Published online Sep 15, 1997. doi: 10.3748/wjg.v3.i3.134
Revised: January 8, 1997
Accepted: February 10, 1997
Published online: September 15, 1997
AIM: To observe the ultrastructural changes of liver tissues on normal rabbit ablated by high-intensity focused ultrasound (HIFU).
METHODS: A single shot of 1.1 MHz focused ultrasound at an intensity of 500 W/cm2 with 20-s duration of continuous exposure was applied intraoperatively in normal rabbit livers. Ultrastructural changes of the sonoablated lesion, as viewed by light and electron microscopy, were observed.
RESULTS: Liver cells at the center of the sonoablated lesion showed irreversible degeneration immediately after HIFU treatment; electron microscopy showed that although the liver cells appeared normal histologically, irregularly shaped cavities of about 0.3-0.5 μm in diameter were present in the cytoplasm.
CONCLUSION: Thermal damages may be the main mechanism of HIFU-induced ablation of liver tissues besides cavitation effect.
- Citation: Cheng SQ, Zhou XD, Tang ZY, Yu Y, Bao SS, Qian DC. Ultrastructural observation of liver tissue ablation induced by high-intensity focused ultrasound. World J Gastroenterol 1997; 3(3): 134-136
- URL: https://www.wjgnet.com/1007-9327/full/v3/i3/134.htm
- DOI: https://dx.doi.org/10.3748/wjg.v3.i3.134
High-intensity focused ultrasound (HIFU) is a new method of ablating non-invasively a selected volume of tissue at a certain depth within the body whilst sparing overlying tissues. This technique was originally developed as a method of selective brain tissue destruction in neurosurgical research[1]. Recently, it has been successfully applied in ophthalmology for the treatment of glaucoma[2] and is undergoing clinical trials for application in the prostate[3]. We have previously used this principle for the ablation of liver tumors in a rabbit model with encouraging results[4]. In this paper, the ultrastructural changes of the ultrasonic lesion in normal rabbit livers, as viewed by light and electron microscopy, are described.
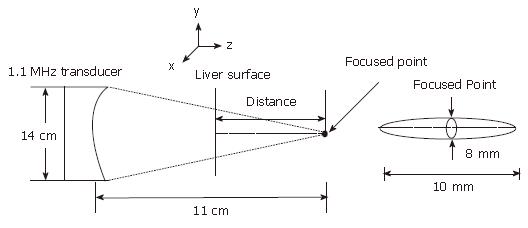
The HIFU instrument developed by the Biomedical Ultrasound Laboratory, Shanghai Jiaotong University, is composed of the following: a signal generator, power amplifier, phase-control system, transducer motion control, microcomputer, and a transducer. The transducer had an array of 16 pistons distributed on the concave spherical surface and was 14 cm in diameter; it produced an acoustic beam with half-intensity axial and lateral dimensions of 10 mm and 8 mm, respectively, at a focal distance of 11 cm (Figure 1).
The energy was generated by the amplifier delivering 500 W/cm2 at a frequency of 1.1 MHz. For experiments on the rabbit, the transducer was fixed to a motorized, three-dimensional coordinate system; this enabled a movement of 0.1 mm in accuracy. A microcomputer controlled the parameters of phase, power, emitting time, and mode.
Five New Zealand white rabbits, weighing 2.5 ± 0.24 kg, were anesthetized by administration of 3% pentobarbital (1 mL/kg of body weight, administered intravenously) and a subxiphoid midline abdominal incision was made. The hepatic lobe of the right center was exteriorized and exposed to the transducer hung by the motorized three-dimensional coordinate system at the focal point of the beam. A thin latex bag filled with thermostatical degassed water, which served as the acoustic coupling medium, was placed between the transducer and the liver. Then, a single shot of 20 s in the continuous wave mode was administered, followed by closure of the abdomen. The animals were allowed to recover. The surviving rabbits were killed at various intervals after HIFU treatment.
After the post-mortem excision of the liver, it was fixed immediately in 10% methacarn, embedded in wax, and thin sections were prepared. They were stained with hematoxylin and eosin (HE) and reticulin and periodic acid Schiff’s base reaction (PAS).
Immediately after HIFU exposure, tissue samples in the centre and edge of the lesion (Figure 2) were placed in 2.5% glutaraldehyde, fixed overnight, and postfixed in 1% osmium tetroxide. The fixed samples were dehydrated using a graded series of ethanol and acetone and were embedded in Epon 618 resin. Ultrathin sections were then cut, double-stained with uranyl acetate and lead citrate, and viewed under a JEM-1200EX transmission electron microscope.
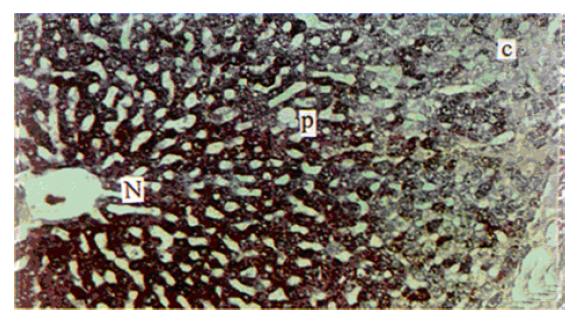
Immediately after HIFU exposure, a sharply defined, grey-white block with diameter 8 mm was noted in the liver. The liver cells showed enlargements and sinus showed stenosis on HE staining, but the liver plate still appeared regular and the cytoplasm and the nucleus appeared normal. PAS staining showed that the cytoplasmic glycogen had decreased but had not disappeared (Figure 2). After 24 h, the cells in this lesion died with cell debris, pyknotic nuclei, karyolysis, or karyorrhexis, and glycogen had almost disappeared completely on PAS stained lesions.
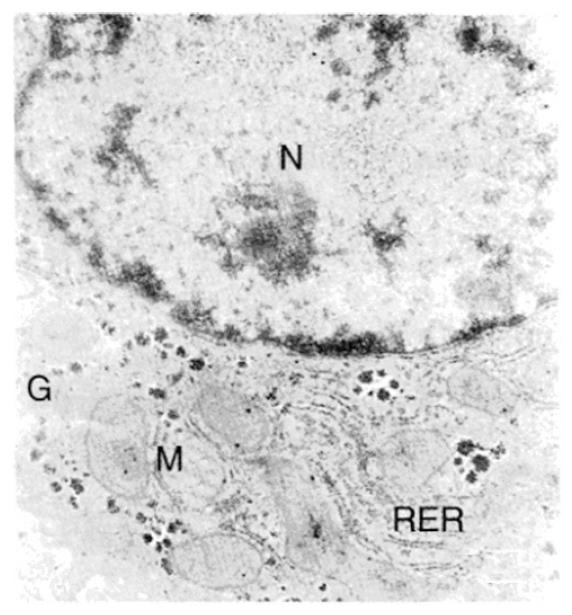
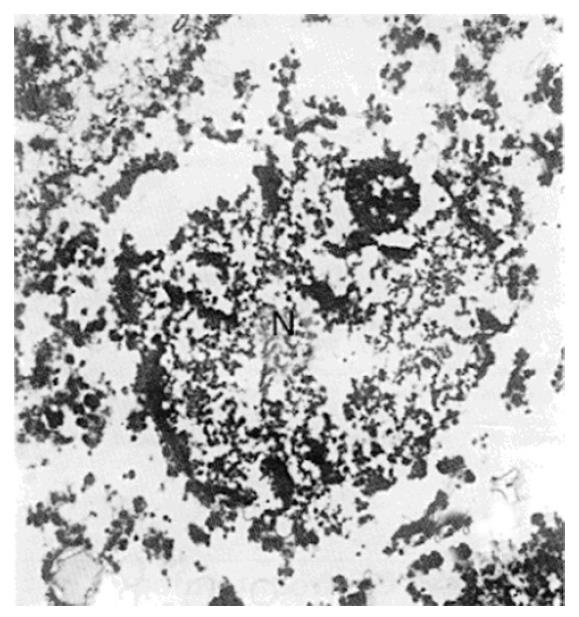
An electron micrograph of a normal rabbit hepatocyte is shown in Figure 3, with a nucleus, mitochondria with intact cristae, glycogen, and rough endoplasmic reticulum. Immediately after insonation, the nucleus appeared irregular, but its membrane was still intact at the edge of the sonoablated lesion (Figure 4), the matrix of mitochondria was reduced and the cristae were disrupted. The ultrastructure of rough endoplasmic reticulum was disordered and some ribosome disappeared. Cavities (about 0.3-0.5 μm) were also found, with no glycogen and irregularly shaped holes in the cytoplasm. These cavities may be the result of mitochondria cavitation. Figure 5 shows that in the centre of the lesion, the nucleus was almost completely disrupted, and the integrity of the structure of mitochondria, the endoplasmic reticulum, and glycogen had disappeared. Again, there were 0.3-0.5 μm cavities in the cytoplasm. The cellular ground substance was amorphous, with many electronic deposits; this indicated typical complete disruption and destruction of the cytoplasmic ultrastructure.
In the previous study on histological changes in rabbit liver tumor treated with HIFU, we found that HIFU insonation at a peak intensity of 500 W/cm2 for 20 s efficiently ablated the liver tumor, inducing coagulation necrosis 24 h later; however, evidence of prompt damage to the target area could not be clearly identified immediately after HIFU exposure[4]. With the same results, this study also revealed that the liver cells in the treated centre appeared histologically normal immediately after HIFU treatment administered at a similar intensity (1.1 MHz, 500 W/cm2, 20 s). However, these cells exhibited irreversible degeneration with disruption of the nuclear membrane and absence of mitochondria and endoplasmic reticulum, as observed by electron microscopy. It is indicated that coagulation necrosis of the targeted cells occurred as the last stage of the cell death, and that in fact, the liver cells were significantly damaged by HIFU insonation.
Although some recent reports have examined HIFU ablation of experimental liver tumors, few of them have focused on the resultant ultrastructural changes and the underlying mechanism, which remains to be fully determined[5]. Generally, it is believed that the two main mechanisms involved in tissue sonoablation are as follows: (1) a thermal effect (rapid elevation of local tissue temperature to over 80 °C) that promptly coagulates the cell protein and (2) a cavitation effect (generation of violently expending and collapsing gaseous bubbles) that mechanically disrupts the cellular and tissue structure. Thermal damage is usually assumed to occur in association with long exposure times of over 1 s and relatively low intensities (below 1000 W/cm2), while cavitation occurs with high-peak intensities and brief exposure periods. There is a range of levels of intensities and exposure periods for which both phenomena may not be distinctly identified[6,7]. In our previous study[8], we found that the peak tissue temperature at the sonic focal region was > 90 °C, with the same energy; this thermal effect could provoke tissue destruction by coagulation necrosis. Therefore, thermal necrosis may play a dominant role in HIFU tumor ablation.
ter Haar et al[9] used HIFU (1.7 MHz, 3000 W/cm2, 10 s) and performed a similar study in normal rat livers. They found that the cells in the rim of the ultrasonic lesion appeared histologically normal immediately after HIFU treatment; however, these cells were functionally impaired, for their cellular glycogen was missing. These findings are in agreement with our results. Moreover, cavities (about 4 μm) could be observed by electron microscopy, but it remains to be determined whether it is due to vaporization or acoustic cavitation. In our study, we also found many 0.3-0.5 μm cavities in the cytoplasm and deduced that it may be due to the cavitation of mitochondria or other organelles. Therefore, it is suggested that the cavitation effect may also play a significant role in inducing irreversible cell death immediately after HIFU treatment when applied under a relatively low-intensity condition.
Above all, our results show that the thermal effect and cavitation due to ablation probably occur simultaneously as the HIFU is directed at the liver tissues when the intensity is relatively low. This findings will be helpful in improving the focusing and selectiveness of liver cancer ablation extracorporeally if the cavitation could be much more enhanced, which worth investigating in the future.
Shu Qun Cheng, MD and PhD, having 18 papers published, now being a medical postdoctor in the East Institute of Hepatobiliary Surgery, Second Military Medical University, China.
Supported in part by the China Medical Board Grant #93-583, New York, and China Postdoctoral Science Foundation.
Original title:
S- Editor: Filipodia L- Editor: Jennifer E- Editor: Hu S
| 1. | FRY WJ, MOSBERG WH, BARNARD JW, FRY FJ. Production of focal destructive lesions in the central nervous system with ultrasound. J Neurosurg. 1954;11:471-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 268] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Silverman RH, Vogelsang B, Rondeau MJ, Coleman DJ. Therapeutic ultrasound for the treatment of glaucoma. Am J Ophthalmol. 1991;111:327-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 42] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Foster RS, Bihrle R, Sanghvi NT, Fry FJ, Donohue JP. High-intensity focused ultrasound in the treatment of prostatic disease. Eur Urol. 1993;23 Suppl 1:29-33. [PubMed] |
| 4. | Cheng SQ, Zhou XD, Tang ZY, Yu Y, Wang HZ, Bao SS, Qian DC, Zhang HY. Histological changes in rabbit liver tumour treated with high-intensity focused ultrasound (in Chinese). Chinese Journal of Experimental Surgery. 1996;13:136-137. |
| 5. | Prat F, Chapelon JY, Abou el Fadil F, Sibille A, Theillière Y, Ponchon T, Cathignol D. Focused liver ablation by cavitation in the rabbit: a potential new method of extracorporeal treatment. Gut. 1994;35:395-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Yang R, Sanghvi NT, Rescorla FJ, Kopecky KK, Grosfeld JL. Liver cancer ablation with extracorporeal high-intensity focused ultrasound. Eur Urol. 1993;23 Suppl 1:17-22. [PubMed] |
| 7. | Sibille A, Prat F, Chapelon JY, Abou el Fadil F, Henry L, Theillère Y, Ponchon T, Cathignol D. Extracorporeal ablation of liver tissue by high-intensity focused ultrasound. Oncology. 1993;50:375-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 46] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Cheng SQ, Zhou XD, Tang ZY, Yu Y, Wang HZ, Bao SS, Qian DC. Liver tissue ablation induced by high-intensity phased focused ultrasound: preliminary results of experimental study (in Chinese). Chinese Journal of Ultrasound in Medicine. 1996;12:1-4. |