Published online Mar 14, 2024. doi: 10.3748/wjg.v30.i10.1466
Peer-review started: January 6, 2024
First decision: January 16, 2024
Revised: January 23, 2024
Accepted: February 25, 2024
Article in press: February 25, 2024
Published online: March 14, 2024
Processing time: 67 Days and 19.4 Hours
For ulcerative colitis (UC), the variability in inflammatory activity along the colon poses a challenge in management. The focus on achieving endoscopic healing in UC is evident, where the UC Endoscopic Index of Severity and Mayo Endoscopic Subscore are commonly used for evaluation. However, these indices primarily consider the most severely affected region. Liu et al recent study validates the Toronto Inflammatory Bowel Disease Global Endoscopic Reporting (TIGER) score offering a comprehensive assessment of inflammatory activity across diverse segments of the colon and rectum and a reliable index correlating strongly with UC Endoscopic Index of Severity and moderately with Mayo Endoscopic Subscore (MES). Despite recommendation, certain aspects warrant further investigation. Fecal calprotectin, an intermediate target, correlates with TIGER and should be explored. Determining TIGER scores defining endoscopic remission and response, evaluating agreement with histological activity, and assessing inter-endoscopist agreement for TIGER require scrutiny. Exploring the correlation between TIGER and intestinal ultrasound, akin to MES, adds value.
Core Tip: For ulcerative colitis (UC), the degree of inflammatory activity can vary along the length of the colon, ranging from the rectum to the proximal colon. Currently, achieving endoscopic healing is a long-term goal in the management of UC, with the UC Endoscopic Index of Severity score and Mayo Endoscopic Subscore being the most suggested indices to evaluate this target. However, both scores only consider the most severely affected area in their final assessment. Recently, the Toronto Inflammatory Bowel Disease Global Endoscopic Reporting score has shown its usefulness in determining the extent and severity of inflammatory activity across various segments of the colon and rectum. Despite this, there is no consensus regarding the endoscopic method (total colonoscopy or sigmoidoscopy) for evaluating the achievement of endoscopic healing in UC patients.
- Citation: Quera R, Núñez F P. Are we ready to use new endoscopic scores for ulcerative colitis? World J Gastroenterol 2024; 30(10): 1466-1469
- URL: https://www.wjgnet.com/1007-9327/full/v30/i10/1466.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i10.1466
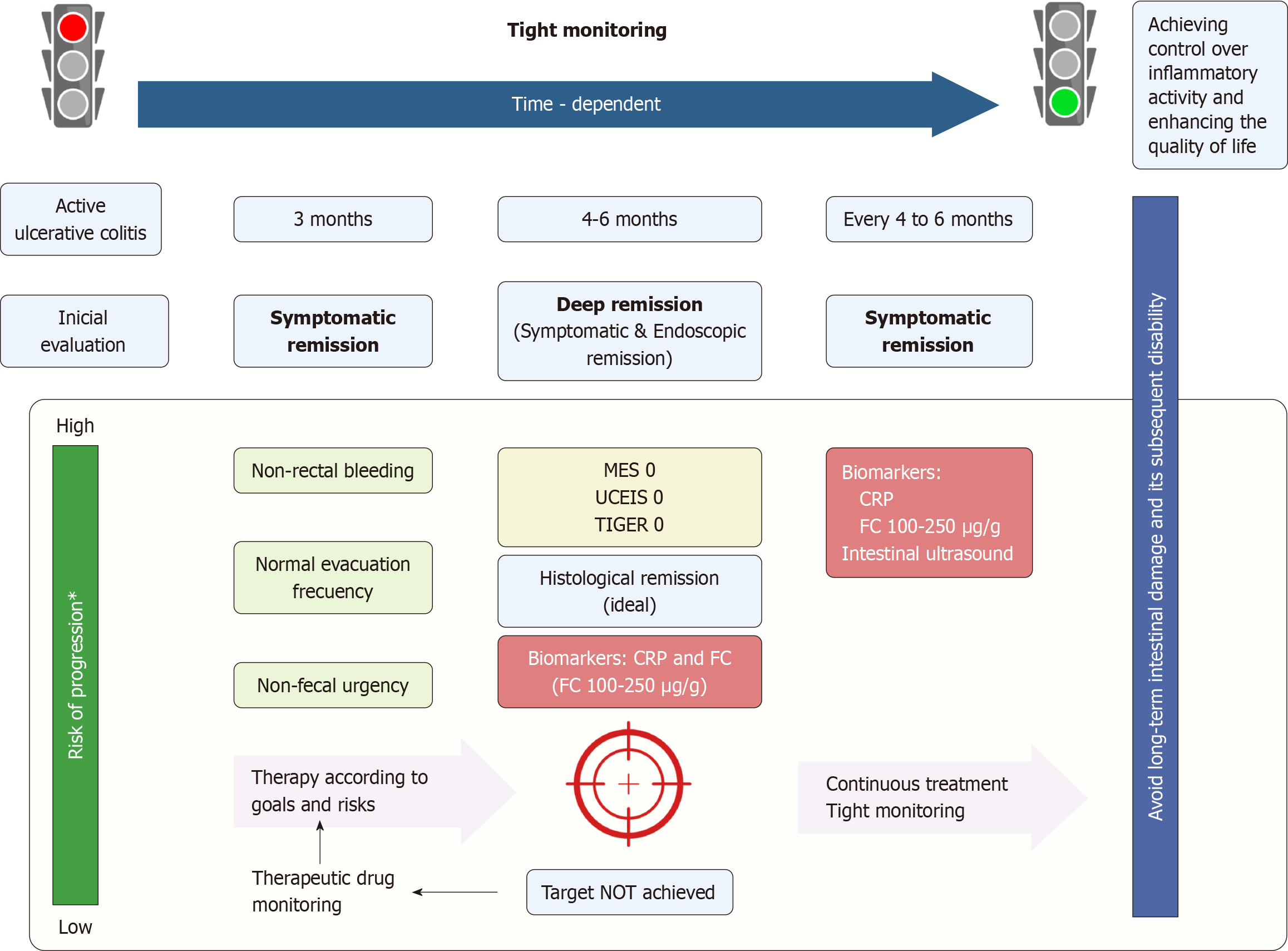
Early recognition of inflammatory activity, prompt intervention, along with tight monitoring constitute the cornerstones of the treat-to-target approach in ulcerative colitis (UC)[1,2]. Recently, the Therapeutic Goals Consensus in Inflammatory Bowel Disease (STRIDE-II) has highlighted that achieving mucosa healing in the rectum and colon is the long-term goal for patients with UC (Figure 1)[2]. Total colonoscopy provides comprehensive information about the extent and severity of inflammatory activity in patients with UC. This approach enhances the precision of UC management, whether conducted via colonoscopy or sigmoidoscopy[3]. With this in mind, we would like to extend our congratulations to Liu et al[4] for their article published last month in the World Journal of Gastroenterology. Their results confirm that the Toronto Inflammatory Bowel Disease Global Endoscopic Reporting (TIGER) score is a reliable and straightforward endoscopic index for UC patients to assess the overall endoscopic disease burden[4]. In a retrospective study involving 166 patients with UC, the authors demonstrated a strong correlation between the TIGER index and the UC Endoscopic Index of Severity (UCEIS) score (r = 0.721, P < 0.001) and a moderate correlation with the Mayo Endoscopic Subscore (MES) (r = 0.626, P < 0.001). UCEIS and MES are widely used indices in UC. Furthermore, a TIGER score ≥ 317 was identified as an independent risk factor for advanced treatment. This includes, the use of systemic corticosteroids, biologics, immunomodulators, thalidomide, and surgery. Nonetheless, there are certain aspects that warrant further investigation in subsequent studies.
To commence, fecal calprotectin is regarded as an intermediate target in UC[2]. While Liu et al[4] did not incorporate this biomarker in their research, other studies have established a correlation between fecal calprotectin levels and TIGER.
Secondly, it is important to determinate the TIGER scores that define endoscopic remission and endoscopic response. While the UCEIS score and MES 0 have been proposed as definitions for endoscopic remission, a decrease in UCEIS by ≥ 2 points or a decrease in Mayo endoscopic score by ≥ 1 grade is suggested for defining endoscopic response in UC[5].
Thirdly, exploring the agreement between the TIGER score and histological activity in UC is crucial. Previous studies have established correlations between the endoscopic scores (UCEIS score and MES) and histological indices[6,7].
Fourthly, it is essential to assess the agreement among endoscopists for the TIGER score. Studies have demonstrated adequate, though not perfect, correlation between different endoscopists when using MES or UCEIS in UC patients[8], but agreement among endoscopists for the TIGER score has not been conclusively demonstrated[9].
Finally, considering the potential of intestinal ultrasound as a tool for assessing inflammatory activity in UC patients, like MES[10], it would be valuable to explore whether there is a correlation between the TIGER score and the intestinal ultrasound index.
As previously mentioned, there is currently a lack of consensus regarding the preferred endoscopic method for evaluating the goal of endoscopic healing in UC patients. Some studies have suggested that sigmoidoscopy might be sufficient, given the highest inflammatory activity is typically observed in the distal colon[11,12]. However, this recommendation has not been universally confirmed, as some UC patients may exhibit higher inflammatory activity in the ascending colon. In such cases, total colonoscopy becomes the most appropriate endoscopic examination to assess inflammation in UC patients[13,14]. Although sigmoidoscopy is limited to evaluating inflammatory activity from the rectum to the descending colon, it is essential to recognize some benefits of this procedure. Sigmoidoscopy is safer, requires reduced preparation, has a lower cost, and takes less time to perform compared to a total colonoscopy. Moreover, some patients may find this procedure preferable.
Given the current lack of consensus and the need for confirmation through prospective multicenter studies, a personalized approach should be recommended for the evaluation of activity and severity of inflammatory activity in UC patients using the TIGER score. Total colonoscopy is likely the preferred method in scenarios where sigmoidoscopy results are inconsistent with clinical setting or biomarkers. This is particularly applicable in patients with UC and primary sclerosing cholangitis and during surveillance for the development of colorectal neoplasia.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Chile
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Knudsen T, Denmark S-Editor: Chen YL L-Editor: A P-Editor: Zheng XM
| 1. | Nuñez F P, Mahadevan U, Quera R, Bay C, Ibañez P. Treat-to-target approach in the management of inflammatory Bowel disease. Gastroenterol Hepatol. 2021;44:312-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Turner D, Ricciuto A, Lewis A, D'Amico F, Dhaliwal J, Griffiths AM, Bettenworth D, Sandborn WJ, Sands BE, Reinisch W, Schölmerich J, Bemelman W, Danese S, Mary JY, Rubin D, Colombel JF, Peyrin-Biroulet L, Dotan I, Abreu MT, Dignass A; International Organization for the Study of IBD. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology. 2021;160:1570-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 1644] [Article Influence: 411.0] [Reference Citation Analysis (1)] |
