Published online Feb 14, 2023. doi: 10.3748/wjg.v29.i6.1026
Peer-review started: November 19, 2022
First decision: November 30, 2022
Revised: December 29, 2023
Accepted: January 29, 2023
Article in press: January 29, 2023
Published online: February 14, 2023
Processing time: 82 Days and 14 Hours
One of the significant health issues in the world is the prevalence of ulcerative colitis (UC). UC is a chronic disorder that mainly affects the colon, beginning with the rectum, and can progress from asymptomatic mild inflammation to extensive inflammation of the entire colon. Understanding the underlying molecular mechanisms of UC pathogenesis emphasizes the need for innovative therapeutic approaches based on identifying molecular targets. Interestingly, in response to cellular injury, the NLR family pyrin domain containing 3 (NLRP3) inflammasome is a crucial part of the inflammation and immunological reaction by promoting caspase-1 activation and the release of interleukin-1β. This review discusses the mechanisms of NLRP3 inflammasome activation by various signals and its regulation and impact on UC.
Core Tip: Ulcerative colitis (UC) is a common chronic type of inflammatory bowel disease that affects a significant number of populations. Needing to counteract the UC prevalence, attract scientists to study its pathological mechanism deeply. NLR family pyrin domain containing 3 (NLRP3) inflammasome has been observed to have a crucial role in the pathological features of UC. Targeting NLRP3 inflammasome signals with phytochemicals, plants, probiotics, and chemical agents could be promising candidates for fixing the UC problem.
- Citation: Ali FE, Ibrahim IM, Ghogar OM, Abd-alhameed EK, Althagafy HS, Hassanein EH. Therapeutic interventions target the NLRP3 inflammasome in ulcerative colitis: Comprehensive study. World J Gastroenterol 2023; 29(6): 1026-1053
- URL: https://www.wjgnet.com/1007-9327/full/v29/i6/1026.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i6.1026
Inflammatory bowel diseases (IBD), most referred to as Crohn’s disease and ulcerative colitis (UC), are chronic, idiopathic disorders characterized by intestinal inflammation that have increased incidence and prevalence over time and in various countries worldwide[1-3]. The global prevalence is expected to impact up to 30 million by 2025[4]. Several factors are known to play a considerable role in disease pathogenesis. IBD has been linked to genetic etiology and other variables such as pathogens, appendectomy, stress, and air pollution[5,6]. UC is a chronic disorder that mainly affects the colon, beginning with the rectum and perhaps progressing to inflammation of the entire large intestine[7]. UC can begin with severe illnesses such as melena, diarrhea, and mucus production[8,9], and often between the ages of 50 years and 80 years[3,10,11].
A family history of IBD elevates the potential risk of UC, and first-degree relatives are four times more likely to acquire the disease, suggesting that genetic factors are likely related to UC[12,13]. Also, environmental factors play a crucial role in its development[14]. About 110 of the 163 susceptibility loci (67%) encode innate and adaptive immunity pathways, cytokine signaling, and immunological sensing. Several other autoimmune diseases, including psoriasis and ankylosing spondylitis, share many of these genes[15,16]. Additionally, many UC-specific genes have a role in controlling epithelial barrier function[17,18].
The gastrointestinal mucosa is susceptible to many antigens from food, the environment, and the microbiome[19]. The mucin layer that covers the epithelium, the mucosa’s outer layer, serves as the gut immune system’s first line of defense since it physically separates antigens from gut immune cells and has antimicrobial capabilities. Patients with active UC have been demonstrated to have a thinner mucin-containing mucosal layer in the colon, primarily due to a reduction in mucin 2 production[20]. In addition, exposure to potentially harmful stimuli such as nonsteroidal anti-inflammatory drugs and food components such as emulsifiers may prompt colitis[21,22]. Moreover, dysbiosis reduces short-chain fatty acid synthesis, which is required for epithelial energy supply and mucus formation[23]. On the same approach, UC has been attributed to a decrease in short-chain fatty acid-producing Ruminococcaceae and Lachnospiraceae and an increase in pro-inflammatory microorganisms such as Enterobacteriaceae[24,25].
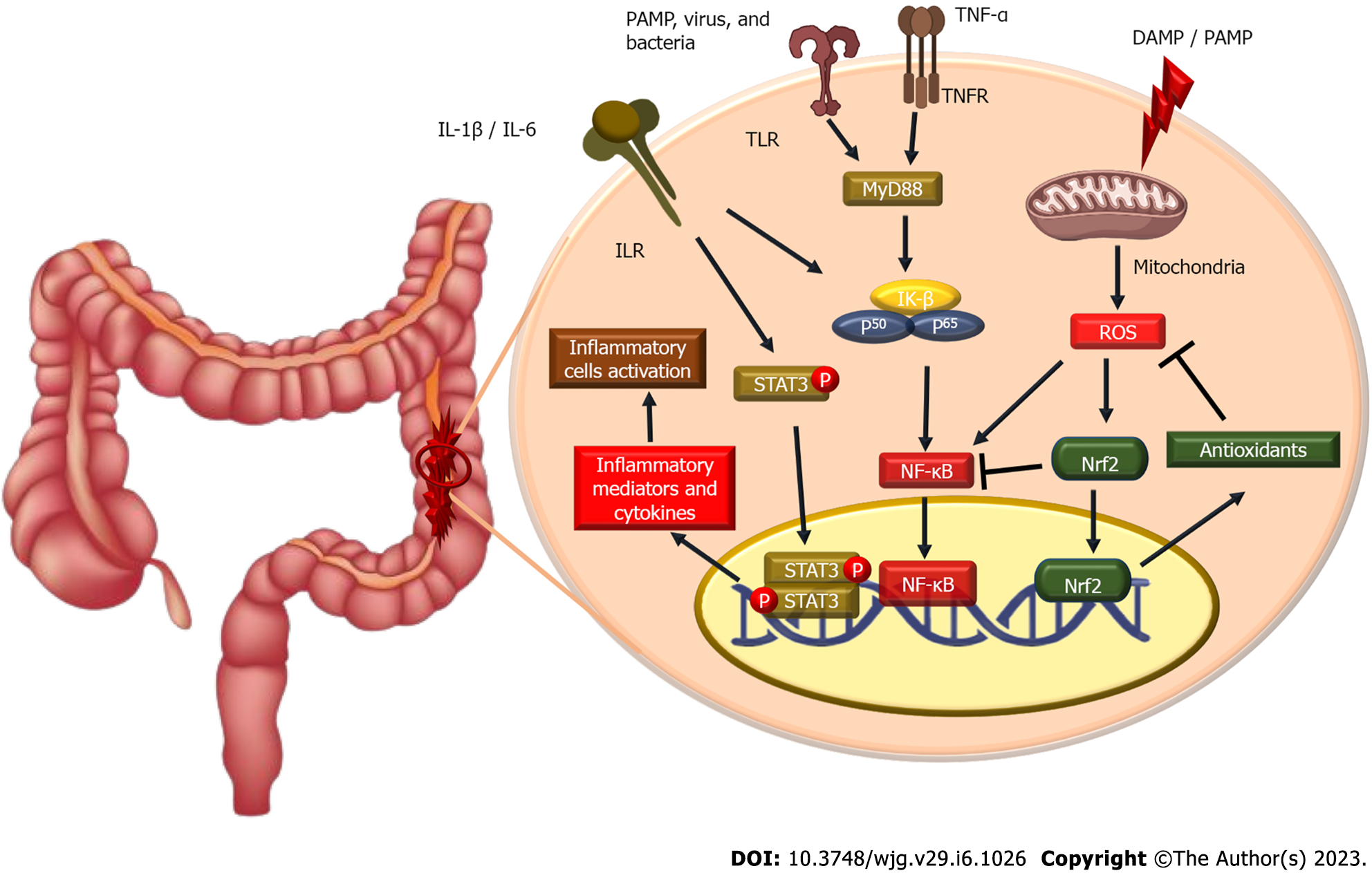
When the epithelium is damaged, the mucosa becomes more permeable to luminal pathogens, increasing the absorption of these antigens and possibly activating gastrointestinal tract immunity[26,27]. Since it is the link between the host immune response repertoire and the intestinal microbiota, the intestinal epithelium plays a crucial role in the innate immune system[28]. Antigens activate the innate immune response via antigen-presenting cells and T cells, which evoke an inflammatory response that activates the adaptive immune system[27,29]. Mechanistically, mature dendritic cells express a lot of Toll-like receptors (TLR), which activate multiple transcription factors like nuclear factor-κB (NF-κB), which in turn causes an inflammatory response in UC[30,31]. These inflammatory cascades result in the production of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukins (IL)[26,27,29]. These pro-inflammatory cytokines have critical roles in signaling through intracellular proteins, including Janus kinase (Jak), which enhances lymphocyte activation and proliferation[32,33]. Interestingly, innate immune signaling via cytokine receptors, TNF receptors, and TLR-adaptor myeloid differentiation as a primary response promotes NLRP3 transcription and oligomerization through NF-kB activation[34,35]. Figure 1 outlines this inflammatory response inside the colon in IBDs, including UC.
The innate immune system is the primary mechanism by which most organisms respond quickly to diseases or injury. Pattern-recognition receptors (PRRs) are activated by the host and identify chemicals released by infections or damaged cells. Pathogen-associated molecular patterns and damage-associated molecular patterns are the names of these molecular signals[36]. Numerous intracellular DNA sensors, TLRs, nucleotide-binding and oligomerization domain (NOD)-like receptors, C-type lectin receptors, RLRs, and NOD-inducible gene-I-like receptors are among the numerous members of the PRR family[37].
A multi-protein complex NLRP3 inflammasome is a type of PRR essential for the host’s innate defenses against bacterial, fungal, and viral infections[38-41]. However, when it is dysregulated, it has been connected to the pathogenesis of many inflammatory-associated diseases, including cancer, neuroinflammation, retinopathy, stroke, diabetes, atherosclerosis, and autoinflammatory diseases[42-46]. The three protein components that make up NLRP3 are an amino-terminal pyrin domain (PYD), a NOD, and a leucine-rich repeat domain at the protein’s C-terminus[47]. Apoptosis-associated speck-like protein (ASC) is composed of two proteins, pyrin and a caspase-recruitment domain (CARD), but their interaction to initiate inflammasome assembly facilitates it to recruit pro-caspase-1 to the inflammasome complex[48,49].
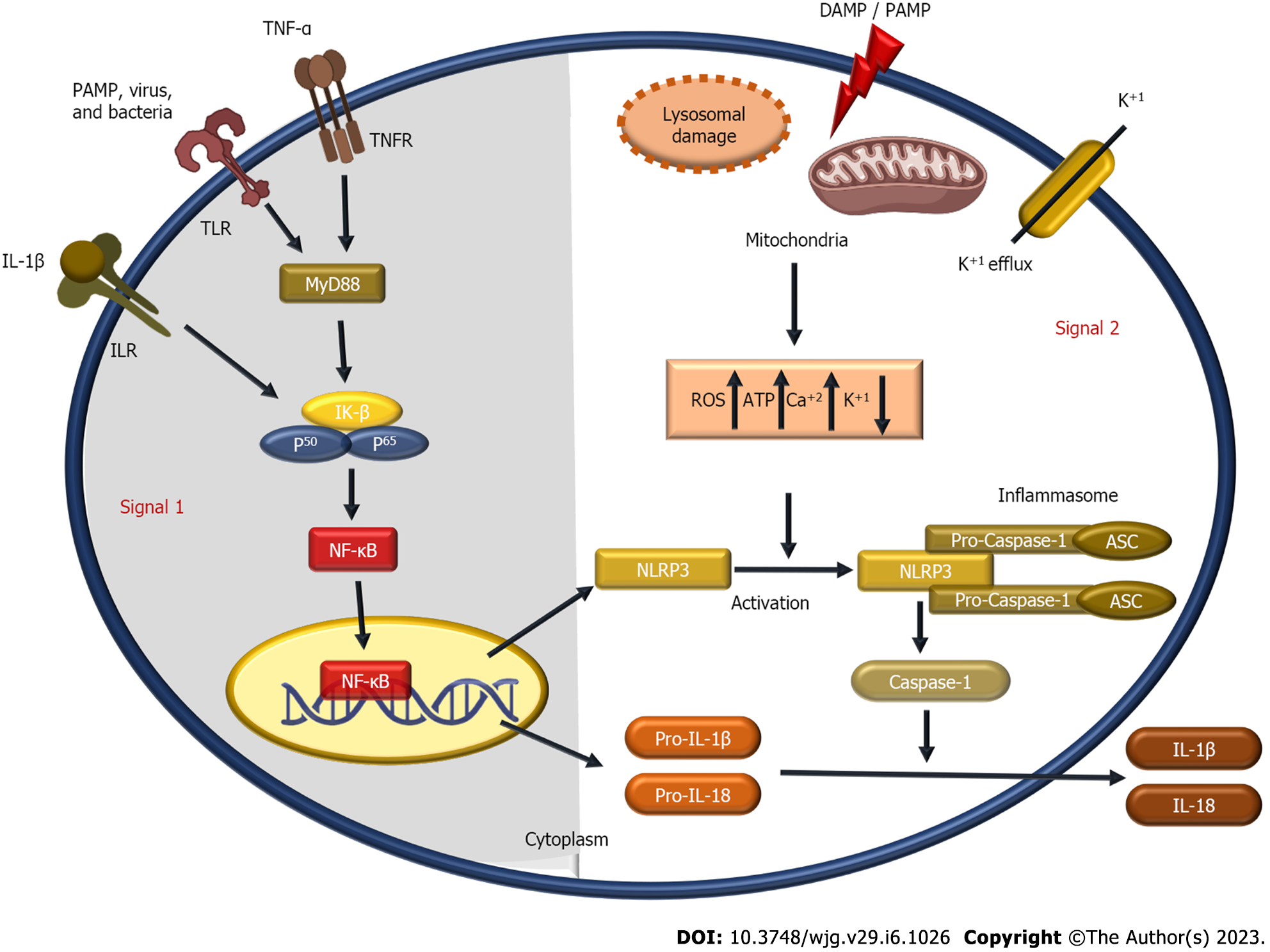
The activation of the NLRP3 inflammasome is essentially caspase-1 autocatalysis. Once turned on, NLRP3 functions as a sensor molecule that self-oligomerizes and recruits ASC through homotypic PYD-PYD interaction, causing ASC to assemble into sizable speck-like formations. Caspase-1 is then autocatalytically activated as aggregated ASC recruits pro-caspase-1 through CARD-CARD interaction. Proteolytic activation of the pro-inflammatory cytokines IL-1β and IL-18 and the soluble cytosolic protein gasdermin D (GSDMD) is the function of activated caspase-1 heterotetramers. Following proteolysis, the oligomerized gasdermin N can bind membrane lipids and create membrane pores to mediate the release of IL-1β and IL-18 outside the normal secretory pathway. In parallel, cells go through pyroptosis, a pro-inflammatory cell death[50-53]. IL-1β stimulates endothelial cell response by allowing immune cell infiltration into infected or injured tissues via the activation of genes that regulate temperature, pain threshold, vasodilation, and hypotension[54]. IL-18 is a co-stimulatory cytokine necessary for mediating adaptive immunity through the influence of interferon-gamma production[54].
It is believed that several cellular signals, including ion fluxes (K+ efflux, Cl− efflux, Ca2+ influx, and Na+ influx), mitochondrial dysfunction, and reactive oxygen species (ROS) generation, are responsible for NLRP3 inflammasome activation[55-60]. In addition, heme[61,62], particulate matter[56,63,64], pathogen-associated RNA[65-68], and bacterial and fungal toxins[69,70] are also considered NLRP3 activators.
NLRP3 activators require a priming signal (signal 1) to be activated[71]. TLRs, NOD-like receptors (e.g., NOD1 and NOD2), or cytokine receptors such as TNF-α and IL-1β, bind to stimuli in the priming signal, which is crucial for macrophage stimulation. Pro-IL-1β, which is not constitutively produced in inactive macrophages, is upregulated by NF-κB. In addition, NF-κB also upregulates the expression of NLRP3, which is assumed to exist in quantities insufficient for starting inflammasome activation during rest[71,72]. In contrast, priming signals are not crucial for ASC, pro-caspase-1, and pro-IL-18 production levels[71]. Inflammasome activation and NLRP3 self-association are regulated by the priming signal’s induction of Jun N-terminal 1-mediated phosphorylation of NLRP3[73].
Ca2+ is necessary for NLRP3 inflammasome activation by interacting with inositol 1,4,5-trisphosphate, a byproduct of phosphatidylinositol 4,5-bisphosphate hydrolysis catalyzed by phospholipase C, and the inositol 1,4,5-trisphosphate receptor on the endoplasmic reticulum, which promotes Ca2+ mobilization and NLRP3 inflammasome activation[60]. The NLRP3 inflammasome is activated by excessive ER Ca2+ release associated with mitochondrial damage and results in ROS production and an excess of Ca2+[74-76]. Additionally, K+ efflux and a reduction in intracellular K+ are upstream events in NLRP3 activation[59,77]. The NLRP3 inflammasome also needs K+ efflux for NLRP3 formation, according to a recently discovered component called NIMA-related kinase 7 (NEK7), which may directly bind to NLRP3 protein[78,79]. Various substances can increase ROS generation and induce NLRP3 activation without requiring K+ efflux[80,81]. Na+ influx-induced NLRP3 activation also relies on K+ efflux[59]. Reducing the concentration of extracellular Cl- activates caspase-1 and the generation of IL-β1[82]. An anion channel called the intracellular chloride channel has the potential to activate the volume-regulated anion channel. It is intriguing since the K+ efflux-mitochondrial ROS axis was shown to be a downstream event of chloride intracellular channel-dependent chloride efflux. Intracellular chloride channel-mediated chloride efflux can encourage NEK7-NLRP3 association and subsequent ASC oligomerization[83].
ROS are one of the earliest identified triggers to engage the NLRP3 inflammasome. Lysosomal NADPH oxidase was once believed to be the source of ROS formation, even though mitochondria are the primary site of ROS production[59,84]. According to several studies, most NLRP3 inflammasome agonists have been shown to produce mitochondrial ROS in various cell types[63,85-88]. NADPH oxidase 4 (NOX4) has also been shown to influence carnitine palmitoyl transferase 1A and promote fatty acid oxidation, which aids NLRP3 activation[89].
Lysosomal destabilization contributes to NLRP3 activation in both phases (signal 2) and the priming step (signal 1); in palmitate-induced NLRP3 activation. By controlling the stability of the IL-1 mRNA, lysosomal calcium signaling controls the production of pro-IL-1β (signal 1), whereas cathepsin B, a lysosomal protease, contributes to the NLRP3 activation[90]. It has been hypothesized that acidic conditions are necessary for monosodium urate crystals to activate the inflammasome within lysosomes as the significant Na+ release raises the cellular osmolality and water influx and lowers intracellular K+ concentration[91].
Protein folding, localization, and functional activity are all controlled by post-translational modifications. Numerous post-translational modifications have been shown to affect innate immunity by affecting immune cells’ activation, survival, differentiation, and migration[92]. NLRP3 is ubiquitylated in dormant macrophages and deubiquitylated following activation and priming[93]. For the NLRP3 inflammasome to activate, the linear ubiquitin assembly complex must selectively ubiquitylate NLRP3 and ASC[94]. TLR priming enhances NLRP3 self-association and activation by causing Jun N-terminal kinase 1 to phosphorylate NLRP3[95]. The mechanism of NLRP3 inflammasome activation is outlined in Figure 2.
Naringin: Naringin is a flavonoid glycoside, rich in citrus fruits such as grapefruit and orange[96,97]. Naringin protects mice from obesity, azoxymethane/dextran sodium sulfate (DSS)-induced carcinogenesis, and acetic acid (AA)-induced colitis[98-100]. In 2018, Cao et al[101] revealed that naringin protects mice from DSS-induced UC owing to its NLRP3 inflammasome and mitogen-activated protein kinase (MAPK) inhibitory effects. Furthermore, it upregulated peroxisome proliferator-activated receptor γ (PPARγ) while suppressing NF-κB activation.
Chlorogenic acid: Chlorogenic acid (CGA) is a phenolic acid found in foods and many plants[102,103]. Zeng et al[104] reported that CGA prevented DSS-induced mice colitis by down-regulating miR-155 expression and suppressing NLRP3 and NF-κB in macrophages. CGA downregulates caspase-1, pro-cleaved-IL-1β, and pro-cleaved-IL-18 in mice with colitis. In line with this study, CGA ameliorates NLRP3 inflammasome in different models, such as CCl4-induced acute liver injury[105] and lipopolysaccharide (LPS)-induced mice with acute lung injury[106].
1, 25-dihydroxy vitamin D3: Vitamin D (Vit D3) is a fat-soluble vitamin that maintains calcium and phosphorus homeostasis[107]. Significantly, Vit D3 ameliorates the NLRP3 inflammasome in variant mouse models like periodontitis[108], diabetes-induced retinal vascular damage[109], nitrogen mustard-induced cutaneous inflammation[110], hyperosmotic stress-induced injury[111], cisplatin-induced acute renal injury[112], and allergy inhibition[113]. Cao et al[114] reported that it suppresses NLRP3 inflammasome activation, NLRP3-mediated ASC oligomerization, caspase-1 activation, and IL-1β production. Also, NLRP3 binding to NEK7 was inhibited in DSS-induced UC.
Ginsenosides: Ginsenosides are the most significant phytochemical of ginseng obtained from the Panax species family Araliaceae[115,116]. Ginsenoside Rk3 (Rk3) is a tetracyclic triterpenoid reportedly found in Radix notoginseng herbs[117]. Tian et al[118] revealed that Rk3 protects against DSS-induced UC by suppressing NLRP3 expression. Rk3 administration significantly attenuated myeloperoxidase (MPO), inducible nitric oxide synthase activities, and downregulated NLRP3, ASC, and caspase-1 expression. Ginsenoside Rd (Rd) is another ginsenoside that inhibits NLRP3 in UC[119]. Rd suppresses NLRP3 activation and decreases caspase-1 production and IL-1β levels in DSS-induced UC. Also, Rd inhibits MPO and inducible nitric oxide synthase activities while increasing antioxidant glutathione content[120].
Phloretin: Phloretin (PHL) is a flavonoid found in fruit, leaves, and roots of the apple tree Malus domestica[121,122]. Zhang et al[123] demonstrated that PHL suppressed DSS-induced NLRP3 inflammasome activations and regulated the NF-κB, TLR4, and PPARγ pathways. Moreover, PHL attenuated oxidative stress and regulated the expression of ZO-1 and occludin. In the same context, Wu et al[124] found that PHL ameliorated UC by suppressing NF-κB and NLRP3 activation. Additionally, it decreases cytokines and oxidative injury while maintaining intestinal barrier integrity.
Cinnamaldehyde: Cinnamaldehyde (CA) is the principal constituent of the bark of Cinnamomum cassia and C. verum[125]. CA suppresses NLRP3 in several models like rheumatoid arthritis[126], endotoxin-poisoned mice[127], cardiac ischemia-reperfusion[128], fructose-induced cardiac inflammation and fibrosis[129], and LPS-induced renal inflammation[130]. CA mitigates DSS-induced colitis by suppressing NLRP3 activation and miR-21, miR-155, IL-1β, and IL-6 levels. Furthermore, the suppression of NLRP3, miR-21, and miR-155 was observed in CA-treated, LPS-stimulated RAW264.7 cells. The production ROS and protein kinase B/mammalian target of rapamycin phosphorylation was also reduced[131].
Palmatine: Palmatine is an isoquinoline alkaloid found in traditional Chinese medicine (TCM), including Fibraurea spp., Corydalis spp., Berberis spp., and Phellodendron spp[132-134]. Palmatine suppresses NLRP3 inflammasomes in different models, like hyperuricemia-induced kidney injury[135] and monosodium uric acid-induced gouty arthritis[136]. Mai et al[137] revealed that palmatine attenuates DSS-induced colitis in mice by suppressing NLRP3 inflammasomes and decreasing the colonic levels of MPO, IL-1β, and TNF-α. In 2022, Cheng and co-workers recently suggested that 8-oxypalmatine exerted an appreciable protective effect on DSS-induced colitis, suppressing the NLRP3 inflammasome and activating the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway[138].
L-fucose: L-fucose, a naturally safe monosaccharide found in animals, is often utilized as a food ingredient[139,140]. L-fucose attenuates colitis by suppressing NLRP3 inflammasome and NF-κB activation, decreasing pro-inflammatory cytokines, and reducing neutrophil and macrophage infiltration[141].
Genistein: Genistein (Gen) is a significant isoflavone in soy[142]. Gen suppresses the NLRP3 inflammasome in macrophages by activating the G protein–coupled receptor 5-cAMP signal in DSS-induced UC mice. Additionally, the Gen administration boosted intracellular cAMP levels and decreased caspase-1 and IL-1β levels[143]. In agreement with this study, Gen suppressed the NLRP3 inflammasome in different models, such as cerebral ischemia in mice[144], H2O2-induced senescence of human umbilical vein endothelial cells[145], hippocampal neurons in aging rat brain tissue[146], and alloxan-induced diabetes in mice[147].
Sinapic acid: Sinapic acid (SA) is a phytochemical abundant in various food plants, including spices, citrus and berry fruits, cereals, and others[148-150]. SA was shown to have potential therapeutic efficacy in inhibiting NLRP3-associated inflammatory disorders[151]. In conjunction with that, SA upregulated tight junction protein-1 (ZO-1) and claudin-1 in DSS-induced UC, suppressing the NLRP3 activation and attenuating intestinal permeability. Additionally, SA attenuates oxidative injury by boosting antioxidants superoxide dismutase, glutathione peroxidase, and catalase activity while lowering MPO and pro-inflammatory cytokine mRNA levels in circulation and colonic tissue[152].
Terpinen-4-ol: Terpinen-4-ol (TER) is a primary ingredient in Zanthoxylum bungeanum Maxim’s essential oil[153]. Zhang et al[154] investigated whether the protective effect of TER may be substantially related to its suppression of NLRP3 activation in DSS-induced colitis. TER balanced the amounts of Lactobacillus and Escherichia coli while lowering the plasmatic LPS content. Additionally, TER inhibited the breakdown of the colon epithelial barrier by controlling the expression of ZO-1 and occluding and decreasing IL-1β production.
Apigenin: Apigenin (API) is a flavone found in many vascular plants’ leaves, vegetables, stems, and fruits[155]. According to Márquez-Flores et al[156], API anti-inflammatory activity was attributed to a suppression of the NLRP3 inflammasome by lowering levels of pro-inflammatory cytokines and oxidative stress as a result of controlling the activity of cleaved caspase-1 and caspase-11 enzymes. In agreement with this study, API suppresses NLRP3 inflammasome activation in high-fat diet-induced non-alcoholic fatty liver disease[157], palmitic acid-induced liver injury[158], arteriosclerosis[159], human umbilical vein endothelial cells inflammation[160], depression in chronic unpredictable mild stress rats[161], and doxorubicin-induced renal injury[162].
Rosmarinic acid: Rosmarinic acid (RA) is a natural polyphenol found in plants from the Labiatae family[163]. RA down-regulates the NRLP3-inflammasome in neuroinflammation[164], acetaminophen-induced liver injury[165], and nicotine-induced atherosclerosis[166]. In 2021, Marinho et al[167] demonstrated that RA-loaded nanovesicles enhanced Nrf2 and heme oxygenase-1 expression while decreasing NLRP3 inflammasome, ASC, and caspase-1 protein expression and IL-1β levels. RA also reduces TNF-α and MPO activity.
Lonicerin: A flavonoid glycoside, lonicerin, is extracted from the plant Lonicera japonica Thunb. It treats infectious and inflammatory diseases[168,169]. Numerous investigations have shown that lonicerin is among the main ingredients with anti-inflammatory and immunomodulatory properties[170]. Lv et al[165] suggested that lonicerin reduces colitis through NLRP3-ASC-pro-caspase-1 complex assembly. Lonicerin has a therapeutic impact on intestinal inflammation by directly binding to the histone methyltransferase enhancer of zeste homolog 2, which promotes autophagy and speeds up the destruction of NLRP3 by autolysosomes.
Magnesium lithospermate B: Magnesium lithospermate B (MLB) is the primary ingredient of Salviae miltiorrhizae[171]. It has been reported that MLB treats acute colitis[172,173]. Jiang et al[174] found that MLB might treat acute and chronic colitis by downregulating NLRP3, ASC, and caspase-1 levels.
Fumigaclavine C: Fumigaclavine C is an indole alkaloid obtained from endophytic Aspergillus terreus and Rhizophora stylosa[175,176]. According to Guo et al[177], fumigaclavine C attenuates DSS-induced colitis by decreasing pro-inflammatory cytokines TNF-α, IL-1β, and IL-17A, mediated by suppressing NLRP3 inflammasome and caspase-1 activation.
Nigeglanine: Nigeglanine is a phytochemical obtained from Nigella sativa oil[178]. According to a study conducted in 2019 by Gao et al[179], nigeglanine inhibits colon epithelial cell pyroptosis by suppressing the NLRP3 inflammasome, NF-κB, and MAPK in DSS-induced colitis in mice. Surprisingly, nigeglanine supplementation enhanced ZO-1 and occludin protein levels, indicating that nigeglanine protects barrier integrity.
Mycophenolate mofetil: Mycophenolate mofetil (MMF) is a fermentation byproduct of Penicillium stoloniferum[180-182]. MMF is an immunosuppressant commonly used to treat systemic lupus erythematosus, lupus nephritis, and other autoimmune disorders[183]. Serrya et al[184] observed that MMF had coloprotective effects against AA-induced UC by suppressing the NLRP3 inflammasome and consequent release of IL-1β, IL-18, and INF-γ. In colon tissues, MMF considerably reduced oxidative stress and boosted antioxidants glutathione, catalase, and superoxide dismutase levels as well as total antioxidant capacity mediated by upregulating Nrf-2.
Evodiamine: Evodiamine (EVO), a significant alkaloidal substance discovered from Evodia rutaecarpa, has long been used in TCM to treat various infection-related diseases, such as diarrhea, beriberi, and oral ulcers[185,186]. Additionally, EVO effectively treats nausea and abdominal discomfort[187]. Furthermore, EVO inhibits NLRP3 inflammasome activation, indicating the application of EVO in treating IBD[188] and enhancing innate immunity against bacterial infection[189]. In DSS-induced UC mice, EVO reduced inflammation by controlling the NF-κB signal and NLRP3 inflammasome, which reduced the production of TNF-α, IL-1β, and IL-6 and altered the expression of ZO-1 and occludin[190]. Similarly, Ding et al[188] showed that EVO inhibits NLRP3 inflammasome activation by enhancing autophagosome degradation and inhibiting the NF-κB pathway.
Brusatol: Brusatol is a major quassinoid of Brucea javanica used in treating inflammatory disorders, particularly intestinal inflammation, such as colitis and dysentery[191]. Zhou et al[192] revealed that the anti-UC activity of brusatol is closely related to the suppression of NF-κB and NLRP3 signals and the activation of Nrf2 expression-mediated anti-oxidative effects. The TNF-α, pro- IL-1β, IL-18, prostaglandin E2, and nitric oxide levels were significantly reduced by brusatol in LPS-stimulated macrophages.
Bergenin: Bergenin, a C-glycoside produced from 4-O-methyl gallic acid, is one of the primary active components of plants in the genus Peltophorum[193]. Bergenin reduces palmitic acid-induced pancreatic injury by suppressing NLRP3 activation[194]. Likewise, Lopes de Oliveira et al[195] showed that bergenin attenuates 2,4,6-trinitrobenzene sulfonic acid-induced acute colitis in rats. Bergenin decreased inflammatory cytokines levels mediated by suppressing phosphorated signal transducer and activator of transcription 3 (STAT3), NF-κB, and NLRP3/ASC inflammasome signals.
Wedelolactone: Wedelolactone is a coumarin obtained from plants found in Wedelia chinensis[196,197]. Wei et al[198] revealed that wedelolactone administration significantly suppressed NLRP3 and caspase-1 activation to reduce IL-1β production in DSS-induced UC. Wedelolactone also successfully maintains intestinal barrier function by activating AMP-activated protein kinase (AMPK).
Walnut oil: Walnut oil is commonly recommended as a nutritious, premium food oil and is used extensively in TCM[199]. Walnut oil has anti-inflammatory and antioxidant effects[200-202]. Miao et al[203] explored how walnut oil inhibits the NLRP3 inflammasome activation and modifies gut microbiota in DSS-induced colitis in mice. Walnut oil enhanced the levels of short-chain fatty acids and blocked apoptosis while reducing ROS generation and pro-inflammatory cytokines release by sup
Nigakinone: Nigakinone is the principal active ingredient of Ramulus et Folium Picrasmae, and it is one of the TCM substances frequently used to treat diarrhea, colitis, and dysentery[204]. In DSS-induced colitis, nigakinone alleviated symptoms by regulating the farnesoid X receptor/NLRP3 pathway[205].
Oroxindin: Oroxindin is a natural bioflavonoid that was identified in Huang Qin. Oroxindin has been shown in several studies to have a variety of bioactivities, including anti-inflammatory, antioxidant, and anticancer effects[206-208]. According to Liu et al[209], oroxindin reduces inflammatory responses by suppressing NLRP3 inflammasome activation and reducing colonic IL-1β and IL-18 Levels. Oroxindin modulated the production of thioredoxin interacting protein (TXNIP), reducing LPS-induced NLRP3 inflammasome and caspase-1 activation in cultured macrophages, hence decreasing TXNIP-dependent NF-κB activation.
Schisandrin B: Schisandrin B is the most significant component in Schisandra chinensis[210]. Schisandrin B suppresses NLRP3 inflammasome activation in LPS-induced airway inflammation and airway remodeling[211] and propionibacterium acnes-induced pyroptosis[212]. In 2021, Zhang et al[213] demonstrated schisandrin B reduces NLRP3 inflammasome activation mediated by IL-1β release in intestinal epithelial cells of a colitis model.
Trifolirhizin: Trifolirhizin is a pterocarpan identified from Trifolium pratense and Sophora flavescens[214,215]. Trifolirhizin treatment successfully controlled the balance of T helper/T regulatory (Th17/Treg) cells and inflammation in DSS-induced UC mice by suppressing NLRP3 activation by TXNIP. Trifolirhizin has also been shown to control the AMPK-TXNIP pathway[216].
Sanguinarine: Sanguinarine, benzylisoquinoline alkaloids, is obtained from Sanguinaria canadensis and Fumaria[217]. According to Li et al[218], sanguinarine showed a potential therapeutic impact on DSS-induced UC mice by suppressing NLRP3-(Caspase-1)/IL-1β pathway and enhancing gut microbial dysbiosis. Additionally, sanguinarine decreased the expression of NLRP3 and the activation of caspase-1 and IL-1β in THP-1 cells induced by LPS[218].
Geniposide: The primary active ingredient of Gardenia jasminoides is geniposide, an iridoid glycoside extracted from the Gardenia used in TCM[219]. Geniposide was shown by Pu et al[220] to reduce macrophage differentiation in mice with acute colitis induced by DSS by inhibiting the NLRP3. In addition, geniposide augmented AMPK/Sirt1 signal and inhibited the NLRP3 inflammasome in LPS-induced BMDM cell or RAW264.7 cell cultures. In line with this study, geniposide suppressed the activation of the NLRP3 inflammasome in different in vivo and in vitro models, such as myocardial ischemia/reperfusion injury[221], sepsis-induced myocardial dysfunction[222], and cholestatic liver inflammatory injury[223].
Sinomenine hydrochloride: Sinomenine is a pure alkaloid derived from the plant Sinomenium acutum of the Menispermaceae family[224]. According to Zhou et al[225], sinomenine may reduce the symptoms of experimental colitis by altering the production of the gut microbiota and reducing the activity of the NLRP3 inflammasome and pro-inflammatory mediators levels in mice. In line with this study, sinomenine inhibits the NLRP3 inflammasome, which has a favorable effect on cartilage degradation[226], autoimmune encephalomyelitis[227], ischemic stroke[228], and others.
Picroside II: Picroside II is a kind of iridoid chemical that is used in TMC. There are now three known iridoids in Picrorhiza scrophularii flora pennell[229]. Picroside II possesses hepatoprotective, immune-regulating, anti-inflammatory, and antioxidant properties[230-232]. Yao et al[233] demonstrated that picroside II alleviated DDS-induced UC by significantly suppressing NLRP3 inflammasomes and inflammatory components in vivo induced by DDS.
Hydroxytyrosol: Hydroxytyrosol is a prominent and typical phenolic component in olive oil and leaves[234]. Miao[235] demonstrated that hydroxytyrosol supplementation has a coloprotective effect on DSS-induced UC by suppressing NLRP3, caspase-1, and ASC expression levels and downregulating IL-18 and IL-1β levels. Additionally, hydroxytyrosol modifies the in vivo gut microbiota while boosting colonic antioxidant functionality.
Trans-10-hydroxy-2-decenoic acid: The most prevalent fatty acid and main lipid in royal jelly is 10-hydroxy-2-decenoic acid, which also has antibacterial[236], antioxidant[236], immunomodulatory[237], anti-inflammatory[238] properties, and others. According to Huang et al[239], trans-10-hydroxy-2-decenoic acid improves colonic barrier function, decreases colonic TXNIP, ASC, caspase-1, GSDMD, IL-1β, and IL-18 Levels, and regulates the NLRP3 inflammasome-mediated pyroptosis to treat DSS-induced colitis.
Canna x generalis (CG) LH Bailey is a hybrid of two closely related species that belongs to the Canna genus[240]. With its enormous leaves and gorgeous blooms, CG may grow without special care and can adapt to various soil conditions[241,242]. CG exhibits anti-inflammatory[243] and antioxidant[244] activities. CG extract exhibits a protective effect against DSS-induced UC mice by suppressing colonic activation of NLRP3 and NF-κB/TLR4 signals. CG extract reduces pro-inflammatory mediators, oxidative stress, and the inflammatory cascade in colonic tissues. Additionally, CG extract downregulates caspase-1, ASC, TLR4, and caspase-3 expressions[245].
Kuijieling decoction: An empirical formula known as Kuijieling decoction (KJL) has been introduced in clinical conditions for several years and is effective in treating UC[246,247]. KJL showed anti-inflammatory and antioxidant bioactivities[248,249]. KJL effectively attenuates UC symptoms in the UC rat model[250]. Jie et al[251] revealed that KJL suspension attenuates UC by decreasing NLRP3, caspase 1, and pro-inflammatory cytokines levels. They also showed that in DSS-induced UC mice, KJL downregulates NLRP3, caspase-1, GSDMD-N, IL-1β, and IL-18. Also, the expression levels of ASC, caspase-1, IL-1β, IL-18, and Mir-223 were downregulated in colon tissue.
Huaier: For more than 1600 years, Trametes robiniophila Murr (Huaier), a sandy beige fungus that grows on tree trunks, has been extensively employed in TCM[252-254]. Wang et al[255] showed that Huaier has anti-inflammatory activity in DSS-induced murine colitis in mice by downregulating NLRP3 expression and preventing NLRP3 inflammasome activation-induced IL-1β production and caspase-1 cleavage.
Shaoyao decoction: Shaoyao decoction (SYD) is a TCM that has demonstrated that it has anti-inflammatory[256] and anticancer[257] effects, among others. In addition, a recent study revealed that SYD had been successfully utilized to treat IBD and other disorders linked to the damp-heat syndrome in the intestines[258]. Wei et al[259] reported the protective effects of SYD on DSS-induced UC and pyroptosis by suppressing the NLRP3 and NF-κB/P38 signals. Moreover, SYD supplementation decreases caspase-1 activity and the release of ASC and GSDMD, preventing the formation of NLRP3 and protecting the intestinal barrier integrity.
Smilax china L. is one of the TCM that is effective in reducing inflammation[260], nociception[261], and cancer[262]. S. china L. polysaccharide (also known as SCLP) was isolated from the rhizome of S. china L. and is a TCM used to treat inflammatory disorders[263]. SCLP treats UC, according to Pan et al[264], by inhibiting the galectin-3 expression and its connection with NLRP3 activation and IL-1β production, which in turn decreases the release of inflammatory mediators in the DSS mice model.
Dendrobium officinale Kimura et Migo is the second-biggest genus of Orchidaceae[265,266]. Polysaccharides, the main active constituents in D. officinale, have received much interest due to their numerous biological activities[267-269]. Moreover, a prior investigation showed that D. officinale polysaccharides (DOPS) could successfully alleviate DSS-induced colitis in mice[270]. In 2018, Liang et al[270] found that DOPS has a notable therapeutic effect in treating DSS-induced acute UC mice. They also examined whether DOPS administration might significantly reduce the activation of the NLRP3 inflammasome and the β-arrestin1 signaling pathways. DOPS significantly decreases colonic pathological damage, relieves clinical signs and symptoms, and restores the pro- and anti-inflammatory cytokines balances.
As a result of its various pharmacological properties, the fruit of Schisandra chinensis, a member of the Magnoliaceae family, has long been utilized as an herbal remedy in TCM[271,272]. These fruits were often utilized as wholesome meals and pharmaceuticals for several chronic diseases, such as cancer, liver injuries, and gastrointestinal disorders[210,273]. S. chinensis mitigates ferroptosis and NLRP3 inflammasome-mediated pyroptosis in diabetic nephrosis[274]. Recently, Bian et al[275] found that S. chinensis extract can reduce the symptoms of DSS-induced colitis by TLR4/NF-κB and NLRP3 inflammasome suppression. Additionally, S. chinensis extract might correct the gut microbiota imbalance brought on by UC while maintaining gut barrier function by raising ZO-1 and occludin levels.
Tripterygium wilfordii Hook. f., commonly referred to as Lei Gong Teng, is a TCM herb frequently used to treat autoimmune and inflammatory diseases[276-278]. The root bark of T. wilfordii demonstrated pharmacological actions against inflammation[279], autoimmune disorders[280], Crohn’s disease[281], liver cancer[282], and others. T. wilfordii polycoride is the primary active ingredient of T. wilfordii[283]. Fangxiao et al[284] reported the anti-inflammatory effects of T. wilfordii polycoride on 2,4,6-trinitrobenzenesulfonic acid-induced colitis by downregulating the NOXs-ROS-NLRP3 signal. T. wilfordii polycoride decreased ROS production, and NOXs’ activity was mediated by downregulating colonic NLRP3, ASC, and caspase-1.
Rubia cordifolia L. is a perennial climbing vine from the Rubiaceae family[285,286]. Its aerial portion possesses a range of bioactivities, including anti-inflammatory[287], antioxidant[288,289], and others. Recent findings by Qin et al[290] showed that using R. cordifolia extract inhibits the NLRP3 inflammasome and IL-6/Jak-2/STAT3 signal activation in DSS-induced UC. R. cordifolia extract improves the symptoms, diminishes colonic mucosal injury and macrophage infiltration, inhibits the production of inflammatory cytokines, and lowers mortality.
Mulberry fruit extract (MFE) is obtained from the fully ripened fruits of Morus macroura[291,292]. Salama et al[293] recently reported the involvement of NLRP3, miRNA-223, and the TNF-α/NF-κB pathway in the coloprotective effects of MFE against AA-induced UC in rats. MFE also reduces levels of TNFR1, NLRP3, NF-κB p65, TNF-α, IL-1β, IL-18, and caspase-1, while increases miRNA-223 expression.
Akkermansia muciniphila, a Gram-negative and anaerobes bacterium, is a member of the Verrucomicrobia phylum, which has been discovered to be common in the human gut[294-296]. According to investigations, A. muciniphila may protect against major diseases like atherosclerosis[297], amyotrophic lateral sclerosis[298], and immune-mediated liver damage[299]. Additionally, it was reported that A. muciniphila improves chronic colitis[300]. Recently, Qu et al[301] revealed that oral treatment of A. muciniphila strain BAA-835 effectively reduces the signs and symptoms of DSS-induced acute colitis dependent on NLRP3 activation. The expression of NLRP3, caspase-1, and IL-1β was elevated in mouse macrophage cells in A. muciniphila-treated animals, as well as pro-inflammatory cytokines such as TNF-α, IL-6, and monocyte chemoattractant protein 1 (MCP-1).
A facultative anaerobic fungus called Saccharomyces cerevisiae has been extensively employed in medicine to create modified carriers and oral vaccinations[302,303]. According to a study by Sun et al[304], modified S. cerevisiae reduced the severity of DSS-induced colitis in mice by inhibiting macrophage pyroptosis and regulating the intestinal microbiota. Lactic acid-produced S. cerevisiae modulated macrophage polarisation, prevented the production of pro-inflammatory cytokines in vivo and in vitro, and decreased NLRP3 inflammasome and caspase-1 levels.
Lactobacillus acidophilus is one of the most common commercial species of lactic acid bacteria, found in various dairy products and nutritional supplements with probiotic indications[305]. According to studies, L. acidophilus may have immunomodulatory, anti-inflammatory, and antioxidant[306-308] activities. Furthermore, it improves the intestinal epithelial barrier function[309,310]. Additionally, it was demonstrated to be efficient in preventing colitis caused by Citrobacter rodentium[309] and relieving DSS-induced colitis[311]. According to Li et al[312], L. acidophilus attenuates UC in rats by inhibiting the NLRP3 inflammasome pathway, increasing the short-chain fatty acids level, and promoting autophagy.
Grape seed proanthocyanidin extract (GSPE) is a grape seed extract that contains catechin, epicatechin gallate, and epigallocatechin[313,314]. According to Sheng et al[315], GSPE supplementation reduces inflammatory cytokines and oxidative stress, maintains the intestinal barrier, and enhances the microbiome in DSS-induced colitis. GSPE decreases the NLRP3 inflammasome and increases ZO-1, occludin, and claudin-1 levels. Furthermore, GSPE treatment of colon tissues resulted in a considerable decrease in TNF-α and IL-1β levels.
Brilliant blue G (BBG) is a triarylmethane dye with a modest hydrophilicity that has been proven to stain the inner limiting membrane selectively[316]. In 2021, Saber et al[317] revealed that the BBG/OLT1177 combination produced complementary effects and significantly alleviated DSS-induced UC by downregulating NLRP3, caspase-1, IL-1β, and IL-18 levels. In addition to reducing purinergic receptor (P2X7R) and oxidative stress levels, BBG treatment downregulated myeloid differentiation primary response 88, NF-κB, IL-6, TNF-α, the recruitment of the NLRP3 inflammasome, and the consequent activation of caspase-1, IL-1β, and IL-18.
Forsythia suspensa (Thunb.) Vahl is well-known in TCM[318]. Chao et al[319] showed that Forsythia suspensa extract effectively reduces metabolic dysfunction and DSS-induced UC damage by suppressing the NLRP3 pathway and activating Nrf2.
Kui jie tong is a herbal extract that effectively treats UC[320]. Xue et al[321] reported that Kui jie tong mitigates UC by downregulating NLRP3 and caspase-1 as well as serum IL-1β, IL-18, and IL-33 and enhancing intestinal microbiota.
Jianpi Qingchang decoction, a TCM prescription, is composed of nine Chinese herbs and effectively attenuates moderate or beginning cases of UC[322,323]. Zhang et al[324] stated that Jianpi Qingchang decoction has a protective function by preventing DSS-induced NLRP3 inflammasome activation. Jianpi Qingchang decoction decreases inflammatory cytokine release.
Bioactive extracellular vesicles are present in the milk of all mammalian species[325,326]. Bovine milk exosomes have been shown to support intestinal cell proliferation, boost the number of goblet cells and mucin synthesis, inhibit bacterial growth, and support the intestinal microbiota[327-329]. According to the Tong et al[330] study, milk-derived extracellular vesicles regulate intestinal immunological homeostasis by suppressing TLR4- NF-κB and NLRP3 signaling pathways, re-establishing the balance of Treg/Th17 cells, and altering the gut microbiota. In a mouse UC model, milk-derived extracellular vesicles were proven to have a role in the control of immunological and inflammatory pathways, which decreased intestinal epithelium disruption, blocked the infiltration of inflammatory cells, and reduced tissue fibrosis[330].
Mirtazapine is a well-known antidepressant medication used to treat depression, anxiety, and sleep disorders[331]. In addition, mirtazapine reduces inflammation in diabetic rat kidneys by inhibiting the NLRP3 inflammasome[332]. The same results showed that mirtazapine has a coloprotective effect against UC, which was mediated by suppressing NLRP3 and caspase-1 activation and restoring the antioxidant/oxidant balance in AA-induced UC in rats. Additionally, mirtazapine reduces the levels of NF-κB, TNF-α, IL-1β, and IL-18[333].
Paeoniflorin-6’-O-benzene sulfonate is a new active monomer created by structurally altering paeoniflorin[334] and has anti-inflammatory and immunomodulatory characteristics[335,336]. According to Li et al[337], paeoniflorin-6’-O-benzene sulfonate has anti-colitis properties via suppressing TLR4-NF-κB and NLRP3 signals and G protein-coupled receptor kinase 2 translocations. Furthermore, paeoniflorin-6’-O-benzene sulfonate maintains intestinal barrier function in LPS-treated mice.
Dapagliflozin (DPZ), a sodium-glucose cotransporter-2 inhibitor, is recommended in addition to routine medical treatment for managing type 2 diabetes mellitus that has not been effectively controlled[338]. Interestingly, DPZ modulates the NLRP3 inflammasome in different models, such as diabetic nephropathy[339], LPS-induced lung damage[340], and type 2 diabetes mellitus- induced cardiomyopathy[341]. El-Rous and colleagues reported that DPZ suppressed the activation stage (signal 2) of NLRP3 inflammasome activation and prevented the priming step (signal 1) of that activation in AA-induced UC through altering NF-κB/AMPK interaction and halting NLRP3/caspase-1 communication. Furthermore, DPZ increases the anti-inflammatory cytokine IL-10 and remarkably suppresses caspase-1 activity and IL-1β and IL-18 production[342].
Canagliflozin is a small-molecule hypoglycemic medication that lowers blood glucose levels by blocking the type 2 sodium-glucose cotransporter, which prevents the kidneys from reabsorbing glucose[343]. Nasr et al[344] reported that canagliflozin-loaded chitosan-hyaluronic acid microspheres significantly suppressed NF-κB and NLRP3 activation, resulting in a decrease in caspase-1 cleavage and the inhibition of several inflammatory cytokines, including IL-1β and IL-18, in AA-induced colitis.
Rosuvastatin, an HMG-CoA inhibitor that is relatively new, has a safety and tolerability profile that is comparable to or better than the regularly prescribed dosages of other statins[345]. Rosuvastatin and Lactobacillus inhibit the NLRP3 inflammasome assembly, pro- IL-1β, pro-IL-18, and NF-κB, decreasing caspase-1 activity and IL-1β-driven pyroptotic activity in DSS in high-fat diet-induced colitis in rats[346].
MCC950 is an IL-1β inhibitor and is known to be a potent inhibitor of the NLRP3 inflammasome[347]. MCC950 inhibits NLRP3 among inflammasomes[347,348] and blocks canonical, non-canonical, and alternative NLRP3 activation[80,347,349]. MCC950 showed promising therapeutic potential for inhibiting NLRP3 inflammasome in the atherosclerotic lesion[350], cholestatic liver, non-alcoholic steatohepatitis-induced liver fibrosis[351,352], LPS-induced lung inflammation[353], and others. In 2018, Perera et al[354] revealed that MCC950 effectively treats murine UC. MCC950 significantly suppresses NLRP3 inflammasome activation and decreases colonic cytokines, chemokines, and nitric oxide levels. Moreover, MCC950 suppresses caspase-1 activation in the colon and macrophage cells.
The incidence and prevalence of UC, a recurrent and remitting condition, are rising. The goal of treatment is to increase a patient’s quality of life while achieving quick symptom alleviation and mucosal healing. There are still a lot of unsolved concerns despite the abundance of evidence pointing to genetic and host-related variables in UC. Numerous opportunities exist for further inquiry to improve our understanding of the pathogenesis of UC and uncover possible predictors of disease severity, responsiveness to medication, and novel therapeutic targets as the incidence and prevalence of UC around the world increase. The NLRP3 inflammasome plays a crucial role in the inflammation and immunological reaction by promoting caspase-1 activation and the release of IL-1β. Interestingly, phytochemicals, plant extracts, and synthetic drugs exhibit promising colon protective effects mediated by NLRP3 and caspase-1 activation suppression and, subsequently, the release of the pro-inflammatory cytokine IL-1β, which has a key role in UC-associated inflammation. However, future clinical studies are required to comprehend how NLRP3 inflammasome activation inhibition significantly treats and controls UC.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arumugam VA, India; Zhao G, China S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Shouval DS, Rufo PA. The Role of Environmental Factors in the Pathogenesis of Inflammatory Bowel Diseases: A Review. JAMA Pediatr. 2017;171:999-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 127] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 2. | Jones GR, Lyons M, Plevris N, Jenkinson PW, Bisset C, Burgess C, Din S, Fulforth J, Henderson P, Ho GT, Kirkwood K, Noble C, Shand AG, Wilson DC, Arnott ID, Lees CW. IBD prevalence in Lothian, Scotland, derived by capture-recapture methodology. Gut. 2019;68:1953-1960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 135] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 3. | Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46-54.e42; quiz e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3789] [Cited by in RCA: 3524] [Article Influence: 271.1] [Reference Citation Analysis (5)] |
| 4. | Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12:720-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1157] [Cited by in RCA: 1859] [Article Influence: 185.9] [Reference Citation Analysis (1)] |
| 5. | Rosenstiel P, Sina C, Franke A, Schreiber S. Towards a molecular risk map--recent advances on the etiology of inflammatory bowel disease. Semin Immunol. 2009;21:334-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Frolkis A, Dieleman LA, Barkema HW, Panaccione R, Ghosh S, Fedorak RN, Madsen K, Kaplan GG; Alberta IBD Consortium. Environment and the inflammatory bowel diseases. Can J Gastroenterol. 2013;27:e18-e24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 7. | Feuerstein JD, Moss AC, Farraye FA. Ulcerative Colitis. Mayo Clin Proc. 2019;94:1357-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 271] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 8. | Ford AC, Moayyedi P, Hanauer SB. Ulcerative colitis. BMJ. 2013;346:f432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (2)] |
| 9. | Dignass A, Eliakim R, Magro F, Maaser C, Chowers Y, Geboes K, Mantzaris G, Reinisch W, Colombel JF, Vermeire S, Travis S, Lindsay JO, Van Assche G. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J Crohns Colitis. 2012;6:965-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 640] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 10. | Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 1559] [Article Influence: 111.4] [Reference Citation Analysis (1)] |
| 11. | Sýkora J, Pomahačová R, Kreslová M, Cvalínová D, Štych P, Schwarz J. Current global trends in the incidence of pediatric-onset inflammatory bowel disease. World J Gastroenterol. 2018;24:2741-2763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 271] [Cited by in RCA: 262] [Article Influence: 37.4] [Reference Citation Analysis (6)] |
| 12. | Halme L, Paavola-Sakki P, Turunen U, Lappalainen M, Farkkila M, Kontula K. Family and twin studies in inflammatory bowel disease. World J Gastroenterol. 2006;12:3668-3672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 191] [Cited by in RCA: 192] [Article Influence: 10.1] [Reference Citation Analysis (1)] |
| 13. | Moller FT, Andersen V, Wohlfahrt J, Jess T. Familial risk of inflammatory bowel disease: a population-based cohort study 1977-2011. Am J Gastroenterol. 2015;110:564-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 162] [Article Influence: 16.2] [Reference Citation Analysis (1)] |
| 14. | Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, Kaplan GG. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769-2778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2677] [Cited by in RCA: 4079] [Article Influence: 509.9] [Reference Citation Analysis (110)] |
| 15. | Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, Essers J, Mitrovic M, Ning K, Cleynen I, Theatre E, Spain SL, Raychaudhuri S, Goyette P, Wei Z, Abraham C, Achkar JP, Ahmad T, Amininejad L, Ananthakrishnan AN, Andersen V, Andrews JM, Baidoo L, Balschun T, Bampton PA, Bitton A, Boucher G, Brand S, Büning C, Cohain A, Cichon S, D'Amato M, De Jong D, Devaney KL, Dubinsky M, Edwards C, Ellinghaus D, Ferguson LR, Franchimont D, Fransen K, Gearry R, Georges M, Gieger C, Glas J, Haritunians T, Hart A, Hawkey C, Hedl M, Hu X, Karlsen TH, Kupcinskas L, Kugathasan S, Latiano A, Laukens D, Lawrance IC, Lees CW, Louis E, Mahy G, Mansfield J, Morgan AR, Mowat C, Newman W, Palmieri O, Ponsioen CY, Potocnik U, Prescott NJ, Regueiro M, Rotter JI, Russell RK, Sanderson JD, Sans M, Satsangi J, Schreiber S, Simms LA, Sventoraityte J, Targan SR, Taylor KD, Tremelling M, Verspaget HW, De Vos M, Wijmenga C, Wilson DC, Winkelmann J, Xavier RJ, Zeissig S, Zhang B, Zhang CK, Zhao H; International IBD Genetics Consortium (IIBDGC), Silverberg MS, Annese V, Hakonarson H, Brant SR, Radford-Smith G, Mathew CG, Rioux JD, Schadt EE, Daly MJ, Franke A, Parkes M, Vermeire S, Barrett JC, Cho JH. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3979] [Cited by in RCA: 3594] [Article Influence: 276.5] [Reference Citation Analysis (0)] |
| 16. | Goyette P, Boucher G, Mallon D, Ellinghaus E, Jostins L, Huang H, Ripke S, Gusareva ES, Annese V, Hauser SL, Oksenberg JR, Thomsen I, Leslie S; International Inflammatory Bowel Disease Genetics Consortium; Australia and New Zealand IBDGC; Belgium IBD Genetics Consortium; Italian Group for IBD Genetic Consortium; NIDDK Inflammatory Bowel Disease Genetics Consortium; United Kingdom IBDGC; Wellcome Trust Case Control Consortium; Quebec IBD Genetics Consortium, Daly MJ, Van Steen K, Duerr RH, Barrett JC, McGovern DP, Schumm LP, Traherne JA, Carrington MN, Kosmoliaptsis V, Karlsen TH, Franke A, Rioux JD. High-density mapping of the MHC identifies a shared role for HLA-DRB1*01:03 in inflammatory bowel diseases and heterozygous advantage in ulcerative colitis. Nat Genet. 2015;47:172-179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 292] [Cited by in RCA: 243] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 17. | Cattin AL, Le Beyec J, Barreau F, Saint-Just S, Houllier A, Gonzalez FJ, Robine S, Pinçon-Raymond M, Cardot P, Lacasa M, Ribeiro A. Hepatocyte nuclear factor 4alpha, a key factor for homeostasis, cell architecture, and barrier function of the adult intestinal epithelium. Mol Cell Biol. 2009;29:6294-6308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 132] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 18. | Asano K, Matsushita T, Umeno J, Hosono N, Takahashi A, Kawaguchi T, Matsumoto T, Matsui T, Kakuta Y, Kinouchi Y, Shimosegawa T, Hosokawa M, Arimura Y, Shinomura Y, Kiyohara Y, Tsunoda T, Kamatani N, Iida M, Nakamura Y, Kubo M. A genome-wide association study identifies three new susceptibility loci for ulcerative colitis in the Japanese population. Nat Genet. 2009;41:1325-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 219] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 19. | Sands BE, Kaplan GG. The role of TNFalpha in ulcerative colitis. J Clin Pharmacol. 2007;47:930-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 161] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 20. | Van Klinken BJ, Van der Wal JW, Einerhand AW, Büller HA, Dekker J. Sulphation and secretion of the predominant secretory human colonic mucin MUC2 in ulcerative colitis. Gut. 1999;44:387-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 117] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Klein A, Eliakim R. Non Steroidal Anti-Inflammatory Drugs and Inflammatory Bowel Disease. Pharmaceuticals (Basel). 2010;3:1084-1092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE, Gewirtz AT. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;519:92-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1086] [Cited by in RCA: 1371] [Article Influence: 137.1] [Reference Citation Analysis (0)] |
| 23. | Lloyd-Price J, Arze C, Ananthakrishnan AN, Schirmer M, Avila-Pacheco J, Poon TW, Andrews E, Ajami NJ, Bonham KS, Brislawn CJ, Casero D, Courtney H, Gonzalez A, Graeber TG, Hall AB, Lake K, Landers CJ, Mallick H, Plichta DR, Prasad M, Rahnavard G, Sauk J, Shungin D, Vázquez-Baeza Y, White RA 3rd; IBDMDB Investigators, Braun J, Denson LA, Jansson JK, Knight R, Kugathasan S, McGovern DPB, Petrosino JF, Stappenbeck TS, Winter HS, Clish CB, Franzosa EA, Vlamakis H, Xavier RJ, Huttenhower C. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019;569:655-662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1850] [Reference Citation Analysis (0)] |
| 24. | Duvallet C, Gibbons SM, Gurry T, Irizarry RA, Alm EJ. Meta-analysis of gut microbiome studies identifies disease-specific and shared responses. Nat Commun. 2017;8:1784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 498] [Cited by in RCA: 666] [Article Influence: 83.3] [Reference Citation Analysis (0)] |
| 25. | Gevers D, Kugathasan S, Denson LA, Vázquez-Baeza Y, Van Treuren W, Ren B, Schwager E, Knights D, Song SJ, Yassour M, Morgan XC, Kostic AD, Luo C, González A, McDonald D, Haberman Y, Walters T, Baker S, Rosh J, Stephens M, Heyman M, Markowitz J, Baldassano R, Griffiths A, Sylvester F, Mack D, Kim S, Crandall W, Hyams J, Huttenhower C, Knight R, Xavier RJ. The treatment-naive microbiome in new-onset Crohn's disease. Cell Host Microbe. 2014;15:382-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1945] [Cited by in RCA: 2346] [Article Influence: 213.3] [Reference Citation Analysis (0)] |
| 26. | Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Bürgel N, Fromm M, Zeitz M, Fuss I, Strober W, Schulzke JD. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 806] [Cited by in RCA: 895] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 27. | Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012;380:1606-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1151] [Cited by in RCA: 1537] [Article Influence: 118.2] [Reference Citation Analysis (5)] |
| 28. | Kaser A, Adolph TE, Blumberg RS. The unfolded protein response and gastrointestinal disease. Semin Immunopathol. 2013;35:307-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 29. | Tak E, Park GC, Kim SH, Jun DY, Lee J, Hwang S, Song GW, Lee SG. Epigallocatechin-3-gallate protects against hepatic ischaemia-reperfusion injury by reducing oxidative stress and apoptotic cell death. J Int Med Res. 2016;44:1248-1262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Zhang FX, Kirschning CJ, Mancinelli R, Xu XP, Jin Y, Faure E, Mantovani A, Rothe M, Muzio M, Arditi M. Bacterial lipopolysaccharide activates nuclear factor-kappaB through interleukin-1 signaling mediators in cultured human dermal endothelial cells and mononuclear phagocytes. J Biol Chem. 1999;274:7611-7614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 468] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 31. | Hart AL, Al-Hassi HO, Rigby RJ, Bell SJ, Emmanuel AV, Knight SC, Kamm MA, Stagg AJ. Characteristics of intestinal dendritic cells in inflammatory bowel diseases. Gastroenterology. 2005;129:50-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 373] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 32. | Danese S, D'Amico F, Bonovas S, Peyrin-Biroulet L. Positioning Tofacitinib in the Treatment Algorithm of Moderate to Severe Ulcerative Colitis. Inflamm Bowel Dis. 2018;24:2106-2112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Danese S, Grisham M, Hodge J, Telliez JB. JAK inhibition using tofacitinib for inflammatory bowel disease treatment: a hub for multiple inflammatory cytokines. Am J Physiol Gastrointest Liver Physiol. 2016;310:G155-G162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 130] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 34. | Próchnicki T, Mangan MS, Latz E. Recent insights into the molecular mechanisms of the NLRP3 inflammasome activation. F1000Res. 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 132] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 35. | Mangan MSJ, Olhava EJ, Roush WR, Seidel HM, Glick GD, Latz E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov. 2018;17:588-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 1193] [Article Influence: 170.4] [Reference Citation Analysis (0)] |
| 36. | Gong T, Liu L, Jiang W, Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol. 2020;20:95-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 1101] [Article Influence: 183.5] [Reference Citation Analysis (0)] |
| 37. | Cao X. Self-regulation and cross-regulation of pattern-recognition receptor signalling in health and disease. Nat Rev Immunol. 2016;16:35-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 459] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 38. | Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ, Ting JP. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30:556-565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 897] [Cited by in RCA: 911] [Article Influence: 56.9] [Reference Citation Analysis (1)] |
| 39. | Gross O, Poeck H, Bscheider M, Dostert C, Hannesschläger N, Endres S, Hartmann G, Tardivel A, Schweighoffer E, Tybulewicz V, Mocsai A, Tschopp J, Ruland J. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 715] [Cited by in RCA: 729] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 40. | Kanneganti TD, Body-Malapel M, Amer A, Park JH, Whitfield J, Franchi L, Taraporewala ZF, Miller D, Patton JT, Inohara N, Núñez G. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J Biol Chem. 2006;281:36560-36568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 554] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 41. | Thomas PG, Dash P, Aldridge JR Jr, Ellebedy AH, Reynolds C, Funk AJ, Martin WJ, Lamkanfi M, Webby RJ, Boyd KL, Doherty PC, Kanneganti TD. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity. 2009;30:566-575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 604] [Cited by in RCA: 602] [Article Influence: 37.6] [Reference Citation Analysis (1)] |
| 42. | Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1530] [Cited by in RCA: 1782] [Article Influence: 137.1] [Reference Citation Analysis (1)] |
| 43. | Rheinheimer J, de Souza BM, Cardoso NS, Bauer AC, Crispim D. Current role of the NLRP3 inflammasome on obesity and insulin resistance: A systematic review. Metabolism. 2017;74:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 208] [Article Influence: 26.0] [Reference Citation Analysis (1)] |
| 44. | Sun HJ, Ren XS, Xiong XQ, Chen YZ, Zhao MX, Wang JJ, Zhou YB, Han Y, Chen Q, Li YH, Kang YM, Zhu GQ. NLRP3 inflammasome activation contributes to VSMC phenotypic transformation and proliferation in hypertension. Cell Death Dis. 2017;8:e3074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 203] [Article Influence: 25.4] [Reference Citation Analysis (1)] |
| 45. | Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21:677-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1797] [Cited by in RCA: 2472] [Article Influence: 247.2] [Reference Citation Analysis (0)] |
| 46. | Menu P, Vince JE. The NLRP3 inflammasome in health and disease: the good, the bad and the ugly. Clin Exp Immunol. 2011;166:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 309] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 47. | Franchi L, Warner N, Viani K, Nuñez G. Function of Nod-like receptors in microbial recognition and host defense. Immunol Rev. 2009;227:106-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 700] [Cited by in RCA: 658] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 48. | Fernandes-Alnemri T, Wu J, Yu JW, Datta P, Miller B, Jankowski W, Rosenberg S, Zhang J, Alnemri ES. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007;14:1590-1604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 841] [Cited by in RCA: 812] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 49. | Vajjhala PR, Mirams RE, Hill JM. Multiple binding sites on the pyrin domain of ASC protein allow self-association and interaction with NLRP3 protein. J Biol Chem. 2012;287:41732-41743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 147] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 50. | De Nardo D, Latz E. NLRP3 inflammasomes link inflammation and metabolic disease. Trends Immunol. 2011;32:373-379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 351] [Cited by in RCA: 340] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 51. | He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, Yang ZH, Zhong CQ, Han J. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015;25:1285-1298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1061] [Cited by in RCA: 1851] [Article Influence: 185.1] [Reference Citation Analysis (0)] |
| 52. | Ali F, Abo-Youssef A, Messiha B, Hemeda R. Protective effects of quercetin and ursodeoxycholic acid on hepatic ischemiareperfusion injury in rats. Clin Pharmacol Biopharm. 2015;4:2. |
| 53. | Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, Sun H, Wang DC, Shao F. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1158] [Cited by in RCA: 1934] [Article Influence: 214.9] [Reference Citation Analysis (0)] |
| 54. | Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2289] [Cited by in RCA: 2620] [Article Influence: 163.8] [Reference Citation Analysis (0)] |
| 55. | Yang Y, Wang H, Kouadir M, Song H, Shi F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019;10:128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 538] [Cited by in RCA: 906] [Article Influence: 151.0] [Reference Citation Analysis (0)] |
| 56. | Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2201] [Cited by in RCA: 2412] [Article Influence: 126.9] [Reference Citation Analysis (0)] |
| 57. | Green JP, Yu S, Martín-Sánchez F, Pelegrin P, Lopez-Castejon G, Lawrence CB, Brough D. Chloride regulates dynamic NLRP3-dependent ASC oligomerization and inflammasome priming. Proc Natl Acad Sci U S A. 2018;115:E9371-E9380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 145] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 58. | Katsnelson M, Dubyak G. Cytosolic K+ and extracellular Na+ as regulators of NLRP3 inflammasome activation and the IL-1β secretion response of macrophages to crystalline stimuli. 2013. Available from: https://faseb.onlinelibrary.wiley.com/doi/10.1096/fasebj.27.1_supplement.138.8. |
| 59. | Muñoz-Planillo R, Kuffa P, Martínez-Colón G, Smith BL, Rajendiran TM, Núñez G. K⁺ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38:1142-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1562] [Cited by in RCA: 1636] [Article Influence: 136.3] [Reference Citation Analysis (0)] |
| 60. | Murakami T, Ockinger J, Yu J, Byles V, McColl A, Hofer AM, Horng T. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc Natl Acad Sci U S A. 2012;109:11282-11287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 718] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 61. | Erdei J, Tóth A, Balogh E, Nyakundi BB, Bányai E, Ryffel B, Paragh G, Cordero MD, Jeney V. Induction of NLRP3 Inflammasome Activation by Heme in Human Endothelial Cells. Oxid Med Cell Longev. 2018;2018:4310816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 62. | Silveira AA, Cunningham C, Corr E, Ferreira Jr WA, Costa FF, Almeida CB, Conran N, Dunne A. Heme Induces NLRP3 Inflammasome Formation in Primary Human Macrophages and May Propagate Hemolytic Inflammatory Processes by Inducing S100A8 Expression. Blood. 2016;128:1256. |
| 63. | Dostert C, Pétrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674-677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2196] [Cited by in RCA: 2084] [Article Influence: 122.6] [Reference Citation Analysis (0)] |
| 64. | Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847-856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2506] [Cited by in RCA: 2357] [Article Influence: 138.6] [Reference Citation Analysis (0)] |
| 65. | Eigenbrod T, Dalpke AH. Bacterial RNA: An Underestimated Stimulus for Innate Immune Responses. J Immunol. 2015;195:411-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 66. | Gupta R, Ghosh S, Monks B, DeOliveira RB, Tzeng TC, Kalantari P, Nandy A, Bhattacharjee B, Chan J, Ferreira F, Rathinam V, Sharma S, Lien E, Silverman N, Fitzgerald K, Firon A, Trieu-Cuot P, Henneke P, Golenbock DT. RNA and β-hemolysin of group B Streptococcus induce interleukin-1β (IL-1β) by activating NLRP3 inflammasomes in mouse macrophages. J Biol Chem. 2014;289:13701-13705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 67. | Kanneganti TD, Ozören N, Body-Malapel M, Amer A, Park JH, Franchi L, Whitfield J, Barchet W, Colonna M, Vandenabeele P, Bertin J, Coyle A, Grant EP, Akira S, Núñez G. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 840] [Cited by in RCA: 917] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 68. | Sha W, Mitoma H, Hanabuchi S, Bao M, Weng L, Sugimoto N, Liu Y, Zhang Z, Zhong J, Sun B, Liu YJ. Human NLRP3 inflammasome senses multiple types of bacterial RNAs. Proc Natl Acad Sci U S A. 2014;111:16059-16064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 69. | Kasper L, König A, Koenig PA, Gresnigt MS, Westman J, Drummond RA, Lionakis MS, Groß O, Ruland J, Naglik JR, Hube B. The fungal peptide toxin Candidalysin activates the NLRP3 inflammasome and causes cytolysis in mononuclear phagocytes. Nat Commun. 2018;9:4260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 204] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 70. | Mathur A, Feng S, Hayward JA, Ngo C, Fox D, Atmosukarto II, Price JD, Schauer K, Märtlbauer E, Robertson AAB, Burgio G, Fox EM, Leppla SH, Kaakoush NO, Man SM. A multicomponent toxin from Bacillus cereus incites inflammation and shapes host outcome via the NLRP3 inflammasome. Nat Microbiol. 2019;4:362-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 71. | Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787-791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2409] [Cited by in RCA: 2334] [Article Influence: 145.9] [Reference Citation Analysis (1)] |
| 72. | Franchi L, Eigenbrod T, Núñez G. Cutting edge: TNF-alpha mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J Immunol. 2009;183:792-796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 483] [Cited by in RCA: 469] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 73. | Allam R, Lawlor KE, Yu EC, Mildenhall AL, Moujalled DM, Lewis RS, Ke F, Mason KD, White MJ, Stacey KJ, Strasser A, O'Reilly LA, Alexander W, Kile BT, Vaux DL, Vince JE. Mitochondrial apoptosis is dispensable for NLRP3 inflammasome activation but non-apoptotic caspase-8 is required for inflammasome priming. EMBO Rep. 2014;15:982-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 190] [Article Influence: 17.3] [Reference Citation Analysis (1)] |
| 74. | Camello-Almaraz C, Gomez-Pinilla PJ, Pozo MJ, Camello PJ. Mitochondrial reactive oxygen species and Ca2+ signaling. Am J Physiol Cell Physiol. 2006;291:C1082-C1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 247] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 75. | Csordás G, Hajnóczky G. SR/ER-mitochondrial local communication: calcium and ROS. Biochim Biophys Acta. 2009;1787:1352-1362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 233] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 76. | Lemasters JJ, Theruvath TP, Zhong Z, Nieminen AL. Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta. 2009;1787:1395-1401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 527] [Cited by in RCA: 499] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 77. | Pétrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14:1583-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 995] [Cited by in RCA: 1132] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 78. | He Y, Zeng MY, Yang D, Motro B, Núñez G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature. 2016;530:354-357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 602] [Cited by in RCA: 942] [Article Influence: 104.7] [Reference Citation Analysis (0)] |
| 79. | Shi H, Wang Y, Li X, Zhan X, Tang M, Fina M, Su L, Pratt D, Bu CH, Hildebrand S, Lyon S, Scott L, Quan J, Sun Q, Russell J, Arnett S, Jurek P, Chen D, Kravchenko VV, Mathison JC, Moresco EM, Monson NL, Ulevitch RJ, Beutler B. NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nat Immunol. 2016;17:250-258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 405] [Cited by in RCA: 567] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 80. | Groß CJ, Mishra R, Schneider KS, Médard G, Wettmarshausen J, Dittlein DC, Shi H, Gorka O, Koenig PA, Fromm S, Magnani G, Ćiković T, Hartjes L, Smollich J, Robertson AAB, Cooper MA, Schmidt-Supprian M, Schuster M, Schroder K, Broz P, Traidl-Hoffmann C, Beutler B, Kuster B, Ruland J, Schneider S, Perocchi F, Groß O. K(+) Efflux-Independent NLRP3 Inflammasome Activation by Small Molecules Targeting Mitochondria. Immunity. 2016;45:761-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 391] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 81. | Sanman LE, Qian Y, Eisele NA, Ng TM, van der Linden WA, Monack DM, Weerapana E, Bogyo M. Disruption of glycolytic flux is a signal for inflammasome signaling and pyroptotic cell death. Elife. 2016;5:e13663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 156] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 82. | Verhoef PA, Kertesy SB, Lundberg K, Kahlenberg JM, Dubyak GR. Inhibitory effects of chloride on the activation of caspase-1, IL-1beta secretion, and cytolysis by the P2X7 receptor. J Immunol. 2005;175:7623-7634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 98] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 83. | Tang T, Lang X, Xu C, Wang X, Gong T, Yang Y, Cui J, Bai L, Wang J, Jiang W, Zhou R. CLICs-dependent chloride efflux is an essential and proximal upstream event for NLRP3 inflammasome activation. Nat Commun. 2017;8:202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 270] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 84. | Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, Fitzgerald KA, Ryter SW, Choi AM. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2499] [Cited by in RCA: 2400] [Article Influence: 171.4] [Reference Citation Analysis (0)] |
| 85. | Heid ME, Keyel PA, Kamga C, Shiva S, Watkins SC, Salter RD. Mitochondrial reactive oxygen species induces NLRP3-dependent lysosomal damage and inflammasome activation. J Immunol. 2013;191:5230-5238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 429] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 86. | Sorbara MT, Girardin SE. Mitochondrial ROS fuel the inflammasome. Cell Res. 2011;21:558-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 216] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 87. | Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3253] [Cited by in RCA: 4236] [Article Influence: 282.4] [Reference Citation Analysis (0)] |
| 88. | Cruz CM, Rinna A, Forman HJ, Ventura AL, Persechini PM, Ojcius DM. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem. 2007;282:2871-2879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 631] [Cited by in RCA: 621] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 89. | Moon JS, Nakahira K, Chung KP, DeNicola GM, Koo MJ, Pabón MA, Rooney KT, Yoon JH, Ryter SW, Stout-Delgado H, Choi AM. NOX4-dependent fatty acid oxidation promotes NLRP3 inflammasome activation in macrophages. Nat Med. 2016;22:1002-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 263] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 90. | Weber K, Schilling JD. Lysosomes integrate metabolic-inflammatory cross-talk in primary macrophage inflammasome activation. J Biol Chem. 2014;289:9158-9171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 91. | Schorn C, Frey B, Lauber K, Janko C, Strysio M, Keppeler H, Gaipl US, Voll RE, Springer E, Munoz LE, Schett G, Herrmann M. Sodium overload and water influx activate the NALP3 inflammasome. J Biol Chem. 2011;286:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 92. | Liu J, Qian C, Cao X. Post-Translational Modification Control of Innate Immunity. Immunity. 2016;45:15-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 437] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 93. | Juliana C, Fernandes-Alnemri T, Kang S, Farias A, Qin F, Alnemri ES. Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J Biol Chem. 2012;287:36617-36622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 607] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 94. | Rodgers MA, Bowman JW, Fujita H, Orazio N, Shi M, Liang Q, Amatya R, Kelly TJ, Iwai K, Ting J, Jung JU. The linear ubiquitin assembly complex (LUBAC) is essential for NLRP3 inflammasome activation. J Exp Med. 2014;211:1333-1347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 204] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 95. | Song N, Liu ZS, Xue W, Bai ZF, Wang QY, Dai J, Liu X, Huang YJ, Cai H, Zhan XY, Han QY, Wang H, Chen Y, Li HY, Li AL, Zhang XM, Zhou T, Li T. NLRP3 Phosphorylation Is an Essential Priming Event for Inflammasome Activation. Mol Cell. 2017;68:185-197.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 368] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 96. | Wong KC, Pang WY, Wang XL, Mok SK, Lai WP, Chow HK, Leung PC, Yao XS, Wong MS. Drynaria fortunei-derived total flavonoid fraction and isolated compounds exert oestrogen-like protective effects in bone. Br J Nutr. 2013;110:475-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 97. | Manners GD. Citrus limonoids: analysis, bioactivity, and biomedical prospects. J Agric Food Chem. 2007;55:8285-8294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 139] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 98. | Sui GG, Xiao HB, Lu XY, Sun ZL. Naringin Activates AMPK Resulting in Altered Expression of SREBPs, PCSK9, and LDLR To Reduce Body Weight in Obese C57BL/6J Mice. J Agric Food Chem. 2018;66:8983-8990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 99. | Kumar VS, Rajmane AR, Adil M, Kandhare AD, Ghosh P, Bodhankar SL. Naringin ameliorates acetic acid induced colitis through modulation of endogenous oxido-nitrosative balance and DNA damage in rats. J Biomed Res. 2014;28:132-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 100. | Zhang YS, Wang F, Cui SX, Qu XJ. Natural dietary compound naringin prevents azoxymethane/dextran sodium sulfate-induced chronic colorectal inflammation and carcinogenesis in mice. Cancer Biol Ther. 2018;19:735-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 101. | Cao H, Liu J, Shen P, Cai J, Han Y, Zhu K, Fu Y, Zhang N, Zhang Z, Cao Y. Protective Effect of Naringin on DSS-Induced Ulcerative Colitis in Mice. J Agric Food Chem. 2018;66:13133-13140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 144] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 102. | Venditti A, Bianco A, Frezza C, Conti F, Bini LM, Giuliani C, Bramucci M, Quassinti L, Damiano S, Lupidi GJIc, products. Essential oil composition, polar compounds, glandular trichomes and biological activity of Hyssopus officinalis subsp. aristatus (Godr. Nyman from central Italy. 2015;77:353-363. |
| 103. | Upadhyay R, Mohan Rao LJ. An outlook on chlorogenic acids-occurrence, chemistry, technology, and biological activities. Crit Rev Food Sci Nutr. 2013;53:968-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 235] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 104. | Zeng J, Zhang D, Wan X, Bai Y, Yuan C, Wang T, Yuan D, Zhang C, Liu C. Chlorogenic Acid Suppresses miR-155 and Ameliorates Ulcerative Colitis through the NF-κB/NLRP3 Inflammasome Pathway. Mol Nutr Food Res. 2020;e2000452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 105. | Shi A, Shi H, Wang Y, Liu X, Cheng Y, Li H, Zhao H, Wang S, Dong L. Activation of Nrf2 pathway and inhibition of NLRP3 inflammasome activation contribute to the protective effect of chlorogenic acid on acute liver injury. Int Immunopharmacol. 2018;54:125-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 106. | Zhang L, Fan Y, Su H, Wu L, Huang Y, Zhao L, Han B, Shu G, Xiang M, Yang JM. Chlorogenic acid methyl ester exerts strong anti-inflammatory effects via inhibiting the COX-2/NLRP3/NF-κB pathway. Food Funct. 2018;9:6155-6164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 107. | Mouli VP, Ananthakrishnan AN. Review article: vitamin D and inflammatory bowel diseases. Aliment Pharmacol Ther. 2014;39:125-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 174] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 108. | Li H, Zhong X, Li W, Wang Q. Effects of 1,25-dihydroxyvitamin D3 on experimental periodontitis and AhR/NF-κB/NLRP3 inflammasome pathway in a mouse model. J Appl Oral Sci. 2019;27:e20180713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 109. | Lu L, Lu Q, Chen W, Li J, Li C, Zheng Z. Vitamin D(3) Protects against Diabetic Retinopathy by Inhibiting High-Glucose-Induced Activation of the ROS/TXNIP/NLRP3 Inflammasome Pathway. J Diabetes Res. 2018;2018:8193523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 112] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 110. | Dong X, He Y, Ye F, Zhao Y, Cheng J, Xiao J, Yu W, Zhao J, Sai Y, Dan G, Chen M, Zou Z. Vitamin D3 ameliorates nitrogen mustard-induced cutaneous inflammation by inactivating the NLRP3 inflammasome through the SIRT3-SOD2-mtROS signaling pathway. Clin Transl Med. 2021;11:e312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 111. | Dai Y, Zhang J, Xiang J, Li Y, Wu D, Xu J. Calcitriol inhibits ROS-NLRP3-IL-1β signaling axis via activation of Nrf2-antioxidant signaling in hyperosmotic stress stimulated human corneal epithelial cells. Redox Biol. 2019;21:101093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 139] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 112. | Jiang S, Zhang H, Li X, Yi B, Huang L, Hu Z, Li A, Du J, Li Y, Zhang W. Vitamin D/VDR attenuate cisplatin-induced AKI by down-regulating NLRP3/Caspase-1/GSDMD pyroptosis pathway. J Steroid Biochem Mol Biol. 2021;206:105789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 84] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 113. | Huang H, Hong JY, Wu YJ, Wang EY, Liu ZQ, Cheng BH, Mei L, Liu ZG, Yang PC, Zheng PY. Vitamin D receptor interacts with NLRP3 to restrict the allergic response. Clin Exp Immunol. 2018;194:17-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 114. | Cao R, Ma Y, Li S, Shen D, Yang S, Wang X, Cao Y, Wang Z, Wei Y, Liu G, Zhang H, Wang Y. 1,25(OH)(2) D(3) alleviates DSS-induced ulcerative colitis via inhibiting NLRP3 inflammasome activation. J Leukoc Biol. 2020;108:283-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 115. | Kim DS, Song M, Kim SH, Jang DS, Kim JB, Ha BK, Lee KJ, Kang SY, Jeong IY. The improvement of ginsenoside accumulation in Panax ginseng as a result of γ-irradiation. J Ginseng Res. 2013;37:332-340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 116. | Kim HJ, Kim P, Shin CY. A comprehensive review of the therapeutic and pharmacological effects of ginseng and ginsenosides in central nervous system. J Ginseng Res. 2013;37:8-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 178] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 117. | Lau AJ, Seo BH, Woo SO, Koh HL. High-performance liquid chromatographic method with quantitative comparisons of whole chromatograms of raw and steamed Panax notoginseng. J Chromatogr A. 2004;1057:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 79] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 118. | Tian M, Ma P, Zhang Y, Mi Y, Fan D. Ginsenoside Rk3 alleviated DSS-induced ulcerative colitis by protecting colon barrier and inhibiting NLRP3 inflammasome pathway. Int Immunopharmacol. 2020;85:106645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 119. | Liu H, Yang J, Du F, Gao X, Ma X, Huang Y, Xu F, Niu W, Wang F, Mao Y, Sun Y, Lu T, Liu C, Zhang B, Li C. Absorption and disposition of ginsenosides after oral administration of Panax notoginseng extract to rats. Drug Metab Dispos. 2009;37:2290-2298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 306] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 120. | Liu C, Wang J, Yang Y, Liu X, Zhu Y, Zou J, Peng S, Le TH, Chen Y, Zhao S, He B, Mi Q, Zhang X, Du Q. Ginsenoside Rd ameliorates colitis by inducing p62-driven mitophagy-mediated NLRP3 inflammasome inactivation in mice. Biochem Pharmacol. 2018;155:366-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 121. | Lee KW, Kim YJ, Kim DO, Lee HJ, Lee CY. Major phenolics in apple and their contribution to the total antioxidant capacity. J Agric Food Chem. 2003;51:6516-6520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 322] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 122. | Tsao R, Yang R, Young JC, Zhu H. Polyphenolic profiles in eight apple cultivars using high-performance liquid chromatography (HPLC). J Agric Food Chem. 2003;51:6347-6353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 330] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 123. | Zhang Z, Li S, Cao H, Shen P, Liu J, Fu Y, Cao Y, Zhang N. The protective role of phloretin against dextran sulfate sodium-induced ulcerative colitis in mice. Food Funct. 2019;10:422-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (1)] |
| 124. | Wu M, Li P, An Y, Ren J, Yan D, Cui J, Li D, Li M, Wang M, Zhong G. Phloretin ameliorates dextran sulfate sodium-induced ulcerative colitis in mice by regulating the gut microbiota. Pharmacol Res. 2019;150:104489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 190] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 125. | Doyle AA, Stephens JC. A review of cinnamaldehyde and its derivatives as antibacterial agents. Fitoterapia. 2019;139:104405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 203] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 126. | Liu P, Wang J, Wen W, Pan T, Chen H, Fu Y, Wang F, Huang JH, Xu S. Cinnamaldehyde suppresses NLRP3 derived IL-1β via activating succinate/HIF-1 in rheumatoid arthritis rats. Int Immunopharmacol. 2020;84:106570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 127. | Xu F, Wang F, Wen T, Sang W, Wang D, Zeng N. Inhibition of NLRP3 inflammasome: a new protective mechanism of cinnamaldehyde in endotoxin poisoning of mice. Immunopharmacol Immunotoxicol. 2017;39:296-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 128. | Luan F, Lei Z, Peng X, Chen L, Peng L, Liu Y, Rao Z, Yang R, Zeng N. Cardioprotective effect of cinnamaldehyde pretreatment on ischemia/ reperfusion injury via inhibiting NLRP3 inflammasome activation and gasdermin D mediated cardiomyocyte pyroptosis. Chem Biol Interact. 2022;368:110245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 129. | Kang LL, Zhang DM, Ma CH, Zhang JH, Jia KK, Liu JH, Wang R, Kong LD. Cinnamaldehyde and allopurinol reduce fructose-induced cardiac inflammation and fibrosis by attenuating CD36-mediated TLR4/6-IRAK4/1 signaling to suppress NLRP3 inflammasome activation. Sci Rep. 2016;6:27460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 130. | Ka SM, Kuoping Chao L, Lin JC, Chen ST, Li WT, Lin CN, Cheng JC, Jheng HL, Chen A, Hua KF. A low toxicity synthetic cinnamaldehyde derivative ameliorates renal inflammation in mice by inhibiting NLRP3 inflammasome and its related signaling pathways. Free Radic Biol Med. 2016;91:10-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 131. | Qu S, Shen Y, Wang M, Wang X, Yang Y. Suppression of miR-21 and miR-155 of macrophage by cinnamaldehyde ameliorates ulcerative colitis. Int Immunopharmacol. 2019;67:22-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 132. | Kumar P, Srivastava V, Chaturvedi R, Sundar D, Bisaria VJPC, Tissue, Culture O. Elicitor enhanced production of protoberberine alkaloids from in vitro cell suspension cultures of Tinospora cordifolia. Miers ex Hook. F. Thoms. 2017;130:417-426. |
| 133. | Wang YQ, Zhang HM, Zhang GC. Studies of the interaction between palmatine hydrochloride and human serum albumin by fluorescence quenching method. J Pharm Biomed Anal. 2006;41:1041-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 134. | Pustovidko AV, Rokitskaya TI, Severina II, Simonyan RA, Trendeleva TA, Lyamzaev KG, Antonenko YN, Rogov AG, Zvyagilskaya RA, Skulachev VP, Chernyak BV. Derivatives of the cationic plant alkaloids berberine and palmatine amplify protonophorous activity of fatty acids in model membranes and mitochondria. Mitochondrion. 2013;13:520-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 135. | Ai G, Huang R, Xie J, Zhong L, Wu X, Qin Z, Su Z, Chen J, Yang X, Dou Y. Hypouricemic and nephroprotective effects of palmatine from Cortex Phellodendri Amurensis: A uric acid modulator targeting Keap1-Nrf2/NLRP3 axis. J Ethnopharmacol. 2023;301:115775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 136. | Cheng JJ, Ma XD, Ai GX, Yu QX, Chen XY, Yan F, Li YC, Xie JH, Su ZR, Xie QF. Palmatine Protects Against MSU-Induced Gouty Arthritis via Regulating the NF-κB/NLRP3 and Nrf2 Pathways. Drug Des Devel Ther. 2022;16:2119-2132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 54] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 137. | Mai CT, Wu MM, Wang CL, Su ZR, Cheng YY, Zhang XJ. Palmatine attenuated dextran sulfate sodium (DSS)-induced colitis via promoting mitophagy-mediated NLRP3 inflammasome inactivation. Mol Immunol. 2019;105:76-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 146] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 138. | Cheng J, Ma X, Zhang H, Wu X, Li M, Ai G, Zhan R, Xie J, Su Z, Huang X. 8-Oxypalmatine, a novel oxidative metabolite of palmatine, exhibits superior anti-colitis effect via regulating Nrf2 and NLRP3 inflammasome. Biomed Pharmacother. 2022;153:113335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 139. | Becerra JE, Yebra MJ, Monedero V. An L-Fucose Operon in the Probiotic Lactobacillus rhamnosus GG Is Involved in Adaptation to Gastrointestinal Conditions. Appl Environ Microbiol. 2015;81:3880-3888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 140. | Choi SS, Lynch BS, Baldwin N, Dakoulas EW, Roy S, Moore C, Thorsrud BA, Röhrig CH. Safety evaluation of the human-identical milk monosaccharide, l-fucose. Regul Toxicol Pharmacol. 2015;72:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 141. | He R, Li Y, Han C, Lin R, Qian W, Hou X. L-Fucose ameliorates DSS-induced acute colitis via inhibiting macrophage M1 polarization and inhibiting NLRP3 inflammasome and NF-kB activation. Int Immunopharmacol. 2019;73:379-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 142. | Walter E. Genistin (an isoflavone glucoside) and its aglucone, genistein, from soybeans. J Am Chem Soc. 1941;63:3273-3276. |
| 143. | Chen Y, Le TH, Du Q, Zhao Z, Liu Y, Zou J, Hua W, Liu C, Zhu Y. Genistein protects against DSS-induced colitis by inhibiting NLRP3 inflammasome via TGR5-cAMP signaling. Int Immunopharmacol. 2019;71:144-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 144. | Wang S, Wang J, Wei H, Gu T, Wu Z, Yang Q. Genistein Attenuates Acute Cerebral Ischemic Damage by Inhibiting the NLRP3 Inflammasome in Reproductively Senescent Mice. Front Aging Neurosci. 2020;12:153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 145. | Wu G, Li S, Qu G, Hua J, Zong J, Li X, Xu F. Genistein alleviates H(2)O(2)-induced senescence of human umbilical vein endothelial cells via regulating the TXNIP/NLRP3 axis. Pharm Biol. 2021;59:1388-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 146. | Zhao K, Li S, Chen J, Jin Q. Inhibitory Effect of Trihydroxy Isoflavone on Neuronal Apoptosis in Natural Aging Rats. Dis Markers. 2022;2022:4688203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 147. | Eo H, Lee HJ, Lim Y. Ameliorative effect of dietary genistein on diabetes induced hyper-inflammation and oxidative stress during early stage of wound healing in alloxan induced diabetic mice. Biochem Biophys Res Commun. 2016;478:1021-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 148. | Kuwahara H, Kanazawa A, Wakamatu D, Morimura S, Kida K, Akaike T, Maeda H. Antioxidative and antimutagenic activities of 4-vinyl-2,6-dimethoxyphenol (canolol) isolated from canola oil. J Agric Food Chem. 2004;52:4380-4387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 149. |
Moreno DA, Pérez-Balibrea S, Ferreres F, Gil-Izquierdo Á, García-Viguera CJFC.
|
| 150. | Sawa T, Nakao M, Akaike T, Ono K, Maeda H. Alkylperoxyl radical-scavenging activity of various flavonoids and other phenolic compounds: implications for the anti-tumor-promoter effect of vegetables. J Agric Food Chem. 1999;47:397-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 218] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 151. | Lee EH, Shin JH, Kim SS, Seo SR. Sinapic Acid Controls Inflammation by Suppressing NLRP3 Inflammasome Activation. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 152. | Qian B, Wang C, Zeng Z, Ren Y, Li D, Song JL. Ameliorative Effect of Sinapic Acid on Dextran Sodium Sulfate- (DSS-) Induced Ulcerative Colitis in Kunming (KM) Mice. Oxid Med Cell Longev. 2020;2020:8393504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 153. | Carson CF, Hammer KA, Riley TV. Melaleuca alternifolia (Tea Tree) oil: a review of antimicrobial and other medicinal properties. Clin Microbiol Rev. 2006;19:50-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 791] [Cited by in RCA: 629] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 154. | Zhang Z, Shen P, Lu X, Li Y, Liu J, Liu B, Fu Y, Cao Y, Zhang N. In Vivo and In Vitro Study on the Efficacy of Terpinen-4-ol in Dextran Sulfate Sodium-Induced Mice Experimental Colitis. Front Immunol. 2017;8:558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 155. | Yan J, Yu L, Xu S, Gu W, Zhu WJSH. Apigenin accumulation and expression analysis of apigenin biosynthesis relative genes in celery. Scientia Horticulturae. 2014;165:218-224. |
| 156. | Márquez-Flores YK, Villegas I, Cárdeno A, Rosillo MÁ, Alarcón-de-la-Lastra C. Apigenin supplementation protects the development of dextran sulfate sodium-induced murine experimental colitis by inhibiting canonical and non-canonical inflammasome signaling pathways. J Nutr Biochem. 2016;30:143-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 157. | Lv Y, Gao X, Luo Y, Fan W, Shen T, Ding C, Yao M, Song S, Yan L. Apigenin ameliorates HFD-induced NAFLD through regulation of the XO/NLRP3 pathways. J Nutr Biochem. 2019;71:110-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 158. | Meng Z, Zhu B, Gao M, Wang G, Zhou H, Lu J, Guan S. Apigenin alleviated PA-induced pyroptosis by activating autophagy in hepatocytes. Food Funct. 2022;13:5559-5570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 159. | Yamagata K, Hashiguchi K, Yamamoto H, Tagami M. Dietary Apigenin Reduces Induction of LOX-1 and NLRP3 Expression, Leukocyte Adhesion, and Acetylated Low-Density Lipoprotein Uptake in Human Endothelial Cells Exposed to Trimethylamine-N-Oxide. J Cardiovasc Pharmacol. 2019;74:558-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 160. | Yu J, Jiang Q, Liu N, Fan D, Wang M, Zhao Y. Apigenin and apigenin-7, 4'-O-dioctanoate protect against acrolein-aggravated inflammation via inhibiting the activation of NLRP3 inflammasome and HMGB1/MYD88/NF-κB signaling pathway in Human umbilical vein endothelial cells (HUVEC). Food Chem Toxicol. 2022;168:113400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 161. | Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12:325-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1512] [Cited by in RCA: 2528] [Article Influence: 280.9] [Reference Citation Analysis (0)] |
| 162. | Wu Q, Li W, Zhao J, Sun W, Yang Q, Chen C, Xia P, Zhu J, Zhou Y, Huang G, Yong C, Zheng M, Zhou E, Gao K. Apigenin ameliorates doxorubicin-induced renal injury via inhibition of oxidative stress and inflammation. Biomed Pharmacother. 2021;137:111308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 163. | Guan C, Parrot D, Wiese J, Sönnichsen FD, Saha M, Tasdemir D, Weinberger F. Identification of rosmarinic acid and sulfated flavonoids as inhibitors of microfouling on the surface of eelgrass Zostera marina. Biofouling. 2017;33:867-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 164. | Wei Y, Chen J, Hu Y, Lu W, Zhang X, Wang R, Chu K. Rosmarinic Acid Mitigates Lipopolysaccharide-Induced Neuroinflammatory Responses through the Inhibition of TLR4 and CD14 Expression and NF-κB and NLRP3 Inflammasome Activation. Inflammation. 2018;41:732-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 165. | Lv Q, Xing Y, Liu J, Dong D, Liu Y, Qiao H, Zhang Y, Hu L. Lonicerin targets EZH2 to alleviate ulcerative colitis by autophagy-mediated NLRP3 inflammasome inactivation. Acta Pharm Sin B. 2021;11:2880-2899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 130] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 166. | Yao Y, Mao J, Xu S, Zhao L, Long L, Chen L, Li D, Lu S. Rosmarinic acid inhibits nicotine-induced C-reactive protein generation by inhibiting NLRP3 inflammasome activation in smooth muscle cells. J Cell Physiol. 2019;234:1758-1767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 167. | Marinho S, Illanes M, Ávila-Román J, Motilva V, Talero E. Anti-Inflammatory Effects of Rosmarinic Acid-Loaded Nanovesicles in Acute Colitis through Modulation of NLRP3 Inflammasome. Biomolecules. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 168. | He S, Hu Q, Yang G. Research of honeysuckle. Yunnan Chem Technol. 2010;37:72-75. |
| 169. | Shang X, Pan H, Li M, Miao X, Ding H. Lonicera japonica Thunb.: ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J Ethnopharmacol. 2011;138:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 392] [Cited by in RCA: 383] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 170. | Lee SJ, Shin EJ, Son KH, Chang HW, Kang SS, Kim HP. Anti-inflammatory activity of the major constituents of Lonicera japonica. Archives of Pharm Res. 1995;18:133-135. |
| 171. | Bu Y, Lee K, Jung HS, Moon SK. Therapeutic effects of traditional herbal medicine on cerebral ischemia: a perspective of vascular protection. Chin J Integr Med. 2013;19:804-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 172. | Jiang X, Jiang Y, Sun D, Rong L. Protective effect of magnesium lithospermate B against dextran sodiumsulfate induced ulcerative colitis in mice. Environ Toxicol Pharmacol. 2013;36:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 173. | Quan W, Liu F, Zhang Y, Xie C, Wu B, Yin J, Wang L, Zhang W, Zhang X, Wu Q. Antidepressant-like effects of magnesium lithospermate B in a rat model of chronic unpredictable stress. Pharm Biol. 2015;53:1168-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 174. | Jiang X, Zhong L, Sun D, Rong L. Magnesium lithospermate B acts against dextran sodiumsulfate-induced ulcerative colitis by inhibiting activation of the NRLP3/ASC/Caspase-1 pathway. Environ Toxicol Pharmacol. 2016;41:72-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 175. | Fujiki H, Mori M, Nakayasu M, Terada M, Sugimura T, Moore RE. Indole alkaloids: dihydroteleocidin B, teleocidin, and lyngbyatoxin A as members of a new class of tumor promoters. Proc Natl Acad Sci U S A. 1981;78:3872-3876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 152] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 176. | Wang Y, Zhu H, Tam NFY. Polyphenols, tannins and antioxidant activities of eight true mangrove plant species in South China. Plant and soil. 2014;374:549-563. |
| 177. | Guo W, Hu S, Elgehama A, Shao F, Ren R, Liu W, Zhang W, Wang X, Tan R, Xu Q, Sun Y, Jiao R. Fumigaclavine C ameliorates dextran sulfate sodium-induced murine experimental colitis via NLRP3 inflammasome inhibition. J Pharmacol Sci. 2015;129:101-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 178. | Mohammed NK, Meor Hussin AS, Tan CP, Abdul Manap MY, Alhelli AMJIjofp. Quality changes of microencapsulated Nigella sativa oil upon accelerated storage. Int J Food Prop. 2017;20:S2395-S2408. |
| 179. | Gao XJ, Tang B, Liang HH, Yi L, Wei ZG. The protective effect of nigeglanine on dextran sulfate sodium-induced experimental colitis in mice and Caco-2 cells. J Cell Physiol. 2019;234:23398-23408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 180. | Placebo-controlled study of mycophenolate mofetil combined with cyclosporin and corticosteroids for prevention of acute rejection. European Mycophenolate Mofetil Cooperative Study Group. Lancet. 1995;345:1321-1325. [PubMed] |
| 181. | Sollinger HW. Mycophenolate mofetil for the prevention of acute rejection in primary cadaveric renal allograft recipients. U.S. Renal Transplant Mycophenolate Mofetil Study Group. Transplantation. 1995;60:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1040] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 182. | A blinded, randomized clinical trial of mycophenolate mofetil for the prevention of acute rejection in cadaveric renal transplantation. The Tricontinental Mycophenolate Mofetil Renal Transplantation Study Group. Transplantation. 1996;61:1029-1037. [PubMed] |
| 183. | Liu Z, Yuan X, Luo Y, He Y, Jiang Y, Chen ZK, Sun E. Evaluating the effects of immunosuppressants on human immunity using cytokine profiles of whole blood. Cytokine. 2009;45:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 184. | Serrya MS, El-Sheakh AR, Makled MN. Evaluation of the therapeutic effects of mycophenolate mofetil targeting Nrf-2 and NLRP3 inflammasome in acetic acid induced ulcerative colitis in rats. Life Sci. 2021;271:119154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 185. | Wu WS, Chien CC, Liu KH, Chen YC, Chiu WT. Evodiamine Prevents Glioma Growth, Induces Glioblastoma Cell Apoptosis and Cell Cycle Arrest through JNK Activation. Am J Chin Med. 2017;45:879-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 186. | Liao JF, Chiou WF, Shen YC, Wang GJ, Chen CF. Anti-inflammatory and anti-infectious effects of Evodia rutaecarpa (Wuzhuyu) and its major bioactive components. Chin Med. 2011;6:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 187. | Zhao Z, Gong S, Wang S, Ma C. Effect and mechanism of evodiamine against ethanol-induced gastric ulcer in mice by suppressing Rho/NF-кB pathway. Int Immunopharmacol. 2015;28:588-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 188. | Ding W, Ding Z, Wang Y, Zhu Y, Gao Q, Cao W, Du R. Evodiamine Attenuates Experimental Colitis Injury Via Activating Autophagy and Inhibiting NLRP3 Inflammasome Assembly. Front Pharmacol. 2020;11:573870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 189. | Li CG, Zeng QZ, Chen MY, Xu LH, Zhang CC, Mai FY, Zeng CY, He XH, Ouyang DY. Evodiamine Augments NLRP3 Inflammasome Activation and Anti-bacterial Responses Through Inducing α-Tubulin Acetylation. Front Pharmacol. 2019;10:290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 190. | Shen P, Zhang Z, Zhu K, Cao H, Liu J, Lu X, Li Y, Jing Y, Yuan X, Fu Y, Cao Y, Zhang N. Evodiamine prevents dextran sulfate sodium-induced murine experimental colitis via the regulation of NF-κB and NLRP3 inflammasome. Biomed Pharmacother. 2019;110:786-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 191. | Zhou J, Tan L, Xie J, Lai Z, Huang Y, Qu C, Luo D, Lin Z, Huang P, Su Z, Xie Y. Characterization of brusatol self-microemulsifying drug delivery system and its therapeutic effect against dextran sodium sulfate-induced ulcerative colitis in mice. Drug Deliv. 2017;24:1667-1679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 192. | Zhou J, Wang T, Dou Y, Huang Y, Qu C, Gao J, Huang Z, Xie Y, Huang P, Lin Z, Su Z. Brusatol ameliorates 2, 4, 6-trinitrobenzenesulfonic acid-induced experimental colitis in rats: Involvement of NF-κB pathway and NLRP3 inflammasome. Int Immunopharmacol. 2018;64:264-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 193. | Bajracharya GB. Diversity, pharmacology and synthesis of bergenin and its derivatives: potential materials for therapeutic usages. Fitoterapia. 2015;101:133-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 194. | Lei D, Sun Y, Liu J, Chi J. Bergenin inhibits palmitic acid-induced pancreatic β-cell inflammatory death via regulating NLRP3 inflammasome activation. Ann Transl Med. 2022;10:1058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 195. | Lopes de Oliveira GA, Alarcón de la Lastra C, Rosillo MÁ, Castejon Martinez ML, Sánchez-Hidalgo M, Rolim Medeiros JV, Villegas I. Preventive effect of bergenin against the development of TNBS-induced acute colitis in rats is associated with inflammatory mediators inhibition and NLRP3/ASC inflammasome signaling pathways. Chem Biol Interact. 2019;297:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 196. | Yuan F, Chen J, Sun PP, Guan S, Xu J. Wedelolactone inhibits LPS-induced pro-inflammation via NF-kappaB pathway in RAW 264.7 cells. J Biomed Sci. 2013;20:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 197. | Mors WB, do Nascimento MC, Parente JP, da Silva MH, Melo PA, Suarez-Kurtz G. Neutralization of lethal and myotoxic activities of South American rattlesnake venom by extracts and constituents of the plant Eclipta prostrata (Asteraceae). Toxicon. 1989;27:1003-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 122] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 198. | Wei W, Ding M, Zhou K, Xie H, Zhang M, Zhang C. Protective effects of wedelolactone on dextran sodium sulfate induced murine colitis partly through inhibiting the NLRP3 inflammasome activation via AMPK signaling. Biomed Pharmacother. 2017;94:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 199. | Hayes D, Angove MJ, Tucci J, Dennis C. Walnuts (Juglans regia) Chemical Composition and Research in Human Health. Crit Rev Food Sci Nutr. 2016;56:1231-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 200. | Gao P, Liu R, Jin Q, Wang X. Comparative study of chemical compositions and antioxidant capacities of oils obtained from two species of walnut: Juglans regia and Juglans sigillata. Food Chem. 2019;279:279-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 201. | Laubertová L, Koňariková K, Gbelcová H, Ďuračková Z, Žitňanová I. Effect of walnut oil on hyperglycemia-induced oxidative stress and pro-inflammatory cytokines production. Eur J Nutr. 2015;54:291-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 202. | Zhao H, Li J, Zhao J, Chen Y, Ren C. Antioxidant effects of compound walnut oil capsule in mice aging model induced by D-galactose. Food Nutr Res. 2018;62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 203. | Miao F, Shan C, Ma T, Geng S, Ning D. Walnut oil alleviates DSS-induced colitis in mice by inhibiting NLRP3 inflammasome activation and regulating gut microbiota. Microb Pathog. 2021;154:104866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 204. | Liu F, Zhang Q, Lin C, Yao Y, Wang M, Liu C, Zhu C. A comparative study on pharmacokinetics and tissue distribution of 5-hydroxy-4-methoxycanthin-6-one and its metabolite in normal and dextran sodium sulfate-induced colitis rats by HPLC-MS/MS. J Pharm Pharmacol. 2020;72:1761-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 205. | Liu F, Yao Y, Wang Q, Zhang F, Wang M, Zhu C, Lin C. Nigakinone alleviates DSS-induced experimental colitis via regulating bile acid profile and FXR/NLRP3 signaling pathways. Phytother Res. 2023;37:15-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 206. | Yang YZ, Tang YZ, Liu YH. Wogonoside displays anti-inflammatory effects through modulating inflammatory mediator expression using RAW264.7 cells. J Ethnopharmacol. 2013;148:271-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 207. | Li H, Hui H, Xu J, Yang H, Zhang X, Liu X, Zhou Y, Li Z, Guo Q, Lu N. Wogonoside induces growth inhibition and cell cycle arrest via promoting the expression and binding activity of GATA-1 in chronic myelogenous leukemia cells. Arch Toxicol. 2016;90:1507-1522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 208. | Li-Weber M. New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat Rev. 2009;35:57-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 634] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 209. | Liu Q, Zuo R, Wang K, Nong FF, Fu YJ, Huang SW, Pan ZF, Zhang Y, Luo X, Deng XL, Zhang XX, Zhou L, Chen Y. Oroxindin inhibits macrophage NLRP3 inflammasome activation in DSS-induced ulcerative colitis in mice via suppressing TXNIP-dependent NF-κB pathway. Acta Pharmacol Sin. 2020;41:771-781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 210. | Panossian A, Wikman G. Pharmacology of Schisandra chinensis Bail.: an overview of Russian research and uses in medicine. J Ethnopharmacol. 2008;118:183-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 374] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 211. | Chen X, Xiao Z, Jiang Z, Jiang Y, Li W, Wang M. Schisandrin B Attenuates Airway Inflammation and Airway Remodeling in Asthma by Inhibiting NLRP3 Inflammasome Activation and Reducing Pyroptosis. Inflammation. 2021;44:2217-2231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 212. | Guo M, An F, Yu H, Wei X, Hong M, Lu Y. Comparative effects of schisandrin A, B, and C on Propionibacterium acnes-induced, NLRP3 inflammasome activation-mediated IL-1β secretion and pyroptosis. Biomed Pharmacother. 2017;96:129-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 213. | Zhang W, Wang W, Shen C, Wang X, Pu Z, Yin Q. Network pharmacology for systematic understanding of Schisandrin B reduces the epithelial cells injury of colitis through regulating pyroptosis by AMPK/Nrf2/NLRP3 inflammasome. Aging (Albany NY). 2021;13:23193-23209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 214. | Fujise Y, Toda T, Itô S. Isolation of trifolirhizin from Ononis spinosa L. Chem Pharm Bull (Tokyo). 1965;13:93-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 215. | Zhou H, Lutterodt H, Cheng Z, Yu LL. Anti-Inflammatory and antiproliferative activities of trifolirhizin, a flavonoid from Sophora flavescens roots. J Agric Food Chem. 2009;57:4580-4585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 216. | Zhang Q, Wang S, Ji S. Trifolirhizin regulates the balance of Th17/Treg cells and inflammation in the ulcerative colitis mice through inhibiting the TXNIP-mediated activation of NLRP3 inflammasome. Clin Exp Pharmacol Physiol. 2022;49:787-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 217. | Reagan-Shaw S, Breur J, Ahmad N. Enhancement of UVB radiation-mediated apoptosis by sanguinarine in HaCaT human immortalized keratinocytes. Mol Cancer Ther. 2006;5:418-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 218. | Li X, Wu X, Wang Q, Xu W, Zhao Q, Xu N, Hu X, Ye Z, Yu S, Liu J, He X, Shi F, Zhang Q, Li W. Sanguinarine ameliorates DSS induced ulcerative colitis by inhibiting NLRP3 inflammasome activation and modulating intestinal microbiota in C57BL/6 mice. Phytomedicine. 2022;104:154321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 219. | Kim YS, Lee CJ, Ma JY. Enhancement of active compound, genipin, from Gardeniae Fructus using immobilized glycosyl hydrolase family 3 β-glucosidase from Lactobacillus antri. AMB Express. 2017;7:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 220. | Pu Z, Liu Y, Li C, Xu M, Xie H, Zhao J. Using Network Pharmacology for Systematic Understanding of Geniposide in Ameliorating Inflammatory Responses in Colitis Through Suppression of NLRP3 Inflammasome in Macrophage by AMPK/Sirt1 Dependent Signaling. Am J Chin Med. 2020;48:1693-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 221. | Li H, Yang DH, Zhang Y, Zheng F, Gao F, Sun J, Shi G. Geniposide suppresses NLRP3 inflammasome-mediated pyroptosis via the AMPK signaling pathway to mitigate myocardial ischemia/reperfusion injury. Chin Med. 2022;17:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 222. | Song P, Shen DF, Meng YY, Kong CY, Zhang X, Yuan YP, Yan L, Tang QZ, Ma ZG. Geniposide protects against sepsis-induced myocardial dysfunction through AMPKα-dependent pathway. Free Radic Biol Med. 2020;152:186-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 223. | Song M, Chen Z, Qiu R, Zhi T, Xie W, Zhou Y, Luo N, Fuqian Chen, Liu F, Shen C, Lin S, Zhang F, Gao Y, Liu C. Inhibition of NLRP3-mediated crosstalk between hepatocytes and liver macrophages by geniposidic acid alleviates cholestatic liver inflammatory injury. Redox Biol. 2022;55:102404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 224. | Chan K, Liu ZQ, Jiang ZH, Zhou H, Wong YF, Xu HX, Liu L. The effects of sinomenine on intestinal absorption of paeoniflorin by the everted rat gut sac model. J Ethnopharmacol. 2006;103:425-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 225. | Zhou Y, Chen S, Gu W, Sun X, Wang L, Tang L. Sinomenine hydrochloride ameliorates dextran sulfate sodium-induced colitis in mice by modulating the gut microbiota composition whilst suppressing the activation of the NLRP3 inflammasome. Exp Ther Med. 2021;22:1287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 226. | Dong HC, Li PN, Chen CJ, Xu X, Zhang H, Liu G, Zheng LJ, Li P. Sinomenine Attenuates Cartilage Degeneration by Regulating miR-223-3p/NLRP3 Inflammasome Signaling. Inflammation. 2019;42:1265-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 227. | Kiasalari Z, Afshin-Majd S, Baluchnejadmojarad T, Azadi-Ahmadabadi E, Fakour M, Ghasemi-Tarie R, Jalalzade-Ogvar S, Khodashenas V, Tashakori-Miyanroudi M, Roghani M. Sinomenine Alleviates Murine Experimental Autoimmune Encephalomyelitis Model of Multiple Sclerosis through Inhibiting NLRP3 Inflammasome. J Mol Neurosci. 2021;71:215-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 228. | Qiu J, Wang M, Zhang J, Cai Q, Lu D, Li Y, Dong Y, Zhao T, Chen H. The neuroprotection of Sinomenine against ischemic stroke in mice by suppressing NLRP3 inflammasome via AMPK signaling. Int Immunopharmacol. 2016;40:492-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 117] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 229. | Huang Y, Zhou M, Li C, Chen Y, Fang W, Xu G, Shi X. Picroside II protects against sepsis via suppressing inflammation in mice. Am J Transl Res. 2016;8:5519-5531. [PubMed] |
| 230. | Gao H, Zhou YW. Anti-lipid peroxidation and protection of liver mitochondria against injuries by picroside II. World J Gastroenterol. 2005;11:3671-3674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 231. | Verma PC, Basu V, Gupta V, Saxena G, Rahman LU. Pharmacology and chemistry of a potent hepatoprotective compound Picroliv isolated from the roots and rhizomes of Picrorhiza kurroa royle ex benth. (kutki). Curr Pharm Biotechnol. 2009;10:641-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 232. | Guo Y, Xu X, Li Q, Li Z, Du F. Anti-inflammation effects of picroside 2 in cerebral ischemic injury rats. Behav Brain Funct. 2010;6:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 233. | Yao H, Yan J, Yin L, Chen W. Picroside II alleviates DSS-induced ulcerative colitis by suppressing the production of NLRP3 inflammasomes through NF-κB signaling pathway. Immunopharmacol Immunotoxicol. 2022;44:437-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 234. | Manna C, Galletti P, Cucciolla V, Montedoro G, Zappia V. Olive oil hydroxytyrosol protects human erythrocytes against oxidative damages. J Nutr Biochem. 1999;10:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 235. | Miao F. Hydroxytyrosol alleviates dextran sodium sulfate-induced colitis by inhibiting NLRP3 inflammasome activation and modulating gut microbiota in vivo. Nutrition. 2022;97:111579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 236. | Zheng J, Lai W, Zhu G, Wan M, Chen J, Tai Y, Lu C. 10-Hydroxy-2-decenoic acid prevents ultraviolet A-induced damage and matrix metalloproteinases expression in human dermal fibroblasts. J Eur Acad Dermatol Venereol. 2013;27:1269-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 237. | Sugiyama T, Takahashi K, Kuzumaki A, Tokoro S, Neri P, Mori H. Inhibitory mechanism of 10-hydroxy-trans-2-decenoic acid (royal jelly acid) against lipopolysaccharide- and interferon-β-induced nitric oxide production. Inflammation. 2013;36:372-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 238. | You M, Miao Z, Tian J, Hu F. Trans-10-hydroxy-2-decenoic acid protects against LPS-induced neuroinflammation through FOXO1-mediated activation of autophagy. Eur J Nutr. 2020;59:2875-2892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 239. | Huang S, Tao R, Zhou J, Qian L, Wu J. Trans-10-Hydroxy-2-Decenoic Acid Alleviates Dextran Sulfate Sodium-Induced Colitis in Mice via Regulating the Inflammasome-Mediated Pyroptotic Pathway and Enhancing Colonic Barrier Function. Mol Nutr Food Res. 2022;66:e2100821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 240. | Khoshoo TN, Mukherjee I. Genetic-evolutionary studies on cultivated cannas : VI. Origin and evolution of ornamental taxa. Theor Appl Genet. 1970;40:204-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 241. | Doi M, Nakamura N, Takizawa Y, Wakita M, Shimizu F, Kitamura Y, Hosokawa M. Harvest characteristics of Canna× generalis LH Bailey leaves. Scientia Horticulturae. 2013;150: 441-447. |
| 242. | Singh R, Dubey AK, Sanyal I. Optimisation of adventitious shoot regeneration and agrobacterium-mediated transformation in canna× generalis (Canna Lily). Horticult Plant J. 2019;5:39-46. |
| 243. | Al-Snafi A. Medical importance of Cichorium intybus–A review. IOSR J Pharm. 2016;6:41-56. |
| 244. | Le HL, Nguyen TMH, Vu TT, Nguyen TTO, Ly HDT, Le NT, Nguyen TVA. Potent antiplatelet aggregation, anticoagulant and antioxidant activity of aerial Canna x generalis LH Bailey & EZ Bailey and its phytoconstituents. South African Journal of Botany. 2022;147:882-893. |
| 245. | Mahmoud TN, El-Maadawy WH, Kandil ZA, Khalil H, El-Fiky NM, El Alfy TSMA. Canna x generalis L.H. Bailey rhizome extract ameliorates dextran sulfate sodium-induced colitis via modulating intestinal mucosal dysfunction, oxidative stress, inflammation, and TLR4/ NF-ҡB and NLRP3 inflammasome pathways. J Ethnopharmacol. 2021;269:113670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 246. | Li J, Ma XP, Yu CM, Ou WJ, Zhang MS, Liang QC, Zhao JB. [A case-control study on the duration of sleeping and cerebral infarction]. Zhonghua Liu Xing Bing Xue Za Zhi. 2013;34:914-916. [PubMed] |
| 247. | Li H, Du Q, Wang WJ, Wang RJ, Wang JH, Liu SS. [Effect of kuijieling decoction on gene expression of TLR2, TLR4 in colonic mucosa of UC rats]. Zhong Yao Cai. 2007;30:56-59. [PubMed] |
| 248. | Hossen MJ, Chou JY, Li SM, Fu XQ, Yin C, Guo H, Amin A, Chou GX, Yu ZL. An ethanol extract of the rhizome of Atractylodes chinensis exerts anti-gastritis activities and inhibits Akt/NF-κB signaling. J Ethnopharmacol. 2019;228:18-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 249. | Yang B, Li H, Ruan Q, Xuan S, Chen X, Cui H, Liu Z, Jin J, Zhao Z. Effects of Gut Microbiota and Ingredient-Ingredient Interaction on the Pharmacokinetic Properties of Rotundic Acid and Pedunculoside. Planta Med. 2019;85:729-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 250. | Long Y, Li S, Qin J, Xie L, Gan L, Jie F, Wu Y, Li Y, Du Q. Kuijieling regulates the differentiation of Treg and Th17 cells to ameliorate experimental colitis in rats. Biomed Pharmacother. 2018;105:781-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 251. | Jie F, Xiao S, Qiao Y, You Y, Feng Y, Long Y, Li S, Wu Y, Li Y, Du Q. Kuijieling decoction suppresses NLRP3-Mediated pyroptosis to alleviate inflammation and experimental colitis in vivo and in vitro. J Ethnopharmacol. 2021;264:113243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 252. | Song X, Li Y, Zhang H, Yang Q. The anticancer effect of Huaier (Review). Oncol Rep. 2015;34:12-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 253. | Wu T, Chen W, Liu S, Lu H, Wang H, Kong D, Huang X, Kong Q, Ning Y, Lu Z. Huaier suppresses proliferation and induces apoptosis in human pulmonary cancer cells via upregulation of miR-26b-5p. FEBS Lett. 2014;588:2107-2114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 254. | Zhang N, Kong X, Yan S, Yuan C, Yang Q. Huaier aqueous extract inhibits proliferation of breast cancer cells by inducing apoptosis. Cancer Sci. 2010;101:2375-2383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 255. | Wang L, Yu Z, Wei C, Zhang L, Song H, Chen B, Yang Q. Huaier aqueous extract protects against dextran sulfate sodium-induced experimental colitis in mice by inhibiting NLRP3 inflammasome activation. Oncotarget. 2017;8:32937-32945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 256. | Lin X, Yi Z, Diao J, Shao M, Zhao L, Cai H, Fan Q, Yao X, Sun X. ShaoYao decoction ameliorates colitis-associated colorectal cancer by downregulating proinflammatory cytokines and promoting epithelial-mesenchymal transition. J Transl Med. 2014;12:105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 257. | Wang X, Saud SM, Zhang X, Li W, Hua B. Protective effect of Shaoyao Decoction against colorectal cancer via the Keap1-Nrf2-ARE signaling pathway. J Ethnopharmacol. 2019;241:111981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 258. | Chen X. The Paeoniae decoction on the treatment of 36 cases of ulcerative colitis clinical observation. J Pract Traditional Chin Intern Med. 2014;28:39-40. |
| 259. | Wei YY, Fan YM, Ga Y, Zhang YN, Han JC, Hao ZH. Shaoyao decoction attenuates DSS-induced ulcerative colitis, macrophage and NLRP3 inflammasome activation through the MKP1/NF-κB pathway. Phytomedicine. 2021;92:153743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 99] [Article Influence: 24.8] [Reference Citation Analysis (1)] |
| 260. | Li X, Chu L, Liu S, Zhang W, Lin L, Zheng G. Smilax china L. flavonoid alleviates HFHS-induced inflammation by regulating the gut-liver axis in mice. Phytomedicine. 2022;95:153728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 261. | Li X, Yang L, Xu M, Qiao G, Li C, Lin L, Zheng G. Smilax china L. polyphenols alleviates obesity and inflammation by modulating gut microbiota in high fat/high sucrose diet-fed C57BL/6J mice. J of Func Foods. 2021;77:104332. |
| 262. | Tettey CO, Yang I, Shin HM. Smilax china leaf extracts suppress pro-inflammatory adhesion response in human umbilical vein endothelial cells and proliferation of HeLa cells. Arch Physiol Biochem. 2020;126:287-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 263. | Zhang Y, Pan X, Ran S, Wang K. Purification, structural elucidation and anti-inflammatory activity in vitro of polysaccharides from Smilax china L. Int J Biol Macromol. 2019;139:233-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 264. | Pan X, Wang H, Zheng Z, Huang X, Yang L, Liu J, Wang K, Zhang Y. Pectic polysaccharide from Smilax china L. ameliorated ulcerative colitis by inhibiting the galectin-3/NLRP3 inflammasome pathway. Carbohydr Polym. 2022;277:118864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 265. | Jin XH, Huang LQ. Investigation of original materials of Chinese medicine "Shihu" and "Tiepishihu". Zhongguo Zhong Yao Za Zhi. 2015;40:2475-2479. [PubMed] |
| 266. | Yang S, Gong Q, Wu Q, Li F, Lu Y, Shi J. Alkaloids enriched extract from Dendrobium nobile Lindl. attenuates tau protein hyperphosphorylation and apoptosis induced by lipopolysaccharide in rat brain. Phytomedicine. 2014;21:712-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 267. | Gao Y, Zhou S, Wang F, Zhou Y, Sheng S, Qi D, Huang JH, Wu E, Lv Y, Huo X. Hepatoprotective effects of limb ischemic post-conditioning in hepatic ischemic rat model and liver cancer patients via PI3K/ERK pathways. Int J Biol Sci. 2018;14:2037-2050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 268. | Zeng YJ, Yang HR, Ou XY, Su HH, Zong MH, Yang JG, Lou WY. Fungal polysaccharide similar with host Dendrobium officinale polysaccharide: Preparation, structure characteristics and biological activities. Int J Biol Macromol. 2019;141:460-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 269. | Huang K, Li Y, Tao S, Wei G, Huang Y, Chen D, Wu C. Purification, Characterization and Biological Activity of Polysaccharides from Dendrobium officinale. Molecules. 2016;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 270. | Liang J, Chen S, Chen J, Lin J, Xiong Q, Yang Y, Yuan J, Zhou L, He L, Hou S, Li S, Huang S, Lai X. Therapeutic roles of polysaccharides from Dendrobium Officinaleon colitis and its underlying mechanisms. Carbohydr Polym. 2018;185:159-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 271. | Liu GT. Pharmacological actions and clinical use of fructus schizandrae. Chin Med J (Engl). 1989;102:740-749. [PubMed] |
| 272. | Wei H, Sun L, Tai Z, Gao S, Xu W, Chen W. A simple and sensitive HPLC method for the simultaneous determination of eight bioactive components and fingerprint analysis of Schisandra sphenanthera. Anal Chim Acta. 2010;662:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 273. | Choi YW, Takamatsu S, Khan SI, Srinivas PV, Ferreira D, Zhao J, Khan IA. Schisandrene, a dibenzocyclooctadiene lignan from Schisandra chinensis: structure-antioxidant activity relationships of dibenzocyclooctadiene lignans. J Nat Prod. 2006;69:356-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 138] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 274. | Wang X, Li Q, Sui B, Xu M, Pu Z, Qiu T. Schisandrin A from Schisandra chinensis Attenuates Ferroptosis and NLRP3 Inflammasome-Mediated Pyroptosis in Diabetic Nephropathy through Mitochondrial Damage by AdipoR1 Ubiquitination. Oxid Med Cell Longev. 2022;2022:5411462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 45] [Reference Citation Analysis (0)] |
| 275. | Bian Z, Qin Y, Li L, Su L, Fei C, Li Y, Hu M, Chen X, Zhang W, Mao C, Yuan X, Lu T, Ji D. Schisandra chinensis (Turcz.) Baill. Protects against DSS-induced colitis in mice: Involvement of TLR4/NF-κB/NLRP3 inflammasome pathway and gut microbiota. J Ethnopharmacol. 2022;298:115570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 276. | Li Y, Wang J, Xiao Y, Wang Y, Chen S, Yang Y, Lu A, Zhang S. A systems pharmacology approach to investigate the mechanisms of action of Semen Strychni and Tripterygium wilfordii Hook F for treatment of rheumatoid arthritis. J Ethnopharmacol. 2015;175:301-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 277. | Han R, Rostami-Yazdi M, Gerdes S, Mrowietz U. Triptolide in the treatment of psoriasis and other immune-mediated inflammatory diseases. Br J Clin Pharmacol. 2012;74:424-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 278. | Graziose R, Lila MA, Raskin I. Merging traditional Chinese medicine with modern drug discovery technologies to find novel drugs and functional foods. Curr Drug Discov Technol. 2010;7:2-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 279. | Xue M, Jiang ZZ, Wu T, Li J, Zhang L, Zhao Y, Li XJ, Zhang LY, Yang SY. Anti-inflammatory effects and hepatotoxicity of Tripterygium-loaded solid lipid nanoparticles on adjuvant-induced arthritis in rats. Phytomedicine. 2012;19:998-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 280. | Chen YZ, Gao Q, Zhao XZ, Chen XM, Zhang F, Chen J, Xu CG, Sun LL, Mei CL. Meta-analysis of Tripterygium wilfordii Hook F in the immunosuppressive treatment of IgA nephropathy. Intern Med. 2010;49:2049-2055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 281. | Zhu W, Li Y, Gong J, Zuo L, Zhang W, Cao L, Gu L, Guo Z, Li N, Li J. Tripterygium wilfordii Hook. f. versus azathioprine for prevention of postoperative recurrence in patients with Crohn's disease: a randomized clinical trial. Dig Liver Dis. 2015;47:14-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 282. | Sun YY, Xiao L, Wang D, Ji YC, Yang YP, Ma R, Chen XH. Triptolide inhibits viability and induces apoptosis in liver cancer cells through activation of the tumor suppressor gene p53. Int J Oncol. 2017;50:847-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 283. | Qin DP, Sun PN, Zhou YJ, Chen FM, Zhang CL, Han JX, Yang XJ. [Effect of Tripterygium wilfordii polycoride upon inflammation and TLR4/MyD88 signaling pathway in ulcerative colitis rats model]. Zhonghua Yi Xue Za Zhi. 2016;96:1444-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 284. | Fangxiao M, Yifan K, Jihong Z, Yan S, Yingchao L. Effect of Tripterygium wilfordii Polycoride on the NOXs-ROS-NLRP3 Inflammasome Signaling Pathway in Mice with Ulcerative Colitis. Evid Based Complement Alternat Med. 2019;2019:9306283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 285. | Gupta D, Kumari S, Gulrajani M. Dyeing studies with hydroxyanthraquinones extracted from Indian madder. Part 1: Dyeing of nylon with purpurin. Coloration Technology. 2001;117:328-332. |
| 286. | Yusuf M, Shahid M, Khan SA, Khan MI, Islam S-U, Mohammad F, Khan MA. Eco-dyeing of wool using aqueous extract of the roots of Indian madder (Rubia cordifolia) as natural dye. J of Natural Fibers. 2013;10:14-28. |
| 287. | López-Expósito I, Castillo A, Yang N, Liang B, Li XM. Chinese herbal extracts of Rubia cordifolia and Dianthus superbus suppress IgE production and prevent peanut-induced anaphylaxis. Chin Med. 2011;6:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 288. | Lodi S, Sharma V, Kansal L. The protective effect of Rubia cordifolia against lead nitrate-induced immune response impairment and kidney oxidative damage. Indian J Pharmacol. 2011;43:441-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 289. | Shilpa PN, Sivaramakrishnan V, Niranjali Devaraj S. Induction of apoptosis by methanolic extract of Rubia cordifolia Linn in HEp-2 cell line is mediated by reactive oxygen species. Asian Pac J Cancer Prev. 2012;13:2753-2758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 290. | Qin W, Luo H, Yang L, Hu D, Jiang SP, Peng DY, Hu JM, Liu SJ. Rubia cordifolia L. ameliorates DSS-induced ulcerative colitis in mice through dual inhibition of NLRP3 inflammasome and IL-6/JAK2/STAT3 pathways. Heliyon. 2022;8:e10314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 291. | Nile SH, Park SW. Edible berries: bioactive components and their effect on human health. Nutrition. 2014;30:134-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 434] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 292. | Sánchez-Salcedo EM, Sendra E, Carbonell-Barrachina ÁA, Martínez JJ, Hernández F. Fatty acids composition of Spanish black (Morus nigra L.) and white (Morus alba L.) mulberries. Food Chem. 2016;190:566-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 293. | Salama RM, Darwish SF, El Shaffei I, Elmongy NF, Fahmy NM, Afifi MS, Abdel-Latif GA. Morus macroura Miq. Fruit extract protects against acetic acid-induced ulcerative colitis in rats: Novel mechanistic insights on its impact on miRNA-223 and on the TNFα/NFκB/NLRP3 inflammatory axis. Food Chem Toxicol. 2022;165:113146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 294. | Derrien M, Collado MC, Ben-Amor K, Salminen S, de Vos WM. The Mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Appl Environ Microbiol. 2008;74:1646-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 500] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 295. | Collado MC, Derrien M, Isolauri E, de Vos WM, Salminen S. Intestinal integrity and Akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the elderly. Appl Environ Microbiol. 2007;73:7767-7770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 534] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 296. | Miller RS, Hoskins LC. Mucin degradation in human colon ecosystems. Fecal population densities of mucin-degrading bacteria estimated by a "most probable number" method. Gastroenterology. 1981;81:759-765. [PubMed] |
| 297. | Li J, Lin S, Vanhoutte PM, Woo CW, Xu A. Akkermansia Muciniphila Protects Against Atherosclerosis by Preventing Metabolic Endotoxemia-Induced Inflammation in Apoe-/- Mice. Circulation. 2016;133:2434-2446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 539] [Article Influence: 59.9] [Reference Citation Analysis (0)] |
| 298. | Blacher E, Bashiardes S, Shapiro H, Rothschild D, Mor U, Dori-Bachash M, Kleimeyer C, Moresi C, Harnik Y, Zur M, Zabari M, Brik RB, Kviatcovsky D, Zmora N, Cohen Y, Bar N, Levi I, Amar N, Mehlman T, Brandis A, Biton I, Kuperman Y, Tsoory M, Alfahel L, Harmelin A, Schwartz M, Israelson A, Arike L, Johansson MEV, Hansson GC, Gotkine M, Segal E, Elinav E. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature. 2019;572:474-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 483] [Article Influence: 80.5] [Reference Citation Analysis (0)] |
| 299. | Wu W, Lv L, Shi D, Ye J, Fang D, Guo F, Li Y, He X, Li L. Protective Effect of Akkermansia muciniphila against Immune-Mediated Liver Injury in a Mouse Model. Front Microbiol. 2017;8:1804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 188] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 300. | Zhai R, Xue X, Zhang L, Yang X, Zhao L, Zhang C. Strain-Specific Anti-inflammatory Properties of Two Akkermansia muciniphila Strains on Chronic Colitis in Mice. Front Cell Infect Microbiol. 2019;9:239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 253] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 301. | Qu S, Fan L, Qi Y, Xu C, Hu Y, Chen S, Liu W, Si J. Akkermansia muciniphila Alleviates Dextran Sulfate Sodium (DSS)-Induced Acute Colitis by NLRP3 Activation. Microbiol Spectr. 2021;9:e0073021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 149] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 302. | Schiller JT, Müller M. Next generation prophylactic human papillomavirus vaccines. Lancet Oncol. 2015;16:e217-e225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 303. | Cen Q, Gao T, Ren Y, Lu X, Lei H. Immune evaluation of a Saccharomyces cerevisiae-based oral vaccine against Helicobacter pylori in mice. Helicobacter. 2021;26:e12772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 304. | Sun S, Xu X, Liang L, Wang X, Bai X, Zhu L, He Q, Liang H, Xin X, Wang L, Lou C, Cao X, Chen X, Li B, Wang B, Zhao J. Lactic Acid-Producing Probiotic Saccharomyces cerevisiae Attenuates Ulcerative Colitis via Suppressing Macrophage Pyroptosis and Modulating Gut Microbiota. Front Immunol. 2021;12:777665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 136] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 305. | Anjum N, Maqsood S, Masud T, Ahmad A, Sohail A, Momin A. Lactobacillus acidophilus: characterization of the species and application in food production. Crit Rev Food Sci Nutr. 2014;54:1241-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 306. | Foye OT, Huang IF, Chiou CC, Walker WA, Shi HN. Early administration of probiotic Lactobacillus acidophilus and/or prebiotic inulin attenuates pathogen-mediated intestinal inflammation and Smad 7 cell signaling. FEMS Immunol Med Microbiol. 2012;65:467-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 307. | Kumar A, Anbazhagan AN, Coffing H, Chatterjee I, Priyamvada S, Gujral T, Saksena S, Gill RK, Alrefai WA, Borthakur A, Dudeja PK. Lactobacillus acidophilus counteracts inhibition of NHE3 and DRA expression and alleviates diarrheal phenotype in mice infected with Citrobacter rodentium. Am J Physiol Gastrointest Liver Physiol. 2016;311:G817-G826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 308. | Amdekar S, Singh V, Kumar A, Sharma P, Singh R. Lactobacillus casei and Lactobacillus acidophilus regulate inflammatory pathway and improve antioxidant status in collagen-induced arthritic rats. J Interferon Cytokine Res. 2013;33:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 309. | Al-Sadi R, Nighot P, Nighot M, Haque M, Rawat M, Ma TY. Lactobacillus acidophilus Induces a Strain-specific and Toll-Like Receptor 2-Dependent Enhancement of Intestinal Epithelial Tight Junction Barrier and Protection Against Intestinal Inflammation. Am J Pathol. 2021;191:872-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 310. | Qi H, Li Y, Yun H, Zhang T, Huang Y, Zhou J, Yan H, Wei J, Liu Y, Zhang Z, Gao Y, Che Y, Su X, Zhu D, Zhang Y, Zhong J, Yang R. Lactobacillus maintains healthy gut mucosa by producing L-Ornithine. Commun Biol. 2019;2:171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 311. | Zhang Y, Zhao X, Zhu Y, Ma J, Ma H, Zhang H. Probiotic Mixture Protects Dextran Sulfate Sodium-Induced Colitis by Altering Tight Junction Protein Expressions and Increasing Tregs. Mediators Inflamm. 2018;2018:9416391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 312. | Li P, Chen G, Zhang J, Pei C, Chen Y, Gong J, Deng S, Cai K, Li H, Wang D, Shen B, Xie Z, Liao Q. Live Lactobacillus acidophilus alleviates ulcerative colitis via the SCFAs/mitophagy/NLRP3 inflammasome axis. Food Funct. 2022;13:2985-2997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 313. | Monagas M, Quintanilla-López JE, Gómez-Cordovés C, Bartolomé B, Lebrón-Aguilar R. MALDI-TOF MS analysis of plant proanthocyanidins. J Pharm Biomed Anal. 2010;51:358-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 314. | Prasain JK, Peng N, Dai Y, Moore R, Arabshahi A, Wilson L, Barnes S, Michael Wyss J, Kim H, Watts RL. Liquid chromatography tandem mass spectrometry identification of proanthocyanidins in rat plasma after oral administration of grape seed extract. Phytomedicine. 2009;16:233-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 315. | Sheng K, Zhang G, Sun M, He S, Kong X, Wang J, Zhu F, Zha X, Wang Y. Grape seed proanthocyanidin extract ameliorates dextran sulfate sodium-induced colitis through intestinal barrier improvement, oxidative stress reduction, and inflammatory cytokines and gut microbiota modulation. Food Funct. 2020;11:7817-7829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 316. | Enaida H, Hisatomi T, Hata Y, Ueno A, Goto Y, Yamada T, Kubota T, Ishibashi T. Brilliant blue G selectively stains the internal limiting membrane/brilliant blue G-assisted membrane peeling. Retina. 2006;26:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 317. | Saber S, Youssef ME, Sharaf H, Amin NA, El-Shedody R, Aboutouk FH, El-Galeel YA, El-Hefnawy A, Shabaka D, Khalifa A, Saleh RA, Osama D, El-Zoghby G, Gobba NA. BBG enhances OLT1177-induced NLRP3 inflammasome inactivation by targeting P2X7R/NLRP3 and MyD88/NF-κB signaling in DSS-induced colitis in rats. Life Sci. 2021;270:119123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 318. | Wang Z, Xia Q, Liu X, Liu W, Huang W, Mei X, Luo J, Shan M, Lin R, Zou D, Ma Z. Phytochemistry, pharmacology, quality control and future research of Forsythia suspensa (Thunb.) Vahl: A review. J Ethnopharmacol. 2018;210:318-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 210] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 319. | Chao L, Lin J, Zhou J, Du H, Chen X, Liu M, Qu Q, Lv W, Guo S. Polyphenol Rich Forsythia suspensa Extract Alleviates DSS-Induced Ulcerative Colitis in Mice through the Nrf2-NLRP3 Pathway. Antioxidants (Basel). 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 320. | GU S-z, XUE Y, ZHANG Y-l, GAO Y, DOU D-b, CAI G. Clinical Efficacy of Kuijietong Against Mild to Moderate Active Ulcerative Colitis. Chinese Journal of Experimental Traditional Medical Formulae 2021: 106-111. |
| 321. | Xue S, Xue Y, Dou D, Wu H, Zhang P, Gao Y, Tang Y, Xia Z, Yang S, Gu S. Kui Jie Tong Ameliorates Ulcerative Colitis by Regulating Gut Microbiota and NLRP3/Caspase-1 Classical Pyroptosis Signaling Pathway. Dis Markers. 2022;2022:2782112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 322. | Dai YC, Zhang YL, Wang LJ, Guo Q, Yang K, Ye RH, Tang ZP. Clinical presentation and treatment strategies for ulcerative colitis: A retrospective study of 247 inpatients. Chin J Integr Med. 2016;22:811-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 323. | Zheng L, Zhang YL, Dai YC, Chen X, Chen DL, Dai YT, Tang ZP. Jianpi Qingchang decoction alleviates ulcerative colitis by inhibiting nuclear factor-κB activation. World J Gastroenterol. 2017;23:1180-1188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 324. | Zhang J, Kang X, Sun M, Zhang S. Qingre Jianpi decoction attenuates inflammatory responses by suppressing NOD-like receptor family pyrin domain-containing 3 inflammasome activation in dextran sulfate sodium-induced colitis mice. J Tradit Chin Med. 2021;41:68-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 325. | Alsaweed M, Lai CT, Hartmann PE, Geddes DT, Kakulas F. Human milk miRNAs primarily originate from the mammary gland resulting in unique miRNA profiles of fractionated milk. Sci Rep. 2016;6:20680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 130] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 326. | Benmoussa A, Laugier J, Beauparlant CJ, Lambert M, Droit A, Provost P. Complexity of the microRNA transcriptome of cow milk and milk-derived extracellular vesicles isolated via differential ultracentrifugation. J Dairy Sci. 2020;103:16-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 327. | Gao HN, Guo HY, Zhang H, Xie XL, Wen PC, Ren FZ. Yak-milk-derived exosomes promote proliferation of intestinal epithelial cells in an hypoxic environment. J Dairy Sci. 2019;102:985-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 328. | Hock A, Miyake H, Li B, Lee C, Ermini L, Koike Y, Chen Y, Määttänen P, Zani A, Pierro A. Breast milk-derived exosomes promote intestinal epithelial cell growth. J Pediatr Surg. 2017;52:755-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 173] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 329. | Zhou F, Paz HA, Sadri M, Cui J, Kachman SD, Fernando SC, Zempleni J. Dietary bovine milk exosomes elicit changes in bacterial communities in C57BL/6 mice. Am J Physiol Gastrointest Liver Physiol. 2019;317:G618-G624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 330. | Tong L, Hao H, Zhang Z, Lv Y, Liang X, Liu Q, Liu T, Gong P, Zhang L, Cao F, Pastorin G, Lee CN, Chen X, Wang JW, Yi H. Milk-derived extracellular vesicles alleviate ulcerative colitis by regulating the gut immunity and reshaping the gut microbiota. Theranostics. 2021;11:8570-8586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 170] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 331. | Anttila SA, Leinonen EV. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001;7:249-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 372] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 332. | Sahin E, Bektur E, Burukoglu Donmez D, Baycu C, Can OD, Sahinturk V. Mirtazapine suppresses sterile inflammation through NLRP3-inflammasome in diabetic rat kidney. Acta Histochem. 2019;121:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 333. | Hafez HM, Ibrahim MA, Yehia Abdelzaher W, Gad AA, Mohammed Naguib Abdel Hafez S, Abdel-Gaber SA. Protective effect of mirtazapine against acetic acid-induced ulcerative colitis in rats: Role of NLRP3 inflammasome pathway. Int Immunopharmacol. 2021;101:108174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 334. | Chang Y, Jia X, Wei F, Wang C, Sun X, Xu S, Yang X, Zhao Y, Chen J, Wu H, Zhang L, Wei W. CP-25, a novel compound, protects against autoimmune arthritis by modulating immune mediators of inflammation and bone damage. Sci Rep. 2016;6:26239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 335. | Jia XY, Chang Y, Wei F, Dai X, Wu YJ, Sun XJ, Xu S, Wu HX, Wang C, Yang XZ, Wei W. CP-25 reverses prostaglandin E4 receptor desensitization-induced fibroblast-like synoviocyte dysfunction via the G protein-coupled receptor kinase 2 in autoimmune arthritis. Acta Pharmacol Sin. 2019;40:1029-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 336. | Wu H, Chen X, Gu F, Zhang P, Xu S, Liu Q, Zhang Q, Wang X, Wang C, Körner H, Wei W. CP-25 alleviates antigen-induced experimental Sjögren's syndrome in mice by inhibiting JAK1-STAT1/2-CXCL13 signaling and interfering with B-cell migration. Lab Invest. 2021;101:1084-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 337. | Li Y, Jiang MY, Chen JY, Xu ZW, Zhang JW, Li T, Zhang LL, Wei W. CP-25 exerts therapeutic effects in mice with dextran sodium sulfate-induced colitis by inhibiting GRK2 translocation to downregulate the TLR4-NF-κB-NLRP3 inflammasome signaling pathway in macrophages. IUBMB Life. 2021;73:1406-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 338. | Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, Huikuri HV, Johansson I, Jüni P, Lettino M, Marx N, Mellbin LG, Östgren CJ, Rocca B, Roffi M, Sattar N, Seferović PM, Sousa-Uva M, Valensi P, Wheeler DC; ESC Scientific Document Group. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1670] [Cited by in RCA: 2633] [Article Influence: 658.3] [Reference Citation Analysis (0)] |
| 339. | Birnbaum Y, Chen H, Tran D, Nylander S, Ye Y. Ticagrelor and Dapagliflozin Have Additive Effects in Ameliorating Diabetic Nephropathy in Mice with Type-2 Diabetes Mellitus. Cardiovasc Drugs Ther. 2022;36:829-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 340. | Abd El-Fattah EE, Saber S, Mourad AAE, El-Ahwany E, Amin NA, Cavalu S, Yahya G, Saad AS, Alsharidah M, Shata A, Sami HM, Kaddah MMY, Ghanim AMH. The dynamic interplay between AMPK/NFκB signaling and NLRP3 is a new therapeutic target in inflammation: Emerging role of dapagliflozin in overcoming lipopolysaccharide-mediated lung injury. Biomed Pharmacother. 2022;147:112628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 56] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 341. | Ye Y, Bajaj M, Yang HC, Perez-Polo JR, Birnbaum Y. SGLT-2 Inhibition with Dapagliflozin Reduces the Activation of the Nlrp3/ASC Inflammasome and Attenuates the Development of Diabetic Cardiomyopathy in Mice with Type 2 Diabetes. Further Augmentation of the Effects with Saxagliptin, a DPP4 Inhibitor. Cardiovasc Drugs Ther. 2017;31:119-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 296] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 342. | El-Rous MA, Saber S, Raafat EM, Ahmed AAE. Dapagliflozin, an SGLT2 inhibitor, ameliorates acetic acid-induced colitis in rats by targeting NFκB/AMPK/NLRP3 axis. Inflammopharmacology. 2021;29:1169-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 343. | Nomura S, Sakamaki S, Hongu M, Kawanishi E, Koga Y, Sakamoto T, Yamamoto Y, Ueta K, Kimata H, Nakayama K, Tsuda-Tsukimoto M. Discovery of canagliflozin, a novel C-glucoside with thiophene ring, as sodium-dependent glucose cotransporter 2 inhibitor for the treatment of type 2 diabetes mellitus. J Med Chem. 2010;53:6355-6360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 286] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 344. | Nasr M, Cavalu S, Saber S, Youssef ME, Abdelhamid AM, Elagamy HI, Kamal I, Gaafar AGA, El-Ahwany E, Amin NA, Girgis S, El-Sandarosy R, Mahmoud F, Rizk H, Mansour M, Hasaballah A, El-Rafi AA, El-Azez RA, Essam M, Mohamed D, Essam N, Mohammed OA. Canagliflozin-loaded chitosan-hyaluronic acid microspheres modulate AMPK/NF-κB/NLRP3 axis: A new paradigm in the rectal therapy of ulcerative colitis. Biomed Pharmacother. 2022;153:113409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 345. | Zacà V, Rastogi S, Imai M, Wang M, Sharov VG, Jiang A, Goldstein S, Sabbah HN. Chronic monotherapy with rosuvastatin prevents progressive left ventricular dysfunction and remodeling in dogs with heart failure. J Am Coll Cardiol. 2007;50:551-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 346. | Saber S, Abd El-Fattah EE, Yahya G, Gobba NA, Maghmomeh AO, Khodir AE, Mourad AAE, Saad AS, Mohammed HG, Nouh NA, Shata A, Amin NA, Abou El-Rous M, Girgis S, El-Ahwany E, Khalaf EM, El-Kott AF, El-Baz AM. A Novel Combination Therapy Using Rosuvastatin and Lactobacillus Combats Dextran Sodium Sulfate-Induced Colitis in High-Fat Diet-Fed Rats by Targeting the TXNIP/NLRP3 Interaction and Influencing Gut Microbiome Composition. Pharmaceuticals (Basel). 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 347. | Coll RC, Robertson AA, Chae JJ, Higgins SC, Muñoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A, Croker DE, Butler MS, Haneklaus M, Sutton CE, Núñez G, Latz E, Kastner DL, Mills KH, Masters SL, Schroder K, Cooper MA, O'Neill LA. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21:248-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1446] [Cited by in RCA: 2076] [Article Influence: 207.6] [Reference Citation Analysis (0)] |
| 348. | Van Gorp H, Saavedra PH, de Vasconcelos NM, Van Opdenbosch N, Vande Walle L, Matusiak M, Prencipe G, Insalaco A, Van Hauwermeiren F, Demon D, Bogaert DJ, Dullaers M, De Baere E, Hochepied T, Dehoorne J, Vermaelen KY, Haerynck F, De Benedetti F, Lamkanfi M. Familial Mediterranean fever mutations lift the obligatory requirement for microtubules in Pyrin inflammasome activation. Proc Natl Acad Sci U S A. 2016;113:14384-14389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 139] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 349. | Gaidt MM, Ebert TS, Chauhan D, Schmidt T, Schmid-Burgk JL, Rapino F, Robertson AA, Cooper MA, Graf T, Hornung V. Human Monocytes Engage an Alternative Inflammasome Pathway. Immunity. 2016;44:833-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 604] [Article Influence: 67.1] [Reference Citation Analysis (0)] |
| 350. | van der Heijden T, Kritikou E, Venema W, van Duijn J, van Santbrink PJ, Slütter B, Foks AC, Bot I, Kuiper J. NLRP3 Inflammasome Inhibition by MCC950 Reduces Atherosclerotic Lesion Development in Apolipoprotein E-Deficient Mice-Brief Report. Arterioscler Thromb Vasc Biol. 2017;37:1457-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 270] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 351. | Mridha AR, Wree A, Robertson AAB, Yeh MM, Johnson CD, Van Rooyen DM, Haczeyni F, Teoh NC, Savard C, Ioannou GN, Masters SL, Schroder K, Cooper MA, Feldstein AE, Farrell GC. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J Hepatol. 2017;66:1037-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 823] [Article Influence: 102.9] [Reference Citation Analysis (0)] |
| 352. | Qu J, Yuan Z, Wang G, Wang X, Li K. The selective NLRP3 inflammasome inhibitor MCC950 alleviates cholestatic liver injury and fibrosis in mice. Int Immunopharmacol. 2019;70:147-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 353. | Wang L, Lei W, Zhang S, Yao L. MCC950, a NLRP3 inhibitor, ameliorates lipopolysaccharide-induced lung inflammation in mice. Bioorg Med Chem. 2021;30:115954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 354. | Perera AP, Fernando R, Shinde T, Gundamaraju R, Southam B, Sohal SS, Robertson AAB, Schroder K, Kunde D, Eri R. MCC950, a specific small molecule inhibitor of NLRP3 inflammasome attenuates colonic inflammation in spontaneous colitis mice. Sci Rep. 2018;8:8618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 233] [Article Influence: 33.3] [Reference Citation Analysis (0)] |