Published online Apr 7, 2024. doi: 10.3748/wjg.v30.i13.1791
Peer-review started: December 30, 2023
First decision: January 16, 2024
Revised: February 3, 2024
Accepted: March 14, 2024
Article in press: March 14, 2024
Published online: April 7, 2024
Processing time: 94 Days and 17.4 Hours
Liver transplantation (LT) has become the most efficient treatment for pediatric and adult end-stage liver disease and the survival time after transplantation is becoming longer due to the development of surgical techniques and perioperative management. However, long-term side-effects of immunosuppressants, like infection, metabolic disorders and malignant tumor are gaining more attention. Immune tolerance is the status in which LT recipients no longer need to take any immunosuppressants, but the liver function and intrahepatic histology maintain normal. The approaches to achieve immune tolerance after transplantation include spontaneous, operational and induced tolerance. The first two means require no specific intervention but withdrawing immunosuppressant gradually during follow-up. No clinical factors or biomarkers so far could accurately predict who are suitable for immunosuppressant withdraw after transplantation. With the understanding to the underlying mechanisms of immune tolerance, many strategies have been developed to induce tolerance in LT recipients. Cellular strategy is one of the most promising methods for immune tolerance induction, including chimerism induced by hematopoietic stem cells and adoptive transfer of regulatory immune cells. The safety and efficacy of various cell products have been evaluated by prospective preclinical and clinical trials, while obstacles still exist before translating into clinical practice. Here, we will summarize the latest perspectives and concerns on the clinical application of cellular strategies in LT recipients.
Core Tip: Immune tolerance after liver transplantation could significantly reduce the long-term side-effects of immunosuppressants. Compared with operational and spontaneous tolerance, induced tolerance by cellular therapy could reduce immunosuppressant dosage at early stage after transplantation. Regulatory immune cells could suppress the inflammatory response, which are widely explored in preclinical and clinical trials. So far, regulatory CD4+ T cells, mesenchymal stromal cells and regulatory dendritic cells are mostly studied. However, even the safety and tolerability of cellular therapy in transplantation recipients have been validated, the overall efficacy of tolerance induction is unsatisfactory. Detailed exploration is required in the future.
- Citation: Zhou AW, Jin J, Liu Y. Cellular strategies to induce immune tolerance after liver transplantation: Clinical perspectives. World J Gastroenterol 2024; 30(13): 1791-1800
- URL: https://www.wjgnet.com/1007-9327/full/v30/i13/1791.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i13.1791
With development of surgical techniques and perioperative management, liver transplantation (LT) has become the most efficient treatment for end-stage liver diseases, with 75%-90% recipients owning the chance to survival over 5 years after transplantation[1-3]. Most recipients need lifelong immunosuppression to prevent acute rejection and achieve ideal long-term outcomes[4]. However, the long-term side-effects caused by immunosuppressant usage, like opportunistic infection, malignant tumor, metabolic disorders and renal dysfunction have become the dominant obstacle to the long-term survival rates and life quality of LT recipients, especially for pediatric ones[5]. When matching by gender and age, LT recipients suffer a 2.4-fold higher risk of death and a 5.8-fold higher risk of premature death than the general population[6]. Therefore, strategies facilitating reduction or discontinuation of immunosuppressant are highly desirable.
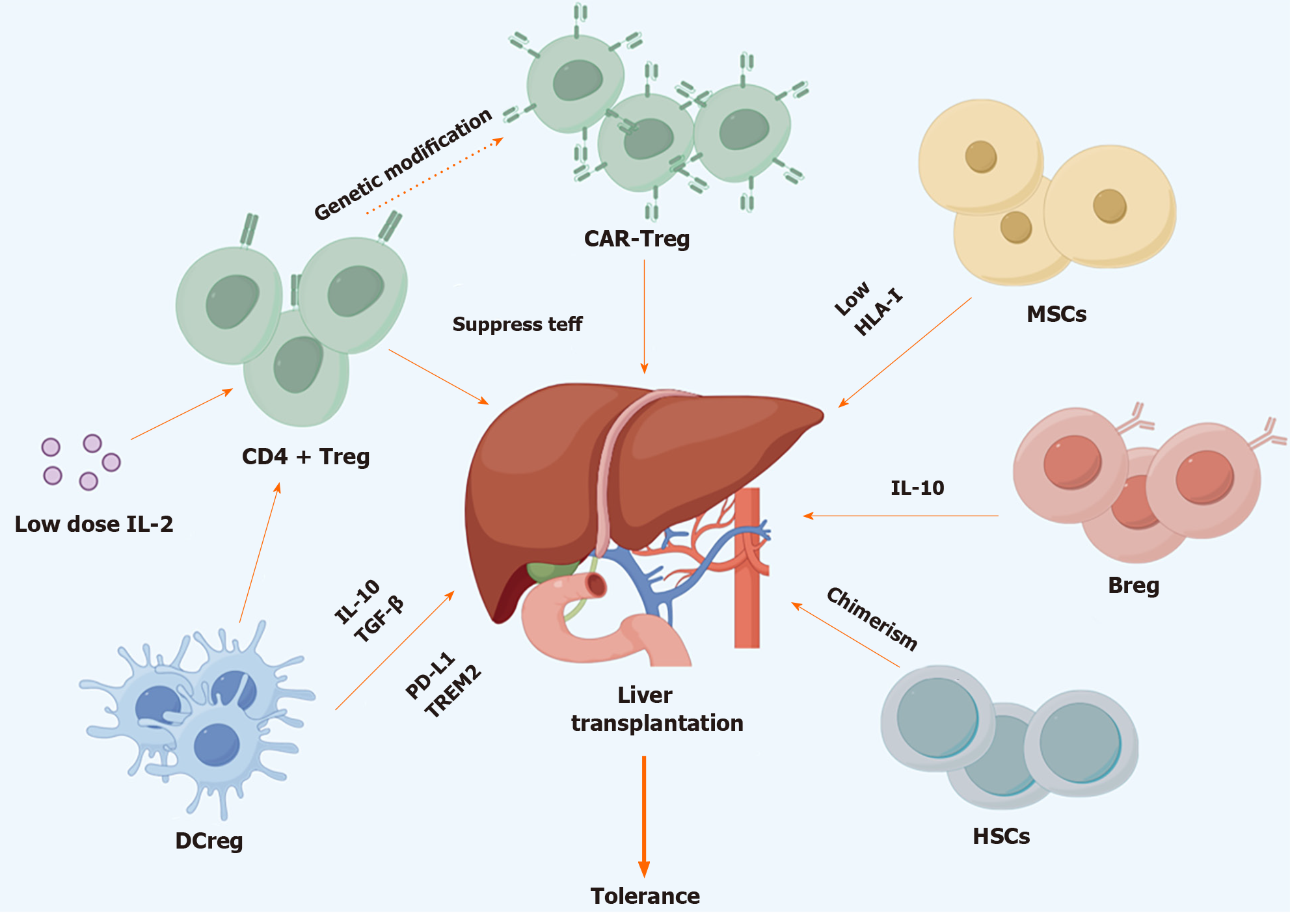
Safely minimizing or discontinuing immunosuppressant without compromising allograft function could be an attractive strategy to improve the long-term post-LT survival[7,8]. The liver is considered a tolerogenic organ as LT recipients require less immunosuppressants and suffer lower risk of immune rejection when comparing with other solid organ recipients[9-11]. Anatomically, antigen-rich blood from the gastrointestinal tract flow through the intrahepatic sinusoids and scanned by antigen-presenting cells (APCs) and lymphocytes, while liver sinusoidal endothelial cells (LSECs) and hepatocytes act as scavenger cells contributing to the clearance of antigens[12-15]. Apoptosis of cytotoxic T lymphocyte (CTL) that induced by FasL and Programmed death ligand 1 (PD-L1) expressed by LSECs and hepatic stellate cells facilities the maintenance of the tolerogenic state[16,17]. Regulatory immune cells inside the liver like regulatory CD4+ T cell (Treg), Regulatory B cell (Breg) and regulatory dendritic cell (DCreg) also contribute to the development of tolerance by suppressing intrahepatic immune assault[18]. Traditionally, tolerance could be achieved through spontaneous, operational and induced ways. The first two means for tolerance were generally conducted in long-term follow-up recipients, while induced tolerance could be finished at early stage after transplantation, regardless of recipient’s medical background, which makes it more applicable in clinical practices. Cellular strategy by infusion of ex vivo regulatory immune cell to create suppressive immune environment is the mainstream to achieve inducible tolerance. So far, many clinical and preclinical trials have been conducted to prove the efficacy of induced tolerance in LT recipients. Although promising preclinical and early-stage clinical results have proven the safety and feasibility of cellular therapy, its application in clinical practices requires more validation (Figure 1).
