Published online May 7, 2024. doi: 10.3748/wjg.v30.i17.2343
Revised: March 9, 2024
Accepted: April 11, 2024
Published online: May 7, 2024
Processing time: 86 Days and 4.5 Hours
The GALAD score has improved early hepatocellular carcinoma (HCC) detection rate. The role of the GALAD score in staging and predicting tumor characteristics or clinical outcome of HCC remains of particular interest.
To determine the diagnostic/prognostic performances of the GALAD score at various phases of initial diagnosis, tumor features, and 1-year mortality of HCC and compare the performance of the GALAD score with those of other serum biomarkers.
This prospective, diagnostic/prognostic study was conducted among patients with newly diagnosed HCC at the liver center of Vajira Hospital. Eligible patients had HCC staging allocation using the Barcelona Clinic Liver Cancer (BCLC) categorization. Demographics, HCC etiology, and HCC features were recorded. Biomarkers and the GALAD score were obtained at baseline. The performance of the GALAD score and biomarkers were prospectively assessed.
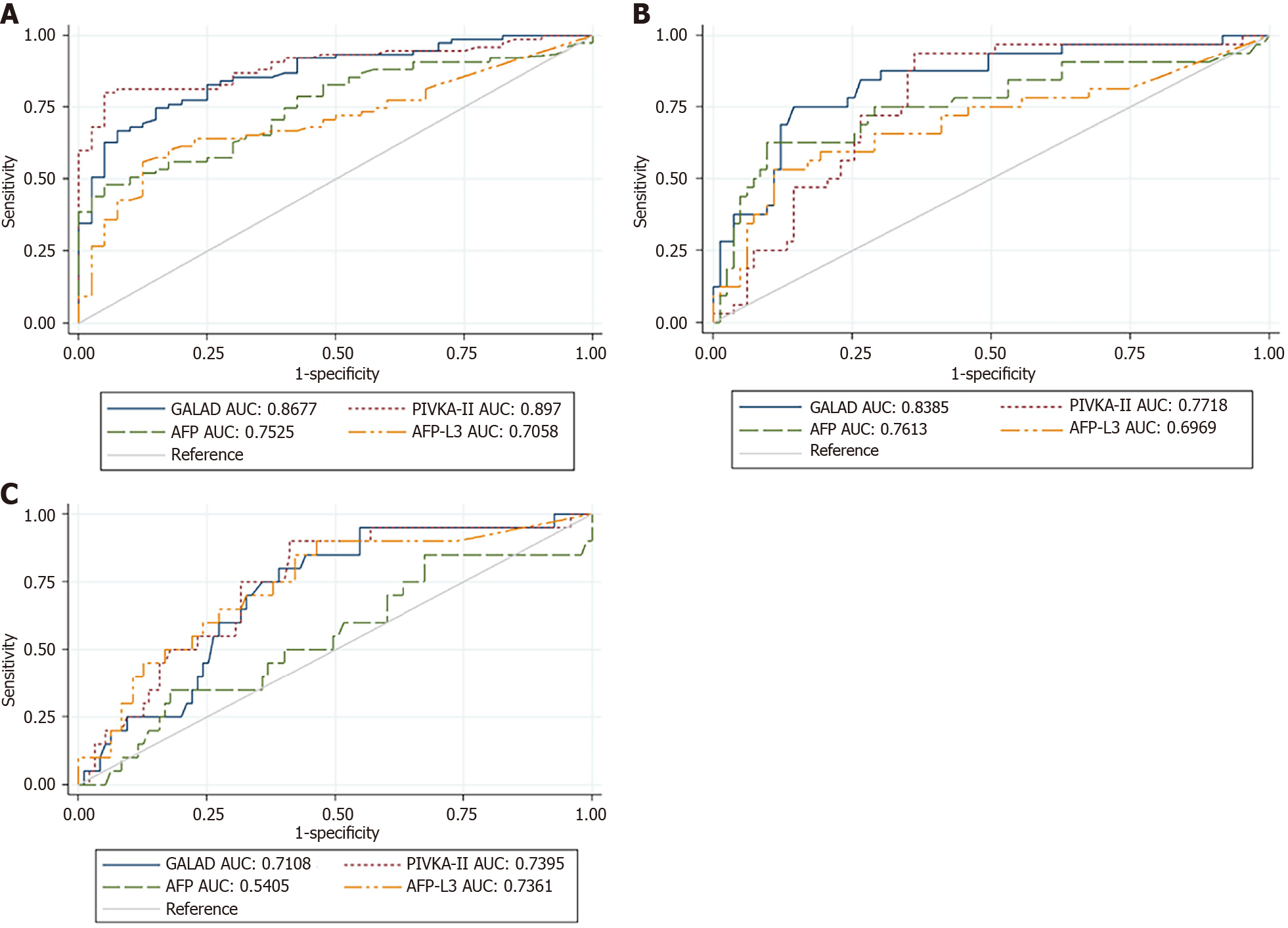
Exactly 115 individuals were diagnosed with HCC. The GALAD score increased with disease severity. Between BCLC-0/A and BCLC-B/C/D, the GALAD score predicted HCC staging with an area under the curve (AUC) of 0.868 (95%CI: 0.80–0.93). For identifying the curative HCC, the AUC of GALAD score was significantly higher than that of Alpha-fetoprotein (AFP) (0.753) and Lens culinaris agglutinin-reactive fraction of AFP-L3 (0.706), and as good as that of Protein induced by vitamin K absence-II (PIVKA-II) (0.897). For detecting aggressive features, the GALAD score gave an AUC of 0.839 (95%CI: 0.75–0.92) and significantly outperformed compared to that of AFP (0.761) and AFP-L3 (0.697), with a trend of superiority to that of PIVKA-II (0.772). The performance to predict 1-year mortality of GALAD score (AUC: 0.711, 95%CI: 0.60–0.82) was better than that of AFP (0.541) and as good as that of PIVKA-II (0.736). The optimal cutoff value of GALAD score was ≥ 6.83, with a specificity of 72.63% for exhibiting substantial reduction in the 1-year mortality.
The GALAD model can diagnose HCC at the curative stage, including the characteristic of advanced disease, more than that by AFP and AFP-L3, but not PIVKA-II. The GALAD score can be used to predict the 1-year mortality of HCC.
Core Tip: The GALAD score performance showed a benefit not only in the accuracy of curative hepatocellular carcinoma (HCC) staging but also in the characteristic of advanced disease. Incorporating the GALAD model may increase the opportunity for prognosis prioritization for patients with HCC to predict the 1-year mortality.
