Published online Sep 15, 1997. doi: 10.3748/wjg.v3.i3.198
Revised: January 6, 1997
Accepted: January 30, 1997
Published online: September 15, 1997
- Citation: Hu CJ, Yang DL. Detection method for peripheral venous AFP mRNA in hepatocellular carcinoma. World J Gastroenterol 1997; 3(3): 198-199
- URL: https://www.wjgnet.com/1007-9327/full/v3/i3/198.htm
- DOI: https://dx.doi.org/10.3748/wjg.v3.i3.198
Hepatocellular carcinoma (HCC) is a major cause of death in patients with chronic liver disease. One of the difficulties in managing HCC is its complex character: intrahepatic metastasis, venous invasion, and distant metastasis can occur. In metastasis, tumor cells are scattered from the original site, spread hematogenously, and are arrested at the small vessels. Thus, detecting tumor cells in the circulation might predict tumor metastasis[1]. However, the number of cells in circulation could be too low to be detected morphologically.
Recently, tumor-associated genes in circulating tumor cells (predicting the presence of tumor cells in the circulation) could be detected by PCR in patients with prostate cancer with distant metastasis or in patients with neuroblastoma[2,3]. In HCC cells, the human alpha-fetoprotein (AFP) gene is transcribed prominently, but not in normal adult cells[4]. Thus, detecting HCC-associated gene transcription (AFP mRNA) in the circulation might be related to the hematogenous metastasis of HCC, even though overt metastasis might be obscure.
In the present study, a sensitive nested reverse transcription–PCR (RT-PCR) assay for detecting HCC cells in the blood was investigated.
The BEL-7402 HCC cell line[5] (a gift from Dr. Liu from the Shandong Medical Institute cell bank) was maintained in RPMI 1640 medium containing 15% fetal bovine serum. It served as the positive control for AFP mRNA expression.
(70 g/L, made by our laboratory).
Peripheral blood from healthy volunteers was collected in a disposable syringe containing heparin. One thousand BEL-7402 cells were added to 5 mL whole blood, and serial dilutions of tumor cells were made. Each dilution contained 1000, 100, 10, and 1 BEL-7402 cell per 5 mL whole blood.
After adding an equal volume of 3% gelatin solution to the tube containing 5 mL blood, the tube was incubated for 5 min at room temperature. The supernatant was collected and centrifuged at 500 ×g for 5 min. Residual erythrocytes were lysed by adding distilled water, and isotonicity was restored after 30 sec by adding the same volume of 1.8% NaCl solution. After 5-min centrifugation at 500 ×g, the cells were immediately frozen using liquid nitrogen and stored until used.
Using an RNA extraction system (prepared by our laboratory), total RNA was extracted from the nuclear cell component of the peripheral blood. About 5 μg RNA was extracted from 5 mL blood and 1 μg RNA was extracted from 10.5 BEL-7402 cells.
RNA (1 μg), which was heated at 95 °C for 10 min and rapidly cooled in ice water, was mixed with 3 μL 10 × buffer (pH 8.3, Tris–HCl), 0.6 μL 10 mmol/L dNTPs, 2.5 μL 25 mmol/L MgCl2, 0.5 μL each of primer #1 (upstream, 5′-ACTGAATCCAGAACACTGCATAG-3′) and primer #2 (downstream, 5′-TGCAGTCAATGCATCTTTCACCA-3′), and 2 U reverse transcriptase, and the volume was adjusted to 30 μL by adding diethylpyrocarbonate-treated water and covered with liquid paraffin. The cDNA was synthesized by incubating the mixture at 37 °C for 30 min. After initial denaturation at 94 °C for 5 min, RT-PCR was performed according to the temperature profile (94 °C for 45 sec, 54 °C for 60 sec, 72 °C for 45 sec) for 35 cycles. The reaction was terminated by 5-min heating at 72 °C and was then cooled to 4 °C.
The primers used in the nested PCR for AFP mRNA were #3 (upstream, 5′-TGGAATAGCTTCCATATTGGATTC-3′) and #4 (downstream, 5′-AAGTGGCTTCTTGAACAAACTGC-3′).
The amplified RT-PCR product (2 μL) was mixed with the second PCR buffer [3 μL; 100 mmol/L Tris–HCl (pH 8.3), 10 mmol/L MgCl2], 0.35 μL 42 mmol/L each primer (#3 and #4), 1.5 U Taq DNA polymerase, and the mixture was diluted to 30 μL with distilled water and covered with liquid paraffin. The temperature profile of the nested PCR was the same as that of the RT-PCR described above.
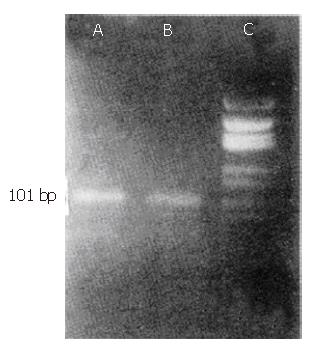
The amplified product was electrophoresed on 3.8% agarose gel and stained with ethidium bromide. The size of the amplified nested PCR product of AFP mRNA was 101 bp.
The nested PCR did not detect AFP mRNA in the nuclear cell component of the peripheral blood from the healthy volunteers.
(Figure 1).
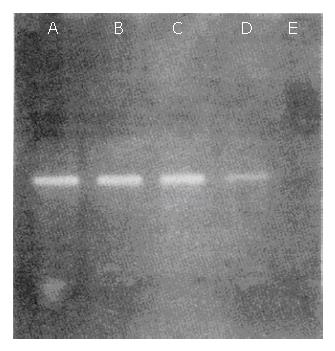
BEL-7402 cells (104) were suspended in 5 mL whole blood from healthy volunteers and sequentially diluted with blood. AFP mRNA–specific PCR products (101 bp) could be observed in each lane, which represented 1, 10, 100, and 1000 BEL-7402 cells in 5 mL normal blood, respectively (Figure 2).
To detect tumor cells in blood, the demonstration of tumor-associated genes has been developed using PCR. In a recent study, albumin mRNA in blood was detected as a marker of circulating hepatocytes[6]. Some researchers have indicated that AFP production is increased in HCC cell lines such as BEL-7402, and AFP gene expression is increased during carcinogenesis[4]. Thus, we developed a nested PCR assay to detect the HCC-associated tumor gene transcript AFP mRNA in the nuclear cell component of blood, as the number of circulating tumor cells in patients with HCC accompanying metastasis to the distant organs might be too low. To explain the sensitivity of nested PCR for detecting HCC cells in the blood, BEL-7402 cells, established from hepatoblastoma and that produce adequate amounts of AFP, were mixed with blood[5]. The nested PCR could detect AFP mRNA when 1–10 tumor cells were present in 5 mL blood. Therefore, if BEL-7402 cells were circulating in a body (total volume of blood: around 5000 mL), it would be possible to detect AFP mRNA when more than 1000 tumor cells are present in the circulation. Although a moderate number of circulating tumor cells might be present, as assessed from the detection of the BEL-7402 cells, there might be several steps for the establishment of metastasis[1] such as tumor cell adhesion to the vascular endothelium, migration into the extracellular space, and proliferation at the metastatic foci. During this process, host organ immunological reactions take place, and some tumor cells might be damaged by immunocompetent cells, the mechanical force of the blood flow, or by platelet aggregation.
These results suggest that the highly sensitive nested PCR assay is useful for demonstrating the hematogenous spread of tumor cells. Further studies are being carried out in our laboratory, focusing particularly on predicting metastasis and post-therapeutic changes in patients with HCC by detecting circulating tumor cells.
We thank Mr. Li-Zhen Yong and Guo-Ying Song, Sino American Biotechnology Company, for their excellent technical assistance.
Original title:
S- Editor: Filipodia L- Editor: Jennifer E- Editor: Hu S
| 1. | Hart IR, Saini A. Biology of tumour metastasis. Lancet. 1992;339:1453-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 235] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 2. | Moreno JG, Croce CM, Fischer R, Monne M, Vihko P, Mulholland SG, Gomella LG. Detection of hematogenous micrometastasis in patients with prostate cancer. Cancer Res. 1992;52:6110-6112. [PubMed] |
| 3. | Mattano LA, Moss TJ, Emerson SG. Sensitive detection of rare circulating neuroblastoma cells by the reverse transcriptase-polymerase chain reaction. Cancer Res. 1992;52:4701-4705. [PubMed] |
| 4. | Matsumura M, Niwa Y, Kato N, Komatsu Y, Shiina S, Kawabe T, Kawase T, Toyoshima H, Ihori M, Shiratori Y. Detection of alpha-fetoprotein mRNA, an indicator of hematogenous spreading hepatocellular carcinoma, in the circulation: a possible predictor of metastatic hepatocellular carcinoma. Hepatology. 1994;20:1418-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 88] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Zhu HD. Human hepatocellular carcinoma cell line BEL-7402. Chinese Journal of Cell Biology. 1984;6:91. |
| 6. | Hillaire S, Barbu V, Boucher E, Moukhtar M, Poupon R. Albumin messenger RNA as a marker of circulating hepatocytes in hepatocellular carcinoma. Gastroenterology. 1994;106:239-242. [PubMed] |