Published online Dec 15, 1997. doi: 10.3748/wjg.v3.i4.238
Revised: April 12, 1997
Accepted: September 22, 1997
Published online: December 15, 1997
AIM: To compare the efficacy of a combined chemotherapy regimen of 5-fluouracil (5-FU) and adriamycin (ADM) with nimustine hydrochloride (ACNU; brand name Nidran), a new nitrosourea agent, or with methyl-CCNU for advanced gastric cancer.
METHODS: One-hundred-and-three cases of advanced gastric cancer were randomly allocated into Group A (Me-CCNU, 5-FU and ADM combination) and Group B (ACNU, 5-FU and ADM combination). The quality of life (QOL) questionnaire, composed of 11 ordinal categorical items, was used to collect data from these patients.
RESULTS: Group A had no case of complete remission (CR) or partial remission (PR), while Group B had no CR but 8 PR (8/46 cases), for a response rate of 0% in Group A and 17.4% in Group B. The median survival time in Group A was 108 d and in Group B was 112 d. Both groups tolerated the treatment well and there were no serious adverse effects. QOL evaluations showed better psychological and physical feelings of tiredness for Group B than for Group A, and scores based on facial scaling showed a more pleasant inclination for the former.
CONCLUSION: ACNU combination is superior to the Me-CCNU combination for advanced gastric cancer patients.
- Citation: Xiao SD, Li DH, Zhang DZ, Shen MJ, Zhu XT, He GF, Zhao TP, Li LP, Deng XC, Wang M, Wang XL, Chen Q, Zhang YP, Yao CL, Bao JG, Tong GW, Zhu LF, Jiang H, Minoru K. Multicenter randomized study on Me-CCNU, 5-FU and ADM vs ACNU, 5-FU and ADM for treatment of advanced gastric cancer. World J Gastroenterol 1997; 3(4): 238-241
- URL: https://www.wjgnet.com/1007-9327/full/v3/i4/238.htm
- DOI: https://dx.doi.org/10.3748/wjg.v3.i4.238
In the 1960s, combined chemotherapy regimens with 5-fluouracil (5-FU), adriamycin (ADM) and mitomycin C, known as FAM, and with 5-FU, ADM and methyl (Me)-CCNU, known as FAMe, were regarded as effective for the treatment of advanced gastric cancer. However, later massive serial studies of FAM showed low response rates, namely 33% for FAM[1] and 18% for FAMe[2].
ACNU or nimustine hydrochloride (brand name Nidran, manufactured by Sankyo Co. Ltd., Japan) is a new nitrosourea anti-tumor agent applicable to various malignant tumors. It becomes even more effective when used in combination with other chemical agents. The Shanghai nimustine hydrochloride (ACNU) Cooperative Study Group carried out a randomized controlled study on Me-CCNU, 5-FU and ADM combination versus ACNU, 5-FU and ADM combination for advanced gastric cancer, and evaluated the efficacies by observing response rates, toxicities, adverse effects, and quality of life (QOL).
A study on ACNU combination therapy versus Me-CCNU combination therapy was carried out by the Shanghai ACNU Cooperative Study Group from October 1992 through April 1994, using 103 patients with advanced gastric cancer. Among them, 102 cases were eligible and 93 of them had complete records; therefore, the participation rate was 90.3%. The patient data for age, sex, performance status (PS), and histological classification are presented in Table 1.
| Parameter | Group A | Group B |
| No. of cases | 47 | 46 |
| Sex, M | 36 | 32 |
| Sex, F | 11 | 14 |
| Age (yr), -40 | 4 | 7 |
| 41-50 | 9 | 4 |
| 51-60 | 8 | 14 |
| 61-70 | 16 | 17 |
| 71-80 | 10 | 4 |
| Performance status grade 1 | 1 | 0 |
| Performance status grade 2 | 5 | 11 |
| Performance status grade 3 | 5 | 5 |
| Performance status grade 4 | 35 | 26 |
| Unclear | 1 | 4 |
| Histology | ||
| Papillary adenocarcinoma | 3 | 1 |
| Tubular adenocarcinoma 1 | 4 | 8 |
| Tubular adenocarcinoma 2 | 17 | 12 |
| Low differentiated adenocarcinoma | 13 | 8 |
| Mucocellular carcinoma | 2 | 1 |
| Signet ring cell carcinoma | 0 | 2 |
| Others | 8 | 14 |
Patients in the study had primary gastric cancers that were histologically confirmed by biopsy and no patient had received chemotherapy and/or radiotherapy prior to study enrollment. All patients had unresectable or recurrent primary foci with metastatic lesions. The age of the patients ranged from 35-75 years and had PS of 1-4. All of these patients had no active duplicate cancers, no severe disturbance in bone marrow, hepatic, renal or cardiac functions, and were able to be treated with oral medication.
Allocation Patients meeting the requirements of the study from Zeach were enrolled and their data obtained and recorded using standardized forms and telephone interview conducted by the cooperative study group office. After all the reported details were checked for comprehensiveness, the patient participants were randomly allocated into either treatment Group A or Group B.
Treatment methods Group A (control group) consisted of patients who received Me-CCNU 100 mg/d p.o. on day 1, ADM 30 mg/m2i.v. on day 1, and 5-FU 300 mg/m2i.v. from days 1-5. Group B (treatment group) consisted of patients who received ACNU 50-75 mg/d i.v. on day 1, ADM 30 mg/m2i.v. on day 1, and 5-FU 300 mg/m2i.v. from days 1-5. Thus, 5 d were taken as one course and successive courses were repeated at an interval of 4-5 wk. The total ADM doses did not exceed 500 mg/m2.
QOL questionnaire[3] Measurement of QOL was made by a self-assessment report by the patients. The patients themselves, their relatives, or care nurses filled in the QOL questionnaire once a week at regular intervals. Doctors were forbidden from filling in the questionnaire in order to avoid confounding by subjective assumption. The QOL questionnaire was composed of 11 ordinal categorical items, consisting of 3 basic items covering mental feelings (appetite, psychology and sleepiness), physical feelings (tiredness, pain and worry) and social relationships (family, friends or co workers).
Question 1. How good is your appetite
a. Very good
b. Pretty good
c. OK
d. Not too good
e. Not good at all
Question 2. How well do you sleep
Question 3. How often do you feel tired (psychological)
Question 4. How often do you feel tired (physical)
Question 5. How much and how often do you feel pain
Question 6. How much does your family understand and support you
Question 7. What is your relationship with your family and co-workers
Question 8. How often do you worry about your disease
Question 9. Would you like to continue the current therapy
Question 10. How much help do you need to accomplish your daily activity
Question 11. Please rate your overall condition today by marking 0 around the number.
Observations Blood cell counting was performed every week. Hepatic and renal functions were tested every 2 wk. Imaging examination, including barium X-ray surveys or endoscopic and ultrasonic B and/or CT scanning, were performed every 4-8 wk. Patients who had considerable adverse effects and could not proceed to a second course were given a barium X-ray check-up 4 wk after the first course.
Details of all examinations, as well as of any adverse effects, were carefully recorded by means of standardized forms. Each patient was followed-up until death.
Evaluation of efficacy A clinical evaluation committee was organized to estimate the following 4 items periodically in accordance with the Criteria of Evaluating the Efficacy of Chemotherapy for Solid Tumors proposed by the Japanese Research Society for Gastric Cancer: Qualification and completion of materials; extent of development of lesions and response to the treatment; survival time; and quality of life.
The data of treatment efficacy was compared using the Chi-square test and Fisher’s exact test. Principal component analysis and correlation analysis were used to assess the quality of life data.
Each case underwent at least one course of combination therapy. In Group A 44 cases underwent a total of 85 courses, and in Group B 41 cases received a total of 95 courses (Table 2).
| Groups | No. of courses | Total | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| Group A | 16 | 20 | 5 | 2 | 0 | 1 | 0 | 0 | 47 |
| Group B | 8 | 23 | 6 | 2 | 0 | 0 | 1 | 1 | 46 |
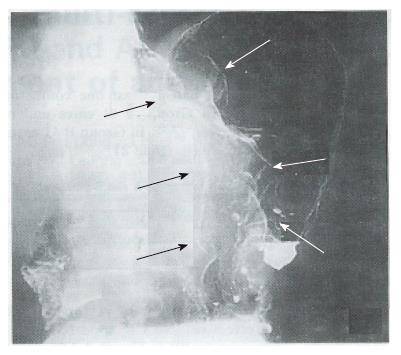
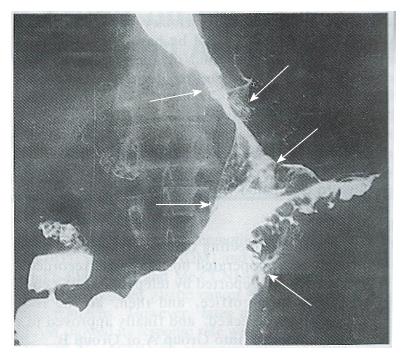
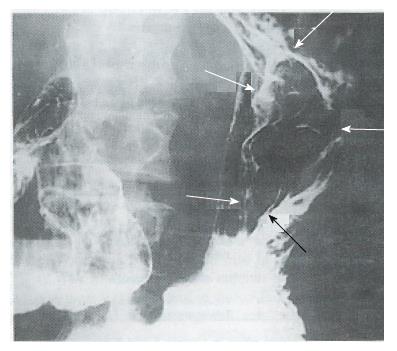
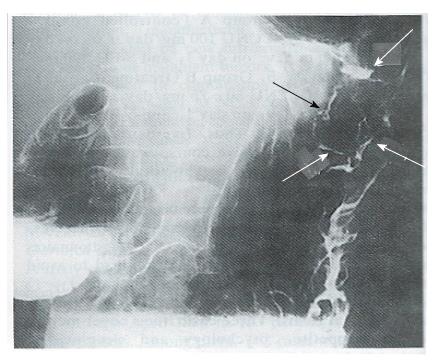
Complete response (CR) and partial response (PR) were regarded as effective. No CR occurred in either groups. As shown in Table 3, there was no PR in Group A (0/47 cases) and 8 in Group B (8/46; for a response rate of 17.4%, P < 0.01). The anti-tumor efficacy of the treatments in the two groups is shown in Table 4. The X-ray barium examinations of 2 PR cases before and after ACNU combination therapy are shown in Figures 1-4.
| Case No. | Group | Sex | Age | Performance status | Pathohistology | No. of courses | Effective site |
| 22 | B | M | 54 | 4 | Tubular adenocarcinoma | 4 | Liver |
| 35 | B | M | 46 | 4 | Tubular adenocarcinoma | 3 | Stomach |
| 45 | B | M | 65 | 4 | Low differentiated adenocarcinoma | 7 | Liver |
| 67 | B | F | 60 | 4 | Low differentiated adenocarcinoma | 4 | Spleen |
| 71 | B | F | 66 | 3 | Tubular adenocarcinoma | 3 | Stomach |
| 82 | B | F | 72 | 2 | Low differentiated adenocarcinoma | 2 | Liver spleen |
| 83 | B | M | 74 | 4 | Tubular adenocarcinoma | 8 | Stomach |
| 100 | B | M | 65 | 4 | Tubular adenocarcinoma | 3 | Liver |
| Group | A | B |
| Partial response (%) | 0 (0) | 8 (17.4) |
| No change (moderate response) | 16 (1) | 17 (2) |
| Progressive lesion | 31 | 21 |
| 95% reliability | 0, 6.2% | 6.4%, 28.4% |
| Fisher’s test | P > 0.01 | P > 0.01 |
| Median survival time | 108 d | 112 d |
| Average course | 1.9 | 2.3 |
In group A the median survival time from the beginning of the treatment was 108 d. Three out of 47 cases in Group A, survived > 1 year, with the longest survival time being 740 d (Case No. 36). In Group B the median survival time was 112 d. Of the 46 total cases, 6 survived > 1 year, with the longest survival time being 690 d (Case No. 53).
Both groups tolerated the respective treatment well and very few adverse effects were seen and those occurred only in the early days of chemotherapy. No remarkable leukopenia, thrombopenia or decreased hemoglobin occurred, and there were no serious impairments of hepatic, renal or cardiac functions (Table 5).
| Group A | Group B | |||
| Cases | % | Cases | % | |
| Loss of appetite | 3 | 6.5 | 4 | 8.7 |
| Nausea | 3 | 6.5 | 3 | 6.5 |
| Vomiting | 9 | 19.6 | 8 | 17.4 |
| Fatigue | 1 | 2.2 | 1 | 2.2 |
| Muscular disability | 4 | 8.7 | 3 | 8.7 |
| Alopecia | 4 | 8.7 | 3 | 6.5 |
| Fever | 1 | 2.2 | 2 | 4.3 |
| EKG abnormality | 1 | 2.2 | 1 | 2.2 |
| Leukopenia | 2 | 4.3 | 5 | 10.9 |
| Decrease of hemoglobin | 1 | 2.2 | 0 | 0.0 |
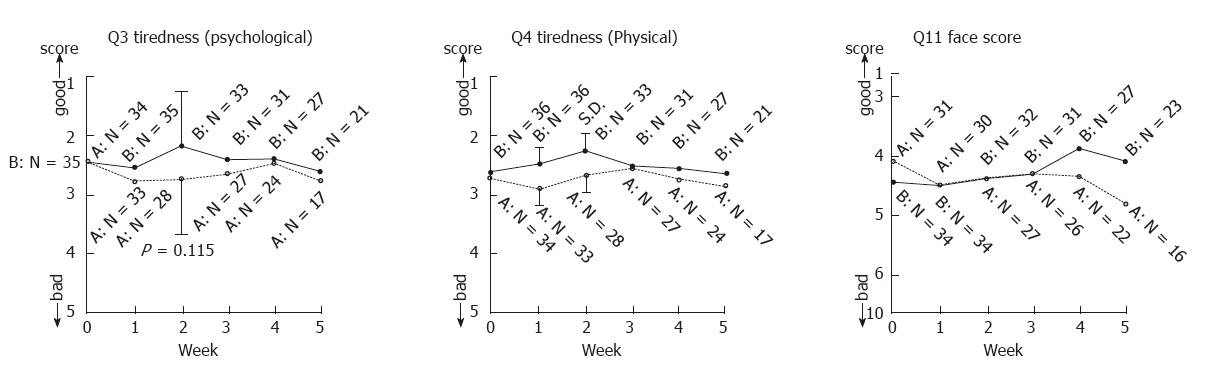
An overall investigation into appetite, sleepiness, tiredness (psychological and physical), pain, social relationships, mood, daily activities and facial expressions showed that the reports of psychological tiredness (Q3), physical tiredness (Q4) and facial expression (Q11) were better for Group B than for Group A (Figure 5).
ACNU is a newly developed nitrosourea anti-cancer agent. Different from other kinds of nitrosourea agents, it is water-soluble, capable of being administered through the vein and artery. Moreover, it is able to penetrate the blood brain barrier to reach into brain cancerous tissues and exert a full anti-tumor action. The mechanism of ACNU acts strongly across a broad spectrum against various tumors by way of alkylation of cellular DNA, which causes depolymerization of DNA and results in inhibition of DNA synthesis. Laboratory experiments showed that it could rapidly distribute systemically to organs including brain. Administration of ACNU to mice solid FM3A tumors inhibited tumor growth and reduced tumor size[5].
Our clinical study on advanced gastric cancer showed that the Group B treatment (ACNU combination) was significantly superior to the Group A treatment (Me-CCNU combination) in terms of efficacy, QOL, survival time, etc. As for survival time, 6 cases in Group B survived > 1 year, but only 3 cases did so in Group A. The median survival time was 112 d for patients in Group B, but 108 d for those in Group A. However, the survival time in neither group was long, which might be related to the fact that 70% of the cases had performance status of Grade 4 upon enrollment.
As to dosage of ACNU, 14 cases were given 50 mg/d, and 2 cases reached PR, accounting for a response rate of 14.3%. When the dosage was increased to 75 mg/d for 32 cases, 6 reached PR, accounting for a response rate of 18.3%. Neither dosage caused significant adverse effects. Therefore, we suggest that 75 mg/d should be used at the very beginning or at least on condition that no significant adverse effects appear, dosage could be adjusted to 75 mg/d. Moreover, an even higher dosage could be considered as an approach to yield more ideal results.
Recently QOL has become accepted worldwide as an important assessment tool for use in clinical practice. It is also regarded as an important factor for evaluating anti-cancer agents. The QOL questionnaire that we used had been proven clinically at the Tokyo Women’s Medical College for its practicability, suitability and reliability. The current study proved that the overall QOL features of Group B were better than those of Group A, which is consistent with the results of efficacy of ACNU.
Original title:
S- Editor: A L- Editor: Filipodia E- Editor: Liu WX
| 1. | Chen Q. The present status and advances of chemotherapy for gastric cancer. Zhonghua Xiaohua Zazhi. 1994;14:187-189. |
| 2. | Lacave A, Wils J, Bleiberg H, Diaz-Rubio E, Duez N, Dalesio O. An EORTC Gastrointestinal Group phase evaluation of combinations of methyl-CCNU, 5-fluorouracil, and adriamycin in advanced gastric cancer. J Clin Oncol. 1987;5:1387-1393. [PubMed] |
| 3. | Ishihara Y, Nagai A and Kagawa J. Development of a core questionaire on the quality of life for advanced cancer patients in palliative therapy. Biotherapy. 1992;6:1467-1473. |
| 4. | Kurhara M, Izumi T. Evaluation of the effect of chemotherapy in gastric cancer patients with an intact primary tumor according to the criteria of the Japanese Research Society for Gastric Cancer. J Jpn Soc Cancer Therapy. 1991;26:644-654. |