- Citation: Jitpraphawan O, Ruamtawee W, Treewatchareekorn M, Sethasine S. Diagnostic and prognostic performances of GALAD score in staging and 1-year mortality of hepatocellular carcinoma: A prospective study. World J Gastroenterol 2024; 30(17): 2343-2353
- URL: https://www.wjgnet.com/1007-9327/full/v30/i17/2343.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i17.2343
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer-related mortality worldwide. In Thailand, it was the leading cause of cancer-related deaths among males and the third-highest cause among females[1]. Despite the rapid introduction of novel and efficient HCC treatments, screening for HCC using a combination of serum alpha-fetoprotein (AFP) and ultrasonography, slightly improved the early detection rate of HCC[2,3].
Due to the suboptimal improvements obtained from combining AFP with ultrasound, the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases recommended only biannual ultrasound for HCC surveillance[3-5]. Other biomarkers including AFP-L3, the Lens culinaris agglutinin-reactive fraction of AFP, which appears to be more specific for HCC[6,7]; and des-carboxy-prothrombin (DCP), a prothrombin precursor produced by HCC, have been investigated as potential tumor markers for HCC in several ethnic populations[8-10]. Additionally, AFP, AFP-L3, and DCP levels have been extensively studied in relation to prognosis[11-15]. According to a meta-analysis, combining these serum biomarkers may boost sensitivity and specificity compared to that from using each biomarker alone[16].
Previous research has presented a novel diagnostic algorithm, the GALAD score, which considers gender, age, AFP-L3, AFP, and DCP. This model shows enhanced early-stage HCC detection sensitivity[17-20]. As such, the role of the GALAD score in terms of tumor stage or clinical outcomes of HCC was intriguing. Thus, this study aimed to determine the diagnostic/prognostic performances of the GALAD score at various phases of the initial HCC diagnosis, tumor features, and 1-year mortality of patients with HCC and compare the performance of the GALAD score with that of individual serum biomarkers.
This prospective study was conducted at Faculty of Medicine Vajira Hospital, Navamindradhiraj University, Bangkok, Thailand. Participants were required to be at least 18 years old and diagnosed with HCC for the first time between September 2021 and March 2022. Hepatitis B and C virus (HBV and HCV, respectively) infections were verified based on the presence of hepatitis B surface antigen and anti-HCV antibodies. Alcohol consumption was considered a cause of HCC when there was a significant and documented history of alcohol abuse in the patient[21]. Metabolic-associated fatty liver disease (MAFLD) was diagnosed based on either an increase in liver ultrasound echogenicity or steatosis, as determined by a transient liver stiffness test in the presence of metabolic syndrome[22]. Cirrhosis was defined by: (1) Histology; (2) cirrhosis characteristics in imaging studies using ultrasound or computed tomography (CT) imaging (nodular configuration of the liver, dilated portal vein (PV), splenomegaly with or without ascites); or (3) transient elastography with a cut point of liver stiffness greater than 12.5 kPa. The predictive prognosis of cirrhosis was evaluated using both the Child-Pugh and Model for End-Stage Liver Disease scores. HCC was diagnosed based on: (1) Histology; or (2) presence of cirrhosis with the radiologic characteristics of HCC on a CT scan. In chronic HBV infection, HCC diagnosis is determined by the presence of both radiologic hallmarks and an AFP level > 200 ng/mL[23-25]. Patients with other primary liver malignancies, patients with liver metastases, and patients with HCC who did not complete the informed consent form were excluded. All individuals who receive a diagnosis of HCC will be eligible for standard treatment according to the Barcelona Clinic Liver Cancer (BCLC) guideline. Each individual provided informed consent prior to enrollment, after the researchers had thoroughly explained them the research topic. Participants’ demographic information, including age, sex, body mass index, symptoms at initial presentation, HCC etiology, performance status, tumor burden and characteristics of advanced disease, was collected. The following biochemical data were collected: Total blood count, blood urea nitrogen, creatinine level, levels of electrolytes, coagulogram, liver function test, and viral markers.
Individual blood samples (10 mL) for AFP, AFP-L3, and Protein induced by vitamin K absence- II (PIVKA-II) (stored at 20 °C) were measured using the µTASWako i30 fully automated immune analyzer (Fujifilm Wako Pure Chemical Corporation, Osaka, Japan)[26]. Microfluidic chips were analyzed using liquid-phase binding assays, followed by capillary electrophoresis and fluorescence detection. This machine had lower limited detections of 0.3 ng/mL for AFP and 5 mAU/mL for PIVKA-II for each biomarker. The percentage of AFP-L3 was measured when the AFP value was more than 0.3 ng/mL.
The GALAD score was computed as follows: Z = -10.08 + 0.09 × age + 1.67 × sex + 2.34 Log (AFP) + 0.04 × (AFP-L3) + 1.33 Log (DCP). The formula × 0.012 was used to convert the DCP (ng/mL) to the PIVKA-II value (mAU/mL)[27]. https://www.mayoclinic.org/medical-professionals/model-end-stage-liver-disease/GALAD is a web-based calculator. The definition of sex was 1 for males and 0 for females. The BCLC staging system was used to categorize tumor stages[28]. Participants who were eligible received conventional HCC treatment. Mortality was measured from the initial HCC diagnosis until death or at the end of follow-up at 1 year.
The sample size determined the number of participants for estimating accuracy index formula using the area under the curve (AUC)[29]:
n = Z2α/2V (AUC)/d2
Where n is the sample size for each group with disease/endpoint and non-disease/non-endpoint, V(AUC)= (0.0099 x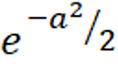 ) x (6a2 + 16), a = φ-1(AUC)x 1.414, and φ-1 is the inverse of standard cumulative normal distribution or ZAUC.
) x (6a2 + 16), a = φ-1(AUC)x 1.414, and φ-1 is the inverse of standard cumulative normal distribution or ZAUC.
