Published online Feb 14, 2023. doi: 10.3748/wjg.v29.i6.967
Peer-review started: October 16, 2022
First decision: November 3, 2022
Revised: November 14, 2022
Accepted: January 30, 2023
Article in press: January 30, 2023
Published online: February 14, 2023
Processing time: 116 Days and 18.6 Hours
A growing body of evidence from multiple areas proposes that periodontal disease, accompanied by oral inflammation and pathological changes in the microbiome, induces gut dysbiosis and is involved in the pathogenesis of nonalcoholic fatty liver disease (NAFLD). A subgroup of NAFLD patients have a severely progressive form, namely nonalcoholic steatohepatitis (NASH), which is characterized by histological findings that include inflammatory cell infiltration and fibrosis. NASH has a high risk of further progression to cirrhosis and hepatocellular carcinoma. The oral microbiota may serve as an endogenous reservoir for gut microbiota, and transport of oral bacteria through the gastro-intestinal tract can set up a gut microbiome dysbiosis. Gut dysbiosis increases the production of potential hepatotoxins, including lipopolysaccharide, ethanol, and other volatile organic compounds such as acetone, phenol and cyclopentane. Moreover, gut dysbiosis increases intestinal permeability by disrupting tight junctions in the intestinal wall, leading to enhanced translocation of these hepatotoxins and enteric bacteria into the liver through the portal circulation. In particular, many animal studies support that oral administration of Porphyromonas gingivalis, a typical periodontopathic bacterium, induces disturbances in glycolipid metabolism and inflammation in the liver with gut dysbiosis. NAFLD, also known as the hepatic phenotype of metabolic syndrome, is strongly associated with metabolic complications, such as obesity and diabetes. Periodontal disease also has a bidirectional relationship with metabolic syndrome, and both diseases may induce oral and gut microbiome dysbiosis with insulin resistance and systemic chronic inflammation cooperatively. In this review, we will describe the link between periodontal disease and NAFLD with a focus on basic, epidemiological, and clinical studies, and discuss potential mechanisms linking the two diseases and possible therapeutic approaches focused on the microbiome. In conclusion, it is presumed that the pathogenesis of NAFLD involves a complex crosstalk between periodontal disease, gut microbiota, and metabolic syndrome. Thus, the conventional periodontal treatment and novel microbiome-targeted therapies that include probiotics, prebiotics and bacteriocins would hold great promise for preventing the onset and progression of NAFLD and subsequent complications in patients with periodontal disease.
Core Tip: A growing body of evidence from multiple areas highlights that periodontal disease, accompanied by oral inflammation and pathological changes in the microbiome, induces gut dysbiosis and is involved in the pathogenesis of Nonalcoholic fatty liver disease (NAFLD). Thus, the conventional periodontal treatment and microbiome-targeted therapies that include probiotics, prebiotics and bacteriocin would hold great promise for preventing the onset and progression of NAFLD. In this review, we will describe the link between periodontal disease and NAFLD with a focus on basic, epidemiological, and clinical studies, and discuss potential mechanisms linking the two diseases and possible therapeutic approaches focused on the microbiome.
- Citation: Kuraji R, Shiba T, Dong TS, Numabe Y, Kapila YL. Periodontal treatment and microbiome-targeted therapy in management of periodontitis-related nonalcoholic fatty liver disease with oral and gut dysbiosis. World J Gastroenterol 2023; 29(6): 967-996
- URL: https://www.wjgnet.com/1007-9327/full/v29/i6/967.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i6.967
Periodontal disease is an inflammatory disease that is induced by a structural and metabolic imbalance of the oral microbiome, namely an oral dysbiosis, and is characterized by significant periodontal tissue destruction and tooth loss[1-3]. Until recently, specific periodontopathic bacteria, such as the Red Complex, were thought to be the major pathogens of periodontal disease[4,5], but the success of novel methods focused on the studying the oral microbiome with next-generation sequencing approaches has revealed the possibility that a variety of previously unknown bacteria, fungi, and even viruses are associated with the periodontal disease[6-9].
Periodontal disease has also been reported to adversely affect the pathogenesis of various systemic diseases, including diabetes, coronary vascular disease, rheumatoid arthritis, and cancer[10,11]. As a major mechanism, it has been proposed that pro-inflammatory cytokines, periodontal pathogens, and their microbial components spread out from damaged periodontal tissues sites into the systemic circulation and thereby reach and affect other organ sites[1,12-14]. Another idea has also emerged in the last decade that there is enteric translocation following the development of gut dysbiosis that originates from oral bacteria, and this is another pathway linking periodontitis and systemic disease[11]. Many studies have shown that swallowed periodontopathic bacteria can pass through the gastric acid barrier and reach the gut, thereby shifting the gut microbiome to an unhealthy state[15-19]. In addition, even oral commensal bacteria that are not normally pathogenic may proliferate and become established in the gut environment due to periodontal inflammation, and may even manifest pathogenic properties[20,21].
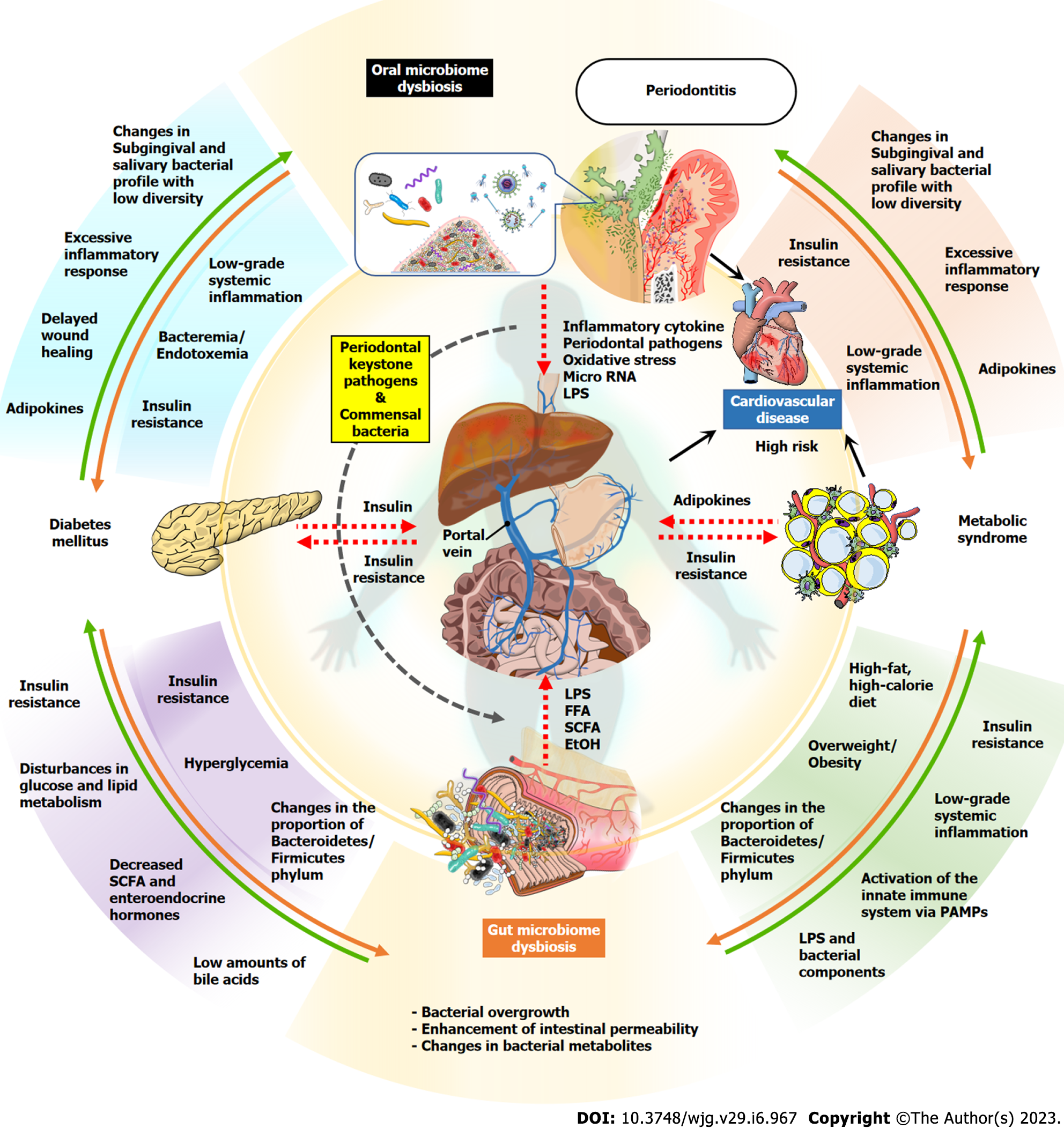
In the context of the association between periodontal disease and gut dysbiosis, nonalcoholic fatty liver disease (NAFLD) has received particular attention in recent years[11,22] (Figure 1). NAFLD is currently the most prevalent chronic liver disease worldwide, with a marked increase in individuals with metabolic syndrome[23], and it is defined as a fatty liver condition diagnosed upon histological observation or image analysis, but other liver diseases/conditions, such as a history of alcohol consumption, viral hepatitis, and drug-induced liver injury are excluded[24,25]. The majority of NAFLD patients (approximately 80%) have a good prognosis and simply have a fatty liver (NAFL), whereas the remaining 10%-20% have a severely progressive form, namely nonalcoholic steatohepatitis (NASH)[25]. In addition to lipid deposition, NASH is characterized by histological findings that include inflammatory cell infiltration, ballooning degeneration and fibrosis of hepatocytes, and a reported high risk of further progression to end-stage liver disease, such as cirrhosis and hepatocellular carcinoma[26,27]. NAFLD/NASH is considered a liver phenotype within various metabolic syndrome components and has a robust and bidirectional association with metabolic complications such as diabetes, obesity, and cardiovascular disease[28].
Several animal studies have demonstrated that periodontopathic bacteria, such as Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans cause increased insulin resistance and glucose intolerance through gut dysbiosis, as well as liver inflammation and fat deposition and fibrosis[29-33]. These relationships between periodontal bacteria and NAFLD are also supported by epidemiological studies[30,34-37], including cross-sectional and cohort studies, and further strengthened by clinical studies reporting that periodontal treatment improved NAFLD[36,38].
The liver is in a unique anatomic location and as such it has a dual blood supply. The liver gets blood from the systemic circulation and the gut, with the portal vein responsible for most of this blood supply, and it transports nutrients, bacterial metabolites, toxins, and drugs absorbed from the gut to liver[39,40]. In gut dysbiosis, abnormal growth and structural changes in intestinal bacteria result in an increase in hepatotoxic substances, such as endotoxin and ethanol, which reach the liver via the portal circulation[41-44]. In addition, increased intestinal permeability due to gut dysbiosis accelerates these hepatic exposures[45,46].
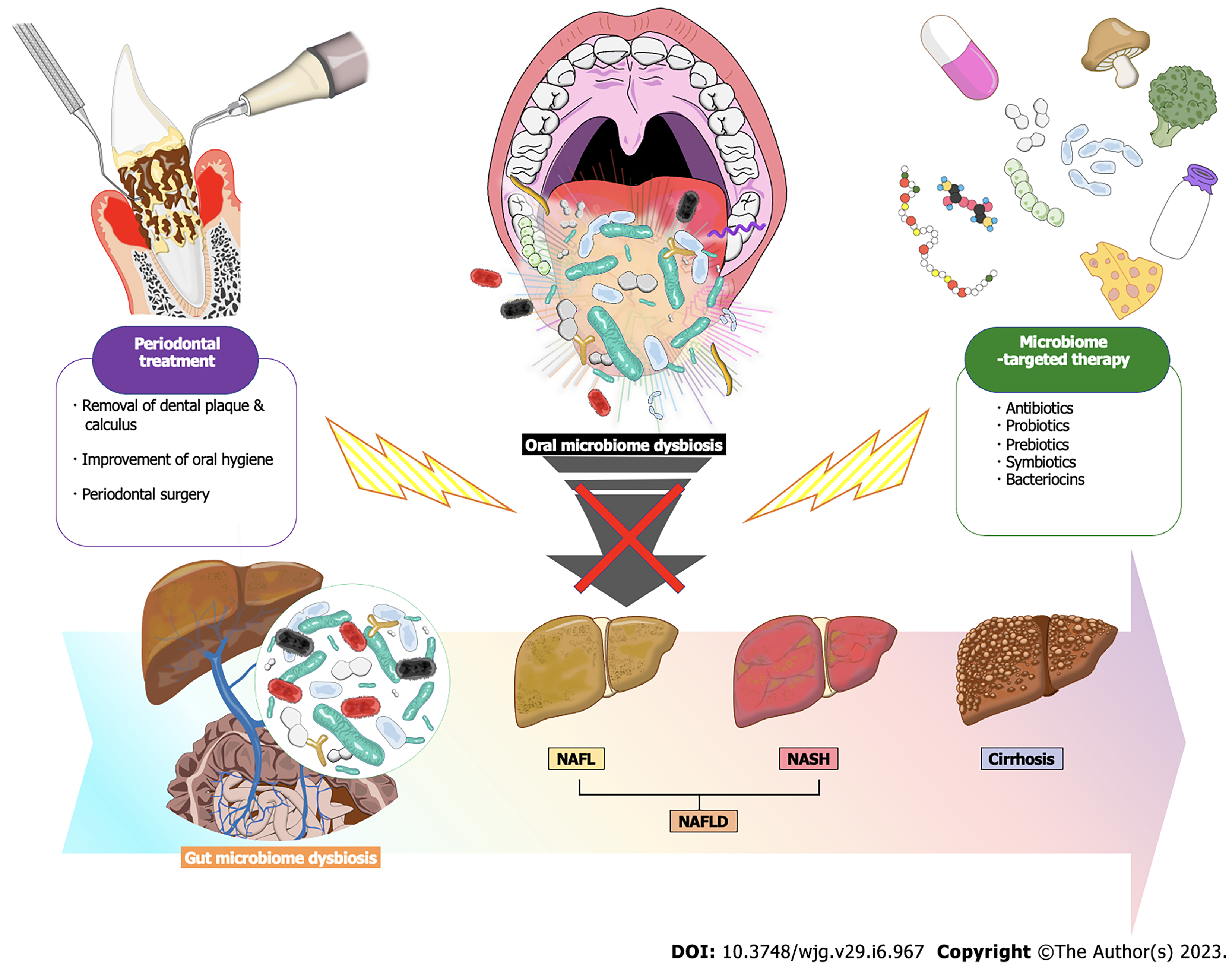
Thus, management of the oral and gut microbiome may be an important preventive strategy for patients with periodontal disease and NAFLD (Figure 2). In this regard, besides traditional therapies, the use of adjunctive therapies that include probiotics and prebiotics may be beneficial in normalizing dysbiotic microbiomes present in periodontal tissues, the liver, and the gut. This review will describe the link between periodontal disease and NAFLD with a focus on basic, epidemiological, and clinical studies. Potential mechanisms linking the two diseases and possible therapeutic approaches focused on the microbiome will also be discussed.
Periodontitis, a chronic inflammatory disease is characterized by a microbial dysbiosis and a progressive destruction of the tooth supporting structures[47]. In the United States, Periodontitis affects 42.2% of the population over the age of 30 and 59.8% over the age of 65[48]. Periodontitis is the major cause of tooth loss in adults, according to data from the World Health Organization[49]. Periodontitis has a multifactorial pathogenesis that includes environmental, microbial, and host factors which affect disease outcomes. Several systemic diseases and conditions have been associated with periodontal disease, including cardiovascular disease, diabetes mellitus, and metabolic syndrome[11,50-54].
The impact of systemic diseases and disorders on the periodontal tissues is well known, and the evidence indicates that periodontal disease may significantly enhance the risk for certain systemic diseases or conditions or alter their natural course[55-60]. Many systemic conditions have been associated with periodontal diseases, although the level of evidence for each condition varies. Conditions for which the influence of periodontal disease is well documented include coronary heart disease and related events, diabetes mellitus, preterm and low-birth-weight delivery, and preeclampsia; and respiratory conditions, such as chronic obstructive pulmonary disease[55-57]. A smaller but growing evidence base supports an association between poor oral health, tooth loss, or periodontitis and conditions, such as chronic kidney disease and renal insufficiency[61-65]; certain forms of cancer[66-70] affecting the liver, pancreas, and colorectal region; rheumatoid arthritis[71,72] and altered cognitive function, dementia, and Alzheimer disease[22,55,73-77]. Here we will discuss the influence of periodontal disease in the context of diabetes and metabolic syndrome (Figure 1).
In terms of diabetes, a variety of studies have examined the effects of diabetes on the periodontal tissues, whereas others have examined the effect of periodontal disease on the control of diabetes[56]. A review of several studies including those with more than 22000 patients found that the incidence of type 2 diabetes (i.e., new diagnoses) was significantly greater in individuals with periodontal disease than in those without periodontal disease[78]. Severe periodontal disease was associated with a significant worsening of glycemic control over time in patients with type 2 diabetes[79]. Those with severe periodontitis at baseline had a greater incidence of worsening glycemic control over a 2- to 4-year period as compared with those without periodontitis at baseline. Furthermore, periodontitis was known to have preceded the worsening of glycemic control in this study. In addition, numerous systematic reviews and meta-analyses have consistently shown that periodontal therapy is associated with a significant and clinical improvement in glycemic control in patients with diabetes and periodontitis[80-82]. Furthermore, meta-analyses also indicate a higher prevalence and severity of periodontal disease in diabetic patients and vice versa[83,84].
Understanding the mechanisms that underlie other infections is helpful to understanding the mechanisms whereby periodontitis influences glycemic control. Systemic inflammation is well known to play a major role in insulin sensitivity and glucose dynamics. Periodontitis can trigger or sustain an elevated systemic chronic inflammatory state, as shown by increased levels of serum C-reactive protein (CRP), interleukin (IL)-6, and fibrinogen in individuals with periodontal disease[79,85,86]. Inflammation triggers insulin resistance, which often accompanies systemic infections. For example, acute viral and bacterial infections can increase insulin resistance and aggravate glycemic control[87,88]. Systemic infections increase insulin resistance through several mechanisms. The insulin resistance, in turn, prevents glucose from entering target cells, causing elevated blood glucose levels. The elevated blood glucose then requires increased pancreatic insulin production to maintain normal glycemic levels. The insulin resistance can persist for weeks or months after the individual has recovered from the illness. Individuals with type 2 diabetes who already have significant insulin resistance, may experience additional tissue resistance to insulin as a result of infection, and thus exhibit an exacerbated poor glycemic control. In addition, studies suggest a relationship between the inflammatory status associated with diabetes or metabolic syndrome and the inflammatory status of periodontal disease[89,90]. Indeed chronic tissue inflammation has been recognized in the onset of metabolic diseases[91].
Chronic gram-negative periodontal infections may also lead to increased insulin resistance and poor glycemic control[92]. In individuals with periodontal disease, persistent systemic exposure to periodontopathic bacteria and their products results in an up-regulation of the immunoinflammatory response, with elevation in serum levels of proinflammatory mediators, such as IL-1β, tumor necrosis factor alpha (TNF-α), and IL-6, similar to well-recognized systemic infections, but on a more persistent, chronic basis. Increased serum levels of several cytokines, including TNF-α and IL-6, are associated with increased insulin resistance. These processes would help explain the worsening glycemic control associated with severe periodontal disease. Periodontal treatment, which is meant to decrease the bacterial insult and reduce inflammation, may lead to decreased systemic inflammation that may restore insulin sensitivity over time, and thereby result in improved metabolic control. This hypothesis would be supported by the many studies showings the improved glycemic control following periodontal therapy.
Much focus has been placed on the fact that chronic conditions can change the microbiome. As an example, individuals exhibiting obesity or type 2 diabetes demonstrate a modified gastrointestinal microbiome (reviewed by[93-96]). In addition, rodents exposed to Enterobacter cloacae B29, an intestinal microbe obtained from obese subjects with diabetes, develop insulin resistance and obesity[97]; indicating that bacteria can also directly induce diabetes-related symptoms. Also, modulating the microbial-mediated mucosal immunity and inflammation may help attenuate type 2 diabetes; by mechanisms that include modulation of T lymphocytes[98,99]. Since there are associations between type 2 diabetes, periodontal disease, and changes in the gut microbiome of individuals with type 2 diabetes, additional focus has been placed on microbiome changes in the periodontal tissues of individuals with type 2 diabetes.
Studies have shown that type 2 diabetes alters the subgingival and salivary microbial profile by lowering richness and diversity[100-105]. These findings support the observations found in the gut microbiome[93-96,106]. Studies show that the subgingival microbiome diversity is not only decreased in individuals with type 2 diabetes, but when separated by adequate or inadequate glycemic control, there is a further decrease in the microbiome diversity in those with inadequate glycemic control (hemoglobin A1c 8)[107]. However, another study which assessed the microbiome present in the saliva of 17 subjects with periodontal disease and type 2 diabetes was not able to find a relationship between glycemic control and changes in the oral microbiota. Although, the study found that in individuals with type 2 diabetes, the microbial composition varied significantly between obese (body mass index = 30) and non-obese individuals with type 2 diabetes, such that obesity reduced the oral microbial diversity[108]. Furthermore, although the subgingival and supragingival microbial diversity decreases when subjects with type 2 diabetes are compared to normoglycemic individuals, the microbial shift is less in individuals with periodontal disease with type 2 diabetes subjects than in normoglycemic individuals[101,102,109].
In summary, the subgingival and salivary microbial profile is altered by type 2 diabetes by decreasing microbial diversity and richness (Figure 1). In terms of glycemic control, there is a further decrease in microbial diversity when there is inadequate glycemic control. Furthermore, the microbial shift observed in individuals with periodontal disease is less prominent in individuals with type 2 diabetes compared to normoglycemic controls. In addition, microbial diversity is further reduced in smokers. Additional research is needed to examine a potential two-way relationship between diabetes and the periodontal microbiome; namely that periodontal microbes may directly induce diabetes-related pathology, as data indicate that microbes derived from the gastrointestinal tract of obese patients with diabetes can themselves mediate symptoms related to diabetes in animal models.
We will next discuss the association between metabolic syndrome and periodontal disease. Metabolic syndrome is a characterized by constellation of conditions that elevate the risk for cardiovascular disease and double the risk for type 2 diabetes[110-113]. Approximately 34% of the US population[114] and 10% of US adolescents are affected by Metabolic syndrome[115]. Metabolic syndrome prevalence increases with age and varies with ethnicity and gender[116]. Metabolic syndrome is defined slightly differently by different agencies. That conveyed by the National Cholesterol Education Program Adult Treatment Panel III is the most frequently used definition; an individual has to exhibit at a minimum, three out of the five risk factors: (1) Low plasma levels of high density lipoprotein cholesterol; (2) Increased values for plasma triglycerides; (3) Increased abdominal circumference; (4) Elevated blood pressure; and (5) Elevated glucose levels[117]. Pre-diabetes is included in metabolic syndrome as it presents with insulin resistance and is highly predictive of new-onset type 2 diabetes[118].
Abdominal obesity and insulin resistance are primary risk factors for metabolic syndrome are. Physical inactivity, aging, and hormonal imbalance also contribute to metabolic syndrome[119]. A primary trigger and risk factor for the majority of mechanisms in metabolic syndrome is visceral adiposity[120]. Precise processes that underlie the systemic response seen in metabolic syndrome have not been well elucidated, but data highlight that the inflammatory response present this disease mediates endothelial dysfunction that may, in turn, promote type 2 diabetes and cardiovascular disease[121-123].
There has been a significant interest in the association between metabolic syndrome and periodontal disease, since both are characterized by insulin resistance and systemic inflammation[124,125]; common pathways whereby they could impact each other. Cross-sectional, longitudinal, and meta-analyses studies have evaluated the association between metabolic syndrome and periodontal disease. Most data indicates that there is an association between metabolic syndrome and periodontal disease[126-130]. Three meta-analyses, discussed here, found an association between periodontal disease and metabolic syndrome (odds ratio from 1.38 to 1.99)[131-133]. One meta-analysis[133] that discussed 39 studies showed an association between metabolic syndrome and periodontal disease [crude odds ratio of 1.99 (95%CI: 1.75-2.25) and an adjusted odds ratio of 1.46 (95%CI: 1.31-1.61)]. A subgroup analysis focused on different countries was also included in this study and the results showed a combined odds ratio of 1.75 (95%CI: 1.31-2.34) for the United States; 1.68 (95%CI: 1.41-2) for Japan; 1.81 (95%CI: 1.35-2.42) for Korea, and 2.29 (95%CI: 1.53-3.41) for China[133]. Another meta-analysis[131] of 26 manuscripts that included radiographic and clinical exam data reported an association between these two diseases [odds ratio of 1.38 (95%CI: 1.26-1.51)]. This suggested that patients with metabolic syndrome are 38% more likely to exhibit periodontal disease[131]. A systematic review/meta-analysis of multiple manuscripts that reported on 36,337 individuals, found a positive association between periodontal disease and metabolic syndrome [odds ratio of 1.71 (95%CI: 1.42-2.03)][132]. A comprehensive review further found a positive association between metabolic syndrome and periodontal disease[134]. Furthermore, various animal studies that employed different periodontal disease models demonstrated that rodents with metabolic syndrome or obesity due to a high-fat diet, also exhibited exacerbated periodontal bone loss[135-139].
In terms of assessing metabolic syndrome’s role in contributing to periodontal disease, two longitudinal studies found that metabolic syndrome increases the risk of periodontal disease development and progression[140,141]. Specifically, one study found that individuals with metabolic syndrome were 2.6 times more likely to develop periodontal disease. Furthermore, with increased attributes of metabolic syndrome, the periodontal disease exhibits a higher prevalence and a more extensive presentation[141,142]. Even though most studies found an association between periodontal disease and metabolic syndrome, some reports found a minimal association for these diseases[143-148]. However, most of the studies that found no association were either cross-sectional or shorter longitudinal studies (only one year), and one study was performed on a young cohort of subjects[147], that had low levels of both metabolic syndrome and periodontal disease. Furthermore, in longitudinal study of three years, researchers found that toothbrushing frequency was inversely related to the incidence of metabolic syndrome[149].
Most studies have concluded that periodontal disease may contribute to the development or exacerbation of metabolic syndrome[150-152]. In a cross-sectional study of 190 individuals, researchers found that periodontal disease and periodontal bone loss may contribute to the development of metabolic syndrome[150]. A four-year longitudinal study of 1023 adults found deeper periodontal pockets associated with a positive conversion of metabolic components (odds ratio 1.6; 95%CI: 1.1-2.2)[151]. Furthermore, another study reported that in individuals with metabolic syndrome a decrease in periodontal inflammation reduced C-reactive protein levels in those subjects[152].
There has been an increasing interest in the role of the microbiome in metabolic diseases and disturbances. Metabolic diseases change the gut microbiome (reviewed by[96]), and the oral microbiome varies significantly between individuals with a healthy periodontium and those with periodontal disease[153]. Furthermore, changes in the gastrointestinal microbiome are associated with metabolic syndrome and obesity[154]. Obesity affects the oral microbiome in the context of type 2 diabetes[108], plus it lowers the diversity of the microbiome within the distal gut[155,156]. Further, subjects with decreased microbial diversity exhibit significantly more insulin resistance, adiposity, and dyslipidemia in contrast to individuals with elevated microbial richness[156]. A study focused on 17 subjects with type 2 diabetes and with advanced periodontal disease found that the composition of the oral microbiome varied significantly between non-obese and obese (BMI = 30) subjects. Those findings suggested that obesity is related to a decreased oral microbial diversity[108]. Our research group also showed that the oral microbiome is altered in individuals with metabolic syndrome as compared to healthy individuals[157]. In addition, there are a growing number of studies suggesting potential links between the gut or oral microbiome and obesity[158-160].
In vitro and in vivo (animal) studies have shown how dyslipidemia (high-fat diet) compounds the effects of metabolic syndrome on periodontitis[135-139,161], and a variety of studies have assessed the role of impaired glucose in periodontal disease. A study on obese mice (metabolic syndrome model) inoculated with P. gingivalis and fed a high-fat diet that did not exhibit diabetes, revealed that these mice had 40% more alveolar bone loss and higher levels of P. gingivalis versus control mice[135]. Using the same model, another group of researchers found that the high-fat diet group exhibited metabolic syndrome, including dyslipidemia, obesity, hyperinsulinemia, and insulin-resistance. Further, the group with metabolic syndrome exhibited significantly higher osteoclast formation and alveolar bone loss. Similarly, our previous study showed that the ligature-induced periodontitis with P. gingivalis infection in rats fed a high-fat diet not only exacerbated the alveolar bone resorption, but also induced increase in levels of fasting blood glucose and liver damage markers, and systemic inflammation[138,139].
Moreover, lipopolysaccharide (LPS) induced-periodontitis exacerbated inflammatory cytokine expression (IL-6, monocyte chemoattractant protein-1, receptor activator of NF-kappaB ligand, and macrophage colony-stimulating factor), osteoclast formation, and alveolar bone loss[137]. Studies that used osteoblasts from obese mice showed a reduction in cell proliferation and an elevated osteoblast cell death following P. gingivalis treatment vs controls[162]. Furthermore, obese rodents with metabolic syndrome exhibited significant elevated levels of A.actinomycetemcomitans-LPS-induced alveolar bone loss versus the non-obese, non-metabolic syndrome animals. In addition, a cholesterol-lowering medication frequently given to patients with metabolic syndrome[163], attenuated alveolar bone loss in both groups, further supporting the role of dyslipidemia in periodontal inflammation[164]. In this regard, our research found that submucosal administration of radio-labeled P. gingivalis LPS to the palatal gingiva of high-fat diet-induced obese rats led to a significant accumulation of LPS in the liver and gingiva compared to other organs, including the spleen, kidney, and brain. Furthermore, the gingiva and fatty liver of obese rats demonstrated an increased level of P. gingivalis LPS and a prolonged accumulation time compared to mice fed a normal diet. In addition, a high-fat diet further delayed the metabolic clearance of P. gingivalis LPS from the gingiva and liver[165].
The role of lipids in periodontal disease was further explored using in vitro studies, and these studies showed that fatty acids augmented the LPS-mediated expression of markers involved in periodontal disease, for example IL1-a, IL1-b, C-X-C motif chemokine 10, cluster of differentiation (CD) 86, colony stimulating factor 2, monocyte chemoattractant protein-1, toll-like receptor 2, TNF-α, and CD14[137]. Further in vitro studies performed on macrophages demonstrated a significant increase in CD36, a major fatty acid receptor, upon treatment with LPS plus palmitate in comparison to macrophages only treated with LPS or palmitate alone[161]. Periodontal disease and metabolic syndrome, independently and significantly increased the levels of CD36, and when metabolic syndrome and periodontal disease were present simultaneously, there was an additive effect. The expression of CD36 in periodontal tissues also positively correlated with osteoclast formation.
Taken in aggregate, human studies and animal models show an association between metabolic syndrome and periodontal disease, and metabolic syndrome can change the oral microbiome and potentiate the harmful effects of periodontal disease.
Periodontal disease has been associated with liver diseases, such as NAFLD, liver cirrhosis, and hepatocellular carcinoma, and even a possible worse prognosis for liver transplantation (Figure 1). The relationship between periodontitis and NAFLD is supported by a number of epidemiological studies[30,34-36,166-170]. Cross-sectional studies have also shown that the severity of periodontal disease correlates significantly with levels of blood liver injury markers [e.g. alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma-glutamyl transpeptidase[171-173], hepatic imaging by ultrasound and computerized tomography, and a formula-based scoring system for estimating the degree of fatty liver and liver fibrosis[166,174,175]. Furthermore, several cohort studies have stren
Periodontal disease has been reported to exacerbate metabolic diseases including diabetes, dyslipidemia and obesity through disturbance of energy homeostasis, and given the close association between NAFLD and metabolic syndrome, this underscores the importance of understanding the periodontal-metabolic relationship in the NAFLD pathogenesis. Two-thirds of patients with obesity and type 2 diabetes have been found to have fatty liver, and both excessive body mass index and visceral obesity are recognized as risk factors for NAFLD[177,178]. Metabolic syndrome is also related to an increased risk of more progressive liver disease, such as NASH, cirrhosis, and future liver failure, in patients with NAFLD[179-181]. A 5 year-follow up cohort study reported by Kuroe et al[182] demonstrated that in 341 Japanese subjects with NAFL without complications of liver fibrosis, stratified analysis by obesity showed that in obese subjects, severity of periodontal destruction was significantly correlated with liver fibrosis with an odds ratio of 2.87, whereas in non-obese subjects they were not correlated. Moreover, in metabolic diseases animal models induced by high-fat diet or high-cholesterol diet, the ligature-induced experimental periodontitis and periodontopathic bacterial infection exacerbated disturbances of glycolipid-metabolism in the liver and enhance the NAFLD progression[138,183,184]. Although not all mechanisms explaining the interaction between periodontal disease and NAFLD via metabolic diseases are known, diffusion of inflammatory mediators and periodontopathic bacteria and their components, and release of reactive oxygen species from inflamed periodontal tissues into the circulation can mediate low-glade systemic inflammation and, thereby exacerbate insulin resistance in obesity and diabetes[185].
Data over the last 10 years strongly suggest that P. gingivalis is involved in NAFLD and NASH. P. gingivalis has many virulence factors, such as collagenase, trypsin-like gingipain enzymes, LPS and fimbriae, that are also known to trigger intracellular signaling events[186]. Yoneda et al[36] analyzed various periodontopathic bacteria in saliva collected from non-NAFLD control subjects and NAFLD patients using polymerase chain reaction assays and showed that the detection frequency of P. gingivalis was significantly higher in the NAFLD patients. In addition, injection of P. gingivalis via the jugular vein dramatically accelerated NAFLD progression in mice fed a high fat diet. We have previously reported that P. gingivalis infection combined with ligature-induced periodontitis increased serum ALT and LPS levels and hepatic fat deposition in high-fat diet-induced obese rats with insulin resistance[138,139]. Conversely, hyperglycemia can promote translocation of P. gingivalis from the oral cavity to the liver and reduce hepatic insulin-induced glycogen biosynthesis in mice[187]. For other periodontopathic bacteria, enteral infection with Aggregatibacter actinomycetemcomitans and Prevotella intermedia as well as P.gingivalis may contribute to NAFLD by altering the gut bacterial flora and metabolome in mice[30,32].
At the present time, dual pathways have been proposed to explain the connection between periodontal disease and liver pathology, namely hematogenous and enteral diffusion of hepatotoxic components[11]. The liver is the largest parenchymal organ and, due to its unique anatomical location, it has a dual blood supply coming from the systemic circulation and the gastrointestinal tract[39], which helps explain how toxic factors diffuse from periodontal tissue and reach the liver. Eighty % of the total blood supply received by the liver is derived from the enteric portal vein, which is rich in nutrients, bacterial metabolites, and food antigens. The remaining 20% is supplied by the hepatic artery, which is the nutrient vessel of the liver and branches off the abdominal aorta. Thus, the liver is the hemodynamic confluence in the human body, and its unique characteristics allow for the maintenance of a diverse intrahepatic cell population composed of metabolically active hepatic parenchymal cells, connective tissue cells, and various immune cells[40].
One of the pathways linking the oral cavity and liver is thought to be hematogenous diffusion, which is caused by inflammatory mediators and microorganisms passing through the systemic circulation due to hyperpermeability of the pocket epithelium and micro-ulceration in the periodontal inflamed tissue[1,12]. For example, the serum levels of reactive oxygen species and inflammatory cytokines are elevated in periodontitis patients, suggesting a mild chronic inflammatory state systemically[188,189]. In fact, epidemiological studies have shown that Elevated blood level of CRP, which is secreted by hepatocytes upon stimulation of proinflammatory cytokines, are a common modifier of periodontitis and NAFLD[167,190]. With regard to microbiological invasion, mechanical injuries such as daily oral hygiene activities, chewing movements, and calculus removal as periodontal treatment have been reported to increase the frequency of bacteremia and endotoxemia in patients with periodontal disease compared to healthy subjects[191,192].
Another possible pathway linking the oral cavity to the liver is the transport of oral bacteria through the gastrointestinal tract, resulting in abnormalities in the gut microbiota[42,193,194]. Patients with periodontitis have a characteristic oral microbiome, and they continuously and unknowingly swallow pathogens present in saliva and dental plaque[19]. A part of oral bacteria can reach the intestinal tract, even in systemically healthy individuals and regardless of the harsh acidic environment in the stomach. Thus, the oral microbiome may function as an endogenous reservoir that supply novel bacteria to the gut microbiome[195]. It is widely known that gut dysbiosis is closely associated with the pathogenesis of NAFLD through changes in gut bacterial composition and metabolites[193,196,197]. In a state of gut dysbiosis, the production of choline which is an essential substance for lipolysis is decreased, whereas LPS, ethanol and volatile organic compounds (e.g. acetone, phenol and cyclopentane) are increased, which are potential hepatotoxin[41,42,44]. Moreover, the gut dysbiosis enhanced intestinal permeability by disrupting intercellular junctions in the intestinal mucosa, leading to increased translocation of enteric bacteria and their metabolites to the liver. Therefore, the liver is constantly exposed to various substances of intestinal derivation, which diffuse into the liver through the enterohepatic portal circulation.
A study by Lourenςo et al[19] reported that numerous oral taxa related to periodontal inflammation and destruction were detected in the gut microbiome of individuals, regardless of periodontal condition. Patients with periodontal disease were characterized by a higher ratio of Firmicutes/ Bacteroidetes, an enrichment in Euryarcheota, Verrucomicrobiota, and Proteobacteria, and a less diverse gut microbiota than those with healthy periodontal status. Kawamoto et al[16] found that fecal samples from patients with severe periodontitis were enriched in Acidaminococcus, Clostridium, Lactobacillus, Bifidobacterium, Megasphaera, and Romboutsiacompared to those from healthy subjects. However, few studies have observed changes in the gut microbiota in patients with periodontal disease, and consistent trends in the gut dysbiosis remain unclear, with wide variations. In the future, it would be interesting to compare the modifications of the gut microbiota in the state of periodontal disease with those observed in NAFLD, diabetes, and obesity to elucidate the complex relationship between periodontal disease and systemic diseases.
Many animal studies have shown that orally administration of periodontal pathogens caused insulin resistance and hepatic fat deposition in rat and mice, accompanied by alterations in gut microbiota and glycolipid-metabolism[18,29-32]. In particular, P. gingivalis, known as a keystone pathogen, causes mucosal inflammation via contributing to oral and gut microbiome dysbiosis[29,198]. Yamazaki et al[32] showed that in an NAFLD mouse model fed a high-fat diet, oral administration of P. gingivalis or P. intermedia changed the composition of gut microbiota and blood metabolome, resulting in a shift in liver transcriptome expression toward the NAFLD pathogenic form. In contrast, application of commensal oral bacteria Actinomyces naeslundii and Veillonella rogosae did not affect NAFLD progression. Komazaki et al[31] reported that oral infection with A.actinomycetemcomitans may contribute to NAFLD by altering the bacterial flora and glucose metabolism in mice. This result is also consistent with the fact that Anti-A. actinomycetemcomitans antibodies were positively correlated with visceral fat area, insulin resistance, and serum AST level in 52 Japanese NAFLD patients.
Recent studies have revealed that inflammation of periodontal tissue alters gut bacteria and metabolites even without specific periodontopathic bacterial infections[21,199,200]. Even commensal oral bacteria, which are not notably pathogenic in the oral cavity, such as Enterobacter spp. and Klebsiella spp. when stimulated to proliferate by ligature-induced periodontitis, can mediate ectopic intestinal colonization and may play an important role in the exacerbation of enteritis[21]. Therefore, future studies on the pathogenesis of NAFLD should not only focus on individual periodontal pathogens, but also comprehensively evaluate the relationship between changes in the oral and gut microbiota as a whole.
Polymicrobial diseases represent clinical and pathological conditions caused by 2 or more microorganisms[201]. The bacterial microbiome in the oral cavity is composed of more than 700 bacterial species[202] and is associated with the development and progression of oral diseases[203]. Periodontal diseases are representative oral polymicrobial diseases that involve a microbiome imbalance known as dysbiosis[204,205], leading to dysregulated host-microbial crosstalk that induces periodontal inflammation and alveolar bone loss[206]. The 2009–2010 and 2011–2012 cycles of the National Health and Nutrition Examination Survey reported that almost 50% of adults aged ≥ 30 years in the United States were affected by periodontitis[207]. In addition, periodontitis is associated with systemic diseases, such as type 2 diabetes, obesity, cardiovascular disease, preterm low birth weight, and NAFLD[30,208-212]. Therefore, a complete understanding of periodontal disease is essential to prevent a decline in quality of life.
The current theory suggests that gingival lesions are precursors of periodontitis[213]. Loe et al[214] demonstrated that the accumulation of bacterial plaque causes gingivitis in humans, and gingival inflammation is resolved by the removal of the plaque[235]. In addition, continued plaque accumulation in animal models induced gingivitis and gradually developed into periodontitis[215]. These results clearly indicate that bacterial plaque is involved in periodontitis. This belief was called the “nonspecific plaque hypothesis” which states that a sufficient accumulation of microorganisms results in destructive inflammation in periodontal tissues[216]. The contradiction between the nonspecific plaque hypothesis and actual clinical findings led scientists to propose the “specific plaque hypothesis”. This hypothesis was that specific bacterial species were involved in the etiology of periodontitis[217]. However, potential periodontal pathogens could not be identified because approximately 30–100 bacterial species inhabit the periodontal pocket, and plenty of other bacterial species may have been unculturable[218].
Although culture methods have traditionally been useful for the identification of causative bacteria, DNA-based bacterial detection methods using molecular biology techniques have also contributed to the characterization of oral bacterial composition. Socransky et al[4] reported on findings relating to the specific plaque hypothesis where they targeted 40 subgingival bacterial species using the checkerboard DNA-DNA hybridization method. These bacteria were mainly divided into five groups: Red, orange, green, yellow, and purple complexes. P. gingivalis, Tannerella forsythia, and Treponema denticola were defined as periodontal pathogens and classified as the red complex because they exhibited a strong relationship with probing pocket depth and bleeding on probing. The orange complex was also associated with probing pocket depth and appeared to be closely related to the red complex. Red and orange complexes were detected more frequently in patients with periodontitis than in healthy subjects[219]. Periodontitis has been reported to be associated with many systemic diseases[220], especially diabetes caused by metabolic syndrome. The prevalence of periodontitis in diabetic subjects was higher than that in non-diabetic subjects[221]. In addition, poorly controlled diabetic subjects have more severe periodontitis[222]. In periodontitis sites, a higher quantity of P. gingivalis in poor controlled diabetic subjects was observed than that of non-diabetic subjects[223]. Haffajee et al[224] reported that a reduction in the red complex was observed at sites where periodontal treatment with scaling and root planning alone or with systemically administered azithromycin, metronidazole, or a sub-antimicrobial dose of doxycycline was performed. In another study, Duarte et al[225] also found a reduction in red and orange complexes after periodontal treatment. Therefore, members of the red complex are still considered periodontal pathogens; however, some inconsistency exists in this concept. Previous studies demonstrated that Streptococcus mutans and Streptococcus salivarius, which were not defined as periodontal pathogens in the complex-based classification, causing attachment loss of periodontal tissues in animal studies[226,227]. Another example of contradictory findings was that periodontal bacterial species in a complex-based classification were detected, but periodontitis was not observed[218].
Recently, high-throughput DNA sequencing technology using next-generation sequencing (NGS) methods has been used to comprehensively analyze bacterial composition in different environments[228]. In addition, bioinformatic techniques and pipelines have been improved to analyze data obtained from NGS[229]. In particular, NGS technology is suitable for bacterial analysis because the 16S rRNA genes from bacteria critically define all bacterial species and have an approximate length of 1 kbp of nucleotide sequences that are highly conserved among bacterial species[230]. Therefore, NGS technology combined with bioinformatic techniques is a useful tool for scientists as it allows for a comprehensive evaluation of microbial composition within the microbiome. Griffen et al[231] compared the bacterial composition of subgingival plaque between patients with chronic periodontitis and periodontally healthy controls using 16S pyrosequencing. They reported that the proportion of red complex microbes was high at the periodontitis sites, but the microbiome included bacterial species that were not previously regarded as being involved in the etiology of periodontitis. This result showed that conventional methods, such as culture methods, real-time polymerase chain reaction, or checkerboard DNA-DNA hybridization, were limited by the number of bacteria that could be detected. However, current methods using NGS are not limited to the number of bacterial species that can be detected. A few studies using NGS with the 16S rRNA gene region suggested that several taxa, including Filifactor alocis and TM7, showed greater abundance in periodontitis and seemed to act as the core bacteria in the subgingival microbiome of disease in addition to the bacteria thought to be conventional periodontal pathogens[231,232]. In other words, these results suggest that bacteria other than conventional periodontal pathogens, such as red complex members, also contribute to periodontitis, and dysbiosis within the microbiome potentially induces periodontal inflammation.
Hajishengallis et al[241] proposed the “keystone-pathogen hypothesis,” wherein a specific bacterial species is considered a keystone periodontal pathogen, such as P. gingivalis, which changes the microbial balance and induces inflammation through dysbiosis, despite the low abundance of the pathogen[19,233]. Several problems with DNA-based analyses alone have indicated that the 16S rDNA profile may include dead bacteria[234]. On the other hand, only viable bacteria can be analyzed by conducting experiments using 16S rRNA[235]. However, analysis of only 16S rRNA is not a reliable index for bacterial activity and may be misleading in some cases because the relationship between rRNA and growth rate differs significantly in subspecies, even if these bacteria belong to the same bacteria at the species level[236]. Therefore, the 16S rRNA/16S rDNA ratio has been useful as a bacterial activity index for detecting highly active bacterial species, which, despite the low abundance of the pathogen, can induce dysbiosis in certain environments[237,238]. Kachi et al[237] clarified that the activity of P. gingivalis was lower than that of other species in periodontitis sites, although P. gingivalis was abundant in both DNA and RNA. In contrast, T. forsythia, Fusobacterium nucleatum subsp. Vincentii, Streptococcus oralis, T. denticola, F. alocis, and Streptococcus salivarius showed high activity in periodontitis sites. By analyzing both DNA and RNA using the 16S rRNA region, it may be easier to detect keystone pathogens because this analysis can lead to the understanding of not only the abundance of each bacterium but also their activities. However, these analyses alone cannot reveal the functional composition of the microbiome. Tools such as Piphillin or Piecrust can predict the functional composition of the microbiome using the information of the 16S rRNA region[239,240]. Ikeda et al[241] reported that pathways for phenylpropanoid and GPI-anchor biosynthesis and metabolism of alanine, arginine, aspartate, butanoate, cyanoamino acid, fatty acid, glutamate, methane, proline, and vitamin B6 were significantly over-represented in periodontitis subjects compared with healthy subjects using Piphillin. Thus, we might understand the essence of polymicrobial disease by determining not only the composition of the microbiome but also the functional gene composition.
In terms of bacterial composition analysis, the 16S rRNA region is highly conserved among prokaryotes, such as bacteria and archaea, and the length of the 16S rRNA region is suitable to provide sufficient information for phylogenetic analysis[230]. However, the length of the 16S rRNA region is almost 1600 base pairs. Among bacteria, Mycoplasma, which is the smallest parasitic bacterium, has 580000 base pairs[242]. This also indicates that the 16S rRNA region alone is insufficient for a complete understanding of the microbiome. In addition, 16S rDNA and 16S rRNA sequencing has caused PCR bias[243] and the different results depend on primer type[244]. To compensate for these shortcomings, metagenomic and metatranscriptomic analyses, which decode all DNA and RNA information, have attracted attention. Both metagenomic and metatranscriptomic analyses can analyze bacterial and functional composition, but metagenomic analyses focus more on species-level profiling, metatranscriptomic analyses concentrate more on gene expression levels[245]. A report by Dabdoub et al[246], using metagenomic analysis, reported the microbial and functional differences between healthy and periodontal sites. Porphyromonas, Fusobacterium, Fretibacterium, Filifactor, Parvimonas, Selenomonas, Treponema, and Kingella were more abundant in periodontitis sites than in healthy sites. In addition, most functional genes in healthy sites were dedicated to energy utilization through oxidative pathways, while fermentation and methanogenesis were the predominant energy transfer mechanisms in the disease site[246]. A study using metatranscriptomic analysis reported that T. forsythia and P. gingivalis upregulated different TonB-dependent receptors, peptidases, proteases, aerotolerance genes, iron transport genes, hemolysins, and clustered regularly interspaced short palindromic repeats-associated genes in periodontitis sites. Interestingly, bacteria that have not been usually implicated in periodontitis, such as S. oralis, S. mutans, S. intermedius, Streptococcus mitis, Veillonella parvula, and Pseudomonas fluorescens, were highly active in transcribing putative virulence factors in periodontitis sites[247]. Another report using metatranscriptomic analysis revealed that bacteria, such as Bacteroides massiliensis and Leptotrichia hofstadii had high transcriptional activity in addition to that of the red complex[8]. Furthermore, combining metagenomic and metatranscriptomic analyses help understand the activity or enrichment of a given gene set in the microbiome[245]. The combination of metagenomic and metatranscriptomic analyses by Duran-Pinedo et al[248] showed that P. gingivalis, T. forsythia, and T. denticola exhibited a higher mean abundance and gene expression in periodontitis sites than in healthy sites. However, Bacteroidetes oral taxon 274, Corynebacterium matruchotii, and L. hofstadii in periodontitis sites showed a decrease in relative abundance but represented a high proportion of gene expression, when comparing healthy and periodontitis sites. In addition, the vast majority of virulence factors upregulated in periodontitis sites originate from bacteria that are not considered major periodontal pathogens, such as Neisseria flavescens, C. matruchotii, Rothia dentocariosa. Komatsu et al[249] combined metagenomic and metatranscriptomic analyses with a network analysis for periodontitis. Their results showed that Fusobacterium nucleatum subsp. vincentii had the highest activity in periodontitis sites, followed by Peptostreptococcus stomatis and Leptotrichia sp. according to the RNA/DNA ratio which are similar to the 16S rRNA/16S rDNA ratio. Additionally, Fretibacterium fastidious, and F. alocis, Eubac
Periodontitis is not an infectious disease caused by a single bacterium, such as diphtheria, tetanus, typhoid fever, and leprosy, but a dysbiotic disease caused by alterations in the abundance of keystone and/or accessory pathogens within the polymicrobial community, leading to inflammatory responses (Figure 1). In addition, dysbiotic microbiota further develops and stimulate inflammatory responses[6]. However, it is difficult to comprehensively understand the etiology of periodontitis, although exhaustive DNA and RNA analyses have been performed. There are several possible explanations for this reason. First, many microorganisms, including bacteria, unculturable bacteria, fungi, and viruses, are present in the oral cavity[250]. Second, the same bacteria can act as homeostatic commensals in one environment and accessory pathogens in another. Furthermore, some bacteria can act as keystone or accessory pathogens depending on their condition[6]. Recently, trans-omics has been attracting attention and has focused on connecting multi-omics data, including not only the genome and transcriptome, but also the proteome and metabolome[251]. Trans-omics has been shown to be effective in elucidating various complex mechanisms, such as insulin sensitivity in liver cells and muscle adaptation[252,253]. These techniques might be applied to unravel the full picture of periodontal disease as a polymicrobial disease.
Periodontal disease is characterized by a complex relationship between the host inflammation and polymicrobial dysbiosis of the oral-dental biofilm. This leads to chronic inflammation of the periodontal tissue, ultimately leading to bone destruction and tooth loss. However, over the last several years, this dysbiosis of the oral microbiome has been also associated with multiple systemic inflammatory conditions, such as cardiovascular disease, obesity, metabolic syndrome, and rheumatoid arthritis[71,254,255]. As mentioned previously, there are two main prevailing hypotheses regarding the potential for localized chronic periodontal infection to cause systemic inflammation. Namely: (1) Periodontal dysbiosis and inflammation leads to increase bacterial translocation into the systemic circulation causing inflammatory mediators and immune complexes to be circulated to other organ systems[256,257], and (2) Chronic periodontal dysbiosis leads to disturbances and changes to the gut microbiome via oral ingestion of periodontopathic organisms.
The concept that the oral microbiome drives the gut microbiome space is highlighted by the fact that some of the first bacteria to colonize the gut come from the mouth[258]. Infants born from vaginal deliveries are exposed to the microbiome of the mother’s vaginal tract and gastrointestinal tract during labor, while breastfeeding introduces the skin microbiome to the infant[258]. As the gut microbiome matures over time, new bacterial strains are introduced into the gut via the environment and foods that we are exposed to in our childhood and young adulthood.
Yet, the established gut microbiome of adults is not stagnant, but rather can be change drastically by the environment, food, and/or medications. The connection of the oral microbiome and periodontal inflammation causing changes in the gut microbiome is highlighted in several animal and human studies. In a study of 44 patients, which included 7 healthy controls, authors found that patients with either gingivitis (n = 14) or chronic periodontitis (n = 23) had lower gut bacterial diversity as compared to healthy controls[19]. Specifically, they saw that patients with chronic periodontitis had higher levels of Firmicutes, Proteobacteria, Verrucomicrobia and Euryarchaeota and lower levels of Bacteroidetes as compared to controls[19]. In patients with gingivitis, Prevotella, Comamonadaceae and Lactobacillales were elevated and Bacteroidales was decreased as compared to healthy control[19]. Utilizing a random forest classifier, Mogibacteriaceae, Ruminococcaceae, and Prevotella were able to discriminate individuals with periodontal diseases from healthy controls with an accuracy of 84%. Because of the cross-sectional design of this study, causality could not be inferred. However, several animal models have shown that the oral administration of P. gingivalis into mice leads to significant alteration of the gut microbiome, increase in serum endotoxemia, and a reduction of gut barrier function[20,32]. The oral administration of P. gingivalis in mice led to increases in Bacteroidetes and a reduction of Firmicutes[29,31]. These results in mice were recently mirrored in a translational study in humans. Bao et al[18] took salivary and fecal samples from 16 healthy participants and 21 patients with severe periodontitis and transplanted their salivary microbiome into wildtype C57BL6 mice. The authors showed that patients with severe periodontitis had a significantly different gut microbiome than those of healthy controls, with more saliva-sourced microbiome found in the gut of patients with severe periodontitis as compared to controls[18]. When transplanted into mice, the authors showed that by using utilizing carboxyfluorescein diacetate succinimidyl ester-stained bacteria the salivary microbiota persist in the gut for at least 24 h[18]. They also showed that the salivary microbiome of patients with severe periodontitis when transplanted into mice lead to an increase in gut levels of Porphyromonadaceae and Fusobacterium, while at the same time leading to a decreased expression of gut epithelial tight junction proteins and increased levels of pro-inflammatory cytokines. These findings alongside our prior works[11,22], demonstrate the important role of the oral-gut axis in other systemic diseases.
Because of the interplay between the gut microbiome and inflammation, many studies have examined the role of the gut microbiome in liver disease. As part of this review, we will only focus on the relationship between the gut microbiome and NAFLD. The term NAFLD encompasses a spectrum of diseases that range from bland steatosis or NAFL to NASH and advanced hepatic fibrosis or cirrhosis. Several studies have linked gut dysbiosis to each phase of NAFLD.
There have been several studies linking the gut microbiome to NAFL with variable results. In adult patients with NAFL, the most common genera that have been elevated were Lactobacillus and Escherichia, and Coprococcus and Prevotella were the most common to be reduced as compared to weight matched controls[259-262]. While these studies looked only at composition, several others examined NAFL utilizing a multiomics approach, which combined sequencing data with metabolomics. Raman et al[43] have shown that 18 stool metabolites, including increased levels of acetic acid, butanoic acid, and propanoic acid, are associated with NAFL in adult patients. Da Silva et al[261] also found a characteristic enrichment of isobutyric acid and propionic acid in the feces of NAFL patients. However, one of the most convincing data that has shown the causative relationship between the gut microbiome and NAFL comes from the translational study performed by Hoyles et al[262]. Utilizing hepatic transcriptome, gut metagenome, and serum and urine metabolome, they saw NAFL was associated with an increased serum level of several branched-chain and aromatic compounds, including phenylacetic acid[262]. In addition, administration of phenylacetic acid into mice transplanted with human fecal microbiota caused hepatic steatosis[262].
While studies examining the profile of NAFL were slightly mixed based on the population being investigated, studies examining the profile of NASH to obese controls showed more consistent findings[263]. In adults, NASH patients had lower levels of Faecalibacterium, Ruminococcus and Bifidobacterium[261,264] and a higher level of Lactobacillus[265]. Few studies have examined the metabolomics of NASH patients separate from NAFL, likely due to the reason that a diagnosis of NASH requires a liver biopsy to confirm. However, in one study of 16 adults diagnosed with NASH by biopsy, NASH patients had an increased ratio of primary to secondary bile acids which correlated with increase hepatic injury and inflammation[266].
Advanced fibrosis is defined as a fibrosis stage of 3 or more and is associated with higher risk for morbidity and mortality. In general, microbiome studies of NAFLD-related advanced fibrosis usually see a decrease in microbial diversity marked by an increase in gram-negative bacteria[267-270]. Studies have found that NAFLD-related fibrosis was associated with an increase level of Bacteroides and Escherichia[267-269] with mixed results with other genera like Prevotella[268,270,271]. Metabolite studies found that 3-phenylproanoate[270] and 3-(4-hydroxyphenyl) lactate[272] were associated with NAFLD-related advanced fibrosis. 3-(4-hydroxyphenyl) lactate was also strongly correlated with several bacterial species that were also associated with hepatic fibrosis, such as Escherichia coli, Bacteroides caccae and Clostridium sp[272].
The mechanisms by which the gut microbiome can cause steatosis, inflammation, and hepatic fibrosis is highlighted in animal models. Patients with NAFLD and obesity are associated with a microbiome that leads to decreased gut epithelial barrier function[273]. This disruption in the gut epithelium leads to increased bacterial translocation, higher levels of endotoxemia, and subsequent increased activation of toll-like receptor signaling[274,275]. However, a clinical trial utilizing toll-like receptor inhibitor did not show any benefit in patients with NASH[276]. Other mechanisms, include alterations of short-chain fatty acids[277] and bile acid metabolism[278] and signaling[279].
Periodontal treatment is based on the mechanical and chemical removal of dental plaque and pathogenic factors from the root surfaces by the patient or by a specialist to eliminate inflammation in the periodontal tissues and promote wound healing in the host. It is known that patients who achieve good oral hygiene and a healthy morphology of periodontal tissues through periodontal treatment show a decrease in the total number of bacteria and the proportion of periodontal pathogens in saliva and plaque, as well as a change in the composition of the oral microbiota to a healthy state. Therefore, given the relationship between periodontal disease and NAFLD supported by many epidemiological studies, periodontal therapeutic interventions may be a preventive measure for NAFLD (Figure 2).
In a cross-sectional study of 6352 adults participating in the Korean National Health and Nutrition Examination Survey, Kim et al[280] reported that there was an inverse association between frequency of tooth brushing and NAFLD prevalence (fatty liver index ≥ 60), with a modified odds ratio of 0.56 for NAFLD in the group that brushed three or more times a day compared with the group that brushed once a day or less. A few intervention studies have examined the effect of periodontal treatment on NAFLD. Yoneda et al[36] reported that in a single-arm intervention study, non-surgical periodontal therapy, including oral hygiene instruction and scaling/root planing (SRP) to 10 patients with NAFLD who had 4 or more sites with periodontal pockets ≥ 5 mm led to a significant improvement in AST and ALT at 3 mo after treatment. Interestingly, a recent multicenter randomized controlled trial by Kamata et al[38,281] demonstrated the efficacy of periodontal therapy for patients with NAFLD and periodontal disease. The control group (n = 20) received oral hygiene instruction only, while the experimental group (n = 20) received oral hygiene instruction and SRP. Serum levels of ALT and anti-P. gingivalis immunoglobulin antibody titers at 12 and 60 wk after treatment were measured as primary endpoints. Results showed significant reductions in ALT levels, endotoxin levels, and liver fat content in the SRP group compared to the toothpaste group. In addition, the decrease in P. gingivalis immunoglobulin G (IgG) antibody titer was significantly higher in the SRP group than in the toothpaste group. These decreased ALT levels and P. gingivalis IgG-antibody titers in the SRP group were sustained until 60 wk from baseline. The SRP group showed significantly greater reductions in blood lipid parameters, including total cholesterol, low density lipoprotein cholesterol, and triglycerides at 12 wk post-treatment, while no significant changes in glycometabolism parameters such as glucose and insulin.
Several studies presented in this section support the potential effect of periodontal treatment in improving outcomes in NAFLD. They also strengthen the causal relationship between periodontal disease and NAFLD. However, only one high-quality randomized controlled trial has been reported to date to assess treatment efficacy, and the relationship between the two is not clear. In the future, more evidence will need to be accumulated, and these controlled trials will need to be systematically meta-analyzed.
If opportunistic pathogens and periodontopathic bacteria that predominate in oral microbiome changes cause subsequent gut microbiome dysbiosis, an approach of microbiome-targeted therapy with antibiotics, probiotics and prebiotics may help prevent the onset and progression of NAFLD for patients with periodontal disease[11,22] (Figure 2). Probiotics are defined as live cultured microorganisms that provide health benefits in humans and animals when consumed, generally by improving or restoring the gut microbiota. While, prebiotics are defined as compounds in food that induce the growth or activity of beneficial microorganisms such as bacteria and fungi by altering the composition of organisms in the gut microbiome. When probiotics and prebiotics are used in combination, this is referred to as symbiotics. On the other hand, antibiotics exert beneficial effects on metabolic disorders by non-specifically suppressing the microbiome but may be accompanied by harmful side effects and potential emergence of antibiotic-resistant bacterial strains. Thus, currently, supplementation with probiotics and prebiotics in the treatment of NAFLD and periodontal disease has been favorably accepted due to a potential improved safety for humans and the environment[282-284].
As for treatment of NAFLD, multi-strain probiotic VSL#3 (contains eight species of Streptococcus thermophilus,Bifidobacteria [B. breve, B. infantis, B. longum], Lactobacillus acidophilus, L. plantarum,L. paracasei,L. bulgaricus) was originally developed to treat inflammatory bowel disease, but VSL#3 administration has also been reported to improve fatty liver and fibrosis for NAFLD[285-287]. A randomized controlled trial by Eslamparast et al[288] in patients with NAFLD showed that compared to the placebo group, a symbiotic consisting of a probiotic cocktail similar to VSL#3 and fructo-oligosaccharides given twice daily for 28 wk significantly improved liver function, inflammation, and fibrosis grade. Furthermore, a systematic meta-analysis of 21 randomized clinical trial concluded that the consumption of probiotics or symbiotics in NAFLD patients is an effective treatment modality to improve liver function, liver hardness, and blood markers of liver damage[282].
The effect of probiotics on periodontal treatment have also been reported to have several benefits in animal and clinical studies[289]. In studies using probiotics as monotherapy or as adjunctive therapy, Lactobacillus salivarius WB21, L. plantarum, L. reuteri, L. rhamnosus SP1, Bacillus lactis, and various Streptococci showed some efficacy in suppressing periodontopathic bacteria or other anaerobic bacteria. In particular, some Lactobacillus spp. have been noted to play a protective role against both NAFLD and periodontal disease[290-292]. Despite limited, beneficial effects were also observed in improving clinical parameters of periodontal disease, such as plaque score, gingival inflammation, and host immune response factors.
In recent years, our research team has reported multiple times that the use of nisin, an antimicrobial peptide produced by Lactococcus lactis, is effective in treating periodontal disease[289,293-296]. Nisin is a type of bacteriocin that is widely used in the food industry as well as in the medical field because of its potent and broad-spectrum antimicrobial activity even in small amounts, despite its low cytotoxicity and low incidence of bacterial resistance[297-299]. Nisin is classified as a Class I bacteriocin, and is a lantibiotic (lanthionine-containing antibiotics) based on its chemical structure, because it has abnormal amino acids that are caused by translational modifications[300-302]. With regard to the mechanism of action as an antimicrobial substance, nisin attaches to lipid II present in the bacterial cell membrane, and eight molecules of nisin form a tunnel-like pore with four molecules of lipid II, thereby promoting the outflow of intracellular ions, adenosine triphosphate and other substances from the bacteria[303-306]. The production of bacteriocins, such as nisin, is strictly regulated by bacterial signaling via quorum sensing, which works together with the self-tolerance capacity of bacteriocin-producing bacteria to selectively control the bacterial flora[307].
Interestingly, we found that in artificial oral biofilms derived from saliva, administration of the nisin-producing probiotic L. lactis or nisin significantly inhibited the biofilm formation and bacterial viability, reducing periodontal pathogens while maintaining endogenous oral bacteria such as Neisseria spp[294]. Similarly, wild type L. lactis or nisin dose-dependently reduced the viability of artificial biofilms formed on titanium alloy discs mimicking peri-implantitis and shifted the composition, relative abundance, and diversity level of the biofilms toward a healthy state with improved proportions of Proteobacteria and Firmicutes phylum[295]. Furthermore, in a polymicrobial mouse model of periodontal disease infected with multiple periodontopathic bacteria, we have shown that oral administration of nisin-producing L. lactis or nisin suppressed periodontal inflammation and alveolar bone loss[293]. These microbial treatments improved oral dysbiosis and decreased serum antibody responses to periodontal pathogens. On the other hand, non-nisin-producing L. lactis also reduced the oral and systemic impacts of the periodontal polymicrobial infection, but its effects were not as pronounced as those of nisin-producing L. lactis. Surprisingly, nisin and the L. lactis probiotic also induced a reparative phenotype in vivo in this polymicrobial disease model of periodontal disease. These results suggest that the combined interaction of living bacteria and their bacteriocins is important for probiotic use to be effective.
However, the significance of microbiome-targeted therapy in the treatment of periodontal disease-related NAFLD has been studied to a limited degree. In this regard, we are currently exploring the prophylactic effects of nisin on periodontitis-induced gut dysbiosis and liver disease using the polymicrobial infection mouse model. Detailed analysis of NAFLD-related genes and glycolipid metabolism in the liver is still ongoing in our lab, but we found that nisin shifts the oral and gut microbial composition to a healthy state and mitigates inflammatory responses. Nisin may also prevent hepatic lipid deposition by regulating mitochondrial functions and oxidative stress production in liver tissue.
Few studies have evaluated the effects of L. lactis probiotics or nisin in NAFLD, but some evidence based on in vivo experiments has been reported[308-310]. Lee et al[309] (2020) showed that oral administration of L. lactis NZ3900 strain pre-stimulated with nisin significantly protected fatty liver formation and progression of early atherosclerosis in a rabbit model fed a high cholesterol diet. In a study by Naudin et al[310] (2020) using mice fed a high calorie Western diet, compared to control mice taking the beneficial bacterium Lactobacillus rhamnosus GGL, oral administration of L. lactis subsp cremoris improved glucose tolerance and reduced serum cholesterol levels, weight gain, and obesity, resulting in less hepatic lipid deposition and inflammation. Jena et al[308] also reported that Lactococcus plays a protective role against liver inflammation in mice whose intestinal environment has been compromised by a Western diet. Although human studies on this topic are very limited, Ansari et al[311] showed that a fermented herbal formula containing L. lactis effectively improved serum liver function markers and liver fat deposition.
In summary, the use of probiotics, prebiotics, and bacteriocins may be a potential therapeutic strategy for patients with periodontal disease and NAFLD. These microbiome-targeted therapies have been shown to be safe for human health and easy to use on a daily basis in an environmentally friendly manner, so they may be applied alone or in combination with periodontal therapy. The fact that adjuvant therapy with probiotics in periodontal treatment has already been applied clinically will also support the practical application of this new strategy. However, the development of microbiome-targeted therapies for NAFLD is in its infancy and further elucidation is warranted regarding the effectiveness of microbiome-targeted therapies on host immunomodulatory mechanisms, efficient intestinal delivery methods, effective combinations of various probiotics and bacteriocins, and methods for long-term maintenance of microbial composition and functional changes.
A growing body of evidence from multiple areas proposes that periodontal disease, accompanied by oral inflammation and pathological changes in the oral microbiome, induces gut dysbiosis and is involved in the pathogenesis of NAFLD[11,22] (Figure 1). The emerging concept of periodontitis-associated oral dysbiosis strongly implicates the role of the oral-gut-liver axis in the pathogenesis of NAFLD, based on the close relationship between the gut and liver connected by the enterohepatic circulation. This is due to the advancement of innovative technologies that enable comprehensive exploration of the microbiota through the widespread use of next-generation sequencing, which has fundamentally changed the definition of periodontopathic bacteria that has persisted in periodontology, and now highlights a turning point in rethinking the relationship between periodontal disease and systemic disease.
The liver is not merely a passive organ; NAFLD itself enhances insulin resistance and increases the risk of diabetes, atherosclerosis, and myocardial infarction with exacerbation of metabolic syndrome[312-314]. This means that even if NAFLD does not lead to end-stage liver disease, such as cirrhosis or cancer in individuals with metabolic syndrome, it can promote extrahepatic complications that can lead to significant mortality-related disease. Thus, both periodontal disease and NAFLD are interrelated with various elements of metabolic syndrome, leading to a vicious cycle of systemic inflammation and metabolic disturbance through amplification of these disease triangles[315]. Furthermore, in recent years, the liver from patients with NAFLD, as in the elderly and post-cardiac arrest donors, is a marginal organ that increases the risk of poor prognosis after liver resection or liver transplantation, the main treatment modalities for liver disease[316,317]. The detailed pathomechanisms of hepatic ischemia-reperfusion injury and post-transplant rejection in these operations are not known, but periodontal disease may be a potential risk factor for these hepatotoxic events as well[316,318-320].
Thus, oral and gut microbiome-targeted therapy based on management of periodontal infections would hold great promise for preventing the onset and progression of NAFLD and subsequent complications (Figure 2). Studies suggesting the efficacy of conventional periodontal treatment and micro
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: The Japanese Society of Periodontology; The International Association for Dental Research.
Specialty type: Dentistry, oral surgery and medicine
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bukhari SM, Saudi Arabia; Galmiche M, France S-Editor: Liu GL L-Editor: A P-Editor: Liu GL
| 1. | Page RC. The pathobiology of periodontal diseases may affect systemic diseases: inversion of a paradigm. Ann Periodontol. 1998;3:108-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 214] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 2. | Lamont RJ, Koo H, Hajishengallis G. The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol. 2018;16:745-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 949] [Cited by in RCA: 1294] [Article Influence: 184.9] [Reference Citation Analysis (0)] |
| 3. | Williams RC. Periodontal disease. N Engl J Med. 1990;322:373-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 409] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 4. | Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL, Jr. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3166] [Cited by in RCA: 3393] [Article Influence: 125.7] [Reference Citation Analysis (0)] |
| 5. | Socransky SS, Haffajee AD. Dental biofilms: difficult therapeutic targets. Periodontol 2000. 2002;28:12-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 701] [Cited by in RCA: 724] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 6. | Hajishengallis G, Lamont RJ. Polymicrobial communities in periodontal disease: Their quasi-organismal nature and dialogue with the host. Periodontol 2000. 2021;86:210-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 161] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 7. | Lamont RJ, Hajishengallis G. Polymicrobial synergy and dysbiosis in inflammatory disease. Trends Mol Med. 2015;21:172-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 377] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 8. | Shiba T, Watanabe T, Kachi H, Koyanagi T, Maruyama N, Murase K, Takeuchi Y, Maruyama F, Izumi Y, Nakagawa I. Distinct interacting core taxa in co-occurrence networks enable discrimination of polymicrobial oral diseases with similar symptoms. Sci Rep. 2016;6:30997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 9. | Gao L, Kang M, Zhang MJ, Reza Sailani M, Kuraji R, Martinez A, Ye C, Kamarajan P, Le C, Zhan L, Rangé H, Ho SP, Kapila YL. Polymicrobial periodontal disease triggers a wide radius of effect and unique virome. NPJ Biofilms Microbiomes. 2020;6:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 10. | Kapila YL. Oral health's inextricable connection to systemic health: Special populations bring to bear multimodal relationships and factors connecting periodontal disease to systemic diseases and conditions. Periodontol 2000. 2021;87:11-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 188] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 11. | Kuraji R, Sekino S, Kapila Y, Numabe Y. Periodontal disease-related nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: An emerging concept of oral-liver axis. Periodontol 2000 2021; 87: 204-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 12. | Hujoel PP, White BA, García RI, Listgarten MA. The dentogingival epithelial surface area revisited. J Periodontal Res. 2001;36:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 132] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Frank RM. Bacterial penetration in the apical pocket wall of advanced human periodontitis. J Periodontal Res. 1980;15:563-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 137] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Konkel JE, O'Boyle C, Krishnan S. Distal Consequences of Oral Inflammation. Front Immunol. 2019;10:1403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 15. | Amado PPP, Kawamoto D, Albuquerque-Souza E, Franco DC, Saraiva L, Casarin RCV, Horliana ACRT, Mayer MPA. Oral and Fecal Microbiome in Molar-Incisor Pattern Periodontitis. Front Cell Infect Microbiol. 2020;10:583761. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Kawamoto D, Borges R, Ribeiro RA, de Souza RF, Amado PPP, Saraiva L, Horliana ACRT, Faveri M, Mayer MPA. Oral Dysbiosis in Severe Forms of Periodontitis Is Associated With Gut Dysbiosis and Correlated With Salivary Inflammatory Mediators: A Preliminary Study. Front Oral Health. 2021;2:722495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 17. | Atarashi K, Suda W, Luo C, Kawaguchi T, Motoo I, Narushima S, Kiguchi Y, Yasuma K, Watanabe E, Tanoue T, Thaiss CA, Sato M, Toyooka K, Said HS, Yamagami H, Rice SA, Gevers D, Johnson RC, Segre JA, Chen K, Kolls JK, Elinav E, Morita H, Xavier RJ, Hattori M, Honda K. Ectopic colonization of oral bacteria in the intestine drives T(H)1 cell induction and inflammation. Science. 2017;358:359-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 648] [Article Influence: 92.6] [Reference Citation Analysis (0)] |
| 18. | Bao J, Li L, Zhang Y, Wang M, Chen F, Ge S, Chen B, Yan F. Periodontitis may induce gut microbiota dysbiosis via salivary microbiota. Int J Oral Sci. 2022;14:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 105] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 19. | Lourenςo TGB, Spencer SJ, Alm EJ, Colombo APV. Defining the gut microbiota in individuals with periodontal diseases: an exploratory study. J Oral Microbiol. 2018;10:1487741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 108] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 20. | Kitamoto S, Nagao-Kitamoto H, Hein R, Schmidt TM, Kamada N. The Bacterial Connection between the Oral Cavity and the Gut Diseases. J Dent Res. 2020;99:1021-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 242] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 21. | Kitamoto S, Nagao-Kitamoto H, Jiao Y, Gillilland MG 3rd, Hayashi A, Imai J, Sugihara K, Miyoshi M, Brazil JC, Kuffa P, Hill BD, Rizvi SM, Wen F, Bishu S, Inohara N, Eaton KA, Nusrat A, Lei YL, Giannobile WV, Kamada N. The Intermucosal Connection between the Mouth and Gut in Commensal Pathobiont-Driven Colitis. Cell. 2020;182:447-462.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 416] [Article Influence: 83.2] [Reference Citation Analysis (0)] |
| 22. | Kuraji R, Kapila Y, Numabe Y. Periodontal Disease and Nonalcoholic Fatty Liver Disease: New Microbiome-Targeted Therapy Based on the Oral–Gut–Liver Axis Concept. Curr Oral Health Rep. 2022;9:89-102. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Rinella M, Charlton M. The globalization of nonalcoholic fatty liver disease: Prevalence and impact on world health. Hepatology. 2016;64:19-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 166] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 24. | Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3655] [Cited by in RCA: 3719] [Article Influence: 161.7] [Reference Citation Analysis (2)] |
| 25. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2413] [Cited by in RCA: 2613] [Article Influence: 201.0] [Reference Citation Analysis (1)] |
| 26. | Calzadilla Bertot L, Adams LA. The Natural Course of Non-Alcoholic Fatty Liver Disease. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 339] [Cited by in RCA: 457] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 27. | Pais R, Charlotte F, Fedchuk L, Bedossa P, Lebray P, Poynard T, Ratziu V; LIDO Study Group. A systematic review of follow-up biopsies reveals disease progression in patients with non-alcoholic fatty liver. J Hepatol. 2013;59:550-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 384] [Article Influence: 32.0] [Reference Citation Analysis (1)] |
| 28. | Younossi ZM. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: Implications for liver transplantation. Liver Transpl. 2018;24:166-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 29. | Arimatsu K, Yamada H, Miyazawa H, Minagawa T, Nakajima M, Ryder MI, Gotoh K, Motooka D, Nakamura S, Iida T, Yamazaki K. Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota. Sci Rep. 2014;4:4828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 263] [Cited by in RCA: 401] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 30. | Komazaki R, Katagiri S, Takahashi H, Maekawa S, Shiba T, Takeuchi Y, Kitajima Y, Ohtsu A, Udagawa S, Sasaki N, Watanabe K, Sato N, Miyasaka N, Eguchi Y, Anzai K, Izumi Y. Periodontal pathogenic bacteria, Aggregatibacter actinomycetemcomitans affect non-alcoholic fatty liver disease by altering gut microbiota and glucose metabolism. Sci Rep. 2017;7:13950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 31. | Nakajima M, Arimatsu K, Kato T, Matsuda Y, Minagawa T, Takahashi N, Ohno H, Yamazaki K. Oral Administration of P. gingivalis Induces Dysbiosis of Gut Microbiota and Impaired Barrier Function Leading to Dissemination of Enterobacteria to the Liver. PLoS One. 2015;10:e0134234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 274] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 32. | Yamazaki K, Kato T, Tsuboi Y, Miyauchi E, Suda W, Sato K, Nakajima M, Yokoji-Takeuchi M, Yamada-Hara M, Tsuzuno T, Matsugishi A, Takahashi N, Tabeta K, Miura N, Okuda S, Kikuchi J, Ohno H, Yamazaki K. Oral Pathobiont-Induced Changes in Gut Microbiota Aggravate the Pathology of Nonalcoholic Fatty Liver Disease in Mice. Front Immunol. 2021;12:766170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 33. | Kashiwagi Y, Aburaya S, Sugiyama N, Narukawa Y, Sakamoto Y, Takahashi M, Uemura H, Yamashita R, Tominaga S, Hayashi S, Nozaki T, Yamada S, Izumi Y, Kashiwagi A, Bamba T, Ishihama Y, Murakami S. Porphyromonas gingivalis induces entero-hepatic metabolic derangements with alteration of gut microbiota in a type 2 diabetes mouse model. Sci Rep. 2021;11:18398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 34. | Helenius-Hietala J, Suominen AL, Ruokonen H, Knuuttila M, Puukka P, Jula A, Meurman JH, Åberg F. Periodontitis is associated with incident chronic liver disease-A population-based cohort study. Liver Int. 2019;39:583-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 35. | Nakahara T, Hyogo H, Ono A, Nagaoki Y, Kawaoka T, Miki D, Tsuge M, Hiraga N, Hayes CN, Hiramatsu A, Imamura M, Kawakami Y, Aikata H, Ochi H, Abe-Chayama H, Furusho H, Shintani T, Kurihara H, Miyauchi M, Takata T, Arihiro K, Chayama K. Involvement of Porphyromonas gingivalis in the progression of non-alcoholic fatty liver disease. J Gastroenterol. 2018;53:269-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 36. | Yoneda M, Naka S, Nakano K, Wada K, Endo H, Mawatari H, Imajo K, Nomura R, Hokamura K, Ono M, Murata S, Tohnai I, Sumida Y, Shima T, Kuboniwa M, Umemura K, Kamisaki Y, Amano A, Okanoue T, Ooshima T, Nakajima A. Involvement of a periodontal pathogen, Porphyromonas gingivalis on the pathogenesis of non-alcoholic fatty liver disease. BMC Gastroenterol. 2012;12:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 206] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 37. | Sato S, Kamata Y, Kessoku T, Shimizu T, Kobayashi T, Kurihashi T, Takashiba S, Hatanaka K, Hamada N, Kodama T, Higurashi T, Taguri M, Yoneda M, Usuda H, Wada K, Nakajima A, Morozumi T, Minabe M. A cross-sectional study assessing the relationship between non-alcoholic fatty liver disease and periodontal disease. Sci Rep. 2022;12:13621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 38. | Kamata Y, Kessoku T, Shimizu T, Sato S, Kobayashi T, Kurihashi T, Morozumi T, Iwasaki T, Takashiba S, Hatanaka K, Hamada N, Kodama T, Higurashi T, Taguri M, Yoneda M, Usuda H, Wada K, Nakajima A, Minabe M. Periodontal Treatment and Usual Care for Nonalcoholic Fatty Liver Disease: A Multicenter, Randomized Controlled Trial. Clin Transl Gastroenterol. 2022;13:e00520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 39. | Gao B, Jeong WI, Tian Z. Liver: An organ with predominant innate immunity. Hepatology. 2008;47:729-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 721] [Article Influence: 42.4] [Reference Citation Analysis (1)] |
| 40. | Velázquez-Miranda E, Díaz-Muñoz M, Vázquez-Cuevas FG. Purinergic signaling in hepatic disease. Purinergic Signal. 2019;15:477-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 41. | Volynets V, Küper MA, Strahl S, Maier IB, Spruss A, Wagnerberger S, Königsrainer A, Bischoff SC, Bergheim I. Nutrition, intestinal permeability, and blood ethanol levels are altered in patients with nonalcoholic fatty liver disease (NAFLD). Dig Dis Sci. 2012;57:1932-1941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 207] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 42. | Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, Gill SR. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57:601-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 1281] [Article Influence: 106.8] [Reference Citation Analysis (1)] |
| 43. | Raman M, Ahmed I, Gillevet PM, Probert CS, Ratcliffe NM, Smith S, Greenwood R, Sikaroodi M, Lam V, Crotty P, Bailey J, Myers RP, Rioux KP. Fecal microbiome and volatile organic compound metabolome in obese humans with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2013;11:868-75.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 520] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 44. | Spencer MD, Hamp TJ, Reid RW, Fischer LM, Zeisel SH, Fodor AA. Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. Gastroenterology. 2011;140:976-986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 498] [Cited by in RCA: 544] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 45. | Wigg AJ, Roberts-Thomson IC, Dymock RB, McCarthy PJ, Grose RH, Cummins AG. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001;48:206-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 610] [Cited by in RCA: 629] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 46. | Farhadi A, Gundlapalli S, Shaikh M, Frantzides C, Harrell L, Kwasny MM, Keshavarzian A. Susceptibility to gut leakiness: a possible mechanism for endotoxaemia in non-alcoholic steatohepatitis. Liver Int. 2008;28:1026-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 183] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 47. | Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, Fine DH, Flemmig TF, Garcia R, Giannobile WV, Graziani F, Greenwell H, Herrera D, Kao RT, Kebschull M, Kinane DF, Kirkwood KL, Kocher T, Kornman KS, Kumar PS, Loos BG, Machtei E, Meng H, Mombelli A, Needleman I, Offenbacher S, Seymour GJ, Teles R, Tonetti MS. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Clin Periodontol. 2018;45 Suppl 20:S162-S170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 724] [Article Influence: 120.7] [Reference Citation Analysis (1)] |
| 48. | Eke PI, Thornton-Evans GO, Wei L, Borgnakke WS, Dye BA, Genco RJ. Periodontitis in US Adults: National Health and Nutrition Examination Survey 2009-2014. J Am Dent Assoc. 2018;149:576-588.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 408] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 49. | Petersen PE. The World Oral Health Report 2003: continuous improvement of oral health in the 21st century--the approach of the WHO Global Oral Health Programme. Community Dent Oral Epidemiol. 2003;31 Suppl 1:3-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1254] [Cited by in RCA: 1433] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 50. | Lalla E, Lamster IB, Drury S, Fu C, Schmidt AM. Hyperglycemia, glycoxidation and receptor for advanced glycation endproducts: potential mechanisms underlying diabetic complications, including diabetes-associated periodontitis. Periodontol 2000. 2000;23:50-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 101] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 51. | Genco R, Offenbacher S, Beck J. Periodontal disease and cardiovascular disease: epidemiology and possible mechanisms. J Am Dent Assoc. 2002;133 Suppl:14S-22S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 177] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 52. | Hujoel PP, Drangsholt M, Spiekerman C, DeRouen TA. Periodontal disease and coronary heart disease risk. JAMA. 2000;284:1406-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 292] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 53. | Stewart R, West M. Increasing Evidence for an Association Between Periodontitis and Cardiovascular Disease. Circulation. 2016;133:549-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 54. | Minagawa K, Iwasaki M, Ogawa H, Yoshihara A, Miyazaki H. Relationship between metabolic syndrome and periodontitis in 80-year-old Japanese subjects. J Periodontal Res. 2015;50:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 55. | Linden GJ, Lyons A, Scannapieco FA. Periodontal systemic associations: review of the evidence. J Periodontol. 2013;84:S8-S19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 133] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 56. | Mealey BL, Oates TW; American Academy of Periodontology. Diabetes mellitus and periodontal diseases. J Periodontol. 2006;77:1289-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 537] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 57. | Monsarrat P, Blaizot A, Kémoun P, Ravaud P, Nabet C, Sixou M, Vergnes JN. Clinical research activity in periodontal medicine: a systematic mapping of trial registers. J Clin Periodontol. 2016;43:390-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 114] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 58. | Papapanou PN. Systemic effects of periodontitis: lessons learned from research on atherosclerotic vascular disease and adverse pregnancy outcomes. Int Dent J. 2015;65:283-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 59. | Seymour GJ, Ford PJ, Cullinan MP, Leishman S, Yamazaki K. Relationship between periodontal infections and systemic disease. Clin Microbiol Infect. 2007;13 Suppl 4:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 436] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 60. | Williams RC. Understanding and managing periodontal diseases: a notable past, a promising future. J Periodontol. 2008;79:1552-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 61. | Chen LP, Chiang CK, Peng YS, Hsu SP, Lin CY, Lai CF, Hung KY. Relationship between periodontal disease and mortality in patients treated with maintenance hemodialysis. Am J Kidney Dis. 2011;57:276-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 62. | Fisher MA, Taylor GW. A prediction model for chronic kidney disease includes periodontal disease. J Periodontol. 2009;80:16-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 63. | Fisher MA, Taylor GW, West BT, McCarthy ET. Bidirectional relationship between chronic kidney and periodontal disease: a study using structural equation modeling. Kidney Int. 2011;79:347-355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 64. | Kshirsagar AV, Offenbacher S, Moss KL, Barros SP, Beck JD. Antibodies to periodontal organisms are associated with decreased kidney function. The Dental Atherosclerosis Risk In Communities study. Blood Purif. 2007;25:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 65. | Shultis WA, Weil EJ, Looker HC, Curtis JM, Shlossman M, Genco RJ, Knowler WC, Nelson RG. Effect of periodontitis on overt nephropathy and end-stage renal disease in type 2 diabetes. Diabetes Care. 2007;30:306-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 191] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 66. | Ahn J, Segers S, Hayes RB. Periodontal disease, Porphyromonas gingivalis serum antibody levels and orodigestive cancer mortality. Carcinogenesis. 2012;33:1055-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 260] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 67. | Fitzpatrick SG, Katz J. The association between periodontal disease and cancer: a review of the literature. J Dent. 2010;38:83-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 68. | Michaud DS, Joshipura K, Giovannucci E, Fuchs CS. A prospective study of periodontal disease and pancreatic cancer in US male health professionals. J Natl Cancer Inst. 2007;99:171-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 210] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 69. | Michaud DS, Liu Y, Meyer M, Giovannucci E, Joshipura K. Periodontal disease, tooth loss, and cancer risk in male health professionals: a prospective cohort study. Lancet Oncol. 2008;9:550-558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 300] [Cited by in RCA: 290] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 70. | Tamaki N, Takaki A, Tomofuji T, Endo Y, Kasuyama K, Ekuni D, Yasunaka T, Yamamoto K, Morita M. Stage of hepatocellular carcinoma is associated with periodontitis. J Clin Periodontol. 2011;38:1015-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 71. | de Pablo P, Chapple IL, Buckley CD, Dietrich T. Periodontitis in systemic rheumatic diseases. Nat Rev Rheumatol. 2009;5:218-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 329] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 72. | de Pablo P, Dietrich T, McAlindon TE. Association of periodontal disease and tooth loss with rheumatoid arthritis in the US population. J Rheumatol. 2008;35:70-76. [PubMed] |
| 73. | Kamer AR, Morse DE, Holm-Pedersen P, Mortensen EL, Avlund K. Periodontal inflammation in relation to cognitive function in an older adult Danish population. J Alzheimers Dis. 2012;28:613-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 74. | Kaye EK, Valencia A, Baba N, Spiro A 3rd, Dietrich T, Garcia RI. Tooth loss and periodontal disease predict poor cognitive function in older men. J Am Geriatr Soc. 2010;58:713-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 199] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 75. | Sparks Stein P, Steffen MJ, Smith C, Jicha G, Ebersole JL, Abner E, Dawson D 3rd. Serum antibodies to periodontal pathogens are a risk factor for Alzheimer's disease. Alzheimers Dement. 2012;8:196-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 314] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 76. | Stewart R, Sabbah W, Tsakos G, D'Aiuto F, Watt RG. Oral health and cognitive function in the Third National Health and Nutrition Examination Survey (NHANES III). Psychosom Med. 2008;70:936-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 77. | Yu YH, Kuo HK. Association between cognitive function and periodontal disease in older adults. J Am Geriatr Soc. 2008;56:1693-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 78. | Borgnakke WS, Ylöstalo PV, Taylor GW, Genco RJ. Effect of periodontal disease on diabetes: systematic review of epidemiologic observational evidence. J Periodontol. 2013;84:S135-S152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 193] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 79. | Taylor GW, Burt BA, Becker MP, Genco RJ, Shlossman M, Knowler WC, Pettitt DJ. Severe Periodontitis and Risk for Poor Glycemic Control in Patients with Non-Insulin-Dependent Diabetes Mellitus. J Periodontol. 1996;67 Suppl 10S:1085-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 373] [Article Influence: 12.9] [Reference Citation Analysis (1)] |
| 80. | Engebretson S, Kocher T. Evidence that periodontal treatment improves diabetes outcomes: a systematic review and meta-analysis. J Periodontol. 2013;84:S153-S169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 81. | Sgolastra F, Severino M, Pietropaoli D, Gatto R, Monaco A. Effectiveness of periodontal treatment to improve metabolic control in patients with chronic periodontitis and type 2 diabetes: a meta-analysis of randomized clinical trials. J Periodontol. 2013;84:958-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 82. | Teeuw WJ, Gerdes VE, Loos BG. Effect of periodontal treatment on glycemic control of diabetic patients: a systematic review and meta-analysis. Diabetes Care. 2010;33:421-427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 373] [Cited by in RCA: 316] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 83. | Wu CZ, Yuan YH, Liu HH, Li SS, Zhang BW, Chen W, An ZJ, Chen SY, Wu YZ, Han B, Li CJ, Li LJ. Epidemiologic relationship between periodontitis and type 2 diabetes mellitus. BMC Oral Health. 2020;20:204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 216] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 84. | Zheng M, Wang C, Ali A, Shih YA, Xie Q, Guo C. Prevalence of periodontitis in people clinically diagnosed with diabetes mellitus: a meta-analysis of epidemiologic studies. Acta Diabetol. 2021;58:1307-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 85. | Loos BG, Craandijk J, Hoek FJ, Wertheim-van Dillen PM, van der Velden U. Elevation of systemic markers related to cardiovascular diseases in the peripheral blood of periodontitis patients. J Periodontol. 2000;71:1528-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 547] [Article Influence: 21.9] [Reference Citation Analysis (1)] |
| 86. | D'Aiuto F, Parkar M, Andreou G, Suvan J, Brett PM, Ready D, Tonetti MS. Periodontitis and systemic inflammation: control of the local infection is associated with a reduction in serum inflammatory markers. J Dent Res. 2004;83:156-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 472] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 87. | Sammalkorpi K. Glucose intolerance in acute infections. J Intern Med. 1989;225:15-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 88. | Yki-Järvinen H, Sammalkorpi K, Koivisto VA, Nikkilä EA. Severity, duration, and mechanisms of insulin resistance during acute infections. J Clin Endocrinol Metab. 1989;69:317-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 88] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 89. | Rapone B, Ferrara E, Corsalini M, Qorri E, Converti I, Lorusso F, Delvecchio M, Gnoni A, Scacco S, Scarano A. Inflammatory Status and Glycemic Control Level of Patients with Type 2 Diabetes and Periodontitis: A Randomized Clinical Trial. Int J Environ Res Public Health. 2021;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 90. | Torumtay G, Kırzıoğlu FY, Öztürk Tonguç M, Kale B, Calapoğlu M, Orhan H. Effects of periodontal treatment on inflammation and oxidative stress markers in patients with metabolic syndrome. J Periodontal Res. 2016;51:489-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 91. | Lee YS, Olefsky J. Chronic tissue inflammation and metabolic disease. Genes Dev. 2021;35:307-328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 174] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 92. | Taylor JJ, Preshaw PM, Lalla E. A review of the evidence for pathogenic mechanisms that may link periodontitis and diabetes. J Periodontol. 2013;84:S113-S134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 93. | Tilg H, Moschen AR. Microbiota and diabetes: an evolving relationship. Gut. 2014;63:1513-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 559] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 94. | Tai N, Wong FS, Wen L. The role of gut microbiota in the development of type 1, type 2 diabetes mellitus and obesity. Rev Endocr Metab Disord. 2015;16:55-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 186] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 95. | Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, Nielsen J, Bäckhed F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1766] [Cited by in RCA: 2146] [Article Influence: 178.8] [Reference Citation Analysis (0)] |
| 96. | Karlsson F, Tremaroli V, Nielsen J, Bäckhed F. Assessing the human gut microbiota in metabolic diseases. Diabetes. 2013;62:3341-3349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 304] [Cited by in RCA: 311] [Article Influence: 25.9] [Reference Citation Analysis (1)] |
| 97. | Fei N, Zhao L. An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. ISME J. 2013;7:880-884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 422] [Cited by in RCA: 541] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 98. | Zhao L, Zhang F, Ding X, Wu G, Lam YY, Wang X, Fu H, Xue X, Lu C, Ma J, Yu L, Xu C, Ren Z, Xu Y, Xu S, Shen H, Zhu X, Shi Y, Shen Q, Dong W, Liu R, Ling Y, Zeng Y, Zhang Q, Wang J, Wang L, Wu Y, Zeng B, Wei H, Zhang M, Peng Y, Zhang C. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 2018;359:1151-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1058] [Cited by in RCA: 1542] [Article Influence: 220.3] [Reference Citation Analysis (68)] |
| 99. | Li Q, Gao Z, Wang H, Wu H, Liu Y, Yang Y, Han L, Wang X, Zhao L, Tong X. Intestinal Immunomodulatory Cells (T Lymphocytes): A Bridge between Gut Microbiota and Diabetes. Mediators Inflamm. 2018;2018:9830939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 100. | Casarin RC, Barbagallo A, Meulman T, Santos VR, Sallum EA, Nociti FH, Duarte PM, Casati MZ, Gonçalves RB. Subgingival biodiversity in subjects with uncontrolled type-2 diabetes and chronic periodontitis. J Periodontal Res. 2013;48:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 170] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 101. | Farina R, Severi M, Carrieri A, Miotto E, Sabbioni S, Trombelli L, Scapoli C. Whole metagenomic shotgun sequencing of the subgingival microbiome of diabetics and non-diabetics with different periodontal conditions. Arch Oral Biol. 2019;104:13-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 102. | Yang Y, Liu S, Wang Y, Wang Z, Ding W, Sun X, He K, Feng Q, Zhang X. Changes of saliva microbiota in the onset and after the treatment of diabetes in patients with periodontitis. Aging (Albany NY). 2020;12:13090-13114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 103. | Matsha TE, Prince Y, Davids S, Chikte U, Erasmus RT, Kengne AP, Davison GM. Oral Microbiome Signatures in Diabetes Mellitus and Periodontal Disease. J Dent Res. 2020;99:658-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 102] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 104. | Sabharwal A, Ganley K, Miecznikowski JC, Haase EM, Barnes V, Scannapieco FA. The salivary microbiome of diabetic and non-diabetic adults with periodontal disease. J Periodontol. 2019;90:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 105. | Ogawa T, Honda-Ogawa M, Ikebe K, Notomi Y, Iwamoto Y, Shirobayashi I, Hata S, Kibi M, Masayasu S, Sasaki S, Kawabata S, Maeda Y. Characterizations of oral microbiota in elderly nursing home residents with diabetes. J Oral Sci. 2017;59:549-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 106. | Lambeth SM, Carson T, Lowe J, Ramaraj T, Leff JW, Luo L, Bell CJ, Shah VO. Composition, Diversity and Abundance of Gut Microbiome in Prediabetes and Type 2 Diabetes. J Diabetes Obes. 2015;2:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 107. | Longo PL, Dabdoub S, Kumar P, Artese HPC, Dib SA, Romito GA, Mayer MPA. Glycaemic status affects the subgingival microbiome of diabetic patients. J Clin Periodontol. 2018;45:932-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 108. | Tam J, Hoffmann T, Fischer S, Bornstein S, Gräßler J, Noack B. Obesity alters composition and diversity of the oral microbiota in patients with type 2 diabetes mellitus independently of glycemic control. PLoS One. 2018;13:e0204724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 109. | Shi B, Lux R, Klokkevold P, Chang M, Barnard E, Haake S, Li H. The subgingival microbiome associated with periodontitis in type 2 diabetes mellitus. ISME J. 2020;14:519-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 110. | Simmons RK, Alberti KG, Gale EA, Colagiuri S, Tuomilehto J, Qiao Q, Ramachandran A, Tajima N, Brajkovich Mirchov I, Ben-Nakhi A, Reaven G, Hama Sambo B, Mendis S, Roglic G. The metabolic syndrome: useful concept or clinical tool? Diabetologia. 2010;53:600-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 295] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 111. | Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008;28:629-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 939] [Cited by in RCA: 1001] [Article Influence: 58.9] [Reference Citation Analysis (0)] |
| 112. | Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, Montori VM. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 1318] [Article Influence: 73.2] [Reference Citation Analysis (0)] |
| 113. | Lorenzo C, Williams K, Hunt KJ, Haffner SM. The National Cholesterol Education Program - Adult Treatment Panel III, International Diabetes Federation, and World Health Organization definitions of the metabolic syndrome as predictors of incident cardiovascular disease and diabetes. Diabetes Care. 2007;30:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 405] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 114. | Moore JX, Chaudhary N, Akinyemiju T. Metabolic Syndrome Prevalence by Race/Ethnicity and Sex in the United States, National Health and Nutrition Examination Survey, 1988-2012. Prev Chronic Dis. 2017;14:E24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 425] [Cited by in RCA: 632] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 115. | Miller JM, Kaylor MB, Johannsson M, Bay C, Churilla JR. Prevalence of metabolic syndrome and individual criterion in US adolescents: 2001-2010 National Health and Nutrition Examination Survey. Metab Syndr Relat Disord. 2014;12:527-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 116. | Day C. Metabolic syndrome, or What you will: definitions and epidemiology. Diab Vasc Dis Res. 2007;4:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 188] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 117. | Stone NJ, Bilek S, Rosenbaum S. Recent National Cholesterol Education Program Adult Treatment Panel III update: adjustments and options. Am J Cardiol. 2005;96:53E-59E. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 175] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 118. | Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20476] [Cited by in RCA: 20693] [Article Influence: 862.2] [Reference Citation Analysis (2)] |
| 119. | American Heart Association; National Heart, Lung, and Blood Institue, Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Executive summary. Cardiol Rev. 2005;13:322-327. [PubMed] |
| 120. | Matsuzawa Y, Funahashi T, Nakamura T. The concept of metabolic syndrome: contribution of visceral fat accumulation and its molecular mechanism. J Atheroscler Thromb. 2011;18:629-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 308] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 121. | Arcaro G, Cretti A, Balzano S, Lechi A, Muggeo M, Bonora E, Bonadonna RC. Insulin causes endothelial dysfunction in humans: sites and mechanisms. Circulation. 2002;105:576-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 286] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 122. | Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113:1888-1904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1082] [Cited by in RCA: 1182] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 123. | Sjöholm A, Nyström T. Endothelial inflammation in insulin resistance. Lancet. 2005;365:610-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 137] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 124. | Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4273] [Cited by in RCA: 4502] [Article Influence: 225.1] [Reference Citation Analysis (0)] |
| 125. | Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr; International Diabetes Federation Task Force on Epidemiology and Prevention; Hational Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8720] [Cited by in RCA: 10560] [Article Influence: 660.0] [Reference Citation Analysis (0)] |
| 126. | Shimazaki Y, Saito T, Yonemoto K, Kiyohara Y, Iida M, Yamashita Y. Relationship of metabolic syndrome to periodontal disease in Japanese women: the Hisayama Study. J Dent Res. 2007;86:271-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 143] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 127. | Tegelberg P, Tervonen T, Knuuttila M, Jokelainen J, Keinänen-Kiukaanniemi S, Auvinen J, Ylöstalo P. Long-term metabolic syndrome is associated with periodontal pockets and alveolar bone loss. J Clin Periodontol. 2019;46:799-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 128. | Jaramillo A, Contreras A, Lafaurie GI, Duque A, Ardila CM, Duarte S, Osorio L. Association of metabolic syndrome and chronic periodontitis in Colombians. Clin Oral Investig. 2017;21:1537-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 129. | Kikui M, Kokubo Y, Ono T, Kida M, Kosaka T, Yamamoto M, Watanabe M, Maeda Y, Miyamoto Y. Relationship between Metabolic Syndrome Components and Periodontal Disease in a Japanese General Population: the Suita Study. J Atheroscler Thromb. 2017;24:495-507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 130. | Campos JR, Costa FO, Cota LOM. Association between periodontitis and metabolic syndrome: A case-control study. J Periodontol. 2020;91:784-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 131. | Daudt LD, Musskopf ML, Mendez M, Remonti LLR, Leitão CB, Gross JL, Weidlich P, Oppermann RV. Association between metabolic syndrome and periodontitis: a systematic review and meta-analysis. Braz Oral Res. 2018;32:e35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 132. | Nibali L, Tatarakis N, Needleman I, Tu YK, D'Aiuto F, Rizzo M, Donos N. Clinical review: Association between metabolic syndrome and periodontitis: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2013;98:913-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 167] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 133. | Gobin R, Tian D, Liu Q, Wang J. Periodontal Diseases and the Risk of Metabolic Syndrome: An Updated Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne). 2020;11:336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 134. | Watanabe K, Cho YD. Periodontal disease and metabolic syndrome: a qualitative critical review of their association. Arch Oral Biol. 2014;59:855-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 135. | Amar S, Zhou Q, Shaik-Dasthagirisaheb Y, Leeman S. Diet-induced obesity in mice causes changes in immune responses and bone loss manifested by bacterial challenge. Proc Natl Acad Sci U S A. 2007;104:20466-20471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 195] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 136. | Jin J, Machado ER, Yu H, Zhang X, Lu Z, Li Y, Lopes-Virella MF, Kirkwood KL, Huang Y. Simvastatin inhibits LPS-induced alveolar bone loss during metabolic syndrome. J Dent Res. 2014;93:294-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 137. | Li Y, Lu Z, Zhang X, Yu H, Kirkwood KL, Lopes-Virella MF, Huang Y. Metabolic syndrome exacerbates inflammation and bone loss in periodontitis. J Dent Res. 2015;94:362-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 138. | Kuraji R, Fujita M, Ito H, Hashimoto S, Numabe Y. Effects of experimental periodontitis on the metabolic system in rats with diet-induced obesity (DIO): an analysis of serum biochemical parameters. Odontology. 2018;106:162-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 139. | Kuraji R, Ito H, Fujita M, Ishiguro H, Hashimoto S, Numabe Y. Porphyromonas gingivalis induced periodontitis exacerbates progression of non-alcoholic steatohepatitis in rats. Clin Exp Dent Res. 2016;2:216-225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 140. | Kaye EK, Chen N, Cabral HJ, Vokonas P, Garcia RI. Metabolic Syndrome and Periodontal Disease Progression in Men. J Dent Res. 2016;95:822-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 141. | Iwasaki M, Sato M, Minagawa K, Manz MC, Yoshihara A, Miyazaki H. Longitudinal relationship between metabolic syndrome and periodontal disease among Japanese adults aged ≥70 years: the Niigata Study. J Periodontol. 2015;86:491-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 142. | Pham T. The association between periodontal disease severity and metabolic syndrome in Vietnamese patients. Int J Dent Hyg. 2018;16:484-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 143. | Adachi N, Kobayashi Y. One-year follow-up study on associations between dental caries, periodontitis, and metabolic syndrome. J Oral Sci. 2020;62:52-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 144. | Timonen P, Niskanen M, Suominen-Taipale L, Jula A, Knuuttila M, Ylöstalo P. Metabolic syndrome, periodontal infection, and dental caries. J Dent Res. 2010;89:1068-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 145. | Musskopf ML, Daudt LD, Weidlich P, Gerchman F, Gross JL, Oppermann RV. Metabolic syndrome as a risk indicator for periodontal disease and tooth loss. Clin Oral Investig. 2017;21:675-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 146. | LaMonte MJ, Williams AM, Genco RJ, Andrews CA, Hovey KM, Millen AE, Browne RW, Trevisan M, Wactawski-Wende J. Association between metabolic syndrome and periodontal disease measures in postmenopausal women: the Buffalo OsteoPerio study. J Periodontol. 2014;85:1489-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 147. | Nascimento GG, Leite FRM, Peres KG, Demarco FF, Corrêa MB, Peres MA. Metabolic syndrome and periodontitis: A structural equation modeling approach. J Periodontol. 2019;90:655-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 148. | Borges PK, Gimeno SG, Tomita NE, Ferreira SR. [Prevalence and characteristics associated with metabolic syndrome in Japanese-Brazilians with and without periodontal disease]. Cad Saude Publica. 2007;23:657-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 149. | Kobayashi Y, Niu K, Guan L, Momma H, Guo H, Cui Y, Nagatomi R. Oral health behavior and metabolic syndrome and its components in adults. J Dent Res. 2012;91:479-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 150. | Nesbitt MJ, Reynolds MA, Shiau H, Choe K, Simonsick EM, Ferrucci L. Association of periodontitis and metabolic syndrome in the Baltimore Longitudinal Study of Aging. Aging Clin Exp Res. 2010;22:238-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 151. | Morita T, Yamazaki Y, Mita A, Takada K, Seto M, Nishinoue N, Sasaki Y, Motohashi M, Maeno M. A cohort study on the association between periodontal disease and the development of metabolic syndrome. J Periodontol. 2010;81:512-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 152. | López NJ, Quintero A, Casanova PA, Ibieta CI, Baelum V, López R. Effects of periodontal therapy on systemic markers of inflammation in patients with metabolic syndrome: a controlled clinical trial. J Periodontol. 2012;83:267-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 153. | Marchesan J, Jiao Y, Schaff RA, Hao J, Morelli T, Kinney JS, Gerow E, Sheridan R, Rodrigues V, Paster BJ, Inohara N, Giannobile WV. TLR4, NOD1 and NOD2 mediate immune recognition of putative newly identified periodontal pathogens. Mol Oral Microbiol. 2016;31:243-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 154. | Parekh PJ, Balart LA, Johnson DA. The Influence of the Gut Microbiome on Obesity, Metabolic Syndrome and Gastrointestinal Disease. Clin Transl Gastroenterol. 2015;6:e91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 161] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 155. | Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI. A core gut microbiome in obese and lean twins. Nature. 2009;457:480-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6397] [Cited by in RCA: 5666] [Article Influence: 354.1] [Reference Citation Analysis (1)] |
| 156. | Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto JM, Kennedy S, Leonard P, Li J, Burgdorf K, Grarup N, Jørgensen T, Brandslund I, Nielsen HB, Juncker AS, Bertalan M, Levenez F, Pons N, Rasmussen S, Sunagawa S, Tap J, Tims S, Zoetendal EG, Brunak S, Clément K, Doré J, Kleerebezem M, Kristiansen K, Renault P, Sicheritz-Ponten T, de Vos WM, Zucker JD, Raes J, Hansen T; MetaHIT consortium, Bork P, Wang J, Ehrlich SD, Pedersen O. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2727] [Cited by in RCA: 3213] [Article Influence: 267.8] [Reference Citation Analysis (2)] |
| 157. | Pirih FQ, Monajemzadeh S, Singh N, Sinacola RS, Shin JM, Chen T, Fenno JC, Kamarajan P, Rickard AH, Travan S, Paster BJ, Kapila Y. Association between metabolic syndrome and periodontitis: The role of lipids, inflammatory cytokines, altered host response, and the microbiome. Periodontol 2000. 2021;87:50-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 115] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 158. | Gasmi Benahmed A, Gasmi A, Doşa A, Chirumbolo S, Mujawdiya PK, Aaseth J, Dadar M, Bjørklund G. Association between the gut and oral microbiome with obesity. Anaerobe. 2021;70:102248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 159. | Shaalan A, Lee S, Feart C, Garcia-Esquinas E, Gomez-Cabrero D, Lopez-Garcia E, Morzel M, Neyraud E, Rodriguez-Artalejo F, Streich R, Proctor G. Alterations in the Oral Microbiome Associated With Diabetes, Overweight, and Dietary Components. Front Nutr. 2022;9:914715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 160. | Yang Y, Cai Q, Zheng W, Steinwandel M, Blot WJ, Shu XO, Long J. Oral microbiome and obesity in a large study of low-income and African-American populations. J Oral Microbiol. 2019;11:1650597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 161. | Lu Z, Li Y, Brinson CW, Kirkwood KL, Lopes-Virella MF, Huang Y. CD36 is upregulated in mice with periodontitis and metabolic syndrome and involved in macrophage gene upregulation by palmitate. Oral Dis. 2017;23:210-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 162. | Dittmann C, Doueiri S, Kluge R, Dommisch H, Gaber T, Pischon N. Porphyromonas gingivalis Suppresses Differentiation and Increases Apoptosis of Osteoblasts From New Zealand Obese Mice. J Periodontol. 2015;86:1095-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 163. | Gotto AM Jr, Moon JE. Management of cardiovascular risk: the importance of meeting lipid targets. Am J Cardiol. 2012;110:3A-14A. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 164. | Lu SY, Qi SD, Zhao Y, Li YY, Yang FM, Yu WH, Jin M, Chen LX, Wang JB, He ZL, Li HJ. Type 2 diabetes mellitus non-genetic Rhesus monkey model induced by high fat and high sucrose diet. Exp Clin Endocrinol Diabetes. 2015;123:19-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 165. | Fujita M, Kuraji R, Ito H, Hashimoto S, Toen T, Fukada T, Numabe Y. Histological effects and pharmacokinetics of lipopolysaccharide derived from Porphyromonas gingivalis on rat maxilla and liver concerning with progression into non-alcoholic steatohepatitis. J Periodontol. 2018;89:1101-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 166. | Akinkugbe AA, Barritt AS, Cai J, Offenbacher S, Thyagarajan B, Khambaty T, Singer R, Kallwitz E, Heiss G, Slade GD. Periodontitis and prevalence of elevated aminotransferases in the Hispanic Community Health Study/Study of Latinos. J Periodontol. 2018;89:949-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 167. | Iwasaki T, Hirose A, Azuma T, Ohashi T, Watanabe K, Obora A, Deguchi F, Kojima T, Isozaki A, Tomofuji T. Correlation between ultrasound-diagnosed non-alcoholic fatty liver and periodontal condition in a cross-sectional study in Japan. Sci Rep. 2018;8:7496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 168. | Kuroki A, Sugita N, Komatsu S, Yokoseki A, Yoshihara A, Kobayashi T, Nakamura K, Momotsu T, Endo N, Sato K, Narita I, Yoshie H. Association of liver enzyme levels and alveolar bone loss: A cross-sectional clinical study in Sado Island. J Clin Exp Dent. 2018;10:e100-e106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 169. | Zhou Y, Vatsalya V, Gobejishvili L, Lamont RJ, McClain CJ, Feng W. Porphyromonas gingivalis as a Possible Risk Factor in the Development/Severity of Acute Alcoholic Hepatitis. Hepatol Commun. 2019;3:293-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 170. | Shin HS. Association between periodontal status and non-alcoholic fatty liver disease in a Korean adult population: A nationwide cross-sectional study. J Periodontol. 2020;91:524-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 171. | Ahmad A, Furuta M, Shinagawa T, Takeuchi K, Takeshita T, Shimazaki Y, Yamashita Y. Association of periodontal status with liver abnormalities and metabolic syndrome. J Oral Sci. 2015;57:335-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 172. | Furuta M, Ekuni D, Yamamoto T, Irie K, Koyama R, Sanbe T, Yamanaka R, Morita M, Kuroki K, Tobe K. Relationship between periodontitis and hepatic abnormalities in young adults. Acta Odontol Scand. 2010;68:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 173. | Saito T, Shimazaki Y, Koga T, Tsuzuki M, Ohshima A. Relationship between periodontitis and hepatic condition in Japanese women. J Int Acad Periodontol. 2006;8:89-95. [PubMed] |
| 174. | Aberg F, Helenius-Hietala J, Meurman J, Isoniemi H. Association between dental infections and the clinical course of chronic liver disease. Hepatol Res. 2014;44:349-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 175. | Weintraub JA, Lopez Mitnik G, Dye BA. Oral Diseases Associated with Nonalcoholic Fatty Liver Disease in the United States. J Dent Res. 2019;98:1219-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 176. | Akinkugbe AA, Slade GD, Barritt AS, Cole SR, Offenbacher S, Petersmann A, Kocher T, Lerch MM, Mayerle J, Völzke H, Heiss G, Holtfreter B. Periodontitis and Non-alcoholic Fatty Liver Disease, a population-based cohort investigation in the Study of Health in Pomerania. J Clin Periodontol. 2017;44:1077-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 177. | Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L, Day C, Arcaro G. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30:1212-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 700] [Article Influence: 38.9] [Reference Citation Analysis (1)] |
| 178. | Lonardo A, Mantovani A, Lugari S, Targher G. Epidemiology and pathophysiology of the association between NAFLD and metabolically healthy or metabolically unhealthy obesity. Ann Hepatol. 2020;19:359-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 179. | Zhang T, Zhang C, Zhang Y, Tang F, Li H, Zhang Q, Lin H, Wu S, Liu Y, Xue F. Metabolic syndrome and its components as predictors of nonalcoholic fatty liver disease in a northern urban Han Chinese population: a prospective cohort study. Atherosclerosis. 2015;240:144-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 180. | Ryan MC, Wilson AM, Slavin J, Best JD, Jenkins AJ, Desmond PV. Associations between liver histology and severity of the metabolic syndrome in subjects with nonalcoholic fatty liver disease. Diabetes Care. 2005;28:1222-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 181. | Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N, Melchionda N, Rizzetto M. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1907] [Cited by in RCA: 1917] [Article Influence: 87.1] [Reference Citation Analysis (0)] |
| 182. | Kuroe K, Furuta M, Takeuchi K, Takeshita T, Suma S, Shinagawa T, Shimazaki Y, Yamashita Y. Association between periodontitis and fibrotic progression of non-alcoholic fatty liver among Japanese adults. J Clin Periodontol. 2021;48:368-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 183. | Blasco-Baque V, Garidou L, Pomié C, Escoula Q, Loubieres P, Le Gall-David S, Lemaitre M, Nicolas S, Klopp P, Waget A, Azalbert V, Colom A, Bonnaure-Mallet M, Kemoun P, Serino M, Burcelin R. Periodontitis induced by Porphyromonas gingivalis drives periodontal microbiota dysbiosis and insulin resistance via an impaired adaptive immune response. Gut. 2017;66:872-885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 214] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 184. | Andrade RSB, França LFC, Pessoa LDS, Landim BAA, Rodrigues AA, Alves EHP, Lenardo DD, Nascimento HMS, Sousa FBM, Barbosa ALDR, Medeiros JR, Vasconcelos ACCG, Vasconcelos DFP. High-fat diet aggravates the liver disease caused by periodontitis in rats. J Periodontol. 2019;90:1023-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 185. | Watanabe K, Petro BJ, Shlimon AE, Unterman TG. Effect of periodontitis on insulin resistance and the onset of type 2 diabetes mellitus in Zucker diabetic fatty rats. J Periodontol. 2008;79:1208-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 186. | Bostanci N, Belibasakis GN. Porphyromonas gingivalis: an invasive and evasive opportunistic oral pathogen. FEMS Microbiol Lett. 2012;333:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 392] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 187. | Ishikawa M, Yoshida K, Okamura H, Ochiai K, Takamura H, Fujiwara N, Ozaki K. Oral Porphyromonas gingivalis translocates to the liver and regulates hepatic glycogen synthesis through the Akt/GSK-3β signaling pathway. Biochim Biophys Acta. 2013;1832:2035-2043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 188. | Baltacıoğlu E, Kehribar MA, Yuva P, Alver A, Atagün OS, Karabulut E, Akalın FA. Total oxidant status and bone resorption biomarkers in serum and gingival crevicular fluid of patients with periodontitis. J Periodontol. 2014;85:317-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 189. | Gonçalves TE, Zimmermann GS, Figueiredo LC, Souza Mde C, da Cruz DF, Bastos MF, da Silva HD, Duarte PM. Local and serum levels of adipokines in patients with obesity after periodontal therapy: one-year follow-up. J Clin Periodontol. 2015;42:431-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 190. | Akinkugbe AA, Avery CL, Barritt AS, Cole SR, Lerch M, Mayerle J, Offenbacher S, Petersmann A, Nauck M, Völzke H, Slade GD, Heiss G, Kocher T, Holtfreter B. Do Genetic Markers of Inflammation Modify the Relationship between Periodontitis and Nonalcoholic Fatty Liver Disease? J Dent Res. 2017;96:1392-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 191. | Horliana AC, Chambrone L, Foz AM, Artese HP, Rabelo Mde S, Pannuti CM, Romito GA. Dissemination of periodontal pathogens in the bloodstream after periodontal procedures: a systematic review. PLoS One. 2014;9:e98271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 192. | Parahitiyawa NB, Jin LJ, Leung WK, Yam WC, Samaranayake LP. Microbiology of odontogenic bacteremia: beyond endocarditis. Clin Microbiol Rev. 2009;22:46-64, Table of Contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 226] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 193. | Wieland A, Frank DN, Harnke B, Bambha K. Systematic review: microbial dysbiosis and nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2015;42:1051-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 164] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 194. | Mouzaki M, Comelli EM, Arendt BM, Bonengel J, Fung SK, Fischer SE, McGilvray ID, Allard JP. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology. 2013;58:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 556] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 195. | Schmidt TS, Hayward MR, Coelho LP, Li SS, Costea PI, Voigt AY, Wirbel J, Maistrenko OM, Alves RJ, Bergsten E, de Beaufort C, Sobhani I, Heintz-Buschart A, Sunagawa S, Zeller G, Wilmes P, Bork P. Extensive transmission of microbes along the gastrointestinal tract. Elife. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 331] [Cited by in RCA: 366] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 196. | de Faria Ghetti F, Oliveira DG, de Oliveira JM, de Castro Ferreira LEVV, Cesar DE, Moreira APB. Influence of gut microbiota on the development and progression of nonalcoholic steatohepatitis. Eur J Nutr. 2018;57:861-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 197. | Canfora EE, Meex RCR, Venema K, Blaak EE. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat Rev Endocrinol. 2019;15:261-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 933] [Article Influence: 155.5] [Reference Citation Analysis (1)] |
| 198. | Hajishengallis G, Darveau RP, Curtis MA. The keystone-pathogen hypothesis. Nat Rev Microbiol. 2012;10:717-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 946] [Cited by in RCA: 1228] [Article Influence: 94.5] [Reference Citation Analysis (0)] |
| 199. | Wu L, Han J, Nie JY, Deng T, Li C, Fang C, Xie WZ, Wang SY, Zeng XT. Alterations and Correlations of Gut Microbiota and Fecal Metabolome Characteristics in Experimental Periodontitis Rats. Front Microbiol. 2022;13:865191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 200. | Xing T, Liu Y, Cheng H, Bai M, Chen J, Ji H, He M, Chen K. Ligature induced periodontitis in rats causes gut dysbiosis leading to hepatic injury through SCD1/AMPK signalling pathway. Life Sci. 2022;288:120162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 201. | Bakaletz LO. Developing animal models for polymicrobial diseases. Nat Rev Microbiol. 2004;2:552-568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 110] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 202. | Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43:5721-5732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1867] [Cited by in RCA: 2022] [Article Influence: 101.1] [Reference Citation Analysis (0)] |
| 203. | Hajishengallis G, Maekawa T, Abe T, Hajishengallis E, Lambris JD. Complement Involvement in Periodontitis: Molecular Mechanisms and Rational Therapeutic Approaches. Adv Exp Med Biol. 2015;865:57-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 204. | Costalonga M, Herzberg MC. The oral microbiome and the immunobiology of periodontal disease and caries. Immunol Lett. 2014;162:22-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 410] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 205. | Kilian M, Chapple IL, Hannig M, Marsh PD, Meuric V, Pedersen AM, Tonetti MS, Wade WG, Zaura E. The oral microbiome - an update for oral healthcare professionals. Br Dent J. 2016;221:657-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 714] [Article Influence: 102.0] [Reference Citation Analysis (0)] |
| 206. | Hajishengallis G. Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol. 2014;35:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 736] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 207. | Eke PI, Dye BA, Wei L, Slade GD, Thornton-Evans GO, Borgnakke WS, Taylor GW, Page RC, Beck JD, Genco RJ. Update on Prevalence of Periodontitis in Adults in the United States: NHANES 2009 to 2012. J Periodontol. 2015;86:611-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1023] [Cited by in RCA: 1056] [Article Influence: 105.6] [Reference Citation Analysis (0)] |
| 208. | Hajishengallis G, Chavakis T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat Rev Immunol. 2021;21:426-440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 855] [Article Influence: 213.8] [Reference Citation Analysis (0)] |
| 209. | Katagiri S, Nitta H, Nagasawa T, Izumi Y, Kanazawa M, Matsuo A, Chiba H, Fukui M, Nakamura N, Oseko F, Kanamura N, Inagaki K, Noguchi T, Naruse K, Matsubara T, Miyazaki S, Miyauchi T, Ando Y, Hanada N, Inoue S. Effect of glycemic control on periodontitis in type 2 diabetic patients with periodontal disease. J Diabetes Investig. 2013;4:320-325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 210. | Sasaki N, Katagiri S, Komazaki R, Watanabe K, Maekawa S, Shiba T, Udagawa S, Takeuchi Y, Ohtsu A, Kohda T, Tohara H, Miyasaka N, Hirota T, Tamari M, Izumi Y. Endotoxemia by Porphyromonas gingivalis Injection Aggravates Non-alcoholic Fatty Liver Disease, Disrupts Glucose/Lipid Metabolism, and Alters Gut Microbiota in Mice. Front Microbiol. 2018;9:2470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 211. | Udagawa S, Katagiri S, Maekawa S, Takeuchi Y, Komazaki R, Ohtsu A, Sasaki N, Shiba T, Watanabe K, Ishihara K, Sato N, Miyasaka N, Izumi Y. Effect of Porphyromonas gingivalis infection in the placenta and umbilical cord in pregnant mice with low birth weight. Acta Odontol Scand. 2018;76:433-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 212. | Hatasa M, Ohsugi Y, Katagiri S, Yoshida S, Niimi H, Morita K, Tsuchiya Y, Shimohira T, Sasaki N, Maekawa S, Shiba T, Hirota T, Tohara H, Takahashi H, Nitta H, Iwata T. Endotoxemia by Porphyromonas gingivalis Alters Endocrine Functions in Brown Adipose Tissue. Front Cell Infect Microbiol. 2020;10:580577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 213. | Lang NP, Schätzle MA, Löe H. Gingivitis as a risk factor in periodontal disease. J Clin Periodontol. 2009;36 Suppl 10:3-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 205] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 214. | Loe H, Theilade E, Jensen SB. Experimental Gingivitis In Man. J Periodontol (1930). 1965;36:177-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2178] [Cited by in RCA: 2159] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 215. | Hamp SE, Lindhe J, Löe H. Experimental periodontitis in the beagle dog. J Periodontal Res. 1972;13-14. [PubMed] |
| 216. | Schultz-Haudt S, Bruce MA, Bibby BG. Bacterial factors in nonspecific gingivitis. J Dent Res. 1954;33:454-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 217. | Loesche WJ. Chemotherapy of dental plaque infections. Oral Sci Rev. 1976;9:65-107. [PubMed] |
| 218. | Haffajee AD, Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000. 1994;5:78-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1082] [Cited by in RCA: 1086] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 219. | Ximénez-Fyvie LA, Haffajee AD, Socransky SS. Comparison of the microbiota of supra- and subgingival plaque in health and periodontitis. J Clin Periodontol. 2000;27:648-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 370] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 220. | Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15:30-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1219] [Cited by in RCA: 1873] [Article Influence: 187.3] [Reference Citation Analysis (0)] |
| 221. | Nelson RG, Shlossman M, Budding LM, Pettitt DJ, Saad MF, Genco RJ, Knowler WC. Periodontal disease and NIDDM in Pima Indians. Diabetes Care. 1990;13:836-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 260] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 222. | Mealey BL, Rose LF. Diabetes mellitus and inflammatory periodontal diseases. Curr Opin Endocrinol Diabetes Obes. 2008;15:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 223. | Aemaimanan P, Amimanan P, Taweechaisupapong S. Quantification of key periodontal pathogens in insulin-dependent type 2 diabetic and non-diabetic patients with generalized chronic periodontitis. Anaerobe. 2013;22:64-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 224. | Haffajee AD, Patel M, Socransky SS. Microbiological changes associated with four different periodontal therapies for the treatment of chronic periodontitis. Oral Microbiol Immunol. 2008;23:148-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 225. | Duarte PM, Feres M, Yassine LLS, Soares GMS, Miranda TS, Faveri M, Retamal-Valdes B, Figueiredo LC. Clinical and microbiological effects of scaling and root planing, metronidazole and amoxicillin in the treatment of diabetic and non-diabetic subjects with periodontitis: A cohort study. J Clin Periodontol. 2018;45:1326-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 226. | Gibbons RJ, Banghart S. Induction of dental caries in gnotobiotic rats with a levan-forming streptococcus and a streptococcus isolated from subacute bacterial endocarditis. Arch Oral Biol. 1968;13:297-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 227. | Gibbons RJ, Berman KS, Knoettner P, Kapsimalis B. Dental caries and alveolar bone loss in gnotobiotic rats infected with capsule forming streptococci of human origin. Arch Oral Biol. 1966;11:549-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 254] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 228. | Levy SE, Myers RM. Advancements in Next-Generation Sequencing. Annu Rev Genomics Hum Genet. 2016;17:95-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 344] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 229. | Anilkumar Sithara A, Maripuri DP, Moorthy K, Amirtha Ganesh SS, Philip P, Banerjee S, Sudhakar M, Raman K. iCOMIC: a graphical interface-driven bioinformatics pipeline for analyzing cancer omics data. NAR Genom Bioinform. 2022;4:lqac053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 230. | Clarridge JE 3rd. Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin Microbiol Rev. 2004;17:840-862, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1174] [Cited by in RCA: 1115] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 231. | Griffen AL, Beall CJ, Campbell JH, Firestone ND, Kumar PS, Yang ZK, Podar M, Leys EJ. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. ISME J. 2012;6:1176-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 732] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 232. | Abusleme L, Dupuy AK, Dutzan N, Silva N, Burleson JA, Strausbaugh LD, Gamonal J, Diaz PI. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J. 2013;7:1016-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 763] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 233. | Moeseneder MM, Arrieta JM, Herndl GJ. A comparison of DNA- and RNA-based clone libraries from the same marine bacterioplankton community. FEMS Microbiol Ecol. 2005;51:341-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 234. | Treude T, Knittel K, Blumenberg M, Seifert R, Boetius A. Subsurface microbial methanotrophic mats in the Black Sea. Appl Environ Microbiol. 2005;71:6375-6378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 235. | Hirawati, Katoch K, Chauhan DS, Singh HB, Sharma VD, Singh M, Kashyap M, Katoch VM. Detection of M. leprae by reverse transcription- PCR in biopsy specimens from leprosy cases: a preliminary study. J Commun Dis. 2006;38:280-287. [PubMed] |
| 236. | Blazewicz SJ, Barnard RL, Daly RA, Firestone MK. Evaluating rRNA as an indicator of microbial activity in environmental communities: limitations and uses. ISME J. 2013;7:2061-2068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 591] [Cited by in RCA: 474] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 237. | Kachi H, Maruyama N, Maruyama F, Shiba T, Watanabe T, Goda A, Murase K, Michi Y, Takeuchi Y, Izumi Y, Yamaguchi S, Nakagawa I. Active Microbiota Show Specific Correlationships in Peri-implantitis and Periodontitis. The journal of the Stomatological Society, Japan. 2017;84:25-36. |
| 238. | Mei R, Narihiro T, Nobu MK, Kuroda K, Liu WT. Evaluating digestion efficiency in full-scale anaerobic digesters by identifying active microbial populations through the lens of microbial activity. Sci Rep. 2016;6:34090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 239. | Iwai S, Weinmaier T, Schmidt BL, Albertson DG, Poloso NJ, Dabbagh K, DeSantis TZ. Piphillin: Improved Prediction of Metagenomic Content by Direct Inference from Human Microbiomes. PLoS One. 2016;11:e0166104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 201] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 240. | Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Vega Thurber RL, Knight R, Beiko RG, Huttenhower C. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013;31:814-821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5694] [Cited by in RCA: 6299] [Article Influence: 524.9] [Reference Citation Analysis (0)] |
| 241. | Ikeda E, Shiba T, Ikeda Y, Suda W, Nakasato A, Takeuchi Y, Azuma M, Hattori M, Izumi Y. Japanese subgingival microbiota in health vs disease and their roles in predicted functions associated with periodontitis. Odontology. 2020;108:280-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 242. | Larsen B, Hwang J. Mycoplasma, Ureaplasma, and adverse pregnancy outcomes: a fresh look. Infect Dis Obstet Gynecol. 2010;2010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 243. | Sze MA, Schloss PD. The Impact of DNA Polymerase and Number of Rounds of Amplification in PCR on 16S rRNA Gene Sequence Data. mSphere. 2019;4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 244. | Abellan-Schneyder I, Matchado MS, Reitmeier S, Sommer A, Sewald Z, Baumbach J, List M, Neuhaus K. Primer, Pipelines, Parameters: Issues in 16S rRNA Gene Sequencing. mSphere. 2021;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 199] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 245. | Niu SY, Yang J, McDermaid A, Zhao J, Kang Y, Ma Q. Bioinformatics tools for quantitative and functional metagenome and metatranscriptome data analysis in microbes. Brief Bioinform. 2018;19:1415-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 246. | Dabdoub SM, Ganesan SM, Kumar PS. Comparative metagenomics reveals taxonomically idiosyncratic yet functionally congruent communities in periodontitis. Sci Rep. 2016;6:38993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 247. | Yost S, Duran-Pinedo AE, Teles R, Krishnan K, Frias-Lopez J. Functional signatures of oral dysbiosis during periodontitis progression revealed by microbial metatranscriptome analysis. Genome Med. 2015;7:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 221] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 248. | Duran-Pinedo AE, Chen T, Teles R, Starr JR, Wang X, Krishnan K, Frias-Lopez J. Community-wide transcriptome of the oral microbiome in subjects with and without periodontitis. ISME J. 2014;8:1659-1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 237] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 249. | Komatsu K, Shiba T, Takeuchi Y, Watanabe T, Koyanagi T, Nemoto T, Shimogishi M, Shibasaki M, Katagiri S, Kasugai S, Iwata T. Discriminating Microbial Community Structure Between Peri-Implantitis and Periodontitis With Integrated Metagenomic, Metatranscriptomic, and Network Analysis. Front Cell Infect Microbiol. 2020;10:596490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 250. | Alghamdi S. Isolation and identification of the oral bacteria and their characterization for bacteriocin production in the oral cavity. Saudi J Biol Sci. 2022;29:318-323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 251. | Yugi K, Kuroda S. Metabolism-Centric Trans-Omics. Cell Syst. 2017;4:19-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 252. | Hoshino D, Kawata K, Kunida K, Hatano A, Yugi K, Wada T, Fujii M, Sano T, Ito Y, Furuichi Y, Manabe Y, Suzuki Y, Fujii NL, Soga T, Kuroda S. Trans-omic Analysis Reveals ROS-Dependent Pentose Phosphate Pathway Activation after High-Frequency Electrical Stimulation in C2C12 Myotubes. iScience. 2020;23:101558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 253. | Kawata K, Hatano A, Yugi K, Kubota H, Sano T, Fujii M, Tomizawa Y, Kokaji T, Tanaka KY, Uda S, Suzuki Y, Matsumoto M, Nakayama KI, Saitoh K, Kato K, Ueno A, Ohishi M, Hirayama A, Soga T, Kuroda S. Trans-omic Analysis Reveals Selective Responses to Induced and Basal Insulin across Signaling, Transcriptional, and Metabolic Networks. iScience. 2018;7:212-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 254. | Kholy KE, Genco RJ, Van Dyke TE. Oral infections and cardiovascular disease. Trends Endocrinol Metab. 2015;26:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 133] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 255. | da Silva FG, Pola NM, Casarin M, Silva CFE, Muniz FWMG. Association between clinical measures of gingival inflammation and obesity in adults: systematic review and meta-analyses. Clin Oral Investig. 2021;25:4281-4298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 256. | Li X, Kolltveit KM, Tronstad L, Olsen I. Systemic diseases caused by oral infection. Clin Microbiol Rev. 2000;13:547-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 509] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 257. | Winning L, Patterson CC, Cullen KM, Stevenson KA, Lundy FT, Kee F, Linden GJ. The association between subgingival periodontal pathogens and systemic inflammation. J Clin Periodontol. 2015;42:799-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 258. | Dong TS, Gupta A. Influence of Early Life, Diet, and the Environment on the Microbiome. Clin Gastroenterol Hepatol. 2019;17:231-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 124] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 259. | Shen F, Zheng RD, Sun XQ, Ding WJ, Wang XY, Fan JG. Gut microbiota dysbiosis in patients with non-alcoholic fatty liver disease. Hepatobiliary Pancreat Dis Int. 2017;16:375-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 411] [Article Influence: 51.4] [Reference Citation Analysis (1)] |
| 260. | Jiang W, Wu N, Wang X, Chi Y, Zhang Y, Qiu X, Hu Y, Li J, Liu Y. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci Rep. 2015;5:8096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 473] [Cited by in RCA: 453] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 261. | Da Silva HE, Teterina A, Comelli EM, Taibi A, Arendt BM, Fischer SE, Lou W, Allard JP. Nonalcoholic fatty liver disease is associated with dysbiosis independent of body mass index and insulin resistance. Sci Rep. 2018;8:1466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 202] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 262. | Hoyles L, Fernández-Real JM, Federici M, Serino M, Abbott J, Charpentier J, Heymes C, Luque JL, Anthony E, Barton RH, Chilloux J, Myridakis A, Martinez-Gili L, Moreno-Navarrete JM, Benhamed F, Azalbert V, Blasco-Baque V, Puig J, Xifra G, Ricart W, Tomlinson C, Woodbridge M, Cardellini M, Davato F, Cardolini I, Porzio O, Gentileschi P, Lopez F, Foufelle F, Butcher SA, Holmes E, Nicholson JK, Postic C, Burcelin R, Dumas ME. Molecular phenomics and metagenomics of hepatic steatosis in non-diabetic obese women. Nat Med. 2018;24:1070-1080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 303] [Cited by in RCA: 491] [Article Influence: 70.1] [Reference Citation Analysis (0)] |
| 263. | Dong TS, Jacobs JP. Nonalcoholic fatty liver disease and the gut microbiome: Are bacteria responsible for fatty liver? Exp Biol Med (Maywood). 2019;244:408-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 264. | Wong VW, Tse CH, Lam TT, Wong GL, Chim AM, Chu WC, Yeung DK, Law PT, Kwan HS, Yu J, Sung JJ, Chan HL. Molecular characterization of the fecal microbiota in patients with nonalcoholic steatohepatitis--a longitudinal study. PLoS One. 2013;8:e62885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 249] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 265. | Duarte SMB, Stefano JT, Miele L, Ponziani FR, Souza-Basqueira M, Okada LSRR, de Barros Costa FG, Toda K, Mazo DFC, Sabino EC, Carrilho FJ, Gasbarrini A, Oliveira CP. Gut microbiome composition in lean patients with NASH is associated with liver damage independent of caloric intake: A prospective pilot study. Nutr Metab Cardiovasc Dis. 2018;28:369-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 266. | Mouzaki M, Wang AY, Bandsma R, Comelli EM, Arendt BM, Zhang L, Fung S, Fischer SE, McGilvray IG, Allard JP. Bile Acids and Dysbiosis in Non-Alcoholic Fatty Liver Disease. PLoS One. 2016;11:e0151829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 300] [Article Influence: 33.3] [Reference Citation Analysis (1)] |
| 267. | Million M, Maraninchi M, Henry M, Armougom F, Richet H, Carrieri P, Valero R, Raccah D, Vialettes B, Raoult D. Obesity-associated gut microbiota is enriched in Lactobacillus reuteri and depleted in Bifidobacterium animalis and Methanobrevibacter smithii. Int J Obes (Lond). 2012;36:817-825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 516] [Cited by in RCA: 493] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 268. | Ponziani FR, Bhoori S, Castelli C, Putignani L, Rivoltini L, Del Chierico F, Sanguinetti M, Morelli D, Paroni Sterbini F, Petito V, Reddel S, Calvani R, Camisaschi C, Picca A, Tuccitto A, Gasbarrini A, Pompili M, Mazzaferro V. Hepatocellular Carcinoma Is Associated With Gut Microbiota Profile and Inflammation in Nonalcoholic Fatty Liver Disease. Hepatology. 2019;69:107-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 473] [Article Influence: 78.8] [Reference Citation Analysis (1)] |
| 269. | Loomba R, Seguritan V, Li W, Long T, Klitgord N, Bhatt A, Dulai PS, Caussy C, Bettencourt R, Highlander SK, Jones MB, Sirlin CB, Schnabl B, Brinkac L, Schork N, Chen CH, Brenner DA, Biggs W, Yooseph S, Venter JC, Nelson KE. Gut Microbiome-Based Metagenomic Signature for Non-invasive Detection of Advanced Fibrosis in Human Nonalcoholic Fatty Liver Disease. Cell Metab. 2017;25:1054-1062.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 752] [Article Influence: 94.0] [Reference Citation Analysis (0)] |
| 270. | Dong TS, Katzka W, Lagishetty V, Luu K, Hauer M, Pisegna J, Jacobs JP. A Microbial Signature Identifies Advanced Fibrosis in Patients with Chronic Liver Disease Mainly Due to NAFLD. Sci Rep. 2020;10:2771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 271. | Boursier J, Mueller O, Barret M, Machado M, Fizanne L, Araujo-Perez F, Guy CD, Seed PC, Rawls JF, David LA, Hunault G, Oberti F, Calès P, Diehl AM. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63:764-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 763] [Cited by in RCA: 1050] [Article Influence: 116.7] [Reference Citation Analysis (0)] |
| 272. | Caussy C, Hsu C, Lo MT, Liu A, Bettencourt R, Ajmera VH, Bassirian S, Hooker J, Sy E, Richards L, Schork N, Schnabl B, Brenner DA, Sirlin CB, Chen CH, Loomba R; Genetics of NAFLD in Twins Consortium. Link between gut-microbiome derived metabolite and shared gene-effects with hepatic steatosis and fibrosis in NAFLD. Hepatology. 2018;68:918-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 153] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 273. | Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, Mascianà R, Forgione A, Gabrieli ML, Perotti G, Vecchio FM, Rapaccini G, Gasbarrini G, Day CP, Grieco A. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1133] [Cited by in RCA: 1101] [Article Influence: 68.8] [Reference Citation Analysis (1)] |
| 274. | Ye D, Li FY, Lam KS, Li H, Jia W, Wang Y, Man K, Lo CM, Li X, Xu A. Toll-like receptor-4 mediates obesity-induced non-alcoholic steatohepatitis through activation of X-box binding protein-1 in mice. Gut. 2012;61:1058-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 162] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 275. | Sharifnia T, Antoun J, Verriere TG, Suarez G, Wattacheril J, Wilson KT, Peek RM Jr, Abumrad NN, Flynn CR. Hepatic TLR4 signaling in obese NAFLD. Am J Physiol Gastrointest Liver Physiol. 2015;309:G270-G278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 194] [Article Influence: 19.4] [Reference Citation Analysis (1)] |
| 276. | Diehl AM, Harrison S, Caldwell S, Rinella M, Paredes A, Moylan C, Guy C, Bashir M, Wang Y, Miller L, Chang A, Wu E, Abdelmalek M. JKB-121 in patients with nonalcoholic steatohepatitis: A phase 2 double blind randomized placebo control study. J Hepatol. 2018;68:S103-S103. [DOI] [Full Text] |
| 277. | Mattace Raso G, Simeoli R, Russo R, Iacono A, Santoro A, Paciello O, Ferrante MC, Canani RB, Calignano A, Meli R. Effects of sodium butyrate and its synthetic amide derivative on liver inflammation and glucose tolerance in an animal model of steatosis induced by high fat diet. PLoS One. 2013;8:e68626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 152] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 278. | Ridlon JM, Harris SC, Bhowmik S, Kang DJ, Hylemon PB. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes. 2016;7:22-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 740] [Article Influence: 82.2] [Reference Citation Analysis (0)] |
| 279. | Jiang C, Xie C, Li F, Zhang L, Nichols RG, Krausz KW, Cai J, Qi Y, Fang ZZ, Takahashi S, Tanaka N, Desai D, Amin SG, Albert I, Patterson AD, Gonzalez FJ. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J Clin Invest. 2015;125:386-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 540] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 280. | Kim JY, Park YM, Lee GN, Song HC, Ahn YB, Han K, Ko SH. Association between toothbrushing and non-alcoholic fatty liver disease. PLoS One. 2021;16:e0243686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 281. | Kamata Y, Kessoku T, Shimizu T, Kobayashi T, Kurihashi T, Sato S, Kuraji S, Aoyama N, Iwasaki T, Takashiba S, Hamada N, Kodama T, Tamura T, Ino S, Higurashi T, Taguri M, Yamanaka T, Yoneda M, Usuda H, Wada K, Nakajima A, Minabe M. Efficacy and safety of PERIOdontal treatment versus usual care for Nonalcoholic liver disease: protocol of the PERION multicenter, two-arm, open-label, randomized trial. Trials. 2020;21:291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 282. | Sharpton SR, Maraj B, Harding-Theobald E, Vittinghoff E, Terrault NA. Gut microbiome-targeted therapies in nonalcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression. Am J Clin Nutr. 2019;110:139-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 142] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 283. | Liu L, Li P, Liu Y, Zhang Y. Efficacy of Probiotics and Synbiotics in Patients with Nonalcoholic Fatty Liver Disease: A Meta-Analysis. Dig Dis Sci. 2019;64:3402-3412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 284. | Loman BR, Hernández-Saavedra D, An R, Rector RS. Prebiotic and probiotic treatment of nonalcoholic fatty liver disease: a systematic review and meta-analysis. Nutr Rev. 2018;76:822-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 285. | Li Z, Yang S, Lin H, Huang J, Watkins PA, Moser AB, Desimone C, Song XY, Diehl AM. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 2003;37:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 658] [Cited by in RCA: 702] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 286. | Velayudham A, Dolganiuc A, Ellis M, Petrasek J, Kodys K, Mandrekar P, Szabo G. VSL#3 probiotic treatment attenuates fibrosis without changes in steatohepatitis in a diet-induced nonalcoholic steatohepatitis model in mice. Hepatology. 2009;49:989-997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 204] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 287. | Alisi A, Bedogni G, Baviera G, Giorgio V, Porro E, Paris C, Giammaria P, Reali L, Anania F, Nobili V. Randomised clinical trial: The beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2014;39:1276-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 342] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 288. | Eslamparast T, Poustchi H, Zamani F, Sharafkhah M, Malekzadeh R, Hekmatdoost A. Synbiotic supplementation in nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled pilot study. Am J Clin Nutr. 2014;99:535-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 285] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 289. | Nguyen T, Brody H, Lin GH, Rangé H, Kuraji R, Ye C, Kamarajan P, Radaic A, Gao L, Kapila Y. Probiotics, including nisin-based probiotics, improve clinical and microbial outcomes relevant to oral and systemic diseases. Periodontol 2000. 2020;82:173-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 290. | Jang HR, Park HJ, Kang D, Chung H, Nam MH, Lee Y, Park JH, Lee HY. A protective mechanism of probiotic Lactobacillus against hepatic steatosis via reducing host intestinal fatty acid absorption. Exp Mol Med. 2019;51:1-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 291. | Ritze Y, Bárdos G, Claus A, Ehrmann V, Bergheim I, Schwiertz A, Bischoff SC. Lactobacillus rhamnosus GG protects against non-alcoholic fatty liver disease in mice. PLoS One. 2014;9:e80169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 221] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 292. | Wang Y, Liu Y, Sidhu A, Ma Z, McClain C, Feng W. Lactobacillus rhamnosus GG culture supernatant ameliorates acute alcohol-induced intestinal permeability and liver injury. Am J Physiol Gastrointest Liver Physiol. 2012;303:G32-G41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 195] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 293. | Gao L, Kuraji R, Zhang MJ, Martinez A, Radaic A, Kamarajan P, Le C, Zhan L, Ye C, Rangé H, Sailani MR, Kapila YL. Nisin probiotic prevents inflammatory bone loss while promoting reparative proliferation and a healthy microbiome. NPJ Biofilms Microbiomes. 2022;8:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 294. | Radaic A, Ye C, Parks B, Gao L, Kuraji R, Malone E, Kamarajan P, Zhan L, Kapila YL. Modulation of pathogenic oral biofilms towards health with nisin probiotic. J Oral Microbiol. 2020;12:1809302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 295. | Radaic A, Brody H, Contreras F, Hajfathalian M, Lucido L, Kamarajan P, Kapila YL. Nisin and Nisin Probiotic Disrupt Oral Pathogenic Biofilms and Restore Their Microbiome Composition towards Healthy Control Levels in a Peri-Implantitis Setting. Microorganisms. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 296. | Kamarajan P, Ateia I, Shin JM, Fenno JC, Le C, Zhan L, Chang A, Darveau R, Kapila YL. Periodontal pathogens promote cancer aggressivity via TLR/MyD88 triggered activation of Integrin/FAK signaling that is therapeutically reversible by a probiotic bacteriocin. PLoS Pathog. 2020;16:e1008881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 297. | Fujita K, Ichimasa S, Zendo T, Koga S, Yoneyama F, Nakayama J, Sonomoto K. Structural analysis and characterization of lacticin Q, a novel bacteriocin belonging to a new family of unmodified bacteriocins of gram-positive bacteria. Appl Environ Microbiol. 2007;73:2871-2877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 115] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 298. | Cleveland J, Montville TJ, Nes IF, Chikindas ML. Bacteriocins: safe, natural antimicrobials for food preservation. Int J Food Microbiol. 2001;71:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1209] [Cited by in RCA: 1038] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 299. | Twomey D, Ross RP, Ryan M, Meaney B, Hill C. Lantibiotics produced by lactic acid bacteria: structure, function and applications. Antonie Van Leeuwenhoek. 2002;82:165-185. [PubMed] |
| 300. | Smith L, Hasper H, Breukink E, Novak J, Cerkasov J, Hillman JD, Wilson-Stanford S, Orugunty RS. Elucidation of the antimicrobial mechanism of mutacin 1140. Biochemistry. 2008;47:3308-3314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 301. | Cotter PD, Hill C, Ross RP. Bacteriocins: developing innate immunity for food. Nat Rev Microbiol. 2005;3:777-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1461] [Cited by in RCA: 1429] [Article Influence: 71.5] [Reference Citation Analysis (0)] |
| 302. | Xie L, van der Donk WA. Post-translational modifications during lantibiotic biosynthesis. Curr Opin Chem Biol. 2004;8:498-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 303. | Breukink E, de Kruijff B. Lipid II as a target for antibiotics. Nat Rev Drug Discov. 2006;5:321-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 516] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 304. | Hasper HE, Kramer NE, Smith JL, Hillman JD, Zachariah C, Kuipers OP, de Kruijff B, Breukink E. An alternative bactericidal mechanism of action for lantibiotic peptides that target lipid II. Science. 2006;313:1636-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 381] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 305. | Hsu ST, Breukink E, Tischenko E, Lutters MA, de Kruijff B, Kaptein R, Bonvin AM, van Nuland NA. The nisin-lipid II complex reveals a pyrophosphate cage that provides a blueprint for novel antibiotics. Nat Struct Mol Biol. 2004;11:963-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 419] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 306. | Wiedemann I, Benz R, Sahl HG. Lipid II-mediated pore formation by the peptide antibiotic nisin: a black lipid membrane study. J Bacteriol. 2004;186:3259-3261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 131] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 307. | Mierau I, Kleerebezem M. 10 years of the nisin-controlled gene expression system (NICE) in Lactococcus lactis. Appl Microbiol Biotechnol. 2005;68:705-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 438] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 308. | Jena PK, Sheng L, Liu HX, Kalanetra KM, Mirsoian A, Murphy WJ, French SW, Krishnan VV, Mills DA, Wan YY. Western Diet-Induced Dysbiosis in Farnesoid X Receptor Knockout Mice Causes Persistent Hepatic Inflammation after Antibiotic Treatment. Am J Pathol. 2017;187:1800-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 309. | Lee MF, Chiang CH, Lin SJ, Song PP, Liu HC, Wu TJ, Lin WW. Recombinant Lactococcus lactis Expressing Ling Zhi 8 Protein Ameliorates Nonalcoholic Fatty Liver and Early Atherogenesis in Cholesterol-Fed Rabbits. Biomed Res Int. 2020;2020:3495682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 310. | Naudin CR, Maner-Smith K, Owens JA, Wynn GM, Robinson BS, Matthews JD, Reedy AR, Luo L, Wolfarth AA, Darby TM, Ortlund EA, Jones RM. Lactococcus lactis Subspecies cremoris Elicits Protection Against Metabolic Changes Induced by a Western-Style Diet. Gastroenterology. 2020;159:639-651.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 311. | Ansari A, Bose S, Patra JK, Shin NR, Lim DW, Kim KW, Wang JH, Kim YM, Chin YW, Kim H. A Controlled Fermented Samjunghwan Herbal Formula Ameliorates Non-alcoholic Hepatosteatosis in HepG2 Cells and OLETF Rats. Front Pharmacol. 2018;9:596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 312. | Ghouri N, Preiss D, Sattar N. Liver enzymes, nonalcoholic fatty liver disease, and incident cardiovascular disease: a narrative review and clinical perspective of prospective data. Hepatology. 2010;52:1156-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 208] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 313. | Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1647] [Cited by in RCA: 1708] [Article Influence: 89.9] [Reference Citation Analysis (0)] |
| 314. | Rafiq N, Bai C, Fang Y, Srishord M, McCullough A, Gramlich T, Younossi ZM. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol. 2009;7:234-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 560] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 315. | Stickel F, Hellerbrand C. Non-alcoholic fatty liver disease as a risk factor for hepatocellular carcinoma: mechanisms and implications. Gut. 2010;59:1303-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 316. | Micó-Carnero M, Rojano-Alfonso C, Álvarez-Mercado AI, Gracia-Sancho J, Casillas-Ramírez A, Peralta C. Effects of Gut Metabolites and Microbiota in Healthy and Marginal Livers Submitted to Surgery. Int J Mol Sci. 2020;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 317. | Cornide-Petronio ME, Álvarez-Mercado AI, Jiménez-Castro MB, Peralta C. Current Knowledge about the Effect of Nutritional Status, Supplemented Nutrition Diet, and Gut Microbiota on Hepatic Ischemia-Reperfusion and Regeneration in Liver Surgery. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 318. | Han P, Sun D, Yang J. Interaction between periodontitis and liver diseases. Biomed Rep. 2016;5:267-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 319. | Anand AC, Pardal PK, Sachdev VP. DENTAL CARIES AND PERIODONTAL DISORDERS IN CHRONIC LIVER DISEASE. Med J Armed Forces India. 2001;57:26-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 320. | Novacek G, Plachetzky U, Pötzi R, Lentner S, Slavicek R, Gangl A, Ferenci P. Dental and periodontal disease in patients with cirrhosis--role of etiology of liver disease. J Hepatol. 1995;22:576-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 2.2] [Reference Citation Analysis (0)] |