Published online Feb 7, 2023. doi: 10.3748/wjg.v29.i5.825
Peer-review started: September 27, 2022
First decision: October 20, 2022
Revised: October 29, 2022
Accepted: January 11, 2023
Article in press: January 11, 2023
Published online: February 7, 2023
Processing time: 132 Days and 1 Hours
Given that the liver is involved in many metabolic mechanisms, it is not surprising that chronic liver disease (CLD) could have numerous complications. Secondary osteoporosis and increased bone fragility are frequently overlooked complications in CLD patients. Previous studies implied that up to one-third of these individuals meet diagnostic criteria for osteopenia or osteoporosis. Recent publications indicated that CLD-induced bone fragility depends on the etiology, duration, and stage of liver disease. Therefore, the increased fracture risk in CLD patients puts a severe socioeconomic burden on the health system and urgently requires more effective prevention, diagnosis, and treatment measures. The pathogenesis of CLD-induced bone loss is multifactorial and still insufficiently understood, especially considering the relative impact of increased bone resorption and reduced bone formation in these individuals. It is essential to note that inconsistent findings regarding bone mineral density measurement were previously reported in these individuals. Bone mineral density is widely used as the “golden standard” in the clinical assessment of bone fragility although it is not adequate to predict individual fracture risk. Therefore, microscale bone alterations (bone microstructure, mechanical properties, and cellular indices) were analyzed in CLD individuals. These studies further support the thesis that bone strength could be compromised in CLD individuals, implying that an individualized approach to fracture risk assessment and subsequent therapy is necessary for CLD patients. However, more well-designed studies are required to solve the bone fragility puzzle in CLD patients.
Core Tip: Secondary osteoporosis and increased bone fragility are frequently overlooked complications in patients with chronic liver disease (CLD). Recent publications agree that CLD-induced bone fragility depends on the etiology, duration, and stage of liver disease, but certain ambiguities are still present. Importantly, etiopathogenetic mechanisms leading to CLD-induced bone loss are still insufficiently clarified. Given that available clinical tools for fracture risk assessment are not entirely reliable, evaluating small-length structural bone properties could improve understanding of the multifactorial nature of bone fragility in CLD patients, which could set a base for the development of more effective preventive and therapeutic strategies.
- Citation: Jadzic J, Djonic D. Bone loss in chronic liver diseases: Could healthy liver be a requirement for good bone health? World J Gastroenterol 2023; 29(5): 825-833
- URL: https://www.wjgnet.com/1007-9327/full/v29/i5/825.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i5.825
The importance of a wide range of liver functions in the human body becomes the most visible in chronic liver disease (CLD). The most commonly known CLD complications are portal hypertension, hepatic encephalopathy, ascites, hepatorenal syndrome, variceal bleeding, and hepatocellular carcinoma[1,2]. However, CLD is also associated with changes in the skeleton, previously known as hepatic osteodystrophy[3,4]. Among CLD patients, substantial heterogeneity of skeletal changes was noted depending on the etiology, duration, and stage of the liver disorder[5,6]. Namely, osteoporosis was initially described as a complication of primary biliary cholangitis and primary biliary cirrhosis (cholestatic liver diseases)[7], while skeletal changes were later described in other (non-cholestatic) hepatic disorders as well[8,9]. It has been reported that approximately every second patient with viral hepatitis, hemochromatosis, and Wilson’s disease has osteoporosis or osteopenia[10-12], while up to 55% of patients with alcoholic liver cirrhosis have osteoporotic bone changes[3,13,14]. Interestingly, bone alterations in nonalcoholic fatty liver disease or nonalcoholic steatohepatitis have recently drawn researchers’ attention, revealing that up to one-third of these individuals could develop bone alterations[15,16].
Consequently, CLD individuals are at substantial risk for non-traumatic bone fractures[17-19], with a prevalence between 7% and 35%[20]. Recent data suggest that fracture incidence is two to three times higher in end-stage CLD patients compared to healthy controls[19,21], while others reported an eight-fold increase in the risk of bone fractures in these patients[22]. Regarding fracture localization, data suggest that vertebral fractures are most common in patients with end-stage CLD[19,23-26], given that more than one-third of these individuals experienced at least one vertebral fracture during their lifetime[8,23,27]. Moreover, CLD contributes to the age-associated increase in the risk of femoral fracture and subsequently its life-threatening complications[22]. It is important to emphasize that end-stage CLD patients are experiencing fragility fractures at a significantly younger age than most osteoporotic patients[22], considering that the cumulative fracture risk in CLD patients younger than 45 years corresponds to the risk of healthy controls over 75 years of age[22]. It is important to emphasize that CLD likely changes the sex distribution of fracture risk in the aged population, considering that CLD is more frequent in male patients[28], while osteoporosis and osteoporosis-related bone fractures are more likely to develop in older women[29].
Despite the significant number of studies that have assessed various characteristics of bone deterioration in CLD individuals, many unknowns should be elucidated to understand this topic entirely.
Most studies dealing with bone changes in CLD patients used dual-energy X-ray absorptiometry as the most valuable tool in the clinical assessment of fracture risk[30]. Interestingly, opposite results were yielded. Namely, dual-energy X-ray absorptiometry assessment revealed significantly lower bone mineral density (BMD) in patients with viral, autoimmune, and primary biliary cirrhosis[31-33]. At the same time, other authors failed to show a significant BMD decrease in CLD of the same etiology[34,35]. Multiple studies showed reduced dual-energy X-ray absorptiometry-obtained BMD values, suggesting osteopenia or osteoporotic changes of the lumbar spine and femoral neck in patients with alcohol-induced CLDs[36-38], while other research teams failed to show these bone alterations in individuals prone to chronic alcohol abuse[17,39,40]. Given that the primary source of these contradictory data could be in the study design (cross-sectional study design), selection criteria, and the number of participants included in the study, future well-designed prospective studies are required to fully understand BMD alterations in CLD patients.
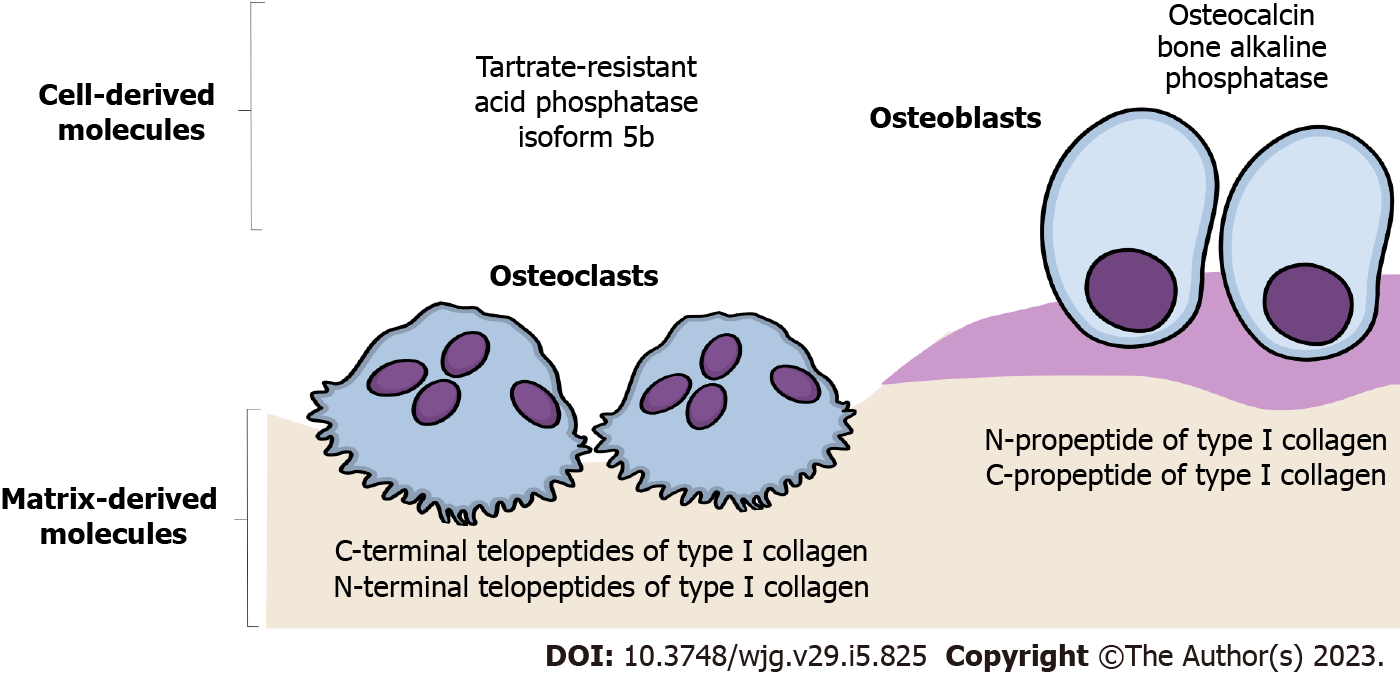
As a non-invasive and cost-effective tool for indirect assessment of bone remodeling dynamics, bone turnover biomarkers (BTMs) are a complementary method in the clinical management and follow-up of the treatment effects in patients with osteoporosis and osteoporosis-related bone fragility[41]. Automated or manual immunoassays using blood or urine samples are utilized to measure a specific combination of these protein or protein-derivative biomarkers[42], which are considered indicative of the dynamic relationship between osteoblast activity (bone formation markers) and osteoclast activity (bone resorption markers)[41,43]. The most frequently investigated bone formation markers are osteocalcin, bone alkaline phosphatase, and N-propeptide of type I collagen[41]. On the other side, commonly interpreted bone resorption markers are C-terminal and N-terminal telopeptides of type I collagen, deoxypyridinoline, and tartrate-resistant acid phosphatase isoform 5b[41] (Figure 1).
The interpretation of BTM levels has been of clinical utility in age-related osteoporosis[43], while its role in the clinical management of CLD-induced bone loss is still modest. Some data suggest that serum levels of osteocalcin and bone alkaline phosphatase are decreased in individuals with CLD[25,36,44], while others failed to show significant differences between individuals with CLD and the control group[45,46]. Moreover, contradictory data regarding the level of β-CTX and deoxypyridinoline were noted in CLD patients[36,45,47,48]. It is important to note that liver dysfunction could affect serum concentrations of BTMs, which reveals excessive bone matrix degradation, indicating that its assessment allows only limited conclusions in CLD individuals[10,49]. Multiple limitations of BTM assessment are among the reasons why CLD-induced bone changes are recognized and treated after a patient experiences non-traumatic fracture[10], suggesting that further investigation is required to elucidate the role of BTMs in developing novel, adequate preventive and treatment strategies.
The World Health Organization recommended BMD as the primary parameter for the diagnosis of osteopenia and osteoporosis and for clinical fracture risk assessment[50]. However, considering that the occurrence of fragility fractures primarily requires the action of several bone strength determinants and their mutual interaction, it is evident that increased bone fragility could not be solely explained by BMD decrease[51,52]. In other words, low BMD should only be considered an applicable and non-invasive clinical surrogate marker of bone fragility[52,53]. Namely, it has been known that only up to one-third of non-traumatic fractures are attributable to low BMD values, indicating that many individuals with bone fractures have BMD in the referent range[54].
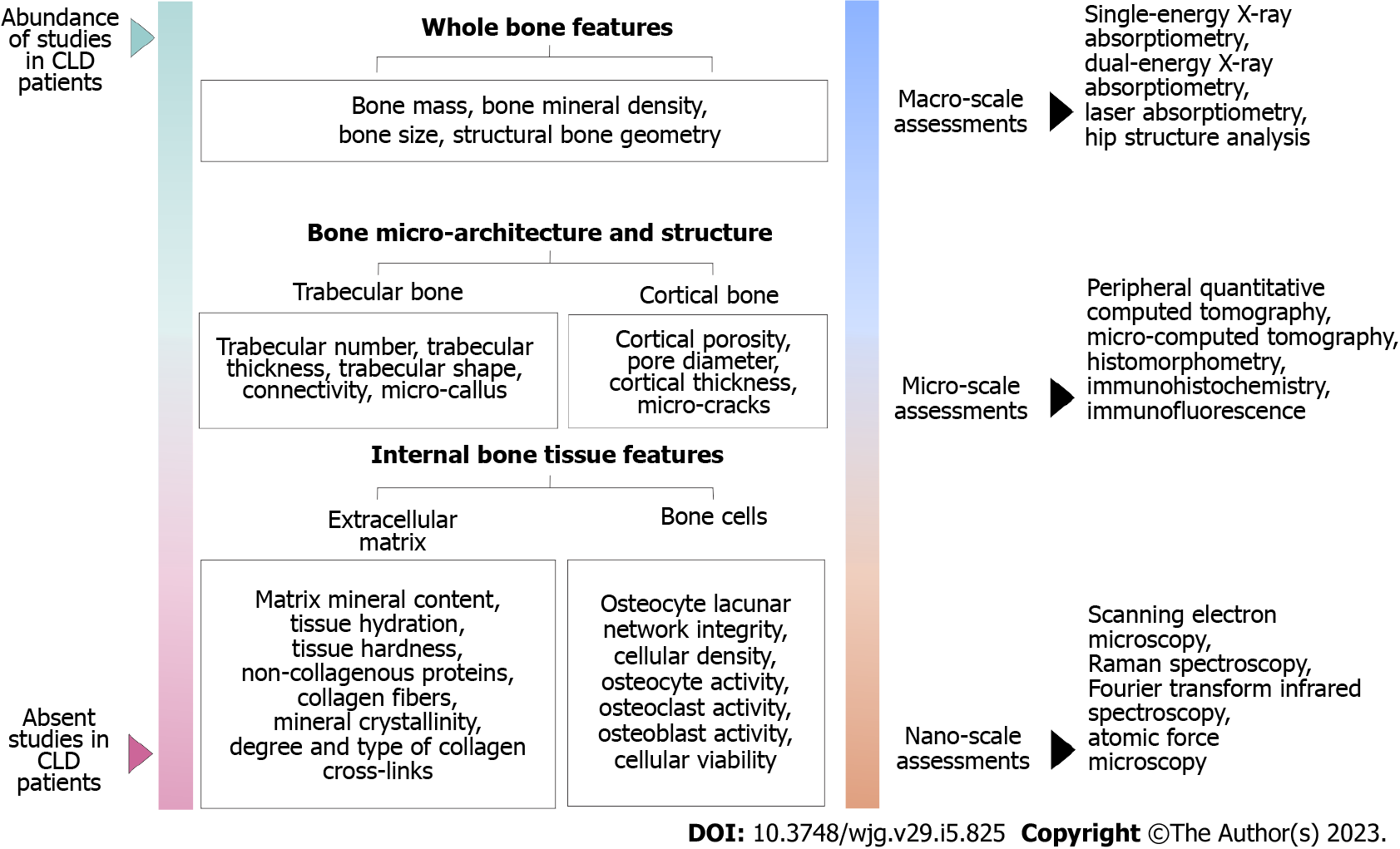
Moreover, various bone properties are recognized as important determinants affecting bone strength (ability to resist fracture)[55]. Thus, current studies suggested that multiscale analysis of various bone properties (with respect to the hierarchical structure of the bone, Figure 2) could contribute to a better understanding of increased bone fragility in elderly individuals with chronic comorbidities, including a variety of CLDs[56]. The importance of assessing these bone properties is highlighted by the fact that some pharmaceutical agents were proven to improve bone strength and reduce fracture risk without increasing BMD[57,58], indicating the potential for developing new and effective treatment strategies[52].
Initially, histomorphometry studies using optic microscopy assessment of iliac bone biopsies showed deteriorated trabecular bone architecture in CLD patients[59,60]. In addition, some novel clinical studies confirmed these results on the tibia and radius of CLD patients, using a newer methodology called peripheral quantitative computed tomography[33,61,62]. Since osteoporosis is not uniform throughout the skeleton[63] it was crucial to assess CLD-induced microstructural decline in lumbar vertebrae and proximal femora[38,64]. Similarly to previous findings, our research group used microcomputed tomography with an isotropic resolution of 10 µm to observe impaired microarchitectural integrity of lumbar vertebrae and proximal femora collected from CLD individuals[9,38,64]. On the trace of altered trabecular and cortical microarchitecture, we demonstrated reduced mechanical bone competence in these individuals[38,65], indicating that altered bone matrix content could be involved in CLD-induced bone fragility.
Future state-of-the-art studies should focus on a precise nanoscale morphostructural estimate of the inorganic (mineral) and organic component of the bone extracellular matrix (collagen fibers) to elucidate its role in increased bone fragility among CLD individuals (Figure 2). Finally, the long-term benefit of small-length bone studies could develop a specific diagnostic algorithm that will help to reliably predict bone strength based on the information available in the clinical context of each patient.
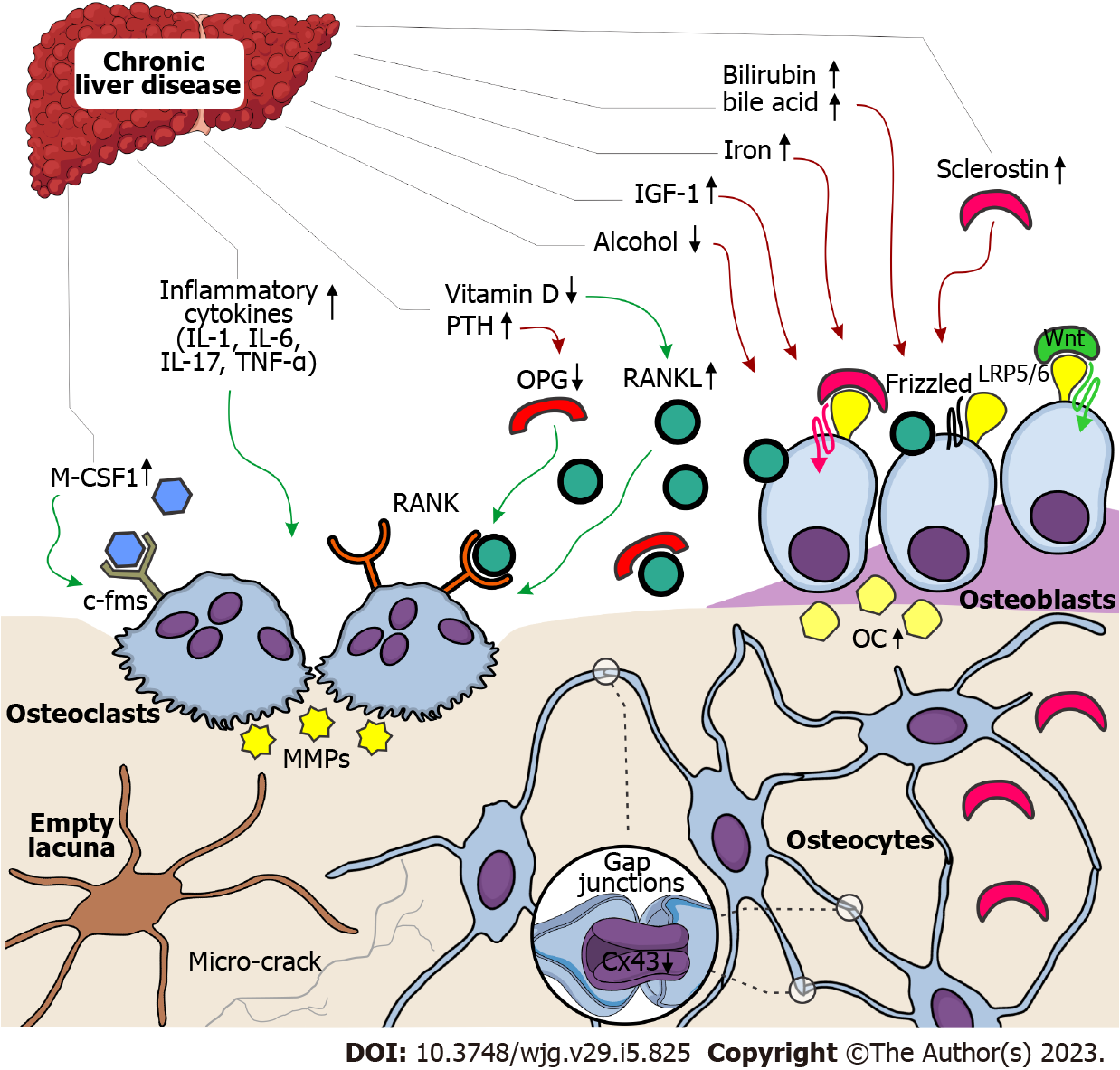
Bone loss in CLD patients is commonly described as a consequence of bone remodeling disturbance[8], but the particular contribution of increased bone resorption and decreased bone formation still needs to be thoroughly explained. Nowadays, a common understanding is that the etiopathogenetic mechanisms of bone loss are dependable on the etiology of liver disease[3,8]. Previous data revealed that osteoblast dysfunction and decreased bone formation play a central role in the etiopathogenesis of bone loss in patients with cholestatic liver disease, Wilson’s disease, and hemochromatosis[7,12,48,66]. Conversely, viral CLD displays a more dominant effect on increased osteoclast activity, inducing high-turnover osteoporosis[21,32,67].
On a molecular level, low-turnover osteoporosis in CLD patients is commonly associated with toxic effects of biliary stasis and copper/iron accumulation on differentiation, maturation, and proliferation of osteoblasts (Figure 3)[68-70]. Also, previous studies suggested that osteoblast dysfunction in patients with cholestatic forms of CLD could be mediated by insulin growth factor-1 or oncofetal fibronectin[66,70,71], while direct toxic effects of alcohol on osteoblastic function contribute to bone loss among patients within alcohol-induced CLD[72,73]. During the process of bone formation, osteoblasts become embedded within the bone matrix, continuing to function as bone remodeling orchestrators or osteocytes[74]. Osteocytes form a global network throughout the bone tissue by intercellular channels (gap junctions), most frequently formed by connexin 43[75]. Reduction in osteocytic expression levels of connexin 43 and minor disruptions in the osteocyte lacunar network was noted in CLD individuals (Figure 3), suggesting that the mechanosensing potential and molecular transduction might be defective in those patients with CLD[65,76]. In addition, increased bone expression levels of sclerostin (an osteocyte-derived negative regulator of bone formation) were noted in CLD individuals[65,76], which was in accordance with previous clinical studies[77,78]. These data indicate that treatment targeting sclerostin may be an interesting strategy to fight osteoporosis in CLD patients[10]. Still, possible therapeutical utilities in CLD patients are yet to be thoroughly investigated in the years ahead.
Previous studies revealed that bone loss in CLD individuals could be explained by a strong link between systemic hyperproduction of inflammatory mediators and increased bone resorption (Figure 3)[21,32,67]. Most commonly, it is understood that tumor necrosis factor-α, interleukin (IL)-1, IL-6, IL-7, IL-11, IL-13, IL-15, and IL-17, produced by immune cells, could directly activate osteoclast precursors or display an indirect effect by osteoblasts[8,10,72]. Namely, increased secretion of receptor activator for nuclear factor kappa B ligand (RANKL), the disturbed ratio between RANKL and osteoprotegerin, matrix metalloproteinases activity, and cathepsin K are described as contributing factors in CLD-induced bone loss via increased bone resorption (Figure 3)[10,79-81]. The recent recommendation for therapy targeting RANKL advocates the importance of the RANK-RANKL-osteoprotegerin system in bone loss among CLD patients[20,82]. In addition, increased circulating macrophage colony-stimulating factor 1 in CLD patients could promote bone resorption due to its role in priming a larger number of monocytes to form osteoclasts in these patients[6].
Lastly, low vitamin D levels, unbalanced diet (low calcium and protein intake), malabsorption, disruption in the homeostasis of the intestinal microbiome, coupled with a variety of hormonal and metabolic disruptions (such as increased levels of parathyroid hormone, hypogonadism, and hypercorticism) were identified as factors that contribute to bone loss in CLD individuals[20,72,83]. Based on these data, new nutritional support guidelines were recently introduced by the European Association for the Study of the Liver[20,84]. However, given that bone changes in CLD patients are undoubtedly present, it is vital to further investigate more direct etiopathogenetic mechanisms involved in the relationship between liver and bone disorders.
Bone alterations are a common complication in patients with CLD, especially in those with liver cirrhosis. Over the previous period, numerous studies have contributed to understanding bone fragility in CLD patients. However, numerous ambiguities are still present due to the modest reliability of clinical diagnostic methods, which could lead clinicians to doubt whether or when it is necessary to start treating CLD-induced skeletal alterations. Thus, evaluating small-length structural bone properties could improve understanding of the multifactorial nature of bone fragility in CLD patients. All these data could set a base for developing a patient-specific diagnostic algorithm that will reliably predict bone strength based on the information available in a clinical context. Additionally, specific clinical guidelines for preventing, diagnosing, and treating skeletal disorders in patients with CLD need to be established in the near future.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Serbia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chuang W, Taiwan; Ferraioli G, Italy; Hakim GD, Turkey S-Editor: Liu GL L-Editor: Filipodia P-Editor: Liu GL
| 1. | Rahimi RS, Rockey DC. Complications and outcomes in chronic liver disease. Curr Opin Gastroenterol. 2011;27:204-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Tarao K, Nozaki A, Ikeda T, Sato A, Komatsu H, Komatsu T, Taguri M, Tanaka K. Real impact of liver cirrhosis on the development of hepatocellular carcinoma in various liver diseases-meta-analytic assessment. Cancer Med. 2019;8:1054-1065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 3. | López-Larramona G, Lucendo AJ, González-Castillo S, Tenias JM. Hepatic osteodystrophy: An important matter for consideration in chronic liver disease. World J Hepatol. 2011;3:300-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Barbu EC, Chițu-Tișu CE, Lazăr M, Olariu C, Bojincă M, Ionescu RA, Ion DA, Bădărău IA. Hepatic Osteodystrophy: A Global (Re)View of the Problem. Acta Clin Croat. 2017;56:512-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Crawford BA, Kam C, Donaghy AJ, McCaughan GW. The heterogeneity of bone disease in cirrhosis: a multivariate analysis. Osteoporos Int. 2003;14:987-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Nakchbandi IA, van der Merwe SW. Current understanding of osteoporosis associated with liver disease. Nat Rev Gastroenterol Hepatol. 2009;6:660-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Parés A, Guañabens N. Primary biliary cholangitis and bone disease. Best Pract Res Clin Gastroenterol. 2018;34-35:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Guañabens N, Parés A. Osteoporosis in chronic liver disease. Liver Int. 2018;38:776-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 9. | Jadzic J, Cvetkovic D, Tomanovic N, Zivkovic V, Nikolic S, Milovanovic P, Djuric M, Djonic D. The severity of hepatic disorder is related to vertebral microstructure deterioration in cadaveric donors with liver cirrhosis. Microsc Res Tech. 2021;84:840-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Ehnert S, Aspera-Werz RH, Ruoß M, Dooley S, Hengstler JG, Nadalin S, Relja B, Badke A, Nussler AK. Hepatic Osteodystrophy-Molecular Mechanisms Proposed to Favor Its Development. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 11. | Weiss KH, Van de Moortele M, Gotthardt DN, Pfeiffenberger J, Seessle J, Ullrich E, Gielen E, Borghs H, Adriaens E, Stremmel W, Meersseman W, Boonen S, Cassiman D. Bone demineralisation in a large cohort of Wilson disease patients. J Inherit Metab Dis. 2015;38:949-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Valenti L, Varenna M, Fracanzani AL, Rossi V, Fargion S, Sinigaglia L. Association between iron overload and osteoporosis in patients with hereditary hemochromatosis. Osteoporos Int. 2009;20:549-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 137] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 13. | Bang UC, Benfield T, Bendtsen F, Hyldstrup L, Beck Jensen JE. The risk of fractures among patients with cirrhosis or chronic pancreatitis. Clin Gastroenterol Hepatol. 2014;12:320-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | López-Larramona G, Lucendo AJ, González-Delgado L. Alcoholic liver disease and changes in bone mineral density. Rev Esp Enferm Dig. 2013;105:609-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Lee SH, Yun JM, Kim SH, Seo YG, Min H, Chung E, Bae YS, Ryou IS, Cho B. Association between bone mineral density and nonalcoholic fatty liver disease in Korean adults. J Endocrinol Invest. 2016;39:1329-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Chen DZ, Xu QM, Wu XX, Cai C, Zhang LJ, Shi KQ, Shi HY, Li LJ. The Combined Effect of Nonalcoholic Fatty Liver Disease and Metabolic Syndrome on Osteoporosis in Postmenopausal Females in Eastern China. Int J Endocrinol. 2018;2018:2314769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Bang CS, Shin IS, Lee SW, Kim JB, Baik GH, Suk KT, Yoon JH, Kim YS, Kim DJ. Osteoporosis and bone fractures in alcoholic liver disease: a meta-analysis. World J Gastroenterol. 2015;21:4038-4047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Asoudeh F, Salari-Moghaddam A, Larijani B, Esmaillzadeh A. A systematic review and meta-analysis of prospective cohort studies on the association between alcohol intake and risk of fracture. Crit Rev Food Sci Nutr. 2022;62:5623-5637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Liang J, Meng WD, Yang JM, Li SL, Zhong MN, Hou XX, Wang R, Long YY, Bao LX, Bao M. The association between liver cirrhosis and fracture risk: A systematic review and meta-analysis. Clin Endocrinol (Oxf). 2018;89:408-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Jeong HM, Kim DJ. Bone Diseases in Patients with Chronic Liver Disease. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 21. | Nakchbandi IA. Osteoporosis and fractures in liver disease: relevance, pathogenesis and therapeutic implications. World J Gastroenterol. 2014;20:9427-9438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 48] [Reference Citation Analysis (0)] |
| 22. | Otete H, Deleuran T, Fleming KM, Card T, Aithal GP, Jepsen P, West J. Hip fracture risk in patients with alcoholic cirrhosis: A population-based study using English and Danish data. J Hepatol. 2018;69:697-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 23. | Wibaux C, Legroux-Gerot I, Dharancy S, Boleslawski E, Declerck N, Canva V, Mathurin P, Pruvot FR, Cortet B. Assessing bone status in patients awaiting liver transplantation. Joint Bone Spine. 2011;78:387-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Ninkovic M, Love SA, Tom B, Alexander GJ, Compston JE. High prevalence of osteoporosis in patients with chronic liver disease prior to liver transplantation. Calcif Tissue Int. 2001;69:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 74] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Culafić Dj, Djonic D, Culafic-Vojinovic V, Ignjatovic S, Soldatovic I, Vasic J, Beck TJ, Djuric M. Evidence of degraded BMD and geometry at the proximal femora in male patients with alcoholic liver cirrhosis. Osteoporos Int. 2015;26:253-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Chen TL, Lin CS, Shih CC, Huang YF, Yeh CC, Wu CH, Cherng YG, Liao CC. Risk and adverse outcomes of fractures in patients with liver cirrhosis: two nationwide retrospective cohort studies. BMJ Open. 2017;7:e017342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Peris P, Guañabens N, Parés A, Pons F, del Rio L, Monegal A, Surís X, Caballería J, Rodés J, Muñoz-Gómez J. Vertebral fractures and osteopenia in chronic alcoholic patients. Calcif Tissue Int. 1995;57:111-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Sagnelli E, Stroffolini T, Sagnelli C, Pirisi M, Babudieri S, Colloredo G, Russello M, Coppola N, Gaeta GB, Cacopardo B, De Luca M, Almasio PL; EPACRON study group. Gender differences in chronic liver diseases in two cohorts of 2001 and 2014 in Italy. Infection. 2018;46:93-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Djonic D, Milovanovic P, Nikolic S, Ivovic M, Marinkovic J, Beck T, Djuric M. Inter-sex differences in structural properties of aging femora: implications on differential bone fragility: a cadaver study. J Bone Miner Metab. 2011;29:449-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Eastell R, Vittinghoff E, Lui LY, McCulloch CE, Pavo I, Chines A, Khosla S, Cauley JA, Mitlak B, Bauer DC, Bouxsein M, Black DM. Validation of the Surrogate Threshold Effect for Change in Bone Mineral Density as a Surrogate Endpoint for Fracture Outcomes: The FNIH-ASBMR SABRE Project. J Bone Miner Res. 2022;37:29-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 31. | Mounach A, Ouzzif Z, Wariaghli G, Achemlal L, Benbaghdadi I, Aouragh A, Bezza A, El Maghraoui A. Primary biliary cirrhosis and osteoporosis: a case-control study. J Bone Miner Metab. 2008;26:379-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Gallego-Rojo FJ, Gonzalez-Calvin JL, Muñoz-Torres M, Mundi JL, Fernandez-Perez R, Rodrigo-Moreno D. Bone mineral density, serum insulin-like growth factor I, and bone turnover markers in viral cirrhosis. Hepatology. 1998;28:695-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 174] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 33. | Wakolbinger R, Muschitz C, Scheriau G, Bodlaj G, Kocijan R, Feichtinger X, Schanda JE, Haschka J, Resch H, Pietschmann P. Bone microarchitecture and bone turnover in hepatic cirrhosis. Osteoporos Int. 2019;30:1195-1204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 34. | González-Calvin JL, Mundi JL, Casado-Caballero FJ, Abadia AC, Martin-Ibañez JJ. Bone mineral density and serum levels of soluble tumor necrosis factors, estradiol, and osteoprotegerin in postmenopausal women with cirrhosis after viral hepatitis. J Clin Endocrinol Metab. 2009;94:4844-4850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Campbell MS, Lichtenstein GR, Rhim AD, Pazianas M, Faust T. Severity of liver disease does not predict osteopenia or low bone mineral density in primary sclerosing cholangitis. Liver Int. 2005;25:311-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Díez-Ruiz A, García-Saura PL, García-Ruiz P, González-Calvin JL, Gallego-Rojo F, Fuchs D. Bone mineral density, bone turnover markers and cytokines in alcohol-induced cirrhosis. Alcohol Alcohol. 2010;45:427-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Mahmoudi A, Sellier N, Reboul-Marty J, Chalès G, Lalatonne Y, Bourcier V, Grando V, Barget N, Beaugrand M, Trinchet JC, Ganne-Carrié N. Bone mineral density assessed by dual-energy X-ray absorptiometry in patients with viral or alcoholic compensated cirrhosis. A prospective study. Clin Res Hepatol Gastroenterol. 2011;35:731-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Jadzic J, Milovanovic P, Cvetkovic D, Ivovic M, Tomanovic N, Bracanovic M, Zivkovic V, Nikolic S, Djuric M, Djonic D. Mechano-structural alteration in proximal femora of individuals with alcoholic liver disease: Implications for increased bone fragility. Bone. 2021;150:116020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 39. | Laitinen K, Lamberg-Allardt C, Tunninen R, Härkönen M, Välimäki M. Bone mineral density and abstention-induced changes in bone and mineral metabolism in noncirrhotic male alcoholics. Am J Med. 1992;93:642-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 77] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | Kanis JA, Johansson H, Johnell O, Oden A, De Laet C, Eisman JA, Pols H, Tenenhouse A. Alcohol intake as a risk factor for fracture. Osteoporos Int. 2005;16:737-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 370] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 41. | Eastell R, Pigott T, Gossiel F, Naylor KE, Walsh JS, Peel NFA. DIAGNOSIS OF ENDOCRINE DISEASE: Bone turnover markers: are they clinically useful? Eur J Endocrinol. 2018;178:R19-R31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 42. | Wheater G, Elshahaly M, Tuck SP, Datta HK, van Laar JM. The clinical utility of bone marker measurements in osteoporosis. J Transl Med. 2013;11:201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 212] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 43. | Greenblatt MB, Tsai JN, Wein MN. Bone Turnover Markers in the Diagnosis and Monitoring of Metabolic Bone Disease. Clin Chem. 2017;63:464-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 238] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 44. | Malik P, Gasser RW, Moncayo R, Kemmler G, Wolfgang Fleischhacker W. Markers of bone resorption and formation during abstinence in male alcoholic patients. Alcohol Clin Exp Res. 2012;36:2059-2064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 45. | Uretmen S, Gol M, Cimrin D, Irmak E. Effects of chronic liver disease on bone mineral density and bone metabolism markers in postmenopausal women. Eur J Obstet Gynecol Reprod Biol. 2005;123:67-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 46. | George J, Ganesh HK, Acharya S, Bandgar TR, Shivane V, Karvat A, Bhatia SJ, Shah S, Menon PS, Shah N. Bone mineral density and disorders of mineral metabolism in chronic liver disease. World J Gastroenterol. 2009;15:3516-3522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 76] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 47. | Wariaghli G, Mounach A, Achemlal L, Benbaghdadi I, Aouragh A, Bezza A, El Maghraoui A. Osteoporosis in chronic liver disease: a case-control study. Rheumatol Int. 2010;30:893-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 48. | Hegedus D, Ferencz V, Lakatos PL, Meszaros S, Lakatos P, Horvath C, Szalay F. Decreased bone density, elevated serum osteoprotegerin, and beta-cross-laps in Wilson disease. J Bone Miner Res. 2002;17:1961-1967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 49. | Liu T, Wang X, Karsdal MA, Leeming DJ, Genovese F. Molecular serum markers of liver fibrosis. Biomark Insights. 2012;7:105-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 50. | Jeon YK, Kim BH, Kim IJ. The diagnosis of osteoporosis. J Korean Med Assoc. 2016;59:842-846. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 51. | Ott SM. Bone strength: more than just bone density. Kidney Int. 2016;89:16-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 52. | Fonseca H, Moreira-Gonçalves D, Coriolano HJ, Duarte JA. Bone quality: the determinants of bone strength and fragility. Sports Med. 2014;44:37-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 284] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 53. | Djonić D, Milovanović P, Djurić M. Basis of bone strength vs. bone fragility: a review of determinants of age-related hip fracture risk. Srp Arh Celok Lek. 2013;141:548-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 54. | Stone KL, Seeley DG, Lui LY, Cauley JA, Ensrud K, Browner WS, Nevitt MC, Cummings SR; Osteoporotic Fractures Research Group. BMD at multiple sites and risk of fracture of multiple types: long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res. 2003;18:1947-1954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 710] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 55. | Ammann P, Rizzoli R. Bone strength and its determinants. Osteoporos Int. 2003;14 Suppl 3:S13-S18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 475] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 56. | Osterhoff G, Morgan EF, Shefelbine SJ, Karim L, McNamara LM, Augat P. Bone mechanical properties and changes with osteoporosis. Injury. 2016;47 Suppl 2:S11-S20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 328] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 57. | Bjarnason NH, Sarkar S, Duong T, Mitlak B, Delmas PD, Christiansen C. Six and twelve month changes in bone turnover are related to reduction in vertebral fracture risk during 3 years of raloxifene treatment in postmenopausal osteoporosis. Osteoporos Int. 2001;12:922-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 165] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 58. | Sarkar S, Mitlak BH, Wong M, Stock JL, Black DM, Harper KD. Relationships between bone mineral density and incident vertebral fracture risk with raloxifene therapy. J Bone Miner Res. 2002;17:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 290] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 59. | Guichelaar MM, Malinchoc M, Sibonga J, Clarke BL, Hay JE. Bone metabolism in advanced cholestatic liver disease: analysis by bone histomorphometry. Hepatology. 2002;36:895-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 60. | Jorge-Hernandez JA, Gonzalez-Reimers CE, Torres-Ramirez A, Santolaria-Fernandez F, Gonzalez-Garcia C, Batista-Lopez JN, Pestana-Pestana M, Hernandez-Nieto L. Bone changes in alcoholic liver cirrhosis. A histomorphometrical analysis of 52 cases. Dig Dis Sci. 1988;33:1089-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 61. | Schmidt T, Schmidt C, Schmidt FN, Butscheidt S, Mussawy H, Hubert J, Hawellek T, Oehler N, Barvencik F, Lohse AW, Schinke T, Schramm C, Amling M, Rolvien T. Disease Duration and Stage Influence Bone Microstructure in Patients With Primary Biliary Cholangitis. J Bone Miner Res. 2018;33:1011-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 62. | Jandl NM, Rolvien T, Schmidt T, Mussawy H, Nielsen P, Oheim R, Amling M, Barvencik F. Impaired Bone Microarchitecture in Patients with Hereditary Hemochromatosis and Skeletal Complications. Calcif Tissue Int. 2020;106:465-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 63. | Choksi P, Jepsen KJ, Clines GA. The challenges of diagnosing osteoporosis and the limitations of currently available tools. Clin Diabetes Endocrinol. 2018;4:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 64. | Jadzic J, Cvetkovic D, Milovanovic P, Tomanovic N, Zivkovic V, Nikolic S, Djuric M, Djonic D. The micro-structural analysis of lumbar vertebrae in alcoholic liver cirrhosis. Osteoporos Int. 2020;31:2209-2217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 65. | Jadzic J, Tomanovic N, Djukic D, Zivkovic V, Nikolic S, Djuric M, Milovanovic P, Djonic D. Micro-scale assessment of bone quality changes in adult cadaveric men with congestive hepatopathy. Histochem Cell Biol. 2022;158:583-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 66. | Kawelke N, Bentmann A, Hackl N, Hager HD, Feick P, Geursen A, Singer MV, Nakchbandi IA. Isoform of fibronectin mediates bone loss in patients with primary biliary cirrhosis by suppressing bone formation. J Bone Miner Res. 2008;23:1278-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 67. | Corazza GR, Trevisani F, Di Stefano M, De Notariis S, Veneto G, Cecchetti L, Minguzzi L, Gasbarrini G, Bernardi M. Early increase of bone resorption in patients with liver cirrhosis secondary to viral hepatitis. Dig Dis Sci. 2000;45:1392-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 68. | Mitchell R, McDermid J, Ma MM, Chik CL. MELD score, insulin-like growth factor 1 and cytokines on bone density in end-stage liver disease. World J Hepatol. 2011;3:157-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 69. | Santori C, Ceccanti M, Diacinti D, Attilia ML, Toppo L, D'Erasmo E, Romagnoli E, Mascia ML, Cipriani C, Prastaro A, Carnevale V, Minisola S. Skeletal turnover, bone mineral density, and fractures in male chronic abusers of alcohol. J Endocrinol Invest. 2008;31:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 70. | Sens C, Altrock E, Rau K, Klemis V, von Au A, Pettera S, Uebel S, Damm T, Tiwari S, Moser M, Nakchbandi IA. An O-Glycosylation of Fibronectin Mediates Hepatic Osteodystrophy Through α4β1 Integrin. J Bone Miner Res. 2017;32:70-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 71. | Danford CJ, Trivedi HD, Papamichael K, Tapper EB, Bonder A. Osteoporosis in primary biliary cholangitis. World J Gastroenterol. 2018;24:3513-3520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 72. | Luo Z, Liu Y, Chen H, Shi S. Cellular and molecular mechanisms of alcohol-induced osteopenia. Cell Mol Life Sci. 2017;74:4443-4453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 73. | Chakkalakal DA. Alcohol-induced bone loss and deficient bone repair. Alcohol Clin Exp Res. 2005;29:2077-2090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 158] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 74. | Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26:229-238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1520] [Cited by in RCA: 1547] [Article Influence: 110.5] [Reference Citation Analysis (0)] |
| 75. | Stains JP, Fontana F, Civitelli R. Intercellular junctions and cell-cell communication in the skeletal system. Principles of Bone Biology (Fourth Edition). 2020;423-442. [DOI] [Full Text] |
| 76. | Jadzic J, Milovanovic PD, Cvetkovic D, Zivkovic V, Nikolic S, Tomanovic N, Djuric MP, Djonic D. The altered osteocytic expression of connexin 43 and sclerostin in human cadaveric donors with alcoholic liver cirrhosis: Potential treatment targets. J Anat. 2022;240:1162-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 77. | Wakolbinger R, Muschitz C, Wallwitz J, Bodlaj G, Feichtinger X, Schanda JE, Resch H, Baierl A, Pietschmann P. Serum levels of sclerostin reflect altered bone microarchitecture in patients with hepatic cirrhosis. Wien Klin Wochenschr. 2020;132:19-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 78. | Guañabens N, Ruiz-Gaspà S, Gifre L, Miquel R, Peris P, Monegal A, Dubrueil M, Arias A, Parés A. Sclerostin Expression in Bile Ducts of Patients With Chronic Cholestasis May Influence the Bone Disease in Primary Biliary Cirrhosis. J Bone Miner Res. 2016;31:1725-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 79. | Khalifa YH, Mourad GM, Stephanos WM, Omar SA, Mehanna RA. Bone Marrow-Derived Mesenchymal Stem Cell Potential Regression of Dysplasia Associating Experimental Liver Fibrosis in Albino Rats. Biomed Res Int. 2019;2019:5376165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 80. | Liang HPH, Xu J, Xue M, Jackson C. Matrix metalloproteinases in bone development and pathology: current knowledge and potential clinical utility. Met Med. 2016;3:93-102. [RCA] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 81. | Hardy E, Fernandez-Patron C. Destroy to Rebuild: The Connection Between Bone Tissue Remodeling and Matrix Metalloproteinases. Front Physiol. 2020;11:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 82. | Camacho PM, Petak SM, Binkley N, Clarke BL, Harris ST, Hurley DL, Kleerekoper M, Lewiecki EM, Miller PD, Narula HS, Pessah-Pollack R, Tangpricha V, Wimalawansa SJ, Watts NB. AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS AND AMERICAN COLLEGE OF ENDOCRINOLOGY CLINICAL PRACTICE GUIDELINES FOR THE DIAGNOSIS AND TREATMENT OF POSTMENOPAUSAL OSTEOPOROSIS - 2016--EXECUTIVE SUMMARY. Endocr Pract. 2016;22:1111-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 134] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 83. | Alvisa-Negrín J, González-Reimers E, Santolaria-Fernández F, García-Valdecasas-Campelo E, Valls MR, Pelazas-González R, Durán-Castellón MC, de Los Angeles Gómez-Rodríguez M. Osteopenia in alcoholics: effect of alcohol abstinence. Alcohol Alcohol. 2009;44:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 84. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70:172-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 675] [Article Influence: 112.5] [Reference Citation Analysis (2)] |