| 3. | Zittan E, Steinhart AH, Aran H, Milgrom R, Gralnek IM, Zelber-Sagi S, Silverberg MS. The Toronto IBD Global Endoscopic Reporting [TIGER] Score: A Single, Easy to Use Endoscopic Score for Both Crohn's Disease and Ulcerative Colitis Patients. J Crohns Colitis. 2022;16:544-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 4. | Liu XY, Tian ZB, Zhang LJ, Liu AL, Zhang XF, Wu J, Ding XL. Clinical value of the Toronto inflammatory bowel disease global endoscopic reporting score in ulcerative colitis. World J Gastroenterol. 2023;29:6208-6221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 5. | Vuitton L, Peyrin-Biroulet L, Colombel JF, Pariente B, Pineton de Chambrun G, Walsh AJ, Panes J, Travis SP, Mary JY, Marteau P. Defining endoscopic response and remission in ulcerative colitis clinical trials: an international consensus. Aliment Pharmacol Ther. 2017;45:801-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 6. | Fluxá D, Simian D, Flores L, Ibáñez P, Lubascher J, Figueroa C, Kronberg U, Pizarro G, Castro M, Piottante A, Vial MT, Quera R. Clinical, endoscopic and histological correlation and measures of association in ulcerative colitis. J Dig Dis. 2017;18:634-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Irani NR, Wang LM, Collins GS, Keshav S, Travis SPL. Correlation Between Endoscopic and Histological Activity in Ulcerative Colitis Using Validated Indices. J Crohns Colitis. 2018;12:1151-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Belvis Jiménez M, Hergueta-Delgado P, Gómez Rodríguez B, Maldonado Pérez B, Castro Laria L, Rodríguez-Téllez M, Morales Barroso ML, Galván Fernández MD, Guerra Veloz M, Jiménez García VA, Romero-Castro R, Benítez-Roladán A, Castro Márquez C, Aparcero López R, Garrido-Serrano A, Caunedo-Álvarez Á, Argüelles-Arias F. Comparison of the Mayo Endoscopy Score and the Ulcerative Colitis Endoscopy Index of Severity and the Ulcerative Colitis Colonoscopy Index of Severity. Endosc Int Open. 2021;9:E130-E136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Belvis Jiménez M, Hergueta-Delgado P, Gómez Rodríguez BJ, Maldonado Pérez B, Castro Laria L, Rodríguez-Téllez M, Morales Barroso ML, Galván Fernández MD, Guerra Veloz MF, Jiménez García VA, Romero Castro R, Benítez Roldán A, Castro Márquez C, Aparcero López R, Garrido Serrano A, Caunedo Álvarez Á, Argüelles Arias F. Index of the Mayo Endoscopy and Ulcerative Colitis Endoscopy Index of Severity: are they equally valid? Rev Esp Enferm Dig. 2020;112:821-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Bots S, Nylund K, Löwenberg M, Gecse K, D'Haens G. Intestinal Ultrasound to Assess Disease Activity in Ulcerative Colitis: Development of a novel UC-Ultrasound Index. J Crohns Colitis. 2021;15:1264-1271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 11. | Colombel JF, Ordás I, Ullman T, Rutgeerts P, Chai A, O'Byrne S, Lu TT, Panés J. Agreement Between Rectosigmoidoscopy and Colonoscopy Analyses of Disease Activity and Healing in Patients With Ulcerative Colitis. Gastroenterology. 2016;150:389-95.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Park SB, Kim SJ, Lee J, Lee YJ, Baek DH, Seo GS, Kim ES, Kim SW, Kim SY. Efficacy of sigmoidoscopy for evaluating disease activity in patients with ulcerative colitis. BMC Gastroenterol. 2022;22:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 13. | Kato J, Kuriyama M, Hiraoka S, Yamamoto K. Is sigmoidoscopy sufficient for evaluating inflammatory status of ulcerative colitis patients? J Gastroenterol Hepatol. 2011;26:683-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Jamil OK, Shaw D, Deng Z, Dinardi N, Fillman N, Khanna S, Krugliak Cleveland N, Sakuraba A, Weber CR, Cohen RD, Dalal S, Jabri B, Rubin DT, Pekow J. Inflammation in the proximal colon is a risk factor for the development of colorectal neoplasia in inflammatory bowel disease patients with primary sclerosing cholangitis. Therap Adv Gastroenterol. 2023;16:17562848231184985. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Calderón P, Núñez P, Nos P, Quera R. Personalized therapy in inflammatory bowel disease. Gastroenterol Hepatol. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 16. | BioRender. [cited 2 February 2024]. Available from: https://www.biorender.com/. |