Published online Sep 15, 1997. doi: 10.3748/wjg.v3.i3.160
Revised: January 25, 1997
Accepted: February 22, 1997
Published online: September 15, 1997
AIM: To evaluate the role of p53 in the development and progression of colorectal cancer and gastric carcinoma by analyzing the loss of heterozygosity (LOH) at 17p13.1 and 17p13.3.
METHODS: LOH at the p53 gene locus and 17p13.3 were examined in 22 cases of gastric carcinoma and 14 cases of colorectal cancer by Southern blot analysis.
RESULTS: Of the 22 gastrocarcinoma cases, 12 (54%) were heterozygous and LOH was detected in 6 (50%) of the 12 informative cases. In the 14 colorectal cancer cases, 10 (71%) were heterozygous, and LOH was detected in 6 (60%) of the 10 informative cases.
CONCLUSION: LOH at the p53 gene locus is a frequent event in multiple step carcinogenesis progression. The high frequency of LOH at 17p13.3 suggests that there may be another tumor suppresser gene in that chromosome region.
- Citation: Wu GJ, Shan XN, Li MF, Shi SL, Zheng QP, Yu L, Zhao SY. Preliminary study on the loss of heterozygosity at 17p13 in gastric and colorectal cancers. World J Gastroenterol 1997; 3(3): 160-162
- URL: https://www.wjgnet.com/1007-9327/full/v3/i3/160.htm
- DOI: https://dx.doi.org/10.3748/wjg.v3.i3.160
Recent molecular biology studies have revealed that carcinogenesis is a multiple-step process involving many factors. The activation of some oncogenes and inactivation of some tumor suppresser genes play key roles in this process[1]. p53 is a widely related tumor suppresser gene, but its function has not been clearly defined[2,3]. In this research, LOH of the p53 gene in two common cancers, gastric cancer and colorectal cancer, was examined by Southern blot hybridization and RFLP analysis.
Twenty-two cases of gastric cancer and 14 cases of colorectal cancer were acquired from the affiliated hospital of Nanjing Railway Medical College. Tumor tissues and their corresponding normal tissues were obtained during surgery. The classification of tumor and normal tissues was performed by the Department of Pathology of Nanjing Railway Medical College.
php53B is a p53 cDNA probe located at 17p13.1. pYNZ22 is a VNTR (variable number of tandem repeats) probe located at 17p13.3. Both probes were obtained from ATCC (American Type Culture Collection).
Genomic DNA was isolated from tumor and normal tissues according to standard methods[4]. It was then completely digested with specific restriction endonucleases, electrophoresed on agarose gels, denatured, and transferred to nylon filters. The filters were prehybridized for 8-12 h, hybridized for 24-36 h in 50% formamide at 42˚C, washed and autoradiographed for 1-4 d at -70˚C. Probes were radiolabeled using the random primer method.
The hybridization results of each tumor tissue were compared to that of its normal tissue. If the normal tissue had two heterozygosity bands and its corresponding tumor tissue had only one homozygosity band, the patient was classified as having p53 LOH.
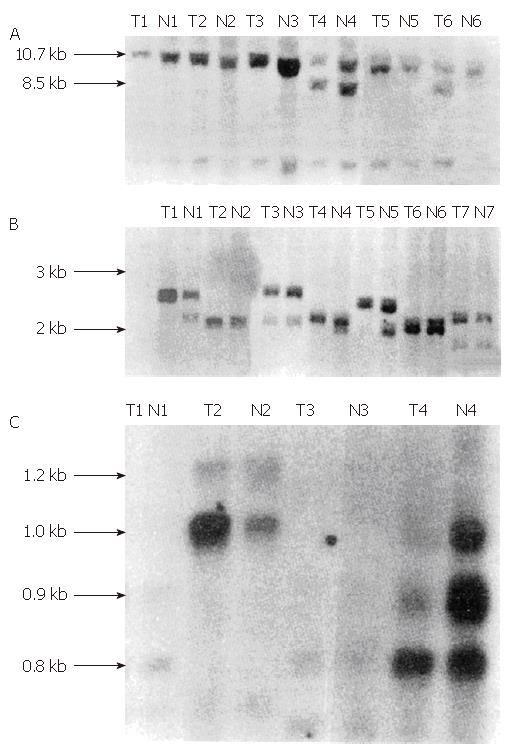
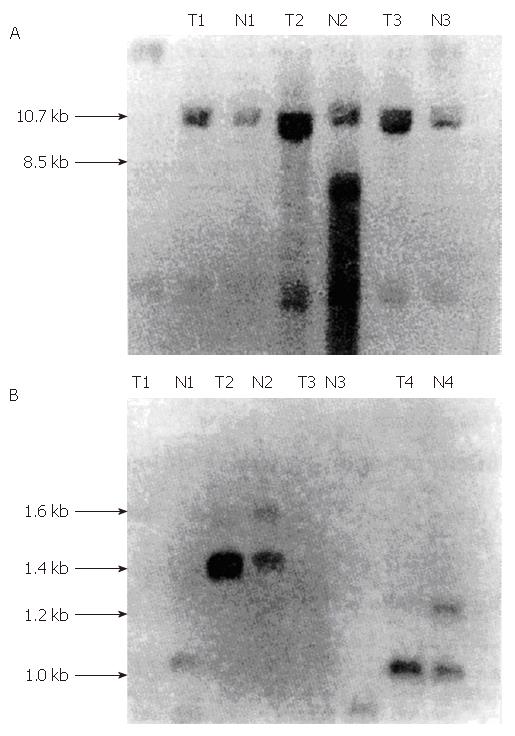
Using the Scal restriction enzyme, we identified 8.5 kb and 10.7 kb allelic fragments with the php53B probe. After use of the MsqI restriction enzyme, we identified a group of alleles 0.5 kb to 1.3 kb with the pYNZ22 probe. While using TaqI, we identified allele fragments ranging from 2 kb to 3 kb with the pYNZ22 probe (Figures 1 and 2).
We identified heterozygosity in all of the normal tissues from the 12 gastric cancer cases and ten colorectal cancer cases. They all showed hybridization bands of different lengths. We determined that six gastric cancer cases and six colorectal cancer cases exhibited LOH. The rate of detection was 50% and 60%, respectively. These details are shown in Tables 1 and 2.
| Locus | Restriction enzyme | Number of sample | Heterozygosity and rate (%) | LOH and rate (%) |
| p53 | Sca I | 7 | 2 (20) | 1 (50) |
| YNZ22 | Msp I | 14 | 8 (57) | 5 (62) |
| 17p13 | 14 | 10 (71) | 6 (60) |
In his “two hit” theory, Knudson showed that the loss of function of tumor suppressor genes generally involves at least two genetic mutation events[5]. These mutations can be detected by Southern blot hybridization and analysis of the LOH occurrence rate. The closely linked polymorphic gene probes located nearby or inside the possible tumor suppressor gene are used to examine the tumor tissue and its normal adjacent tissue. Detection of LOH suggests that there is a tumor suppressor gene located in the region covered by the gene probe, and two mutation events may have occurred in the tumor suppressor gene.
In this study, we detected LOH in two gastric cancer cases and one colorectal cancer case using the php53B probe. As php53B is a p53 cDNA probe, our data suggest that two mutation events occurred in the p53 gene of some gastric cancer and colorectal cancer patients, and that the normal function of the wild type p53 gene was lost. We also detected LOH in five gastric and colorectal cancer cases using the pYNZ22 probe, which is located at 17p13.3 and is tightly linked with p53. These results further suggest that the inactivation of p53 at 17p13.1 is involved in gastric and colorectal carcinogenesis.
To date, p53 is the only tumor suppresser gene that has been assigned to chromosome 17p13. There are conflicting reports on whether the LOH detected by the pYNZ22 probe only reflects p53 inactivation. Studies in breast cancer have shown that LOH detected by pYNZ22 primarily represents p53 inactivation[6]. However, Coles et al[7,8]suggested that there may be certain regulatory genes located between YNZ22 and p53 that control p53 gene expression. Damage to this gene, together with p53 inactivation supposedly contribute to breast cancer carcinogenesis. In our research, only one case of gastric cancer showed LOH in both p53 and the YNZ22 region, while the other cases did not demonstrate LOH in both p53 and the YNZ22 locus. Therefore, further studies are necessary, including collecting more cases and using more restriction endonucleases, to determine whether LOH detected within pYNZ22 in gastric and colorectal cancers represents p53 inactivation or whether there is an additional regulatory gene near the YNZ22 locus that is mutated in gastric and colorectal cancers.
Guo Jun Wu, male, was born on Nov. 2, 1967 in Yuyao County, Zhejiang Province. He graduated from Nanjing Railway Medical College, and is a PhD candidate in genetics at the Institute of Genetics at Fudan University. He has published seven papers.
Presented at the Fifth Congress of Chinese Genetics Society.
Original title:
S- Editor: Filipodia L- Editor: Jennifer E- Editor: Hu S
| 1. | Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8087] [Cited by in RCA: 8005] [Article Influence: 228.7] [Reference Citation Analysis (1)] |
| 2. | Lane DP. Cancer. p53, guardian of the genome. Nature. 1992;358:15-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3502] [Cited by in RCA: 3564] [Article Influence: 108.0] [Reference Citation Analysis (0)] |
| 3. | Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5532] [Cited by in RCA: 5489] [Article Influence: 161.4] [Reference Citation Analysis (0)] |
| 4. | Sambrook J, Fritsch EF, Maniatis T. Molecular cloning Ed 2. New York: Cold Spring Harbor Laboratory Press 1989; 914-923. |
| 5. | Knudson AG. Hereditary cancer, oncogene and antioncogene. Cancer Res. 1985;24:1437-1443. |
| 6. | Singh S, Simon M, Meybohm I, Jantke I, Jonat W, Maass H, Goedde HW. Human breast cancer: frequent p53 allele loss and protein overexpression. Hum Genet. 1993;90:635-640. [PubMed] |
| 7. | Coles C, Thompson AM, Elder PA, Cohen BB, Mackenzie IM, Cranston G, Chetty U, Mackay J, Macdonald M, Nakamura Y. Evidence implicating at least two genes on chromosome 17p in breast carcinogenesis. Lancet. 1990;336:761-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 149] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Kim CJ, Kim WH, Kim CW, Lee JB, Lee CK, Kim YL. Detection of 17p loss in gastric carcinoma using polymerase chain reaction. Lab Invest. 1995;72:232-236. [PubMed] |