The reference AUC values of GALAD score for calculating sample sizes for staging patients with HCC into each of the five stages of BCLC (0/A/B/C/D) and predicting other endpoints were not obtained from any research through our review. Therefore, we used the values from previous research that studied the GALAD performance to diagnose HCC. The reference AUC values were obtained from a systematic review and meta-analysis of the performance of GALAD score for diagnosing HCC in patients with chronic liver diseases, with an AUC of 0.86 for detecting early-stage HCC (BCLC 0/A)[20], and from a multicenter case-control study with an AUC of 0.933 for advanced HCC (BCLC B/C/D)[30]. The reference value used to calculate the sample size for 1-year mortality was obtained from a recent study with an AUC of 0.792[31]. Zα/2 is the Z-score corresponding to a normal distribution defined as 1.96 (α = 0.05) with 95% confidence; and the degree of precision of estimate being about 0.129[20], 0.14[30], and 0.1188[31] (d was set at 15% error of AUC) for statistical significance, with V(AUC) = 0.092483. Therefore, the required sample size obtained by inserting the formula[29] was 44 participants for detecting early-stage HCC, 18 participants for detecting advanced-stage HCC, and 68 participants for predicting 1-year mortality of patients with HCC.
The institutional review board of the Faculty of Medicine at Vajira Hospital (COA 165/2564) authorized the study protocol, which was conducted in accordance with the ethical norms of the 1975 Declaration of Helsinki. All participants provided written informed consent prior to enrollment in the trial.
STATA version 13.0 (Stata Corporation, College Station, TX, United States) was used for statistical analyses. Pearson’s chi-squared or Fisher’s exact tests were used to assess comparable categorical data between HCC stages. A one-way analysis of variance (one-way ANOVA) or Kruskal–Wallis test was used to compare continuous variables. Statistical significance was set at P value < 0.05. The diagnostic and prognostic performance of GALAD score and biomarkers were estimated using the c-statistic, commonly referred to as the area under the receiver operating characteristic (ROC) curve analysis. The area under the ROC curve, sensitivity, specificity, positive and negative predictive values, positive and negative likelihood ratio, correctly classified of the GALAD score and each tumor marker for staging HCC, clinical features of the advanced disease, and 1-year mortality were obtained.
The diagnostic and prognostic performance of the GALAD score and other tumor markers were measured using the AUC that reflects the overall discriminative value of the test. The AUC ranged from 0 (at 0.5 representing “the probability of a false and true diagnosis is both 50%” and such the test is no better than flipping a coin) to 1.0 (indicating excellent discrimination)[32]. Generally, an AUC of 0.75 is considered high enough for use in clinical practice. Therefore, the best cutoff point was the point closest-to- (0,1) corner in the ROC plane (Euclidean distance), that reached 100% sensitivity and 100% specificity. Hence, the cut-points for GALAD score and tumor markers were selected to optimize the values of both sensitivity and specificity based on the Euclidean distance[32,33]. The AUC of the GALAD score and each tumor marker were compared for very early stage (BCLC-0) and early stage (BCLC-A) HCC, curative HCC stage (BCLC-0 to A), and non-curative HCC stage (BCLC B to D), clinical features of poor prognosis, and 1-year mortality.
A total of 115 individuals were diagnosed with HCC, of which 98 (85.2%) were male. The most prevalent symptom was abdominal pain (38.3%), followed by gastrointestinal hemorrhage (10.4%). More than one-third (33.9%) of the patients had no symptoms. The most common cause of chronic hepatitis was hepatitis B (37.4%), followed by chronic hepatitis C (CHC) (12.2%), alcoholic hepatitis (14.8%), and MAFLD (7.8%). The remaining 27.8% of the patients were classified as having mixed etiologies. Eighty percent of the patients with HCC were diagnosed with cirrhosis. Child-Pugh scores A, B, and C were distributed as follows: 56.5%, 34.8%, and 8.7%, respectively. Most participants (68.1%) were in good physical condition. Sixty-four individuals (55.7%) had tumors > 5 cm in size. Fifty patients (43.5%) were diagnosed with a single lesion. The percentage of each stage (0, A, B, C, and D) according to the BCLC criteria was 11.3%, 23.47%, 31.3%, 13.3%, and 20%, respectively. Approximately 65.2% of the patients were in the non-curative stage. Characteristics of advanced-stage HCC (PV thrombosis, macrovascular invasion, and metastasis) were observed in 21.7%, 13.9%, and 8.0% of the cases, respectively. The median levels of AFP, AFP-L3 (%), and PIVKA-II at the time of HCC diagnosis were 38.9 ng/mL (5.4-3305), 7% (1-34.1), and 604 mAU/mL (54-21878), respectively. The median GALAD score for BCLC stages 0, A, B, C, and D were -2.27 (-3.9, 1.2), -0.62 (-1.81, 1.86), 4.15 (1.21, 8.81), 9.59 (6.17, 13.33), and 7.22 (3.7, 10.12), respectively (Table 1). There were no significant differences in median GALAD score and the various etiology of HCC (Supplementary Table 1).
| Total | Stage 0 | Stage A | Stage B | Stage C | Stage D | P valuea | |
| (n = 115) | (n = 13) | (n = 27) | (n = 36) | (n = 16) | (n = 23) | ||
| Age (mean ± SD) | 60.83 ± 12.93 | 60.69 ± 11.64 | 61.07 ± 11.34 | 58.67 ± 15.03 | 62.00 ± 12.73 | 63.22 ± 12.48 | 0.754 |
| Male | 98 (85.2) | 11 (84.6) | 22 (81.5) | 31 (86.1) | 15 (93.8) | 19 (82.6) | 0.849 |
| BMI (kg/m2) | 23.12 ± 3.5 | 24.31 ± 3.12 | 23.43 ± 2.74 | 23.06 ± 3.33 | 22.98 ± 5.06 | 22.26 ± 3.53 | 0.543 |
| Symptom at first presentation | |||||||
| Abdominal pain | 44 (38.3) | 1 (7.7) | 7 (25.9) | 16 (44.4) | 10 (62.5) | 10 (43.5) | 0.02 |
| Jaundice | 7 (6.1) | 1 (7.7) | 0 (0) | 1 (2.8) | 1 (6.3) | 4 (17.4) | 0.106 |
| Anemia | 1 (0.9) | 0 (0) | 0 (0) | 1 (2.8) | 0 (0) | 0 (0) | 0.697 |
| Ascites | 9 (7.8) | 2 (15.4) | 1 (3.7) | 1 (2.8) | 2 (12.5) | 3 (13) | 0.368 |
| Weight loss | 7 (6.1) | 0 (0) | 0 (0) | 5 (13.9) | 0 (0) | 2 (8.7) | 0.102 |
| Fever | 1 (0.9) | 1 (7.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.095 |
| Edema | 4 (3.5) | 0 (0) | 1 (3.7) | 0 (0) | 2 (12.5) | 1 (4.3) | 0.223 |
| Abdominal mass | 3 (2.6) | 0 (0) | 0 (0) | 3 (8.3) | 0 (0) | 0 (0) | 0.154 |
| GI bleeding | 12 (10.4) | 1 (7.7) | 0 (0) | 2 (5.6) | 5 (31.3) | 4 (17.4) | 0.012 |
| Asymptomatic | 39 (33.9) | 8 (61.5) | 17 (63) | 11 (30.6) | 1 (6.3) | 2 (8.7) | < 0.001 |
| Etiology | |||||||
| CHB | 43 (37.4) | 8 (61.5) | 7 (25.9) | 18 (50) | 5 (31.3) | 5 (21.7) | 0.043 |
| CHC | 14 (12.2) | 1 (7.7) | 5 (18.5) | 3 (8.3) | 3 (18.8) | 2 (8.7) | 0.615 |
| Alcohol | 17 (14.8) | 2 (15.4) | 4 (14.8) | 5 (13.9) | 2 (12.5) | 4 (17.4) | 0.995 |
| MAFLD | 9 (7.8) | 1 (7.7) | 4 (14.8) | 2 (5.6) | 1 (6.3) | 1 (4.3) | 0.64 |
| CHB with alcohol | 20 (17.4) | 0 (0) | 4 (14.8) | 4 (11.1) | 5 (31.3) | 7 (30.4) | 0.069 |
| CHC with alcohol | 12 (10.4) | 1 (7.7) | 3 (11.1) | 4 (11.1) | 0 (0) | 4 (17.4) | 0.526 |
| Cirrhosis | 92 (80) | 9 (69.2) | 21 (77.8) | 27 (75) | 12 (75) | 23 (100) | 0.108 |
| CTP-A/B/C (%) | 56.5/34.8/8.7 | 88.9/11.1/0 | 81/19/0 | 70.4/29.6/0 | 50/50/0 | 8.7/56.5/34.8 | < 0.001 |
| MELD (mean ± SD) | 10.31 ± 7.28 | 7.54 ± 7.24 | 8.78 ± 6.73 | 8.28 ± 6.33 | 10.5 ± 6.98 | 16.74 ± 6.17 | < 0.001 |
| Performance status | |||||||
| 0-1 | 79 (68.7) | 12 (92.3) | 26 (96.3) | 36 (100) | 4 (25) | 1 (4.3) | < 0.001 |
| 2 | 2 (1.7) | 0 (0) | 1 (3.7) | 0 (0) | 0 (0) | 1 (4.3) | 0.613 |
| 3 | 15 (13) | 0 (0) | 0 (0) | 0 (0) | 10 (62.5) | 5 (21.7) | < 0.001 |
| 4 | 19 (16.5) | 1 (7.7) | 0 (0) | 0 (0) | 2 (12.5) | 16 (69.6) | < 0.001 |
| Tumor size (cm) | |||||||
| ≤ 2 | 23 (20) | 12 (92.3) | 8 (29.6) | 1 (2.8) | 0 (0) | 2 (8.7) | < 0.001 |
| 2.1-3 | 14 (12.2) | 1 (7.7) | 12 (44.4) | 0 (0) | 0 (0) | 1 (4.3) | < 0.001 |
| 3.1-5 | 14 (12.2) | 0 (0) | 7 (25.9) | 4 (11.1) | 0 (0) | 3 (13) | 0.065 |
| > 5 | 64 (55.7) | 0 (0) | 0 (0) | 31 (86.1) | 16 (100) | 17 (73.9) | < 0.001 |
| Tumor number | |||||||
| 1 | 50 (43.5) | 13 (100) | 14 (51.9) | 17 (47.2) | 2 (12.5) | 4 (17.4) | < 0.001 |
| 2 | 35 (30.4) | 0 (0) | 10 (37) | 8 (22.2) | 10 (62.5) | 7 (30.4) | |
| ≥ 3 | 30 (26.1) | 0 (0) | 3 (11.1) | 11 (30.6) | 4 (25) | 12 (52.2) | |
| Tumor characteristic | |||||||
| Macrovascular invasion | 16 (13.9) | 0 (0) | 0 (0) | 2 (5.6) | 8 (50) | 6 (26.1) | < 0.001 |
| PV thrombosis | 25 (21.7) | 0 (0) | 0 (0) | 1 (2.8) | 11 (68.8) | 13 (56.5) | < 0.001 |
| Metastasis | 10 (8.7) | 0 (0) | 0 (0) | 0 (0) | 6 (37.5) | 4 (17.4) | < 0.001 |
| Liver function test | |||||||
| Albumin (mg/dL) | 3.7 | 3.8 | 4.1 | 3.9 | 3.55 | 2.7 | < 0.001 |
| (3.1, 4.2) | (3.7, 4.4) | (3.5, 4.3) | (3.45, 4.25) | (3.15, 3.7) | (2.3, 3.4) | ||
| Total bilirubin (mg/dL) | 1.04 | 0.91 | 0.69 | 0.86 | 1.35 | 3.44 | < 0.001 |
| (0.63, 1.84) | (0.69, 1.12) | (0.56, 1.64) | (0.55, 1.5) | (1.17, 1.83) | (0.92, 5.19) | ||
| AST (IU/L) | 79 (47, 130) | 34 (29, 67) | 52 (36, 68) | 79 (53, 116.5) | 145 (94.5, 207.5) | 141 (93, 479) | < 0.001 |
| ALT (IU/L) | 41 (24, 65) | 24 (20, 43) | 35 (20, 43) | 53 (38, 71) | 41 (20.5, 124) | 48 (24, 97) | 0.017 |
| ALP (IU/L) | 136 (92, 237) | 86 (71, 108) | 108 (79, 130) | 143.5 (102, 271.5) | 176 (152, 276.5) | 201 (159, 314) | < 0.001 |
| Biomarker (median, 25-75 quartile) | |||||||
| AFP (ng/mL) | 38.9 | 3.1 | 10 | 50 | 22146.3 | 79 | < 0.001 |
| (5.4, 3305.3) | (1.7, 50) | (2.8, 61) | (13.2, 2482.65) | (190.55, 163697) | (17.2, 23475) | ||
| AFP- L3 (%) | 7 | 4.55 | 4.83 | 6.07 | 25.8 | 30.5 | < 0.001 |
| (1, 34.1) | (0.5, 6.76) | (0.5, 8.1) | (0.9, 24.7) | (3.95, 63.9) | (8.16, 74.7) | ||
| |
604 | 26 | 55 | 7820 | 20188 | 4807 | < 0.001 |
| (54, 21878) | (20,35) | (37, 220) | (139, 44019.5) | (761, 91915) | (581, 203031) | ||
| GALAD score | |||||||
| Median (range) | 3.08 | -2.27 | -0.62 | 4.15 | 9.59 | 7.22 | < 0.001 |
| (-0.56, 9.09) | (-3.9, 1.2) | (-1.81, 1.86) | (1.21, 8.81) | (6.17, 13.33) | (3.7, 10.12) | ||
For very early stage (BCLC-0) and early stage (BCLC- A) of HCC: AUC for predicting very early stage HCC was non-significantly superior with GALAD score (0.6097, 95%CI: 0.40 to 0.82) compared to that using individual AFP (0.5655, 95%CI 0.36 to 0.77, P = 0.3721) and AFP-L3 (0.5128, 95%CI 0.32 to 0.70, P = 0.2992), and was comparable with that of PIVKA-II (0.7236, 95%CI 0.54 to 0.91, P = 0.1798). PIVKA-II showed a trend of higher AUC than that of AFP (P = 0.1100) and AFP-L3 (P = 0.0409; Table 2).
| Scores | Barcelona Clinic Liver Cancer staging | Aggressive featuresa,2 | 1-year mortality3 | |
| 0 vs A2 | 0/A vs B/C/D2 | |||
| GALAD | ||||
| AUC (95%CI) | 0.6097 (0.40–0.82) | 0.8677 (0.80–0.93) | 0.8385 (0.75–0.92) | 0.7108 (0.60–0.82) |
| Cutoff values | ≥ -1.95 | ≥ 2.65 | ≥ 7.22 | ≥ 6.83 |
| Sensitivity/Specificity (%) | 81.48/53.85 | 74.67/85.00 | 75.00/85.54 | 60.00/72.63 |
| PPV/NPV (%) | 78.57/58.33 | 90.32/64.15 | 66.67/89.87 | 31.58/89.61 |
| Positive/Negative LR | 1.77/0.34 | 4.98/0.30 | 5.19/0.29 | 2.19/0.55 |
| Correctly classified (%) | 72.50 | 78.26 | 82.61 | 70.43 |
| PIVKA-II | ||||
| AUC (95%CI) | 0.7236 (0.54–0.91) | 0.8970 (0.84–0.95) | 0.7718 (0.68–0.86) | 0.7395 (0.63–0.85) |
| Cutoff values | ≥ 37 | ≥ 354 | ≥ 581 | ≥ 2959 |
| Sensitivity/Specificity (%) | 77.78/76.92 | 81.33/92.50 | 93.75/63.86 | 75.00/68.42 |
| PPV/NPV (%) | 87.50/62.50 | 95.31/72.55 | 50.00/96.36 | 33.33/92.86 |
| Positive/Negative LR | 3.37/0.29 | 10.84/0.20 | 2.59/0.10 | 2.38/0.37 |
| Correctly classified (%) | 77.50 | 85.22 | 72.17 | 69.57 |
| AFP | ||||
| AUC (95%CI) | 0.5655 (0.36–0.77) | 0.7525 (0.67–0.84) | 0.7613 (0.65–0.87) | 0.5405 (0.39–0.69) |
| Cutoff values | ≥ 4.4 | ≥ 16 | ≥ 79 | ≥ 79 |
| Sensitivity/Specificity (%) | 74.07/53.85 | 74.67/60.00 | 75.00/71.08 | 50.00/60.00 |
| PPV/NPV (%) | 76.92/50.00 | 77.78/55.81 | 50.00/88.06 | 20.83/85.07 |
| Positive/Negative LR | 1.60/0.48 | 1.87/0.42 | 2.59/0.35 | 1.25/0.83 |
| Correctly classified (%) | 67.50 | 69.57 | 72.17 | 58.26 |
| AFP-L3 | ||||
| AUC (95%CI) | 0.5128 (0.32–0.70) | 0.7058 (0.61–0.80) | 0.6969 (0.58–0.82) | 0.7361 (0.61–0.86) |
| Cutoff values | ≥ 4.83 | ≥ 7.4 | ≥ 12.3 | ≥ 14.5 |
| Sensitivity/Specificity (%) | 51.85/53.85 | 64.00/77.50 | 65.63/71.08 | 65.00/72.63 |
| PPV/NPV (%) | 70.00/35.00 | 84.21/53.45 | 46.67/84.29 | 33.33/90.79 |
| Positive/Negative LR | 1.12/0.89 | 2.84/0.46 | 2.27/0.48 | 2.38/0.48 |
| Correctly classified (%) | 52.50 | 68.70 | 69.57 | 71.30 |
| Comparison of AUC | ||||
| GALAD and PIVKA-II | P = 0.1798 | P = 0.2353 | P = 0.0683 | P = 0.5349 |
| GALAD and AFP | P = 0.3721 | P < 0.001a | P = 0.0136a | P < 0.001a |
| GALAD and AFP-L3 | P = 0.2992 | P < 0.001a | P = 0.0152a | P = 0.6656 |
| PIVKA-II and AFP | P = 0.1100 | P < 0.001a | P = 0.8476 | P = 0.0086a |
| PIVKA-II and AFP-L3 | P = 0.0409a | P < 0.001a | P = 0.2801 | P = 0.9610 |
For curative HCC stage (BCLC- 0 to A) and non-curative HCC stage (BCLC B to D): The diagnostic performance of the GALAD score in curative HCC stage was more accurate than that in very early stage (0.6097 to 0.8677). For the prediction of BCLC stage 0 to A, AUC of the GALAD score (0.8677, 95%CI 0.80 to 0.93) was significantly higher than that of AFP (0.7525, 95%CI 0.67 to 0.84, P < 0.001) and AFP-L3 (0.7058, 95%CI 0.61 to 0.80, P < 0.001). The performance of the GALAD score was as good as PIVKA-II: AUC at 0.8970 (95%CI 0.84 to 0.95, P = 0.2353) to predict for curative stage of HCC. For predicting curative stage of HCC, the optimal cutoff value of the GALAD score was ≥ 2.65, with 74.67% sensitivity and 85.0% specificity (Table 2 and Figure 1A).
For characteristics of advanced diseases and patient’s mortality: The characteristics of advanced diseases were composed of any one of the following: Macrovascular invasion, 13.9% (n = 16); PV thrombosis, 21.7% (n = 25); and extrahepatic metastasis, 8.7% (n = 10). For predicting aggressive feature of HCC, AUC of the GALAD score (0.8385, 95%CI 0.75 to 0.92) significantly outperformed that of AFP (0.7613, 95%CI 0.65 to 0.87, P = 0.0136) and AFP-L3 (0.6969, 95%CI 0.58 to 0.82, P = 0.0152); moreover, the AUC of the GALAD score showed a superior trend to that of PIVKA (0.7718, 95%CI 0.68 to 0.86, P = 0.0683). There was no significant difference in aggressive feature between PIVKA-II and AFP (P = 0.8476; Table 2 and Figure 1B).
After the diagnosis of HCC, standard therapy was commenced following the BCLC guidelines; however, after 1-year of follow-up, none of the 20 patients (17.39%) survived. Reasons for mortality included severe or terminal disease (12), HCC rupture (3), or sepsis (5). All non-survivors were in advanced or terminal stages, except one patient with BCLC stage B and ruptured HCC.
In terms of predicting the 1-year mortality, the GALAD score (0.7108, 95%CI 0.61–0.82) outperformed AFP (0.5405, 95%CI 0.39–0.69, P < 0.001), but was as good as PIVKA-II (0.7395, 95%CI 0.63–0.85, P = 0.5349). For predicting 1-year mortality of HCC, the optimal cutoff value of the GALAD score was ≥ 6.83, this cutoff value was within intermediate or advanced HCC stage, and gave 60.0% sensitivity and 72.63% specificity. The optimal cutoff value of PIVKA-II for patient’s mortality was ≥ 2959 mAU/mL, which gave a slightly lower specificity (68.42%). Even though the GALAD score gave lower sensitivity, it gave higher specificity to predict 1-year mortality than that of PIVKA-II (Table 2 and Figure 1C).
The prevalence of HCC among all cancer diagnoses in the global population is increasing. Currently, ultrasonography with AFP is the recommended screening method for diagnosing HCC. Other biomarkers, including AFP, AFP-L3, and PIVKA-II, have been shown to boost the diagnostic sensitivity for HCC[34-38]. The GALAD score was developed to enhance the utility of combining various markers derived from sex and age, which has been validated in non-alcoholic fatty liver disease[18,38].
Our investigation revealed a higher median GALAD score in CHC, which was consistent with data from the Chinese population, with the use of the GALAD score in CHC offering greater diagnostic power for HCC than for other etiologies[39]. In contrast to previous studies in which the GALAD utility was established for early HCC diagnosis[16,18], our study demonstrates the novel utility of the GALAD score for accurate HCC staging and prognosis. We emphasized that GALAD has multiple utilities, and it was demonstrated that GALAD has a high AUC for HCC at the curative stage. According to comparable patient’s age for each HCC stage, the increase of the GALAD score in parallel with higher BCLC stage, with the exception of BCLC stage D, reflects liver decompensation itself but not tumor burden.
Serum prothrombin produced by the lack of PIVKA-II is an aberrant prothrombin caused by a deficiency of gamma-glutamyl carboxylase and vitamin K[40]. PIVKA-II is not only more specific than AFP, but a positive result also increases the likelihood of micro- and macrovascular invasion[41,42]. Owing to the distinct synthesis pathway, the benefits of PIVKA-II were complementary to those of AFP[43]. Enrollment in our curative-stage HCC study showed that PIVKA-II performed much better than AFP. Some nations have suggested using both biomarkers for the initial detection of HCC. The cause of HCC in majority of our patients was chronic hepatitis B (CHB), and our PIVKA-II identification of early CHB-related HCC was comparable to that of previous reports[44,45]. According to our data, using PIVKA-II was associated with poor performance in MAFLD-HCC enrollment, which is consistent with the results of a previous study[46]. However, because only a few MAFLD cases were analyzed, our findings may not be definitive. Regarding to the enrollment of substantial number of patients with BCLC stage 0 or A, this may be of interest for prospective clinical outcome prediction research.
The characteristics of advanced disease can be evaluated by utilizing both GALAD scores and PIVKA-II levels. In the present study, after 1-year of mortality monitoring, all non-survivors were in the intermediate and advanced stages of HCC, as established by disease staging and treatment. According to a recent systematic review and meta-analysis, GALAD score was useful for diagnostic performance but not for prognosis of disease progression[20]. We highlight the updated significance of GALAD performance not only in the accuracy of curative HCC staging, but also in the characteristic of advanced diseases and predicting a decline in 1-year mortality. A combination of age and PIVKA-II in the GALAD model may offer an indirect method for determining the aggressiveness of malignancies[47]. It would be intriguing to incorporate the GALAD model in future research for increasing the staging accuracy and for the personalized prognosis of HCC. High pre-treatment serum AFP-L3% levels were also associated with a poor prognosis in our patients with HCC; however, we believe that high AFP-L3% levels may have considerable prognostic value for patients with HCC with low AFP concentrations.
This study had some limitations. Despite the ability to recruit participants based on our sample size calculations, the number of participants in each stage, particularly the initial stage, is very low. This could potentially impact the performance of the GALAD application. If more patients with HCC utilize the GALAD score, the overall advantage in proper disease staging and prognosis may become more apparent. Second, our clinical data was archived with short follow-up periods. The prognostic performance may increase with longer follow-up periods.
The GALAD model can enhance the diagnosis of HCC at the curative stage more than that by AFP and AFP-L3, but not PIVKA-II; moreover, it can also be used to predict the 1-year mortality in non-curative HCC.
We thank Dr. Chadakarn Phaloprakarn for the scientific advice and Miss Kanokwan Sansuk for the reference format.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Thailand
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Zhou S, China S-Editor: Li L L-Editor: A P-Editor: Yu HG
| 1. | Sethasine S, Simasingha N, Ratana-Amornpin S, Mahachai V. Real world for management of hepatocellular carcinoma: a large population-based study. Scand J Gastroenterol. 2023;58:1153-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 2. | Colquhoun SD, Wan YY. Hepatocellular carcinoma diagnosis and treatment: An overview. Liver Res. 2020;4:159-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Tzartzeva K, Obi J, Rich NE, Parikh ND, Marrero JA, Yopp A, Waljee AK, Singal AG. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology. 2018;154:1706-1718.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 638] [Cited by in RCA: 811] [Article Influence: 115.9] [Reference Citation Analysis (0)] |
| 4. | Kim TH, Kim SY, Tang A, Lee JM. Comparison of international guidelines for noninvasive diagnosis of hepatocellular carcinoma: 2018 update. Clin Mol Hepatol. 2019;25:245-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 160] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 5. | Tan CH, Low SC, Thng CH. APASL and AASLD Consensus Guidelines on Imaging Diagnosis of Hepatocellular Carcinoma: A Review. Int J Hepatol. 2011;2011:519783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Moriya S, Morimoto M, Numata K, Nozaki A, Shimoyama Y, Kondo M, Nakano M, Maeda S, Tanaka K. Fucosylated fraction of alpha-fetoprotein as a serological marker of early hepatocellular carcinoma. Anticancer Res. 2013;33:997-1001. [PubMed] |
| 7. | Tateishi R, Yoshida H, Matsuyama Y, Mine N, Kondo Y, Omata M. Diagnostic accuracy of tumor markers for hepatocellular carcinoma: a systematic review. Hepatol Int. 2008;2:17-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 8. | Zhang YS, Chu JH, Cui SX, Song ZY, Qu XJ. Des-γ-carboxy prothrombin (DCP) as a potential autologous growth factor for the development of hepatocellular carcinoma. Cell Physiol Biochem. 2014;34:903-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Ji J, Wang H, Li Y, Zheng L, Yin Y, Zou Z, Zhou F, Zhou W, Shen F, Gao C. Diagnostic Evaluation of Des-Gamma-Carboxy Prothrombin versus α-Fetoprotein for Hepatitis B Virus-Related Hepatocellular Carcinoma in China: A Large-Scale, Multicentre Study. PLoS One. 2016;11:e0153227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 10. | Volk ML, Hernandez JC, Su GL, Lok AS, Marrero JA. Risk factors for hepatocellular carcinoma may impair the performance of biomarkers: a comparison of AFP, DCP, and AFP-L3. Cancer Biomark. 2007;3:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Toyoda H, Kumada T, Tada T, Kaneoka Y, Maeda A, Kanke F, Satomura S. Clinical utility of highly sensitive Lens culinaris agglutinin-reactive alpha-fetoprotein in hepatocellular carcinoma patients with alpha-fetoprotein <20 ng/mL. Cancer Sci. 2011;102:1025-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Matsuda M, Asakawa M, Amemiya H, Fujii H. Lens culinaris agglutinin-reactive fraction of AFP is a useful prognostic biomarker for survival after repeat hepatic resection for HCC. J Gastroenterol Hepatol. 2011;26:731-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Okuda H, Nakanishi T, Takatsu K, Saito A, Hayashi N, Yamamoto M, Takasaki K, Nakano M. Clinicopathologic features of patients with hepatocellular carcinoma seropositive for alpha-fetoprotein-L3 and seronegative for des-gamma-carboxy prothrombin in comparison with those seropositive for des-gamma-carboxy prothrombin alone. J Gastroenterol Hepatol. 2002;17:772-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Li T, Yu Y, Liu J, Tian X, Kong M, Wu L, Tang S, Gu S, Zhao J, Cui Y, Hu J. PIVKA-II level is correlated to development of portal vein tumor thrombus in patients with HBV-related hepatocellular carcinoma. Infect Agent Cancer. 2019;14:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Ma XL, Zhu J, Wu J, Tian L, Gao YY, Zhang CY, Zhou Y, Dai Q, Wang BL, Pan BS, Zhou J, Fan J, Yang XR, Guo W. Significance of PIVKA-II levels for predicting microvascular invasion and tumor cell proliferation in Chinese patients with hepatitis B virus-associated hepatocellular carcinoma. Oncol Lett. 2018;15:8396-8404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Wang X, Zhang Y, Yang N, He H, Tao X, Kou C, Jiang J. Evaluation of the Combined Application of AFP, AFP-L3%, and DCP for Hepatocellular Carcinoma Diagnosis: A Meta-analysis. Biomed Res Int. 2020;2020:5087643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 17. | Yang JD, Addissie BD, Mara KC, Harmsen WS, Dai J, Zhang N, Wongjarupong N, Ali HM, Ali HA, Hassan FA, Lavu S, Cvinar JL, Giama NH, Moser CD, Miyabe K, Allotey LK, Algeciras-Schimnich A, Theobald JP, Ward MM, Nguyen MH, Befeler AS, Reddy KR, Schwartz M, Harnois DM, Yamada H, Srivastava S, Rinaudo JA, Gores GJ, Feng Z, Marrero JA, Roberts LR. GALAD Score for Hepatocellular Carcinoma Detection in Comparison with Liver Ultrasound and Proposal of GALADUS Score. Cancer Epidemiol Biomarkers Prev. 2019;28:531-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 152] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 18. | Best J, Bechmann LP, Sowa JP, Sydor S, Dechêne A, Pflanz K, Bedreli S, Schotten C, Geier A, Berg T, Fischer J, Vogel A, Bantel H, Weinmann A, Schattenberg JM, Huber Y, Wege H, von Felden J, Schulze K, Bettinger D, Thimme R, Sinner F, Schütte K, Weiss KH, Toyoda H, Yasuda S, Kumada T, Berhane S, Wichert M, Heider D, Gerken G, Johnson P, Canbay A. GALAD Score Detects Early Hepatocellular Carcinoma in an International Cohort of Patients With Nonalcoholic Steatohepatitis. Clin Gastroenterol Hepatol. 2020;18:728-735.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 187] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 19. | Best J, Bilgi H, Heider D, Schotten C, Manka P, Bedreli S, Gorray M, Ertle J, van Grunsven LA, Dechêne A. The GALAD scoring algorithm based on AFP, AFP-L3, and DCP significantly improves detection of BCLC early stage hepatocellular carcinoma. Z Gastroenterol. 2016;54:1296-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 20. | Guan MC, Zhang SY, Ding Q, Li N, Fu TT, Zhang GX, He QQ, Shen F, Yang T, Zhu H. The Performance of GALAD Score for Diagnosing Hepatocellular Carcinoma in Patients with Chronic Liver Diseases: A Systematic Review and Meta-Analysis. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 21. | Donato F, Tagger A, Gelatti U, Parrinello G, Boffetta P, Albertini A, Decarli A, Trevisi P, Ribero ML, Martelli C, Porru S, Nardi G. Alcohol and hepatocellular carcinoma: the effect of lifetime intake and hepatitis virus infections in men and women. Am J Epidemiol. 2002;155:323-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 441] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 22. | Lin S, Huang J, Wang M, Kumar R, Liu Y, Liu S, Wu Y, Wang X, Zhu Y. Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver Int. 2020;40:2082-2089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 400] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 23. | Yang JD, Kim WR, Coelho R, Mettler TA, Benson JT, Sanderson SO, Therneau TM, Kim B, Roberts LR. Cirrhosis is present in most patients with hepatitis B and hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2011;9:64-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 193] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 24. | Bargellini I, Battaglia V, Bozzi E, Lauretti DL, Lorenzoni G, Bartolozzi C. Radiological diagnosis of hepatocellular carcinoma. J Hepatocell Carcinoma. 2014;1:137-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6573] [Article Influence: 469.5] [Reference Citation Analysis (1)] |