Treg is a specialized subset of CD4 T cells characterized by the high expression of FoxP3 and interleukin-2 (IL-2) receptor CD25, and low expression of IL-7 receptor CD127[19]. Based on developmental origins, CD4+ Tregs could be divided into thymic Tregs (tTregs) and peripheral Tregs (pTregs). Functionally, tTregs primarily recognize self-antigens, whereas the pTreg could recognize “non-self” pathogens like infectious antigens or gastrointestinal commensal microbiota-derived antigens[20,21]. Tregs induce immune tolerance through a variety of pathways, including direct and indirect pathways. Currently, adoptive transfer of Tregs is becoming an attractive therapy to restore self-tolerance in autoimmune diseases and preventing occurrence of graft vs host disease (GVHD) after hematopoietic transplantation[20,22]. Valuable information has arisen from multiple clinical trials designed to test the safety and efficacy of Treg therapy in solid organ transplantation. Infusion of peripheral polyclonal Tregs in kidney transplantation recipients had proven the safety and feasibility of Treg therapy in solid organ transplantation recipients[23-25]. The first study to describe successful withdrawn of immunosuppressant following Treg therapy was reported by Todo et al[26] (UMIN-000015789), in which 7 out of 10 Living donor liver transplant recipients achieved tolerance[26]. However, less than 20% of the cell product in this study was defined as Tregs, which made it difficult to determine the precise immunoregulatory mechanisms involved. Then Sánchez-Fueyo et al[27] evaluated the safety and applicability of autologous polyclonal Treg adoptive transfer in adult LT recipients through a phase I single-center clinical trial (ThRIL, NCT02166177)[27]. They found that Treg transfer was safe, transiently increased the amount of peripheral circulating Tregs and reduced T cell responses to donor antigens, which might facilitate the reduction or complete discontinuation of immunosuppression following LT. More recently, Tang et al[28] reported the results of a phase I/II trial (ARTEMIS, NCT02474199) of autologous donor alloantigen reactive Treg therapy in living donor liver transplant recipients. Four of five recipients who received sufficient infusion dosage encountered acute rejection during the process of immunosuppressant withdrawal[28]. Therefore, despite the capability of Tregs to ameliorate acute rejection in several preclinical studies, we are far from achieving induced post-LT tolerance in the clinic.
Expanding the circulating Tregs through cytokines treatment has also been tested. Since studies have suggested that Tregs have a reduced IL-2 receptor (IL-2R) signaling threshold than Teff cells, it has been hypothesized that the administration of low doses of IL-2 could preferentially activate Tregs and limit the activation of effector T cells[29,30]. In a murine skin transplantation model, IL-2 treatment with donor-specific Tregs infusion preferentially enhanced the proliferation of Tregs in skin allograft and draining lymph nodes, which prolonged skin allograft survival[31]. Lim et al[32] conducted the first clinical trial of using low-dose IL-2 to induce immune tolerance in adult LT recipients (NCT02949492). Although all participants achieved increased circulating Tregs after treatment, no expansion of donor-reactive Tregs or accumulation of intrahepatic Tregs was found, which was accompanied an interferon-γ dependent inflammatory response[32]. Reasons for the failure of IL-2 induced tolerance includes off-target effects of IL-2 to other immune cells, heterogeneity of IL-2 expanded Tregs and lack of intrahepatic infiltrated Tregs after treatment[33,34]. Therefore, IL-2 mutants or alternative induction approaches should be explored in the future.
Another approach to induce tolerance using Tregs is to generate antigen specific Treg cells by introducing synthetic chimeric antigen receptors (CARs) or engineered T cell receptors, enabling direct antigen recognition in the context of an antigen-major histocompatibility complex (MHC)-peptide complex[20]. In murine model, engineered CAR-Tregs with the ability to directly recognize allogeneic MHC class II molecules could facilitate the long-term acceptance of MHC-mismatched allograft[35]. Human CAR-Tregs targeting the human leukocyte antigen (HLA)-A2 could prevent HLA-A2-positive cells mediated xenogeneic GVHD in mouse models[36]. A multicenter phase I/II clinical trial aiming to evaluate the safety and tolerability of autologous anti-HLA-A2 CAR-Tregs in LT recipients (LIBERATE, NCT05234190) had been launched in Europe, while no further results had been reported so far. Since autologous CD4+ T cells and DCs played an important role in mediated posttransplant rejection, CAR-Treg targeting CD83, which was mainly expressed on alloreactive conventional CD4+ T cells and proinflammatory DCs had been proven to be efficient in preventing GVHD after hematopoietic cell transplantation[37]. Another target protein for CAR-Tregs therapy is GAD65, which had been proved efficient to suppress CTLs in diabetes and islet transplantation mouse model[38]. However, since some studies of CAR effector T cells suggested that the density of the antigen recognized by the CAR must be high on the target cell to trigger activation, the efficiency of CAR-Tregs in the induction of tolerance still need more exploration[39].
Dendritic cells (DCs) are potent APCs linking the innate and adaptive immune process[40]. DCregs are characterized by reduced expression of MHC and co-stimulatory molecules (like CD80 and CD86), and increased level of death-inducing ligands (FasL) and co-inhibitory ligands (PD-L1)[4,41]. Functionally, DCregs are able to produce anti-inflammatory cytokines [IL-10 and Transforming growth factor β (TGF-β)] and impede T cell proliferation[42,43]. Unlike conventional DCs in secondary lymphoid tissue, intrahepatic DCs display tolerogenic properties. Intrahepatic DCs express comparatively low levels of Toll-like receptor 4, leading to limited adaptive immune response[44-46]. DCs express human leukocyte Ig-like receptor B family members result suppression of T cell responses[47]. Murine model indicated that Flt3 and DAP12 regulated liver myeloid DCs maturation and tolerance[46,48]. Meanwhile, donor-derived plasmacytoid DCs express high levels of DAP12, TREM2 and PD-L1 to attenuate graft-infiltrating effector T cell responses, enhance CD4+ Tregs function and promote spontaneous acceptance of allografts[49]. Therefore, application of tolerogenic DCs or DCregs could be an alternative approach to reach the goal of induced tolerance after LT.
The safety and feasibility of autologous DCreg therapy have been confirmed in autoimmune disorders, including rheumatoid arthritis, type I diabetes and Crohn’s disease[50-52]. Many studies in murine transplantation model have confirmed the ability of donor derived DCs to function immunoregulatory properties and enhance organ allograft survival[53,54]. A clinically relevant nonhuman primate model also confirmed the safety and efficacy of donor derived DCs in prolonging MHC mis-matched renal allograft survival[55]. Angus W Thomson performed the first-in-human prospective study of donor-derived DCregs in LT recipients (NCT03164265), which proved the safety of DCreg therapy and changes of immune status after infusion[42]. However, no increase of tolerance rates in LT recipients has been observed so far[56]. One possible reason is the short-lived survival of donor DCreg after infusion, which may be killed by the NK cells. Meanwhile, the influence of donor derived DCreg to the immune status of the recipients is unclear. Even though circulating Treg/Teff ratio witness increase after DCreg infusion, whether the change is sufficient to induce tolerance is questionable. Therefore, although DCs are critical in the balance between allograft rejection and tolerance, extensive data from clinical trials and mechanism study are required before translating DCreg therapy into clinical practice in LT recipients.
Mesenchymal stromal cells (MSCs) are nonhematopoietic multipotent and self-renewing cells with the ability to differentiate into mesodermal lineages like chondrocytes, adipocytes and osteocytes[57]. Surface marker profiles of MSCs include high expression of CD73, CD105 and CD90, and negative expression of CD45, CD34, and CD19[58]. Under normal conditions, MSCs express low levels of HLA-I molecules and do not express HLA-II nor co-stimulatory molecules, which renders MSCs immunoregulatory and anti-inflammatory properties[57,59]. Meanwhile, MSCs can be isolated from diverse tissues and are easy to cultivate, expand and store without losing clinical applicability in vitro[60,61]. In murine models, MSCs polarize both naïve and memory T cells toward Foxp3+ Treg phenotype and induce long-term graft acceptance[62-64]. Based on the preclinical results, lots of clinical trials have been conducted to study the therapeutical potentials of MSCs. Several pilot studies have proved that donor-derived bone marrow MSCs combined with a sparing dose of immunosuppressant dosage could maintain normal allograft function and don’t increase the acute rejection occurrence in kidney transplantation recipients[65,66]. Yves Beguin performed the first human phase I clinical trial (NCT01429038) exploring the safety and tolerability of third-party MSCs infusion in LT recipients[67]. This study showed no toxicity, but a single MSC infusion was not sufficient to allow discontinuition of immunosuppression. Casiraghi et al[68] further revealed that MSCs infusion in LT recipients prior to transplantation was safe and could induce positive changes in peripheral immunoregulatory T and NK cells, but no tolerance data was reported[68]. The MYSTEP1 trial (NCT02957552) is the first clinical trial aiming to investigate the safety and feasibility of donor-derived NSCs in pediatric LT recipients, while no further data is available so far[69]. Pre-clinical studies in transplantation models exhibited a comparable capacity of autologous and allogeneic MSCs to induce Treg expansion and prolong allograft survival[70]. A single-center prospective clinical trial (NCT00658073) to inoculated living kidney transplantation recipients with bone marrow derived autologous MSCs revealed that autologous MSCs therapy resulted in lower incidence of acute rejection, decreased risk of opportunistic infection and better estimated renal function[71]. Modifications of MSCs like cytokine pretreatment, genetic modification or three-dimensional culture can improve the immunoregulatory capacity of MSCs and may be an effective approach to improve the regulatory capacity of MSCs under transplantation circumstance[72]. In rat LT model, infusion of TGF-β overexpressing or HO-1 transduced MSCs could induce a local immunosuppression in liver grafts, ameliorate the acute rejection and reduce the overall mortality[73,74]. However, no genetic modified MSCs have been applied in clinical trial so far. More detailed study to the molecular mechanism to the regulatory feature of MSCs is required before its clinical application (Table 1).
| Ref. | Cellular products | Sample size/stage | Recipients | Status | Trial ID |
| Todo et al[26] | Donor derived Treg | 10/Phase I/II | Adult | 7/10 recipients reached tolerance | UMIN-000015789 |
| Sánchez-Fueyo et al[27] | Recipient derived polyclonal Treg | 6/Phase I | Adult | Safe for recipients, not test tolerance | NCT02166177 |
| Tang et al[28] | Recipient derived darTreg | 5/Phase I/II | Adult | 4/5 encountered acute rejection | NCT02474199 |
| Lim et al[32] | IL-2 infusion | 5/Phase I/II | Adult | All suffered rejection | NCT02949492 |
| Sánchez-Fueyo et al[91] | CAR-Treg targeting HLA-A2 | 18-70/Phase I/II | Adult | Recruiting | NCT05234190 |
| Tran et al[92] | Donor derived DCreg | 13/Phase I/II | Adult | Safe for recipients, no tolerance tested | NCT03164265 |
| Detry et al[67] | Third party MSCs | 10/Phase I/II | Adult | Safe for recipients, no tolerance achieved | NCT01429038 |
| Casiraghi et al[68] | Third party MSCs | 10/Phase I/II | Adult | Safe for recipients | NCT01429038 |
Infusion of hematopoietic stem cells (HSCs) to create mixed chimerism could establish donor-specific tolerance and retain immunocompetence for primary immune responses[75,76]. Kawai et al[77] conducted the first successful application of mixed chimerism in tolerance induction in human kidney transplantation[77]. Four of five recipients who received combined bone marrow and kidney transplants from HLA single-haplotype mismatched living related donors and nonmyeloablative preparative regimen discontinued all immunosuppressive therapy with normal renal function. Patients with end stage renal disease and hematologic malignancies are thought as the most suitable candidates for combined bone marrow and kidney transplant[78]. The idea of hematopoietic chimerism to achieve graft tolerance has also been explored in LT recipients. Spontaneous complete hematopoietic chimerism could be found in deceased donor LT recipient even without HSCs transplant and tolerance was achieved[79]. Tryphonopoulos et al[80] reported that donor bone marrow cell infusion had no influence on the overall survival rates or tolerance of adult LT recipients[80]. Kim et al[81] and Hartleif et al[82] indicated that LT with myeloablative HSC transplant could establish full tolerance in both pediatric and adult recipients, but the life-threatening complication of GVHD couldn’t be avoided[81-83]. Thus, the current dilemma of HSC therapy is that intense myeloablative or non-myeloablative conditioning therapy may not be tolerated by transplantation recipient, while lacking conditioning therapy could compromise the therapeutic efficiency of donor HSC infusion[80]. Therefore, careful selection of recipients might be the key to the safety and efficiency of HSCs therapy.
Bregs are immunosuppressive cells that express immune regulatory cytokines, like IL-10, TGF-β and IL-35, and support immunological tolerance[84]. In autoimmune disease mice model, the most widely investigated Breg population comprises the IL-10 producing B10 cells which could modulate T cell function[85]. It was found that B lymphocytes could interact with allo- and autoreactive effector cells, while selective manipulation of B cell function rather than depletion could be a promising approach to promote tolerance to allografts[86]. In murine heart and islet transplantation models, combined treatment with anti-CD45RB and anti-ICAM/LFA/TIM1 facilitated allograft acceptance via B-cell dependent mechanism[86,87]. A possible explanation is that B cells act as Treg inducing antigen presenting cells to promote Tregs function during this process. Single-cell RNA sequencing data of transplanted murine kidney revealed a shifting from a T cell-dominant to a B cell-rich population at 6 months after transplant with an increased Breg signature, implicating a key role of Bregs in the maintenance of allograft tolerance[88]. Analysis to stable renal transplantation recipients also revealed that B cells from tolerant patients had lower numbers of plasma cells and secreted more IL-10, which reduced production of proinflammatory cytokines and promoted transplantation tolerance[89,90]. However, so far, no clinical trial using Bregs to induce tolerance after transplantation have been conducted. One of the challenges is the lack of lineage marker for Bregs, which impedes the in vitro and ex vivo isolation and expansion of Bregs. Another problem is the unclear underlying mechanism of Bregs in the process of tolerance induction. Therefore, Breg induced tolerance has a long way to go before translation into clinical practice.
Immune tolerance is one of the most promising approaches to avoid the long-term side-effects of immunosuppressants in LT recipients. Cellular therapy could be applied before and after transplantation, which could induce early tolerance. So far, many clinical trials have demonstrated the feasibility and safety of cellular therapies for autoimmune diseases, hematopoietic stem cell transplantation and solid organ transplantation. However, most clinical results for cellular induced tolerance after LT are still very preliminary. The most obstacle is how to improve the efficiency of induced tolerance by cellular therapy. Detailed study to underlying mechanisms of immunoregulatory immune cells, genetic modification and optimal infusion dosage should be conducted in the future.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sanal MG, India S-Editor: Li L L-Editor: A P-Editor: Yu HG
| 1. | Kasahara M, Umeshita K, Eguchi S, Eguchi H, Sakamoto S, Fukuda A, Egawa H, Haga H, Kokudo N, Sakisaka S, Takada Y, Tanaka E, Uemoto S, Ohdan H. Outcomes of Pediatric Liver Transplantation in Japan: A Report from the Registry of the Japanese Liver Transplantation Society. Transplantation. 2021;105:2587-2595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 2. | Bowring MG, Massie AB, Chu NM, Bae S, Schwarz KB, Cameron AM, Bridges JFP, Segev DL, Mogul DB. Projected 20- and 30-Year Outcomes for Pediatric Liver Transplant Recipients in the United States. J Pediatr Gastroenterol Nutr. 2020;70:356-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Elisofon SA, Magee JC, Ng VL, Horslen SP, Fioravanti V, Economides J, Erinjeri J, Anand R, Mazariegos GV; Society of Pediatric Liver Transplantation Research Group. Society of pediatric liver transplantation: Current registry status 2011-2018. Pediatr Transplant. 2020;24:e13605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 4. | Wang P, Jiang Z, Wang C, Liu X, Li H, Xu D, Zhong L. Immune Tolerance Induction Using Cell-Based Strategies in Liver Transplantation: Clinical Perspectives. Front Immunol. 2020;11:1723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Noble J, Terrec F, Malvezzi P, Rostaing L. Adverse effects of immunosuppression after liver transplantation. Best Pract Res Clin Gastroenterol. 2021;54-55:101762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Åberg F, Gissler M, Karlsen TH, Ericzon BG, Foss A, Rasmussen A, Bennet W, Olausson M, Line PD, Nordin A, Bergquist A, Boberg KM, Castedal M, Pedersen CR, Isoniemi H. Differences in long-term survival among liver transplant recipients and the general population: a population-based Nordic study. Hepatology. 2015;61:668-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 7. | Feng S, Bucuvalas JC, Mazariegos GV, Magee JC, Sanchez-Fueyo A, Spain KM, Lesniak A, Kanaparthi S, Perito E, Venkat VL, Burrell BE, Alonso EM, Bridges ND, Doo E, Gupta NA, Himes RW, Ikle D, Jackson AM, Lobritto SJ, Jose Lozano J, Martinez M, Ng VL, Rand EB, Sherker AH, Sundaram SS, Turmelle YP, Wood-Trageser M, Demetris AJ. Efficacy and Safety of Immunosuppression Withdrawal in Pediatric Liver Transplant Recipients: Moving Toward Personalized Management. Hepatology. 2021;73:1985-2004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 8. | Tanimine N, Ohira M, Tahara H, Ide K, Tanaka Y, Onoe T, Ohdan H. Strategies for Deliberate Induction of Immune Tolerance in Liver Transplantation: From Preclinical Models to Clinical Application. Front Immunol. 2020;11:1615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Crispe IN. Immune tolerance in liver disease. Hepatology. 2014;60:2109-2117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 210] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 10. | Thomson AW, Vionnet J, Sanchez-Fueyo A. Understanding, predicting and achieving liver transplant tolerance: from bench to bedside. Nat Rev Gastroenterol Hepatol. 2020;17:719-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 11. | Terrault NA, Francoz C, Berenguer M, Charlton M, Heimbach J. Liver Transplantation 2023: Status Report, Current and Future Challenges. Clin Gastroenterol Hepatol. 2023;21:2150-2166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 134] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 12. | Biswas SK, Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 2009;30:475-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 881] [Cited by in RCA: 1015] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 13. | Heymann F, Tacke F. Immunology in the liver--from homeostasis to disease. Nat Rev Gastroenterol Hepatol. 2016;13:88-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 827] [Article Influence: 91.9] [Reference Citation Analysis (0)] |
| 14. | Limmer A, Ohl J, Kurts C, Ljunggren HG, Reiss Y, Groettrup M, Momburg F, Arnold B, Knolle PA. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nat Med. 2000;6:1348-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 561] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 15. | Klugewitz K, Blumenthal-Barby F, Schrage A, Knolle PA, Hamann A, Crispe IN. Immunomodulatory effects of the liver: deletion of activated CD4+ effector cells and suppression of IFN-gamma-producing cells after intravenous protein immunization. J Immunol. 2002;169:2407-2413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Dangi A, Sumpter TL, Kimura S, Stolz DB, Murase N, Raimondi G, Vodovotz Y, Huang C, Thomson AW, Gandhi CR. Selective expansion of allogeneic regulatory T cells by hepatic stellate cells: role of endotoxin and implications for allograft tolerance. J Immunol. 2012;188:3667-3677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Uchiyama H, Kishihara K, Minagawa R, Hashimoto K, Sugimachi K, Nomoto K. Crucial Fas-Fas ligand interaction in spontaneous acceptance of hepatic allografts in mice. Immunology. 2002;105:450-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Mathew JM, Ansari MJ, Gallon L, Leventhal JR. Cellular and functional biomarkers of clinical transplant tolerance. Hum Immunol. 2018;79:322-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Pilat N, Steiner R, Sprent J. Treg Therapy for the Induction of Immune Tolerance in Transplantation-Not Lost in Translation? Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 20. | Raffin C, Vo LT, Bluestone JA. T(reg) cell-based therapies: challenges and perspectives. Nat Rev Immunol. 2020;20:158-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 491] [Article Influence: 98.2] [Reference Citation Analysis (0)] |
| 21. | Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, Santacruz N, Peterson DA, Stappenbeck TS, Hsieh CS. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478:250-254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 858] [Cited by in RCA: 822] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 22. | Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S, Negrin RS. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9:1144-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1005] [Cited by in RCA: 1023] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 23. | Landwehr-Kenzel S, Zobel A, Hoffmann H, Landwehr N, Schmueck-Henneresse M, Schachtner T, Roemhild A, Reinke P. Ex vivo expanded natural regulatory T cells from patients with end-stage renal disease or kidney transplantation are useful for autologous cell therapy. Kidney Int. 2018;93:1452-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Sawitzki B, Harden PN, Reinke P, Moreau A, Hutchinson JA, Game DS, Tang Q, Guinan EC, Battaglia M, Burlingham WJ, Roberts ISD, Streitz M, Josien R, Böger CA, Scottà C, Markmann JF, Hester JL, Juerchott K, Braudeau C, James B, Contreras-Ruiz L, van der Net JB, Bergler T, Caldara R, Petchey W, Edinger M, Dupas N, Kapinsky M, Mutzbauer I, Otto NM, Öllinger R, Hernandez-Fuentes MP, Issa F, Ahrens N, Meyenberg C, Karitzky S, Kunzendorf U, Knechtle SJ, Grinyó J, Morris PJ, Brent L, Bushell A, Turka LA, Bluestone JA, Lechler RI, Schlitt HJ, Cuturi MC, Schlickeiser S, Friend PJ, Miloud T, Scheffold A, Secchi A, Crisalli K, Kang SM, Hilton R, Banas B, Blancho G, Volk HD, Lombardi G, Wood KJ, Geissler EK. Regulatory cell therapy in kidney transplantation (The ONE Study): a harmonised design and analysis of seven non-randomised, single-arm, phase 1/2A trials. Lancet. 2020;395:1627-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 306] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 25. | Roemhild A, Otto NM, Moll G, Abou-El-Enein M, Kaiser D, Bold G, Schachtner T, Choi M, Oellinger R, Landwehr-Kenzel S, Juerchott K, Sawitzki B, Giesler C, Sefrin A, Beier C, Wagner DL, Schlickeiser S, Streitz M, Schmueck-Henneresse M, Amini L, Stervbo U, Babel N, Volk HD, Reinke P. Regulatory T cells for minimising immune suppression in kidney transplantation: phase I/IIa clinical trial. BMJ. 2020;371:m3734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 115] [Article Influence: 23.0] [Reference Citation Analysis (1)] |
| 26. | Todo S, Yamashita K, Goto R, Zaitsu M, Nagatsu A, Oura T, Watanabe M, Aoyagi T, Suzuki T, Shimamura T, Kamiyama T, Sato N, Sugita J, Hatanaka K, Bashuda H, Habu S, Demetris AJ, Okumura K. A pilot study of operational tolerance with a regulatory T-cell-based cell therapy in living donor liver transplantation. Hepatology. 2016;64:632-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 329] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 27. | Sánchez-Fueyo A, Whitehouse G, Grageda N, Cramp ME, Lim TY, Romano M, Thirkell S, Lowe K, Fry L, Heward J, Kerr A, Ali J, Fisher C, Lewis G, Hope A, Kodela E, Lyne M, Farzaneh F, Kordasti S, Rebollo-Mesa I, Jose Lozano J, Safinia N, Heaton N, Lechler R, Martínez-Llordella M, Lombardi G. Applicability, safety, and biological activity of regulatory T cell therapy in liver transplantation. Am J Transplant. 2020;20:1125-1136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 151] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 28. | Tang Q, Leung J, Peng Y, Sanchez-Fueyo A, Lozano JJ, Lam A, Lee K, Greenland JR, Hellerstein M, Fitch M, Li KW, Esensten JH, Putnam AL, Lares A, Nguyen V, Liu W, Bridges ND, Odim J, Demetris AJ, Levitsky J, Taner T, Feng S. Selective decrease of donor-reactive T(regs) after liver transplantation limits T(reg) therapy for promoting allograft tolerance in humans. Sci Transl Med. 2022;14:eabo2628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 29. | Yu A, Snowhite I, Vendrame F, Rosenzwajg M, Klatzmann D, Pugliese A, Malek TR. Selective IL-2 responsiveness of regulatory T cells through multiple intrinsic mechanisms supports the use of low-dose IL-2 therapy in type 1 diabetes. Diabetes. 2015;64:2172-2183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 30. | Koreth J, Matsuoka K, Kim HT, McDonough SM, Bindra B, Alyea EP 3rd, Armand P, Cutler C, Ho VT, Treister NS, Bienfang DC, Prasad S, Tzachanis D, Joyce RM, Avigan DE, Antin JH, Ritz J, Soiffer RJ. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med. 2011;365:2055-2066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 851] [Cited by in RCA: 877] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 31. | Ratnasothy K, Jacob J, Tung S; Boardman D; Lechler RI, Sanchez-Fueyo A, Martinez-Llordella M, Lombardi G. IL-2 therapy preferentially expands adoptively transferred donor-specific Tregs improving skin allograft survival. Am J Transplant. 2019;19:2092-2100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 32. | Lim TY, Perpiñán E, Londoño MC, Miquel R, Ruiz P, Kurt AS, Kodela E, Cross AR, Berlin C, Hester J, Issa F, Douiri A, Volmer FH, Taubert R, Williams E, Demetris AJ, Lesniak A, Bensimon G, Lozano JJ, Martinez-Llordella M, Tree T, Sánchez-Fueyo A. Low dose interleukin-2 selectively expands circulating regulatory T cells but fails to promote liver allograft tolerance in humans. J Hepatol. 2023;78:153-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 40] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 33. | Liao W, Schones DE, Oh J, Cui Y, Cui K, Roh TY, Zhao K, Leonard WJ. Priming for T helper type 2 differentiation by interleukin 2-mediated induction of interleukin 4 receptor alpha-chain expression. Nat Immunol. 2008;9:1288-1296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 236] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 34. | Bénéchet AP, De Simone G, Di Lucia P, Cilenti F, Barbiera G, Le Bert N, Fumagalli V, Lusito E, Moalli F, Bianchessi V, Andreata F, Zordan P, Bono E, Giustini L, Bonilla WV, Bleriot C, Kunasegaran K, Gonzalez-Aseguinolaza G, Pinschewer DD, Kennedy PTF, Naldini L, Kuka M, Ginhoux F, Cantore A, Bertoletti A, Ostuni R, Guidotti LG, Iannacone M. Dynamics and genomic landscape of CD8(+) T cells undergoing hepatic priming. Nature. 2019;574:200-205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 164] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 35. | Tsang JY, Tanriver Y, Jiang S, Xue SA, Ratnasothy K, Chen D, Stauss HJ, Bucy RP, Lombardi G, Lechler R. Conferring indirect allospecificity on CD4+CD25+ Tregs by TCR gene transfer favors transplantation tolerance in mice. J Clin Invest. 2008;118:3619-3628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 235] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 36. | MacDonald KG, Hoeppli RE, Huang Q, Gillies J, Luciani DS, Orban PC, Broady R, Levings MK. Alloantigen-specific regulatory T cells generated with a chimeric antigen receptor. J Clin Invest. 2016;126:1413-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 379] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 37. | Shrestha B, Walton K, Reff J, Sagatys EM, Tu N, Boucher J, Li G, Ghafoor T, Felices M, Miller JS, Pidala J, Blazar BR, Anasetti C, Betts BC, Davila ML. Human CD83-targeted chimeric antigen receptor T cells prevent and treat graft-versus-host disease. J Clin Invest. 2020;130:4652-4662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 38. | Arjomandnejad M, Kopec AL, Keeler AM. CAR-T Regulatory (CAR-Treg) Cells: Engineering and Applications. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 65] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 39. | Walker AJ, Majzner RG, Zhang L, Wanhainen K, Long AH, Nguyen SM, Lopomo P, Vigny M, Fry TJ, Orentas RJ, Mackall CL. Tumor Antigen and Receptor Densities Regulate Efficacy of a Chimeric Antigen Receptor Targeting Anaplastic Lymphoma Kinase. Mol Ther. 2017;25:2189-2201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 278] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 40. | Mellman I. Dendritic cells: master regulators of the immune response. Cancer Immunol Res. 2013;1:145-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 219] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 41. | Vander Lugt B, Riddell J, Khan AA, Hackney JA, Lesch J, DeVoss J, Weirauch MT, Singh H, Mellman I. Transcriptional determinants of tolerogenic and immunogenic states during dendritic cell maturation. J Cell Biol. 2017;216:779-792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 42. | Thomson AW, Humar A, Lakkis FG, Metes DM. Regulatory dendritic cells for promotion of liver transplant operational tolerance: Rationale for a clinical trial and accompanying mechanistic studies. Hum Immunol. 2018;79:314-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 43. | Bell GM, Anderson AE, Diboll J, Reece R, Eltherington O, Harry RA, Fouweather T, MacDonald C, Chadwick T, McColl E, Dunn J, Dickinson AM, Hilkens CM, Isaacs JD. Autologous tolerogenic dendritic cells for rheumatoid and inflammatory arthritis. Ann Rheum Dis. 2017;76:227-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 229] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 44. | Abe M, Tokita D, Raimondi G, Thomson AW. Endotoxin modulates the capacity of CpG-activated liver myeloid DC to direct Th1-type responses. Eur J Immunol. 2006;36:2483-2493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 45. | De Creus A, Abe M, Lau AH, Hackstein H, Raimondi G, Thomson AW. Low TLR4 expression by liver dendritic cells correlates with reduced capacity to activate allogeneic T cells in response to endotoxin. J Immunol. 2005;174:2037-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 115] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 46. | Kingham TP, Chaudhry UI, Plitas G, Katz SC, Raab J, DeMatteo RP. Murine liver plasmacytoid dendritic cells become potent immunostimulatory cells after Flt-3 ligand expansion. Hepatology. 2007;45:445-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 47. | Young NT, Waller EC, Patel R, Roghanian A, Austyn JM, Trowsdale J. The inhibitory receptor LILRB1 modulates the differentiation and regulatory potential of human dendritic cells. Blood. 2008;111:3090-3096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 48. | Yoshida O, Kimura S, Dou L, Matta BM, Yokota S, Ross MA, Geller DA, Thomson AW. DAP12 deficiency in liver allografts results in enhanced donor DC migration, augmented effector T cell responses and abrogation of transplant tolerance. Am J Transplant. 2014;14:1791-1805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 49. | Nakano R, Yoshida O, Kimura S, Nakao T, Yokota S, Ono Y, Minervini MI, Geller DA, Thomson AW. Donor plasmacytoid dendritic cells modulate effector and regulatory T cell responses in mouse spontaneous liver transplant tolerance. Am J Transplant. 2021;21:2040-2055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 50. | Benham H, Nel HJ, Law SC, Mehdi AM, Street S, Ramnoruth N, Pahau H, Lee BT, Ng J, Brunck ME, Hyde C, Trouw LA, Dudek NL, Purcell AW, O'Sullivan BJ, Connolly JE, Paul SK, Lê Cao KA, Thomas R. Citrullinated peptide dendritic cell immunotherapy in HLA risk genotype-positive rheumatoid arthritis patients. Sci Transl Med. 2015;7:290ra87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 279] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 51. | Jauregui-Amezaga A, Cabezón R, Ramírez-Morros A, España C, Rimola J, Bru C, Pinó-Donnay S, Gallego M, Masamunt MC, Ordás I, Lozano M, Cid J, Panés J, Benítez-Ribas D, Ricart E. Intraperitoneal Administration of Autologous Tolerogenic Dendritic Cells for Refractory Crohn's Disease: A Phase I Study. J Crohns Colitis. 2015;9:1071-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 128] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 52. | Giannoukakis N, Phillips B, Finegold D, Harnaha J, Trucco M. Phase I (safety) study of autologous tolerogenic dendritic cells in type 1 diabetic patients. Diabetes Care. 2011;34:2026-2032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 284] [Cited by in RCA: 325] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 53. | Lutz MB, Suri RM, Niimi M, Ogilvie AL, Kukutsch NA, Rössner S, Schuler G, Austyn JM. Immature dendritic cells generated with low doses of GM-CSF in the absence of IL-4 are maturation resistant and prolong allograft survival in vivo. Eur J Immunol. 2000;30:1813-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 54. | Abe M, Wang Z, de Creus A, Thomson AW. Plasmacytoid dendritic cell precursors induce allogeneic T-cell hyporesponsiveness and prolong heart graft survival. Am J Transplant. 2005;5:1808-1819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 101] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 55. | Ezzelarab MB, Zahorchak AF, Lu L, Morelli AE, Chalasani G, Demetris AJ, Lakkis FG, Wijkstrom M, Murase N, Humar A, Shapiro R, Cooper DK, Thomson AW. Regulatory dendritic cell infusion prolongs kidney allograft survival in nonhuman primates. Am J Transplant. 2013;13:1989-2005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 56. | Macedo C, Tran LM, Zahorchak AF, Dai H, Gu X, Ravichandran R, Mohanakumar T, Elinoff B, Zeevi A, Styn MA, Humar A, Lakkis FG, Metes DM, Thomson AW. Donor-derived regulatory dendritic cell infusion results in host cell cross-dressing and T cell subset changes in prospective living donor liver transplant recipients. Am J Transplant. 2021;21:2372-2386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 57. | Vandermeulen M, Grégoire C, Briquet A, Lechanteur C, Beguin Y, Detry O. Rationale for the potential use of mesenchymal stromal cells in liver transplantation. World J Gastroenterol. 2014;20:16418-16432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 58. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12689] [Article Influence: 704.9] [Reference Citation Analysis (2)] |
| 59. | Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringdén O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1228] [Cited by in RCA: 1222] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 60. | Lim JY, Ryu DB, Lee SE, Park G, Min CK. Mesenchymal Stem Cells (MSCs) Attenuate Cutaneous Sclerodermatous Graft-Versus-Host Disease (Scl-GVHD) through Inhibition of Immune Cell Infiltration in a Mouse Model. J Invest Dermatol. 2017;137:1895-1904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 61. | Abomaray FM, Al Jumah MA, Alsaad KO, Jawdat D, Al Khaldi A, AlAskar AS, Al Harthy S, Al Subayyil AM, Khatlani T, Alawad AO, Alkushi A, Kalionis B, Abumaree MH. Phenotypic and Functional Characterization of Mesenchymal Stem/Multipotent Stromal Cells from Decidua Basalis of Human Term Placenta. Stem Cells Int. 2016;2016:5184601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 62. | Prevosto C, Zancolli M, Canevali P, Zocchi MR, Poggi A. Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell-lymphocyte interaction. Haematologica. 2007;92:881-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 270] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 63. | Xu DM, Yu XF, Zhang D, Zhang MX, Zhou JF, Tan PH, Ding YC. Mesenchymal stem cells differentially mediate regulatory T cells and conventional effector T cells to protect fully allogeneic islet grafts in mice. Diabetologia. 2012;55:1091-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 64. | Casiraghi F, Azzollini N, Todeschini M, Cavinato RA, Cassis P, Solini S, Rota C, Morigi M, Introna M, Maranta R, Perico N, Remuzzi G, Noris M. Localization of mesenchymal stromal cells dictates their immune or proinflammatory effects in kidney transplantation. Am J Transplant. 2012;12:2373-2383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 65. | Peng Y, Ke M, Xu L, Liu L, Chen X, Xia W, Li X, Chen Z, Ma J, Liao D, Li G, Fang J, Pan G, Xiang AP. Donor-derived mesenchymal stem cells combined with low-dose tacrolimus prevent acute rejection after renal transplantation: a clinical pilot study. Transplantation. 2013;95:161-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 66. | Pan GH, Chen Z, Xu L, Zhu JH, Xiang P, Ma JJ, Peng YW, Li GH, Chen XY, Fang JL, Guo YH, Zhang L, Liu LS. Low-dose tacrolimus combined with donor-derived mesenchymal stem cells after renal transplantation: a prospective, non-randomized study. Oncotarget. 2016;7:12089-12101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 67. | Detry O, Vandermeulen M, Delbouille MH, Somja J, Bletard N, Briquet A, Lechanteur C, Giet O, Baudoux E, Hannon M, Baron F, Beguin Y. Infusion of mesenchymal stromal cells after deceased liver transplantation: A phase I-II, open-label, clinical study. J Hepatol. 2017;67:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 68. | Casiraghi F, Perico N, Podestà MA, Todeschini M, Zambelli M, Colledan M, Camagni S, Fagiuoli S, Pinna AD, Cescon M, Bertuzzo V, Maroni L, Introna M, Capelli C, Golay JT, Buzzi M, Mister M, Ordonez PYR, Breno M, Mele C, Villa A, Remuzzi G; MSC-LIVER Study Group. Third-party bone marrow-derived mesenchymal stromal cell infusion before liver transplantation: A randomized controlled trial. Am J Transplant. 2021;21:2795-2809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 69. | Hartleif S, Schumm M, Döring M, Mezger M, Lang P, Dahlke MH, Riethmüller J, Königsrainer A, Handgretinger R, Nadalin S, Sturm E. Safety and Tolerance of Donor-Derived Mesenchymal Stem Cells in Pediatric Living-Donor Liver Transplantation: The MYSTEP1 Study. Stem Cells Int. 2017;2017:2352954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 70. | Wang Y, Zhang A, Ye Z, Xie H, Zheng S. Bone marrow-derived mesenchymal stem cells inhibit acute rejection of rat liver allografts in association with regulatory T-cell expansion. Transplant Proc. 2009;41:4352-4356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 71. | Tan J, Wu W, Xu X, Liao L, Zheng F, Messinger S, Sun X, Chen J, Yang S, Cai J, Gao X, Pileggi A, Ricordi C. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: a randomized controlled trial. JAMA. 2012;307:1169-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 423] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 72. | Li SW, Cai Y, Mao XL, He SQ, Chen YH, Yan LL, Zhou JJ, Song YQ, Ye LP, Zhou XB. The Immunomodulatory Properties of Mesenchymal Stem Cells Play a Critical Role in Inducing Immune Tolerance after Liver Transplantation. Stem Cells Int. 2021;2021:6930263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 73. | Wang R, Shen Z, Yang L, Yin M, Zheng W, Wu B, Liu T, Song H. Protective effects of heme oxygenase-1-transduced bone marrow-derived mesenchymal stem cells on reducedsize liver transplantation: Role of autophagy regulated by the ERK/mTOR signaling pathway. Int J Mol Med. 2017;40:1537-1548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 74. | Tang J, Yang R, Lv L, Yao A, Pu L, Yin A, Li X, Yu Y, Nyberg SL, Wang X. Transforming growth factor-β-Expressing Mesenchymal Stem Cells Induce Local Tolerance in a Rat Liver Transplantation Model of Acute Rejection. Stem Cells. 2016;34:2681-2692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 75. | Mache CJ, Schwinger W, Spendel S, Zach O, Regauer S, Ring E. Skin transplantation to monitor clinical donor-related tolerance in mixed hematopoietic chimerism. Pediatr Transplant. 2006;10:128-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 76. | Ildstad ST, Sachs DH. Reconstitution with syngeneic plus allogeneic or xenogeneic bone marrow leads to specific acceptance of allografts or xenografts. Nature. 1984;307:168-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 575] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 77. | Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, Shaffer J, Preffer FI, Ding R, Sharma V, Fishman JA, Dey B, Ko DS, Hertl M, Goes NB, Wong W, Williams WW Jr, Colvin RB, Sykes M, Sachs DH. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358:353-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 890] [Cited by in RCA: 831] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 78. | Sasaki H, Oura T, Spitzer TR, Chen YB, Madsen JC, Allan J, Sachs DH, Cosimi AB, Kawai T. Preclinical and clinical studies for transplant tolerance via the mixed chimerism approach. Hum Immunol. 2018;79:258-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 79. | Alexander SI, Smith N, Hu M, Verran D, Shun A, Dorney S, Smith A, Webster B, Shaw PJ, Lammi A, Stormon MO. Chimerism and tolerance in a recipient of a deceased-donor liver transplant. N Engl J Med. 2008;358:369-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 80. | Tryphonopoulos P, Tzakis AG, Weppler D, Garcia-Morales R, Kato T, Madariaga JR, Levi DM, Nishida S, Moon J, Selvaggi G, Regev A, Nery C, Bejarano P, Khaled A, Kleiner G, Esquenazi V, Miller J, Ruiz P, Ricordi C. The role of donor bone marrow infusions in withdrawal of immunosuppression in adult liver allotransplantation. Am J Transplant. 2005;5:608-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 96] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 81. | Kim SY, Kim DW, Choi JY, Kim DG, Min WS, Lee JW, Kim CC. Full donor chimerism using stem-cell transplantation for tolerance induction in the human leukocyte antigen-matched liver transplant setting. Transplantation. 2009;88:601-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 82. | Hartleif S, Lang P, Handgretinger R, Feuchtinger T, Fuchs J, Königsrainer A, Nadalin S, Sturm E. Outcomes of pediatric identical living-donor liver and hematopoietic stem cell transplantation. Pediatr Transplant. 2016;20:888-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 83. | Domiati-Saad R, Klintmalm GB, Netto G, Agura ED, Chinnakotla S, Smith DM. Acute graft versus host disease after liver transplantation: patterns of lymphocyte chimerism. Am J Transplant. 2005;5:2968-2973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 84. | Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity. 2015;42:607-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 774] [Cited by in RCA: 1062] [Article Influence: 106.2] [Reference Citation Analysis (0)] |
| 85. | Chong AS, Khiew SH. Transplantation tolerance: don't forget about the B cells. Clin Exp Immunol. 2017;189:171-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 86. | Huang X, Moore DJ, Mohiuddin M, Lian MM, Kim JI, Sonawane S, Wang J, Gu Y, Yeh H, Markmann JF, Deng S. Inhibition of ICAM-1/LFA-1 interactions prevents B-cell-dependent anti-CD45RB-induced transplantation tolerance. Transplantation. 2008;85:675-680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 87. | Lee KM, Kim JI, Stott R, Soohoo J, O'Connor MR, Yeh H, Zhao G, Eliades P, Fox C, Cheng N, Deng S, Markmann JF. Anti-CD45RB/anti-TIM-1-induced tolerance requires regulatory B cells. Am J Transplant. 2012;12:2072-2078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 88. | Guinn MT, Szuter ES, Yokose T, Ge J, Rosales IA, Chetal K, Sadreyev RI, Cuenca AG, Kreisel D, Sage PT, Russell PS, Madsen JC, Colvin RB, Alessandrini A. Intragraft B cell differentiation during the development of tolerance to kidney allografts is associated with a regulatory B cell signature revealed by single cell transcriptomics. Am J Transplant. 2023;23:1319-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 89. | Nova-Lamperti E, Chana P, Mobillo P, Runglall M, Kamra Y, McGregor R, Lord GM, Lechler RI, Lombardi G, Hernandez-Fuentes MP; GAMBIT Study. Increased CD40 Ligation and Reduced BCR Signalling Leads to Higher IL-10 Production in B Cells From Tolerant Kidney Transplant Patients. Transplantation. 2017;101:541-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 90. | Cherukuri A, Rothstein DM, Clark B, Carter CR, Davison A, Hernandez-Fuentes M, Hewitt E, Salama AD, Baker RJ. Immunologic human renal allograft injury associates with an altered IL-10/TNF-α expression ratio in regulatory B cells. J Am Soc Nephrol. 2014;25:1575-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 91. | Sánchez-Fueyo A. Safety and Clinical Activity of QEL-001 in A2-mismatch Liver Transplant Patients (LIBERATE). [accessed 2024 Mar 13]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT05234190 ClinicalTrials.gov Identifier: NCT05234190. |
| 92. | Tran LM, Macedo C, Zahorchak AF, Gu X, Elinoff B, Singhi AD, Isett B, Zeevi A, Sykes M, Breen K, Srivastava A, Ables EM, Landsittel D, Styn MA, Humar A, Lakkis FG, Metes DM, Thomson AW. Donor-derived regulatory dendritic cell infusion modulates effector CD8(+) T cell and NK cell responses after liver transplantation. Sci Transl Med. 2023;15:eadf4287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |