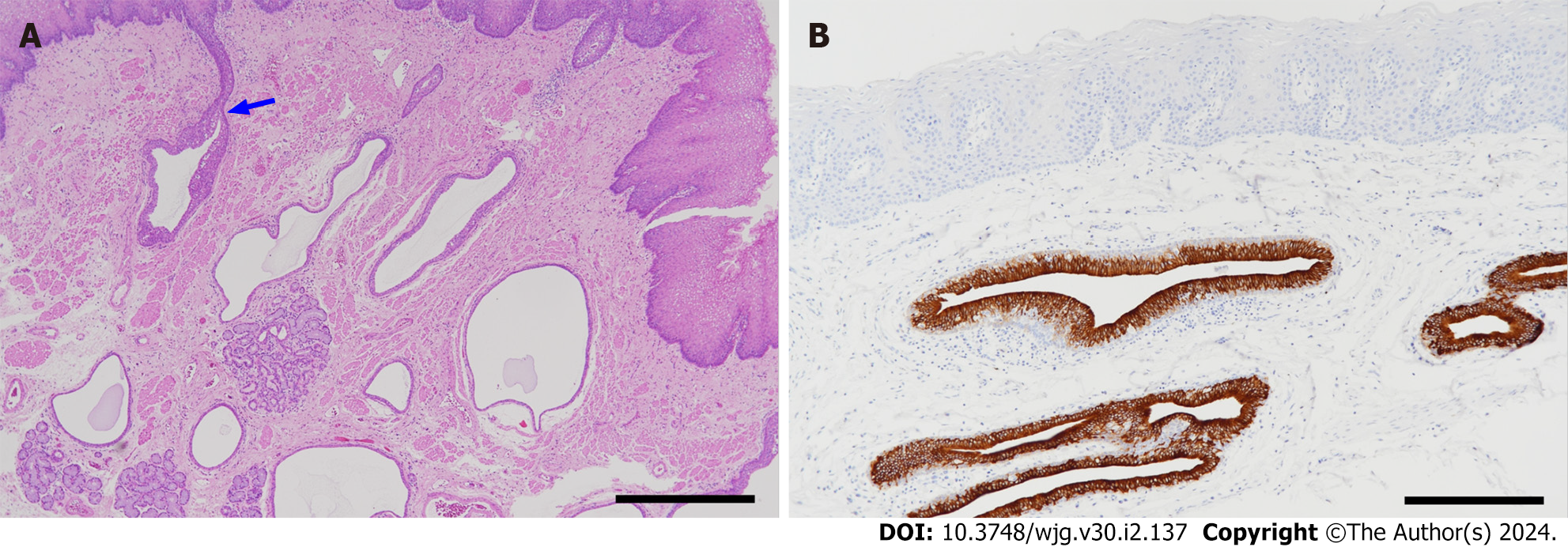
The present author and colleagues reported the results of a histopathological and immunohistochemical study of ductal epithelial cells of the esophageal glands and their pathological changes in two operated cases of EIPD[52]. In the normal esophagus used as a control, the inner surface of the excretory ducts of esophageal gland was lined by simple cuboidal or columnar epithelium, and a single layer of cuboidal, basal cells was seen in the underlying layer. In EIPD, both the surface epithelial cells and basal cells of the ducts showed hyperplasia and stratification of varying degrees, and the ductal walls were thickened (Figure 2A). On immunohistochemical examination, whereas the surface epithelial cells were immunoreactive for cytokeratin 7 (CK7) and negative for CK5/6, basal cells showed the reverse pattern (CK7-negative and CK5/6-positive). The nuclei of basal cells were immunoreactive for p63. The stratified squamous epithelium covering the mucosa of the esophagus was CK7-negative and clearly distinguished from the CK7-positive surface epithelium of the ducts[52]. The immunohistochemical profiles of epithelial cells described above were essentially unchanged in EIPD (Figure 2B). In the acinar portion of esophageal glands located in the submucosa, myoepithelial cells surrounding each acinus were well preserved. This finding supported the endoscopic findings that the orifices of ducts in EIPD periodically opened and closed, thereby discharging mucus onto the mucosal surface.
Figure 2 Pathological findings.
A: Histology of esophageal intramural pseudodiverticulosis (hematoxylin-eosin stain). Many cystically dilated ducts of the esophageal glands are seen in the lamina propria mucosae and submucosa. Some of them are continuous with the superficial epithelium (arrow) (scale bar: 1 mm); B: Cytokeratin 7 (CK7) immunostaining. Epithelial cells lining the ducts are positive for CK7. The epithelium of the duct shows hyperplasia and stratification. The esophageal mucosal epithelium (upper part of the figure) is negative for CK7 (scale bar: 200 µm).
In the cases of EIPD with a long clinical course, inflammatory cells infiltrated into the periductal and periacinar regions of esophageal glands, and the ductal lumina were filled with inflammatory cells, desquamated epithelial cells, and mucus. Extensive periglandular fibrosis was often observed[3,21,28]. Acinar cells were infrequently replaced by squamous cells, thus forming a lesion analogous to the “necrotizing sialometaplasia” of minor salivary glands[52,53].
Esophageal biopsies usually reveal only non-specific, chronic inflammation, edema, and acanthosis of the surface epithelium[10,14]. Although EIPD cannot be diagnosed by biopsy, biopsy is performed to exclude candidiasis, eosinophilic esophagitis, lymphocytic esophagitis, or esophageal cancer, and also to evaluate the degree of inflammatory changes[10].
ETIOLOGY AND PATHOGENESIS
Looking back at the history of the development of the disease concept of EIPD, a pathological condition in which the orifices of esophageal gland ducts are obstructed by inflammation, resulting in the formation of retention cysts that protrude into the esophageal lumen, had been described using the term “cystic esophagitis” in 1899 (according to Piazza and Palma[54]). The first description of EIPD was published in 1960 by Mendl et al[9], who detected an esophageal disease with a radiographic appearance resembling the Rokitansky-Aschoff’s sinus of the gallbladder. They described it as “intramural diverticulosis”[9]. Boyd et al[2], by examining autopsied cases, demonstrated that the disease was not “diverticulosis” but a cystic dilatation of the esophageal gland ducts and termed it “pseudodiverticulosis”. As mentioned previously, EIPD is not a mucosal prolapse or depression, such as seen in pseudodiverticulosis of the large intestine, but a dilatation of the esophageal gland ducts that disturbs mucus excretion. The term “EIPD” therefore does not reflect the true nature of the disease, and a more appropriate term may be needed, such as “systemic dilatation of esophageal gland ducts”.
The etiology and pathogenesis of EIPD remain unknown. It has been generally assumed that the disease is an acquired condition and the neck portion of the ducts is obstructed by inflammatory changes, resulting in cystic dilatation due to an increased internal pressure[1,2]. However, Umlas and Sakhuja[3] found that the ducts are also slightly dilated in autopsy cases used as a normal control, and they stated that it is difficult to determine whether inflammation elicited the dilatation of ducts or the inflammation was only a secondary change caused by EIPD. They considered the esophageal stricture and Candida infection seen in EIPD patients to be secondary changes[3]. Candida infection of the oral cavity and pharynx is a common phenomenon that is found in 30% of normal individuals[55], and Candida infection frequently seen in EIPD patients is now considered a secondary infection rather than a causative agent of EIPD. Medeiros et al[4] found that the number of esophageal glands increased with aging and considered that periglandular chronic inflammation dilates the ducts and leads to the formation of a pseudodiverticulum. Kataoka et al[56] found that, among 27 autopsied cases, 15 cases (56%) showed slight dilatation of esophageal gland ducts. The two-thirds of the cases showing ductal dilatation had chronic inflammatory changes surrounding the glands, and the authors concluded that the changes that had been regarded to be an incipient change of EIPD were commonly seen in the general population[56]. They stated that EIPD is caused by chronic inflammation of the esophageal glands due to various causes, such as infection, GERD, and erosion, and that diabetes mellitus and an excessive ingestion of alcohol might accelerate the inflammation[56].
Candida, bacteria, and refluxing gastric juice or bile easily flow into the pseudodiverticula in EIPD through their dilated orifices. Excessive ingestion of alcohol, smoking, and diabetes mellitus accelerate inflammatory changes. Inflammatory changes cause retention and concentration of secreted mucus within the dilated ducts and further increase dilatation of the ducts[3,21,28].
The etiology and pathogenesis of EIPD remain obscure, but, as mentioned previously, the ducts and acini of esophageal glands in EIPD do not show significant structural differences in comparison with those of normal glands. I suspect that functional abnormalities of the autonomic nerve (vagal nerve) innervating the esophageal glands and regulating their function might play a role in the pathogenesis of EIPD. The functional disturbance of the autonomic nerve caused by inflammation or aging may affect the function of myoepithelial cells in the acini of esophageal glands and aggravate the pathological condition of EIPD.
Regarding pediatric cases of EIPD, Peters et al[57] observed the clinical course of a 5-year-old girl with EIPD for 16 years and found that whereas the pseudodiverticula increased in number and became more pronounced, the patient did not develop any serious complications. Freud et al[36] compared the esophagographic findings obtained from one month after birth to 3 years of a male infant who developed severe vomiting immediately after birth and underwent repeated cardioplasty and dilatation of the esophagus, and stated that his EIPD gradually progressed. Solomon et al[13] reported a female infant who was born with pulmonary dysplasia and was diagnosed with EIPD on day 37 after birth, and presumed that her EIPD was a congenital disorder. A retrospective review of esophagograms from pediatric cases indicated that the number of pseudodiverticula and the sizes of their orifices increased as the children aged[34,57]. In recent years, the number of reported cases of pediatric EIPD has markedly decreased, making comparisons between EIPD in adults and that in the pediatric population more difficult. I suspect that EIPD in pediatric patients may have a different etiopathogenesis from that in adults, and they could actually be distinct entities.
COMPLICATIONS
EIPD, if not treated adequately, can lead to fibrosis of the esophageal mucosa and submucosa as a result of long-lasting, chronic esophagitis caused by infection or gastroesophageal reflux. The fibrosis subsequently causes esophageal stenosis or disturbance of the esophageal motility. Delay of diagnosis can lead to serious complications, such as intra-mediastinal fistula due to progression of the inflammatory changes[26]. There are several reported cases of serious complications of EIPD as follows. For example, a case was reported in which the diagnosis of EIPD was not rendered despite the typical clinical findings, and the patient underwent unnecessary subtotal esophagectomy[58]. In another case, the patient underwent emergency operation because of esophageal perforation due to severe vomiting associated with EIPD[5]. Other cases include EIPD brought about esophageal perforation with mediastinal emphysema or abscess[6], EIPD associated with Mallory-Weiss syndrome[20], and a case in which the patient underwent esophagectomy due to esophageal stenosis[59]. Fatal cases of perforation due to EIPD[60] and the association of EIPD with esophageal cancer[61] have also been reported. Prolongation of the inflammatory changes, excessive intake of alcohol or smoking, and the presence of various underlying disorders are considered risk factors for the complications associated with EIPD[10,23].
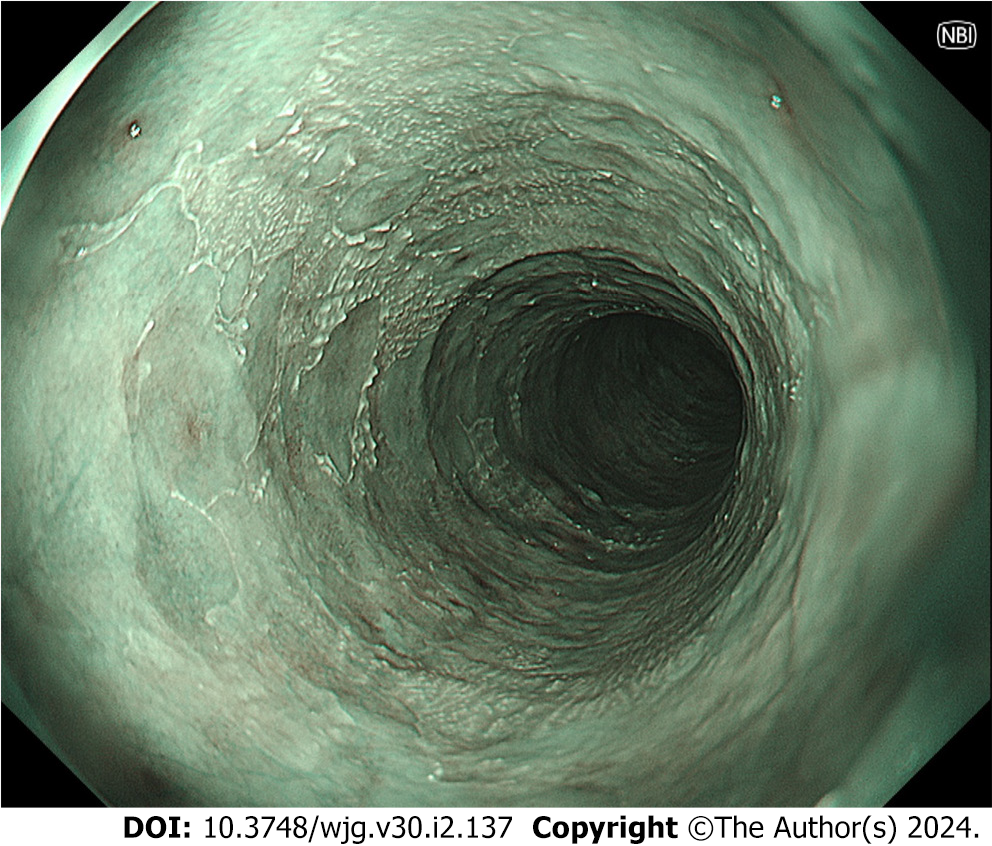
During the observation period of a case of EIPD, my colleagues and I found “epidermization” of the mucosal squamous epithelium of the esophagus[62] (Figure 3). Although the etiology and pathogenesis of “epidermization” have not yet been clarified, it may arise during the repair of damage caused by inflammation[63-65]. Candida esophagitis had persisted for a long time in that case, and we considered that the “epidermization” occurred during the healing process[62]. Since a few cases of esophageal cancer associated with “epidermization” have been reported[65,66], we are carefully following the patient. No significant changes have been found during the 3-year observation period.
Figure 3 Narrow band imaging shows “epidermization”, which was widely spread in the middle esophagus 2.5 years after the first endoscopic examination.
The extent of epidermization has not markedly changed over a 3-year period. Olympus GIF-XZ1200.
TREATMENTS
EIPD is a benign condition, and its treatment is basically conservative, symptomatic therapy. However, to prevent prolongation of the clinical course of EIPD, inflammation associated with EIPD, which can be caused by various factors, including Candida infection and GERD, should be treated. It is also important to be aware of esophageal strictures, perforations, mediastinitis, and lung abscesses that can result from long-term chronic inflammation. Thus, EIPD requires early diagnosis, careful observation, and appropriate treatment. The following factors are worth noting regarding treatment for EIPD: (1) The weaning from the habit of heavy alcohol drinking or smoking is important; (2) For the complication of Candida infection, anti-fungal agents are effective[18,40,44,67,68]; (3) Endoscopic ballooning dilatation is effective when difficulty swallowing due to fibrosis of the esophageal wall develops, but multiple dilatation procedures are often required[17,24,45,69,70]. When the esophageal stenosis is mild, an endoscopic “bougie” procedure is effective and often improves symptoms[16,71]; (4) When EIPD is complicated with GERD, administration of drugs inhibiting the secretion of gastric acid, such as proton pump inhibitors or H2-receptor antagonists, is effective[18,22,24,45]. However, there are no established opinions regarding the dosage or duration of use for these drugs. If the patients are allergic to acid-secretion inhibitors, agents that increase the mucosal protective function are also effective[72]; (5) Isosorbide dinitrate, a smooth muscle relaxant, has been reported to improve symptoms due to strong contraction of the lower esophagus[47]. Inhalation of amyl nitrite is reported to have improved the symptoms in EIPD patients with the complication of esophageal achalasia[48]; (6) Corticosteroids may also alleviate the subjective symptoms of EIPD[56]; and (7) In Europe and the United States, swallowing therapy using inhaled corticosteroids has been studied in EIPD associated with eosinophilic esophagitis[73-75].
CONCLUSION
The etiopathogenesis and natural course of EIPD have not yet been elucidated, and only conservative treatments focusing on symptom management have been employed for patients. In the modern era, EIPD is mainly diagnosed via esophageal endoscopy. A better understanding of EIPD will improve differential diagnoses of this and other disorders, such as dysphagia or a feeling of congestion in the esophagus, and ultimately bring about the early diagnosis and appropriate treatment of EIPD, thereby preventing the development of serious complications.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu YC, China; Pavlidis TE, Greece S-Editor: Chen YL L-Editor: A P-Editor: Yu HG