Published online Sep 15, 1997. doi: 10.3748/wjg.v3.i3.169
Revised: February 25, 1997
Accepted: March 19, 1997
Published online: September 15, 1997
AIM: To investigate the effects of metoclopramide (MCP) action on myoelectric activity in the antrum and small intestine.
METHODS: Ten healthy male Wistar rats, weighing 250-350 g, were anesthetized with ketamine hydrochloride (100 mg/kg, intramuscularly). Four pairs of bipolar stainless steel electrodes 3 mm apart were implanted on the serosal surface of the antrum at one, 10 and 20 cm distal to the pylorus. Five to ten days following the operation, the gastrointestinal myoelectric activity of fasted rats after intramuscular injection of 2.5, six and 12 mg/kg MCP was recorded using a 8-channel EEG machine, and these values were quantitatively compared with the myoelectric activity after saline injection.
RESULTS: In fasted rats, 2.5 mg/kg MCP increased the amplitude of spike activity (402.0 ± 138.4 μV, vs 345 ± 163.4 μV, P < 0.05) and the percentage of the slow wave-containing spike bursts (60.4% ± 22.0% vs 47.4% ± 22.5%, P < 0.01) of small intestine (1 cm distal to the pylorus), but did not affect the myoelectric activity of the antrum. Six and 12 mg/kg MCP increased the amplitude of both the slow wave (332.8 ± 200.1 μV vs 191.2 ± 143.9 μV, P < 0.01; 330.0 ± 197.1 μV vs 191.2 ± 143.9 μV, P < 0.05) and the spike activity of the antrum (180.5 ± 69.7 μV vs 121.8 ± 63.3 μV, P < 0.05; 174.5 ± 71.7 μV vs 123.8 ± 63.3 μV, P < 0.05), while in small intestine (1 cm distal to the pylorus) only the amplitude of spike activity (407.3 ± 179.0 μV vs 345.0 ± 163.4 μV, P < 0.05; 456.0 ± 145.4 μV vs 345.0 ± 163.4 μV, P < 0.05) and the percentage of the slow wave containing spike bursts (61.7% ± 26.5% vs 47.4% ± 22.5%, P < 0.01; 59.1% ± 17.3% vs 47.4% ± 22.5%, P < 0.01) was increased and the latent period significantly prolonged (2.5 ± 0.35 min vs 0.77 ± 0.18 min, P < 0.01).
CONCLUSION: Different mechanisms may be involved in enhancing the myoelectric activity of the antrum and small intestine following MCP administration.
- Citation: Qin XM, Li HF, Wang LD. Effects of metoclopramide on gastrointestinal myoelectric activity in rats. World J Gastroenterol 1997; 3(3): 169-170
- URL: https://www.wjgnet.com/1007-9327/full/v3/i3/169.htm
- DOI: https://dx.doi.org/10.3748/wjg.v3.i3.169
Metoclopramide (MCP) improves gastroduodenal coordination[1], relieves postsurgical and diabetic gastroparesis[2,3], and increases the spike activity during migrating motor complexes (MMC) of small intestine without disrupting the fasting pattern in dogs[4]. While these studies suggest that MCP complicates pharmacological action, few have studied the effects of MCP on gastrointestinal myoelectric activity. It is important to compare how different doses impact MCP’s effect on the myoelectric activity of the antrum and small intestine. Such studies might be useful both for investigating the side effects of MCP and for further understanding the mechanisms controlling gastrointestinal motility.
Ten healthy male Wistar rats, weighing 250-350 g, were anesthetized with ketamine hydrochloride (100 mg/kg, intramuscularly). Four pairs of bipolar stainless steel electrodes (3 mm apart) were implanted onto the serosal surface of the gastrointestinal tract. The first pair was placed on the antrum 0.5 cm from the pylorus and the others on the small intestine at one, 10 and 20 cm distal to the pylorus. The free ends of the electrodes were moved subcutaneously to the back of the neck. Recordings began five to ten days after operation. Electric activity was registered with a 8-channel EEG machine (ND-82B, Shanghai) with a time constant of 0.1 s and a high cutoff frequency of 30 Hz.
In the first, second and third series of experiments, MCP (The Seventh Pharmaceutical Factory of Wuxi) doses were 2.5, six and 12 mg/kg. Each experiment lasted one week and the myoelectric activities were recorded every other day.
The frequency and amplitude of the slow wave and spike activity from the antrum and small intestine were recorded and analyzed 30min following MCP administration, and the number of slow wave-containing spike bursts was also quantified. The results were represented as mean ± SD and analyzed statistically by Student’s t test, with P < 0.05 considered significant.
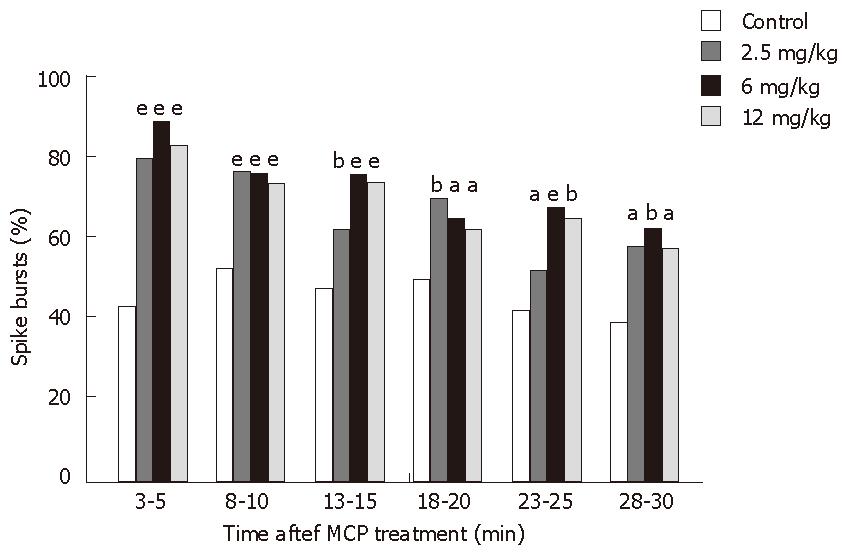
After control recordings following saline injection were obtained at 40 min and 60 min, rats that were fasted for 16-18 h were injected intramuscularly with MCP. The lowest dose of 2.5 mg/kg MCP significantly increased the amplitude of spike activity and the percentage of slow wave-containing spike bursts in the small intestine, however did not affect the myoelectric activity in the gastric antrum (Tables 1 and 2). Nevertheless, the intermediate dose of 6 mg/kg and the highest dose of 12 mg/kg increased the amplitude of spike activity and the percentage of slow wave-containing spike bursts in the small intestine, while also raising the amplitude of both the slow wave and spike activities in the antrum. MCP did not affect the amplitude of the slow wave at any of the four electrode sites (Tables 1 and 2). MCP effects on the electric activity in the small intestine were dose-independent (Table 2, Figure 1).
| Sit | Groups | Slow wave | Spike activity | ||
| Frequency (num/min) | Amplitude (V) | Amplitude (V) | Spike bursts(%) | ||
| 1 cm | Control | 38.2 ± 1.4 | 172.0 ± 101.9 | 345.0 ± 163.4 | 47.4 ± 22.5 |
| 2.5 mg/kg | 28.2 ± 1.6 | 175.0 ± 81.9 | 402.0 ± 138.4a | 60.4 ± 22.0b | |
| 6 mg/kg | 38.0 ± 1.3 | 173.7 ± 110.1 | 407.3 ± 179.0a | 61.7 ± 26.5b | |
| 12 mg/kg | 37.6 ± 1.1 | 179.0 ± 116.4 | 456.0 ± 145.4b | 59.1 ± 17.3b | |
| 10 cm | Control | 37.3 ± 1.4 | 201.2 ± 110.1 | 335.8 ± 138.6 | 38.1 ± 12.9 |
| 2.5 mg/kg | 36.9 ± 1.6 | 201.7 ± 113.3 | 383.6 ± 150.5 | 55.4 ± 16.1b | |
| 6 mg/kg | 37.0 ± 1.2 | 210.7 ± 105.2 | 412.5 ± 158.8b | 54.3 ± 25.1b | |
| 12 mg/kg | 37.2 ± 1.3 | 202.5 ± 103.8 | 452.6 ± 144.3b | 58.8 ± 15.6b | |
| 20 cm | Control | 35.9 ± 1.1 | 153.2 ± 94.7 | 322.0 ± 93.1 | 37.9 ± 14.5 |
| 2.5 mg/kg | 36.6 ± 1.2 | 149.1 ± 69.1 | 338.2 ± 162.7 | 46.3 ± 12.1a | |
| 6 mg/kg | 36.2 ± 1.2 | 150.2 ± 50.2 | 356.4 ± 117.9 | 60.8 ± 22.6e | |
| 12 mg/kg | 35.6 ± 1.4 | 152.9 ± 73.4 | 347.3 ± 159.6 | 59.7 ± 24.2b | |
The latency times of MCP’s effect on the gastric antrum vs the small intestine were significantly different, with 0.7 ± 0.18 min in the antrum and 2.5 ± 0.35 min in the small intestine at 1 cm distal to the pylorus.
Gastrointestinal myoelectric activities are divided into slow wave and spike activity (fast wave). Slow wave pace and direct gastric contraction. Thus, the alteration of slow wave would influence the mechanical contraction[4]. The spike activity can improve gastrointestinal motility[5]. The gastrointestinal myoelectric activity is a sensitivity index of gastrointestinal motility.
In this study, we find that MCP enhances gastrointestinal myoelectric activity, which is consistent with results reported by previous investigations[6]. Our results, however, also show significant differences in drug responses between the antrum and small intestine. Firstly, the drug only increased the spike activity in the small intestine, while both the slow wave and the spike activity in the antrum increased. Secondly, MCP’s effect on the myoelectric activity of the small intestine was not dose-dependent, as reported by 6 Wingate, et al[6] and 7 Achem-Karam, et al[7] in dogs. Nevertheless, the minimal effective doses (or threshold dose) of this drug varied between the antrum and small intestine. For example, a dosage as small as 2.5 mg/kg increased myoelectric activity in only the small intestine, but did not have any effect on the antrum. Thirdly, the latentcy periods of this drug between the antrum and small intestine were markedly different (i.e. 0.70 ± 0.18 min vs 2.50 ± 0.35 min, respectively). These results suggest that the biochemical control of motor activity is not consistent along the gastrointestinal tract. MCP, an analogue of procainamide[8,9], could directly act on the smooth muscle cholinergic M receptor, while also causing acetylcholine release from the postganlionic cholinergic nerve endings[10], as well as increased myoelectric activity in the antrum. However, this increased spiking activity in the small intestine may not be the result of direct action, as other mechanisms are also likely to be involved.
Xiao-Min Qin, Associate professor of physiology, Director of Basic Medical Department, with 30 published papers and seven books.
Original title:
S- Editor: Filipodia L- Editor: Jennifer E- Editor: Hu S
| 1. | Johnson AG. The action of metoclopramide on human gastroduodenal motility. Gut. 1971;12:421-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Reynolds JC, Putnam PE. Prokinetic agents. Gastroenterol Clin North Am. 1992;21:567-596. [PubMed] |
| 3. | Patterson DJ. Prokinetic agents in postgastrectomy patients. Gastroenterol Clin North Am. 1994;23:313-325. [PubMed] |
| 4. | You CH, Chey WY. Study of electromechanical activity of the stomach in humans and in dogs with particular attention to tachygastria. Gastroenterology. 1984;86:1460-1468. [PubMed] |
| 5. | Chow E, Huizinga JD. Myogenic electrical control activity in longitudinal muscle of human and dog colon. J Physiol. 1987;392:21-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Wingate D, Pearce E, Hutton M, Ling A. Effect of metoclopramide on interdigestive myoelectric activity in the conscious dog. Dig Dis Sci. 1980;25:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Achem-Karam SR, Funakoshi A, Vinik AI, Owyang C. Plasma motilin concentration and interdigestive migrating motor complex in diabetic gastroparesis: effect of metoclopramide. Gastroenterology. 1985;88:492-499. [PubMed] |
| 8. | McCallum RW. Review of the current status of prokinetic agents in gastroenterology. Am J Gastroenterol. 1985;80:1008-1016. [PubMed] |
| 9. | Ramirez B, Richter JE. Review article: promotility drugs in the treatment of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 1993;7:5-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Hay AM, Man WK. Effect of metoclopramide on guinea pig stomach: critical dependence on intrinsic stores of acetylcholine. Gastroenterology. 1979;76:492-496. [PubMed] |