Published online May 21, 2024. doi: 10.3748/wjg.v30.i19.2523
Revised: February 20, 2024
Accepted: April 11, 2024
Published online: May 21, 2024
Processing time: 174 Days and 20.2 Hours
Autoimmune enteropathy (AIE) is a rare disease whose diagnosis and long-term prognosis remain challenging, especially for adult AIE patients.
To improve overall understanding of this disease’s diagnosis and prognosis.
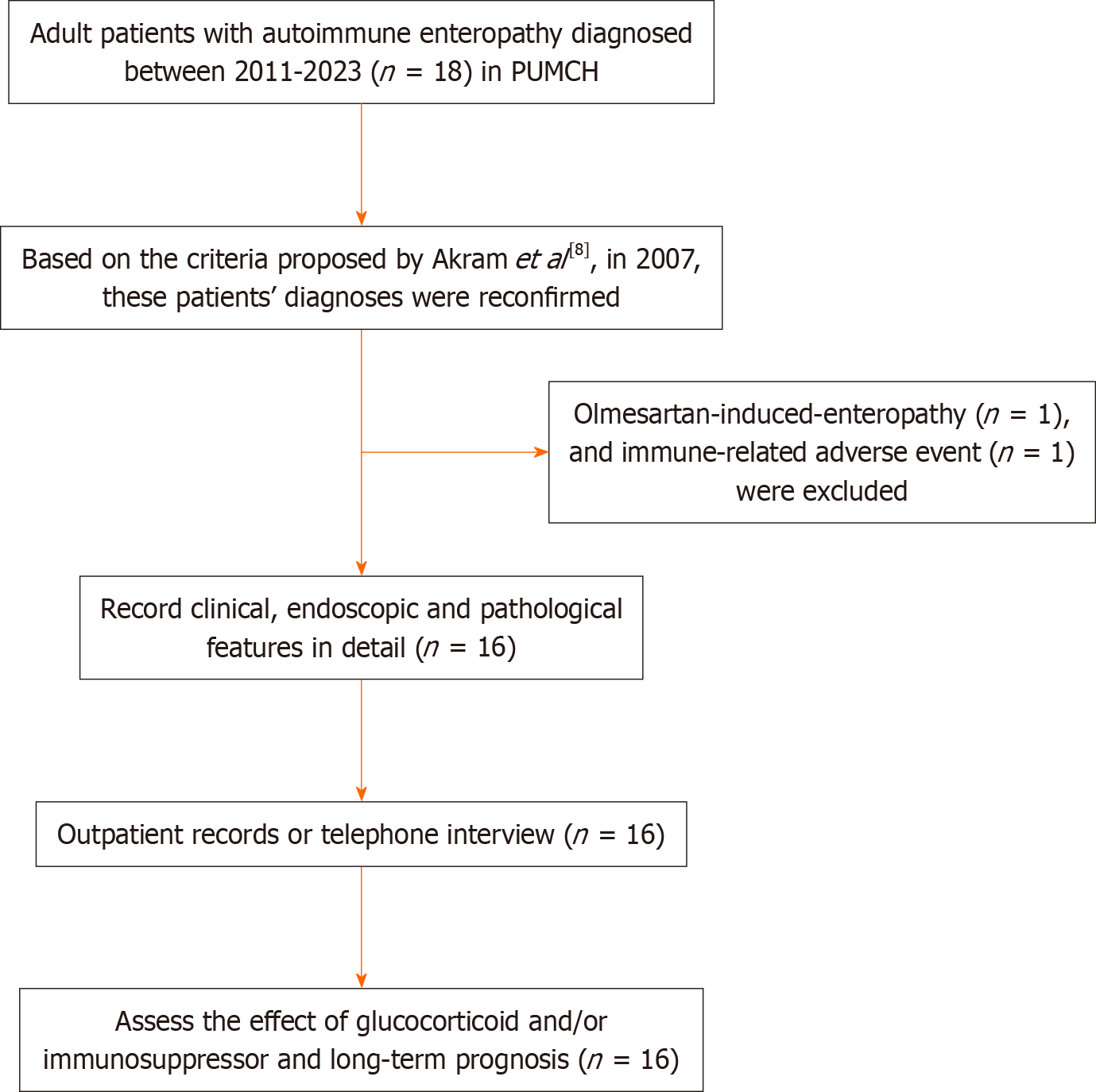
We retrospectively analyzed the clinical, endoscopic and histopathological characteristics and prognoses of 16 adult AIE patients in our tertiary medical center between 2011 and 2023, whose diagnosis was based on the 2007 diagnostic criteria.
Diarrhea in AIE patients was characterized by secretory diarrhea. The common endoscopic manifestations were edema, villous blunting and mucosal hyperemia in the duodenum and ileum. Villous blunting (100%), deep crypt lymphocytic infiltration (67%), apoptotic bodies (50%), and mild intraepithelial lymphocytosis (69%) were observed in the duodenal biopsies. Moreover, there were other remarkable abnormalities, including reduced or absent goblet cells (duodenum 94%, ileum 62%), reduced or absent Paneth cells (duodenum 94%, ileum 69%) and neutrophil infiltration (duodenum 100%, ileum 69%). Our patients also fulfilled the 2018 diagnostic criteria but did not match the 2022 diagnostic criteria due to undetectable anti-enterocyte antibodies. All patients received glucocorticoid therapy as the initial medication, of which 14/16 patients achieved a clinical response in 5 (IQR: 3-20) days. Immunosuppressants were administered to 9 patients with indications of steroid dependence (6/9), steroid refractory status (2/9), or intensified maintenance medication (1/9). During the median of 20.5 months of follow-up, 2 patients died from multiple organ failure, and 1 was diagnosed with non-Hodgkin’s lymphoma. The cumulative relapse-free survival rates were 62.5%, 55.6% and 37.0% at 6 months, 12 months and 48 months, respectively.
Certain histopathological findings, including a decrease or disappearance of goblet and Paneth cells in intestinal biopsies, might be potential diagnostic criteria for adult AIE. The long-term prognosis is still unsatisfactory despite corticosteroid and immunosuppressant medications, which highlights the need for early diagnosis and novel medications.
Core Tip: Autoimmune enteropathy (AIE) is a rare disease with heterogeneous manifestations and continually evolving diagnostic criteria. In adults, the presence of secretory diarrhea alongside specific histopathological findings can indicate the possibility of AIE. Histopathological features such as decreased goblet and Paneth cells might serve as potential diagnostic criteria. Despite treatment involving corticosteroids and immunosuppressants, long-term prognosis remains unsatisfactory, underscoring the pressing need for early diagnosis and innovative therapeutic approaches.
- Citation: Li MH, Ruan GC, Zhou WX, Li XQ, Zhang SY, Chen Y, Bai XY, Yang H, Zhang YJ, Zhao PY, Li J, Li JN. Clinical manifestations, diagnosis and long-term prognosis of adult autoimmune enteropathy: Experience from Peking Union Medical College Hospital. World J Gastroenterol 2024; 30(19): 2523-2537
- URL: https://www.wjgnet.com/1007-9327/full/v30/i19/2523.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i19.2523
Autoimmune enteropathy (AIE) was first reported in a child in 1982[1] and was first described in two adult patients in 1997[2]. As a very rare intestinal disease, AIE occurs more often in infants and young children than in adults, with typical clinical manifestations such as chronic protracted watery diarrhea together with severe malabsorption, weight loss, and no response to any exclusion diet, e.g., a gluten-free diet. Although the incidence of adult-onset AIE is very low, refractory severe diarrhea and secondary conditions not only strongly affect the quality of life of patients but also cause tremendous stress to patients’ families. The current knowledge and understanding of adult-onset AIE are very poor. For example, it was reported that AIE was partially related to anti-enterocyte (AE) and anti-goblet cell (AG) antibodies, but the exact role of these antibodies in the immunologic mechanism of AIE is still unclear[3-5]. In addition, there is still a lack of gold-standard diagnostic criteria, although several criteria have been reported and updated during the past two decades[6-9]. The widely adopted diagnostic criteria were proposed in 2007 based on a summary of 15 adult AIE patients[8], which proposed diagnosis via characteristic histopathological changes in the small intestinal mucosa, including villous blunting, lymphocytosis and apoptotic bodies. However, the differential diagnosis of AIE from other diseases involving villous atrophy, e.g., celiac disease (CD) and common variable immune deficiency, remains challenging and can lead to misdiagnosis and missed diagnosis. Moreover, the medications used were not standardized, and knowledge of the long-term prognosis was limited. To improve the early diagnosis and appropriate treatment of this rare disease, this study retro
This study was approved by the Institutional Review Board of the Peking Union Medical College Hospital (PUMCH) (No. I-23PJ406).
The medical records of patients diagnosed with AIE between January 2011 and April 2023 at our medical center were retrieved through the Hospital Information System. In addition to the basic demographic characteristics, the diarrhea characteristics of the patients were collected in detail, including diarrhea frequency, stool volume, and fasting test results. A decrease of stool volume to less than 500 mL per day in the fasting test indicates positive results and is characteristic of osmotic diarrhea. Otherwise, the results were negative, indicating the presence of secretory diarrhea. Other clinical features, including symptoms other than diarrhea, complications, inflammatory indicators, immunological indicators, and endocrine gland function, were also collected in detail.
Endoscopic images of AIE patients were obtained from the endoscopic database of PUMCH. A professional endo
The histopathological features of all the biopsies, including the degree of villous blunting, the decrease in goblet cells and Paneth cells, the infiltration of lymphocytes and plasma cells, the level of neutrophil infiltration, and the existence of apoptotic bodies, were critically reviewed and collected by two pathologists (Zhang YJ and Zhou WX). Following the criteria proposed by Masia et al[10], duodenal biopsies were classified histologically into four main categories: (1) ACD, active chronic duodenitis; (2) celiac-like, celiac disease-like with increased intraepithelial lymphocytes (IELs); (3) GvHD-like, graft-versus-host disease-like with apoptotic bodies; and (4) mixed pattern.
The diagnostic criteria for adult AIE we adopted were proposed by Akram et al[8] in 2007. These patients’ diagnoses were confirmed by two experienced gastroenterologists (Li J and Ruan GC) based on careful review of retrieved medical re
| Unsworth and Walker-Smith[9] proposed in 1985 | Akram et al[8], proposed in 2007 | Sharma et al[7], proposed in 2018 | Schiepatti et al[6], proposed in 2022 |
| (1) Protracted diarrhea and severe enteropathy; (2) No response to exclusion diet or total parenteral nutrition; (3) Evidence of predisposition to autoimmune disease (presence of circulating autoantibodies and/or associated disease also thought to be autoimmune); and (4) No severe immunodeficiency | (1) Adult-onset chronic diarrhea (> 6 wk’ duration); (2) Malabsorption; (3) Specific small bowel histology, partial/complete villous blunting, deep crypt lymphocytosis, increased crypt apoptotic bodies, minimal intraepithelial lymphocytosis (IEL means > 40 per 100 epithelial cells); (4) Exclusion of other causes of villous atrophy including celiac disease, refractory sprue, and intestinal lymphoma; and (5) Anti-enterocyte (AE) and/or anti–goblet cell (AG) antibodies. Criteria 1-4 are required for a definite diagnosis of AIE. Presence of AE and/or AG antibodies is an important diagnostic support, but their absence does not exclude the diagnosis of AIE | (1) Adult-onset protracted diarrhea (> 6 wk) not responsive to any dietary exclusion; (2) Associated with specific small-bowel histology, villous atrophy, minimal IEL, increased crypt apoptotic bodies, absence of goblet or Paneth cells; and (3) Systematic exclusion of other causes of villous atrophy including CD or RCD (based on combination of celiac serology, HLA typing, histopathology features, and response to GFD), drug-induced enteropathy (based on history of starting medication and its correlation with symptoms, duration of treatment, histopathology and effect after discontinuation), common variable immunodeficiency-associated enteropathy (based on total immunoglobulin titers and histopathology [presence of plasma cells, lymphoid aggregates]), collagenous sprue and colitis (based on histopathology), and intestinal lymphoma | The following criteria must be satisfied for the diagnosis: (1) Severe malabsorption symptoms (chronic diarrhea, weight loss, nutritional deficiencies and electrolyte imbalance) unresponsive to any dietary restriction; (2) Frank villous atrophy unresponsive to any dietary restriction; (3) IgA/IgG positive enterocyte antibodies (indirect immunofluorescence on human/monkey jejunum); (4) Negative celiac serology; and (5) Exclusion of other causes of villous atrophy |
| The following criteria were considered supportive for the diagnosis: (1) History of associated autoimmune conditions; (2) Clinical response to immunosuppressive treatments; (3) Deep crypt lymphocytosis and/or plasma cells infiltration, neutrophilic cryptitis ± crypt microabscesses and lack/decrease of Paneth cells on duodenal histology; (4) Positive serum anti-AIE 75 KD antibodies (ELISA) or nonorgan specific autoantibodies; and (5) Absence of severe immunodeficiencies, diagnostic role of serum antigoblet cells antibodies, involvement of other sites of the GI tract and some duodenal histopathological features (include intraepithelial lymphocytes count, crypt hyperplasia and crypt apoptotic bodies, lack of gamma-delta T cells and depletion of goblet cells) |
The duration of follow-up was defined as the interval between the first diagnosis of AIE and the last follow-up. The endpoints of follow-up were set as the time of the final interview, the time of death, and the time of diagnosis of gastro
The effects of treatment and follow-up information were collected through offline or online outpatient records or telephone interviews. Clinical remission was defined as normal stool and no complication, such as Wernicke encephalopathy and acute kidney injury. A clinical response was defined as diarrhea with a more than 50% decrease in the frequency and volume of stool compared with premedication and improvement of complications. No response was defined as no improvement in diarrhea with an increase or less than a 50% decrease in the frequency and volume of stool and no improvement or worsening of complications. Steroid refractory status was defined as no response to intravenous adequate glucocorticoids [equivalent to prednisone 0.75-1 mg/(kg × d)] within 2 wk. Patients who experienced diarrhea relapse during glucocorticoid tapering or within 3 months after glucocorticoid cessation were then diagnosed as steroid dependent during the follow-up.
Based on endoscopic disease status, the cases were categorized into endoscopic remission and endoscopic activity groups. Endoscopic remission was defined as the absence of abnormalities macroscopically in the duodenum or terminal ileum. All other cases were categorized as endoscopic activity, especially with observable villous blunting.
Descriptive methods were mainly employed for statistical analysis. We presented continuous variables as the median (IQR: 25th percentile point-75th percentile point) with IQR means interquartile range, due to the small sample size. Categorical variables were represented as percentages of the corresponding research population. The difference between the two groups of measurement data conforming to a normal distribution was examined by Student’s t test, and the difference between the two groups of measurement data not conforming to a normal distribution by the Wilcoxon rank sum test. A P < 0.05 was considered to indicate statistical significance in the SPSS 26.0 analysis. Kaplan-Meier survival curves of diarrhea relapse and survival curves at the endpoint of follow-up in our AIE patients were created with GraphPad Prism (version 9.5.0). The log-rank test was subsequently used to identify prognostic factors that might be related to diarrhea relapse.
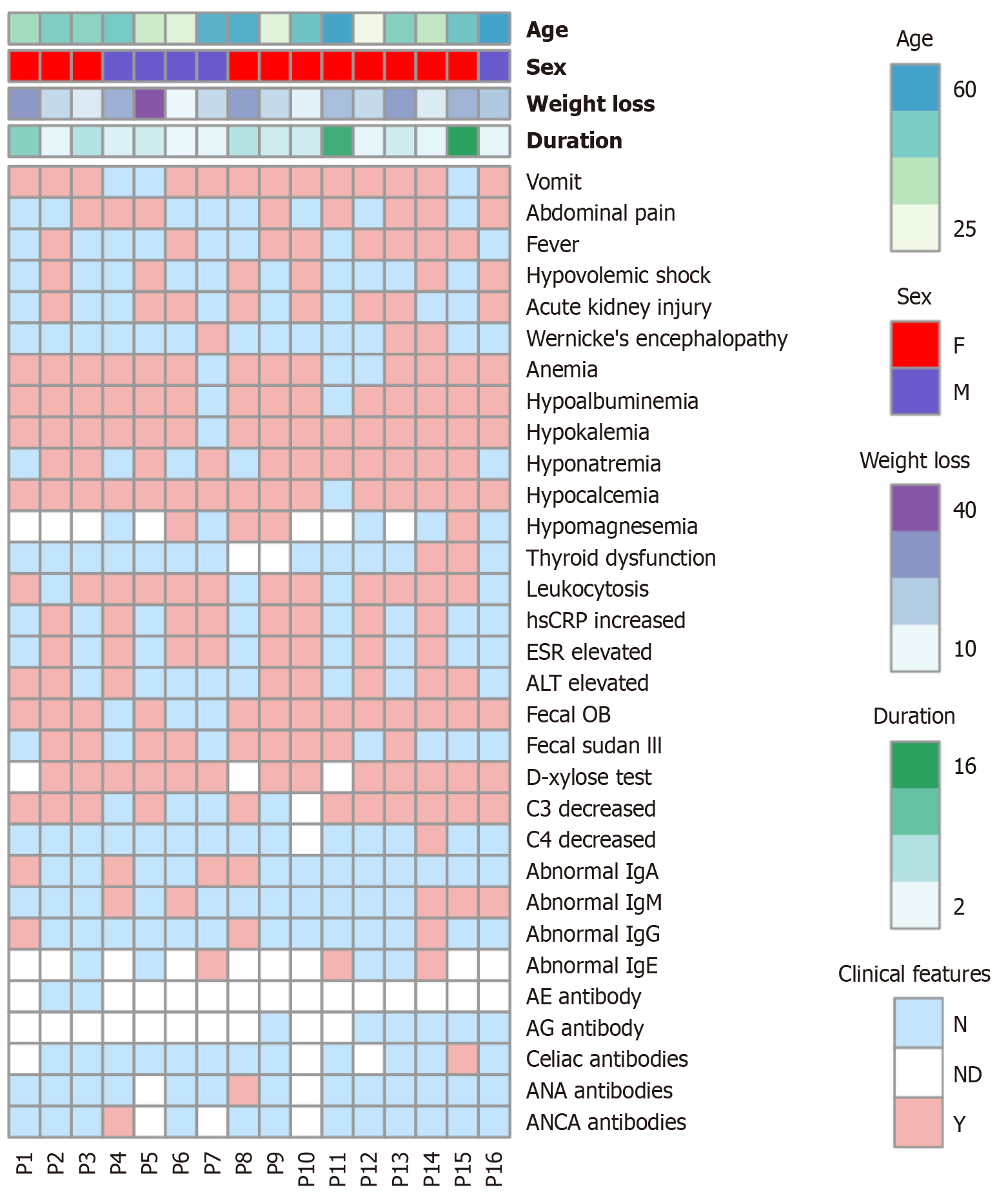
This study included 16 AIE patients (5 males, 11 females) with a median onset age of 45.5 (IQR: 30.8-53.5) years. The median duration of diarrhea before diagnosis was 4.0 (IQR: 2.0-6.0) months. Chronic diarrhea in AIE patients was accompanied by substantial weight loss (17.0 (IQR: 11.3-29.0) kg), and the median BMI was 17.9 (IQR: 16.4-19.1) kg/m2 (Table 2). All patients experienced watery diarrhea with stool volumes ranging from 500 to 5000 mL per day without bloody or purulent stool but with undigested food residue in 25% (4/16) of the patients. Fasting tests were carried out by 81% (13/16) of the patients, resulting in 23% (3/13) positive results and 77% (10/13) negative results. Additionally, 25% (4/16) of patients attempted a gluten-free diet, with 3/4 reporting no response and 1/4 reporting partial response at first and loss of response after several days (Table 3).
| Onset age (yr) | 45.5 (IQR: 30.8-53.5) | |
| Diarrhea duration (months) | 4.0 (IQR: 2.0-6.0) | |
| Weight loss (kg) | 17.0 (IQR: 11.3-29.0) | |
| BMI (kg/m2) | 17.9 (IQR: 16.4-19.1) | |
| Vomit | 81% (13/16) | |
| Abdominal pain | 50% (8/16) | |
| Fever | 50% (8/16) | |
| Hypovolemic shock | 38% (6/16) | |
| Wernicke encephalopathy | 19% (3/16) | |
| Acute kidney injury | 50% (8/16) | |
| Anemia | Anemia | 81% (13/16) |
| Mild | 44% (7/16) | |
| Moderate | 31% (5/16) | |
| Severe | 6% (1/16) | |
| Hypoalbuminemia | 88% (14/16) | |
| Electrolyte disturbance | Hypokalemia | 94% (15/16) |
| Hyponatremia | 69% (11/16) | |
| Hypocalcemia | 94% (15/16) | |
| Hypomagnesemia | 44% (4/9) | |
| Endocrine disorders | Thyroid dysfunction | 14% (2/14) |
| Abnormal blood glucose | 0% (0/14) | |
| Inflammatory indicators | Elevated WBC | 75% (12/16) |
| Elevated hsCRP | 50% (8/16) | |
| Elevated ESR | 50% (8/16) | |
| Liver function | Elevated ALT | 50% (8/16) |
| Fecal OB test | Positive | 81% (13/16) |
| Negative | 19% (3/16) | |
| Fecal Sultan III staining | Positive | 56% (9/16) |
| Negative | 44% (7/16) | |
| D-xylose absorption test | Positive | 100% (13/13) |
| Complement | Decreased C3 | 73% (11/15) |
| Decreased C4 | 7% (1/15) | |
| IgA | Elevated | 25% (4/16) |
| Normal | 75% (12/16) | |
| IgM | Elevated | 6% (1/16) |
| Decreased | 25% (4/16) | |
| Normal | 69% (11/16) | |
| IgG | Elevated | 19% (3/16) |
| Normal | 81% (13/16) | |
| IgE | Elevated | 43% (3/7) |
| Normal | 57% (4/7) | |
| AE antibody | 0% (0/2) | |
| AG antibody | 0% (0/6) | |
| Other antibodies | Celiac | 8% (1/13) |
| ANA | 7% (1/14) | |
| ANCA | 8% (1/13) | |
| Patients | Gender | Age | Stool frequency (/d) | Stool volume (mL/d) | Character of stool | Fasting test | Gluten-free diet |
| P1 | F | 39 | 10+ | 3000-5000 | Watery, with undigested food residue | Positive (100-300 mL) | Partial response |
| P2 | F | 46 | 8-10 | 1000 | Watery, with undigested food residue | Negative | Untested |
| P3 | F | 44 | 2-10 | 500-1000 | Watery | Negative | Untested |
| P4 | M | 48 | 8-10 | 500-3000 | Watery | Positive (50 mL) | Untested |
| P5 | M | 30 | 10+ | 2000-4000 | Watery | Positive (300-400 mL) | Untested |
| P6 | M | 26 | 10-20 | 1000+ | Watery | Negative | Untested |
| P7 | M | 55 | 10+ | 2000-5000 | Watery | Negative | Untested |
| P8 | F | 56 | 4-8 | 1000 | Watery, with undigested food residue | Untested | Untested |
| P9 | F | 26 | 7-20 | 4000-5000 | Watery | Negative | Untested |
| P10 | F | 50 | 10+ | 2000-3000 | Watery | Untested | Untested |
| P11 | F | 59 | 7-8 | 4000-5000 | Watery | Untested | Untested |
| P12 | F | 22 | 10-20 | 1000-2000 | Watery | Negative | No response |
| P13 | F | 45 | 10-20 | 3000-4000 | Watery | Negative | Untested |
| P14 | F | 33 | 10-20 | 1500 | Watery | Negative | Untested |
| P15 | F | 49 | 10+ | 1000-1700 | Watery, with undigested food residue | Negative | No response |
| P16 | M | 60 | 10+ | 3000-5000 | Watery | Negative | No response |
Other prevalent symptoms include vomiting (81%), abdominal pain (50%) and fever (50%). Various complications can result from AIE, including hypovolemic shock (38%), Wernicke encephalopathy (19%), acute kidney injury (50%), hypoalbuminemia (88%) and anemia (81%) (Figure 2). Notably, all patients suffered from electrolyte disturbance (94% hypokalemia, 69% hyponatremia, 94% hypocalcemia, and 44% hypomagnesemia) (Figure 2). In addition, one patient developed hypothyroidism after thyroidectomy, and another had subclinical hypothyroidism; however, all patients measured had normal blood glucose levels. No one showed skin symptoms such as dermatitis. In addition to elevated inflammatory indices, such as WBC (75%), hsCRP (50%) and ESR (50%), half of the patients also exhibited impaired liver function in the form of elevated ALT (Figure 2). The fecal occult blood (OB) test was positive in 81% of patients, and fecal Sultan III staining was positive in 56%. D-xylose absorption tests revealed impaired intestinal absorption in all 13 tested patients. A total of 73% exhibited a reduced C3, while only 7% displayed a reduced C4. The levels of immunoglobulin were mostly normal or elevated. All the patients tested for characteristic AEs and AG antibodies were negative. One patient with weakly positive deamidated gliadin protein IgA was positive for celiac antibody. In addition, ANA and ANCA antibody profiles were positive for 1 patient, respectively (Figure 2, Table 2).
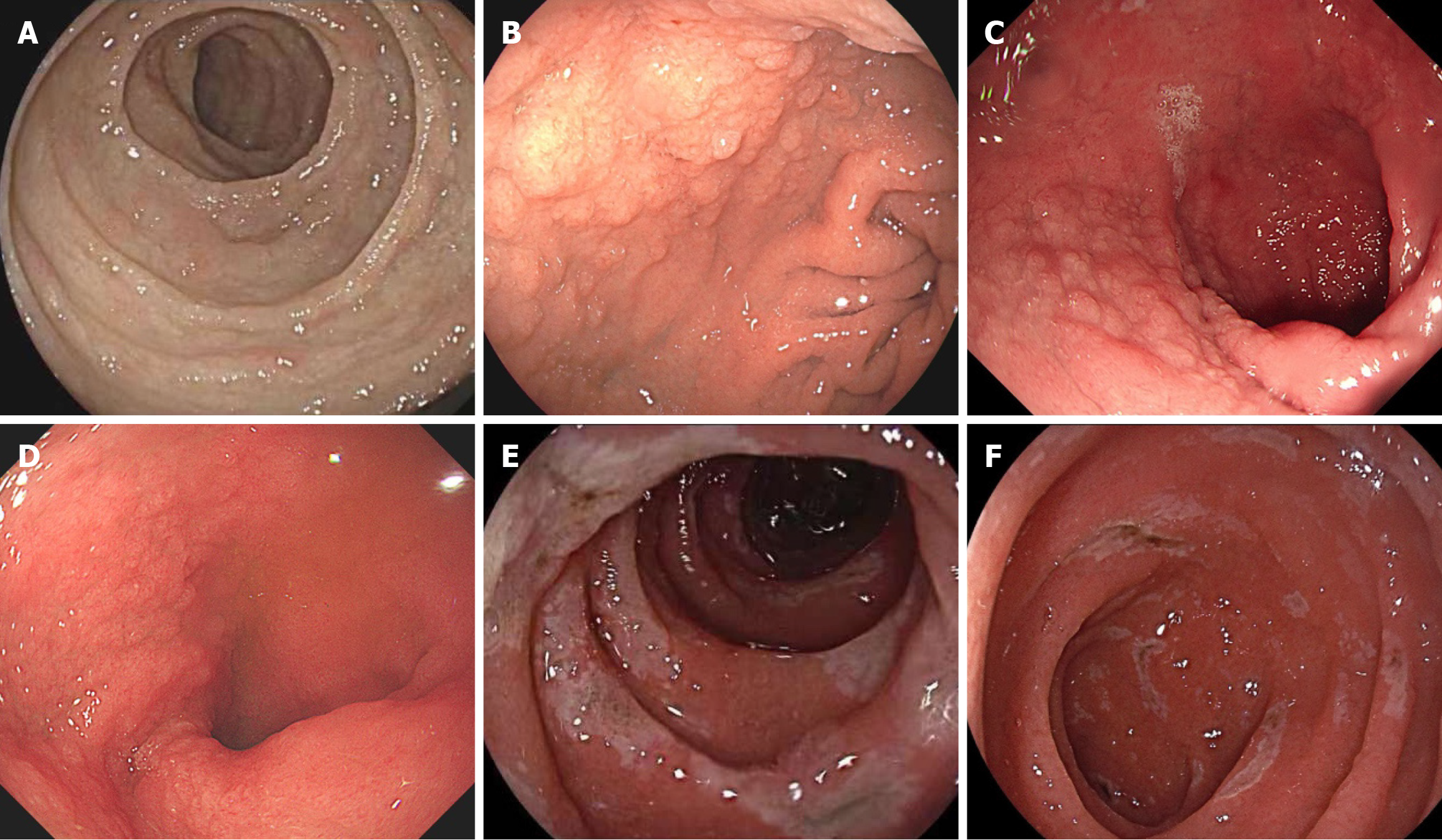
The macroscopic findings of index gastroscopy showed diffuse involvement of the duodenum in 94% of patients, in
| Duodenum (under gastroscopy) | Edema | 94% (15/16) |
| Villous blunting | 94% (15/16) | |
| Hyperemia | 88% (14/16) | |
| Nodular changes | 63% (10/16) | |
| Erosion | 56% (9/16) | |
| Scalloping of plicae | 50% (8/16) | |
| Mosaic mucosa | 31% (5/16) | |
| Ulcer | 13% (2/16) | |
| Small intestine (under enteroscopy) | Edema | 100% (3/3) |
| Villous blunting | 100% (3/3) | |
| Nodular changes | 67% (2/3) | |
| Erosion | 0% (0/3) | |
| Ulcer | 0% (0/3) | |
| Ileum (under colonoscopy) | Villous blunting | 79% (11/14) |
| Edema | 71% (10/14) | |
| Hyperemia | 29% (4/14) | |
| Erosion | 20% (3/14) | |
| Nodular changes | 14% (2/14) | |
| Ulcer | 7% (1/14) | |
| Stomach (under gastroscopy) | Ulcer | 6% (1/16) |
| Colon (under colonoscopy) | Hyperemia | 36% (5/14) |
| Erosion | 29% (4/14) | |
| Edema | 29% (4/14) | |
| Ulcer | 14% (2/14) | |
| Rectum (under colonoscopy) | Erosion | 14% (2/14) |
| Edema | 14% (2/14) | |
| Hyperemia | 14% (2/14) | |
| Ulcer | 7% (1/14) |
Other segments of the digestive tract were also noted to be affected in AIE patients. Only 1 of these AIE patients had gastric involvement, manifested as antral ulcers. The main manifestations of colon involvement were hyperemia (36%), erosion (29%), edema (29%), and ulcers (14%). The rectum was less involved than the colon, with only 2 patients showing erosion, edema, and hyperemia (Figure 3, Table 4).
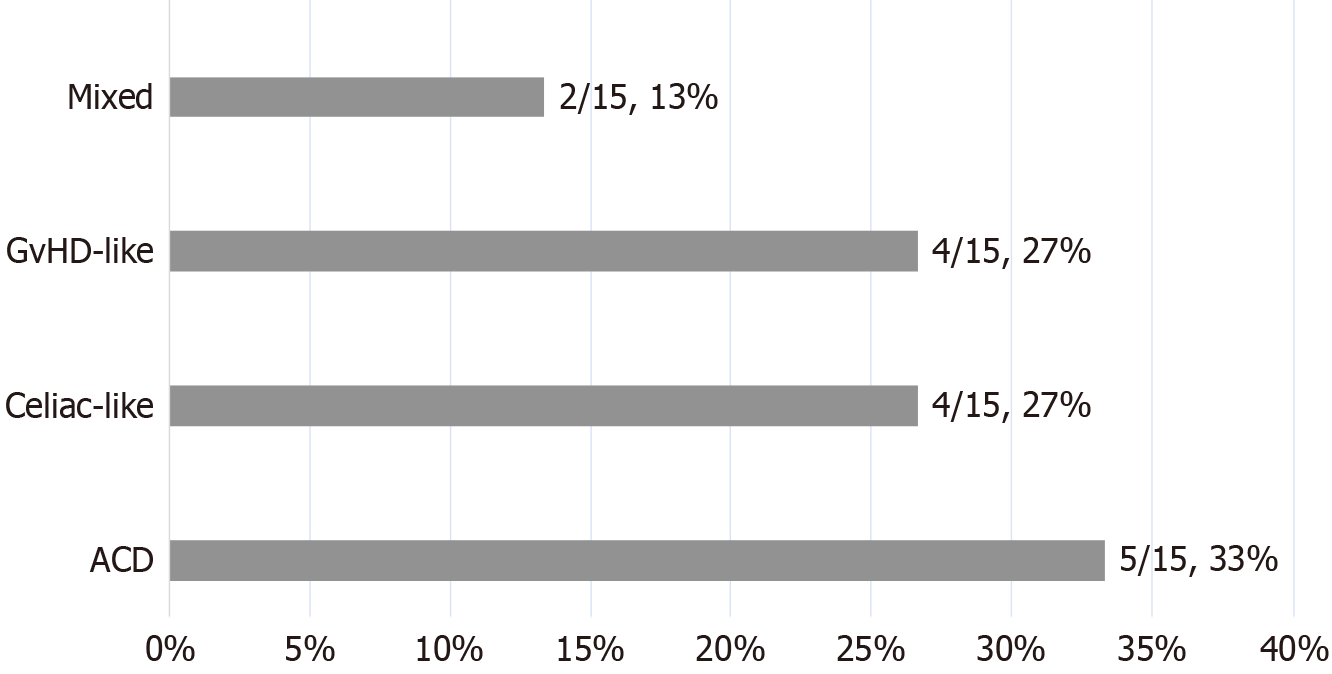
After excluding 1 patient diagnosed in 2011 with unavailable pathological results due to time, the prevailing histopathological classification in the duodenum of AIE patients was ACD (33%), followed by celiac-like (27%) and GvHD-like (27%), with mixed being the least common (13%) (Figure 4).
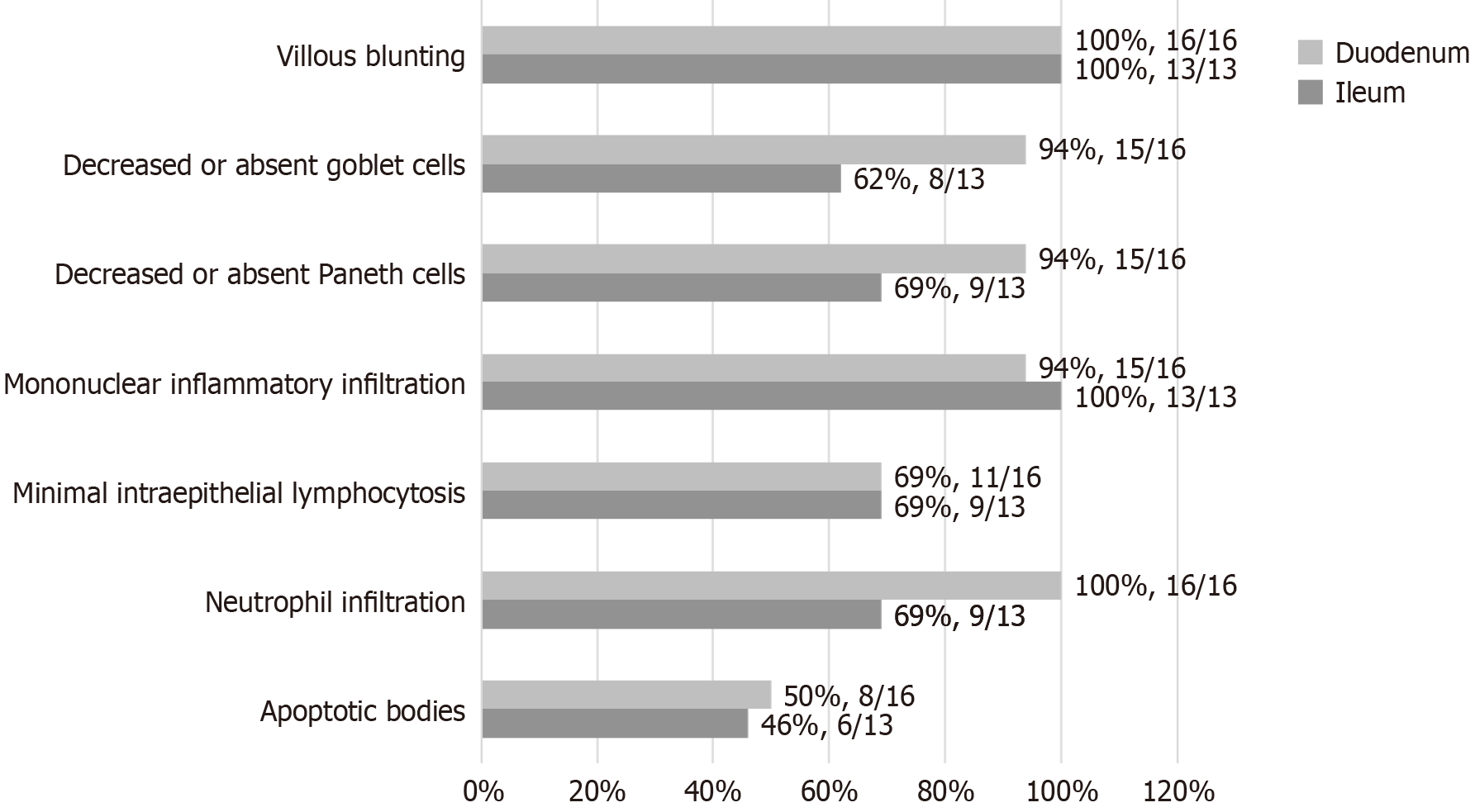
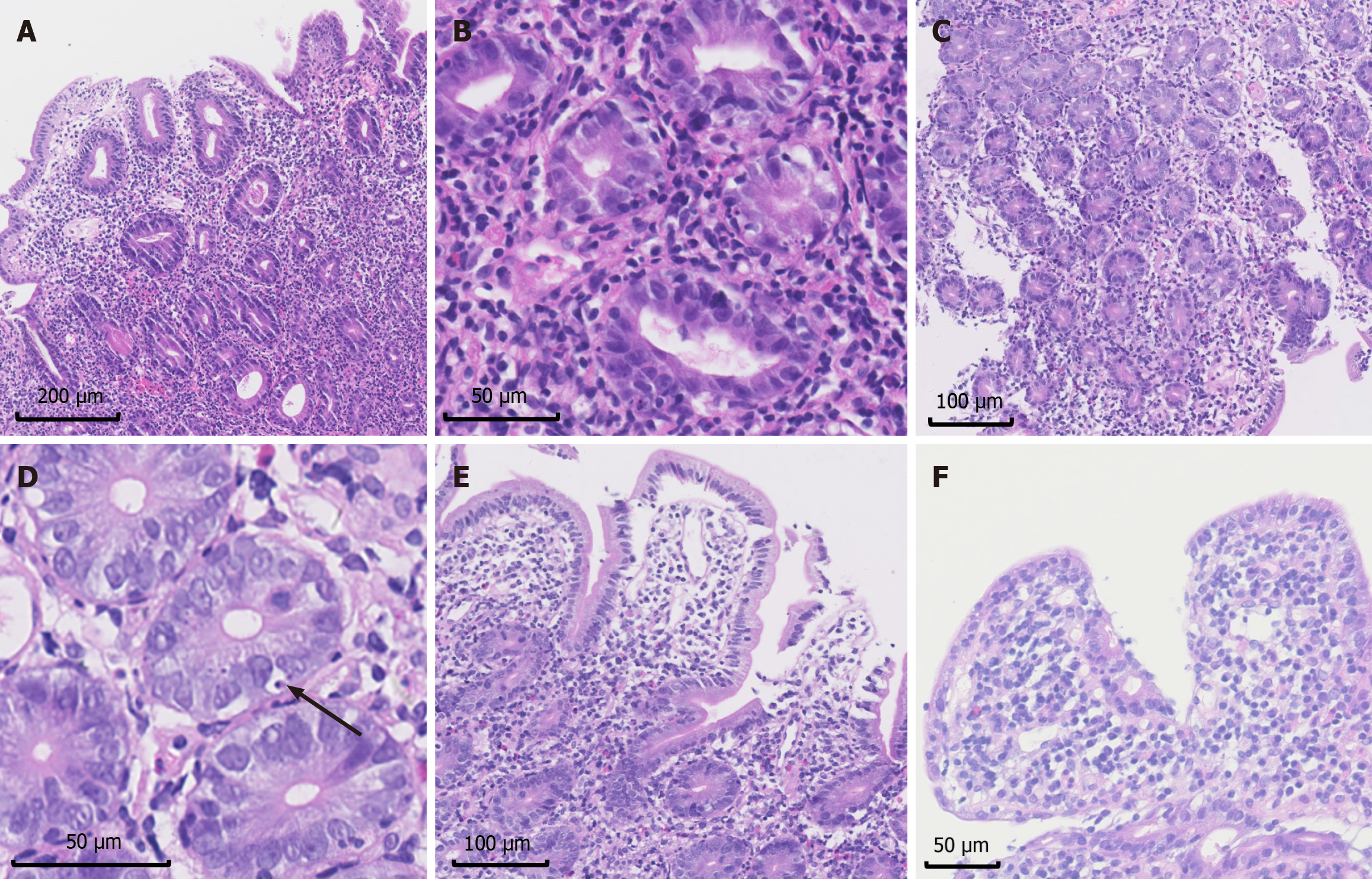
The 2007 diagnostic criteria for AIE involve four main histopathological features. In intestinal biopsies, the positivity rates were as follows: Villous blunting (100%), mononuclear inflammatory infiltration (100%, and 67% deep crypt lymphocytic infiltration), prominent apoptotic bodies (46%-50%), and minimal IEL with less than 40 lymphocytes per 100 epithelial cells (69%) (Figures 5 and 6).
Notably, abnormalities in special intestinal epithelial cells, including reduced or absent goblet cells (duodenum 94%, ileum 62%) and reduced or absent Paneth cells (duodenum 94%, ileum 69%), were common in this group. Neutrophil infiltration was also common (duodenum 100%, ileum 69%), presenting as cryptitis and crypt abscess (Figures 5 and 6). Moreover, some patients exhibit structural disorders or metaplasia in addition to the abovementioned features. For parts of the digestive tract other than the duodenum and ileum, increased epithelial apoptosis can be observed in 3/12 colon biopsies, and neutrophilic microabscesses can also be observed in 5/12 colon biopsies, in addition to acute and/or chronic inflammation.
The median follow-up duration was 20.5 (IQR: 15.3-38.8) months. All patients received glucocorticoid therapy as the initial medication, among which clinical response or remission was achieved in 14/16 patients in 5 (IQR: 3-20) days. Immunosuppressants were added in combination with glucocorticoid therapy to control diarrhea in 9 patients with in
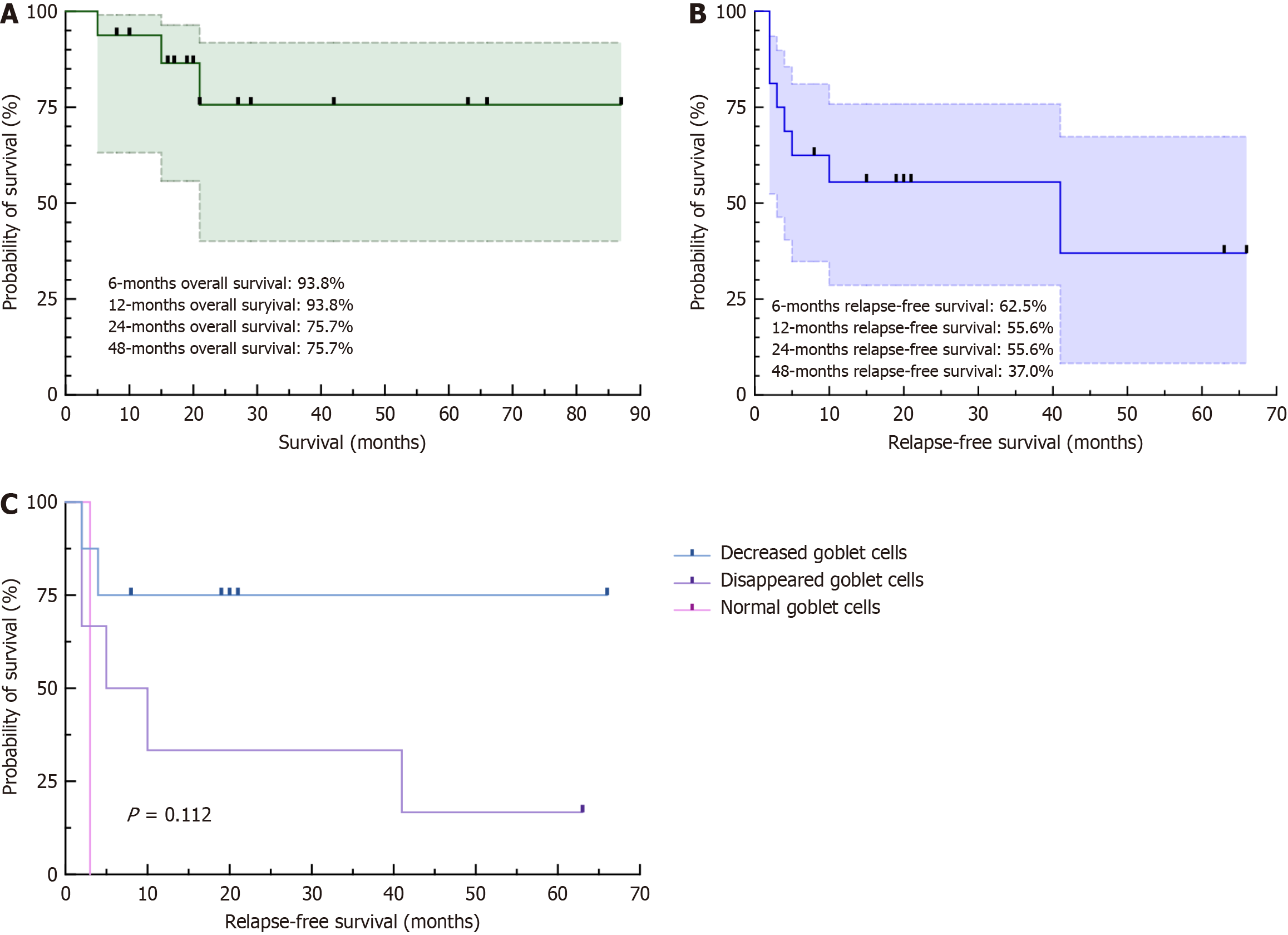
During the follow-up, 2 patients died of multiple-organ failure in 5 and 21 months after diagnosis, and 1 patient was diagnosed with non-Hodgkin lymphoma at 15 months after diagnosis (Figure 7A). Diarrhea relapse was very common (8/16, 50%), as shown in Figure 7B. The relapse-free survival rates were 62.5%, 55.6% and 37.0% at 6 months, 12 months and 48 months, respectively. Kaplan–Meier curves according to prognostic factors for abnormal goblet cells are shown in Figure 7C. No predictive marker for relapse-free survival was found by the log-rank test. At the last follow-up, none of the surviving patients were experiencing disease activity. Seven patients achieved complete clinical remission after the tapering off of steroids or immunosuppressants, and 6 patients achieved remission under maintenance therapy with steroids and immunosuppressants. Moreover, patients who achieved complete clinical remission without medication had less prominent villi blunting than did the other patients (P = 0.023).
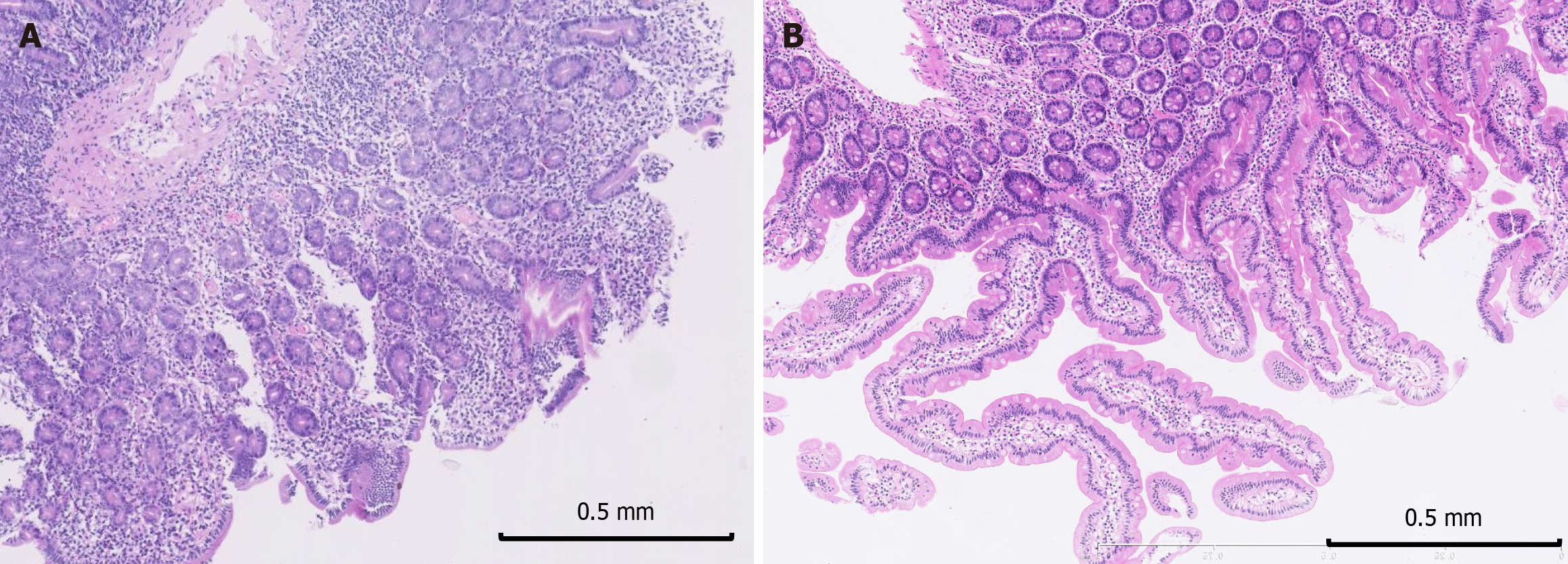
During follow-up, 8/16 patients underwent gastroscopy, 5 had clinical disease activity, and 4 were in clinical remission, with 1 patient undergoing several follow-up endoscopies. Additionally, 3/16 patients underwent follow-up colonoscopy; 2 had disease activity, and 2 had remission, with 1 patient take twice follow-up colonoscopy. Villous blunting remained macroscopically observable in all patients regardless of the clinical disease status, except for one patient, who had normal macroscopic findings and exhibited endoscopic remission. Pathologic abnormalities improved during follow-up but still existed (Figure 8).
The diagnostic criteria for adult AIE have evolved since 1985, but validation between the classic criteria in 2007 and the updated diagnostic criteria in 2018 or 2022 is still lacking. Different versions of diagnostic criteria give variable power to each feature of AIE, making the diagnosis inconsistent. Moreover, many medications, including corticosteroids, immune suppressors and even biologics, have been reported to help induce and maintain the remission of AIE based on case series and case reports. However, the long-term prognosis of AIE is still underdetermined, leaving disease relapse problematic for patients and physicians.
There was no characteristic manifestation in adult AIE patients, but many abnormalities might provide some diagnostic clues to AIE. Patients with AIE mainly present with chronic secretory diarrhea, which is characterized by a large volume and a high frequency of watery diarrhea, and diarrhea cannot be relieved after fasting or a gluten-free diet. The D-xylose test indicated impaired absorption in the small intestine. Weight loss and nutritional deficiencies can be life-threatening, and accompanying symptoms such as vomiting, abdominal pain, and fever are also common. Complications of prolonged diarrhea, particularly hypovolemic shock, acute kidney injury and electrolyte imbalance, require careful and prompt clinical management. In addition to increased WBC, hsCRP, and ESR, decreased C3 but not C4 was very common in our AIE cohort. Unlike IPEX (immunedysregulation, polyendocrinopathy, enteropathy, X-linked) syndrome, none of our patients present characteristic endocrinopathy or skin manifestations, which means that AIE actually is a different disease entity from IPEX syndrome.
Apart from the small intestine, the whole gastrointestinal tract of patients with AIE can sometimes be involved on both microscopic and macroscopic levels, including the stomach and colon[10-14], which was also found in our study. The general features of the duodenum and terminal ileum include edema, villous blunting, mucosal hyperemia, nodular changes (granulated mucosa), erosion, scalloping of the plicae, and mosaic mucosa, similar to what has been observed in other reported AIE series[8,15-17]. In our study, edema and villous blunting were the most prevailing manifestations in the duodenum according to gastroscopy results, in the terminal ileum according to colonoscopy results, and in the small intestine according to very limited enteroscopy results. We suggest that patients with large-volume secretory diarrhea actively undergo endoscopy and biopsy to carefully observe the duodenum and the terminal ileum to avoid overlooking unobvious abnormal changes.
Until now, the clinical value of different histopathologic classifications has not been elucidated. The most common histopathological classification in our adult AIE cohort was ACD, followed by celiac-like and GvHD-like, and the least common was mixed. Similarly, in the cohort of Masia et al[10], who enrolled both pediatric and adult AIE patients, also found that ACD was the most common pattern (52%, 13/25), followed by celiac-like (20%, 5/25), GvHD-like (17%, 4/25), and mixed (13%, 3/25). The most common pattern in the other cohort was celiac-like (50%, 20/40), followed by mixed (35%, 14/40), ACD (10%, 4/40) and GvHD-like (5%, 2/40)[18]. However, the relationship between this histopathological classification and the clinical prognosis of AIE patients was not significantly different, and additional studies with larger sample size are needed.
As the diagnostic criteria for adult AIE evolve, the diagnostic accuracy needs to be validated in general clinical practice. Several versions of the diagnostic criteria proposed for AIE (Table 1) have undergone changes based on progress in understanding these patients. First, the criteria proposed by Unsworth and Walker-Smith[9] did not consider histopathological features; then, the criteria proposed by Akram et al[8] in 2007 included four main histopathological criteria. Villous blunting is the most prominent and well-known pathological characteristic in AIE patients and presents as partial or complete blunting in all of our duodenum and ileum biopsies. This study also suggested that the degree of duodenal villus blunting may be related to patient prognosis. In addition, different degrees of lymphocyte and plasma cell infiltration were generally observed, and we found that infiltration was more severe in the lamina propria than in the deep crypt. Unlike in patients with celiac disease, we observed an increased IEL (> 40 lymphocytes per 100 epithelial cells) in only one-third of the patients with AIE. Likewise, the Mayo Clinic group reported that IELs are increased in 50% of AIE patients[7]. Moreover, IEL might be related to the process of AIE proceeding to enteropathy associated T cell lymphoma[19]. The number of apoptotic bodies is increased in approximately half of our duodenal and ileal biopsies and in approximately half of the AIE cases reported in the literature. Multiple biopsies can improve the likelihood of finding apoptotic bodies, but failure to find them does not rule out a diagnosis of AIE.
However, the 2007 criteria still did not emphasize the importance of the absence of goblet or Paneth cells (Table 1). Paneth cells, goblet cells, and endocrine cells have already been reported to be absent in many pediatric and adult cases under light microscopy and electron microscopy[8,20-29]. Loss of goblet cells has also been observed in colonic biopsies[8,30]. Afterward, the new criteria proposed by Sharma et al[7] in 2018 described AIE-specific small bowel histology as villous atrophy, minimal IEL, increased crypt apoptotic bodies and the absence of goblet or Paneth cells (Table 1). A review of the literature revealed that 40 cases showed decreased or absent goblet cells, and 20 cases showed abnormal Paneth cells. In one of these patients, duodenal biopsy lacked other typical findings and only showed only decreased goblet cells[13]. In fact, abnormalities in the number of goblet and Paneth cells in our cohort are very common, with a higher frequency than currently reported cases in the literature (8%-60%)[31] and contrary to the findings of another cohort[10], which might be due to the ignorance of goblet cells or Paneth cells in many studies. Therefore, our study further highlights the significance of abnormalities in the number of goblet and Paneth cells for the diagnosis of AIE. Although not significant (P = 0.112), which might be due to the small sample size, the decrease in goblet cells might reflect the prognosis of AIE patients. The relationships between decreases in goblet and Paneth cells and disease progression or patient prognosis deserve further exploration[31].
Neutrophil infiltration is detectable in small and large intestinal biopsies of AIE patients[8,32,33] and manifests as cryptitis and crypt abscess, which is considered supportive of AIE diagnosis in the 2022 criteria (Table 1)[6]. Other intestinal diseases characterized by villous atrophy rarely concurrently exhibit neutrophil infiltration. However, the manifestation of neutrophil infiltration is often overlooked and has not been thoroughly described in many published sources. Subsequent case reports or cohort studies should provide a more detailed description and investigation of this pathological feature. Other rare findings include eosinophil infiltration, epithelial metaplasia and granuloma.
According to the diagnostic criteria of 2022, antibodies against enterocytes are defined as the essential diagnostic criterion (Table 1), which still lacks validation. Due to the inconvenience of testing, only a small number of patients in our cohort tested for AE or AG antibodies, and all negative (0%), lower than the detection rates in other cohorts, with 52%-77% for AE[7,34] and 7% for AG[7]. It remains unclear whether AE and AG contribute pathogenically to the development of AIE, and some believe that these antibodies are only byproducts secondary to mucosal injury. AEs can also be found in CD patients (4/52, 7.7%), refractory CD type 2 patients (3/18, 16.7%), and enteropathy-associated T-cell lymphoma patients (2/10, 20%)[34], and AG can be found in CD patients (up to 28%)[3]. In general, autoantibodies, especially AG, are supportive but cannot lead to a definite diagnosis of AIE[35]. Our study indicated that all patients in this cohort who met the 2007 criteria failed to fulfill the 2022 criteria because of undetected or nonexistent AE antibodies, and the AIE diagnosis was missed. Therefore, based on our experience, the presence of AE antibodies should not be a necessary criterion for diagnosis.
Corticosteroids are the mainstay treatment option, followed by immunosuppressants[25] and biological agents[8,17,26] used in steroid-refractory or steroid-dependent therapy or for maintenance therapy. Even if there are problems such as ineffectiveness or dependence on glucocorticoids, the response to immunoregulatory treatment in AIE patients is generally good due to various available treatment options. Early initiation of immunosuppressive therapy can reduce AIE-related morbidity and complications[8]. Therefore, early diagnosis of AIE becomes even more important for mini
During the long-term follow-up, adult AIE patients experienced a waxing and waning disease course, with a high dis
This study has two major strengths. First, our study indicated the inconsistency of diagnostic potential of different diagnostic criteria, implying that additional powerful evidence is needed to further unify the diagnostic criteria for adult AIE. For example, the decrease or disappearance of goblet cells and Paneth cells in biopsies might actually be an important and intriguing characteristic in adult AIE patients. The second one is the description of the prognosis based on long-term follow-up, which arouses interest in discovering novel medications or constructing standardization of medications for this rare disease.
Moreover, there were many shortcomings in this study. First, this study was retrospectively conducted at one center during the long study period, which inevitably led to the omission of some clinical information and selection bias. Second, the relationship between clinical, endoscopic and histopathological changes in AIE patients during long-term follow-up remains unclear because of the limited results in our study and in other cohorts. Many patients with early clinical remission involuntarily underwent follow-up endoscopy, which might overestimate the inconsistency between clinical remission and endoscopic remission. Endoscopic follow-up results are unavailable for some patients who are in long-term clinical remission; thus, it is unclear whether abnormal features such as villous blunting are still present under endoscopy microscopically and macroscopically. More importantly, prognostic factors for survival and relapse-free survival should be further explored for risk stratification, treatment guidance and improved prognosis.
Adult AIE is an important disease for the differential diagnosis of patients with secretory diarrhea. A decrease in or disappearance of goblet and Paneth cells in intestinal biopsies might be a potential diagnostic criterion. The long-term prognosis of adult AIE patients is still poor due to the high incidence of disease relapse despite the use of corticosteroids and immunosuppressant medications, which urgently require early diagnosis and novel medications.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s classification
Scientific Quality: Grade B, Grade C
Novelty: Grade C, Grade C
Creativity or Innovation: Grade B, Grade B
Scientific Significance: Grade B, Grade B
P-Reviewer: Moriya K, Japan S-Editor: Gong ZM L-Editor: A P-Editor: Zheng XM
| 1. | Walker-Smith JA, Unsworth DJ, Hutchins P, Phillips AD, Holborow EJ. Autoantibodies against gut epithelium in child with small-intestinal enteropathy. Lancet. 1982;1:566-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 56] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Corazza GR, Biagi F, Volta U, Andreani ML, De Franceschi L, Gasbarrini G. Autoimmune enteropathy and villous atrophy in adults. Lancet. 1997;350:106-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 107] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Biagi F, Bianchi PI, Trotta L, Corazza GR. Anti-goblet cell antibodies for the diagnosis of autoimmune enteropathy? Am J Gastroenterol. 2009;104:3112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Moes N, Rieux-Laucat F, Begue B, Verdier J, Neven B, Patey N, Torgerson TT, Picard C, Stolzenberg MC, Ruemmele C, Rings EH, Casanova JL, Piloquet H, Biver A, Breton A, Ochs HD, Hermine O, Fischer A, Goulet O, Cerf-Bensussan N, Ruemmele FM. Reduced expression of FOXP3 and regulatory T-cell function in severe forms of early-onset autoimmune enteropathy. Gastroenterology. 2010;139:770-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Ciccocioppo R, D'Alo S, Di Sabatino A, Parroni R, Rossi M, Doglioni C, Cifone MG, Corazza GR. Mechanisms of villous atrophy in autoimmune enteropathy and coeliac disease. Clin Exp Immunol. 2002;128:88-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Schiepatti A, Sanders DS, Baiardi P, Caio G, Ciacci C, Kaukinen K, Lebwohl B, Leffler D, Malamut G, Murray JA, Rostami K, Rubio-Tapia A, Volta U, Biagi F. Nomenclature and diagnosis of seronegative coeliac disease and chronic non-coeliac enteropathies in adults: the Paris consensus. Gut. 2022;71:2218-2225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 7. | Sharma A, Choung RS, Wang XJ, Russo PA, Wu TT, Nehra V, Murray JA. Features of Adult Autoimmune Enteropathy Compared With Refractory Celiac Disease. Clin Gastroenterol Hepatol. 2018;16:877-883.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Akram S, Murray JA, Pardi DS, Alexander GL, Schaffner JA, Russo PA, Abraham SC. Adult autoimmune enteropathy: Mayo Clinic Rochester experience. Clin Gastroenterol Hepatol. 2007;5:1282-90; quiz 1245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 163] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 9. | Unsworth DJ, Walker-Smith JA. Autoimmunity in diarrhoeal disease. J Pediatr Gastroenterol Nutr. 1985;4:375-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 96] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Masia R, Peyton S, Lauwers GY, Brown I. Gastrointestinal biopsy findings of autoimmune enteropathy: a review of 25 cases. Am J Surg Pathol. 2014;38:1319-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 11. | Mitomi H, Tanabe S, Igarashi M, Katsumata T, Arai N, Kikuchi S, Kiyohashi A, Okayasu I. Autoimmune enteropathy with severe atrophic gastritis and colitis in an adult: proposal of a generalized autoimmune disorder of the alimentary tract. Scand J Gastroenterol. 1998;33:716-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Pieplenbosch B, de Leijer JH, van Dop WA, Nagtegaal ID, Witteman EM. Thymoma-associated autoimmune enteropathy with colonic stricture: a diagnostic and histological challenge. Clin J Gastroenterol. 2022;15:112-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Chong A, Kashani A, Ansstas M, Jamil L, Guindi M. Seronegative autoimmune enteropathy with duodenal sparing and colonic clues in an adult female. Clin J Gastroenterol. 2021;14:546-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Gentile NM, Murray JA, Pardi DS. Autoimmune enteropathy: a review and update of clinical management. Curr Gastroenterol Rep. 2012;14:380-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 15. | Gonzalez G, Castro FP, Berho M, Petras R. Autoimmune enteropathy associated with cessation of interferon-alpha therapy in chronic hepatitis C. Dig Dis Sci. 2010;55:1490-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | van Lent AU, Takkenberg RB, van Eeden S, Mulder C, Beuers U. Chronic diarrhea because of villous atrophy unrelated to celiac disease. Endoscopy. 2015;47 Suppl 1 UCTN:E71-E72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | von Hahn T, Stopik D, Koch M, Wiedenmann B, Dignass A. Management of severe refractory adult autoimmune enteropathy with infliximab and tacrolimus. Digestion. 2005;71:141-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Villanacci V, Lougaris V, Ravelli A, Buscarini E, Salviato T, Lionetti P, Salemme M, Martelossi S, De Giacomo C, Falchetti D, Pelizzo G, Bassotti G. Clinical manifestations and gastrointestinal pathology in 40 patients with autoimmune enteropathy. Clin Immunol. 2019;207:10-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Ciccocioppo R, Croci GA, Biagi F, Vanoli A, Alvisi C, Cavenaghi G, Riboni R, Arra M, Gobbi PG, Paulli M, Corazza GR. Intestinal T-cell lymphoma with enteropathy-associated T-cell lymphoma-like features arising in the setting of adult autoimmune enteropathy. Hematol Oncol. 2018;36:481-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Al Khalidi H, Kandel G, Streutker CJ. Enteropathy with loss of enteroendocrine and paneth cells in a patient with immune dysregulation: a case of adult autoimmune enteropathy. Hum Pathol. 2006;37:373-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Hori K, Fukuda Y, Tomita T, Kosaka T, Tamura K, Nishigami T, Kubota A, Shimoyama T. Intestinal goblet cell autoantibody associated enteropathy. J Clin Pathol. 2003;56:629-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Moore L, Xu X, Davidson G, Moore D, Carli M, Ferrante A. Autoimmune enteropathy with anti-goblet cell antibodies. Hum Pathol. 1995;26:1162-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Mais DD, Mulhall BP, Adolphson KR, Yamamoto K. Thymoma-associated autoimmune enteropathy. A report of two cases. Am J Clin Pathol. 1999;112:810-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Hill SM, Milla PJ, Bottazzo GF, Mirakian R. Autoimmune enteropathy and colitis: is there a generalised autoimmune gut disorder? Gut. 1991;32:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Daum S, Sahin E, Jansen A, Heine B, Riecken EO, Zeitz M, Schmidt W. Adult autoimmune enteropathy treated successfully with tacrolimus. Digestion. 2003;68:86-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Elwing JE, Clouse RE. Adult-onset autoimmune enteropathy in the setting of thymoma successfully treated with infliximab. Dig Dis Sci. 2005;50:928-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Lee HE, Yuan L, Wu TT. Neuroendocrine Cells Are Commonly Absent in the Intestinal Crypts in Autoimmune Enteropathy. Am J Surg Pathol. 2020;44:1130-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Greenson JK. The biopsy pathology of non-coeliac enteropathy. Histopathology. 2015;66:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Halabi I, Barohom MN, Peleg S, Trougouboff P, Elias-Assad G, Agbaria R, Tenenbaum-Rakover Y. Case Report: Severe Hypocalcemic Episodes Due to Autoimmune Enteropathy. Front Endocrinol (Lausanne). 2021;12:645279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | León F, Olivencia P, Rodríguez-Pena R, Sánchez L, Redondo C, Alvarez I, Moreira V, Roy G. Clinical and immunological features of adult-onset generalized autoimmune gut disorder. Am J Gastroenterol. 2004;99:1563-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Beck A, Schulte L, Möller P. [Autoimmune enteropathy in adults : A rare and difficult but relevant differential diagnosis of chronic diarrhea]. Pathologe. 2020;41:230-237. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 32. | Trivedi HD, Shannahan SE, Morrow M, Peppercorn MA. Using Adalimumab to Treat Autoimmune Enteropathy. ACG Case Rep J. 2019;6:e00265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 33. | Hasan SS, Siddiqui NS, Chaitanya Arudra SK, de Las Casas L, Akpunonu B, Nawras A. Diffuse Autoimmune Enteropathy and Colopathy in an Adult Patient Successfully Treated With Adalimumab and a Review of the Literature. Am J Ther. 2016;23:e963-e968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | van Wanrooij RLJ, Neefjes-Borst EA, Bontkes HJ, Schreurs MWJ, Langerak AW, Mulder CJJ, Bouma G. Adult-Onset Autoimmune Enteropathy in an European Tertiary Referral Center. Clin Transl Gastroenterol. 2021;12:e00387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Di Sabatino A, Biagi F, Lenzi M, Frulloni L, Lenti MV, Giuffrida P, Corazza GR. Clinical usefulness of serum antibodies as biomarkers of gastrointestinal and liver diseases. Dig Liver Dis. 2017;49:947-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 36. | Xu RY, Wan YP, Zhou YQ, Lu LP, Chen ZQ, Wu YJ, Cai W. Glutamine-supplemented parenteral nutrition and probiotics in four adult autoimmune enteropathy patients. Gut Liver. 2014;8:324-328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 37. | Charbit-Henrion F, Haas M, Chaussade S, Cellier C, Cerf-Bensussan N, Malamut G; Contributors from the AUTOIMMUNE ENTEROPATHY WORKING GROUP, Khater S, Khiat A, Cording S, Parlato M, Dragon-Durey MA, Beuvon F, Brousse N, Terris B, Picard C, Fusaro M, Rieux-Laucat F, Stolzenberg MC, Jannot AS, Mathian A, Allez M, Malphettes M, Fieschi C, Aubourg A, Zallot C, Roblin X, Abitbol V, Belle A, Wils P, Cheminant M, Matysiak-Budnik T, Vuitton L, Pouderoux P, Abramowitz L, Castelle M, Suarez F, Hermine O, Ruemmele F, Mouthon L. Genetic Diagnosis Guides Treatment of Autoimmune Enteropathy. Clin Gastroenterol Hepatol. 2023;21:1368-1371.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Ciccocioppo R, Russo ML, Bernardo ME, Biagi F, Catenacci L, Avanzini MA, Alvisi C, Vanoli A, Manca R, Luinetti O, Locatelli F, Corazza GR. Mesenchymal stromal cell infusions as rescue therapy for corticosteroid-refractory adult autoimmune enteropathy. Mayo Clin Proc. 2012;87:909-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Soma D, Saxena R, Arman ME, Mihaylov P, Ekser B, Kubal CA, Mangus RS. Isolated Intestine Transplantation for Autoimmune Enteropathy: A Case Report. Transplant Proc. 2020;52:2835-2838. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 40. | Casis B, Fernández-Vázquez I, Barnardos E, Saiz A, Ballestín C, Morillas JD, Colina F, Solís-Herruzo JA. Autoimmune enteropathy in an adult with autoimmune multisystemic involvement. Scand J Gastroenterol. 2002;37:1012-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |