Published online May 14, 2024. doi: 10.3748/wjg.v30.i18.2391
Revised: March 9, 2024
Accepted: April 17, 2024
Published online: May 14, 2024
Processing time: 98 Days and 11.5 Hours
This editorial contains comments on the article by Zhao et al in print in the World Journal of Gastroenterology. The mechanisms responsible for hepatic fibrosis are also involved in cancerogenesis. Here, we recapitulated the complexity of the renin-angiotensin system, discussed the role of hepatic stellate cell (HSC) autophagy in liver fibrogenesis, and analyzed the possible implications in the development of hepatocarcinoma (HCC). Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers definitively contribute to reducing hepatic fibrogenesis, whereas their involvement in HCC is more evident in experimental conditions than in human studies. Angiotensin-converting enzyme 2 (ACE2), and its product Angiotensin (Ang) 1-7, not only regulate HSC autophagy and liver fibrosis, but they also represent potential targets for unexplored applications in the field of HCC. Finally, ACE2 overexpression inhibits HSC autophagy through the AMP-activated protein kinase (AMPK)/mammalian target of rapa
Core Tip: In the light of clarifying the link between liver fibrosis and the development of hepatocellular carcinoma, we discussed the renin-angiotensin system involvement in liver fibrosis and hepatocarcinoma development, the specific mechanisms by which angiotensin-converting enzyme 2 (ACE2) regulates hepatic stellate cell (HSC) autophagy and consequently fibrosis, and the ACE2-dependent upstream signals modulating the AMP-activated protein kinase/mammalian target of the rapamycin pathway implicated in the regulation of HSC activation.
- Citation: Barone M. Angiotensin-converting enzyme 2 and AMPK/mTOR pathway in the treatment of liver fibrosis: Should we consider further implications? World J Gastroenterol 2024; 30(18): 2391-2396
- URL: https://www.wjgnet.com/1007-9327/full/v30/i18/2391.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i18.2391
The article by Zhao et al[1] discussing the role of angiotensin-converting enzyme 2 (ACE2) on liver fibrosis prompted further comments on this hot topic since liver fibrosis, inflammation, and hepatocarcinogenesis share some physiopathological mechanisms. In this editorial article, I will discuss the current research status and future directions on the involvement of renin-angiotensin system (RAS) and liver fibrogenesis, focusing on the potential links with hepatocarcinogenesis.
Advanced liver fibrosis is associated with portal hypertension, progressive liver function deterioration, and an increased risk of hepatocellular carcinoma. The mechanisms responsible for hepatic fibrosis and cancerogenesis are related to chronic necroinflammation, compensatory regeneration, and activation of non-parenchymal cells, together with an altered immune response[2,3]. Recent experimental data demonstrate that ACE2 can reduce liver fibrosis and hepatic sinusoidal remodeling by regulating hepatic stellate cell (HSC) autophagy[4]. The molecular mechanism by which ACE2 would regulate HSC autophagy would be mediated by the AMP-activated protein kinase (AMPK)/mammalian target of rapamycin (mTOR) pathway, opening up a new opportunity to develop new potential therapies for the prevention/cure of liver fibrogenesis[4].
Zhao et al[1] have further discussed the role of ACE2 in the reduction of liver fibrosis by implementing the literature data on the role of HSCs and underlining some specific effects of ACE2 overexpression on RAS. They conclude that the pathway described by Wu et al[4] is not entirely independent from the pathway associated with RAS, which could explain alternative mechanisms for liver fibrogenesis reduction.
Here, the role of RAS in liver fibrogenesis, the ACE2-mediated mechanisms regulating autophagy, and the upstream signals regulating the AMPK/mTOR pathway will be further discussed, in the light of the possible link between liver fibrosis and the development of hepatocellular carcinoma.
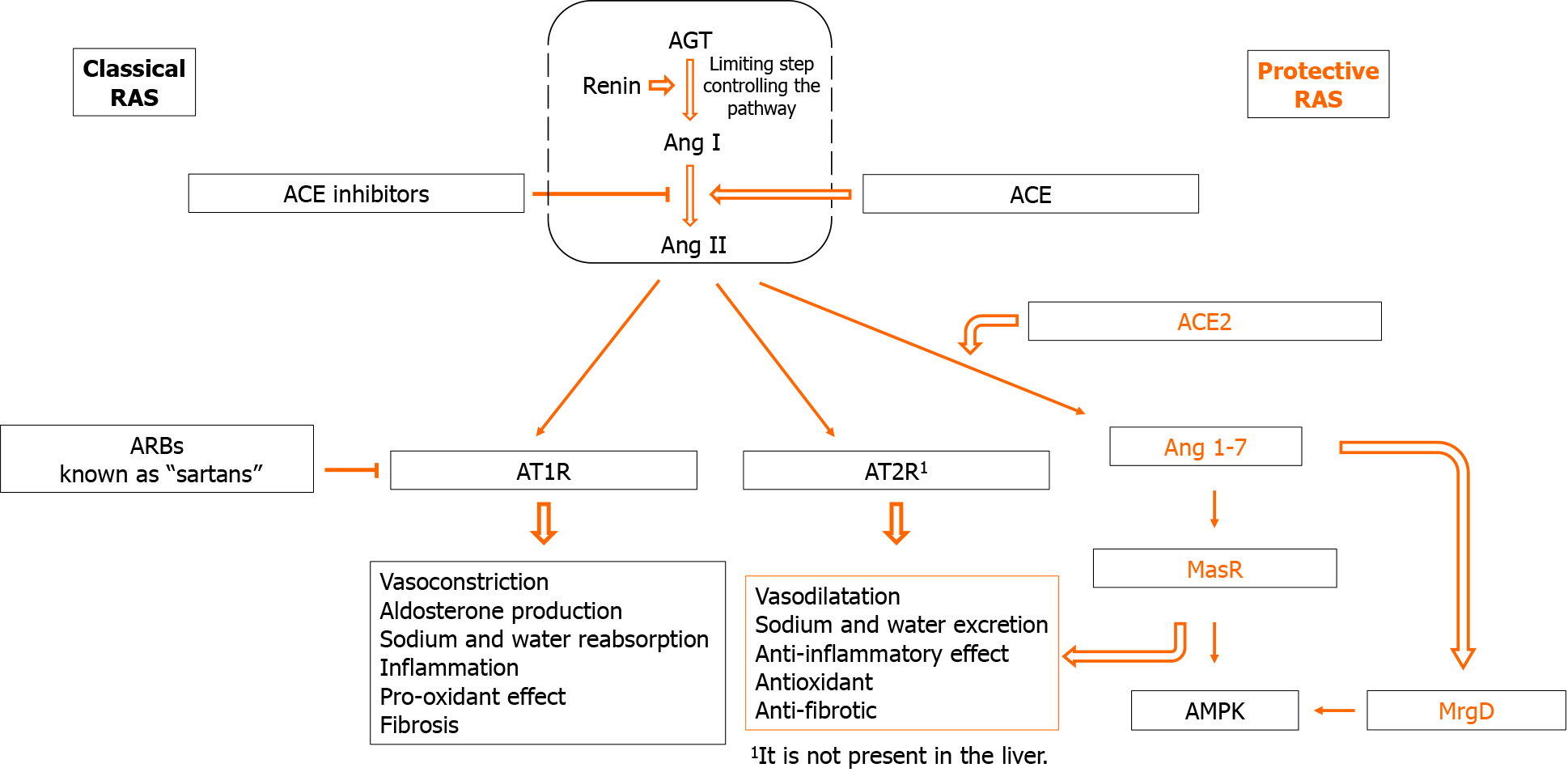
The RAS can be considered a hormonal (tissue-to-tissue), paracrine (cell-to-cell), and intracrine (intracellular/nuclear) system, which is responsible for the control of multiple biological/functional activities. It consists of several components that include angiotensinogen (AGT), renin, ACE, angiotensin I (Ang I), Ang II, AT1R and AT2R, which all together constitute the classical RAS pathway, and ACE-2, angiotensin 1-7 (Ang 1-7), MAS Receptor (MasR), and Mas-related G-protein-coupled receptor D (MrgD), which constitute the alternative so called RAS protective pathway (Figure 1) (for a more comprehensive description of all RAS[5,6]).
As shown in Figure 1, renin is the rate-limiting enzyme and the first recognized component of RAS[7]. AGT, the only precursor of all angiotensin peptides, is a member of the non-inhibitory serine protease inhibitor superfamily, and it is mainly synthesized and released from the liver[8,9].
Ang I is generated from AGT under the action of renin[10]. Angiotensin-converting enzyme is an evolutionarily conserved zinc-metallopeptidase that is expressed on the surface of endothelial and epithelial cells in a wide variety of tissues[11] and is responsible for the formation of Ang II[5], which is the major effector peptide of RAS[12]. The biological function of Ang II is mediated by two receptors with antagonist functions, namely angiotensin type 1 receptors (AT1R) and type 2 receptor (AT2R), the former common in adult tissues such as liver, brain, and kidney, and the latter predominant in fetal tissues, ovary, and uterus, and practically absent in the liver[5,13,14]. AT1R is the dominant receptor that activates intracellular signaling pathways implicated in several biological activities, including inflammation and fibrosis[15]. The hepatic AT1R would be the main target by which Ang II stimulates the proliferation of HSCs and their synthesis of extracellular matrix proteins through induction of transforming growth factor beta (TGF-β)[6,16]. AT2R is practically absent in the liver, and its activation by Ang II has opposite effects, including vasodilation and anti-fibrotic effects[15].
ACE2 represents the initial step for the alternative protective RAS pathway by promoting the degradation of Ang II in Ang 1-7, which in turn becomes a negative regulator of AT1R and AT2R activation. ACE2 is present in two different catalytic forms, the type I transmembrane protein and the soluble form that does not contain the cytosolic and transmembrane domains[17]. Its transmembrane form is expressed by a wide variety of human tissues (alveolar epithelial cells, esophageal epithelial cells, small intestinal epithelial cells, vascular endothelial cells) and at low concentration in the liver, especially in HSCs[18,19]. Ang 1-7 binds to the G protein-coupled receptor named MasR to elicit its cellular responses. It has been shown that MasR can heterooligomerize with the AT1R receptor and inhibit the actions of Ang II, which makes it physiological antagonist of AT1R[20]. Although the knowledge of the signaling mechanisms associated with MasR is essential for therapeutic purposes, there is little data in the literature on this topic, mainly in experimental models hyperexpressing MasR[21]. This receptor is distributed in various tissues and HSCs[22,23].
Overall, the ACE2/Ang- (1-7)/MasR pathway has anti-fibrotic and anti-inflammatory properties[22,15]. In attrition to its activity towards AT1R, Ang 1-7 can reduce inflammation by decreasing the expression of the proinflammatory cytokines, including tumor necrosis factor-alpha (TNF-α) and interleukins (IL)-6[24], whereas its binding with MrgD seems to induce hemodynamic responses[6,25].
Autophagy is a cellular process that degrades damaged organelle or protein aggregation, safeguarding cells against damage or death by eliminating damaged organelles and proteins. However, an excess of autophagy can produce damage, as observed in HSCs where it promotes their activation, which increases liver fibrosis. In fact, autophagy can antagonize apoptosis, can regulate cell death independently from apoptosis, or it can cooperate with apoptosis to promote cell death[26].
Starvation, hypoxia, oxidative stress, protein aggregation, and endoplasmic reticulum stress are the main signals that activate the autophagic process. Autophagy is mainly downregulated by mTOR and its downstream signal, the mTOR complex 1 (mTORC1). The latter is a protein complex regulated by growth factors, energy levels, oxygen, and amino acids. TORC1 controls different cell functions, including inhibition of autophagy, and it can be inhibited by rapamycin[27].
One of the three upstream signaling pathways regulating mTORC1 activation is represented by the AMPK, a Ser/Thr kinase that exists as a heterotrimer composed of catalytic α and regulatory β and γ subunits. AMPK is the key sensor of the AMP:ATP ratio within the cell, which is deputed to transduce this information to mTOR. In the absence of AMP, the autoinhibitory domain of AMPKα maintains the kinase in an inactive conformation. Upon ATP consumption, AMP/ADP directly binds to AMPKγ and promotes the catalytic AMPK subunit phosphorylation, which increases its activity approximately 100 times. AMPK phosphorylation is also mediated by the TGF-β-activated kinase 1[28,29] and several other physiological signals, including cytokines and growth factors[30]. AMPK inhibits mTORC1 both directly and indirectly, while activated mTORC1 inhibits autophagy; therefore, AMPK indirectly leads to the induction of autophagy by inhibiting mTORC1[28]. Interestingly, the main product of ACE2 is Ang 1-7, which seems to have the ability to increase the activation of AMPK[29].
To discuss the link between liver fibrosis, hepatocarcinogenesis, and RAS, we will consider three distinct aspects: The potential antineoplastic use of ACE inhibitors or Angiotensin receptor blokers (ARBs) to reduce the risk of hepatocellular carcinoma through the inhibition of the classical RAS pathway (Ang II/AT1R), the upstream mechanism of HSCs autophagy regulated by ACE2 potentially involving Ang 1-7 agonists, and the downstream mechanism that occurs intracellularly in HSC involving the AMPK/mTOR pathway in the reduction of hepatic fibrosis. In this case, the target for downregulation of autophagy would be the modulation of AMPK activity or the increase of mTORC1 activity.
Interestingly, 15%-20% of all cancer deaths are associated with infections. In addition, the incidence and mortality from cancer decrease in patients treated with both steroidal and non-steroidal anti-inflammatory drugs.
Some of the molecules involved in cancer-related inflammation are IL-1β, IL-6, IL-23 and TNF-α. In the liver, the activation of Kupffer cells increases the of IL-6 production and promotes inflammation, tissue damage, compensatory cell proliferation, and, ultimately, the formation of liver tumors[31].
The mechanism by which the RAS pathway would be involved in liver carcinogenesis would depend essentially on the Ang II/AT1R. The chronically damaged liver increases the production of AGT and Ang II, which stimulates the contraction and proliferation of HSCs[32] and increases the TGF-β expression through AT1R[6,16].
The use of RAS inhibitors in experimental models of liver cancer and some studies on human hepatocellular carcinoma have provided evidence that long-term use of ACE inhibitors and ARBs may protect against cancer[5].
However, although the interest in the potential beneficial effects of RAS inhibitors against HCC started two decades ago, we must wait until 2009 for the first study in humans. Some observational studies, including ours, showed better results with ARBs compared to RAS inhibitors[33,34]. More recently, it has been reported that the potential RAS blockade can be used for cancer metastasis treatment. This antitumoral activity would depend on blockade of AT1R with a reduction of VEGF, angiopoietin 2, fibroblast growth factor, and platelet-derived growth factor, all factors involved in cell proliferation and/or angiogenesis and inhibitory effects on tumor-associated macrophages, cancer-associated fibroblasts, and Kupffer cell activation[14]. Moreover, a large retrospective study showed that ACE inhibitors/ARBs were associated with a lower risk of HCC and cirrhotic complications in patients with non-alcoholic fatty liver disease[35], an effect most likely linked to the antifibrotic activity of these drugs[36]. Interestingly, another possible mechanism linking the antifibrogenic and antitumoral activity of ARBs comes from the experimental study of Gu et al[37]. They demonstrated that losartan ameliorates the immunological response against HCC by reducing peritumoral fibrosis and enhancing HCC infiltration by effector CD8+ T cells. However, it should be considered that two studies report a tumor-promoting effect of ARBs in human lung cancer and melanoma. In the former type of cancer, the authors report that ARBs are associated with a modest increase in risk for a new lung cancer occurrence[38], whereas, in the latter, the authors report that RAS has both oncogenic and tumor suppressor functions in melanoma[39]. Finally, a more recent meta-analysis confirms that treatment with ARBs and ACE inhibitors reduces the risk of hepatocellular carcinoma[40].
The ACE2-regulated HSCs autophagy represents another link between liver fibrosis and hepatocellular carcinoma since ACE2 would increase the production of Ang 1-7, which inhibits the proliferation of activated HSCs. The involvement of ACE2 induced Ang 1-7 up-regulation in the reduction of HCC risk is supported by the study of Barbosa et al[41] in which they describe a novel Ang 1-7 agonist, the A-1317, able to increase both Mas, MRGD, and AMPK mRNA gene expression in the liver, with positive effects on metabolic syndrome-related disorders (altered liver biochemical parameters, increased liver AGT), and AT1R, ACE mRNA gene expression.
To complete our comment on the link between liver fibrosis and carcinogenesis, we are considering the recent study of Wu et al[4] on the involvement AMPK/mTOR pathway in HSC autophagy downregulation to improve liver fibrosis[42]. In their experimental study, they used a liver-specific recombinant viral vector to increase the expression of ACE2 in the liver, and they found reduced fibrogenesis associated with a reduced number of activated HSCs (HSCs contained a lower number of phagosomes compared to controls), and an increased number of apoptotic HSCs. These histological aspects were associated with a reduction of activated AMPK and an increase of p-mTOR. Based on these findings, they concluded that ACE2 overexpression inhibits the expression of HSC autophagy in the liver through the AMPK/mTOR pathway.
Some studies suggest that Ang 1-7 is an activator of AMPK through binding to the MasR[43,44]. Once again, these findings suggest that a possible intervention on this pathway could be mediated by Ang 1-7 agonists. Finally, since AMPK utilizes different targets to suppress mTORC1, i.e., the mTOR downstream complex[28], we must still to unravel the complete pathway to identify other potential targets for therapy.
The studies on the relationship between RAS and fibrogenesis can stimulate new possible therapeutic targets for antifibrotic agents and can also contribute to a deeper comprehension of the mechanisms promoting and inhibiting liver carcinogenesis. It is important to keep in mind that in addition to the mechanisms previously illustrated, angiotensin inhibitors can directly reduce the expression of important mediators of fibrogenesis and inflammation such as TGF-β[6,16,42] and TNFα[22], or indirectly induce downregulation of proinflammatory Th1 and Th17 cytokines, and the production of Treg/Th2 cytokines[45].
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report’s classification
Scientific Quality: Grade B, Grade C
Novelty: Grade C, Grade C
Creativity or Innovation: Grade C, Grade C
Scientific Significance: Grade B, Grade B
P-Reviewer: Liu G, China; Wu L, China S-Editor: Li L L-Editor: A P-Editor: Yuan YY
| 1. | Zhao BW, Chen YJ, Zhang RP, Chen YM, Huang BW. Angiotensin-converting enzyme 2 alleviates liver fibrosis through the renin-angiotensin system. World J Gastroenterol. 2024;30:607-609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 2. | Iredale JP. Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J Clin Invest. 2007;117:539-548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 698] [Cited by in RCA: 699] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 3. | Ringelhan M, Pfister D, O'Connor T, Pikarsky E, Heikenwalder M. The immunology of hepatocellular carcinoma. Nat Immunol. 2018;19:222-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 735] [Article Influence: 105.0] [Reference Citation Analysis (0)] |
| 4. | Wu Y, Yin AH, Sun JT, Xu WH, Zhang CQ. Angiotensin-converting enzyme 2 improves liver fibrosis in mice by regulating autophagy of hepatic stellate cells. World J Gastroenterol. 2023;29:4975-4990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 5. | George AJ, Thomas WG, Hannan RD. The renin-angiotensin system and cancer: old dog, new tricks. Nat Rev Cancer. 2010;10:745-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 406] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 6. | Warner FJ, Rajapaksha H, Shackel N, Herath CB. ACE2: from protection of liver disease to propagation of COVID-19. Clin Sci (Lond). 2020;134:3137-3158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 7. | Li XC, Zhang J, Zhuo JL. The vasoprotective axes of the renin-angiotensin system: Physiological relevance and therapeutic implications in cardiovascular, hypertensive and kidney diseases. Pharmacol Res. 2017;125:21-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 284] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 8. | Clauser E, Gaillard I, Wei L, Corvol P. Regulation of angiotensinogen gene. Am J Hypertens. 1989;2:403-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Takeda Y, Demura M, Yoneda T, Takeda Y. DNA Methylation of the Angiotensinogen Gene, AGT, and the Aldosterone Synthase Gene, CYP11B2 in Cardiovascular Diseases. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Lu H, Cassis LA, Kooi CW, Daugherty A. Structure and functions of angiotensinogen. Hypertens Res. 2016;39:492-500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 132] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 11. | Lambert DW, Clarke NE, Turner AJ. Not just angiotensinases: new roles for the angiotensin-converting enzymes. Cell Mol Life Sci. 2010;67:89-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Warren R. Editorial: Where shall i publish it? Arch Surg. 1976;111:612-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 509] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 13. | Ichiki T. Regulation of angiotensin II receptor expression. Curr Pharm Des. 2013;19:3013-3021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Ishikane S, Takahashi-Yanaga F. The role of angiotensin II in cancer metastasis: Potential of renin-angiotensin system blockade as a treatment for cancer metastasis. Biochem Pharmacol. 2018;151:96-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 15. | Cantero-Navarro E, Fernández-Fernández B, Ramos AM, Rayego-Mateos S, Rodrigues-Diez RR, Sánchez-Niño MD, Sanz AB, Ruiz-Ortega M, Ortiz A. Renin-angiotensin system and inflammation update. Mol Cell Endocrinol. 2021;529:111254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 16. | Chen R, Feng Y, Wu J, Song Y, Li H, Shen Q, Li D, Zhang J, Lu Z, Xiao H, Zhang Y. Metformin attenuates angiotensin II-induced TGFβ1 expression by targeting hepatocyte nuclear factor-4-α. Br J Pharmacol. 2018;175:1217-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Lambert DW, Yarski M, Warner FJ, Thornhill P, Parkin ET, Smith AI, Hooper NM, Turner AJ. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2). J Biol Chem. 2005;280:30113-30119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 571] [Cited by in RCA: 578] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 18. | Hikmet F, Méar L, Edvinsson Å, Micke P, Uhlén M, Lindskog C. The protein expression profile of ACE2 in human tissues. Mol Syst Biol. 2020;16:e9610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 631] [Cited by in RCA: 709] [Article Influence: 141.8] [Reference Citation Analysis (0)] |
| 19. | Wu Y, Li Z, Wang S, Xiu A, Zhang C. Carvedilol Inhibits Angiotensin II-Induced Proliferation and Contraction in Hepatic Stellate Cells through the RhoA/Rho-Kinase Pathway. Biomed Res Int. 2019;2019:7932046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Kostenis E, Milligan G, Christopoulos A, Sanchez-Ferrer CF, Heringer-Walther S, Sexton PM, Gembardt F, Kellett E, Martini L, Vanderheyden P, Schultheiss HP, Walther T. G-protein-coupled receptor Mas is a physiological antagonist of the angiotensin II type 1 receptor. Circulation. 2005;111:1806-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 317] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 21. | Burghi V, Echeverría EB, Sosa MH, Quiroga DT, Muñoz MC, Davio C, Monczor F, Fernández NC, Dominici FP. Participation of Gα(i)-Adenylate Cyclase and ERK1/2 in Mas Receptor Signaling Pathways. Front Pharmacol. 2019;10:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Santos RAS, Sampaio WO, Alzamora AC, Motta-Santos D, Alenina N, Bader M, Campagnole-Santos MJ. The ACE2/Angiotensin-(1-7)/MAS Axis of the Renin-Angiotensin System: Focus on Angiotensin-(1-7). Physiol Rev. 2018;98:505-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 784] [Article Influence: 112.0] [Reference Citation Analysis (0)] |
| 23. | Lubel JS, Herath CB, Tchongue J, Grace J, Jia Z, Spencer K, Casley D, Crowley P, Sievert W, Burrell LM, Angus PW. Angiotensin-(1-7), an alternative metabolite of the renin-angiotensin system, is up-regulated in human liver disease and has antifibrotic activity in the bile-duct-ligated rat. Clin Sci (Lond). 2009;117:375-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Souza LL, Costa-Neto CM. Angiotensin-(1-7) decreases LPS-induced inflammatory response in macrophages. J Cell Physiol. 2012;227:2117-2122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 25. | Tetzner A, Gebolys K, Meinert C, Klein S, Uhlich A, Trebicka J, Villacañas Ó, Walther T. G-Protein-Coupled Receptor MrgD Is a Receptor for Angiotensin-(1-7) Involving Adenylyl Cyclase, cAMP, and Phosphokinase A. Hypertension. 2016;68:185-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 116] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 26. | Khalil MI, Ali MM, Holail J, Houssein M. Growth or death? Control of cell destiny by mTOR and autophagy pathways. Prog Biophys Mol Biol. 2023;185:39-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 27. | Wang H, Liu Y, Wang D, Xu Y, Dong R, Yang Y, Lv Q, Chen X, Zhang Z. The Upstream Pathway of mTOR-Mediated Autophagy in Liver Diseases. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 192] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 28. | Garza-Lombó C, Schroder A, Reyes-Reyes EM, Franco R. mTOR/AMPK signaling in the brain: Cell metabolism, proteostasis and survival. Curr Opin Toxicol. 2018;8:102-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 29. | Liu J, Li X, Lu Q, Ren D, Sun X, Rousselle T, Li J, Leng J. AMPK: a balancer of the renin-angiotensin system. Biosci Rep. 2019;39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 30. | Kim J, Yang G, Kim Y, Kim J, Ha J. AMPK activators: mechanisms of action and physiological activities. Exp Mol Med. 2016;48:e224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 372] [Cited by in RCA: 552] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 31. | Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8581] [Cited by in RCA: 8317] [Article Influence: 489.2] [Reference Citation Analysis (0)] |
| 32. | Kurikawa N, Suga M, Kuroda S, Yamada K, Ishikawa H. An angiotensin II type 1 receptor antagonist, olmesartan medoxomil, improves experimental liver fibrosis by suppression of proliferation and collagen synthesis in activated hepatic stellate cells. Br J Pharmacol. 2003;139:1085-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 99] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 33. | Barone M, Viggiani MT, Losurdo G, Principi M, Leo AD. Systematic review: Renin-angiotensin system inhibitors in chemoprevention of hepatocellular carcinoma. World J Gastroenterol. 2019;25:2524-2538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 34. | Facciorusso A, Del Prete V, Crucinio N, Muscatiello N, Carr BI, Di Leo A, Barone M. Angiotensin receptor blockers improve survival outcomes after radiofrequency ablation in hepatocarcinoma patients. J Gastroenterol Hepatol. 2015;30:1643-1650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 35. | Zhang X, Wong GL, Yip TC, Tse YK, Liang LY, Hui VW, Lin H, Li GL, Lai JC, Chan HL, Wong VW. Angiotensin-converting enzyme inhibitors prevent liver-related events in nonalcoholic fatty liver disease. Hepatology. 2022;76:469-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 20.3] [Reference Citation Analysis (1)] |
| 36. | Goh GB, Pagadala MR, Dasarathy J, Unalp-Arida A, Sargent R, Hawkins C, Sourianarayanane A, Khiyami A, Yerian L, Pai R, McCullough AJ, Dasarathy S. Renin-angiotensin system and fibrosis in non-alcoholic fatty liver disease. Liver Int. 2015;35:979-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 37. | Gu L, Zhu Y, Lee M, Nguyen A, Ryujin NT, Huang JY, Pandit SK, Chamseddine S, Xiao L, Mohamed YI, Kaseb AO, Karin M, Shalapour S. Angiotensin II receptor inhibition ameliorates liver fibrosis and enhances hepatocellular carcinoma infiltration by effector T cells. Proc Natl Acad Sci USA. 2023;120:e2300706120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 38. | Sipahi I, Debanne SM, Rowland DY, Simon DI, Fang JC. Angiotensin-receptor blockade and risk of cancer: meta-analysis of randomised controlled trials. Lancet Oncol. 2010;11:627-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 362] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 39. | Renziehausen A, Wang H, Rao B, Weir L, Nigro CL, Lattanzio L, Merlano M, Vega-Rioja A, Del Carmen Fernandez-Carranco M, Hajji N, Matin R, Harwood C, Li S, Sim VR, O'Neill K, Evans A, Thompson A, Szlosarek P, Fleming C, Stebbing J, Proby C, Tzakos AG, Syed N, Crook T. The renin angiotensin system (RAS) mediates bifunctional growth regulation in melanoma and is a novel target for therapeutic intervention. Oncogene. 2019;38:2320-2336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 40. | Asgharzadeh F, Jafarzadeh-Esfehani R, Hassanian SM, Ferns GA, Avan A, Khazaei M. Renin-angiotensin System Inhibitors and Development of Hepatocellular Carcinoma: A Systematic Review and Meta-analysis. Curr Pharm Des. 2020;26:5079-5085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 41. | Barbosa MA, Barbosa CM, Lima TC, Dos Santos RAS, Alzamora AC. The Novel Angiotensin-(1-7) Analog, A-1317, Improves Insulin Resistance by Restoring Pancreatic β-Cell Functionality in Rats With Metabolic Syndrome. Front Pharmacol. 2020;11:1263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 42. | Hernández-Gea V, Friedman SL. Autophagy fuels tissue fibrogenesis. Autophagy. 2012;8:849-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 43. | Oliveira Andrade JM, Paraíso AF, Garcia ZM, Ferreira AV, Sinisterra RD, Sousa FB, Guimarães AL, de Paula AM, Campagnole-Santos MJ, dos Santos RA, Santos SH. Cross talk between angiotensin-(1-7)/Mas axis and sirtuins in adipose tissue and metabolism of high-fat feed mice. Peptides. 2014;55:158-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 44. | Molaei A, Molaei E, Hayes AW, Karimi G. Mas receptor: a potential strategy in the management of ischemic cardiovascular diseases. Cell Cycle. 2023;22:1654-1674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 45. | Weber J, Tiriveedhi V, Takenaka M, Lu W, Hachem R, Trulock E, Patterson GA, Mohanakumar T. Inhibition of renin angiotensin aldosterone system causes abrogation of obliterative airways disease through inhibition of tumor necrosis factor-α-dependant interleukin-17. J Heart Lung Transplant. 2012;31:419-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |