Published online Mar 21, 2024. doi: 10.3748/wjg.v30.i11.1533
Peer-review started: January 5, 2024
First decision: January 23, 2024
Revised: February 18, 2024
Accepted: March 8, 2024
Article in press: March 8, 2024
Published online: March 21, 2024
Processing time: 76 Days and 3 Hours
Patients with liver cancer complicated by portal hypertension present complex challenges in treatment.
To evaluate the efficacy of radiofrequency ablation in combination with sorafenib for improving liver function and its impact on the prognosis of patients with this condition.
Data from 100 patients with liver cancer complicated with portal hypertension from May 2014 to March 2019 were analyzed and divided into a study group (n = 50) and a control group (n = 50) according to the treatment regimen. The research group received radiofrequency ablation (RFA) in combination with sorafenib, and the control group only received RFA. The short-term efficacy of both the research and control groups was observed. Liver function and portal hypertension were compared before and after treatment. Alpha-fetoprotein (AFP), glypican-3 (GPC-3), and AFP-L3 levels were compared between the two groups prior to and after treatment. The occurrence of adverse reactions in both groups was observed. The 3-year survival rate was compared between the two groups. Basic data were compared between the survival and non-surviving groups. To identify the independent risk factors for poor prognosis in patients with liver cancer complicated by portal hypertension, multivariate logistic regression analysis was employed.
When comparing the two groups, the research group's total effective rate (82.00%) was significantly greater than that of the control group (56.00%; P < 0.05). Following treatment, alanine aminotransferase and aspartate aminotransferase levels increased, and portal vein pressure decreased in both groups. The degree of improvement for every index was substantially greater in the research group than in the control group (P < 0.05). Following treatment, the AFP, GPC-3, and AFP-L3 levels in both groups decreased, with the research group having significantly lower levels than the control group (P < 0.05). The incidence of diarrhea, rash, nausea and vomiting, and fatigue in the research group was significantly greater than that in the control group (P < 0.05). The 1-, 2-, and 3-year survival rates of the research group (94.00%, 84.00%, and 72.00%, respectively) were significantly greater than those of the control group (80.00%, 64.00%, and 40.00%, respectively; P < 0.05). Significant differences were observed between the survival group and the non-surviving group in terms of Child-Pugh grade, history of hepatitis, number of tumors, tumor size, use of sorafenib, stage of liver cancer, histological differentiation, history of splenectomy and other basic data (P < 0.05). Logistic regression analysis demonstrated that high Child-Pugh grade, tumor size (6–10 cm), history of hepatitis, no use of sorafenib, liver cancer stage IIIC, and previous splenectomy were independent risk factors for poor prognosis in patients with liver cancer complicated with portal hypertension (P < 0.05).
Patients suffering from liver cancer complicated by portal hypertension benefit from the combination of RFA and sorafenib therapy because it effectively restores liver function and increases survival rates. The prognosis of patients suffering from liver cancer complicated by portal hypertension is strongly associated with factors such as high Child-Pugh grade, tumor size (6-10 cm), history of hepatitis, lack of sorafenib use, liver cancer at stage IIIC, and prior splenectomy.
Core Tip: The combination of radiofrequency ablation (RFA) and sorafenib shows promise in treating liver cancer with portal hypertension. This approach demonstrated improved short- and long-term efficacy, with significant reduction in portal vein pressure and enhancement of liver function. Patients treated with this combination had higher survival rates compared to those receiving RFA alone. Moreover, the study identified key prognostic factors, such as Child-Pugh grade, tumor size, history of hepatitis, and the use of sorafenib, providing valuable insights for managing liver cancer complicated by portal hypertension. These findings suggest that the RFA and sorafenib combination could be a beneficial therapeutic strategy, but further research with larger sample sizes is warranted to validate these outcomes.
- Citation: Yang LM, Wang HJ, Li SL, Gan GH, Deng WW, Chang YS, Zhang LF. Efficacy of radiofrequency ablation combined with sorafenib for treating liver cancer complicated with portal hypertension and prognostic factors. World J Gastroenterol 2024; 30(11): 1533-1544
- URL: https://www.wjgnet.com/1007-9327/full/v30/i11/1533.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i11.1533
Liver cancer is categorized into two types: primary and metastatic liver cancer. Primary liver cancer is more common than primary liver cancer, and its incidence ranks fifth among malignant tumors. According to epidemiological surveys, there are more than 600000 new cases of liver cancer worldwide. Approximately 85% to 95% of primary liver cancers develop from liver cirrhosis, 15% to 20% of which are complicated with different degrees of portal hypertension[1,2]. The condition of patients suffering from liver cancer complicated with portal hypertension is relatively complex, and since there are no obvious symptoms in the initial stages, most patients visit the hospital when they are typically already in the middle or late stages and have missed the best time for surgical treatment. Moreover, patients suffering from liver cancer complicated with portal hypertension are in poor physical condition and cannot tolerate surgical operation[3,4]. The treatment principle of radiofrequency ablation (RFA) is to increase the temperature of liver tissue to > 60°C and maintain it at that temperature for a certain time to cause degeneration and irreversible necrosis of cellular proteins. Multiple earlier research studies have revealed that RFA effectively treats liver cancer, but studies on its application in patients with liver cancer complicated with portal hypertension are rare[5,6]. Sorafenib, an oral tyrosine kinase inhibitor, can reduce visceral neovascularization and ameliorate portal hypertension via the inhibition of vascular endothelial growth factor receptor (VEGFR) and platelet-derived growth factor receptor (PDGFR) to inhibit neovascularization[7,8]. In the present research, RFA in combination with sorafenib was applied to treat patients suffering from liver cancer complicated by portal hypertension to study its mechanism of action and to analyze patient prognosis. This study provides a reference for the treatment of liver cancer complicated by portal hypertension. The report is detailed below.