| 26. | Kagebayashi C, Yamaguchi I, Akinaga A, Kitano H, Yokoyama K, Satomura M, Kurosawa T, Watanabe M, Kawabata T, Chang W, Li C, Bousse L, Wada HG, Satomura S. Automated immunoassay system for AFP-L3% using on-chip electrokinetic reaction and separation by affinity electrophoresis. Anal Biochem. 2009;388:306-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 27. | Johnson PJ, Pirrie SJ, Cox TF, Berhane S, Teng M, Palmer D, Morse J, Hull D, Patman G, Kagebayashi C, Hussain S, Graham J, Reeves H, Satomura S. The detection of hepatocellular carcinoma using a prospectively developed and validated model based on serological biomarkers. Cancer Epidemiol Biomarkers Prev. 2014;23:144-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 229] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 28. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 2612] [Article Influence: 870.7] [Reference Citation Analysis (59)] |
| 29. | Hajian-Tilaki K. Sample size estimation in diagnostic test studies of biomedical informatics. J Biomed Inform. 2014;48:193-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 647] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 30. | Huang C, Fang M, Xiao X, Wang H, Gao Z, Ji J, Liu L, Gu E, Li Y, Wang M, Gao C. Validation of the GALAD model for early diagnosis and monitoring of hepatocellular carcinoma in Chinese multicenter study. Liver Int. 2022;42:210-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 31. | Wang M, Guo H, Zhang B, Shang Y, Zhang S, Liu X, Cao P, Fan Y, Tan K. Transcription factors-related molecular subtypes and risk prognostic model: exploring the immunogenicity landscape and potential drug targets in hepatocellular carcinoma. Cancer Cell Int. 2024;24:9. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 32. | Grobbee DE, Hoes AW. Clinical Epidemiology Principles, Methods, and Applications for Clinical Research. 2nd ed. Burlington, MA: Jones & Bartlett Learning; 2014. |
| 33. | Perkins NJ, Schisterman EF. The inconsistency of "optimal" cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol. 2006;163:670-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1048] [Cited by in RCA: 1287] [Article Influence: 67.7] [Reference Citation Analysis (0)] |
| 34. | Ertle JM, Heider D, Wichert M, Keller B, Kueper R, Hilgard P, Gerken G, Schlaak JF. A combination of α-fetoprotein and des-γ-carboxy prothrombin is superior in detection of hepatocellular carcinoma. Digestion. 2013;87:121-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 35. | Choi JY, Jung SW, Kim HY, Kim M, Kim Y, Kim DG, Oh EJ. Diagnostic value of AFP-L3 and PIVKA-II in hepatocellular carcinoma according to total-AFP. World J Gastroenterol. 2013;19:339-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 36. | Donati M, Brancato G, Donati A. Clinical biomarkers in hepatocellular carcinoma (HCC). Front Biosci (Schol Ed). 2010;2:571-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Song PP, Xia JF, Inagaki Y, Hasegawa K, Sakamoto Y, Kokudo N, Tang W. Controversies regarding and perspectives on clinical utility of biomarkers in hepatocellular carcinoma. World J Gastroenterol. 2016;22:262-274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 88] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (1)] |
| 38. | Sumida Y, Yoneda M, Seko Y, Ishiba H, Hara T, Toyoda H, Yasuda S, Kumada T, Hayashi H, Kobayashi T, Imajo K, Tada T, Kawaguchi T, Eguchi Y, Oeda S, Takahashi H, Tomita E, Okanoue T, Nakajima A; Japan Study Group Of Nafld Jsg-Nafld. Surveillance of Hepatocellular Carcinoma in Nonalcoholic Fatty Liver Disease. Diagnostics (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 39. | Liu M, Wu R, Liu X, Xu H, Chi X, Wang X, Zhan M, Wang B, Peng F, Gao X, Shi Y, Wen X, Ji Y, Jin Q, Niu J. Validation of the GALAD Model and Establishment of GAAP Model for Diagnosis of Hepatocellular Carcinoma in Chinese Patients. J Hepatocell Carcinoma. 2020;7:219-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 40. | Inagaki Y, Tang W, Makuuchi M, Hasegawa K, Sugawara Y, Kokudo N. Clinical and molecular insights into the hepatocellular carcinoma tumour marker des-γ-carboxyprothrombin. Liver Int. 2011;31:22-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 41. | Fujikawa T, Shiraha H, Yamamoto K. Significance of des-gamma-carboxy prothrombin production in hepatocellular carcinoma. Acta Med Okayama. 2009;63:299-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (1)] |
| 42. | Park H, Park JY. Clinical significance of AFP and PIVKA-II responses for monitoring treatment outcomes and predicting prognosis in patients with hepatocellular carcinoma. Biomed Res Int. 2013;2013:310427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 43. | Fu J, Li Y, Li Z, Li N. Clinical utility of decarboxylation prothrombin combined with α-fetoprotein for diagnosing primary hepatocellular carcinoma. Biosci Rep. 2018;38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 44. | Chen J, Wu G, Li Y. Evaluation of Serum Des-Gamma-Carboxy Prothrombin for the Diagnosis of Hepatitis B Virus-Related Hepatocellular Carcinoma: A Meta-Analysis. Dis Markers. 2018;2018:8906023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 45. | Bertino G, Ardiri AM, Calvagno GS, Bertino N, Boemi PM. Prognostic and diagnostic value of des-γ-carboxy prothrombin in liver cancer. Drug News Perspect. 2010;23:498-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 46. | Hashimoto E, Tokushige K. Hepatocellular carcinoma in non-alcoholic steatohepatitis: Growing evidence of an epidemic? Hepatol Res. 2012;42:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 47. | Yang Y, Li G, Lu Z, Liu Y, Kong J, Liu J. Progression of Prothrombin Induced by Vitamin K Absence-II in Hepatocellular Carcinoma. Front Oncol. 2021;11:726213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |