Published online Mar 14, 2024. doi: 10.3748/wjg.v30.i10.1420
Peer-review started: October 17, 2023
First decision: January 15, 2024
Revised: January 17, 2024
Accepted: February 21, 2024
Article in press: February 21, 2024
Published online: March 14, 2024
Processing time: 149 Days and 12.7 Hours
Various animal models have been used to explore the pathogenesis of choledochal cysts (CCs), but with little convincing results. Current surgical techniques can achieve satisfactory outcomes for treatment of CCs. Consequently, recent studies have focused more on clinical issues rather than basic research. Therefore, we need appropriate animal models to further basic research.
To establish an appropriate animal model that may contribute to the investigation of the pathogenesis of CCs.
Eighty-four specific pathogen-free female Sprague-Dawley rats were randomly allocated to a surgical group, sham surgical group, or control group. A rat model of CC was established by partial ligation of the bile duct. The reliability of the model was confirmed by measurements of serum biochemical indices, morpho
Dilation classified as mild (diameter, ≥ 1 mm to < 3 mm), moderate (≥ 3 mm to < 10 mm), and severe (≥ 10 mm) was observed in 17, 17, and 2 rats in the surgical group, respectively, while no dilation was observed in the control and sham surgical groups. Serum levels of alanine aminotransferase, aspartate aminotransferase, total bilirubin, direct bilirubin, and total bile acids were significantly elevated in the surgical group as compared to the control group 7 d after surgery, while direct bilirubin, total bilirubin, and gamma-glutamyltransferase were further increased 14 d after surgery. Most of the biochemical indices gradually decreased to normal ranges 28 d after surgery. The protein expression trend of signal transducer and activator of transcription 3 in rat model was consistent with the human CC tissues.
The model of partial ligation of the bile duct of juvenile rats could morphologically simulate the cystic or fusiform CC, which may contribute to investigating the pathogenesis of CC.
Core Tip: Recent studies have focused more on clinical issues rather than etiology and pathogenesis of choledochal cyst (CC). In this study, our partial ligation of the bile duct of juvenile rats successfully simulated the pathological processes of recanalization after incomplete obstruction of the distal bile duct. The postoperative disease progression of this model was more consistent with the natural course of CC formation which may assist in further basic research on the pathogenesis of CC.
- Citation: Zhang SH, Zhang YB, Cai DT, Pan T, Chen K, Jin Y, Luo WJ, Huang ZW, Chen QJ, Gao ZG. Preliminary exploration of animal models of congenital choledochal cysts. World J Gastroenterol 2024; 30(10): 1420-1430
- URL: https://www.wjgnet.com/1007-9327/full/v30/i10/1420.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i10.1420
Choledochal cyst (CC) is a congenital hepatic malformation characterized by cystic or fusiform dilation of the common bile duct either with or without dilation of the intrahepatic bile duct. The hypothesis of pancreaticobiliary maljunction (PBM) proposed by Babbit[1] in 1969 is the most likely cause of CC, emphasizing that pancreatic juice could cause segmental stricture, fibrosis, and thinning of the common bile duct. However, with the increased proportion of prenatal diagnosis of CCs, researchers have found that pancreatic acini are just beginning to appear, enzymes are immature during that time period, and there is no evidence of pancreatic secretion observed by electron microscopy[2]. Therefore, the role of fetal pancreatic juice in the formation of CCs remains controversial because the pancreas of newborns does not yet produce functional enzymes[3]. Accumulating evidence from clinical cases suggests that PBM cannot fully explain the pathogenesis of CCs.
Various animal models to simulate the disease process in humans have been used to investigate the pathogenesis of cystic and fusiform CCs. However, anastomosis of the pancreatic and biliary ducts, which allow pancreatic juice to enter the bile duct, only resulted in mild dilation of the common bile duct without cystic dilatation[4]. Moreover, even in large animal models, such as minipigs, no one was observed to have dilatation of the bile ducts[5]. In contrast, complete ligation of the lower segment of the common bile duct in neonatal lambs[6] and infant rats[7] can successfully create a model of cystic dilation, while in mature sheep, rats, dogs or rabbits, no cystic dilation was observed through the ligation of the lower segment of the common bile duct. However, it is not recognized as a conventional CC animal model because it does not conform to the mainstream hypothesis of PBM. Some researchers also constructed an animal model by combining anastomosis of the pancreatic and biliary ducts with ligation of the lower segment of the common bile duct. However, it is still unclear whether dilation of the common bile duct is caused solely by ligation of the lower segment or by the combination of both factors. Satisfactory outcomes achieved with current surgical techniques for CCs have led to a pause in basic research on the etiology of CCs, and recent studies have primarily focused on reporting large clinical series, improvements of surgical techniques and the management of postoperative complications[8]. Hence, appropriate animal models should be established to further basic research. In our study, our partial ligation of the bile duct of juvenile rats successfully simulated the pathological processes of recanalization after incomplete obstruction of the distal bile duct. The postoperative changing trends of biochemical indexes in rat model was similar to those observed in CCs, and the changing trend of signal transducer and activator of transcription 3 (STAT3) in the rat model was consistent with that observed in human CC tissues. Therefore, our study has established an animal model that may contribute to the investigation of the pathogenesis of CCs.
Specific pathogen-free female SD rats (n = 84; mean body weight, 200 g ± 10 g) were purchased from Sibeifu Biotechnology Co., Ltd. (Beijing, China) and housed in an animal care facility operated by Zhejiang Yingyang Pharmaceutical Co., Ltd., (Hangzhou, China) at a constant temperature of 22 °C ± 2 °C and humidity of 50%-60% under a 12-h light-dark cycle with total air exchange at 15-20 times/h.
The 84 rats were randomly allocated to one of 7 groups of 12 rats each. Rats in groups A, B, and C were dissected on days 7, 14, and 28 after partial ligation of the bile duct, respectively. Rats in group D were dissected on day 28 after sham surgery without bile duct ligation. Rats in groups E, F, and G were dissected after 7 d, 14 d, and 28 d of feeding, respectively.
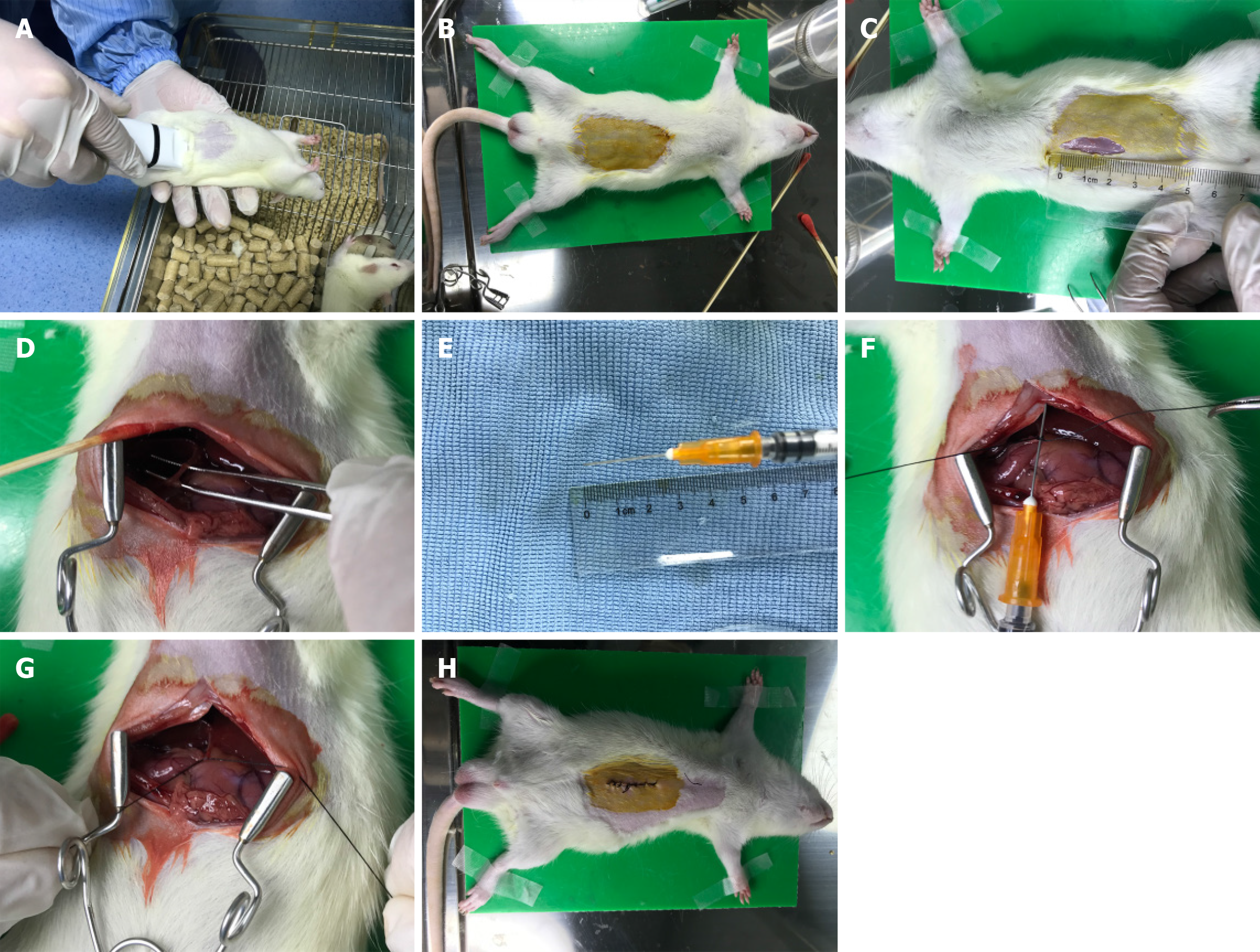
(1) Anesthesia: 2.5% pentobarbital sodium (0.25 mL/100 g) was injected into the abdominal cavity; (2) Positioning: After anesthesia, the rats were fixed with a supine position on the surgical table; (3) Skin preparation: The hair below the xiphoid process in the middle of the abdomen was removed with an electric shaver, exposing about 5 cm × 3 cm of skin, followed by iodine disinfection; (4) Skin incision: A midline longitudinal incision was made on the abdomen with surgical scissors, about 2 cm in length; (5) Bile duct isolation: Wet cotton swabs were used to separate the organs and locate the duodenum and pancreas, and then the bile duct was located with forceps; (6) Partial ligation: Place a 1 mL needle parallel to the bile duct and use 6-0 absorbable suture to ligate the bile duct and needle together at the lower segment of the bile duct. Then the needle was removed carefully after tightening the absorbable suture; and (7) Closure of the incision: After ligation, and the incision was intermittently sutured with 5-0 silk thread. Penicillin was administered intraperitoneally to prevent infection (0.3 mL, 400000 units/mL). Detailed surgical procedures were shown in Figure 1.
An adequate amounts of human bile duct tissues were sent for lncRNA sequencing analysis. The pretreatment of tissue specimens, filtration of sequencing data, obtainment of clean reads, calculation of gene expression levels and differential expression analysis were performed by BGI Genomics CO., Ltd (seen in previous reported study[9]). To gain insights into the phenotypic changes, GO (http://www.geneontology.org/) and KEGG (https://www.kegg.jp/) enrichment analysis of annotated differentially expressed genes was performed using Phyper (https://en.wikipedia.org/wiki/Hypergeometric_distribution) based on the Hypergeometric test. The significant levels of terms and pathways were rigorously corrected using a Q value threshold of ≤ 0.05 by Bonferroni[10].
The rat blood samples were centrifuged at 3000-4000 rpm for 10 min at 4 °C and the plasma was frozen for further ana
RNA Isolation and quantitative reverse transcription-polymerase chain reaction: Total cellular RNA was extracted from CC patients’ common bile ducts and rats’ common bile ducts with TRIzol reagent (Invitrogen, cat. 15596026/15596018) and subjected to reverse transcription with PrimeScript RT reagent Kit (Takara, cat. RR037A) according to the manufacturers’ instructions. The expressions of IL-6 and STAT3 were analyzed via quantitative reverse transcription-polymerase chain reaction (RT-qPCR) with a SYBR Premix EX Taq (Tli RNaseH plus, Takara, cat. RR420A) with primers listed in Table 1. For analysis, expression levels of the genes were normalized to the values of GAPDH. Analysis of relative gene expression data using real-time quantitative PCR was calculated with the 2-11Ct method[11].
| Gene | Gene ID | Forward primers | Reverse primers |
| IL-6 | 3569 | GGTGGGTGTGTCCTCATTCC | GGCATTGCATCCCTGAGTTG |
| STAT3 | 6774 | GTGGGAAGAATCACGCCTTC | AGATCCTGCACTCTCTTCCG |
| GAPDH | 2597 | GAACGGGAAGCTCACTGG | GCCTGCTTCACCACCTTCT |
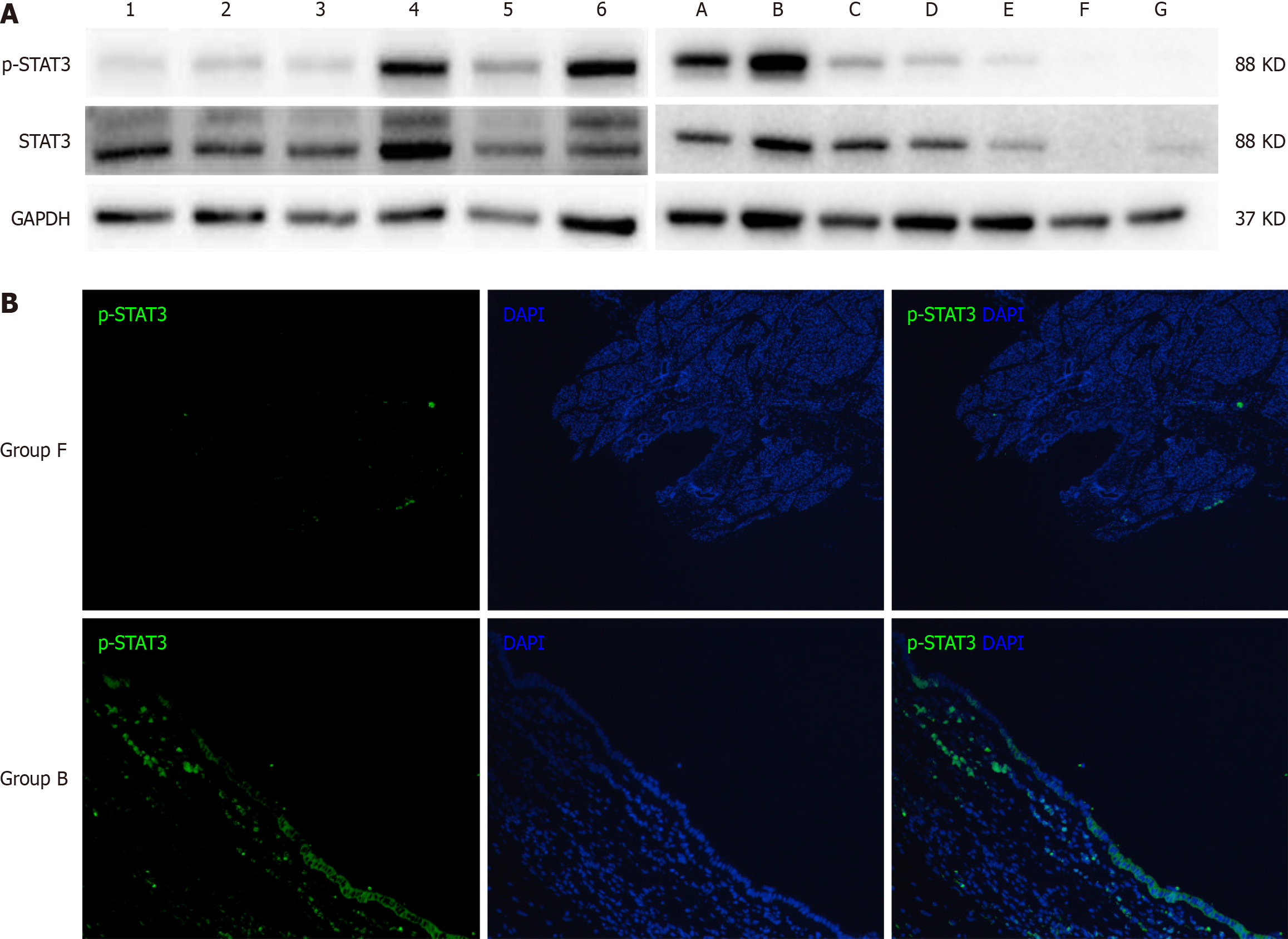
Western blot: Tissue samples (50 mg) were washed with Tris-buffered saline to remove residual blood and ground into small fragments, which were lysed with radioimmunoprecipitation assay on ice for 30 min. After addition of protein inhibitors, the mixture was centrifuged at 13000 rpm for 10-15 min and the supernatant was collected. Then, 5 × loading buffer was added and the mixture was heated in a metal bath at 95 °C for 5 min to fully denature the proteins. Protein levels were determined against standards. Protein expression levels of STAT3 and phosphorylated (p)-STAT3 and were normalized to expression of glyceraldehyde 3-phosphate dehydrogenase.
Immunofluorescence histochemistry: The common bile duct sections were deparaffinized in three changes of xylene and two changes of 100% ethanol and subsequent gradation of 95%, 80%, and 70% alcohol for 3 min each. After being heat-induced epitope retrieval with a preheated epitope retrieval solution (pH 8.0, Enzo Life Sciences, Inc. United States), endogenous peroxidase was inactivated by incubation in 3% H2O2 for 20 min. Next, the sections were pre-incubated with 10% normal goat serum and then incubated overnight with primary antibodies: p-STAT3 (Abcam, ab76315). The next day, after washing, sections were incubated with Alexa Fluoro 488 Goat Anti-Rabbit IgG (Jackson, 111-545-144). At last, sections were counterstained with DAPI (Sigma-Aldrich). Slides were imaged using the Carl Zeiss Scope.A1.
Data were shown as the medians ± IQRs or mean ± SEM depending on data characteristics. Statistical analysis was performed with SPSS18.0 and Graphpad Prism 6. Statistical P values were analyzed by a two-tailed Student’s t-test. P values < 0.05 were considered statistically significant.
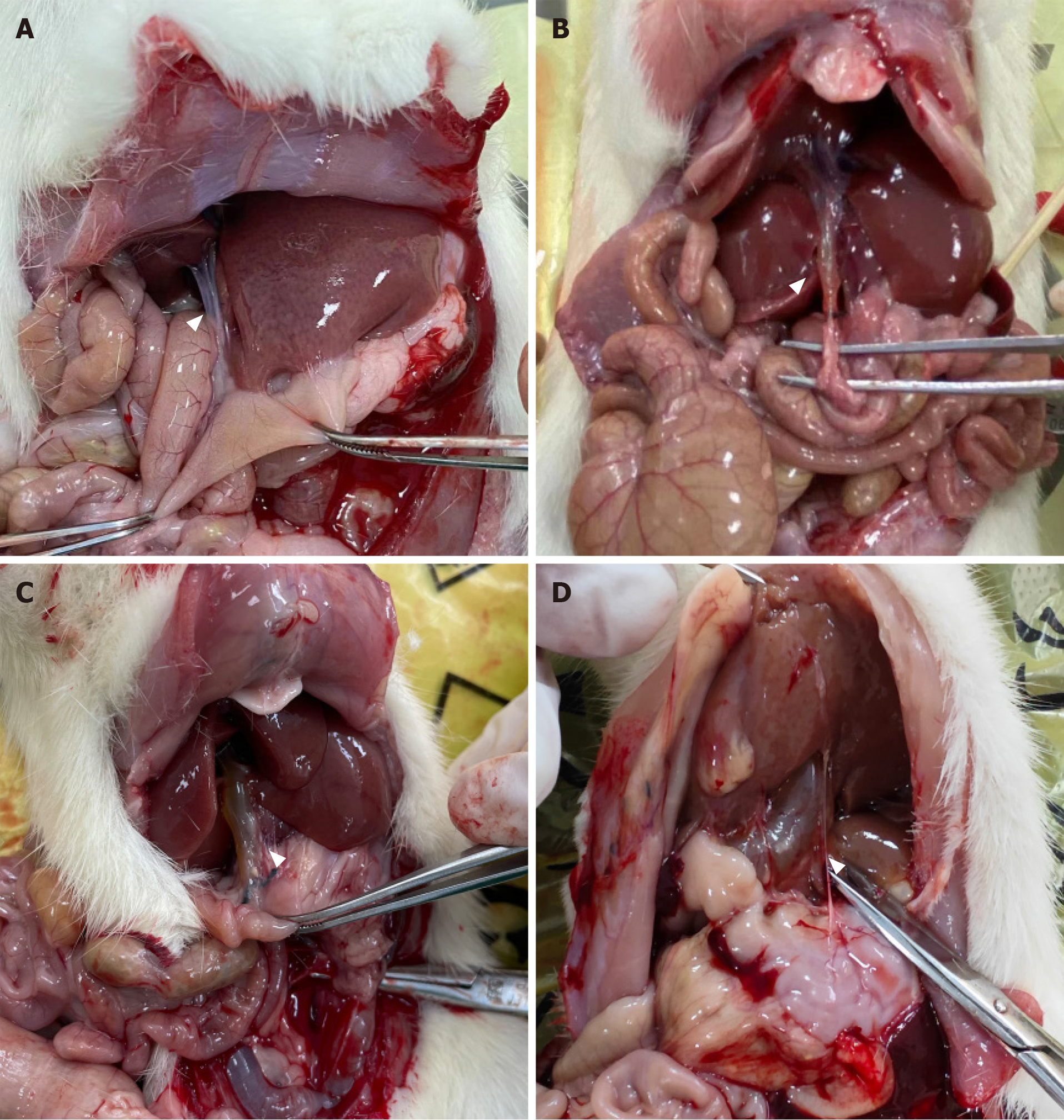
All 84 rats survived to the time of dissection. Rats in groups A-C showed varying degrees of dilation of the common bile ducts with slight damage to the liver, but no significant cholestasis or cirrhosis. Rats in group D developed slight adhesions around the surgical site and those in groups D-G showed no dilation of the common bile ducts. For normal rats, the diameter of the common bile duct is less than 1 mm. Based on measurements taken during dissection, dilation of the common bile duct was classified as none (< 1 mm), mild (≥ 1 mm to < 3 mm), moderate (≥ 3 mm to < 10 mm), and severe (≥ 10 mm). Mild to moderate dilation resembled fusiform CC, while severe dilation resembled cystic CC11 (Figure 2). Bile duct dilation for each group is summarized in Table 2.
| Group | No dilation | Mild dilation | Moderate dilation | Severe dilation |
| (φ < 1 mm) | (1 mm ≤ φ < 3 mm) | (3 mm ≤ φ < 10 mm) | (φ ≥ 10 mm) | |
| A | 0 | 10 | 2 | 0 |
| B | 0 | 5 | 7 | 0 |
| C | 0 | 2 | 8 | 2 |
| D | 11 | 1 | 0 | 0 |
| E-G | 36 | 0 | 0 | 0 |
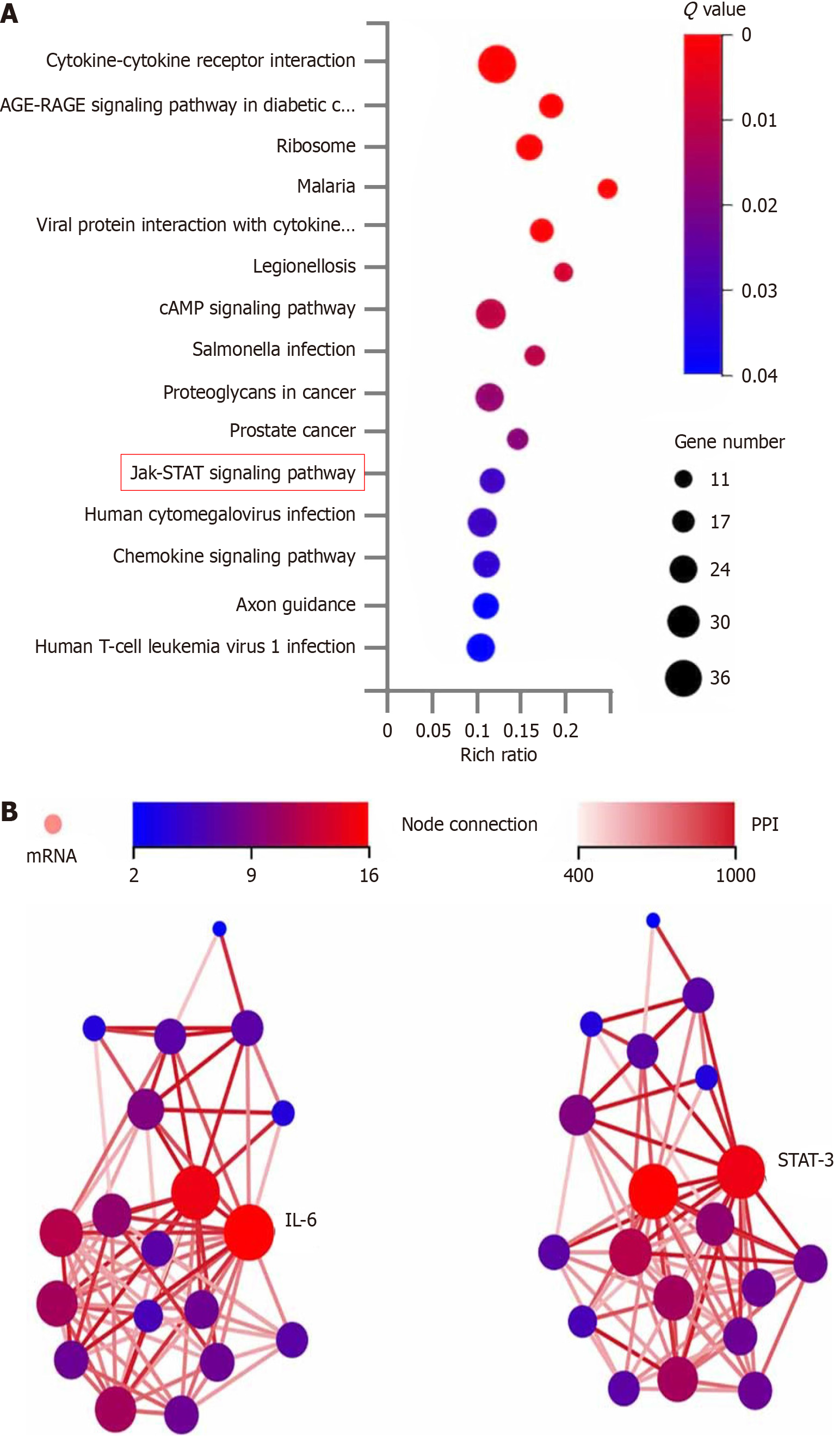
Of 103116 genes detected in the bile duct tissues between cystic and fusiform CCs, 993 were identified as differentially expressed genes (DEGs). Reference to the Kyoto Encyclopedia of Genes and Genomes (https://www.genome.jp/kegg/) revealed that 19 of the DEGs were enriched in the JAK-STAT signaling pathway (Figure 3). A protein-protein interaction network of the 19 DEGs in the JAK-STAT signaling pathway demonstrated that interleukin (IL)-6 had the highest node connectivity in the network, followed by the key factor STAT3 (Figure 3).
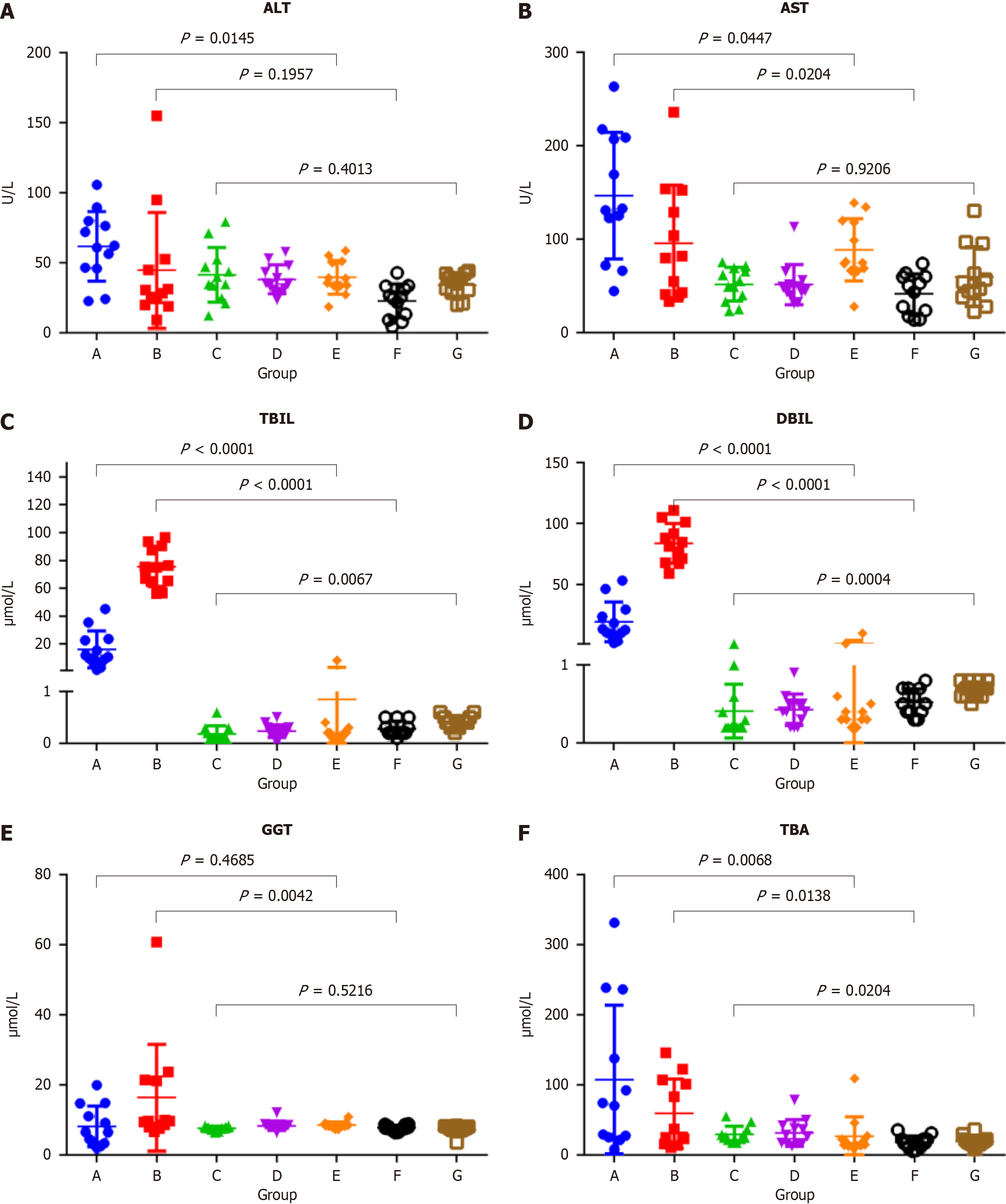
The mean normal serum concentrations (ranges) of ALT, AST, TBIL, DBIL, GGT, and TBA for female SD rats (groups E-G) were 33.55 (24.50-39.10) U/L, 59.60 (38.95-75.35) U/L, 0.60 (0.40-0.70) μmol/L, 0.30 (0.20-0.40) μmol/L, 7.95 (7.30-8.40) μmol/L, and 17.45 (13.55-22.27) μmol/L, respectively. Serum ALT levels were significantly higher in group A than group E (P = 0.0145) and decreased to the normal range in group C. Serum AST levels were significantly higher in groups A and B than groups E and F (P = 0.0447 and 0.0204, respectively), and decreased to the normal range in group C. Serum TBIL levels were significantly higher in groups A and B than groups E and F (both, P < 0.0001), significantly higher in group B than group A (P < 0.0001), and decreased to the normal range in group C. Serum DBIL levels were significantly higher in groups A and B than groups E and F (both, P < 0.0001), significantly higher in group B than group A (P < 0.0001), and decreased to the normal range in group C. Serum GGT levels were significantly higher in group B than group F (P = 0.0042) and decreased to the normal range in group C. Serum TBA levels gradually decreased in group A-C, but were slightly higher than the normal range (P < 0.0204) (Figure 4 and Table 3).
| Biochemical indices | Group A | Group B | Group C | Group D | Group E | Group F | Group G |
| ALT (U/L) | 61.55 | 29.30 | 37.40 | 35.20 | 37.15 | 24.80 | 35.90 |
| (45.95-78.90) | (20.85-50.68) | (27.13-51.25) | (30.65-46.60) | (33.53-50.68) | (10.13-32.70) | (29.03-41.27) | |
| AST (U/L) | 131.80 | 80.60 | 52.30 | 46.60 | 75.20 | 46.75 | 50.20 |
| (85.50-208.15) | (41.23-146.28) | (35.05-68.48) | (40.50-54.75) | (66.93-119.18) | (18.90-59.75) | (38.95-87.25) | |
| TBIL (μmol/L) | 12.90 | 82.90 | 0.20 | 0.40 | 0.35 | 0.50 | 0.70 |
| (9.48-27.85) | (68.45-98.70) | (0.20-0.55) | (0.23-0.50) | (0.30-0.58) | (0.40-0.70) | (0.60-0.80) | |
| DBIL (μmol/L) | 11.25 | 75.20 | 0.10 | 0.20 | 0.20 | 0.20 | 0.40 |
| (7.68-23.35) | (64.95-89.65) | (0.10-0.28) | (0.13-0.30) | (0.10-0.28) | (0.20-0.45) | (0.33-0.50) | |
| GGT (μmol/L) | 6.45 | 9.65 | 7.75 | 8.15 | 8.35 | 7.65 | 7.50 |
| (3.15-13.78) | (8.08-21.33) | (7.38-7.90) | (7.83-8.45) | (8.05-8.90) | (7.18-8.48) | (7.00-8.08) | |
| TBA (μmol/L) | 72.05 | 31.25 | 25.55 | 27.45 | 16.45 | 17.05 | 18.10 |
| (25.60-211.53) | (17.33-105.60) | (21.93-34.93) | (16.40-40.65) | (13.03-25.70) | (11.45-22.28) | (14.90-22.30) |
The Western blot results of human bile duct tissues confirmed significant differences in the expression levels of p-STAT3 between cystic and fusiform CCs (Figure 4). Meanwhile, the Western blot results of the rat bile duct tissue (Figure 5A) showed upregulated expression of p-STAT3 on day 7 after partial ligation surgery, which further increased on day 14. However, p-STAT3 expression was significantly decreased on day 28. The expression level of p-STAT3 protein in group D-G were extremely low. And the expression level of STAT3 was significantly higher in groups A-D than groups E-G. Representative images of immunofluorescence staining of p-STAT3 in the bile duct tissues of groups B and F are shown in Figure 5B. Notably, p-STAT3 expression was significantly greater in the epithelial cells of group B as compared to group F, indicating potential involvement of the JAK-STAT signaling pathway in bile duct dilation after partial ligation.
Although the etiology of CC has been investigated for over a century, there is still a lack of appropriate animal models. In 1977, Spitz[6] reported an animal model of CC which involved distal ligation of the common bile duct in 2-d-old lambs. Subsequently, in 1979, Miyano et al[7] performed distal ligation of the common bile duct in 2-wk-old rats, thereby successfully establishing an animal model with dilated extrahepatic bile duct, while maintaining the normal intrahepatic bile duct. However, after the PBM hypothesis gained widespread recognition, subsequent animal models were mostly based on the anatomical structure of PBM. A dog model of PBM was initially constructed because of the similarity to the anatomical structures of the human pancreatic and bile ducts. Although anastomosis of the pancreatic and bile ducts causes abnormal anatomical structure and reflux of pancreatic juice, the refluxed pancreatic juice did not result in stenosis or typical cystic dilatation of the bile duct[12,13]. Improvements to the dog model have been attempted, such as partial ligation of the distal end of the common bile duct after pancreaticobiliary anastomosis[14]. However, it remains unclear whether dilation of the bile duct was caused by reflux of pancreatic juice, distal ligation, or both. In addition, most previous studies failed to provide an image of the model.
In our center, over 95% of CCs diagnosed prenatally are the cystic type and over 90% of cystic CCs were diagnosed in infants aged < 1 year. And several previous studies of CCs had reported the existence of distal stenosis of the bile duct[2,15]. Babbitt[1], who proposed the hypothesis of PBM, also claimed that distal stenosis played a key role in the pathogenesis of bile duct dilation. Based on previous animal models, we conclude that morphological mimicry of CCs requires the use of an infant animal and stenosis of the distal bile duct. Therefore, we established a juvenile rat model by partial ligation of the distal bile duct. In the present study, all 36 rats in groups A-C and all 12 in group D survived to the day of dissection. The bile ducts of rats in groups A-C exhibited various degrees of dilation, but not those of the rats in group D. Notably, all rats with severe bile duct dilation were in group C. Therefore, this model is relatively stable and repeatable.
In this study, the cystic and fusiform dilations of the bile duct in the partially ligated rat model were morphologically similar to the cystic or fusiform CCs. Further analysis of biochemical indices showed that the serum levels of ALT, AST, DBIL, TBIL, and TBA were significantly elevated at 7 d after surgery, indicating that partial ligation of the bile duct simulated the pathological process of bile stasis caused by biliary obstruction. Meanwhile, serum levels of DBIL, TBIL, and GGT increased to higher levels at 14 d after surgery, and most indices, with the exception of TBA, gradually decreased to normal ranges at 28 d after surgery. These findings indicate that partial ligation of the bile duct results in increasing intra-cystic pressure and dilation of the bile ducts until bile production and outflow reached a dynamic equilibrium. Then, the indices returned to normal or near-normal levels. During this process, the bile duct continued to dilate and eventually formed cystic or fusiform CCs. Hence, partial ligation was more consistent with the natural course of CC formation than complete ligation. Meanwhile, the reliability of the partial ligation rat model was further validated by comparison to the characteristics of the human bile duct. Notably, activation of the IL-6/JAK/STAT3 signaling pathway, which is involved in cell proliferation and differentiation[16], was significantly elevated in cystic CCs. Also, immunofluorescence staining showed that expression of p-STAT3 was significantly elevated in the subepithelial layer of the bile duct, which may cause accelerated cell proliferation in the bile duct and make it easier for the formation of cystic dilation. In the rat model, p-STAT3 expression was also increased after partial ligation and gradually decreased at 28 days after surgery but was still higher than normal rats, which was consistent with the results of biochemical indices.
In summary, our partial ligation of the bile duct in juvenile rats successfully morphologically simulated the cystic or fusiform human CCs. The postoperative disease progression was more consistent with the natural disease course of CC formation compared to complete ligation. Therefore, this stable and repeatable model can be utilized to investigate the pathogenesis of CC and further explore the causes of cystic and fusiform bile duct dilatations.
Pancreaticobiliary maljunction (PBM) is the main hypothesis of choledochal cyst (CC). However, accumulating clinical evidence suggests that PBM cannot fully explain the pathogenesis of CC. Previously reported animal models, including models of anastomosis of the pancreatic and biliary ducts, models of complete ligation of the lower segment of the common bile duct, have been unable to adequately support basic researches on CCs.
Satisfactory outcomes achieved with current surgical techniques for CCs have led to a pause in basic research on the pathogenesis of CCs. Thus, we need appropriate animal models of CCs to further basic researches.
To establish a stable and repeatable animal model of CC based on partial ligation of the bile duct to investigate the pathogenesis of CCs.
Specific pathogen-free female SD rats were randomly allocated to a surgical group (partial ligation of the bile duct), sham surgical group, or control group. The partial ligation of the bile duct was performed by ligating a 1 mL needle and the bile duct together, followed by the careful removal of the needle after tightening the absorbable suture. The reliability of the model was confirmed through measurements of serum biochemical indices, the morphology of common bile duct and molecular biology experiments in rat and human tissues.
All 84 rats survived to the time of dissection. Rats in the surgical group (groups A-C) showed varying degrees of dilation of the common bile ducts with slight damage to the liver and those in the sham surgical and control groups (groups D-G) showed no dilation of the common bile ducts. The changing trends of biochemical indexes indicated that the partially ligated bile ducts experienced a pathological process of recanalization after incomplete obstruction of the distal bile duct. And the reliability of the model was also confirmed by molecular biology experiments in rat and human tissues.
The model of partial ligation of the bile duct of juvenile rats could morphologically simulate the cystic or fusiform CCs. This stable and repeatable model was more consistent with the natural disease course of CC formation than complete ligation which may assist in the basic researches of CCs.
We hope our partial ligation of the bile duct of juvenile rats can promote the basic research of CCs and provide a reliable animal model for further research on the formation of cystic CCs or fusiform CCs.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kordzaia D, Georgia; Mijwil MM, Iraq S-Editor: Chen YL L-Editor: A P-Editor: Zheng XM
| 1. | Babbitt DP. [Congenital choledochal cysts: new etiological concept based on anomalous relationships of the common bile duct and pancreatic bulb]. Ann Radiol (Paris). 1969;12:231-240. [PubMed] |
| 2. | Greenholz SK. Antenatal diagnosis of choledochal cyst at 15 weeks' gestation: etiologic implications and management. J Pediatr Surg. 1990;25:584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Kolacek S, Puntis JW, Lloyd DR, Brown GA, Booth IW. Ontogeny of pancreatic exocrine function. Arch Dis Child. 1990;65:178-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Ohkawa H, Sawaguchi S, Yamazaki Y, Sakaniwa M, Ishikawa A. The production of anomalous pancreaticobiliary ductal union in canine models. Z Kinderchir. 1981;32:328-336. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Benhidjeb T, Said S, Rudolph B, Siegmund E. Anomalous pancreatico-biliary junction--report of a new experimental model and review of the literature. J Pediatr Surg. 1996;31:1670-1674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Spitz L. Experimental production of cystic dilatation of the common bile duct in neonatal lambs. J Pediatr Surg. 1977;12:39-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Miyano T, Suruga K, Suda K. Abnormal choledocho-pancreatico ductal junction related to the etiology of infantile obstructive jaundice diseases. J Pediatr Surg. 1979;14:16-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Friedmacher F, Ford KE, Davenport M. Choledochal malformations: global research, scientific advances and key controversies. Pediatr Surg Int. 2019;35:273-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Huang J, Qiu ZY, He J, Xu HS, Wang K, Du HY, Gao D, Zhao WN, Sun QG, Wang YS, Wen PZ, Li Q, Dong XO, Xie XZ, Jiang L, Wang HY, Liu YQ, Wan JM. Phytochrome B mediates dim-light-reduced insect resistance by promoting the ethylene pathway in rice. Plant Physiol. 2023;191:1272-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Abdi H. The Bonferonni and Šidák Corrections for Multiple Comparisons. encyclopedia of measurement & statistics. 2006. [cited 12 January 2023]. Available from: https://www.semanticscholar.org/paper/The-Bonferonni-and-%C5%A0id%C3%A1k-Corrections-for-Multiple-Abdi/8b4300f253644d49c778c037ee614b9cf42a908c. |
| 11. | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149116] [Cited by in RCA: 133628] [Article Influence: 5567.8] [Reference Citation Analysis (1)] |
| 12. | Diao M, Li L, Cheng W. Congenital biliary dilatation may consist of 2 disease entities. J Pediatr Surg. 2011;46:1503-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Oguchi Y. [Histopathological studies on congenital dilatation of the bile duct--with special reference to the regurgitation of pancreatic juice into the bile duct]. Nihon Geka Gakkai Zasshi. 1986;87:547-557. [PubMed] |
| 14. | Qian D, Jin BX. Experimental Study on the Etiology of Congenital Biliary Dilatation. Chin J Pediatr Surg. 1990;11:257-259. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 15. | Tsang TM, Tam PK, Chamberlain P. Obliteration of the distal bile duct in the development of congenital choledochal cyst. J Pediatr Surg. 1994;29:1582-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Huang B, Lang X, Li X. The role of IL-6/JAK2/STAT3 signaling pathway in cancers. Front Oncol. 2022;12:1023177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 204] [Reference Citation Analysis (0)] |