Data from 100 patients with liver cancer complicated with portal hypertension from May 2014 to March 2019 were analyzed and divided into study groups (n = 50) and control groups (n = 50) according to the treatment regimen. The research group comprised 23 women and 27 men aged 44-69 (55.46 ± 6.31) years; portal hypertension symptoms: 30 patients with hemorrhage, 9 patients with ascites, and 11 patients with hemorrhage and ascites. The control group included 31 men and 19 women aged 40-69 (57.40 ± 5.69) years; portal hypertension symptoms were 22 hemorrhages, 13 ascites, and 15 hemorrhages and ascites. The two groups’ general data were comparable (P > 0.05).
(1) Patients who satisfied the relevant standards in the “Guidelines for the Diagnosis and Treatment of Primary Liver Cancer”[9]; (2) Patients were diagnosed with liver cancer complicated with portal hypertension by clinicopathological and imaging examinations, and gastroscopy revealed active gastroesophageal venous active bleeding and a hepatic venous pressure gradient > 5 mmHg; and (3) Complete clinical data.
(1) Patients with diffuse liver cancer, extrahepatic metastasis, or history of liver cancer surgery; (2) Expected survival time < 3 months; (3) Patients suffering from other cancerous tumors; (4) Individuals suffering from systemic infections; (5) Individuals who experienced disturbance of consciousness; and (6) Patients who experienced allergies triggered by the drugs utilized in this study.
The enrolled patients were screened for one month at our hospital before being included in the study, and each included patient was numbered. The research group received RFA in combination with sorafenib, while the control group received RFA. All the data were collected after admission and were accessed for study purposes in January 2023.
A radiofrequency therapeutic instrument (CTRF220, Covidien, United States) was used for treatment, the output power was 200 W, the frequency was set to 480 kHz, and the electrode diameter was set to 1.2 mm. Patients with multiple tumors were treated with a multihook probe. Patients were placed in the supine or prone position, and multislice spiral CT was used to locate the tumor site. The puncture point on the body surface and the puncture direction were selected, and the puncture site was anesthetized with 10 mL of 2% lidocaine. RFA treatment was performed according to the tumor size after the lesion was punctured with the ablation electrode needle, and the treatment time was 8-12 min. The ablation area was 1-2 cm larger than the lesion area to ensure that the tumor tissue could be completely ablated and that the infiltrated part was killed. After RFA treatment, a CT scan was again performed to observe the effect of tumor ablation.
Sorafenib (Chongqing Yaoyou Pharmaceutical Co., Ltd., SFDA approval number: H20203403) was given orally 14 days before RFA treatment (400 mg/time) twice daily. After oral administration of sorafenib, adverse reactions were assessed as per the Common Terminology Criteria for Adverse Events of National Cancer Institutes[10]. If there was no adverse reaction, the drug dose was maintained until 1-2 d before the operation; if there was an adverse reaction, the dose was halved; if there was a grade 3 or 4 adverse reaction, the drug was stopped, and RFA was performed after 1-2 d of drug withdrawal. If the Child-Pugh grade was A or B after RFA and there was no serious complication, sorafenib was given orally 3-7 d after the operation (400 mg once a day). If no symptoms of discomfort occurred, a dose of 400 mg/time was given 7 d later, two times a day. If there were grade 3-4 adverse reactions, the drug was suspended, and when the adverse reactions were reduced to grade 2 or below, the drug was restored to 400 mg/time, twice/day or 400 mg/time, once/day.
(1) Short-term efficacy; (2) Comparison of liver function and portal hypertension status. The detection of aspartate aminotransferase (AST) and glutamate alanine aminotransferase (ALT) was performed via an automatic biochemical analyzer. The AST and ALT levels before and after treatment were compared between the two groups. The portal vein pressure was compared between the two groups; (3) Comparison of liver cancer markers The levels of alpha-fetoprotein (AFP), glypican-3 (GPC-3) and AFP-L3 were determined via ELISA. The levels of AFP, GPC-3 and AFP-L3 before and after treatment were compared between the two groups; (4) Adverse reactions; and (5) Comparison of the 3-year survival rate between the two groups. Univariate analysis of the survival and non-surviving groups Basic data such as age, Child-Pugh grade, history of hepatitis, number of tumors, tumor size, use of sorafenib, stage of liver cancer, histological differentiation, and history of splenectomy were compared between the survival and non-surviving groups. Multivariate analysis of the survival and non-surviving groups. To analyze the independent risk factors for poor prognosis in patients with liver cancer complicated by portal hypertension, multivariate logistic regression was employed.
The efficacy of the WHO solid tumor evaluation criteria[11] was used to evaluate the efficacy of the treatment. Complete remission (CR) was defined as follows: Tumor disappeared completely; partial response (PR): Tumor regression area > 50% and no new lesions; no response: Tumor regression area ≤ 50% or increased area ≤ 25%; and progressed disease: Increased area > 50%. The total effectiveness was calculated as CR + PR.
SPSS 20.0 statistical software was used to analyze and process the collected data. The measurement data are presented as mean ± SD, and for comparisons between the groups, the independent sample t test was used, while the paired t test was used for comparisons within the groups prior to and following the treatment. Count data are presented as the frequency or composition ratio, and the χ2 test was used for comparison. Logistic multivariate regression was used to analyze the independent risk factors for poor prognosis in patients suffering from liver cancer complicated by portal hypertension. A value of P < 0.05 indicated a statistically significant difference.
The research group’s total effective rate (82.00%) was greater than that of the control group (56.00%), with statistically significant differences between the two groups (P < 0.05). As illustrated in Table 1.
| Group | CR | PR | NR | PD | Total effective rate |
| Research group (n = 50) | 7 (14.00) | 34 (68.00) | 6 (12.00) | 3 (6.00) | 41 (82.00) |
| Control group (n = 50) | 4 (8.00) | 24 (48.00) | 17 (34.00) | 5 (10.00) | 28 (56.00) |
| χ2 value | 7.901 | ||||
| P value | 0.005 |
Prior to treatment, there were no considerable differences in ALT or AST levels or portal venous pressure between the two groups (P > 0.05). Following treatment, the ALT and AST levels in both groups increased, and the portal venous pressure was reduced. The improvement in each index was greater in the research group than in the control group. The differences were statistically significant (P < 0.05). As illustrated in Table 2.
| Group | ALT (U/L) | AST (U/L) | Portal venous pressure (cm H2O) | |||
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | |
| Research group (n = 50) | 40.06 ± 6.15 | 71.45 ± 9.85a | 53.16 ± 6.98 | 75.90 ± 10.09a | 39.71 ± 7.56 | 28.93 ± 5.98a |
| Control group (n = 50) | 40.99 ± 7.51 | 89.27 ± 11.26a | 51.21 ± 9.32 | 95.45 ± 9.29a | 39.83 ± 5.15 | 31.51 ± 5.88a |
| t value | 0.676 | 8.425 | 1.184 | 10.076 | 0.089 | 2.174 |
| P value | 0.501 | < 0.001 | 0.240 | <0.001 | 0.929 | 0.032 |
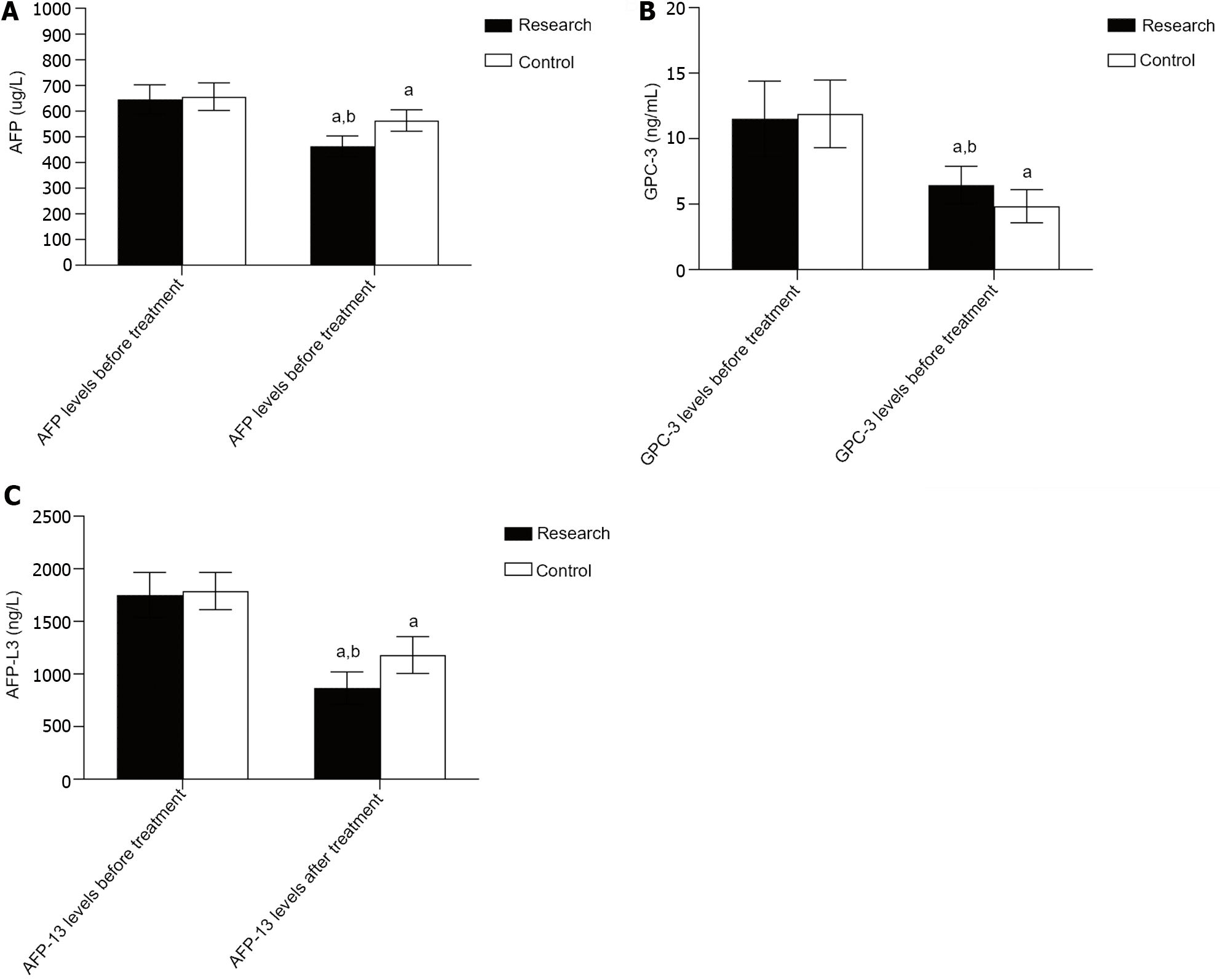
Prior to treatment, there was no considerable difference in the AFP, GPC-3, or AFP-L3 Levels (P > 0.05); following treatment, the AFP, GPC-3 and AFP-L3 Levels decreased in both groups, and the levels in the research group were significantly lower than those in the control group (P < 0.05). As illustrated in Table 3, Figure 1.
| Group | AFP (ug/L) | GPC-3 (ng/mL) | AFP-L3 (ng/L) | |||
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | |
| Research group (n = 50) | 645.88 ± 56.05 | 463.12 ± 40.45a | 11.52 ± 2.88 | 6.46 ± 1.43a | 1751.54 ± 214.99 | 867.26 ± 153.14a |
| Control group (n = 50) | 655.80 ± 53.69 | 563.21 ± 41.46a | 11.89 ± 2.58 | 4.84 ± 1.26a | 1787.74 ± 177.19 | 1179.48 ± 175.10a |
| t value | 0.904 | 12.220 | 0.665 | 5.975 | 0.919 | 9.491 |
| P value | 0.368 | < 0.001 | 0.508 | < 0.001 | 0.360 | < 0.001 |
Instances of diarrhea, rash, nausea, vomiting and fatigue were significantly greater in the research group than in the control group (P < 0.05). As demonstrated in Table 4.
| Group | Diaphragm injury | Diarrhea | Rash | Portal vein and biliary tract injury | Gastrointestinal bleeding | Nausea and vomiting | Fatigue |
| Research group (n = 50) | 3 (6.00) | 14 (28.00) | 20 (40.00) | 13 (26.00) | 5 (10.00) | 9 (18.00) | 33 (66.00) |
| Control group (n = 50) | 1 (2.00) | 2 (4.00) | 2 (4.00) | 10 (20.00) | 3 (6.00) | 1 (2.00) | 10 (20.00) |
| χ2 value | 1.042 | 10.714 | 18.881 | 0.508 | 0.543 | 7.111 | 21.583 |
| P value | 0.307 | 0.001 | < 0.001 | 0.476 | 0.461 | 0.008 | < 0.001 |
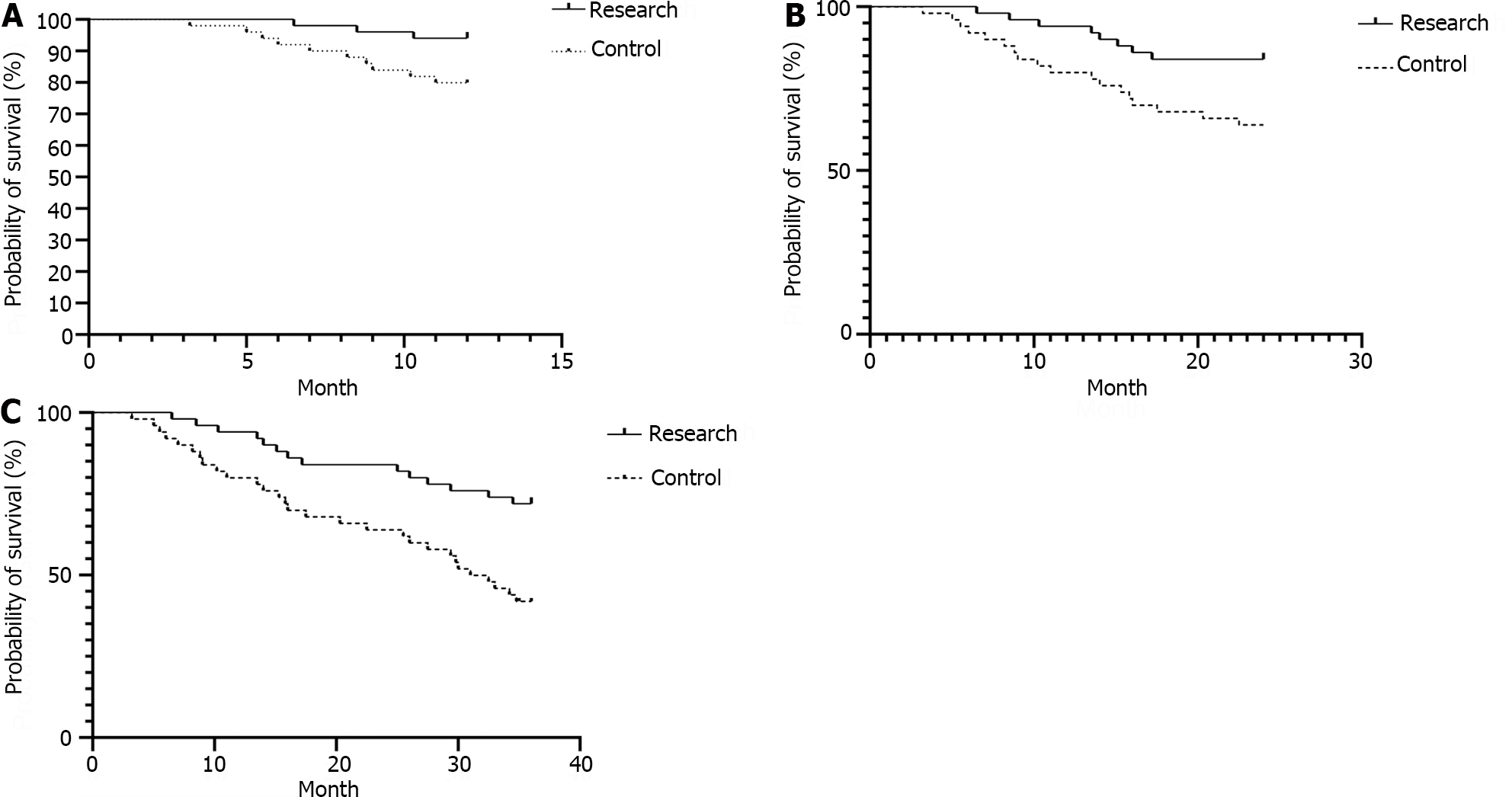
The 1-, 2-, and 3-year survival rates of the research group (94.00%, 84.00%, and 72.00%, respectively) were significantly greater than those of the control group (80.00%, 64.00%, and 40.00%, respectively; P < 0.05). As illustrated in Table 5, Figure 2.
| Group | 1-yr survival rate | 2-yr survival rate | 3-yr survival rate | |||
| Number of cases | Survival rate (%) | Number of cases | Survival rate (%) | Number of cases | Survival rate (%) | |
| Research group (n = 50) | 47 | 94.00 | 42 | 84.00 | 36 | 72.00 |
| Control group (n = 50) | 40 | 80.00 | 32 | 64.00 | 20 | 40.00 |
| Log-χ2 value | 4.465 | 5.337 | 9.223 | |||
| P value | 0.035 | 0.021 | 0.002 | |||
Significant differences were observed between the survival group and the non-surviving group in terms of basic data such as Child-Pugh grade, history of hepatitis, number of tumors, tumor size, use of sorafenib, stage of liver cancer, histological differentiation, and previous splenectomy (P < 0.05). As illustrated in Table 6.
| Item | Survival group (n = 56) | Death group (n = 44) | χ2 value | P value |
| Age | ||||
| ≤ 60 yr | 43 (76.79) | 29 (65.91) | 1.446 | 0.229 |
| > 60 yr | 13 (23.21) | 15 (34.09) | ||
| Child-Pugh grade | ||||
| Grade A | 45 (80.36) | 23 (52.27) | 8.931 | 0.003 |
| Grade B | 11 (19.64) | 21 (47.73) | ||
| History of hepatitis | ||||
| Yes | 18 (32.14) | 29 (65.91) | 11.278 | 0.001 |
| None | 38 (67.86) | 15(34.09) | ||
| Number of tumors | ||||
| 1 | 35 (62.50) | 15 (34.09) | 8.266 | 0.016 |
| 2 | 16 (28.57) | 20 (45.45) | ||
| 3 | 5 (8.93) | 9 (20.45) | ||
| Tumor size (cm) | ||||
| < 6 | 49 (87.50) | 15 (34.09) | 30.506 | <0.001 |
| 6-10 | 7 (12.50) | 29 (65.91) | ||
| Use of sorafenib | ||||
| Yes | 36 (64.29) | 14 (31.82) | 10.390 | 0.001 |
| No | 20 (35.71) | 30 (68.18) | ||
| Stage of liver cancer | ||||
| IIIB | 47 (83.93) | 20 (45.45) | 16.496 | < 0.001 |
| IIIC | 9 (16.07) | 24 (54.55) | ||
| Histological differentiation | ||||
| High | 28 (50.00) | 14 (31.82) | 6.810 | 0.033 |
| Low-moderate | 18 (32.14) | 12 (27.27) | ||
| Necrosis | 10 (17.86) | 18 (40.91) | ||
| Previous splenectomy | ||||
| Yes | 18 (32.14) | 30 (68.18) | 12.822 | < 0.001 |
| None | 38 (67.86) | 14 (31.82) |
The items with statistically significant differences in the above factors were included in the multivariate logistic regression model, with survival at three years of follow-up as the dependent variable and the items with statistically significant differences as the independent variable. The values were assigned as follows: Child-Pugh grade (grade A = 0, grade B = 1), history of hepatitis (none = 0, yes = 1), number of tumors (1 = 0, ≥ 2 = 1), tumor size (< 6 = 0, 6-10 = 1), use of sorafenib (yes = 0, no = 1), stage of liver cancer (III B = 0, III C = 1), histological differentiation (high = 0, low-moderator necrosis = 1), and previous splenectomy (none = 0, yes = 1). Logistic regression analysis demonstrated that high Child-Pugh grade, tumor size (6–10 cm), history of hepatitis, no use of sorafenib, liver cancer stage IIIC, and previous splenectomy were independent risk factors for poor prognosis in patients with liver cancer complicated with portal hypertension (P < 0.05). As demonstrated in Table 7.
| Item | β | SE | Wald | P value | Exp (B) | 95%CI |
| High Child-Pugh grade | 1.470 | 0.738 | 3.970 | 0.046 | 4.349 | 1.024-18.469 |
| History of hepatitis | 2.286 | 0.803 | 8.098 | 0.004 | 9.833 | 2.037-47.463 |
| Tumor size (6-10 cm) | 2.399 | 0.788 | 9.268 | 0.002 | 11.008 | 2.350-51.567 |
| No use of sorafenib | 2.483 | 0.829 | 8.963 | 0.003 | 11.981 | 2.357-60.884 |
| Liver cancer of stage IIIC | 1.900 | 0.719 | 6.988 | 0.008 | 6.683 | 1.634-27.329 |
| Previous splenectomy | 1.629 | 0.741 | 4.835 | 0.028 | 5.101 | 1.194-21.800 |
| Constant | 6.685 | 1.486 | 20.226 | < 0.001 | 0.001 |
Currently, the occurrence of liver cancer is increasing annually, and approximately 70%-90% of liver cancer patients are complicated with cirrhosis[12,13]. The common causes of liver cancer complicated with portal hypertension are as follows: Liver cancer usually develops from cirrhosis, which can cause portal hypertension; the formation of arteriovenous fistula in the tumor body can lead to increased portal vein load; and impaired portal vein patency can increase blood flow resistance. Patients suffering from liver cancer complicated with portal hypertension are at high risk of surgery, and hepatectomy can further lead to increased portal vein pressure. For this reason, the clinical treatment of patients with liver cancer complicated by portal hypertension relies mainly on alleviating portal vein symptoms. RFA is a kind of local ablation therapy. The treatment principle of RFA is to increase the temperature of liver tissue to > 60°C and maintain it at that temperature for a certain time to cause degeneration and irreversible necrosis of cellular proteins. It is suitable for patients with unresectable liver cancer complicated with portal hypertension. Sorafenib is a tyrosinase inhibitor that can reduce the generation of visceral neovascularization and ameliorate portal hypertension. Sorafenib can improve portal hypertension by improving hemodynamics, inhibiting the activation of HSCs, and reducing neovascularization. Several previous studies have applied sorafenib to patients suffering from liver cancer complicated with portal hypertension, and the effect of this treatment is good[14-16]. In the present study, the research group received RFA in combination with sorafenib, while the control group received RFA alone. The outcomes revealed that the research group’s total effective rate (82.00%) was greater than that of the control group (56.00%). Following treatment, the ALT and AST levels of both groups were elevated, and the portal vein pressure was reduced. The degree of improvement for every index in the research group was substantially greater than that in the control group (P < 0.05). The results indicate that RFA in combination with sorafenib effectively treats liver cancer patients with portal hypertension and can effectively reduce portal vein pressure and improve liver function. This may be because, on the basis of RFA for the treatment of liver cancer, sorafenib, a molecularly targeted drug, blocks the further growth of tumor cells and inhibits the development of tumors and the generation of neovascularization. In addition, sorafenib improved portal hypertension, and the two treatment methods had synergistic effects; thus, the treatment effect was better.
AFP is a common marker of liver cancer and is strongly expressed in the serum of liver cancer patients and is directly associated with their prognosis[17,18]. GPC-3, a heparan sulfate glycoprotein, is expressed at low levels in normal liver tissues and nodular hyperplasia tissues and is overexpressed in patients with liver cancer. The specificity and sensitivity of the serum GPC-3 concentration for diagnosing liver cancer are greater than those of the AFP concentration[19,20]. AFP-L3 is a variant of AFP. According to relevant studies, the value of AFP-L3 in assessing the prognosis of liver cancer patients is greater than that of AFP, and high serum AFP-L3 levels can indicate the occurrence and poor prognosis of liver cancer[21,22]. According to the present research, the improved serum AFP, GPC-3, and AFP-L3 Levels in the present study were greater than those in the control group, implying that RFA in combination with sorafenib is capable of more efficiently protecting the liver function of patients suffering from liver cancer complicated with portal hypertension. Compared to those in the control group, the incidences of diarrhea, rash, nausea, vomiting, and fatigue in the research group were greater than those in the control group. These conditions are all typical adverse reactions to sorafenib, suggesting that changes in patients during the course of their clinical treatment should be closely monitored and that effective measures should be taken for patients with serious adverse reactions in time. In this study, the 1-, 2-, and 3-year survival rates of the individuals in the research group (94.00%, 84.00%, 72.00%) were greater than those of the individuals in the control group (80.00%, 64.00%, 40.00%), indicating that the long-term efficacy of RFA in combination with sorafenib for treating liver cancer patients with portal hypertension is better. Sorafenib can dramatically increase the survival duration of patients who have advanced liver cancer, according to numerous earlier studies[23-25]. The outcomes of the present research are in line with these findings and are related to the antitumor effect of sorafenib and the effect of reducing portal hypertension.
The observed efficacy of combined therapy involving RFA and sorafenib in the treatment of liver cancer complicated by portal hypertension can be attributed to the synergistic actions of these modalities, each targeting specific aspects of disease pathophysiology. RFA, a local ablation therapy, exerts its effects by inducing thermal damage to liver tissue, leading to cellular degeneration and irreversible necrosis. This approach is particularly advantageous in patients with unresectable liver cancer complicated by portal hypertension, where surgical intervention may not be feasible due to the patient's clinical condition. The localized tissue destruction achieved through RFA contributes to a reduction in tumor burden, thereby alleviating portal vein pressure and improving liver function, as evidenced by the observed reduction in transaminase levels and portal venous pressure in the study population.
Concurrently, the incorporation of sorafenib, an oral tyrosine kinase inhibitor, complements the effects of RFA by targeting critical molecular pathways involved in neovascularization and tumor progression. The mechanism of action of sorafenib includes the inhibition of VEGFR and PDGFR, which are known to play pivotal roles in the promotion of tumor angiogenesis and vascular remodeling. By disrupting these signaling pathways, sorafenib not only impedes tumor neovascularization but also exerts modulatory effects on portal hypertension, thereby contributing to the overall improvement in clinical outcomes observed in the present study.
Moreover, the synergy between RFA and sorafenib may extend beyond their individual mechanisms of action. It is plausible that the localized tissue injury caused by RFA creates an environment conducive to the antitumor effects of sorafenib, potentially enhancing its penetration and efficacy within the tumor microenvironment. This interplay between the two treatment modalities underscores the importance of combination strategies in addressing the complex interplay of factors associated with liver cancer complicating portal hypertension, with the potential to offer a more comprehensive and efficacious approach to disease management.
In the present research, all patients underwent a three-year follow-up to observe their prognosis, and based on their survival status, they were separated into a survival group and a death group. The basic data of the patients were analyzed via univariate analysis. Considerable differences were found in Child-Pugh grade, history of hepatitis, number of tumors, tumor size, use of sorafenib, stage of liver cancer, histological differentiation, previous splenectomy, and other basic data between the survival and death groups (P < 0.05), suggesting that Child-Pugh grade, history of hepatitis, number of tumors, tumor size, use of sorafenib, stage of liver cancer, histological differentiation and previous splenectomy are strongly associated with the prognosis of liver cancer patients complicated with portal hypertension. Logistic multivariate regression analysis demonstrated that high Child-Pugh grade, tumor size (6–10 cm), history of hepatitis, no use of sorafenib, liver cancer stage IIIC, and previous splenectomy were independent risk factors for poor prognosis in patients with liver cancer complicated with portal hypertension. A high Child-Pugh grade, large tumor diameter, history of hepatitis, and liver cancer stage IIIC indicate severe disease, so the prognosis is poor. The patients who did not use sorafenib composed the control group in this research, and the treatment effect in the control group was worse than that in the research group; thus, the prognosis was poor. Patients with portal hypertension often exhibit hypersplenism, and a history of previous splenectomy indicates that portal hypertension is more serious in these patients, so the prognosis is poor. It is suggested that effective treatment and nursing measures be taken to improve the prognosis of patients with high Child-Pugh grade, large tumor size (6-10 cm), history of hepatitis, no use of sorafenib, liver cancer of stage IIIC, or previous splenectomy.
The findings of this study contribute to elucidating the efficacy and potential challenges associated with combined therapy comprising RFA and sorafenib for the treatment of liver cancer complicated by portal hypertension. While the results indicate a promising improvement in patient outcomes, it is essential to acknowledge the observed increase in adverse reactions, particularly in the form of diarrhea, rash, nausea, vomiting, and fatigue, within the research group. These adverse reactions have been documented as common side effects of sorafenib therapy. Therefore, in light of these findings, it is imperative to address potential strategies for mitigating these adverse events to ensure the overall well-being and treatment adherence of patients.
The management of adverse reactions related to sorafenib therapy is paramount for ensuring the continued effectiveness of the treatment approach. Given the adverse reactions identified in the research group, it is crucial for health care providers to proactively monitor and manage these side effects to optimize patient tolerance and compliance. Strategies for mitigation may include personalized patient education on potential side effects, proactive symptom management, dose adjustments based on individual tolerability, and prompt intervention for severe adverse events. Additionally, comprehensive supportive care measures, such as nutritional support and psychological counseling, can play a significant role in contributing to the overall well-being of patients receiving this combined therapeutic approach.
Furthermore, future research endeavors should focus on investigating novel approaches to reduce the incidence and severity of these adverse events, potentially through the exploration of alternative dosing regimens, the use of adjunctive medications for symptom management, or the identification of predictive markers for susceptibility to specific adverse reactions. By addressing these challenges, health care providers can work toward optimizing the therapeutic benefits of RFA in combination with sorafenib while minimizing the impact of treatment-associated adverse reactions on patient quality of life.
It is also necessary to acknowledge the limitation of the sample size, which underscores the need for a more comprehensive investigation to establish stronger conclusions. While the present study offers valuable insights, a larger-scale investigation is warranted to reinforce the robustness and generalizability of the findings. Therefore, conducting a study with a larger sample size would address this limitation and ensure broader applicability of the results, enhancing the overall strength of the conclusions.
In conclusion, RFA in combination with sorafenib can successfully enhance patient liver function with good short- and long-term efficacy and has clinical therapeutic potential in the treatment of liver cancer complicated by portal hypertension. The disadvantage of the present research is the small sample size, which may produce a risk of selection bias; therefore, further research should be conducted with a larger sample size.
Liver cancer, frequently arising from cirrhosis, presents with accompanying portal hypertension in a substantial portion of cases. Current treatments are limited due to the challenging nature of surgical interventions and poor physical tolerance of affected patients. Radiofrequency ablation (RFA) is a known therapeutic approach, but its application in liver cancer complicated by portal hypertension has been insufficiently explored.
Given the complexity and limited treatment options for patients with liver cancer and portal hypertension, investigating novel therapeutic strategies is crucial. Understanding the potential benefits of combining RFA with sorafenib in this context could offer improved efficacy and survival outcomes.
This study aimed to assess the effectiveness of RFA in combination with sorafenib for patients with liver cancer complicated by portal hypertension, discern prognostic factors, and evaluate their impact on patient outcomes. The study also sought to analyze the potential synergistic effects of both treatments and their impact on liver function and survival rates.
A total of 100 patients were analyzed and categorized into a research group (RFA with sorafenib) and a control group (RFA alone). Short-term efficacy, liver function, portal hypertension, cancer markers, adverse reactions, and survival rates were assessed. Multivariate logistic regression analysis was employed to identify independent risk factors for poor patient prognosis.
The combined RFA and sorafenib treatment demonstrated a significantly higher total effective rate compared to RFA alone, effectively reducing portal vein pressure, improving liver function, and lowering liver cancer markers. Patients in the combined treatment group exhibited higher survival rates at 1-, 2-, and 3-year follow-ups, highlighting the potential long-term benefits of this approach.
The combination of RFA and sorafenib yields promising results in treating liver cancer with portal hypertension, offering improved short- and long-term efficacy. Prognostic factors such as Child-Pugh grade, tumor size, history of hepatitis, and the use of sorafenib were identified as significant predictors of patient outcomes, providing valuable insights for clinical management.
These findings underscore the potential clinical therapeutic value of combining RFA with sorafenib for liver cancer complicated by portal hypertension. However, further research with larger sample sizes is warranted to validate these outcomes and establish guidelines for optimizing treatment protocols and patient care.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Moore LW, United States; Mroweh M, France S-Editor: Lin C L-Editor: A P-Editor: Cai YX
| 1. | Anwanwan D, Singh SK, Singh S, Saikam V, Singh R. Challenges in liver cancer and possible treatment approaches. Biochim Biophys Acta Rev Cancer. 2020;1873:188314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 762] [Cited by in RCA: 900] [Article Influence: 180.0] [Reference Citation Analysis (2)] |
| 2. | Xu F, Jin T, Zhu Y, Dai C. Immune checkpoint therapy in liver cancer. J Exp Clin Cancer Res. 2018;37:110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 284] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 3. | Shen ZF, Liang X. Current status of radical laparoscopy for treating hepatocellular carcinoma with portal hypertension. World J Clin Cases. 2021;9:2419-2432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Azoulay D, Ramos E, Casellas-Robert M, Salloum C, Lladó L, Nadler R, Busquets J, Caula-Freixa C, Mils K, Lopez-Ben S, Figueras J, Lim C. Liver resection for hepatocellular carcinoma in patients with clinically significant portal hypertension. JHEP Rep. 2021;3:100190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 5. | Bai XM, Cui M, Yang W, Wang H, Wang S, Zhang ZY, Wu W, Chen MH, Yan K, Goldberg SN. The 10-year Survival Analysis of Radiofrequency Ablation for Solitary Hepatocellular Carcinoma 5 cm or Smaller: Primary versus Recurrent HCC. Radiology. 2021;300:458-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 6. | Lawal G, Xiao Y, Rahnemai-Azar AA, Tsilimigras DI, Kuang M, Bakopoulos A, Pawlik TM. The Immunology of Hepatocellular Carcinoma. Vaccines (Basel). 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 7. | Zhang FW, Guo PX, Wang X. The analysis of short and long term efficacy of sorafenib combined with TACE in patients with hepatocellular carcinoma complicated with microvascular invasion. Shanxi Yiyao Zazhi. 2020;49:798-802. |
| 8. | Scheiner B, Pomej K, Kirstein MM, Hucke F, Finkelmeier F, Waidmann O, Himmelsbach V, Schulze K, von Felden J, Fründt TW, Stadler M, Heinzl H, Shmanko K, Spahn S, Radu P, Siebenhüner AR, Mertens JC, Rahbari NN, Kütting F, Waldschmidt DT, Ebert MP, Teufel A, De Dosso S, Pinato DJ, Pressiani T, Meischl T, Balcar L, Müller C, Mandorfer M, Reiberger T, Trauner M, Personeni N, Rimassa L, Bitzer M, Trojan J, Weinmann A, Wege H, Dufour JF, Peck-Radosavljevic M, Vogel A, Pinter M. Prognosis of patients with hepatocellular carcinoma treated with immunotherapy - development and validation of the CRAFITY score. J Hepatol. 2022;76:353-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 188] [Article Influence: 62.7] [Reference Citation Analysis (0)] |
| 9. | Chinese guidelines for diagnosis and treatment of primary lung cancer 2018 (English version). Chin J Cancer Res. 2019;31:1-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 10. | Freites-Martinez A, Santana N, Arias-Santiago S, Viera A. Using the Common Terminology Criteria for Adverse Events (CTCAE - Version 5.0) to Evaluate the Severity of Adverse Events of Anticancer Therapies. Actas Dermosifiliogr (Engl Ed). 2021;112:90-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 405] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 11. | Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50 Suppl 1:122S-150S. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2916] [Cited by in RCA: 2800] [Article Influence: 175.0] [Reference Citation Analysis (0)] |
| 12. | Mohr R, Özdirik B, Lambrecht J, Demir M, Eschrich J, Geisler L, Hellberg T, Loosen SH, Luedde T, Tacke F, Hammerich L, Roderburg C. From Liver Cirrhosis to Cancer: The Role of Micro-RNAs in Hepatocarcinogenesis. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Moon AM, Singal AG, Tapper EB. Contemporary Epidemiology of Chronic Liver Disease and Cirrhosis. Clin Gastroenterol Hepatol. 2020;18:2650-2666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 805] [Cited by in RCA: 720] [Article Influence: 144.0] [Reference Citation Analysis (0)] |
| 14. | Hidaka H, Uojima H, Nakazawa T, Shao X, Hara Y, Iwasaki S, Wada N, Kubota K, Tanaka Y, Shibuya A, Kanoh Y, Kokubu S, Koizumi W. Portal hemodynamic effects of lenvatinib in patients with advanced hepatocellular carcinoma: A prospective cohort study. Hepatol Res. 2020;50:1083-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Ma R, Chen J, Liang Y, Lin S, Zhu L, Liang X, Cai X. Sorafenib: A potential therapeutic drug for hepatic fibrosis and its outcomes. Biomed Pharmacother. 2017;88:459-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 16. | Cerrito L, Annicchiarico BE, Iezzi R, Gasbarrini A, Pompili M, Ponziani FR. Treatment of hepatocellular carcinoma in patients with portal vein tumor thrombosis: Beyond the known frontiers. World J Gastroenterol. 2019;25:4360-4382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 82] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (1)] |
| 17. | Liu S, Wang M, Zheng C, Zhong Q, Shi Y, Han X. Diagnostic value of serum glypican-3 alone and in combination with AFP as an aid in the diagnosis of liver cancer. Clin Biochem. 2020;79:54-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Özdemir F, Baskiran A. The Importance of AFP in Liver Transplantation for HCC. J Gastrointest Cancer. 2020;51:1127-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 19. | Liu Y, Tan M, Fang C, Chen X, Liu H, Feng Y, Zhang Y, Min W. A novel multifunctional gold nanorod-mediated and tumor-targeted gene silencing of GPC-3 synergizes photothermal therapy for liver cancer. Nanotechnology. 2021;32:175101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Makkouk A, Yang XC, Barca T, Lucas A, Turkoz M, Wong JTS, Nishimoto KP, Brodey MM, Tabrizizad M, Gundurao SRY, Bai L, Bhat A, An Z, Abbot S, Satpayev D, Aftab BT, Herrman M. Off-the-shelf Vδ1 gamma delta T cells engineered with glypican-3 (GPC-3)-specific chimeric antigen receptor (CAR) and soluble IL-15 display robust antitumor efficacy against hepatocellular carcinoma. J Immunother Cancer. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 123] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 21. | Park SJ, Jang JY, Jeong SW, Cho YK, Lee SH, Kim SG, Cha SW, Kim YS, Cho YD, Kim HS, Kim BS, Park S, Bang HI. Usefulness of AFP, AFP-L3, and PIVKA-II, and their combinations in diagnosing hepatocellular carcinoma. Medicine (Baltimore). 2017;96:e5811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 22. | Zhou JM, Wang T, Zhang KH. AFP-L3 for the diagnosis of early hepatocellular carcinoma: A meta-analysis. Medicine (Baltimore). 2021;100:e27673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 23. | Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, Li Q, Lu Y, Chen Y, Guo Y, Chen Z, Liu B, Jia W, Wu J, Wang J, Shao G, Zhang B, Shan Y, Meng Z, Gu S, Yang W, Liu C, Shi X, Gao Z, Yin T, Cui J, Huang M, Xing B, Mao Y, Teng G, Qin Y, Xia F, Yin G, Yang Y, Chen M, Wang Y, Zhou H, Fan J; ORIENT-32 study group. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol. 2021;22:977-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 673] [Article Influence: 168.3] [Reference Citation Analysis (1)] |
| 24. | Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, Izumi N, Yamasaki T, Nojiri S, Hino K, Tsumura H, Kuzuya T, Isoda N, Yasui K, Aino H, Ido A, Kawabe N, Nakao K, Wada Y, Yokosuka O, Yoshimura K, Okusaka T, Furuse J, Kokudo N, Okita K, Johnson PJ, Arai Y; TACTICS study group. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69:1492-1501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 504] [Article Influence: 100.8] [Reference Citation Analysis (0)] |
| 25. | He M, Li Q, Zou R, Shen J, Fang W, Tan G, Zhou Y, Wu X, Xu L, Wei W, Le Y, Zhou Z, Zhao M, Guo Y, Guo R, Chen M, Shi M. Sorafenib Plus Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin vs Sorafenib Alone for Hepatocellular Carcinoma With Portal Vein Invasion: A Randomized Clinical Trial. JAMA Oncol. 2019;5:953-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 381] [Article Influence: 76.2] [Reference Citation Analysis (0)] |