Published online May 21, 2024. doi: 10.3748/wjg.v30.i19.2564
Revised: April 5, 2024
Accepted: April 18, 2024
Published online: May 21, 2024
Processing time: 110 Days and 22.6 Hours
Cell division cyclin 25C (CDC25C) is a protein that plays a critical role in the cell cycle, specifically in the transition from the G2 phase to the M phase. Recent re
To explore the impact of CDC25C on cell proliferation and apoptosis, as well as its regulatory mechanisms in HCC development.
Hepa1-6 and B16 cells were transduced with a lentiviral vector containing shRNA interference sequences (LV-CDC25C shRNA) to knock down CDC25C. Subse
CDC25C was stably suppressed in Hepa1-6 and B16 cells through LV-CDC25C shRNA transduction. A xenograft model with CDC25C knockdown was successfully established and that downregulation of CDC25C expression significantly inhibited HCC growth in mice. CDC25C knockdown not only inhibited cell proliferation and mig
The regulatory mechanism of CDC25C in HCC development may involve the activation of ER stress and the ER stress-induced apoptosis signaling pathway.
Core Tip: In the current study, cell division cyclin 25C (CDC25C) is an important cell cycle regulatory protein and a potential target for cancer treatment. CDC25C knockdown not only inhibited cell proliferation and migration but also significantly increased the endoplasmic reticulum (ER) stress response. Furthermore, CDC25C knockdown promoted ER stress-induced apoptosis in hepatocellular carcinoma (HCC) cells. The regulatory mechanism of CDC25C in HCC development might be related to the activation of ER stress and the ER stress-induced apoptosis signaling pathway.
- Citation: Li YF, Zheng FY, Miao XY, Liu HL, Zhang YY, Chao NX, Mo FR. Cell division cyclin 25C knockdown inhibits hepatocellular carcinoma development by inducing endoplasmic reticulum stress. World J Gastroenterol 2024; 30(19): 2564-2574
- URL: https://www.wjgnet.com/1007-9327/full/v30/i19/2564.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i19.2564
Hepatocellular carcinoma (HCC) is a prevalent form of cancer and is the third leading cause of cancer-related deaths worldwide[1]. The development of HCC is attributed primarily to oxidative stress or inflammation, and a majority of patients are diagnosed with advanced tumors[2]. Tumor cells exhibit uncontrolled proliferation, which is a key characteristic of an aberrant cell cycle. Cell cycle regulation is influenced by Cell division cyclin 25C (CDC25C), a crucial protein that plays an important role in the initiation, progression, and prognosis of cancer. Numerous studies have reported elevated levels of CDC25C expression in various malignancies[3]. Furthermore, CDC25C has been recognized as a potential gene associated with tumor antigens and has demonstrated efficacy in hepatoma immunotherapy[4]. However, the specific regulatory mechanisms underlying the role of CDC25C in HCC tumorigenesis and development remain incompletely understood.
Hepatocytes contain a very large amount of endoplasmic reticulum (ER), which is essential for various cellular pro
In this study, we aimed to determine the roles of CDC25C in cell proliferation, migration, and apoptosis by conducting CDC25C knockdown in Hepa1-6 cells and subcutaneous xenografts established with these cells. Furthermore, we investigated the potential involvement of CDC25C in activating ER stress. The findings from our study will be beneficial for future molecular research on the regulatory mechanism of CDC25C in the development of HCC.
The mouse HCC cell line Hepa1-6 and the mouse melanoma cell line B16, which were derived from C57BL/6 mice, were obtained from the Cell Bank of the Chinese Academy of Medical Sciences in Beijing, China. Hepa1-6 cells were cultured in Dulbecco's modified Eagle's medium (HyClone, Los Angeles, CA, United States) supplemented with 10% fetal bovine serum (FBS) (Dojindo, Tokyo, Japan), 100 U/mL penicillin (Wisent, Nanjing, China), and 100 μg/mL streptomycin (Wisent). B16 cells were cultured in RPMI 1640 medium (Wisent) supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. The cells were incubated at 37 °C with 5% CO2 and 95% humidity.
The lentiviral empty vector (LV3H1/GFP and Puro) and the lentiviral vector containing the CDC25C shRNA interference sequence (LV-CDC25C shRNA) were purchased from GenePharma (Suzhou, China). Hepa1-6 and B16 cells were seeded in 24-well plates at a density of 5 × 104 cells/well and cultured until they reached 70%-80% confluence. CDC25C expression was suppressed by transducing cells with LV-CDC25C shRNA, and these cells served as the experimental (shRNA-CDC25C) group. The empty lentiviral vector was transduced to establish the negative control (shRNA-NC) group, and uninfected cells were used as the blank control (MOCK) group. The transduction efficiency was monitored using fluorescence microscopy. Stably transduced cells were selected using 4 μg/mL puromycin (Solarbio, Beijing, China) and expanded for further experiments.
C57BL/6 mice (age: 4 wk; weight: 14-16 g) were obtained from Changsha Tianqin Bioscience Co., Inc. (Changsha, China). The mice were housed in a specific pathogen-free animal room at a temperature of 22 °C-25 °C with ad libitum access to water and food. To establish the experimental group, infected Hepa1-6 cells (1 × 107) were subcutaneously injected into mice in the anterior right subaxillary region (N = 15). The body weight and tumor volume were measured every 5 d. Twenty days post-injection, the tumor tissues were harvested and weighed. All animal experiments were reviewed and approved by the Ethics Review Committee for Animal Experiments at Guangxi Medical University (Approval Number: 202201093).
Total RNA was isolated from cells/tissues using TRIzol reagent (TaKaRa, Dalian, China) following the manufacturer's protocol. Subsequently, the isolated RNAs were reverse transcribed into cDNAs using a PrimeScript RT Reagent Kit (TaKaRa). mRNA expression levels were measured using quantitative real-time PCR (qRT-PCR) with a SYBR Green Master Mix Kit (TaKaRa) in a Step One Real-time PCR System (Applied Biosystems, Waltham, MA, United States). The sequences of the primers used for amplification are provided in Table 1. To ensure accuracy, GAPDH was used as an internal control. The amplification reaction consisted of an initial denaturation step at 95 °C for 5 s followed by 40 cycles of denaturation at 95 °C for 5 s and annealing/extension at 60 °C for 30 s. Negative controls without template DNA were included, and each reaction was performed in triplicate. The relative expression levels of the target genes were calculated using the 2-ΔΔCt method.
| Gene | Forward | Reverse |
| CDC25C | 5′-CCTTGGATTCATCTGGACCTCTG-3′ | 5′-ATGTGCTACGCTTTGCAATCTTC-3′ |
| GRP78 | 5′-GAAGAGCTGTGCTCGGACCT-3′ | 5′-CAGCAGCTTCTGCACCTTGG-3′ |
| XBP-1 | 5′-CAGCAAGTGGTGGATTTGGAAG-3′ | 5′-TCTTAACTCCTGGTTCTCAACCACA-3′ |
| CHOP | 5′-GTCACACGCACATCCCAAA-3′ | 5′-GTTCTTCCTTGCTCTTCCTCCTC-3′ |
| Caspase 9 | 5′-GGGACAGTGGCAGTAATGTCTTCA-3′ | 5′-AACTCAATGCCCAGTGTTCCAG-3′ |
| Caspase 3 | 5′-TGAGCAGAGTCAAAGGCTGGAA-3′ | 5′-AGCTATAATGCATGCTATGGTGGTC-3′ |
| GAPDH | 5′-TGTGTCCGTCGTGGATCTGA-3′ | 5′-TTGCTGTTGAAGTCGCAGGAG-3′ |
A Cell Counting Kit-8 (CCK-8) assay kit (Dojindo) was used to assess the proliferation of Hepa1-6 and B16 cells. Transduced cells were seeded in 96-well plates at a density of 2 × 103 cells per well. Then, 0, 24, 48, and 72 h post-transfection, the cells were incubated with 10 µL of CCK-8 solution for 2 h at 37 °C. The optical density at 450 nm was then measured using a microplate reader (BioTek Synergy H1, Winooski, United States).
The migration ability of Hepa1-6 and B16 cells was assessed using a wound healing assay. After transduction for 24 h, cells were seeded in 24-well plates at a density of 2 × 105 cells/well and cultured for an additional 24 h. Subsequently, a uniform scratch was made in the cell layer on the bottom of the plate, and the detached cells were removed by washing using 1 × phosphate-buffered saline (PBS) buffer. The cells were then cultured at 37 °C with 5% CO2, and the scratch wounds were photographed at the 0, 12, and 24 h time points. The migration rate was determined by measuring the scratch width using ImageJ online software.
Total protein was isolated from cells/tissues using a total protein extraction kit (Vazyme, Nanjing, China) following the manufacturer’s protocol. The proteins in the samples were separated by SDS-PAGE and transferred to polyvinylidene fluoride membranes (BD, United States). After completion of the blocking step, the membranes were incubated overnight at 4 °C with diluted primary antibodies (Table 2). GAPDH was used as a loading control. Subsequently, the membranes were incubated at room temperature for 2 h with the appropriate secondary antibody (rabbit, FDR007, Fdbio) at a 1:5000 dilution. Finally, the protein bands were visualized using an ECL kit (Fdbio, Hangzhou, China) in a FluorChem FC3 System (ProteinSimple, United States). Protein expression was quantified using ImageJ online software and is expressed as a ratio of target protein expression to GAPDH expression.
| Antibody | Species | Catalog | Company | Dilution |
| CDC25C | Rabbit | E302 | Cambridge, MA | 1:1000 |
| GRP78 | Rabbit | 3177S | Cell Signaling Technology | 1:500 |
| XBP-1 | Rabbit | AF5110 | Affinity Biosciences | 1:500 |
| CHOP | Mouse | 66741-1-lg | Proteintech | 1:200 |
| Caspase 9 | Rabbit | abs145753 | Absin Bioscience | 1:2000 |
| Caspase 3 | Rabbit | ab184787 | Abcam Cambridge, United Kingdom | 1:500 |
| GAPDH | Rabbit | FD0063 | Fdbio | 1:2000 |
The apoptosis of Hepa1-6 cells was detected using a FITC Annexin V apoptosis detection kit (Keygen, Nanjing, China). After transduction, cells were fully detached with 0.25% EDTA-free trypsin and then washed twice with 1 × PBS at 4 °C. Subsequently, the cells were seeded in 6-well plates at a density of 1 × 106 cells/well and stained with 5 mL of FITC-Annexin V and 5 mL of PI for 15 minutes at room temperature in the dark, and each reaction was repeated three times. The apoptosis rate was analyzed using a flow cytometer (Tree Star, Ashland, OR, United States).
Statistical analyses were conducted using SPSS Statistics 22.0 (SPSS Inc., Chicago, IL, United States). Quantitative data are presented as the mean ± SD values. Student’s t test was used for statistical analyses. A P value less than 0.05 was considered to indicate statistical significance.
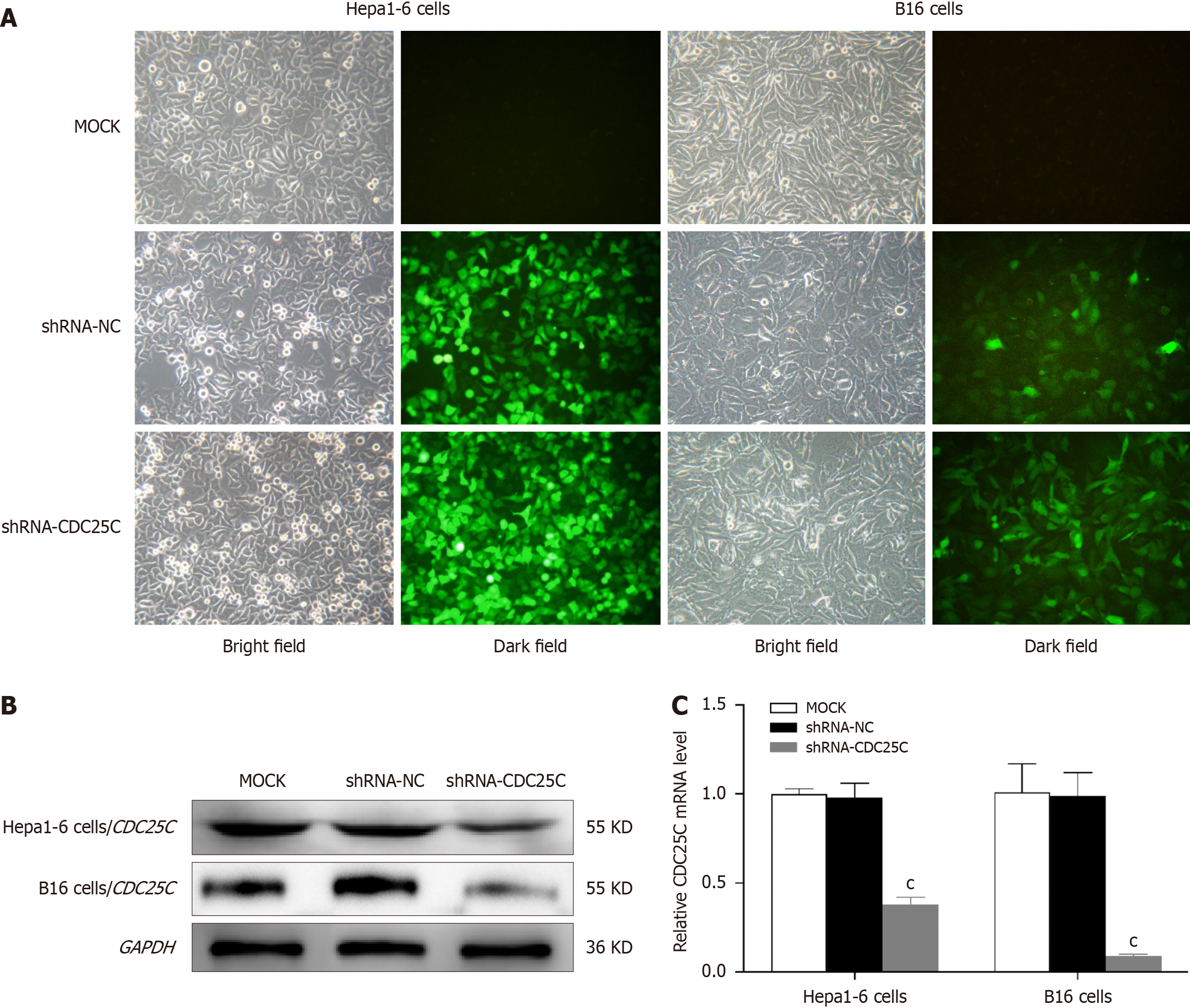
To investigate the role of CDC25C in HCC development, we established a CDC25C knockdown model in the Hepa1-6 cell line by transduction of the LV-CDC25C shRNA plasmid. The B16 cell line was subjected to the same process and served as a positive control. After 72 h of transduction, we observed the Hepa1-6 and B16 cells under an inverted fluorescence microscope to assess the expression of green fluorescent protein (GFP). High levels of GFP were detected in the shRNA-CDC25C and shRNA-NC groups but not in the MOCK group (Figure 1A). After 10 d of puromycin selection, the transduction efficiency of the lentiviral vector in Hepa1-6 cells was found to exceed 90% (Figure 1A). Immunoblotting using an anti-CDC25C antibody confirmed the presence of a 55 kDa band in the total protein lysate, and the size of this band was reduced in the shRNA-CDC25C group (Figure 1B). CDC25C mRNA expression was further evaluated using qRT-PCR, which showed a significant decrease in the shRNA-CDC25C group (P < 0.001) (Figure 1C). These results demonstrated that CDC25C was stably suppressed in Hepa1-6 and B16 cells through LV-CDC25C shRNA transduction.
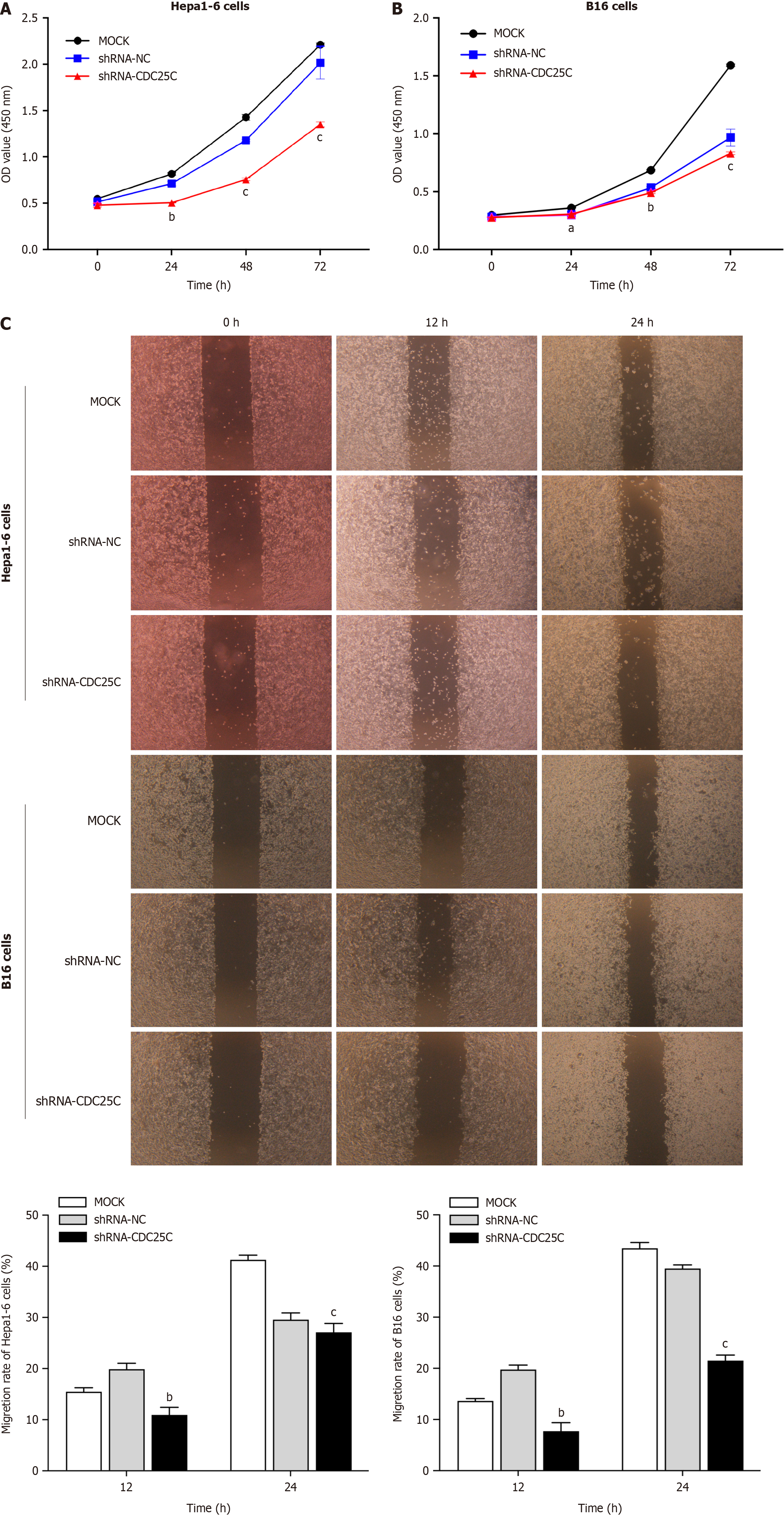
After establishing CDC25C knockdown cells, we performed CCK-8 cell proliferation and wound healing assays to assess the proliferation and migration, respectively, of the Hepa1-6 and B16 cells. In the CCK-8 assay, we observed that the cells in the shRNA-CDC25C group had significantly attenuated proliferation compared to those in the MOCK and shRNA-NC groups at 24, 48, and 72 h post-transduction (Figure 2A and B). Additionally, the wound healing assay revealed that CDC25C knockdown reduced the migration ability of the cells. At both 12 and 24 h post-wounding, the migration rate in the shRNA-CDC25C group was significantly lower than that in either the MOCK or shRNA-NC group (Figure 2C). These findings collectively indicate that CDC25C knockdown inhibits the proliferation and migration of Hepa1-6 and B16 cells.
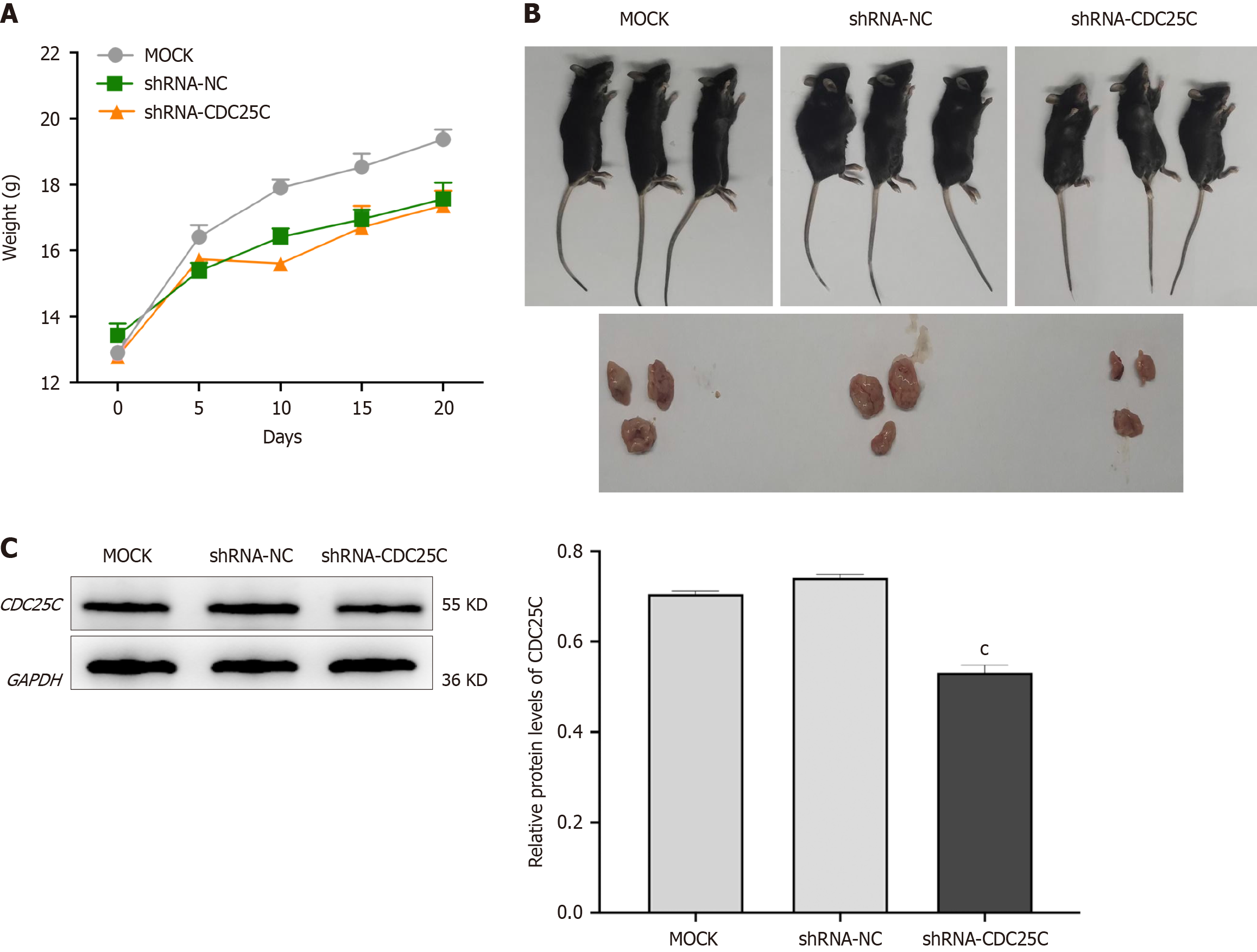
To further investigate the role of CDC25C knockdown in HCC development in vivo, we established a xenograft mouse model by subcutaneously injecting transduced Hepa1-6 cells into mice. The weights of the mice were recorded, and tumor growth curves were plotted every five days after xenografting. The body weight of mice in the shRNA-CDC25C group was significantly lower than that of mice in the MOCK group, but the difference in body weight between the mice in the shRNA-CDC25C group and those in the shRNA-NC group was not statistically significant (Figure 3A). Addi
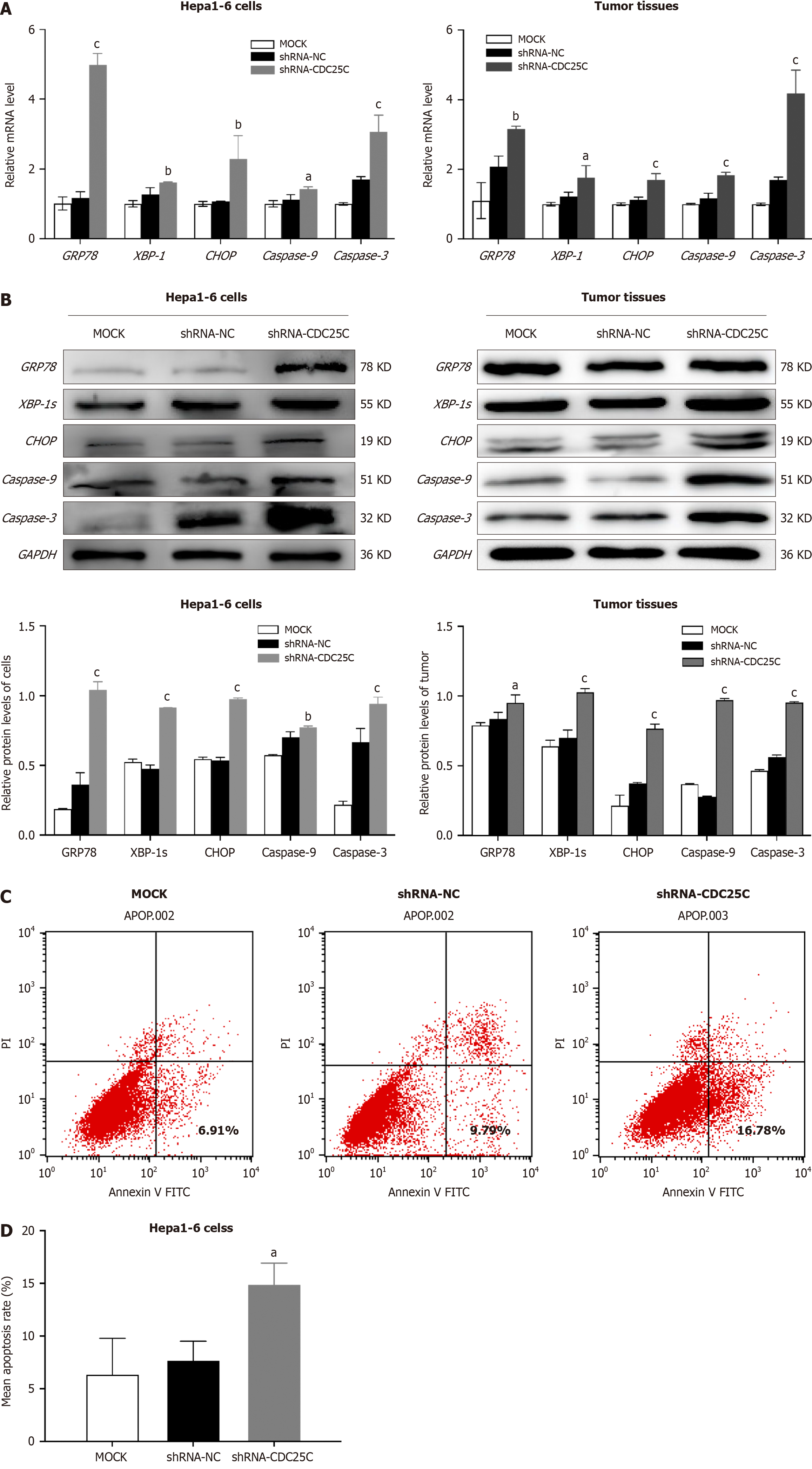
To further investigate the regulatory mechanism of CDC25C in HCC development, we examined the expression of ER stress-related proteins in CDC25C-knockdown cells and subcutaneous xenografts formed from these cells using qRT-PCR and western blotting. The results revealed increased levels of GRP78, XBP-1/XBP-1s (spliced form in the western blot analysis), and CHOP in the shRNA-CDC25C group (Figure 4A and B). Among these ER-related molecules, GRP78 exhibited the highest expression level, indicating its importance as an ER chaperone. Furthermore, the increase in GRP78 expression after CDC25C downregulation was greater in Hepa1-6 cells than in the corresponding xenograft tumor tissues. As CHOP plays a crucial role in ER stress-induced apoptosis, we conducted flow cytometric analysis of apoptosis. The flow cytometric analysis results demonstrated a significantly greater number of apoptotic cells in the shRNA-CDC25C group (Figure 4C). The mean apoptosis rates of the MOCK, shRNA-NC and shRNA-CDC25C groups were 6.423 ± 3.376, 7.763 ± 1.762, and 14.953 ± 1.980, respectively (P < 0.05) (Figure 4D). We also examined the expression of apoptotic proteins at the transcriptional and translational levels and found increased expression of Caspase 9 and Caspase 3 after CDC25C knockdown (Figure 4A and B). In conclusion, these data suggest that CDC25C knockdown induces ER stress and apoptosis in HCC cells.
One of the key characteristics of tumor cells is their abnormal cell cycle, which is often accompanied by abnormal expression of cell cycle-related proteins such as CDC25C. In tumor cells, this abnormal expression has been linked to metastatic potential, drug resistance, and the ability to evade cell death[3]. In this study, our focus was on investigating the role of CDC25C in the development of HCC. To this end, we established a CDC25C knockdown model in Hepa1-6 cells and used a similarly established B16 cell model as a positive control. Previous research has shown that the B16 cell model is suitable for studying the antitumor effects of inducing cell cycle arrest[14]. Our results demonstrated that CDC25C knockdown significantly inhibited the proliferation and migration of the experimental cells. Furthermore, we observed that CDC25C knockdown also inhibited tumor growth in mice. These findings suggest that CDC25C could be a promising therapeutic target for HCC.
GRP78, the most important ER chaperone, is a major inducer of ER stress[7]. In recent years, numerous researchers have focused on therapeutic targeting of GRP78, particularly its role in tumor development[15]. For instance, polyphyllin I was found to effectively suppress the proliferation, invasion, and metastasis of HCC cells by modulating GRP78 activity[16]. Furthermore, researchers discovered that a GRP78-targeted drug delivery system significantly suppressed HCC tumor growth and metastasis in mice[17]. Consistent with previous reports, our findings demonstrated increased GRP78 expression in both cells and tissues after CDC25C knockdown, leading to inhibited HCC growth in mice. XBP-1 is also commonly used as a marker for ER stress[8,18]. It has been observed that silymarin attenuates nonalcoholic fatty liver disease by regulating GRP78 and XBP-1 in mice[19]. In our study, we observed much greater expression of GRP78 than of XBP-1 in the CDC25C knockdown model. This finding aligns with existing literature suggesting that GRP78 has a greater impact on inducing ER stress than does XBP-1. However, after downregulation of CDC25C expression, the increase in GRP78 expression in Hepa1-6 cells was considerably greater than that in xenograft tumor tissues. We attribute this difference to the tumor microenvironment, which may lead to a decrease in GRP78 stoichiometry in xenograft tumor tissues. This finding is similar to that in another report indicating that UPR signaling was downregulated in murine models of prostate cancer[20].
Upon high-intensity or prolonged ER stress, homeostasis is not restored, and apoptosis is induced through ER-related molecules. ER stress-induced apoptosis is generally activated through three primary pathways: The IRE1-JNK pathway, the caspase-12 kinase pathway, and the CHOP pathway[9]. CHOP plays a critical role in ER stress-induced apoptosis. Recent studies have shown that kaempferol can protect hepatocytes from ER stress-induced apoptosis by downregulating CHOP expression[21]. This finding suggested that both the overexpression of CHOP and ER stress can induce tumor cell apoptosis and inhibit tumor development. Our results indicated that the expression of GRP78, XBP-1, and CHOP was significantly elevated in the CDC25C knockdown model. These findings suggest that CDC25C knockdown may induce ER stress and lead to apoptosis through the CHOP pathway.
In addition, we also assessed the apoptosis rate of Hepa1-6 cells using flow cytometry. After CDC25C knockdown, the number of apoptotic cells in the shRNA-CDC25C group was significantly increased. Additionally, both the mRNA and protein levels of Caspase 9 and Caspase 3 were significantly elevated. These results align with a previous report suggesting that downregulating CDC25C expression can induce ER stress and mitochondrial damage, ultimately leading to auto
In conclusion, our data indicate that the activation of the ER stress response is involved in the apoptosis of hepatoma cells. The regulatory mechanism of CDC25C in HCC development might be associated with the activation of ER stress and the ER stress-induced apoptosis signaling pathway. Our findings are anticipated to provide a reference for further molecular studies on the regulatory mechanism of CDC25C in HCC development.
However, we verified the anti-hepatoma effect of CDC25C knockdown only in mouse HCC cells and xenograft tumors formed from these cells. Despite the high sequence homology between the mouse and human genomes, it remains unclear whether the effect of downregulating CDC25C expression in HCC differs due to species variation. Additionally, the ER stress-induced apoptosis signaling pathway is just one of the mechanisms underlying the role of CDC25C in HCC development. In the future, we plan to further investigate the effect of CDC25C on human HCC cells and its potential correlation with ER stress and to explore other molecular mechanisms involved.
The regulatory mechanism of CDC25C in HCC development may involve the activation of ER stress and the ER stress-induced apoptosis signaling pathway. This finding suggests that targeting CDC25C and the associated ER stress response may be a potential strategy for cancer treatment, particularly in the context of HCC.
We would like to thank Central Laboratory, School of Basic Medical Sciences for technical assistance.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Chinese Society for Anatomical Sciences, No. M074450020M.
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Thandassery RB, United States S-Editor: Qu XL L-Editor: A P-Editor: Zheng XM
| 1. | Ji B, Cai H, Yang Y, Peng F, Song M, Sun K, Yan F, Liu Y. Hybrid membrane camouflaged copper sulfide nanoparticles for photothermal-chemotherapy of hepatocellular carcinoma. Acta Biomater. 2020;111:363-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 2. | De Mattia E, Cecchin E, Guardascione M, Foltran L, Di Raimo T, Angelini F, D'Andrea M, Toffoli G. Pharmacogenetics of the systemic treatment in advanced hepatocellular carcinoma. World J Gastroenterol. 2019;25:3870-3896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 3. | Liu K, Zheng M, Lu R, Du J, Zhao Q, Li Z, Li Y, Zhang S. The role of CDC25C in cell cycle regulation and clinical cancer therapy: a systematic review. Cancer Cell Int. 2020;20:213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 183] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 4. | Li CM, Li YF, Tian L, Zhang QH, Zheng FY, Mo FR. Anti-hepatoma Effect of DC2.4 Cells Transfected with Tumor-Associated Antigen Cdc25C In Vitro. Curr Med Sci. 2022;42:491-497. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Kim C, Kim B. Anti-Cancer Natural Products and Their Bioactive Compounds Inducing ER Stress-Mediated Apoptosis: A Review. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 334] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 6. | Wires ES, Trychta KA, Kennedy LM, Harvey BK. The Function of KDEL Receptors as UPR Genes in Disease. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Dudek J, Benedix J, Cappel S, Greiner M, Jalal C, Müller L, Zimmermann R. Functions and pathologies of BiP and its interaction partners. Cell Mol Life Sci. 2009;66:1556-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 147] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 8. | van Schadewijk A, van't Wout EF, Stolk J, Hiemstra PS. A quantitative method for detection of spliced X-box binding protein-1 (XBP1) mRNA as a measure of endoplasmic reticulum (ER) stress. Cell Stress Chaperones. 2012;17:275-279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 9. | Hu H, Tian M, Ding C, Yu S. The C/EBP Homologous Protein (CHOP) Transcription Factor Functions in Endoplasmic Reticulum Stress-Induced Apoptosis and Microbial Infection. Front Immunol. 2018;9:3083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 330] [Cited by in RCA: 792] [Article Influence: 132.0] [Reference Citation Analysis (0)] |
| 10. | Nagesh PKB, Hatami E, Chowdhury P, Kashyap VK, Khan S, Hafeez BB, Chauhan SC, Jaggi M, Yallapu MM. Tannic Acid Induces Endoplasmic Reticulum Stress-Mediated Apoptosis in Prostate Cancer. Cancers (Basel). 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Fang C, Weng T, Hu S, Yuan Z, Xiong H, Huang B, Cai Y, Li L, Fu X. IFN-γ-induced ER stress impairs autophagy and triggers apoptosis in lung cancer cells. Oncoimmunology. 2021;10:1962591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 12. | Wang L, Hu T, Shen Z, Zheng Y, Geng Q, Li L, Sha B, Li M, Sun Y, Guo Y, Xue W, Xuan D, Chen P, Zhao J. Inhibition of USP1 activates ER stress through Ubi-protein aggregation to induce autophagy and apoptosis in HCC. Cell Death Dis. 2022;13:951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 13. | Hsiao YP, Tsai CH, Wu PP, Hsu SC, Liu HC, Huang YP, Yang JH, Chung JG. Cantharidin induces G2/M phase arrest by inhibition of Cdc25c and Cyclin A and triggers apoptosis through reactive oxygen species and the mitochondria‑dependent pathways of A375.S2 human melanoma cells. Int J Oncol. 2014;45:2393-2402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Pan Z, Zhang X, Yu P, Chen X, Lu P, Li M, Liu X, Li Z, Wei F, Wang K, Zheng Q, Li D. Cinobufagin Induces Cell Cycle Arrest at the G2/M Phase and Promotes Apoptosis in Malignant Melanoma Cells. Front Oncol. 2019;9:853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 15. | Lu G, Luo H, Zhu X. Targeting the GRP78 Pathway for Cancer Therapy. Front Med (Lausanne). 2020;7:351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 16. | Du H, Wu H, Kang Q, Liao M, Qin M, Chen N, Huang H, Huang D, Wang P, Tong G. Polyphyllin I attenuates the invasion and metastasis via downregulating GRP78 in drug-resistant hepatocellular carcinoma cells. Aging (Albany NY). 2023;15:12251-12263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 17. | Jiang B, Zhang R, Zhang J, Hou Y, Chen X, Zhou M, Tian X, Hao C, Fan K, Yan X. GRP78-targeted ferritin nanocaged ultra-high dose of doxorubicin for hepatocellular carcinoma therapy. Theranostics. 2019;9:2167-2182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 18. | Barua D, Gupta A, Gupta S. Targeting the IRE1-XBP1 axis to overcome endocrine resistance in breast cancer: Opportunities and challenges. Cancer Lett. 2020;486:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 19. | Sahin E, Bagci R, Bektur Aykanat NE, Kacar S, Sahinturk V. Silymarin attenuated nonalcoholic fatty liver disease through the regulation of endoplasmic reticulum stress proteins GRP78 and XBP-1 in mice. J Food Biochem. 2020;44:e13194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 20. | Ramirez MU, Hernandez SR, Soto-Pantoja DR, Cook KL. Endoplasmic Reticulum Stress Pathway, the Unfolded Protein Response, Modulates Immune Function in the Tumor Microenvironment to Impact Tumor Progression and Therapeutic Response. Int J Mol Sci. 2019;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 21. | Wang H, Chen L, Zhang X, Xu L, Xie B, Shi H, Duan Z, Zhang H, Ren F. Kaempferol protects mice from d-GalN/LPS-induced acute liver failure by regulating the ER stress-Grp78-CHOP signaling pathway. Biomed Pharmacother. 2019;111:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 22. | Chan ML, Liang JW, Hsu LC, Chang WL, Lee SS, Guh JH. Zerumbone, a ginger sesquiterpene, induces apoptosis and autophagy in human hormone-refractory prostate cancers through tubulin binding and crosstalk between endoplasmic reticulum stress and mitochondrial insult. Naunyn Schmiedebergs Arch Pharmacol. 2015;388:1223-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |