Published online Feb 21, 2023. doi: 10.3748/wjg.v29.i7.1173
Peer-review started: October 6, 2022
First decision: November 15, 2022
Revised: November 28, 2022
Accepted: January 30, 2023
Article in press: January 30, 2023
Published online: February 21, 2023
Processing time: 137 Days and 10 Hours
Post-surgical leaks and fistulas are the most feared complication of bariatric surgery. They have become more common in clinical practice given the increasing number of these procedures and can be very difficult to treat. These two related conditions must be distinguished and characterized to guide the appropriate treatment. Leak is defined as a transmural defect with communication between the intra and extraluminal compartments, while fistula is defined as an abnormal communication between two epithelialized surfaces. Traditionally, surgical treatment was the preferred approach for leaks and fistulas and was associated with high morbidity with significant mortality rates. However, with the development of novel devices and techniques, endoscopic therapy plays an increasingly essential role in managing these conditions. Early diagnosis and endoscopic therapy initiation after clinical stabilization are crucial to success since clinical success rates are higher for acute leaks and fistulas when compared to late and chronic leaks and fistulas. Several endoscopic techniques are available with different mechanisms of action, including direct closure, covering/diverting or draining. The treatment should be individualized by considering the characteristics of both the patient and the defect. Although there is a lack of high-quality studies to provide standardized treatment algorithms, this narrative review aims to provide a summary of the current scientific evidence and, based on this data and our extensive experience, make recommendations to help choose the best endoscopic approach for the management of post-bariatric surgical leaks and fistulas.
Core Tip: Post-surgical leaks and fistulas are the most feared complications of bariatric surgery. Endoscopic therapy is essential for effective management of these conditions. Several endoscopic techniques are available, and this review aims to clarify their mechanisms of action, basic principles, and optimal approach for each situation based on a detailed literature review as well as the authors’ personal experience.
- Citation: de Oliveira VL, Bestetti AM, Trasolini RP, de Moura EGH, de Moura DTH. Choosing the best endoscopic approach for post-bariatric surgical leaks and fistulas: Basic principles and recommendations. World J Gastroenterol 2023; 29(7): 1173-1193
- URL: https://www.wjgnet.com/1007-9327/full/v29/i7/1173.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i7.1173
Obesity is now a pandemic, and the prevalence of people living with obesity continues to increase. As a chronic and multifactorial disease with several associated comorbidities, a multidisciplinary approach is required to prevent, treat, and reverse obesity-related complications and to improve quality and length of life for people with obesity[1].
Bariatric and metabolic surgery (BMS) remains the most effective and durable therapy for weight loss and improvement of associated comorbidities. Unsurprisingly, the number of bariatric surgeries performed has grown progressively worldwide. In 2019, about 256000 BMS were performed in the United States. The most performed procedure is laparoscopic sleeve gastrectomy (LSG) followed by Roux-en-Y gastric bypass (RYGB), and revisional surgery[2]. With the increasing number of BMS, familiarity with management of procedure related complications is increasingly important for endoscopists.
Although rare, especially in referral centers, complications can occur, such as leaks and fistulas[3]. Traditionally, leaks and fistulas were treated with surgery. However, surgical management of these defects is usually challenging and has been associated with high morbidity and mortality rates[4]. Less invasive approaches are preferred, when possible, to reduce associated morbidity. Endoscopic therapies play an essential role in the management of leaks and fistulas. Several endoscopic devices and techniques, with different mechanisms of action have been developed, with considerable clinical success for what is often morbid and refractory disease[3,4].
In this narrative review we discuss the pathophysiology, characteristics, diagnosis, and management of post-bariatric surgical leaks and fistulas, focusing on endoscopic therapies. We include descriptions of the mechanism of action, indications, contraindications, tips, tricks, and outcomes for different techniques, in order to facilitate choosing the best approach for each unique case.
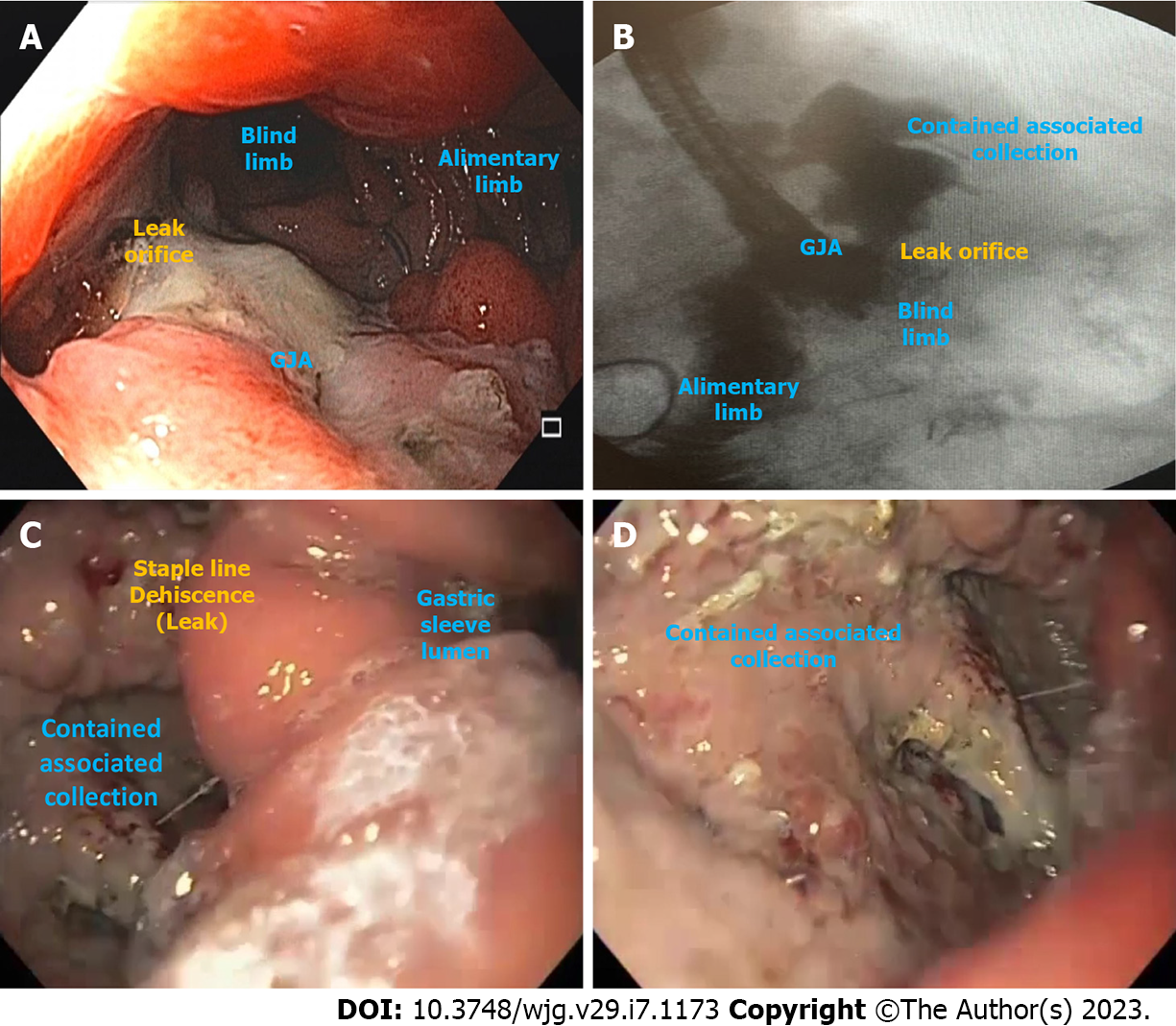
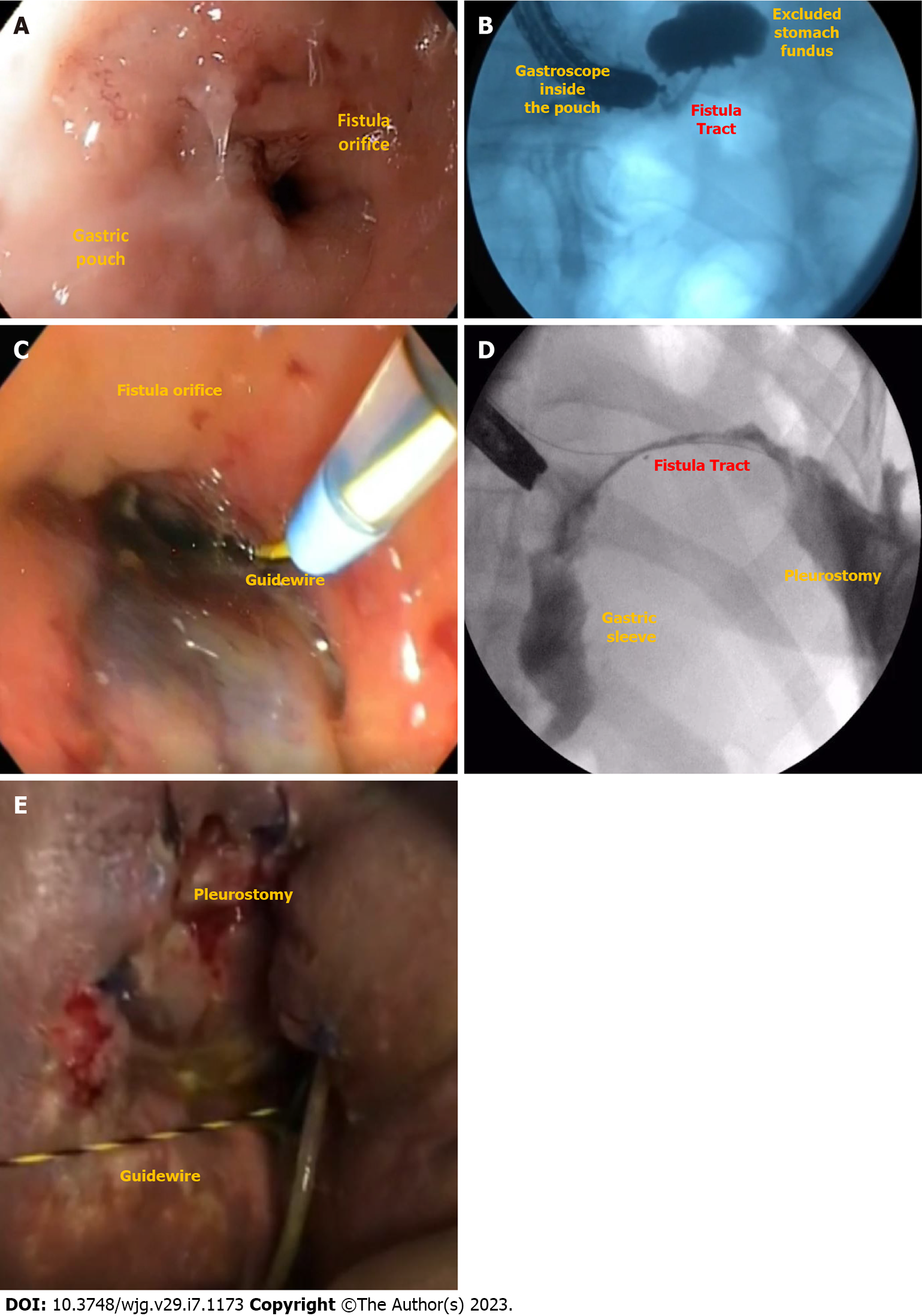
Leak is defined as a transmural defect with communication between the intra and extraluminal compartments (Figure 1). A fistula is defined as an abnormal communication between two epithelialized surfaces. Fistulas can be divided into internal and external. An internal fistula occurs between two internal epithelialized organs, whereas an external fistula is a communication between an internal organ and the skin surface (Figure 2)[5].
Despite the increasing number of BMS, the rate of mortality and adverse events (AEs), including leaks and fistulas, has decreased over the past two decades[6] due to improvement in surgical techniques, such as laparoscopy and robotic surgery, improved materials, and surgeons’ expertise.
Leaks and fistulas occurred with similar incidence among the most common bariatric surgeries, ranging from 0.4% to 5.6% in RYGB and 1.9% to 5.3% in LSG, with higher rates after revisional surgeries[7-10]. This incidence is considerably lower in high-volume specialized centers; as low as 0.5%[11]. Post-BMS mortality is very low, ranging from 0.2% to 0.4%, and is mainly related to late diagnosis and management of complications[7-10].
Leaks usually occur after surgery and are often located at the suture (staple) line and/or anastomosis, while fistulas are mainly caused by untreated leaks[5].
The causes of transmural defects after BMS are related to several factors, including the patient related risk factors and technical risk factors. Patient related risk factors for transmural defects include high body mass index, age, smoking, alcohol use, malnutrition, and other related comorbidities [e.g., type 2 diabetes mellitus (T2DM)][12]. Technical risk factors include mechanical or ischemic insults, both of which contribute to a mechanism of high intraluminal pressure that overcomes the integrity of the tissue. Meticulous surgical technique, with careful attention to tissue handling and inadvertent excessive tension on anastomoses, luminal narrowing, suturing/stapling ischemic areas, and torsion of blood vessels or organs is mandatory to avoid undesirable outcomes such as downstream stenosis, leaks, and fistulas. Additionally, stapling failure is also a cause of BMS leaks[13,14].
The pathophysiology of leaks and fistulas after RYGB and LSG differ; given distinct technical and anatomic particularities of each surgery. Most commonly in LSG, the creation of a long and narrow conduit combined with pylorus preservation creates a high-pressure region proximally leading to stress on the proximal staple line. In RYGB, the gastric pouch is a low-pressure system with low resistance to gastric emptying through the gastrojejunal anastomosis (GJA) into the small bowel, though high-pressure areas can develop in cases of GJA stenosis or excluded stomach gastroparesis. The anatomy of LSG favors leak and fistula formation at the proximal portion of the staple line, especially near the angle of His. Here the thin gastric wall, high pressure, and borderline vascularization secondary to take down of the short gastric arteries all contribute to comprised wall integrity[14-16].
Of note, for both LSG and RYGB, it has been shown that laparoscopic surgery reduces the incidence of leaks and fistulas as well as mortality rates compared to open surgery[6,17]. Intraoperative leak assessment using methylene blue, endoscopy, or air insufflation allows for immediate repair of defects though this practice has never been proven to reduce the incidence of leak or fistula formation after surgery[14,18].
Stapling technology has evolved in recent years with the development of different staple heights capable of accommodating variable gastric wall thickness according to the anatomic portions of the stomach. To take advantage of this technology, the surgeon must be aware of which one is the most appropriate in each case to avoid mismatch[14]. Moreover, several techniques of staple line reinforcement have been studied, from oversewing the staple line with different suture techniques to the use of biologic or synthetic materials, such as glues and tissue sealants, but none of these interventions have been proven to reduce the incidence of post-surgical leaks and fistulas[19-22].
The routine placement of drains near the anastomosis is controversial and there is no consensus in the literature and between surgeons on whether this intervention prevents the development of uncontrolled leakage or increases the risk of developing leaks[23,24].
Leaks and fistulas can be classified based on several parameters, including time of onset and location.
The definition of acute vs chronic leaks and fistulas varies between 30 and 45 d in the literature (acute < 30-45 d and chronic > 30-45 d)[25,26]. In our practice, we use the classification described in the international LSG consensus, which classified the defects into acute (< 7 d), early (between 7 d and 6 wk or 45 d), late (between 6 wk or 45 d and 3 mo or 90 d), and chronic (> 3 mo or 90 d)[27]. Up to 20% of acute and early leaks and fistulas become late and chronic leaks and fistulas, mainly when the defect is untreated[26,28].
Based on the location, post-RYGB leaks are classified into Type I to VI. The locations include: The gastric pouch (Type I), GJA (Type II), blind jejunal stump (Type III), jejunojejunal anastomosis (Type IV), excluded stomach (Type V), and the blind stump of the biliopancreatic limb (Type VI). The first three topographies are easily accessible by endoscopy and can be treated with endoscopic techniques, unlike the others[29].
LSG leaks mostly occur at the level of the angle of His due to poor vascularization (more than 90% of the cases) but can also occur in the distal body and antrum. Both locations can be treated with an endoscopic approach[27].
The diagnosis of leaks and fistulas is based on clinical history and physical examination. Complementary studies, including laboratory tests and imaging are usually required to establish a precise diagnosis.
Clinical manifestations vary according to individual factors related to the level of inflammatory response as well as particularities of the leak or fistula, such as the onset time, defect location, and the placement of a post-surgical drain. If an external drain is placed, the leak can be identified early due to increased drainage[5]. On the other hand, patients without external drainage usually present with infectious manifestations, from mild signs and symptoms, such as low-grade tachycardia, fever, and abdominal pain, to sepsis[30].
Clinical manifestations of fistulas depend on the location of the defect. Respiratory symptoms should be an alarm to consider gastrointestinal (GI)-respiratory fistulas, although patients with peritonitis can also present with pleural effusion[31]. GI-cutaneous fistulas are easily diagnosed if secretions are observed coming out through the skin[32]. Gastrogastric fistula is usually a late complication of RYGB and should be investigated in patients with weight regain and/or gastroesophageal reflux[33].
Laboratory tests are important in acute infection and include leukocytosis, elevated C-reactive protein, and changes indicating organ dysfunction[14]. Additionally, it may be important in patients with long-term fistulas to evaluate nutritional state or electrolyte depletion, for example, in chronic gastrocolic fistulas.
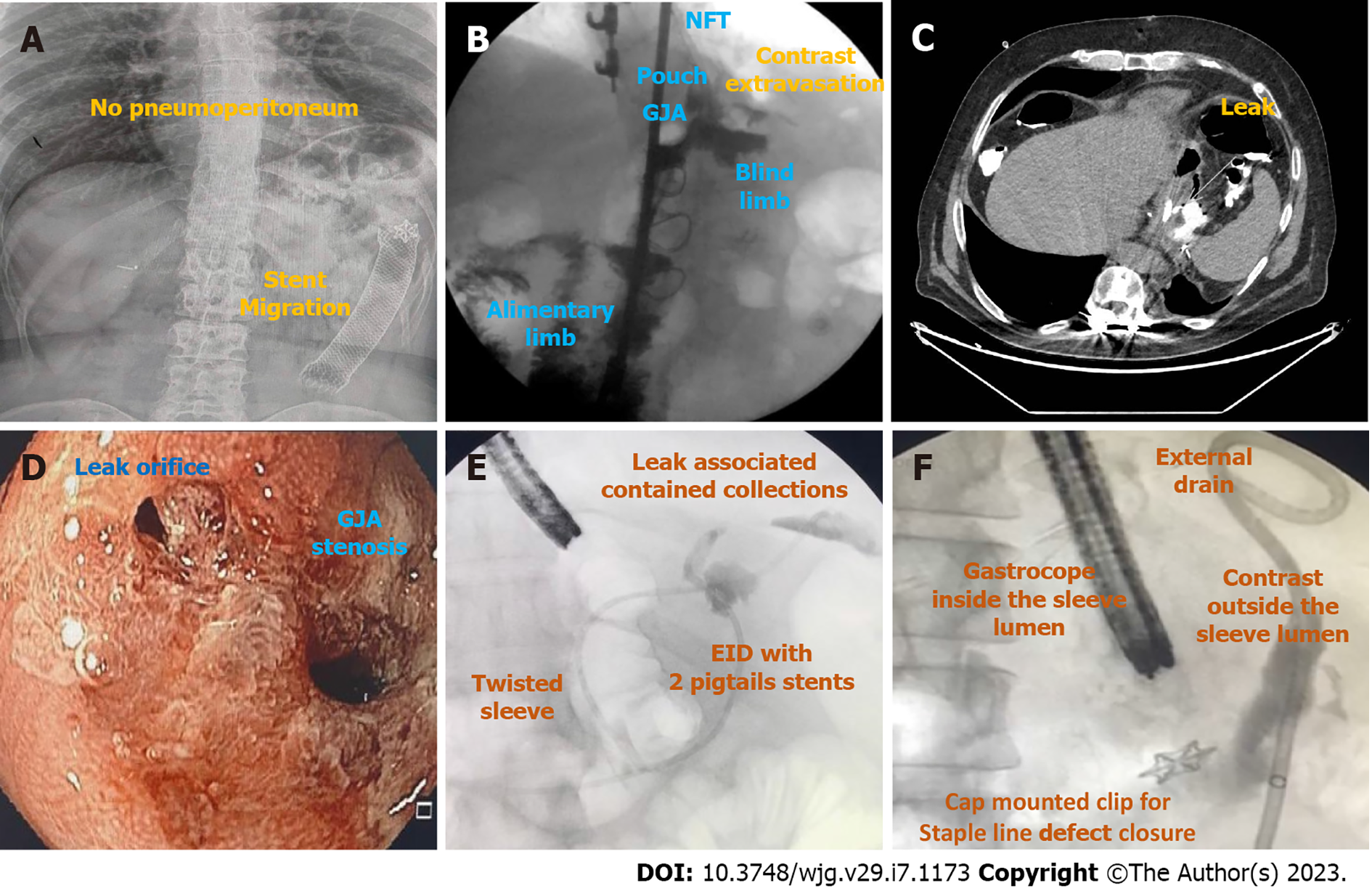
Once suspicion is established, diagnosis is usually confirmed with imaging exams, including radiography of the abdomen, upper gastro-intestinal transit (upper GI series) and computed tomography (CT) scan with administration of water-soluble oral contrast, esophagogastroduodenoscopy (EGD), and/or fistulogram[34] (Figure 3).
Radiography of the abdomen is frequently requested as it can rapidly evaluate for pneumoperitoneum (Figure 3A).
Upper GI transit should be performed with water-soluble contrast if leak or fistula are suspected (Figure 3B). It is important to locate the fistulous orifice and to understand the post-surgical anatomy, including the presence of downstream stenosis for successful treatment. The use of barium is not recommended as it may delay endoscopic treatment. When GI transit findings are doubtful or clinically discordant, CT scan should be used to clarify the results due to its lower sensitivity[35,36].
CT scan allows the evaluation of a fistulous path as well as indirect signs of the source of a leak or fistula, presence of associated collections, pneumoperitoneum, and free fluid. Additionally, it provides a broad evaluation of the intracavitary organs for procedural planning (Figure 3C)[4,37].
EGD is needed to evaluate the fistulous orifice (Figure 3D) surrounding tissue, presence of foreign bodies, and downstream stenosis. Fluoroscopy can be critically useful, especially for orifices smaller than the diameter of the gastroscope. In these cases, a fistulogram can be performed by injecting water-soluble contrast through a catheter or the gastroscope working channel (Figure 3E). Additionally, injection of water-soluble contrast, methylene blue, or a bubble test can be performed if there is an external drain (Figure 3F)[5]. In patients with LSG and suspicion of a leak or fistula without an evident defect, an important reminder is to follow the staple line superiorly looking for small orifices as these can be difficult to locate. A fistulogram can also be performed percutaneously in cases of GI-cutaneous fistulas allowing for a better understanding (size and location) of the fistula tract.
The treatment of leaks and fistulas is based on four pivotal principles: (1) Clinical management; (2) Drainage; (3) Treatment of associated factors; and (4) Closure of the transmural defect.
First, unstable patients must be clinically stabilized, including fluid resuscitation, packed blood cells transfusion when indicated, and administration of vasoactive drugs if necessary.
Infectious source control including intravenous antibiotic therapy and drainage must be performed to control sepsis. Drainage can be performed surgically, percutaneously by image guidance, or endoscopically. For unstable patients with peritonitis and free infected fluid in the cavity, surgical lavage and drainage, with or without defect repair, is the preferred approach.
Nutrition is also key for defect healing and is often neglected as most patients are kept nil per os initially. Therefore, enteral or parenteral nutrition should be introduced as early as feasible. Enteral nutrition is the preferred option, through a nasoenteral feeding tube placed distal to the defect[5].
Defect related factors must be treated to achieve successful closure, such as dilation of downstream stenosis, and foreign body removal (staples, suture, and drains, etc.)[38,39].
After clinical stabilization, endoscopic evaluation is recommended. The endoscopist must communicate with other specialty teams and review the operative report thoroughly before performing the initial endoscopic evaluation. The role of endoscopy in the management of leaks and fistulas includes both diagnosis and treatment. Early diagnosis and endoscopic management are key for success, with closure efficacy as high as 90% when defects are treated within 3 wk of surgery and about 70% after this period[25,26]. Endoscopic treatment of leaks is associated with higher rates of successful closure when compared to fistulas[40]. Despite the lower efficacy of endoscopic treatment for chronic leaks and fistulas compared to acute and early transmural defects, it should be attempted exhaustively before referral for definitive surgical treatment as surgery is often challenging and associated with more morbidity[41]. In this setting, the most commonly proposed revision surgeries are fistulojejunostomy, conversion of LSG to RYGB (without gastrectomy), and total/near total gastrectomy with esophagojejunal anastomosis. Direct surgical repair of the fistula site is not effective and not advised.
Endoscopic evaluation of leaks must be performed under carbon dioxide insufflation or underwater to reduce the risk of pneumoperitoneum or pneumomediastinum, especially when there is no external drainage. Careful endoscopic assessment is essential to avoid wall rupture of a contained collection[37].
The procedure can be performed in the operating room, endoscopy suite, or at the bedside[4]. The endoscopy suite is preferable due to its lower costs when compared to the operating room and greater resources when compared to the bedside. However, for unstable patients, the operating room or at bedside in an intensive care unit may be preferred.
General anesthesia with orotracheal intubation to reduce the risk of aspiration during fluoroscopy is advised, especially in the initial evaluation. General anesthesia often allows a more detailed inspection of the transmural defect, including defect size, fistula path, and presence of extraluminal collection[4]. The need for general anesthesia and fluoroscopy is not mandatory but can be tailored to the case at hand.
Endoscopic therapies utilize several mechanisms of action and can be classified into closure, covering, and draining techniques[5].
Closure techniques include clips [through-the-scope clips (TTSC) and cap mounted clips], endoloop, endoscopic suture, and tissue sealants/glues. Despite some studies[42,43] reporting successful closure of leaks and fistulas using the widely available and simple-to-place TTSC, sometimes combined with endoloop, this technique requires robust and healthy tissue around the defect for successful primary closure. Therefore, this approach is not effective in closing leaks and fistulas and should not be recommended[5]. Hence, this closure technique will not be discussed in this review. Common closure techniques are summarized in Table 1.
| Endoscopic techniques | Indications/advantages | Not indicated/disadvantages | Authors experience |
| Cap-mounted clips | (1) Acute/ early/ late/ chronic; (2) Small orifices (< 20 mm); and (3) Safe | (1) Orifices > 20 mm; (2) Need for external drainage; and (3) Variable efficacy | (1) Acute/ early/ late/ chronic; (2) Safe; (3) < 10 mm: > efficacy; (4) > 10mm: very low efficacy; (5) Combined therapy improves its efficacy; and (6) Can be removed when fails to close the defect (not easy to remove) |
| Glues/ tissue sealants | (1) Acute/ early/ late/ chronic; (2) Diameter < 10 mm; (3) Low drainage (< 200 ml/24 h); and (4) Safe | (1) Multiple sessions are usually required; (2) Need for external drainage; and (3) Variable efficacy | (1) Late/ chronic; (2) Low efficacy; (3) Safe; (4) Helpful as an adjunctive therapy; (5) Never use it as a single therapy; (6) Multiple sessions; (7) Cytology brushing or APC is useful to loosen granulation tissue before application; (8) Delivery via endoscopic or percutaneous access; and (9) High cost (tissue sealants) |
| Endoscopic suturing | (1) Acute/ early/ late/ chronic; (2) High technical success; and (3) Safe | (1) Need for external drainage; (2) Low efficacy (need for robust and healthy tissue for primary closure); (3) Challenging: Previous experience with the device is needed; and (4) High costs (most countries) | (1) Very poor long-term clinical success; (2) Helpful for other devices fixation; (3) Not recommended for chronic defects; and (4) High cost |
Covering techniques utilize cardiac septal defect occluders (CSDO) or self-expandable metal stents (SEMS), including conventional (esophageal) stents and custom (bariatric) stents (Table 2). Closure and covering techniques do not allow internal drainage; thus, external drainage is required when an associated collection or intracavitary infected fluid is identified.
| Endoscopic techniques | Indications/advantages | Not indicated/disadvantages | Authors experience |
| Conventional (esophageal) stents | (1) Acute/ early; (2) Satisfactory efficacy; (3) Very popular; (4) Widely available; (5) International guidelines support; (6) Easy placement; (7) Early oral intake; and (8) Low number of repeated procedures | (1) Late/ chronic; (2) High migration rates; (3) Need for external drainage; (4) Mild symptoms related to the stent; and (5) Possible “surprise” when removing it | (1) Acute/ early; (2) Satisfactory efficacy; (3) Easy placement/ not expensive; (4) Helpful for complete dehiscence; (5) PCSEMS > FCSEMS (challenging removal – do not keep it for > 3 wk); and (6) High migration rates (FCSEMS) |
| Bariatric stents | (1) Acute/Early; (2) Satisfactory efficacy; (3) “Perfect” shape for LSG leaks; (4) Low number of repeated procedures ; and (5) Easy placement | (1) Late/ chronic; (2) High migration rates; (3) Need for external drainage; (4) Severe symptoms related to the stent; and (5) Possible “surprise” when removing it | (1) Acute/ early; (2) Similar efficacy to the conventional stent; (3) More expensive than the conventional stent; (4) Helpful for downstream stenosis and complete dehiscence; (5) Pre-pyloric position: more symptoms; (6) Post-pyloric position: more migration; (7) High rates of adverse events (ulcers and perforations); and (8) Intolerance due to symptoms related to the stent (GERD, pain, and emesis) |
| Cardiac septal defect occluder | (1) Late/ chronic; (2) High efficacy; (3) Safe; and (4) Epithelialized surface is required for device fixation | (1) Need for external drainage; (2) Off-label use; (3) High cost; and (4) Acute and early: enlargement and migration if no epithelialized surface | (1) Very high efficacy for late/ chronic defects with epithelialized tract without associated collection; (2) Safe; (3) High cost; (4) Off-label; (5) Indicated after conventional techniques failure; and (6) Size selection based on defect size (2:1) |
Endoscopic drainage techniques include endoscopic vacuum therapy (EVT), endoscopic internal drainage (EID) with double pigtail stent (DPS), and septotomy (Table 3).
| Endoscopic techniques | Indications/advantages | Not indicated/disadvantages | Authors experience |
| Endoscopic vacuum therapy | (1) Acute/ early/ late/ chronic; (2) High efficacy in leaks and fistulas with or without associated collection; (3) No need for external drainage; (4) Superior to stent in upper GI tract; and (5) Unique mechanism of action: macro-deformation/ micro-deformation, changes in perfusion/ angiogenesis/exudate control/bacterial clearance | (1) Inability to achieve negative pressure; (2) No endoscopic access; (3) Patient discomfort related to nasogastric tube; (4) Usually repeated procedures are needed (especially when traditional sponge is used); and (5) Longer hospital stay/ high costs (?) | (1) Acute/ early/ late/ chronic; (2) Very high clinical success rates; (3) You must place the EVT system in intracavitary position when an associated collection is identified; (4) Placement of both intracavitary and intraluminal EVT appears to be the best approach; (5) Traditional sponge: challenging placement and removal (mouth), prolonged procedures, and need for multiple exchanges (6) Low-cost modified EVT: easy placement and removal, reduction in procedure time, longer interval between EVT system exchanges, low cost, and low AEs rates; and (7) Modified triple-lumen EVT: drainage and nutrition with one tube through the nares |
| Endoscopic internal drainage with double pigtail stent | (1) Acute/early/late/chronic; (2) High efficacy; (3) No need for external drainage; (4) Need of an associated collection; (4) Easy placement (7fr – gastroscope); (5) Small or large orifices; and (6) Short hospital stay | (1) Defects without an associated collection; (2) No place to accommodate the DPS (small collection: < 2 cm); (3) Long period for complete healing; (4) Risk of migration, perforation and bleeding; and (5) Usually, fluoroscopy is needed | (1) Acute/ early/ late/ chronic; (2) High clinical success rates; (3) Small orifices with associated collection; (4) Easy placement; (5) Shorter hospital stay/ electives procedures for DPS exchanges; (6) Faster oral intake (start with clear liquids); (7) Better patient acceptance – no symptoms; (8) Long period for complete healing; and (9) Ureteral stents appear to be safer with similar efficacy |
| Septotomy | (1) Early/late/chronic (> 15 d); (2) High efficacy; (3) Safe; (4) Septum between the orifice/ collection and the gastric lumen; and (5) Must do it when a septum is identified | It is only performed when a septum is identified | (1) Early/late/chronic (> 15 d); (2) Very high clinical success; (3) Usually more than 1 session is required; (4) Cut until the staple line; (5) APC or Knife (APC < bleeding); (6) Always dilate after septotomy; (7) Outpatient procedure; and (8) Septum is the cause of most late/chronic refractory defects treated in a center without experience |
All endoscopic techniques are summarized in Tables 1, 2, and 3 discussed in detail below.
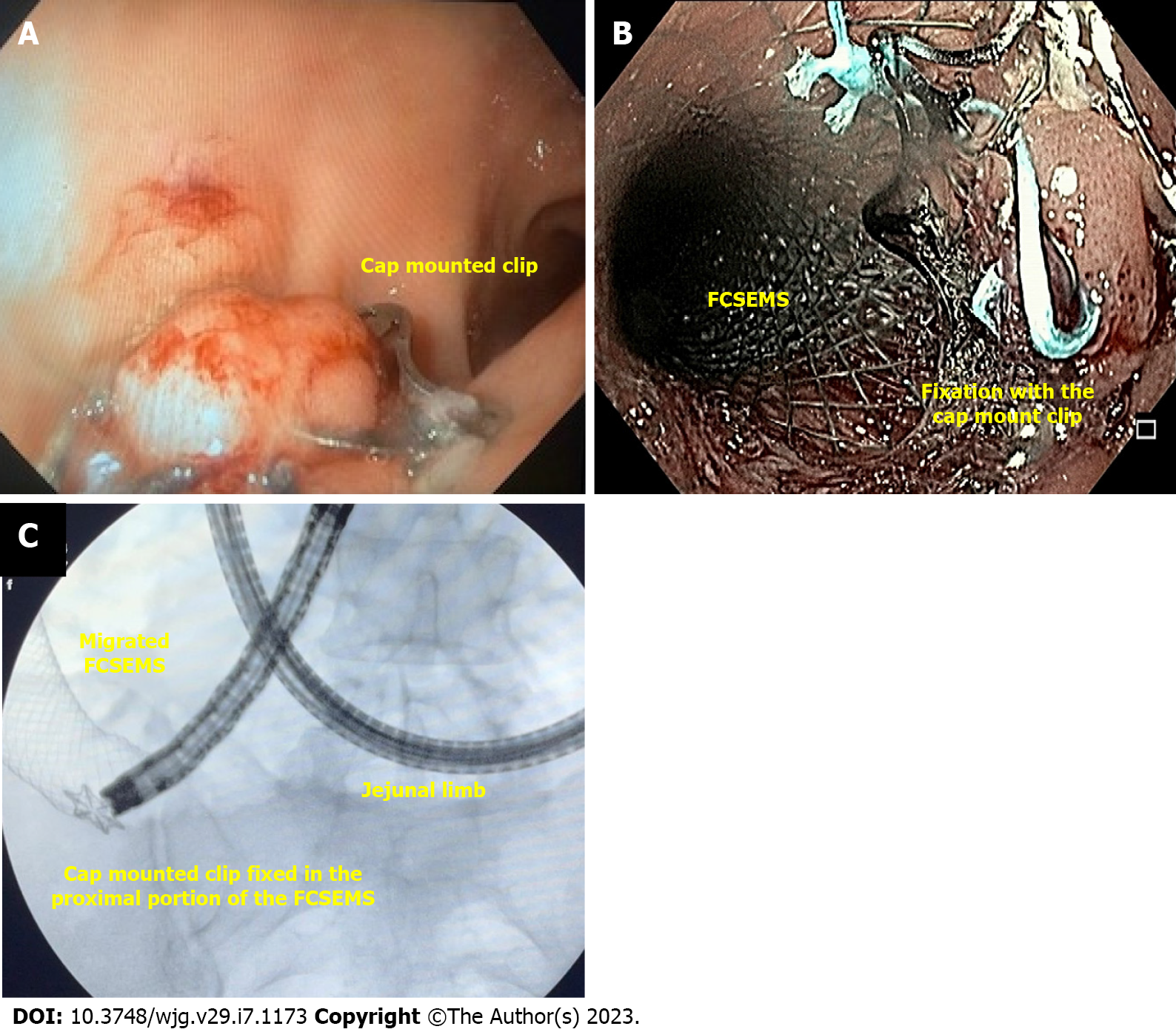
Cap-mounted clips (Figure 4) are usually referred to as over the scope clips (OTSC®, Ovesco Endoscopy GmbH, Tübingen, Germany) as this is the commercial name of the first cap mounted clip. However, it is important to state that the name OTSC® is trademarked and other devices are available.
Cap mounted clip use is increasing due to its benefits compared to the TTSC, including the ability to close leaks and fistulas as it can approximate a larger volume of tissue.
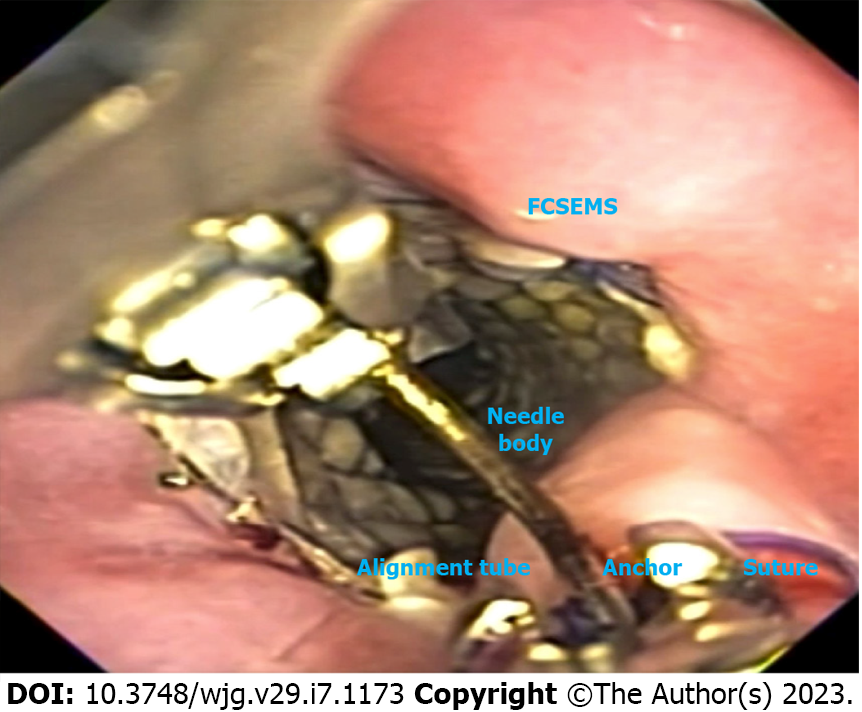
The device is placed into the distal tip of the endoscope and deployment is similar to band ligation, a procedure widely performed by endoscopists, helping to shorten the learning curve[44]. It can only be used in defects smaller than 20 mm.
A meta-analysis including 73 patients from 9 studies evaluating cap-mounted clips for treatment of leaks and fistulas after LSG, including both acute and chronic defects showed a clinical success rate of 63.5% when used as a single therapy and 86.3% when combined with other therapies[45].
In our experience, cap-mounted clips can be used in patients with small orifices without undrained associated collection and always combine with other therapy (Figure 4A). Additionally, cap-mounted clips can be used for stent fixation (Figure 4B and C).
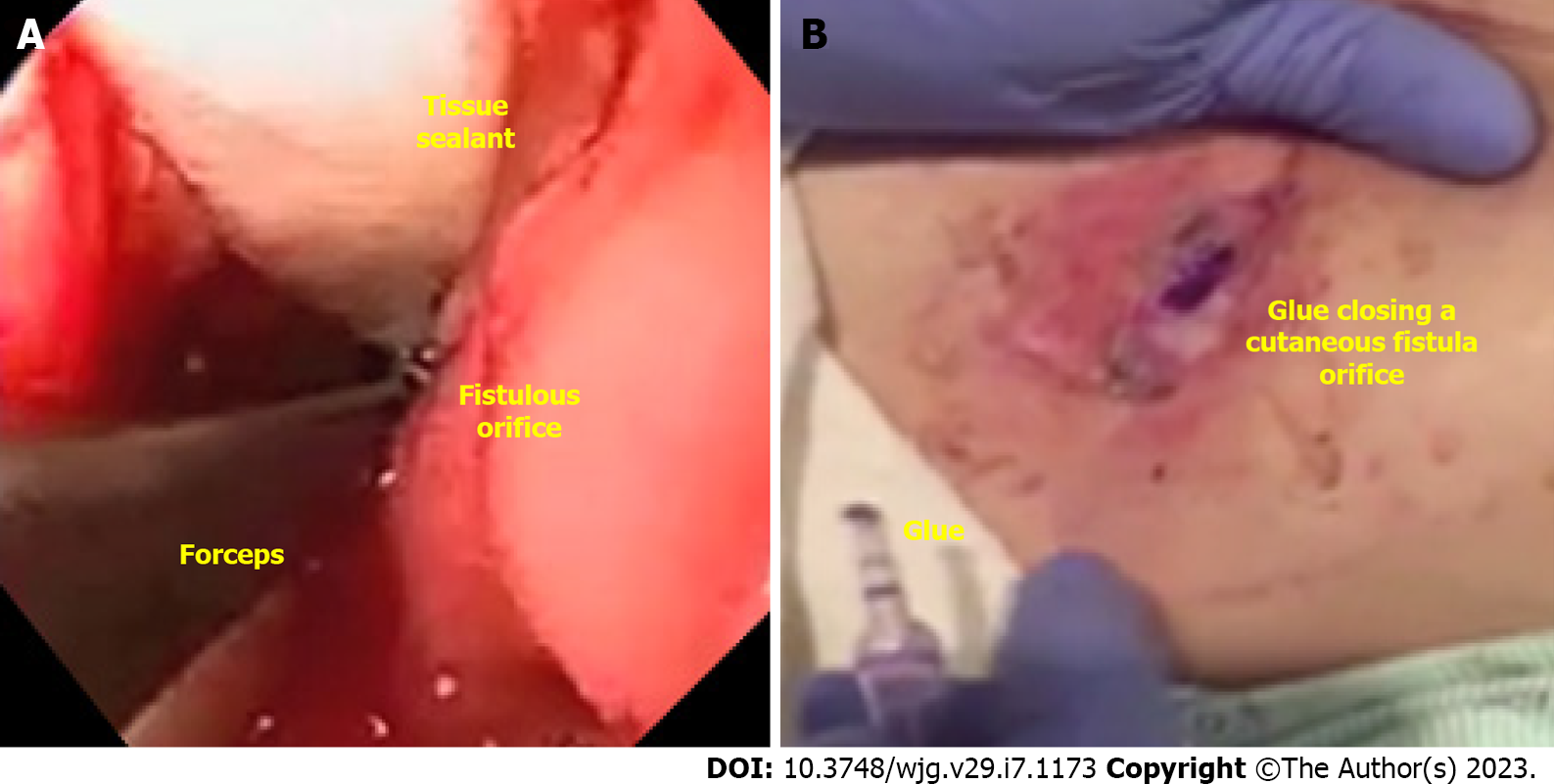
Tissue sealants (Figure 5A) and glues (Figure 5B) include fibrin glue, acellular matrix biomaterial (SurgiSIS® – Cook Medical, Winston-Salem, North Carolina, United States), and cyanoacrylate. These materials are primarily used to close fistulous tracts and are usually used as an adjunctive therapy.
Despite the closure effect of both glues and tissue sealants, the last group also play a role in tissue healing. Fibrin glue induces a cellular response, extracellular matrix formation and neovascularization, while acellular matrix biomaterial induces fibroblast proliferation[46,47].
Although these techniques have a well-established safety profile, reported clinical success is variable and multiple applications are usually required. In a recent meta-analysis evaluating 10 case series involving 63 patients, the number of sessions needed for treatment ranged from 1 to 9, with a pooled successful closure rate of 96.8%[48]. Regardless of the excellent result shown in this meta-analysis, our experience does not reflect this high efficacy. It is important to analyze this data with care given significant limitations and risk of bias for retrospective case series. Furthermore, clinical success is related to the characteristics of the leak or fistula, including defect site, chronicity, and size. Therefore, all these variables should be considered in a treatment plan.
In our experience, the best indication for tissue sealants and glues are GI-cutaneous fistulas, with thin tract and low output and always combined with additional therapies[31]. Tissue sealants and glues can be delivered via endoscopic or percutaneous tract, facilitating the procedure[49]. Cytology brushing or ablation of the epithelialized (chronic) tract to induce granulation is recommended.
Endoscopic suturing (Figure 6) allows for full-thickness closure. However, similar to TTSC, any type of suturing (surgical or endoscopic) needs robust and healthy tissue around the defect for primary closure. Therefore, this technique is not effective in closing most leaks and fistulas and should not be recommended[5]. In a multicenter study evaluating fistula closure by endoscopic suturing, 56 patients underwent the procedure with 100% technical success. Despite the high technical success, only 22.4% of patients achieved clinical success at one year follow-up[50]. Additionally, endoscopic suturing is expensive in most countries and requires specialized training.
In our experience, we only use endoscopic suturing as an adjunctive therapy, for example to fix a stent aiming to reduce the risk of migration.
SEMS (Figure 7) are the most used technique for the treatment of post-bariatric surgical leaks and fistulas worldwide and is recommended by the American Society for Metabolic and Bariatric Surgery in a position statement from 2015[14]. SEMS are widely available, easy to place and usually with a low cost.
SEMS are indicated for acute and early leaks and should be avoided in late and chronic defects and late stenosis due to reduced efficacy and increased AE rates, especially migration[51]. The American Gastroenterology Association suggests the use of SEMS for leaks and fistulas earlier than 6 wk, preferably in defects < 10 mm[15].
Despite satisfactory efficacy in acute and early leaks as shown in a recent meta-analysis (RYGB: 76.1% and LSG: 72.8%), high migration rates (RYGB: 30.5% and LSG: 28.2%) must be considered, as an urgent surgery due to device migration may be catastrophic[52]. It is important to remember that SEMS placement requires drainage of any associated collections before or promptly after stent placement.
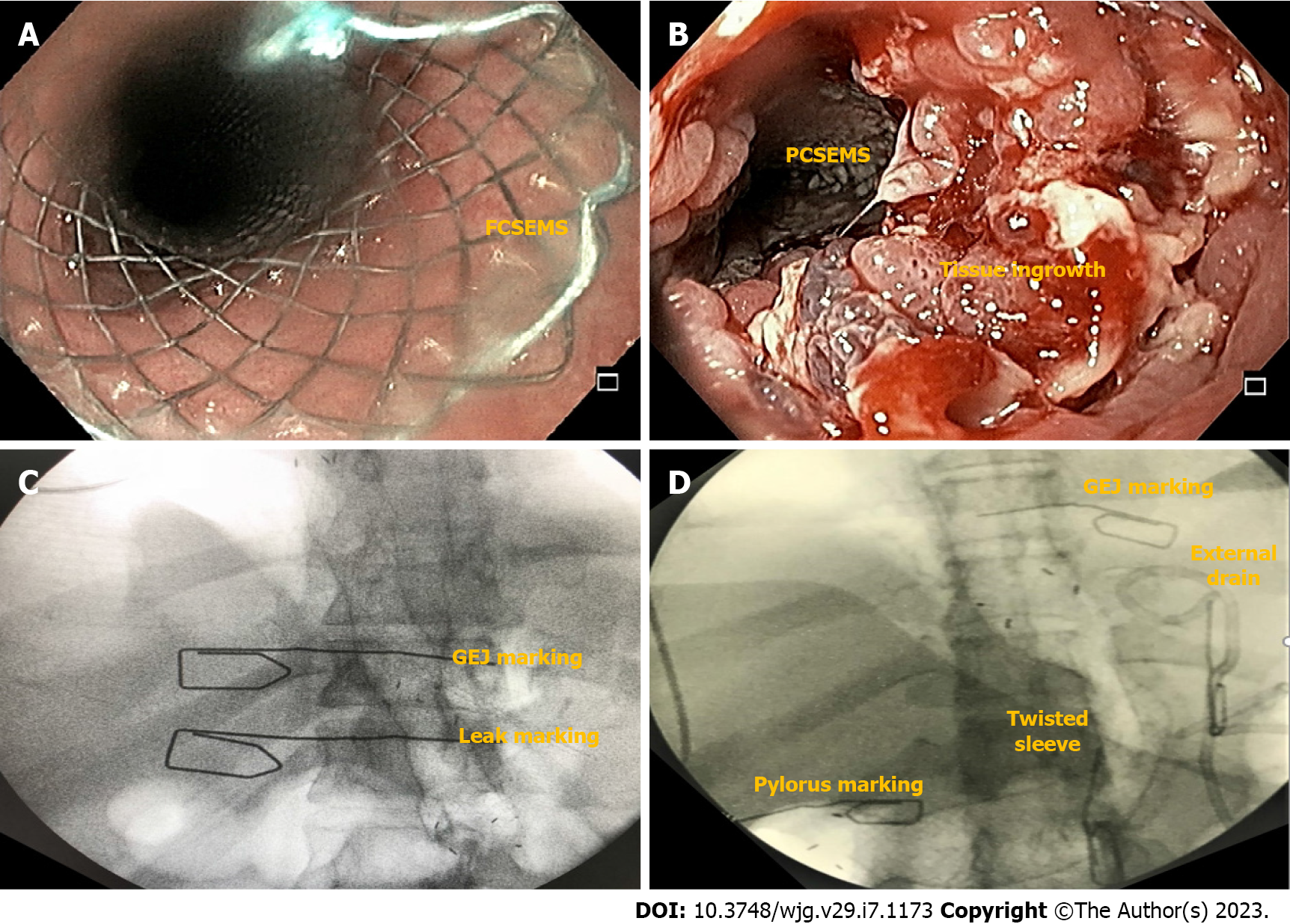
Choosing the correct SEMS facilitates successful therapy. Fully-covered SEMS (FCSEMS) are easy to remove but have a high rate of migration (Figure 7A). Fixation of the FCSEMS with suturing, cap mounted clips or the nasal bridle technique is recommended[53]. On the other hand, partially-covered SEMS (PCSEMS) are associated with low rates of migration, with challenging removal, particularly after 3 wk due to tissue ingrowth (Figure 7B). Nevertheless, some groups argue that the PCSEMS may be better than the FCSEMS as tissue ingrowth prevents leakage between the esophageal wall and the stent. In cases of stent removal failure, several rescue techniques may be used such as the stent in stent technique, ablation and/or endoscopic resection of the tissue ingrowth[15].
Recently, due to the non-negligible migration rate of the esophageal SEMS (Figure 7C), novel models of stents (longer and with a larger diameter) have been developed, focused on the gastric sleeve anatomy. These are customized bariatric stents (Figure 7D). In a retrospective multicenter study[26], 37 patients with early and acute post-LSG leaks were treated with the 24-cm length customized bariatric stent showing similar efficacy and migration rates reported with conventional esophageal SEMS[52]. However, more severe AEs were reported, including two urgent surgeries due to stent migration[54], esophageal perforation[55], contained gastric perforation, and symptoms such as pain and gastroesophageal reflux. In this study, post-pyloric position was associated with high rates of migration and pre-pyloric position with more stent related symptoms[26]. In a meta-analysis comparing conventional esophageal SEMS and the customized bariatric stents, there were no statistical difference in terms of efficacy (93% vs 82%) and migration (15% vs 32%) rates[56]. Therefore, this novel customized bariatric stent should be used with caution, preferably in specialized centers with close follow up[57].
In our experience, SEMS are best applied in acute and early leaks with complete dehiscence and/or with associated downstream stenosis, always in conjunction with external drainage. Moreover, we prefer the esophageal PCSEMS with dwell time of no more than 3 wk, over customized bariatric stents.
The non-surgical closure of a cardiac septal defect was first described in 1976 and was considered a revolution in the treatment of cardiac defects[58]. Although developed for the closure of cardiac defects, the off-label use of the CSDO has been reported for the management of bronchopleural and GI fistulas[59].
The CSDO is a self-expanding, double-disc closure device composed of nitinol and polyester, with shape-memory and impressive expansion force (Figure 8)[28,59]. The thick waist portion serves to self-center the device during deployment to close the defect. The disc diameter varies from 9 mm to 54 mm, and the waist size varies from 4 mm to 38 mm.
The appropriate device size (“waist diameter”) should be 50% larger than the defect orifice. Regarding placement, first, the distal flange is released either into the GI lumen (percutaneous placement) or the fistula tract (endoscopic placement), then, after adequate position is confirmed, the proximal flange is deployed[28].
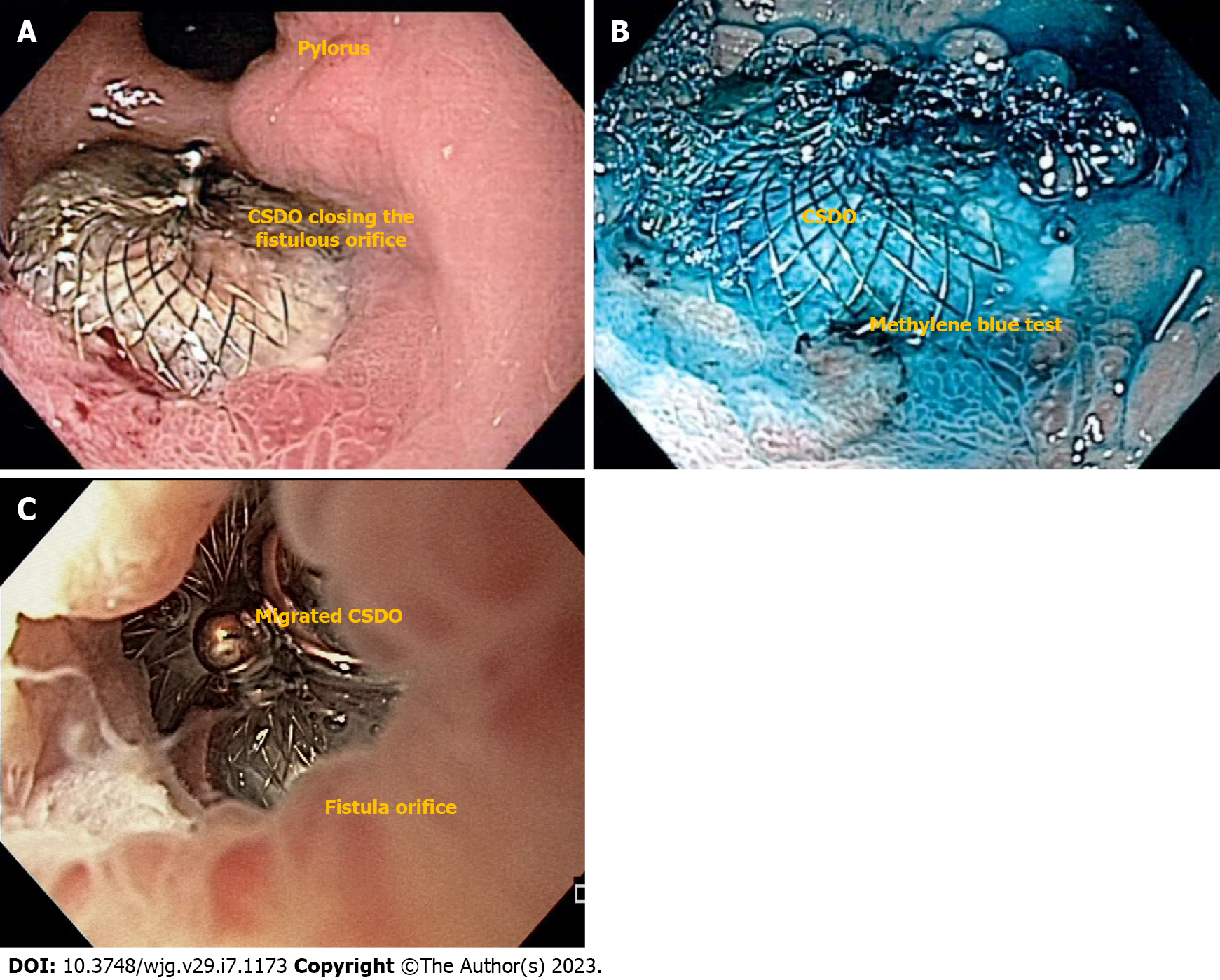
In a multicenter study, clinical success rate for late and chronic defects after BMS was 97.1% vs 62.5% in acute and early scenarios[28]. Due to its expansion force, immediate contrast study or methylene blue test after the procedure usually confirms complete occlusion of the fistulous tract allowing hospital discharge after anesthesia recovery (Figure 8A and B)[32]. If immediate, complete closure fails, adjunctive therapies such as glues/tissue sealants can be performed[31]. Therefore, CSDO is recommended for late and chronic defects as an epithelialized tract is needed for device accommodation. The device should not be used in acute and early leaks or fistulas as it can increases the size of the defect due to its significant expansion force (Figure 8C)[3].
In our experience, the CSDO is the best approach for epithelialized fistulas (Figure 5B) tracts but as an off-label high-cost device, we only use it after conventional technique failure. Regarding long-term follow-up, some devices stay in place and other migrate after complete healing of the defect[28,60].
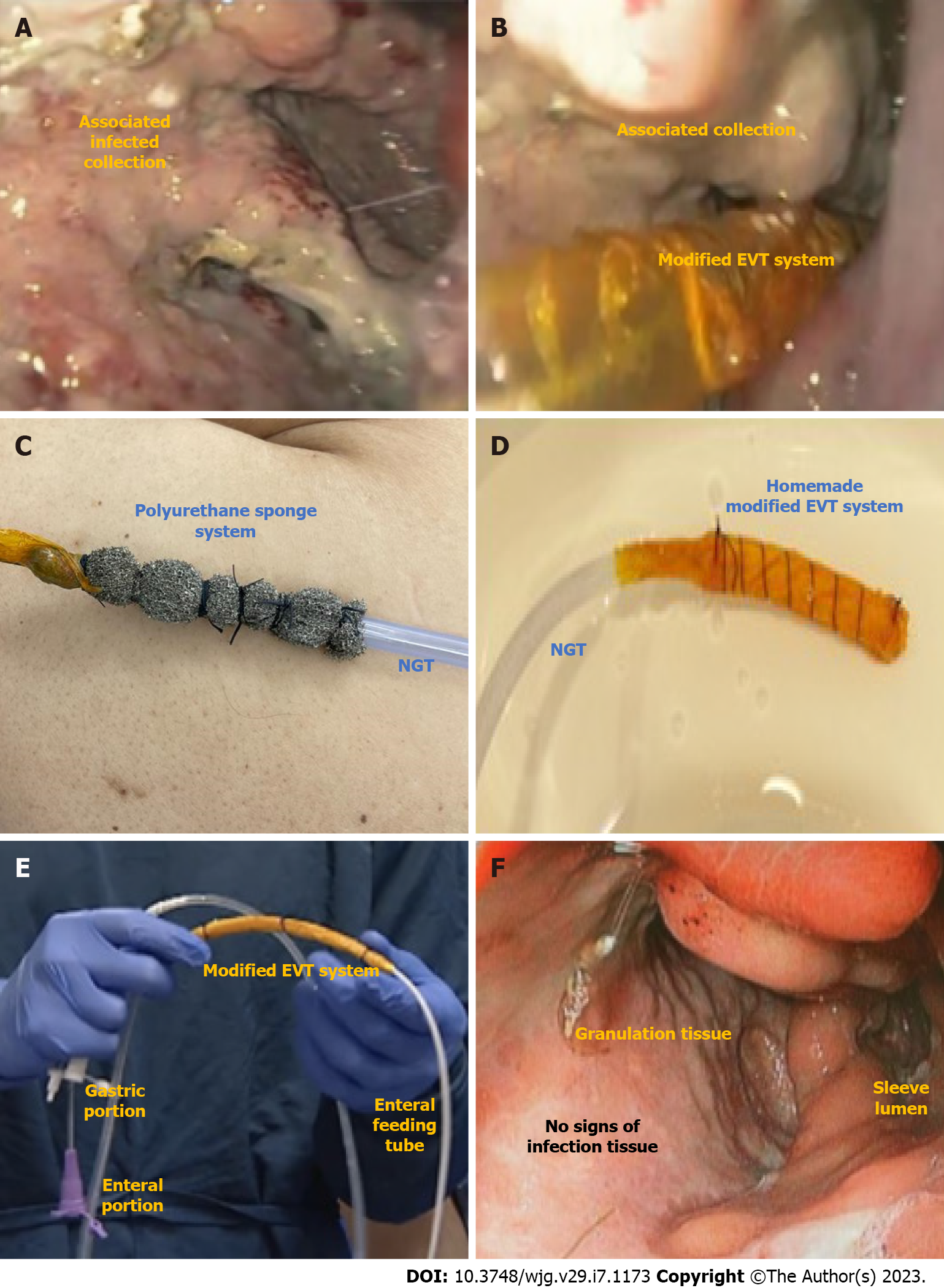
EVT is indicated for GI transmural defects (Figure 9). Its high efficacy is associated with its mechanism of action that involves micro-deformation, macro-deformation, changes in perfusion (angiogenesis), exudate control, and bacterial clearance[4]. The traditional technique involves the use of a polyurethane sponge connected to a nasogastric tube placed into the extraluminal compartment (intracavitary position) or into the GI lumen (intraluminal position). The nasogastric tube is then connected to a vacuum machine with continuous negative pressure (between -125 and -175 mmHg). It is important to understand that, when an associated collection is identified (Figure 9A), the EVT system must be placed inside the collection (intracavitary) (Figure 9B). Despite the high efficacy, the traditional system (Figure 9C) is associated with challenging placement and removal (through the mouth) leading to a prolonged procedure, and the need for frequent exchanges due to tissue ingrowth.
To overcome these limitations, the open pore film system was developed showing several benefits compared to the traditional sponge system, maintaining a high efficacy and safety profile. These advantages include easy placement (through the nares), reduction in procedure time, longer interval between EVT system exchanges, and a lower rate of AEs[61]. Despite these improvements, the high cost and limited availability of the open pore film remain barriers to its use and widespread adoption. Recently, a cost-effective modified-EVT manufactured from widely available materials and utilizing wall suction was described with promising results (Figure 9D)[62-65]. Furthermore, the use of intraluminal EVT via a triple-lumen tube was reported that allowed both drainage (gastric fenestrations) and nutrition (enteral portion) using a single tube through the nares (Figure 9E)[64,66].
Previous studies had already demonstrated very positive outcomes for EVT in the management of general upper GI leaks and fistulas, including with higher success rates and lower AEs rates when compared to SEMS[67]. A recent meta-analysis evaluated the use of the EVT in the management of leaks and fistulas after BMS demonstrating a pooled clinical success rate of 87.2% with 6% AE rate. In this study, the mean number of EVT system exchanges was 6.47 with a mean interval of 4.39 d[68]. Despite the satisfactory results, there is a concern regarding patients’ complaints due to the nasogastric tube and a possible prolonged hospital stay. Additionally, the risk of major bleeding related to the development of a fistula between the wound cavity and major blood vessels, including the aorta or its branches is a reported concern from some experts[4,69,70].
In our practice, the cost-effective modified EVT is the preferred approach due to its high efficacy, satisfactory patient acceptance, and safety benefit of avoiding sponge dislodgement and exchange related bleeding[71]. Based on our experience, the best indication for EVT is acute and early leaks with associated infected collections. In these cases, after clinical improvement and collection clearance associated with granulation tissue (Figure 9F), we do change the intracavitary EVT for EID with DPS allowing earlier hospital discharge or when the associated cavity is smaller than 3 cm, we change the intracavitary EVT for intraluminal EVT.
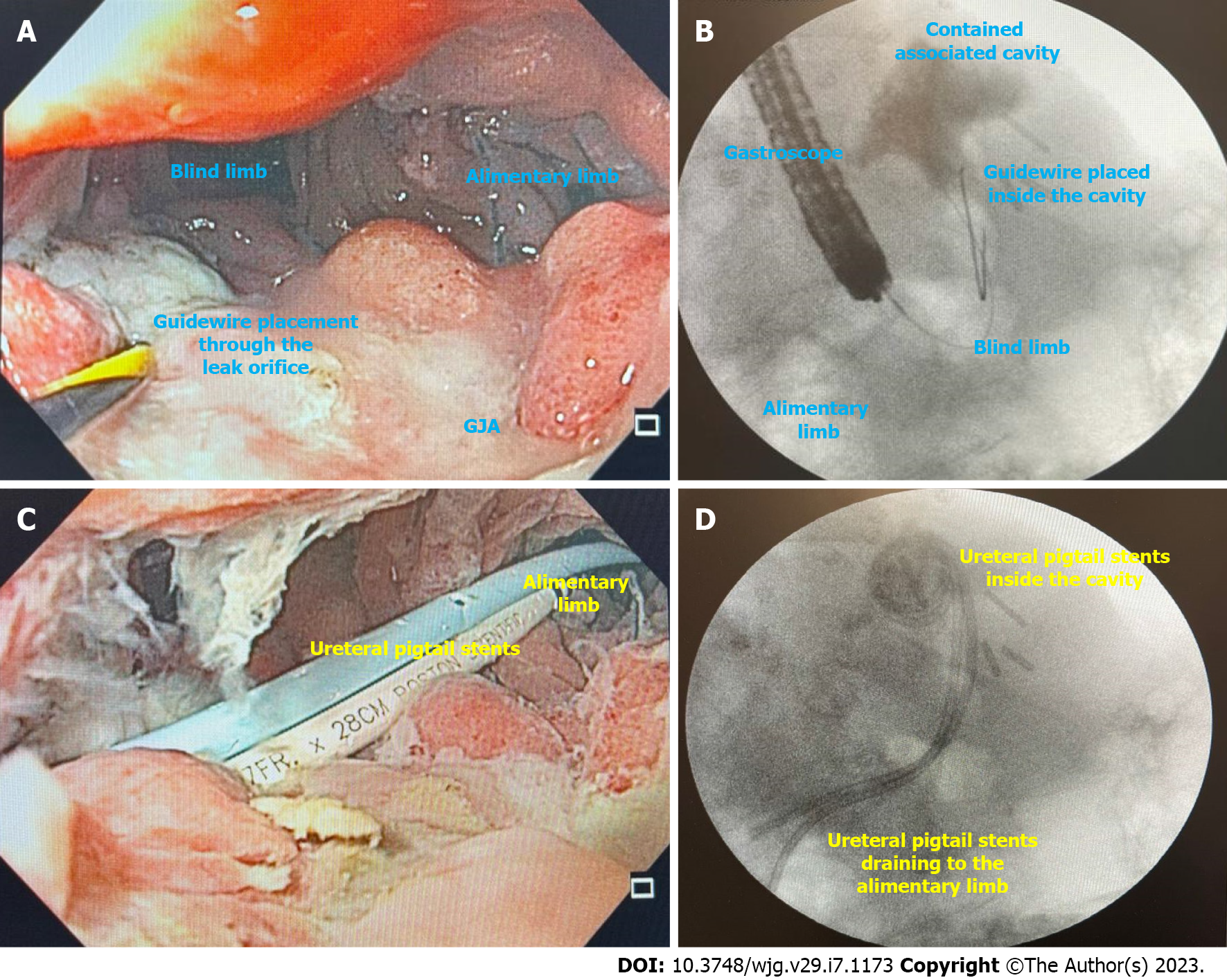
EID with DPS (Figure 10) is indicated for leaks and fistulas with associated fluid collections after BMS. The principle for internal drainage is based on the concept that when the pressure within the gastric lumen is lower than that of the perigastric collection, flow will be directed into the GI tract. Additionally, oral contents will preferentially flow through the gastric lumen.
For EID with DPS to be effective as a sole strategy, the following conditions should be absent: (1) Uncontained perigastric collections; (2) High intragastric pressure secondary to downstream stenosis; and (3) Existence of a gastropleural fistula. With drainage, the perigastric cavity, will typically contract until it is obliterated, resolving the leak. When evaluating the response to therapy, the ability to tolerate diet as well as a decrease in the size of the perigastric cavity are indicative of clinical success[72,73].
EID has been widely adopted, especially in Europe, with high clinical success rates for both acute and chronic leaks and fistulas and a low rate of AEs, allowing a shorter hospital stay.
The largest study evaluating EID with DPS for the treatment of complications after BMS included 617 patients, with a clinical success rate of 89.5% for leaks and 78.5%, for fistulas[74].
Placement of DPS is easy to learn (Figure 10A and B), demonstrated by high clinical success when performed by an endoscopist with (84.71%) or without (83.41%) experience in EID as demonstrated by a recent systematic review[72]. Additionally, it also presents satisfactory clinical success as a rescue therapy (78.05%)[72].
Defect closure is usually achieved after a long period of treatment. However, as most patients are not hospitalized, present no symptoms, receive oral diet, and return to their daily activities, the long treatment time is not considered an issue when compared to other techniques which are associated with symptoms related to the therapy, such as bariatric stents, or need for hospital stay, such as EVT[72].
AEs are reported in about 13.8% of patients, mostly due to stent migration, but also including perforation and bleeding[72].
In our experience, EID with DPS is the best approach for defects with associated collections in patients with no signs of sepsis. As most patients are not hospitalized, close follow-up is needed to avoid complications. Additionally, to minimize the risk of AEs related to the DPS, we prefer to use ureteral stents as they are more flexible and softer than biliary DPS, avoiding damage to tissue and vessels (Figure 10C and D)[75].
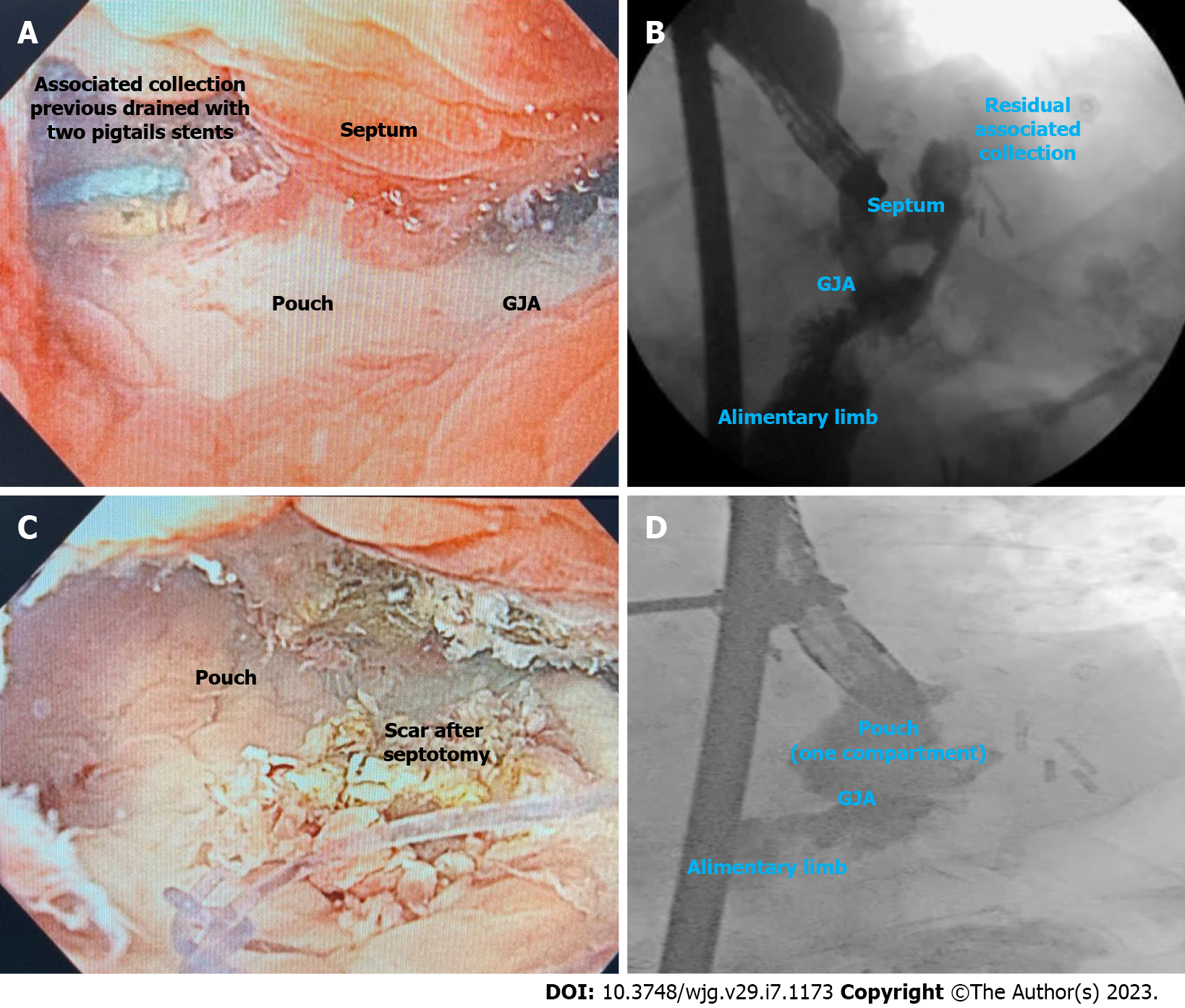
Septotomy (Figure 11) must be performed when a septum between the defect orifice/associated collection and the GI lumen is identified (Figure 11A and B). The principle of this therapy is similar to the Zenker´s diverticulotomy. The septum is sectioned to match the pressure of the leak or fistula orifice within the gastric chamber, providing better drainage (Figure 11C and D). The septotomy can be performed with endoscopic electrosurgical knives, through the scope scissors or argon plasma coagulation (APC), until the depth of the suture line without exceeding this limit (the cut must not extend beyond the base of the perigastric cavity) to avoid perforation[3,76]. It is important to state that in most cases, several sessions are needed.
Septotomy is associated with high clinical success rates and with low AEs in expert hands. In a study involving 27 patients with leaks and fistulas after BMS, including RYGB, LSG, and duodenal switch, the clinical success rate after one to six septotomies was 100%, with an average treatment time of 18.11 d[77]. Our experience reflects the results of this study. We do prefer to use APC as it is associated with a low rate of bleeding.
In our practice, the presence of a septum is the most common cause of late and chronic leaks and fistulas refractory to endoscopic treatment in non-specialized centers referred to our institution. Therefore, when treating a leak or fistula after BMS, attention to the presence of a septum and need for septotomy is required to achieve clinical success.
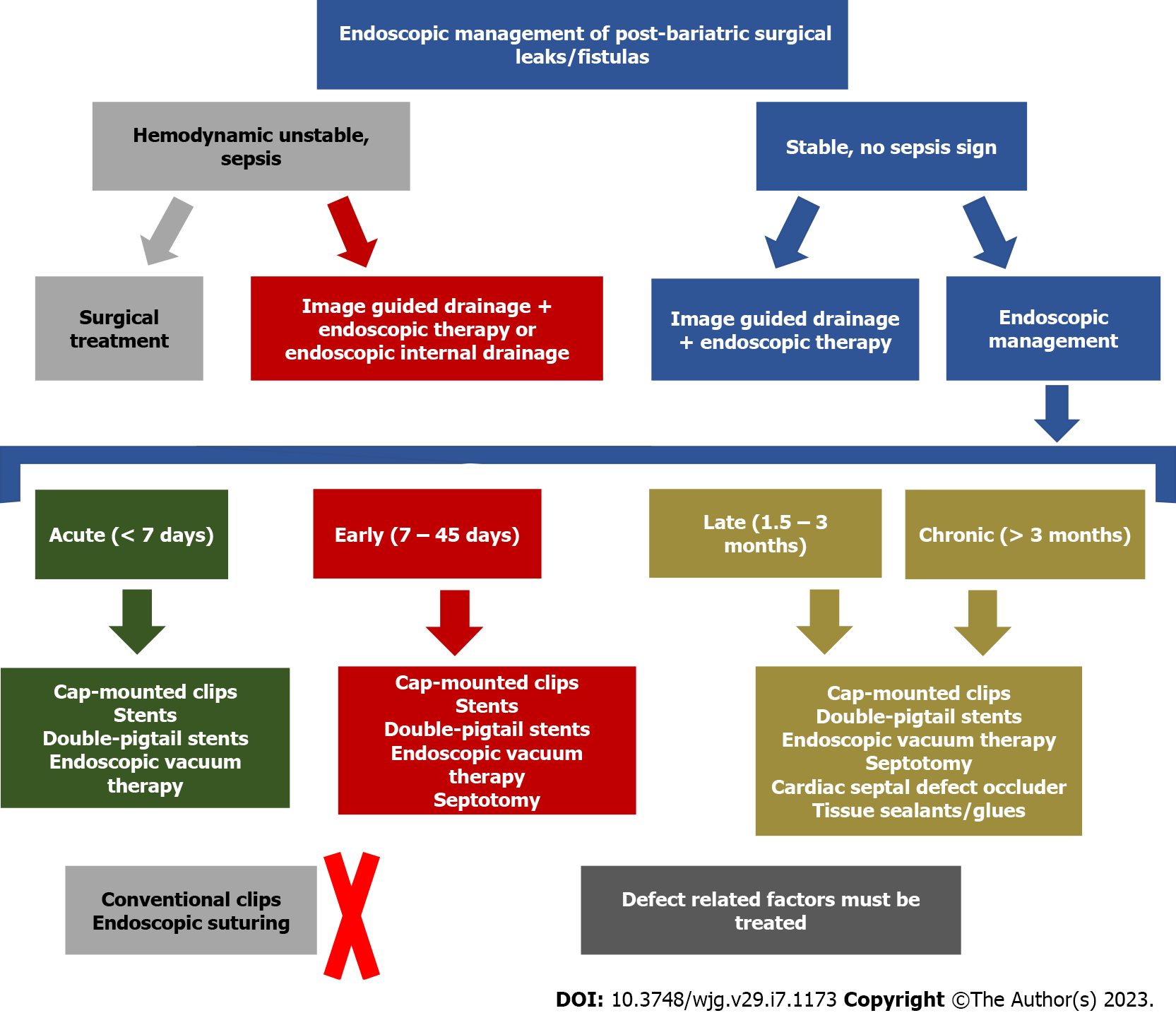
Based on the pathophysiology of leaks and fistulas after BMS, the appropriate therapy should be individualized, considering the patient´s clinical condition, defect characteristics (size, chronicity, associated collections, and health of the surrounding tissue), device availability, cost, patient preference, mechanism of action of each endoscopic therapy, one’s personal experience and comfort and local availability of experienced physicians. Although there is a paucity of high-quality studies to provide standardized treatment algorithms, we summarize our recommendations on the management of these challenging conditions in Table 4 and Figure 12 based on our large referral volume, and review of the available literature.
| Leak or fistula characteristics + patient clinical condition | Recommended management (first line approach) | Possible therapy (second line approach) | Possible endoscopic therapies based on defect characteristics |
| Acute and early leaks with undrained uncontained collection in unstable patients | Surgical lavage + external drainage (surgical placement) ± surgical repair ± endoscopic therapy (see column 4) | Image-guided external drainage + endoscopic therapy (see column 4) OR Intracavitary EVT | Defect < 2 cm: Cap mounted clips OR stents OR intraluminal EVT; Defect > 2 cm: Stents OR intraluminal EVT; If a septum is diagnosed (early): Septotomy must be performed |
| Acute and early leaks with undrained uncontained collections in stable patients (rare condition as most patients with undrained uncontained collections presents with peritonitis/sepsis) | Image-guided external drainage + endoscopic therapy (see column 4) | Surgical lavage + external drainage (surgical placement) ± surgical repair ± endoscopic therapy (see column 4) OR Intracavitary EVT | Defect < 2 cm: Cap mounted clip OR stents (prefer stents if associated with downstream stenosis) OR intraluminal EVT; Defect > 2 cm: Stents OR intraluminal EVT; If a septum is diagnosed (early): Septotomy must be performed |
| Acute and early leaks with undrained contained collections (both unstable or stable patients - most of these patients are stable due to the contained collection) | Endoscopic drainage techniques: Intracavitary EVT OR EID with DPS; If a septum is identified, septotomy must be performed | Image-guided external drainage + endoscopic therapy (see column 4) | Defect < 2 cm: Cap mounted clips OR stents (prefer stents if associate with downstream stenosis) OR intraluminal EVT; Defect > 2 cm: Stents OR intraluminal EVT; If a septum is diagnosed (early): Septotomy must be performed |
| Late and chronic leaks (both unstable or stable patients - most of these patients are stable as uncontained collection are extremely rare in late and chronic leaks) | Endoscopic drainage techniques: EID with DPS OREVT (intracavitary if associated collection > 3 cm); If a septum is identified, septotomy must be performed | Image-guided external drainage + endoscopic therapy (see column 4) OR Surgical approach | Defect < 2 cm: Cap mounted clips OR CSDO OR tissue sealants/glues (as an adjunctive therapy); Defect > 2 cm: CSDO OR tissue sealants/glues (as an adjunctive therapy) |
| Late and chronic fistulas (both unstable or stable patients - most of these patients are stable) | Endoscopic therapy (see column 4); Cytology brushing or APC to loosen granulation tissue before endoscopic therapy is helpful; If a septum is identified, septotomy must be performed | Surgical approach | Defect < 2 cm: CSDO ± tissue sealants/glues OR tissue sealants/glues ± cap mounted clips OR tissue sealants/glues + intraluminal EVT; -Defect > 2 cm: CSDO ± tissue sealants/glues OR tissue sealants/glues + intraluminal EVT, |
| Late and chronic gastro-gastric fistula | Defect < 10 mm: Endoscopic therapy (see column 4); Defect > 10 mm: Surgical approach | Surgical approach after endoscopic management failure | APC ± CSDO ORAPC + suturing OR APC + cap mounted clip |
The management of post bariatric surgery complications is challenging as patients have obesity-mediated chronic pro-inflammatory state and are in a catabolic state related to weight loss. Additionally, most patients have comorbidities, such as T2DM, arterial hypertension, among others. The most feared complication is leak, as an infection in these patients can be life-threatening or severely morbid, especially when early identification and management is not achieved. Although laboratory tests and imaging are important for diagnosis, patient clinical conditions must be the primary consideration. If a patient presents with tachycardia, fever, and intense abdominal pain, a surgical revision should not be postponed.
In cases of acute and early leaks associated with infection, there can be a need for admission to an intensive care unit and long-term hospital stay is often needed. Additionally, some patients cannot take an oral diet and there is a need for enteral nutrition or parenteral nutrition. Unfortunately, long-term inpatient related diseases such as pneumonia, deep venous thrombosis, healthcare-associated infections, and others, can affect these patients. Therefore, multidisciplinary care is required, including surgeons, endoscopists, radiologists, intensivists, infectious disease experts, nutrition experts, nurses, physiotherapists, and other specialists contributing to successful outcomes. Ideally, these patients should be treated in a tertiary referral center given the complexity and relative rarity of this disease state.
For late and chronic leaks and fistulas, almost all patients present in a stable condition, most of them need several procedures and longer treatment time to achieve defect closure. Despite the need for more sessions, as these patients are stable, they do not need to be hospitalized in most cases and can be discharged to attend subsequent elective endoscopic procedures while they carry on with their normal activities.
Endoscopy has evolved to become first line therapy for the treatment of post-bariatric leaks and fistulas, except in unstable patients with undrained, uncontained collections (peritonitis) where a surgical approach is preferred. There are several available endoscopic devices and techniques with different mechanisms of actions, including closing, covering, and draining therapies. When determining the appropriate endoscopic technique, fundamental principles must be considered, such as draining of associated collections and treatment of related factors such as dilation of downstream stenosis and foreign body removal. It is mandatory that endoscopists incorporating management of BMS complications into their clinical practice have expertise in several advanced therapeutic endoscopic techniques as well as knowledge in using fluoroscopy, managing percutaneous drains, and also understanding of post-surgical anatomy and pathophysiology concepts for origin and maintenance of leaks and fistulas. It is also important to understand that there is no gold standard approach for these patients and usually more than one endoscopic intervention is required, especially in a chronic scenario. Furthermore, combined therapies may be required for some patients to achieve clinical success.
In summary, a multidisciplinary team and individualized evaluation considering patient and defect characteristics, available resources, local and personal experience, and knowledge of fundamental principles and mechanisms of action of each technique is essential to choosing the best approach for the management of post-bariatric surgical leaks and fistulas. With a committed approach, high rates of leak and fistula closure can be achieved.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Chen SY, China; Corvino A, Italy; Toskas A, United Kingdom S-Editor: Liu GL L-Editor: A P-Editor: Liu GL
| 1. | Ward ZJ, Bleich SN, Cradock AL, Barrett JL, Giles CM, Flax C, Long MW, Gortmaker SL. Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. N Engl J Med. 2019;381:2440-2450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 756] [Cited by in RCA: 1300] [Article Influence: 216.7] [Reference Citation Analysis (0)] |
| 2. | American Society for Metabolic and Bariatric Surgery (ASMBS). Estimate of bariatric surgery numbers, 2011-2019. [Internet] [accessed 30 May 2019]. Available from: https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers. |
| 3. | de Moura DTH, Dantas ACB, Ribeiro IB, McCarty TR, Takeda FR, Santo MA, Nahas SC, de Moura EGH. Status of bariatric endoscopy-what does the surgeon need to know? World J Gastrointest Surg. 2022;14:185-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | de Moura DTH, de Moura BFBH, Manfredi MA, Hathorn KE, Bazarbashi AN, Ribeiro IB, de Moura EGH, Thompson CC. Role of endoscopic vacuum therapy in the management of gastrointestinal transmural defects. World J Gastrointest Endosc. 2019;11:329-344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 110] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (6)] |
| 5. | de Moura DTH, Sachdev AH, Thompson CC. Endoscopic Full-Thickness Defects and Closure Techniques. Curr Treat Options Gastroenterol. 2018;16:386-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 6. | Rausa E, Bonavina L, Asti E, Gaeta M, Ricci C. Rate of Death and Complications in Laparoscopic and Open Roux-en-Y Gastric Bypass. A Meta-analysis and Meta-regression Analysis on 69,494 Patients. Obes Surg. 2016;26:1956-1963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Jones KB Jr, Afram JD, Benotti PN, Capella RF, Cooper CG, Flanagan L, Hendrick S, Howell LM, Jaroch MT, Kole K, Lirio OC, Sapala JA, Schuhknecht MP, Shapiro RP, Sweet WA, Wood MH. Open versus laparoscopic Roux-en-Y gastric bypass: a comparative study of over 25,000 open cases and the major laparoscopic bariatric reported series. Obes Surg. 2006;16:721-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Burgos AM, Braghetto I, Csendes A, Maluenda F, Korn O, Yarmuch J, Gutierrez L. Gastric leak after laparoscopic-sleeve gastrectomy for obesity. Obes Surg. 2009;19:1672-1677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 193] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 9. | Aurora AR, Khaitan L, Saber AA. Sleeve gastrectomy and the risk of leak: a systematic analysis of 4,888 patients. Surg Endosc. 2012;26:1509-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 437] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 10. | Zellmer JD, Mathiason MA, Kallies KJ, Kothari SN. Is laparoscopic sleeve gastrectomy a lower risk bariatric procedure compared with laparoscopic Roux-en-Y gastric bypass? Am J Surg. 2014;208:903-10; discussion 909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 115] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 11. | Birkmeyer JD, Finks JF, O'Reilly A, Oerline M, Carlin AM, Nunn AR, Dimick J, Banerjee M, Birkmeyer NJ; Michigan Bariatric Surgery Collaborative. Surgical skill and complication rates after bariatric surgery. N Engl J Med. 2013;369:1434-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 961] [Cited by in RCA: 1062] [Article Influence: 88.5] [Reference Citation Analysis (0)] |
| 12. | Mickevicius A, Sufi P, Heath D. Factors predicting the occurrence of a gastrojejunal anastomosis leak following gastric bypass. Wideochir Inne Tech Maloinwazyjne. 2014;9:436-440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Wittgrove AC, Clark GW. Laparoscopic gastric bypass, Roux-en-Y- 500 patients: technique and results, with 3-60 mo follow-up. Obes Surg. 2000;10:233-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 478] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 14. | Kim J, Azagury D, Eisenberg D, DeMaria E, Campos GM; American Society for Metabolic and Bariatric Surgery Clinical Issues Committee. ASMBS position statement on prevention, detection, and treatment of gastrointestinal leak after gastric bypass and sleeve gastrectomy, including the roles of imaging, surgical exploration, and nonoperative management. Surg Obes Relat Dis. 2015;11:739-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 15. | Kumbhari V, Cummings DE, Kalloo AN, Schauer PR. AGA Clinical Practice Update on Evaluation and Management of Early Complications After Bariatric/Metabolic Surgery: Expert Review. Clin Gastroenterol Hepatol. 2021;19:1531-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Benedix F, Benedix DD, Knoll C, Weiner R, Bruns C, Manger T, Stroh C; Obesity Surgery Working Group; Competence Network Obesity. Are there risk factors that increase the rate of staple line leakage in patients undergoing primary sleeve gastrectomy for morbid obesity? Obes Surg. 2014;24:1610-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 17. | Weller WE, Rosati C. Comparing outcomes of laparoscopic versus open bariatric surgery. Ann Surg. 2008;248:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 18. | Aggarwal S, Bhattacharjee H, Chander Misra M. Practice of routine intraoperative leak test during laparoscopic sleeve gastrectomy should not be discarded. Surg Obes Relat Dis. 2011;7:e24-e25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Miller KA, Pump A. Use of bioabsorbable staple reinforcement material in gastric bypass: a prospective randomized clinical trial. Surg Obes Relat Dis. 2007;3:417-21; discussion 422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Shikora SA. The use of staple-line reinforcement during laparoscopic gastric bypass. Obes Surg. 2004;14:1313-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Musella M, Milone M, Maietta P, Bianco P, Pisapia A, Gaudioso D. Laparoscopic sleeve gastrectomy: efficacy of fibrin sealant in reducing postoperative bleeding. A randomized controlled trial. Updates Surg. 2014;66:197-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Aggarwal S, Sharma AP, Ramaswamy N. Outcome of laparoscopic sleeve gastrectomy with and without staple line oversewing in morbidly obese patients: a randomized study. J Laparoendosc Adv Surg Tech A. 2013;23:895-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Kavuturu S, Rogers AM, Haluck RS. Routine drain placement in Roux-en-Y gastric bypass: an expanded retrospective comparative study of 755 patients and review of the literature. Obes Surg. 2012;22:177-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Liscia G, Scaringi S, Facchiano E, Quartararo G, Lucchese M. The role of drainage after Roux-en-Y gastric bypass for morbid obesity: a systematic review. Surg Obes Relat Dis. 2014;10:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Benosman H, Rahmi G, Perrod G, Bruzzi M, Samaha E, Vienne A, Cuenod CA, Chevallier JM, Douard R, Cellier C. Endoscopic Management of Post-bariatric Surgery Fistula: a Tertiary Care Center Experience. Obes Surg. 2018;28:3910-3915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | de Moura DTH, de Moura EGH, Neto MG, Jirapinyo P, Teixeira N, Orso I, Quadros LG, Amorim A, Medeiros F, Neto DR, de Siqueira Neto J, Albano A, de Sousa LH, Almeida D, Marchetti IA, Ivano F, de Lima JHF, Falcão M, Thompson CC. Outcomes of a novel bariatric stent in the management of sleeve gastrectomy leaks: a multicenter study. Surg Obes Relat Dis. 2019;15:1241-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Rosenthal RJ; International Sleeve Gastrectomy Expert Panel, Diaz AA, Arvidsson D, Baker RS, Basso N, Bellanger D, Boza C, El Mourad H, France M, Gagner M, Galvao-Neto M, Higa KD, Himpens J, Hutchinson CM, Jacobs M, Jorgensen JO, Jossart G, Lakdawala M, Nguyen NT, Nocca D, Prager G, Pomp A, Ramos AC, Rosenthal RJ, Shah S, Vix M, Wittgrove A, Zundel N. International Sleeve Gastrectomy Expert Panel Consensus Statement: best practice guidelines based on experience of >12,000 cases. Surg Obes Relat Dis. 2012;8:8-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 713] [Cited by in RCA: 713] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 28. | Baptista A, Hourneaux De Moura DT, Jirapinyo P, Hourneaux De Moura EG, Gelrud A, Kahaleh M, Salinas A, Sabagh LC, Ospina A, Rincones VZ, Doval R, Bandel JW, Thompson CC. Efficacy of the cardiac septal occluder in the treatment of post-bariatric surgery leaks and fistulas. Gastrointest Endosc. 2019;89:671-679.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Csendes A, Burgos AM, Braghetto I. Classification and management of leaks after gastric bypass for patients with morbid obesity: a prospective study of 60 patients. Obes Surg. 2012;22:855-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Larsen M, Kozarek R. Therapeutic endoscopy for the treatment of post-bariatric surgery complications. World J Gastroenterol. 2022;28:199-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (5)] |
| 31. | Bestetti AM, Boghossian MB, Hirsch BS, McCarty TR, Santo MA, de Moura EGH, de Moura DTH. Multiple Endoscopic Therapies for Treatment of Chronic Post-bariatric Surgery Gastropleural Fistula. Obes Surg. 2022;32:3206-3207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 32. | de Moura DTH, Ribeiro IB, Funari MP, Baptista A, Thompson CC, de Moura EGH. Novel use of a cardiac septal occluder to treat a chronic recalcitrant bariatric fistula after Roux-en-Y gastric bypass. Endoscopy. 2019;51:E111-E112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | de Moura DTH, da Ponte-Neto AM, Hathorn KE, do Monte Junior ES, Baptista A, Ribeiro IB, Thompson CC, De Moura EGH. Novel Endoscopic Management of a Chronic Gastro-Gastric Fistula Using a Cardiac Septal Defect Occluder. Obes Surg. 2020;30:3253-3254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Catelli A, Corvino A, Loiudice G, Tucci A, Quarantelli M, Venetucci P. Diagnostic imaging in the diagnosis of acute complications of bariatric surgery. Pol J Radiol. 2021;86:e102-e111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 35. | Tan JT, Kariyawasam S, Wijeratne T, Chandraratna HS. Diagnosis and management of gastric leaks after laparoscopic sleeve gastrectomy for morbid obesity. Obes Surg. 2010;20:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 36. | Gonzalez R, Sarr MG, Smith CD, Baghai M, Kendrick M, Szomstein S, Rosenthal R, Murr MM. Diagnosis and contemporary management of anastomotic leaks after gastric bypass for obesity. J Am Coll Surg. 2007;204:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 162] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 37. | Cho J, Sahakian AB. Endoscopic Closure of Gastrointestinal Fistulae and Leaks. Gastrointest Endosc Clin N Am. 2018;28:233-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | de Moura DTH, de Freitas Júnior JR, de Souza GMV, de Oliveira GHP, McCarty TR, Thompson CC, de Moura EGH. Endoscopic management of acute leak after sleeve gastrectomy: principles and techniques. Endoscopy. 2022;54:E327-E328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Bestetti AM, Santo MA, Trasolini RP, de Freitas Junior JR, Hirsch BS, de Moura EGH, de Moura DTH. Sequential Endoscopic Therapies for Treatment of Complex Gastrointestinal Transmural Leak Following Bariatric Surgery. Obes Surg. 2022;32:4113-4114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 40. | Jaruvongvanich V, Matar R, Storm AC, Beran A, Malandris K, Maselli DB, Vargas EJ, Kellogg TA, Buttar NS, McKenzie TJ, Abu Dayyeh BK. Endoscopic management of refractory leaks and fistulas after bariatric surgery with long-term follow-up. Surg Endosc. 2021;35:2715-2723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 41. | Nedelcu M, Danan M, Noel P, Gagner M, Nedelcu A, Carandina S. Surgical management for chronic leak following sleeve gastrectomy: Review of literature. Surg Obes Relat Dis. 2019;15:1844-1849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 42. | Moon RC, Shah N, Teixeira AF, Jawad MA. Management of staple line leaks following sleeve gastrectomy. Surg Obes Relat Dis. 2015;11:54-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 43. | Merrifield BF, Lautz D, Thompson CC. Endoscopic repair of gastric leaks after Roux-en-Y gastric bypass: a less invasive approach. Gastrointest Endosc. 2006;63:710-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 91] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 44. | Mercky P, Gonzalez JM, Aimore Bonin E, Emungania O, Brunet J, Grimaud JC, Barthet M. Usefulness of over-the-scope clipping system for closing digestive fistulas. Dig Endosc. 2015;27:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 45. | Shoar S, Poliakin L, Khorgami Z, Rubenstein R, El-Matbouly M, Levin JL, Saber AA. Efficacy and Safety of the Over-the-Scope Clip (OTSC) System in the Management of Leak and Fistula After Laparoscopic Sleeve Gastrectomy: a Systematic Review. Obes Surg. 2017;27:2410-2418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 46. | Bonanomi G, Prince JM, McSteen F, Schauer PR, Hamad GG. Sealing effect of fibrin glue on the healing of gastrointestinal anastomoses: implications for the endoscopic treatment of leaks. Surg Endosc. 2004;18:1620-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 47. | Maluf-Filho F, Hondo F, Halwan B, de Lima MS, Giordano-Nappi JH, Sakai P. Endoscopic treatment of Roux-en-Y gastric bypass-related gastrocutaneous fistulas using a novel biomaterial. Surg Endosc. 2009;23:1541-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 48. | Rogalski P, Swidnicka-Siergiejko A, Wasielica-Berger J, Zienkiewicz D, Wieckowska B, Wroblewski E, Baniukiewicz A, Rogalska-Plonska M, Siergiejko G, Dabrowski A, Daniluk J. Endoscopic management of leaks and fistulas after bariatric surgery: a systematic review and meta-analysis. Surg Endosc. 2021;35:1067-1087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 49. | Assalia A, Ilivitzki A, Ofer A, Suissa A, Manassa E, Khamaysi I, Mahajna A. Management of gastric fistula complicating laparoscopic sleeve gastrectomy with biological glue in a combined percutaneous and endoscopic approach. Surg Obes Relat Dis. 2018;14:1093-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 50. | Mukewar S, Kumar N, Catalano M, Thompson C, Abidi W, Harmsen W, Enders F, Gostout C. Safety and efficacy of fistula closure by endoscopic suturing: a multi-center study. Endoscopy. 2016;48:1023-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 51. | Puig CA, Waked TM, Baron TH Sr, Wong Kee Song LM, Gutierrez J, Sarr MG. The role of endoscopic stents in the management of chronic anastomotic and staple line leaks and chronic strictures after bariatric surgery. Surg Obes Relat Dis. 2014;10:613-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 52. | Okazaki O, Bernardo WM, Brunaldi VO, Junior CCC, Minata MK, de Moura DTH, de Souza TF, Campos JM, Santo MA, de Moura EGH. Efficacy and Safety of Stents in the Treatment of Fistula After Bariatric Surgery: a Systematic Review and Meta-analysis. Obes Surg. 2018;28:1788-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 53. | Law R, Prabhu A, Fujii-Lau L, Shannon C, Singh S. Stent migration following endoscopic suture fixation of esophageal self-expandable metal stents: a systematic review and meta-analysis. Surg Endosc. 2018;32:675-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 54. | Madruga Neto AC, Brunaldi VO, Okazaki O, Santo Filho MA, Miranda Neto AA, Anapaz VL, de Moura EGH. Stent migration requiring surgical removal: a serious adverse event after bariatric megastent placement. Endoscopy. 2018;50:E344-E345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 55. | de Moura DTH, Brunaldi VO, Minata M, Riccioppo D, Santo MA, de Moura EGH. Endoscopic vacuum therapy for a large esophageal perforation after bariatric stent placement. VideoGIE. 2018;3:346-348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 56. | Hamid HKS, Emile SH, Saber AA, Dincer M, de Moura DTH, Gilissen LPL, Almadi MA, Montuori M, Vix M, Perisse LGS, Quezada N, Garofalo F, Pescarus R. Customized bariatric stents for sleeve gastrectomy leak: are they superior to conventional esophageal stents? Surg Endosc. 2021;35:1025-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 57. | Sánchez-Luna SA, De Moura EGH, De Moura DTH. Bigger is not always better for the endoscopic treatment of sleeve gastrectomy (SG) leaks using fully covered stents. Obes Surg. 2022;32:1764-1767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 58. | Geva T, Martins JD, Wald RM. Atrial septal defects. Lancet. 2014;383:1921-1932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 227] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 59. | De Moura DTH, Baptista A, Jirapinyo P, De Moura EGH, Thompson C. Role of Cardiac Septal Occluders in the Treatment of Gastrointestinal Fistulas: A Systematic Review. Clin Endosc. 2020;53:37-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 60. | de Moura DTH, Boghossian MB, Hirsch BS, McCarty TR, Baptista AJ, de Moura EGH. Long-term endoscopic follow-up after closure of a post-bariatric surgery fistula with a cardiac septal defect occluder. Endoscopy. 2022;54:E127-E128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 61. | Loske G, Schorsch T, Rucktaeschel F, Schulze W, Riefel B, van Ackeren V, Mueller CT. Open-pore film drainage (OFD): a new multipurpose tool for endoscopic negative pressure therapy (ENPT). Endosc Int Open. 2018;6:E865-E871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 62. | de Moura DTH, Hirsch BS, Do Monte Junior ES, McCarty TR, de Medeiros FS, Thompson CC, de Moura EGH. Cost-effective modified endoscopic vacuum therapy for the treatment of gastrointestinal transmural defects: step-by-step process of manufacturing and its advantages. VideoGIE. 2021;6:523-528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 63. | de Moura DTH, do Monte Junior ES, Hathorn KE, Ribeiro IB, de Medeiros FS, Thompson CC, de Moura EGH. The use of novel modified endoscopic vacuum therapies in the management of a transmural rectal wall defect. Endoscopy. 2021;53:E27-E28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 64. | de Moura DTH, Hirsch BS, Boghossian MB, de Medeiros FS, McCarty TR, Thompson CC, de Moura EGH. Low-cost modified endoscopic vacuum therapy using a triple-lumen tube allows nutrition and drainage for treatment of an early post-bariatric surgery leak. Endoscopy. 2022;54:E376-E377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 65. | de Moura DTH, do Monte Junior ES, Hathorn KE, de Medeiros FS, Thompson CC, de Moura EGH. Modified endoscopic vacuum therapy in the management of a duodenal transmural defect. Endoscopy. 2021;53:E17-E18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 66. | Loske G, Aumiller J, Rucktäschel F, Schorsch T. Spontaneous perforation of an intramural esophageal pseudodiverticulosis treated with intraluminal endoscopic vacuum therapy using a double-lumen vacuum drainage with intestinal feeding tube. Endoscopy. 2016;48 Suppl 1:E154-E155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 67. | do Monte Junior ES, de Moura DTH, Ribeiro IB, Hathorn KE, Farias GFA, Turiani CV, Medeiros FS, Bernardo WM, de Moura EGH. Endoscopic vacuum therapy versus endoscopic stenting for upper gastrointestinal transmural defects: Systematic review and meta-analysis. Dig Endosc. 2021;33:892-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 68. | Intriago JMV, de Moura DTH, do Monte Junior ES, Proença IM, Ribeiro IB, Sánchez-Luna SA, Bernardo WM, de Moura EGH. Endoscopic Vacuum Therapy (EVT) for the Treatment of Post-Bariatric Surgery Leaks and Fistulas: a Systematic Review and Meta-analysis. Obes Surg. 2022;32:3435-3451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 69. | Livingstone I, Pollock L, Sgromo B, Mastoridis S. Current Status of Endoscopic Vacuum Therapy in the Management of Esophageal Perforations and Post-Operative Leaks. Clin Endosc. 2021;54:787-797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 70. | Laukoetter MG, Mennigen R, Neumann PA, Dhayat S, Horst G, Palmes D, Senninger N, Vowinkel T. Successful closure of defects in the upper gastrointestinal tract by endoscopic vacuum therapy (EVT): a prospective cohort study. Surg Endosc. 2017;31:2687-2696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 132] [Article Influence: 14.7] [Reference Citation Analysis (1)] |
| 71. | Sánchez-Luna SA, Thompson CC, De Moura EGH, de Medeiros FS, De Moura DTH. Modified endoscopic vacuum therapy: Are we ready for prime time? Gastrointest Endosc. 2022;95:1281-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 72. | Giuliani A, Romano L, Marchese M, Necozione S, Cianca G, Schietroma M, Carlei F. Gastric leak after laparoscopic sleeve gastrectomy: management with endoscopic double pigtail drainage. A systematic review. Surg Obes Relat Dis. 2019;15:1414-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 73. | Dammaro C, Lainas P, Dumont JL, Tranchart H, Donatelli G, Dagher I. Endoscopic Internal Drainage Coupled to Prompt External Drainage Mobilization Is an Effective Approach for the Treatment of Complicated Cases of Sleeve Gastrectomy. Obes Surg. 2019;29:2929-2935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 74. | Donatelli G, Spota A, Cereatti F, Granieri S, Dagher I, Chiche R, Catheline JM, Pourcher G, Rebibo L, Calabrese D, Msika S, Dammaro C, Tranchart H, Lainas P, Tuszynski T, Pacini F, Arienzo R, Chevallier JM, Trelles N, Lazzati A, Paolino L, Papini F, Torcivia A, Genser L, Arapis K, Soprani A, Randone B, Chosidow D, Bouillot JL, Marmuse JP, Dumont JL. Endoscopic internal drainage for the management of leak, fistula, and collection after sleeve gastrectomy: our experience in 617 consecutive patients. Surg Obes Relat Dis. 2021;17:1432-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 75. | Sánchez-Luna SA, Guimãraes Hourneaux De Moura E, Sena de Medeiros F, Turiani Hourneaux De Moura D. Does it matter which plastic stents we use for the treatment of post-surgical leaks? Rev Esp Enferm Dig. 2022;114:181-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 76. | Mahadev S, Kumbhari V, Campos JM, Galvao Neto M, Khashab MA, Chavez YH, Bessler M, Gonda TA. Endoscopic septotomy: an effective approach for internal drainage of sleeve gastrectomy-associated collections. Endoscopy. 2017;49:504-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 77. | Baretta G, Campos J, Correia S, Alhinho H, Marchesini JB, Lima JH, Neto MG. Bariatric postoperative fistula: a life-saving endoscopic procedure. Surg Endosc. 2015;29:1714-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |