Published online Mar 14, 2024. doi: 10.3748/wjg.v30.i10.1346
Peer-review started: November 7, 2023
First decision: December 27, 2023
Revised: January 12, 2024
Accepted: February 18, 2024
Article in press: February 18, 2024
Published online: March 14, 2024
Processing time: 128 Days and 2.7 Hours
Within the normal range, elevated alanine aminotransferase (ALT) levels are associated with an increased risk of metabolic dysfunction-associated fatty liver disease (MAFLD).
To investigate the associations between repeated high-normal ALT measurements and the risk of new-onset MAFLD prospectively.
A cohort of 3553 participants followed for four consecutive health examinations over 4 years was selected. The incidence rate, cumulative times, and equally and unequally weighted cumulative effects of excess high-normal ALT levels (ehALT) were measured. Cox proportional hazards regression was used to analyse the association between the cumulative effects of ehALT and the risk of new-onset MAFLD.
A total of 83.13% of participants with MAFLD had normal ALT levels. The incidence rate of MAFLD showed a linear increasing trend in the cumulative ehALT group. Compared with those in the low-normal ALT group, the multivariate adjusted hazard ratios of the equally and unequally weighted cumulative effects of ehALT were 1.651 [95% confidence interval (CI): 1.199-2.273] and 1.535 (95%CI: 1.119-2.106) in the third quartile and 1.616 (95%CI: 1.162-2.246) and 1.580 (95%CI: 1.155-2.162) in the fourth quartile, respectively.
Most participants with MAFLD had normal ALT levels. Long-term high-normal ALT levels were associated with a cumulative increased risk of new-onset MAFLD.
Core Tip: Limited evidence exists regarding the association between persistently elevated high-normal alanine transaminase (ALT) levels and the risk of new-onset metabolic dysfunction-associated fatty liver disease (MAFLD). This cohort study analysed 3553 participants followed for four consecutive health examinations between 2017 and 2020 and measured the cumulative effects of excess high-normal ALT (ehALT). Among the participants, the incidence rate of MAFLD showed a linear increasing trend for the cumulative ehALT group. The hazard ratios of new-onset MAFLD were significantly increased in the third and fourth quartiles of the equally and unequally weighted cumulative effects of ehALT. Among Chinese adults, long-term high-normal ALT levels were related to a cumulative increased risk of new-onset MAFLD.
- Citation: Chen JF, Wu ZQ, Liu HS, Yan S, Wang YX, Xing M, Song XQ, Ding SY. Cumulative effects of excess high-normal alanine aminotransferase levels in relation to new-onset metabolic dysfunction-associated fatty liver disease in China. World J Gastroenterol 2024; 30(10): 1346-1357
- URL: https://www.wjgnet.com/1007-9327/full/v30/i10/1346.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i10.1346
Metabolic dysfunction-associated fatty liver disease (MAFLD) is defined as the combination of nonalcoholic fatty liver disease (NAFLD) and metabolic dysfunction and includes overweight/obesity, type 2 diabetes, or other metabolic disorders, as indicated by liver biopsy or imaging examination or even blood biomarker tests suggesting the presence of fatty liver[1,2]. MAFLD has become a growing public health problem, affecting up to a third of the global population, and its burden has grown in parallel with rising rates of type 2 diabetes mellitus and obesity[3,4]. The prevalence of MAFLD is reportedly 25.0% among adults worldwide[5], 29.2% in China[6], and 33.9% in Korea after sex and age standardization[7], with an increasing incidence each year. MAFLD is a multisystemic disease beyond the liver that can increase the risk of heart failure, obstructive sleep apnoea, and malignancy and can result in an increase in cancer-related and cardiovascular disease mortality[8]. Given the high harm and enormous disease burden of MAFLD, a comprehensive analysis of risk factors is essential[9].
Alanine aminotransferase (ALT) has been recognized globally as a reliable indicator reflecting the degree of liver cell damage, such as the damage associated with NAFLD, chronic hepatitis, and cirrhosis[10]. Many studies have suggested that liver damage can occur in the presence of normal ALT levels[11]. Recently, a growing body of evidence has indicated that an ALT level that is within the normal range is an important biomarker for predicting NAFLD; additionally, nonalcoholic steatohepatitis (NASH) or advanced fibrosis is diagnosed in up to 37.5%-59% of patients with NAFLD who have normal ALT levels[12,13]. Our previous work also indicated that an ALT trajectory at a normal level is associated with the risk of new-onset MAFLD based on a cohort study[14]. Thus, we hypothesized that a specific ALT level, particularly a long-term high-normal ALT level, is associated with the risk of new-onset MAFLD.
Some evidence has suggested that the Youden index, a popular summary statistic for receiver-operating characteristic (ROC) curves, provides the optimal cut-off point for a biomarker to distinguish diseased and healthy individuals[15]. In a study of adolescents with obesity, the optimal ALT cut-off points for diagnosing NAFLD were 36 U/L for males and 33 U/L for females[16]. However, limited evidence exists on the determination of optimal ALT cut-off points for diagnosing MAFLD and on the associations between repeated high-normal ALT measurements and both the incidence of MAFLD and risk of new-onset MAFLD. In light of the public health burden of MAFLD in China, we investigated the association between repeated ALT levels that are high-normal and new-onset MAFLD using an ambispective cohort from a health examination population.
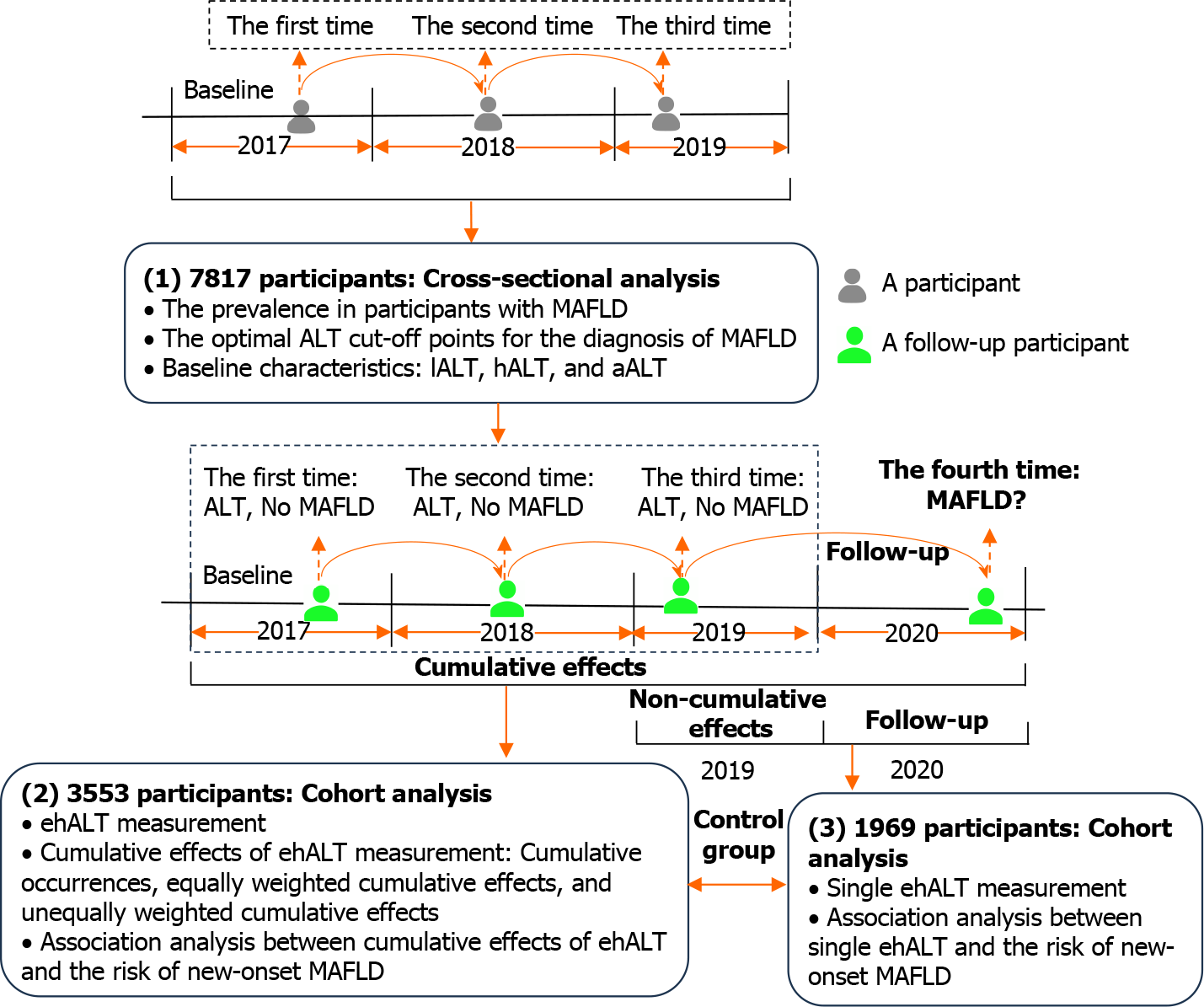
This retrospective and prospective population-based cohort study was based on data from an ambispective cohort from a health examination population in Henan Province. All eligible participants were interviewed by uniformly trained medical staff to gather information about common chronic diseases and factors influencing health. Participants aged ≥ 18 years who underwent a health examination at the First Affiliated Hospital of Zhengzhou University over a period of 3 consecutive years from January 2017 to December 2019 were retrospectively selected. We identified a total of 7817 participants (4975 male and 2842 female individuals); 4521 had no diagnosis of MAFLD according to three consecutive health examinations and were followed up at their fourth health examination in 2020. During the follow-up period, 738 participants did not participate in the health examination for various reasons or had missing information on some of the studied factors; we also excluded 230 participants with viral hepatitis, alcoholic hepatitis, autoimmune hepatitis, severe cardiovascular and cerebrovascular diseases, or malignant tumours. Finally, 3553 eligible participants (1741 male and 1812 female individuals) were selected from the pool of 7817 participants.
The data collected included physical measurements, laboratory test results, abdominal colour Doppler ultrasound results, and diagnostic criteria for MAFLD, as described below: (1) Physical measurements. Participants’ body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), and waist circumference (WC) were measured by clinicians using a uniform measurement instrument; (2) Laboratory tests. An automatic biochemical analyser was used to measure fasting plasma glucose (FPG), glycated haemoglobin (HbA1c), total cholesterol, triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), serum uric acid (SUA), and ALT levels[15]; (3) Abdominal colour Doppler ultrasound. Ultrasound was used to determine the presence of diffuse echogenic changes in the liver; and (4) Diagnostic criteria for MAFLD. These findings included diffuse echogenic changes in the liver as revealed by abdominal colour Doppler ultrasonography and were accompanied by at least one of the following conditions: (1) Overweight/obese (BMI > 23 kg/m2); (2) Type 2 diabetes; and (3) Metabolic dysfunction, defined as the presence of at least two of the following conditions: (1) WC ≥ 90 cm for men and WC ≥ 80 cm for women; (2) Hypertension or use of blood pressure-lowering medication or SBP ≥ 130 mmHg and/or DBP ≥ 85 mmHg; (3) FPG ≥ 5.6 mmol/L or 2-h postprandial glucose ≥ 7.8 mmol/L or HbA1c ≥ 5.7%; (4) TG ≥ 1.7 mmol/L or use of lipid-lowering drugs; and (5) HDL-C < 1.0 mmol/L in men and HDL-C < 1.3 mmol/L in women or use of lipid-lowering medication[16].
In total, 7817 participants with three consecutive health examinations from 2017 to 2019 were analysed to determine the optimal ALT cut-off points for the diagnosis of MAFLD, with ALT within the normal range (0-40 U/L). The participants were divided into three groups: Those with a low-normal ALT (lALT) level, those with a high ALT (hALT) level, and those with an abnormal ALT (aALT) level. A follow-up cohort of 3553 participants who completed their fourth health examination in 2020 was subsequently analysed to calculate the cumulative effects of excess hALT (ehALT) and explore its association with the risk of new-onset MAFLD. In this study, the cumulative effects of ehALT were classified into the following three categories: (1) Cumulative number of ehALT occurrences; (2) Equally weighted cumulative effects of ehALT; and (3) Unequally weighted cumulative effects of ehALT[17]. Finally, a single ehALT occurrence (noncumulative ehALT) was also included as a control for the cumulative effects of ehALT. The study design is shown in Figure 1, and some terms are defined in Table 1.
| Term | Definition |
| lALT group | ALT ≤ optimal ALT cut-off points (U/L) |
| hALT group | Optimal ALT cut-off point < ALT ≤ 40 (U/L) |
| aALT group | ALT > 40 (U/L) |
| ehALT | ALT-optimal ALT cut-off point, if ehALT < 0, redefine ehALT = 0 |
| Cumulative occurrences of ehALT | Sum of times that ehALT > 0 in 2017-2019, time = {0, 1, 2, 3} |
| Equally weighted cumulative effects of ehALT | Sum of ehALT levels with a weight of 1 in 2017-2019, i.e., ehALT2017 + ehALT2018 + ehALT2019 |
| Unequally weighted cumulative effects of ehALT | Sum of ehALT levels with an increasing weight in 2017-2019, i.e., 1 × ehALT2017 + 2 × ehALT2018 + 3 × ehALT2019 |
| Single ehALT occurrence | ehALT2019 along with ehALT2017 = 0 and ehALT2018 = 0 |
The ROC curve with the maximum value of the Youden index (sensitivity + specificity-1) was used to determine the ALT cut-off points for the diagnosis of MAFLD using ALT in 7817 participants from 2017 to 2019. Normally distributed continuous data are presented as the means (SD); comparisons among groups were performed using one-way analysis of variance (ANOVA) along with pairwise comparisons, the least significant difference test for homogeneous variance, and Dunnett’s T3 test for nonhomogeneous variance. Continuous data with a skewed distribution are presented as medians [interquartile ranges (IQRs)], and comparisons were performed using nonparametric tests. Categorical data are described as counts (percentages), and comparisons of rates were performed using Pearson’s χ2 test.
A total of 3553 participants who were followed up in 2020 were used to analyse the association between the cumulative effects of ehALT and new-onset MAFLD via a Cox proportional hazards regression model. MAFLD and follow-up time were regarded as dependent variables, and the cumulative number of ehALT occurrences (four groups: 0, 1, 2, 3 occurrences, with 0 occurrences regarded as the reference group), equally weighted cumulative effect, unequally weighted cumulative effect, and single ehALT occurrence [continuous variable, analysed with the per-SD increase after Z score standardization; discrete variable, five groups, a cumulative effect of 0 as the reference group; more than 0 was divided into four quartile groups (Q1, Q2, Q3, Q4)] were the independent variables. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated, and trend tests were also conducted. Furthermore, we used restricted cubic splines with five knots at the 5th, 35th, 65th, and 95th percentiles to flexibly model the associations of the equally or unequally weighted effects of ehALT with new-onset MAFLD and adopted variance analysis to verify whether there was a nonlinear correlation (12). All the data management and statistical analyses were performed using the statistical software R version 4.2.0 (The R Project for Statistical Computing, Vienna, Austria). P < 0.05 was considered to indicate statistical significance.
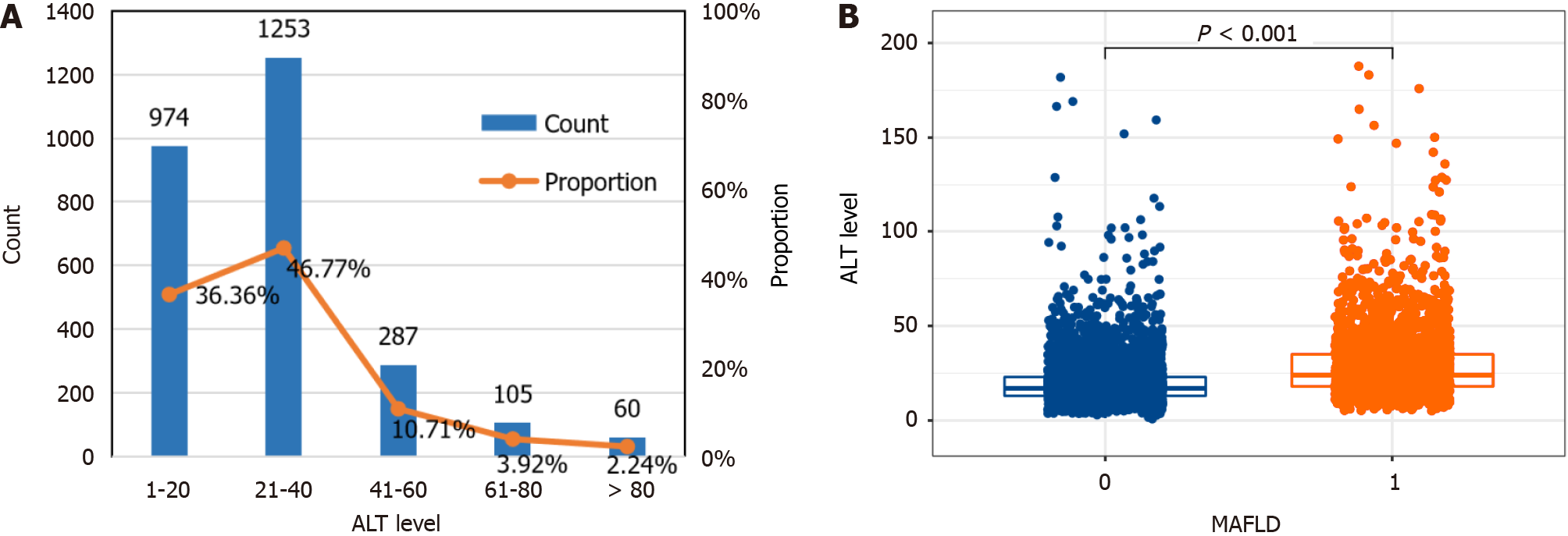
The results of 7817 participants at baseline in 2017 showed that the prevalence of MAFLD was 34.27% (2679/7817), and 83.13% (36.36% + 46.77%) of participants with MAFLD had normal ALT levels (≤ 40 U/L) (Figure 2A). Analysis of differences based on nonparametric tests indicated that ALT levels were significantly greater in MAFLD patients than in healthy individuals (P < 0.001), with median (IQR) ALT levels of 24 (18, 35) U/L and 17 (13, 23) U/L (Figure 2B).
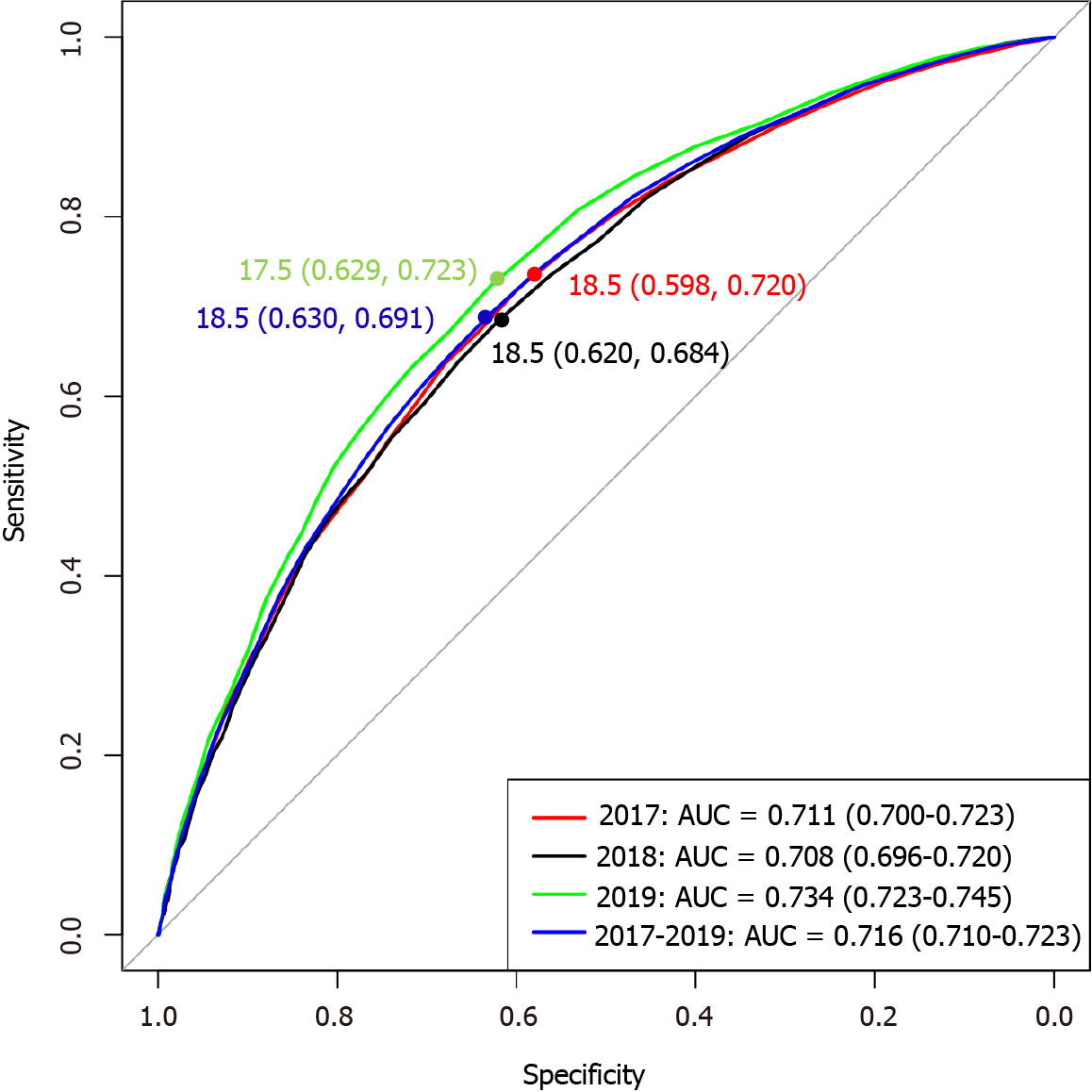
The ROC curve was obtained for the annual and 3-year health examination data of the 7817 participants from 2017 to 2019. The optimal ALT cut-off points and corresponding sensitivity and specificity for diagnosing MAFLD were determined according to the maximum Youden index. The results showed that the optimal ALT cut-off points were 18.5 U/L in 2017, 18.5 U/L in 2018, 17.5 U/L in 2019, and 18.5 U/L in 2017-2019, as shown in Figure 3. Therefore, the optimal ALT cut-off point was 18.5 U/L based on ROC curve and Youden index, and the cut-off point for hALT was 18.6-40 U/L.
According to the definition of hALT (18.6-40 U/L), 3553 participants who were eligible for follow-up were included in the baseline analysis in 2017; their mean age was 48.39 (15.13) years, and 49% were male. The baseline characteristics of the 3553 participants according to hALT level are shown in Table 2. Compared with those in the lALT group, participants in the hALT and aALT groups were significantly more likely to have a higher DBP, BMI, SUA, and TG and lower HDL-C levels (P < 0.001). Compared with those in the hALT group, participants in the aALT group were more likely to be younger and to have lower FPG and HbA1c values.
| Variables | Total (3553) | lALT group (2409) | hALT group (1046) | aALT group (98) | F/χ2/H | P value |
| Sex, n (%) | 264.16 | < 0.001 | ||||
| Male | 1741 (49.00) | 955 (39.64) | 712 (68.07) | 74 (75.51) | ||
| Female | 1812 (51.00) | 1454 (60.36) | 334 (31.93)a | 24 (24.49)a | ||
| Age (yr), mean (SD) | 48.39 (15.13) | 47.82 (15.39) | 50.17 (14.54)a | 43.51 (12.59)a,b | 14.11 | < 0.001 |
| WC (cm), mean (SD) | 85.81 (1.81) | 85.79 (1.67) | 85.85 (1.94) | 85.85 (3.11) | 0.44 | 0.643 |
| SBP (mmHg), mean (SD) | 120.33 (16.66) | 119.18 (16.64) | 122.93 (16.70)a | 121.08 (13.41) | 18.77 | < 0.001 |
| DBP (mmHg), mean (SD) | 72.68 (10.27) | 71.70 (10.03) | 74.81 (10.60)a | 74.07 (8.92)a | 35.09 | < 0.001 |
| BMI (kg/m2), mean (SD) | 22.80 (2.41) | 22.46 (2.39) | 23.46 (2.32)a | 23.94 (2.21)a | 76.76 | < 0.001 |
| SUA (μmol/L), mean (SD) | 290.53 (72.07) | 280.17 (69.87) | 311.33 (71.51)a | 323.01 (74.16)a | 82.00 | < 0.001 |
| TC (mmol/L), mean (SD) | 4.53 (0.84) | 4.51 (0.82) | 4.58 (0.88)a | 4.55 (0.84) | 2.72 | 0.066 |
| TG (mmol/L), median (IQR) | 0.97 (0.72, 1.29) | 0.93 (0.70, 1.26) | 1.06 (0.79, 1.40)a | 1.09 (0.82, 1.52)a | 79.73 | < 0.001 |
| HDL-C (mmol/L), mean (SD) | 1.47 (0.34) | 1.50 (0.34) | 1.42 (0.34)a | 1.39 (0.39)a | 18.51 | < 0.001 |
| LDL-C (mmol/L), mean (SD) | 2.77 (0.75) | 2.74 (0.73) | 2.85 (0.78)a | 2.83 (0.72) | 7.76 | < 0.001 |
| FPG (mmol/L), median (IQR) | 4.96 (4.68, 5.25) | 4.95 (4.67, 5.21) | 5.00 (4.71, 5.30)a | 4.94 (4.65, 5.27)b | 10.65 | 0.005 |
| HbA1c (%), median (IQR) | 5.70 (5.50, 5.70) | 5.70 (5.50, 5.70) | 5.70 (5.40, 5.70) | 5.70 (5.40, 5.70)a,b | 10.69 | 0.005 |
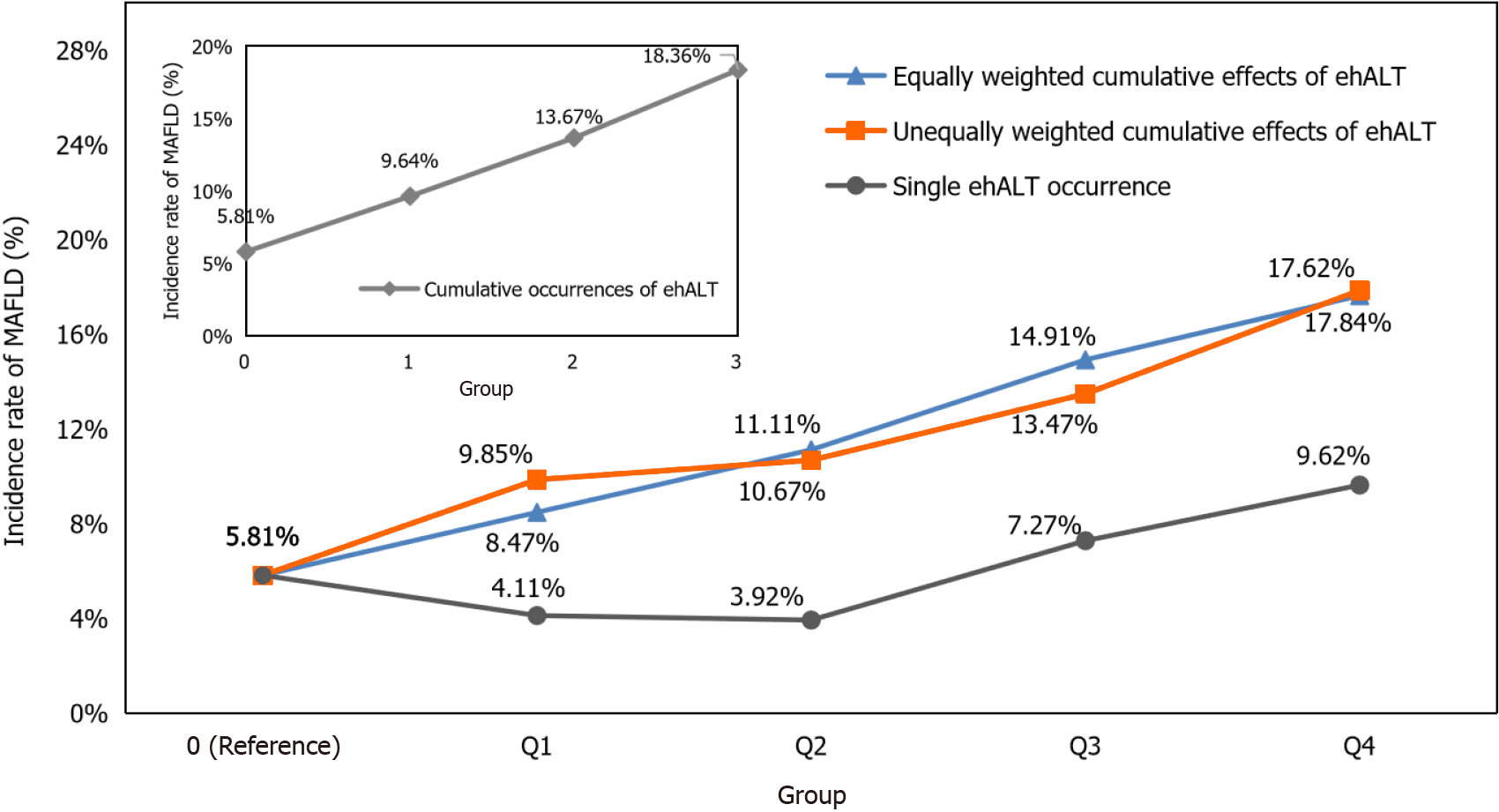
Taking the date of the participants’ health examinations in 2019 as the starting point of follow-up and the occurrence of MAFLD (no = 0, yes = 1) in the health examinations of participants in 2020 as the outcome, we calculated the incidence rate of MAFLD under the cumulative effects of ehALT in different groups and analysed the linear associations, as shown in Figure 4. For the cumulative number of ehALT occurrences of 0, 1, 2, and 3 times before the end of follow-up (by 2020), the incidence rates of MAFLD were 5.81%, 9.64%, 13.67%, and 18.36%, respectively. For the equally weighted cumulative effects of ehALT, the incidence rates of MAFLD in group 0 (reference), Q1, Q2, Q3, and Q4 were 5.81%, 8.47%, 11.11%, 14.91%, and 17.62%, respectively. For the unequally weighted cumulative effects of ehALT, the incidence rates of MAFLD in group 0 (reference), Q1, Q2, Q3, and Q4 were 5.81%, 9.85%, 10.67%, 13.47%, and 17.84%, respectively. Furthermore, P < 0.001 for both the χ2 test and linear association test of the abovementioned cumulative effects, indicating that the incidence rates of MAFLD in the different groups were significantly different, with a linear increasing trend. For a single ehALT occurrence (control group), the incidence rates of MAFLD in group 0 (reference), Q1, Q2, Q3, and Q4 were 5.81%, 4.11%, 3.92%, 7.27%, and 9.62%, respectively. The χ2 test and linear association test showed P = 0.682 and P = 0.434, respectively, indicating that no significant difference existed in the incidence rate of MAFLD among the five groups and that no linear increasing trend was shown.
Taking the cumulative effects of ehALT in different groups and confounders such as age, sex, and WC as independent variables and new-onset MAFLD (no = 0, yes = 1) in the health examinations of participants in 2020 as the outcome, we used a Cox proportional hazards regression model to analyse the association between the cumulative effects of ehALT in different groups and the risk of new-onset MAFLD. The univariate Cox proportional hazards regression model revealed that sex, WC, SBP, DBP, BMI, SUA, TG, LDL-C, FPG, and HbA1c were risk factors for new-onset MAFLD, with an HR > 1 (P < 0.05). HDL-C was a protective factor against new-onset MAFLD, with an HR < 1 (P < 0.05).
The results of a multivariate Cox proportional hazards regression model showed the following: (1) Cumulative number of ehALT occurrences: After adjustment for relevant confounding factors, the risk of new-onset MAFLD in the group of patients with 2 and 3 cumulative episodes of ehALT was 1.443 (95%CI: 1.050-1.982) and 1.551 (95%CI: 1.135-2.119), respectively, higher than that in the group with 0.82% ehALT. Furthermore, the trend test indicated that the risk of new-onset MAFLD showed an increasing trend; (2) Equal and unequally weighted cumulative effects of ehALT: After adjustment for the relevant confounding factors, the risk of new-onset MAFLD increased by 8.8% (95%CI: 0.3%-17.9%) and 9.8% (95%CI: 1.7%-18.5%), respectively, per SD increase in the cumulative effect. For the five grouping variables, compared with those in group 0 (reference), the HRs of new-onset MAFLD in the Q3 and Q4 groups for the equally weighted cumulative effects of ehALT were 1.651 (95%CI: 1.199-2.273) and 1.535 (95%CI: 1.119-2.106), respectively. For the unequally weighted cumulative effects of ehALT, the HRs of new-onset MAFLD in the Q3 and Q4 groups were 1.616 (95%CI: 1.162-2.246) and 1.580 (95%CI: 1.155-2.162), respectively; Q1 and Q2 were not significantly different from those in the reference group. Additionally, the trend test indicated that the risk of new-onset MAFLD showed an increasing trend for all cumulative occurrences of ehALT; and (3) Single ehALT (control group): Compared with those of the reference group, the univariate and multivariate models did not differ significantly for the continuous or categorical variable of a single ehALT occurrence. Additionally, the trend test showed that the risk of new-onset MAFLD did not increase (Table 3).
| Categories | Univariate, HR (95%CI) | P value | Sex-, WC-, SBP-, DBP- and BMI-adjusted1, HR (95%CI) | P value | Multivariate-adjusted2 | P value |
| Cumulative occurrences of ehALT (n = 3553) | ||||||
| 0 (101/1738) | 1.000 | 1.000 | 1.000 | |||
| 1 (82/851) | 1.672 (1.249-2.237) | 0.001 | 1.312 (0.975-1.765) | 0.073 | 1.261 (0.935-1.699) | 0.128 |
| 2 (70/512) | 2.325 (1.714-3.154) | < 0.001 | 1.495 (1.091-2.049) | 0.012 | 1.443 (1.050-1.982) | 0.024 |
| 3 (83/452) | 2.963 (2.215-3.962) | < 0.001 | 1.559 (1.142-2.128) | 0.005 | 1.551 (1.135-2.119) | 0.006 |
| P value for trend3 | < 0.001 | 0.005 | 0.005 | |||
| Equally weighted cumulative effects of ehALT (n = 3553) | ||||||
| Increase per SD4 | 1.227 (1.149-1.310) | < 0.001 | 1.090 (1.006-1.182) | 0.036 | 1.088 (1.003-1.179) | 0.041 |
| 0 (reference) | 1.000 | 1.000 | 1.000 | |||
| Q1 (0.01-3.00 U/L) | 1.541 (1.069-2.225) | 0.020 | 1.139 (0.785-1.652) | 0.493 | 1.083 (0.744-1.575) | 0.678 |
| Q2 (3.01-8.50 U/L) | 1.770 (1.261-2.485) | 0.001 | 1.329 (0.941-1.876) | 0.106 | 1.297 (0.916-1.837) | 0.916 |
| Q3 (8.51-20.50 U/L) | 2.620 (1.926-3.564) | < 0.001 | 1.671 (1.214-2.300) | 0.002 | 1.651 (1.199-2.273) | 0.002 |
| Q4 (≥ 20.51 U/L) | 2.852 (2.120-3.838) | < 0.001 | 1.578 (1.151-2.162) | 0.005 | 1.535 (1.119-2.106) | 0.008 |
| P value for trend3 | < 0.001 | 0.005 | 0.007 | |||
| Unequally weighted cumulative effects of ehALT (n = 3553) | ||||||
| Increase per SD4 | 1.231 (1.154-1.312) | < 0.001 | 1.102 (1.020-1.191) | 0.014 | 1.098 (1.017-1.185) | 0.016 |
| 0 (reference) | 1.000 | 1.000 | 1.000 | |||
| Q1 (0.01-5.00 U/L) | 1.709 (1.209-2.416) | 0.002 | 1.190 (0.835-1.697) | 0.336 | 1.114 (0.779-1.591) | 0.555 |
| Q2 (5.01-15.00 U/L) | 1.749 (1.234-2.478) | 0.002 | 1.283 (0.900-1.830) | 0.168 | 1.278 (0.895-1.826) | 0.177 |
| Q3 (15.01-40.50 U/L) | 2.319 (1.688-3.187) | < 0.001 | 1.636 (1.178-2.273) | 0.003 | 1.616 (1.162-2.246) | 0.004 |
| Q4 (≥ 40.51 U/L) | 2.996 (2.236-4.015) | < 0.001 | 1.626 (1.191-2.220) | 0.002 | 1.580 (1.155-2.162) | 0.004 |
| P value for trend3 | < 0.001 | 0.003 | 0.004 | |||
| Single ehALT occurrence (control group, n = 1969) | ||||||
| Increase per SD4 | 1.055 (0.866-1.286) | 0.594 | 1.010 (0.819-1.245) | 0.929 | 1.012 (0.811-1.262) | 0.917 |
| 0 (reference) | 1.000 | 1.000 | 1.000 | |||
| Q1 (0.01-1.50 U/L) | 0.688 (0.218-2.171) | 0.524 | 0.497 (0.150-1.528) | 0.214 | 0.557 (0.174-1.783) | 0.324 |
| Q2 (1.51-3.50 U/L) | 0.612 (0.151-2.485) | 0.492 | 0.462 (0.113-1.886) | 0.282 | 0.352 (0.085-1.462) | 0.151 |
| Q3 (3.51-8.50 U/L) | 1.250 (0.460-3.397) | 0.662 | 1.326 (0.486-3.620) | 0.582 | 1.043 (0.374-2.908) | 0.936 |
| Q4 (≥ 8.51 U/L) | 1.990 (0.808-4.898) | 0.134 | 1.688 (0.675-4.221) | 0.263 | 1.828 (0.772-4.593) | 0.199 |
| P value for trend3 | 0.142 | 0.259 | 0.264 | |||
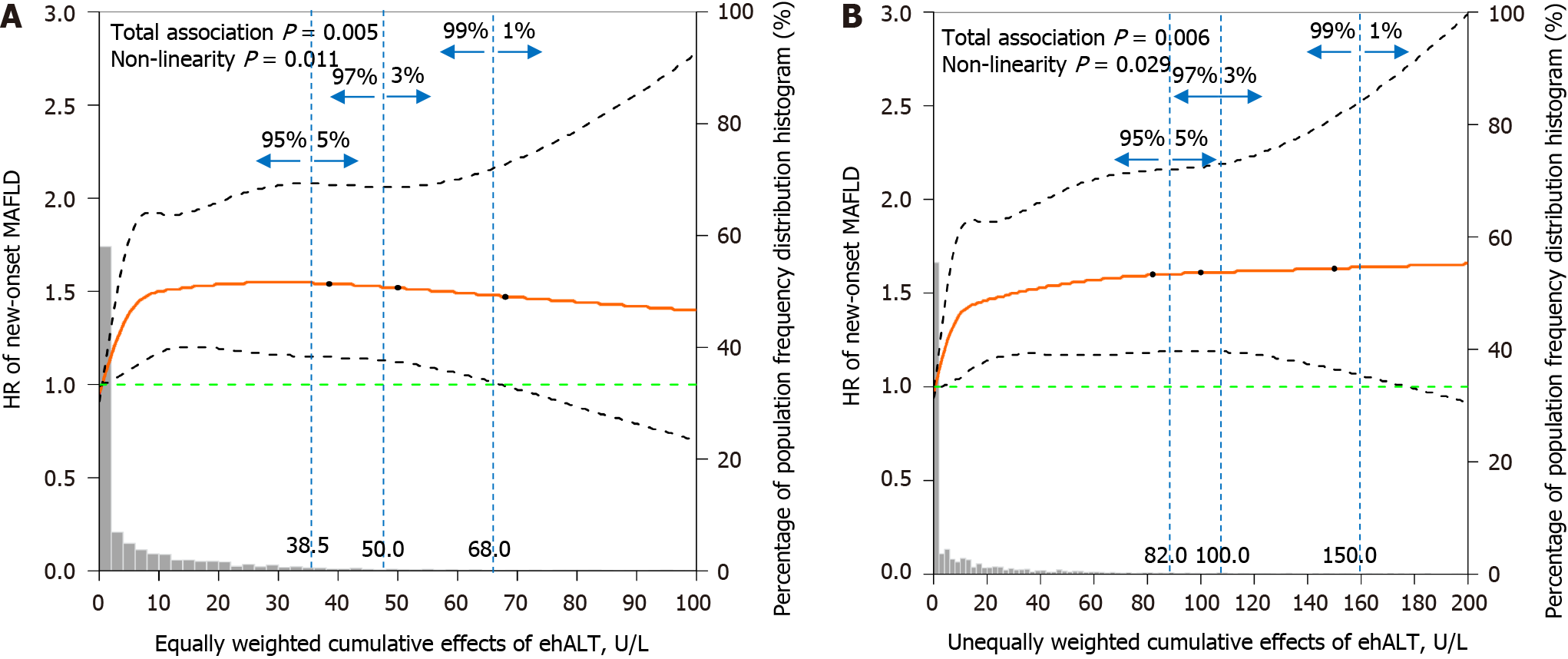
Figure 5 shows the dose-response relationship between the cumulative effects of ehALT and the risk of new-onset MAFLD using restricted cubic splines. After adjustment for sex, WC, SBP, DBP, BMI, SUA, TG, HDL-C, LDL-C, FPG, and HbA1c, the equally and unequally weighted cumulative effects of ehALT had a positive nonlinear relationship with the risk of new-onset MAFLD in approximately 95% of the enrolled participants (i.e., total association P = 0.005 and nonlinearity P = 0.011; total association P = 0.006 and nonlinearity P = 0.029). Specifically, for the equally weighted cumulative effects of ehALT (Figure 5A), the HR increased rapidly to 1.5 with a cumulative effect of 10 U/L and then maintained a steady value thereafter, to a cumulative effect of 38.5 U/L. For the unequally weighted cumulative effects of ehALT (Figure 5B), the HR increased rapidly to 1.5 with a cumulative effect of 20 U/L and then maintained a steady value thereafter, with a cumulative effect of 82.0 U/L.
In this study, a large-scale, longitudinal population-based cohort of 7817 participants in China had a prevalence of MAFLD of 34.27%, and 83.13% of participants with MAFLD had normal ALT levels. The optimal ALT cut-off point for the diagnosis of MAFLD was 18.5 U/L. Our findings indicate that MAFLD has become one of the most common chronic liver diseases and is a growing public health problem. According to a systematic review and meta-analysis, NAFLD and normal ALT levels are closely related to diabetes, hypertension, and metabolic syndrome[18]. Additionally, the highest ALT cut-off points among 526641 participants without excessive alcohol consumption or known liver disease were 32 U/L, 37 U/L for men; 31 U/L for women; 39 U/L for overweight people; and 36 U/L for patients with diabetes, all of which were lower than the upper limit for ALT (40 U/L)[19]. According to the Liver-Bible-2020 cohort study, the best ALT cut-off for steatosis detection was 35 U/L in males and 22 U/L in females, and the best cut-off for fibrosis detection was 27 U/L in males[20]. Wahlang et al[21] reported that elevated ALT within the normal range was a substitute biomarker of NAFLD. However, there is no scientific evidence for whether the long-term, dynamic, or continuous accumulation of ehALT affects new-onset MAFLD (NAFLD accompanied by metabolic disorders).
The incidence rate of MAFLD was significantly different and showed a linear growth trend with the cumulative effects of ehALT in the different groups, whereas there was no such relationship for a single ehALT occurrence (control group). After adjustment for confounding factors, compared with those in the lALT group, the cumulative ehALT level was significantly associated with new-onset MAFLD according to three factors: The cumulative frequency of ehALT and both the equally and unequally weighted cumulative effects of ehALT. Moreover, there was no such relationship for a single ehALT occurrence (control group). This finding suggested that the cumulative effects of ehALT within the long-term normal range will significantly increase the risk of new-onset MAFLD. A prospective study conducted by Gawrieh et al[22] revealed that the histological characteristics of NAFLD, advanced fibrosis, and the frequency and severity of NASH gradually increase when ALT levels increase gradually from < 20 U/L to 20-39 U/L, within the normal range. Our previous study showed that, compared with those in the stable low-ALT subgroup (13.10-13.92 U/L for 3 consecutive years), the stable middle-ALT (22.65-24.08 U/L) and stable high-ALT (32.50-39.78 U/L) groups had a significantly increased risk of MAFLD among men and women in the general population[14]. Thus, long-term hALT intake can increase the risk of developing MAFLD and aggravate the severity of NASH and advanced fibrosis. However, in-depth studies are presently lacking regarding the cumulative effects of and dynamic changes in hALT and the risk of new-onset MAFLD.
With lifestyle changes, a growing number of patients with MAFLD (dominated by NAFLD with metabolic disorders) who were physically asymptomatic had fluctuating ALT levels mostly within the normal range, although liver biopsy results revealed marked inflammation or fibrosis in some patients[23]. ALT is an enzyme that exists widely in the cytoplasm of liver cells. Once hepatocyte apoptosis and damage occur, the serum ALT concentration increases significantly. Therefore, ALT is the most sensitive detection indicator reflecting liver function damage and liver inflammation and is an important marker for detecting steatosis, diagnosing NASH, evaluating NASH-related fibrosis stages, and detecting liver cirrhosis[24]. Clinically, most physicians assess the hepatic risk of NAFLD based on changes in ALT levels, which are often overlooked in patients with MAFLD who have long-standing normal ALT levels, leading to aggravation of the degree of hepatic inflammatory response with insulin resistance and multi-hit pathogenesis, and further resulting in NASH, liver fibrosis, and eventually the development of cirrhosis and even hepatocellular carcinoma[25].
This study used a population-based cohort to explore the cumulative effects of ehALT and the risk of new-onset MAFLD. The main strengths of this study were the determination of the optimal ALT cut-off points and the use of different measurement methods for the cumulative effects of ehALT from three perspectives (i.e., cumulative number of ehALT measurements and equally and unequally weighted cumulative effects). In contrast, previous studies have investigated the association between a single ALT measurement or the ALT trajectory and neglected the effects of new-onset MAFLD by considering the quantitative cumulative effects of ALT on long-term dynamic changes via a lifespan approach.
This study has several limitations. First, the cohort’s follow-up time was short, and the proportion of participants with aALT was low, which led to a decrease in the dose-response relationship and made it difficult to accurately detect a significant difference between the aALT group (> 40 U/L) and the hALT group (18.6-40 U/L) for the cumulative effects on the risk of new-onset MAFLD. Additionally, randomized controlled trials with different lifestyle interventions (including weight loss through diet and physical exercise) will be conducted to explore whether those interventions can improve long-term ALT levels in individuals who are high-normal and ultimately prevent MAFLD.
In conclusion, a large-scale population-based study in Henan Province indicated that a high-normal ALT level was 18.6-40 U/L, that a normal ALT level was common in patients with MAFLD, and that a long-term change in the ALT level had cumulative effects on the risk of new-onset MAFLD. We recommend that individuals in this population, especially those in young adulthood, maintain long-term ALT levels within the normal range.
Additional evidence is needed regarding the association between repeated high-normal alanine transaminase (ALT) measurements and metabolic dysfunction-associated fatty liver disease (MAFLD), and only a few cross-sectional studies have shown that ALT trajectory is associated with the risk of MAFLD. In light of the public health burden of MAFLD in China, we investigated the association between persistently elevated high-normal ALT levels and new-onset MAFLD using an ambispective cohort from a health examination population.
MAFLD has become a growing public health problem and affects up to one-third of the global population, with a heavy disease burden. MAFLD can occur in the presence of normal ALT levels, and a trajectory within the normal range can increase the risk of MAFLD. However, the link between repeated high-normal ALT measurements and new-onset MAFLD has not been well studied.
We investigated the optimal ALT cut-off points for diagnosing MAFLD and the association between repeated high-normal ALT measurements and the risk of new-onset MAFLD in a health examination population in China.
This study used data from an ambispective cohort of individuals from a health examination population in China. Repeated high-normal ALT measurements were assessed by considering equally and unequally weighted cumulative effects of excess high-normal ALT (ehALT), and participants were categorized into quartile groups. We performed multivariable Cox proportional hazards regression analysis to evaluate the association between cumulative ehALT and the risk of new-onset MAFLD and calculated the hazard ratios (HRs) and 95% confidence intervals.
A total of 83.13% of participants with MAFLD had normal ALT levels. The HRs of new-onset MAFLD in the group of patients with 2 or 3 cumulative episodes of ehALT (Q3 and Q4 for the equally and unequally weighted cumulative effects of ehALT) were greater than those in the group with low-normal ALT levels from 2017 to 2019. Additionally, the dose-response relationship indicated that the equally and unequally weighted cumulative effects of ehALT had positive nonlinear relationships with the risk of new-onset MAFLD.
A cohort study of the Chinese adult population revealed that persistently elevated high-normal ALT levels were associated with a dose-dependent increase in the risk of new-onset MAFLD in all participants. The identification and management of high-normal ALT levels for several years may play a crucial role in preventing MAFLD.
Long-term prospective cohort or randomized controlled trials are needed to confirm the relationship between repeated high-normal ALT measurements and new-onset MAFLD. Future studies should focus on whether a healthy lifestyle can improve ALT levels and prevent MAFLD.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gorrell MD, Australia S-Editor: Wang JJ L-Editor: A P-Editor: Yuan YY
| 1. | Lim GEH, Tang A, Ng CH, Chin YH, Lim WH, Tan DJH, Yong JN, Xiao J, Lee CW, Chan M, Chew NW, Xuan Tan EX, Siddiqui MS, Huang D, Noureddin M, Sanyal AJ, Muthiah MD. An Observational Data Meta-analysis on the Differences in Prevalence and Risk Factors Between MAFLD vs NAFLD. Clin Gastroenterol Hepatol. 2023;21:619-629.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 144] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 2. | Eslam M, Sanyal AJ, George J; International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158:1999-2014.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2367] [Cited by in RCA: 2211] [Article Influence: 442.2] [Reference Citation Analysis (1)] |
| 3. | Yang J, Luo S, Li R, Ju J, Zhang Z, Shen J, Sun M, Fan J, Xia M, Zhu W, Liu Y. Sleep Factors in Relation to Metabolic Dysfunction-Associated Fatty Liver Disease in Middle-Aged and Elderly Chinese. J Clin Endocrinol Metab. 2022;107:2874-2882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Eslam M, El-Serag HB, Francque S, Sarin SK, Wei L, Bugianesi E, George J. Metabolic (dysfunction)-associated fatty liver disease in individuals of normal weight. Nat Rev Gastroenterol Hepatol. 2022;19:638-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 144] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 5. | Liu J, Ayada I, Zhang X, Wang L, Li Y, Wen T, Ma Z, Bruno MJ, de Knegt RJ, Cao W, Peppelenbosch MP, Ghanbari M, Li Z, Pan Q. Estimating Global Prevalence of Metabolic Dysfunction-Associated Fatty Liver Disease in Overweight or Obese Adults. Clin Gastroenterol Hepatol. 2022;20:e573-e582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 131] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 6. | Huang YP, Zhang S, Zhang M, Wang Y, Wang WH, Li J, Li C, Lin JN. Gender-specific prevalence of metabolic-associated fatty liver disease among government employees in Tianjin, China: a cross-sectional study. BMJ Open. 2021;11:e056260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Kim M, Yoon EL, Cho S, Lee CM, Kang BK, Park H, Jun DW, Nah EH. Prevalence of advanced hepatic fibrosis and comorbidity in metabolic dysfunction-associated fatty liver disease in Korea. Liver Int. 2022;42:1536-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | Quek J, Ng CH, Tang ASP, Chew N, Chan M, Khoo CM, Wei CP, Chin YH, Tay P, Lim G, Tan DJH, Lim WH, Chan KE, Teng M, Tan E, Tamaki N, Huang DQ, Siddiqui MS, Young DY, Noureddin M, Muthiah MD. Metabolic Associated Fatty Liver Disease Increases the Risk of Systemic Complications and Mortality. A Meta-Analysis and Systematic Review of 12 620 736 Individuals. Endocr Pract. 2022;28:667-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 9. | Lee GB, Huh Y, Lee SH, Han B, Kim YH, Kim DH, Kim SM, Choi YS, Cho KH, Nam GE. Association of low muscle strength with metabolic dysfunction-associated fatty liver disease: A nationwide study. World J Gastroenterol. 2023;29:5962-5973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 10. | Wang M, Wang M, Zhang R, Zhang L, Ding Y, Tang Z, Fan H, Wang H, Zhang W, Chen Y, Wang J. A combined association of serum uric acid, alanine aminotransferase and waist circumference with non-alcoholic fatty liver disease: a community-based study. PeerJ. 2022;10:e13022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 11. | Kang Y, Park S, Kim S, Koh H. Normal serum alanine aminotransferase and non-alcoholic fatty liver disease among Korean adolescents: a cross-sectional study using data from KNHANES 2010-2015. BMC Pediatr. 2018;18:215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Seko Y, Sumida Y, Tanaka S, Mori K, Taketani H, Ishiba H, Hara T, Okajima A, Yamaguchi K, Moriguchi M, Mitsuyoshi H, Kanemasa K, Yasui K, Minami M, Imai S, Itoh Y. Serum alanine aminotransferase predicts the histological course of non-alcoholic steatohepatitis in Japanese patients. Hepatol Res. 2015;45:E53-E61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Wu Y, Yang X, Morris HL, Gurka MJ, Shenkman EA, Cusi K, Bril F, Donahoo WT. Noninvasive Diagnosis of Nonalcoholic Steatohepatitis and Advanced Liver Fibrosis Using Machine Learning Methods: Comparative Study With Existing Quantitative Risk Scores. JMIR Med Inform. 2022;10:e36997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 14. | Chen JF, Qin Q, Wu ZQ, Yan S, Song XQ, Ding SY. A cohort study on the correlation between alanine aminotransferase trajectories and new-onset metabolic fatty liver disease. Zhonghua Liu Xing Bing Xue Za Zhi. 2022;43:234-240. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Zhang YB, Yang G, Bu Y, Lei P, Zhang W, Zhang DY. Development of a machine learning-based model for predicting risk of early postoperative recurrence of hepatocellular carcinoma. World J Gastroenterol. 2023;29:5804-5817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (1)] |
| 16. | Rong Y, Chun-Yan N, Hong-Xin Z, Lu Y, Wen W, Yu T. Association of Adolescent Obesity with Nonalcoholic Fatty Liver Disease and Related Risk Factors in Xi 'an, China. Ann Hepatol. 2018;17:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Li X, Jansen L, Chang-Claude J, Hoffmeister M, Brenner H. Risk of Colorectal Cancer Associated With Lifetime Excess Weight. JAMA Oncol. 2022;8:730-737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Ma X, Liu S, Zhang J, Dong M, Wang Y, Wang M, Xin Y. Proportion of NAFLD patients with normal ALT value in overall NAFLD patients: a systematic review and meta-analysis. BMC Gastroenterol. 2020;20:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 19. | Huang DQ, Yeo YH, Tan E, Takahashi H, Yasuda S, Saruwatari J, Tanaka K, Oniki K, Kam LY, Muthiah MD, Hyogo H, Ono M, Barnett SD, Li J, Zou B, Fung J, Lee TY, Wong VW, Yuen MF, Dan YY, Lim SG, Cheung R, Toyoda H, Eguchi Y, Nguyen MH. ALT Levels for Asians With Metabolic Diseases: A Meta-analysis of 86 Studies With Individual Patient Data Validation. Hepatol Commun. 2020;4:1624-1636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Valenti L, Pelusi S, Bianco C, Ceriotti F, Berzuini A, Iogna Prat L, Trotti R, Malvestiti F, D'Ambrosio R, Lampertico P, Colli A, Colombo M, Tsochatzis EA, Fraquelli M, Prati D. Definition of Healthy Ranges for Alanine Aminotransferase Levels: A 2021 Update. Hepatol Commun. 2021;5:1824-1832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 21. | Wahlang B, Appana S, Falkner KC, McClain CJ, Brock G, Cave MC. Insecticide and metal exposures are associated with a surrogate biomarker for non-alcoholic fatty liver disease in the National Health and Nutrition Examination Survey 2003-2004. Environ Sci Pollut Res Int. 2020;27:6476-6487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 22. | Gawrieh S, Wilson LA, Cummings OW, Clark JM, Loomba R, Hameed B, Abdelmalek MF, Dasarathy S, Neuschwander-Tetri BA, Kowdley K, Kleiner D, Doo E, Tonascia J, Sanyal A, Chalasani N; NASH Clinical Research Network. Histologic Findings of Advanced Fibrosis and Cirrhosis in Patients With Nonalcoholic Fatty Liver Disease Who Have Normal Aminotransferase Levels. Am J Gastroenterol. 2019;114:1626-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 23. | Sun DQ, Zheng KI, Xu G, Ma HL, Zhang HY, Pan XY, Zhu PW, Wang XD, Targher G, Byrne CD, Chen YP, Yuan WJ, Zheng MH. PNPLA3 rs738409 is associated with renal glomerular and tubular injury in NAFLD patients with persistently normal ALT levels. Liver Int. 2020;40:107-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 24. | Kim WR, Flamm SL, Di Bisceglie AM, Bodenheimer HC; Public Policy Committee of the American Association for the Study of Liver Disease. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology. 2008;47:1363-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 585] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 25. | Shim JJ, Kim JW, Oh CH, Lee YR, Lee JS, Park SY, Kim BH, Oh IH. Serum alanine aminotransferase level and liver-related mortality in patients with chronic hepatitis B: A large national cohort study. Liver Int. 2018;38:1751-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |