Published online Jan 28, 2023. doi: 10.3748/wjg.v29.i4.656
Peer-review started: September 13, 2022
First decision: September 29, 2022
Revised: October 13, 2022
Accepted: November 21, 2022
Article in press: November 21, 2022
Published online: January 28, 2023
Processing time: 128 Days and 21.1 Hours
The coronavirus disease 2019 (COVID-19) hit the entire world as a global pandemic and soon became the most important concern for all patients with chronic diseases. An early trend in higher mortality in patients with acute respiratory distress attracted all researchers to closely monitor patients for the involvement of other systems. It soon became apparent that patients with chronic liver diseases are at increased risk of mortality given their cirrhosis-associated immune dysfunction. Additionally, liver function abnormalities were noted in patients with severe COVID-19. Profound cytokine storm, direct viral infection, drugs and reactivation of viral infections were causes of deranged liver functions. Here, we discuss the relation between COVID-19 and chronic liver disease, specifically cirrhosis, hepatitis B, hepatitis C, and non-alcoholic fatty liver disease (NAFLD), as well as the liver manifestations of COVID-19. The metabolic syndrome, obesity, diabetes mellitus and NAFLD were found to worsen outcome in different studies reported worldwide. Decompensated cirrhosis should be considered a risk factor for death and severe COVID-19. Recently, COVID-19 related cholangiopathy has also been reported with changes of secondary sclerosing cholangitis. The long-term persistence of viral antigens in gut epithelia raises concern regarding the future risk of autoimmune liver diseases.
Core Tip: Coronavirus disease 2019 (COVID-19) and liver involvement have been a major concern since the beginning of the COVID-19 pandemic. Deranged liver functions with raised transaminases were reported in patients with severe COVID-19. On the other hand, acute hepatitis or liver failure was uncommon. Severe acute respiratory syndrome coronavirus 2 virus associated cytokine surge, systemic inflammation, direct viral infection, drugs such as remdesivir, steroids, and lopinavir-ritonavir were the main causative factor in raised transaminases. Patients with pre-existing chronic liver diseases especially non-alcoholic fatty liver disease were found to be risk factors for increased mortality in patients with severe COVID-19.
- Citation: Gupta T, Sharma H. COVID-19 and the liver: Are footprints still there? World J Gastroenterol 2023; 29(4): 656-669
- URL: https://www.wjgnet.com/1007-9327/full/v29/i4/656.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i4.656
In December 2019, severe acute respiratory syndrome (SARS) caused by SARS- coronavirus 2 (SARS-CoV2), which belongs to the Coronaviridae family, was first detected in Wuhan, China. It soon spread to the rest of the world, and was declared a global pandemic in March 2020. In mild cases, the symptoms included fever, cough, body aches, malaise, loss of taste and smell. Approximately 15% of patients would eventually have respiratory compromise, hypoxia, and the need for invasive mechanical ventilation. Finally, multi-organ failure, coagulopathy, disseminated intravascular coagulation, acute respiratory distress syndrome, and hypoxia would follow. Over a period of more than 2 years, multiple waves of coronavirus disease 2019 (COVID-19) were observed in different geographical regions. As the virus mutated, there were many shifts in the clinical presentation. New symptoms of predominantly the upper respiratory tract such as sneezing and rhinitis, gastrointestinal symptoms such as diarrhea and non-specific abdominal pain, cardiac symptoms such as arrhythmias, ocular and neurological symptoms were reported. Additionally, as patients underwent more investigations, dysregulated coagulation and thrombosis were documented. Overall, liver involvement such as elevated liver enzymes ranged from 14% to 53% of patients in various studies. However, acute hepatitis or liver failure was uncommon. Furthermore, different studies worldwide have shown that non-alcoholic fatty liver disease (NAFLD), diabetes, hypertension, and obesity are significant risk factors for severe COVID-19.
A lockdown and a ban on air travel during the COVID-19 pandemic helped keep people with chronic illnesses at home. Their overall exposure to COVID-19 and other pathogens was constrained. Mild COVID-19 patients were isolated and quarantined in accordance with protocol and did not frequently undergo examinations. Patients with serious illnesses, however, were the only ones who underwent in-depth examinations. As a result, the majority of research mainly included individuals with serious diseases.
We searched PubMed, Google Scholar, and Google from January 2020 to August 2022, for articles written in English that describe the liver effects of COVID-19, using the search terms “coronaviruses and liver”, “COVID-19 and liver”, “COVID-19 and liver symptoms”, “COVID-19 and hepatic”, “COVID-19 and liver function test”, “COVID-19 and liver inflammation”, “SARS-CoV-2 and liver”, “COVID-19 and NAFLD”, “COVID-19 and non-alcoholic fatty liver disease”, “COVID-19 and non-alcoholic fatty liver disease”, “COVID-19 and hepatitis”, and “COVID-19 and Vaccine”. Reference lists of the articles were scanned to identify any additional studies. The title and abstract of each article were read for the initial selection and then the full-text articles were read on availability. Reference lists of the full-text articles were scanned to identify any additional studies. All types of research articles, including original research articles, reviews, case series, short communications, and case reports were considered. Of the 667 articles identified, 313 were studied for this review.
Due to a lack of significant laboratory testing and tissue biopsies from patients who were actively infected with SARS-CoV2, the mechanism of its replication is still not entirely understood.
SARS-CoV2 is an enveloped positive sense single stranded RNA virus with almost 80% identity with SARS-CoV. It has 4 structural proteins namely nucleocapsid, spike (S), membrane and enveloped proteins. The spike protein has multiple protrusions from the cell surface giving the virus its appearance and name. The angiotensin-converting enzyme 2 (ACE2) receptors are the potential site of entry for SARS-CoV2. ACE2 receptors are abundantly present on alveolar epithelium, lung, nasal epithelium etc. They are also present in fewer numbers in intestinal epithelium and liver[1]. The spike protein having two subunits S1 and S2 interacts with the ACE2 receptor for virus entry. However, ACE2 receptors are not sufficient alone and transmembrane serine proteases 2 (TMPRSS2) in addition to basic amino acid cleaving enzymes (FURIN) are essential for virus entry. According to single cell RNA sequencing analysis, hepatocytes have less co-expression of TMPRSS2 and ACE2 receptors. In the liver, cholangiocytes and sinusoidal endothelial cells have the highest expression of the ACE2 gene in almost 60% of the cell population as compared to hepatocytes (3% cells)[2,3]. Thus, a tissue or organoid model is required to understand the permissibility of liver cell types to SARS CoV-2 infection. Zhao et al[4] created human liver ductal organoids that were able to replicate SARS-CoV2 infection and expressed ACE2 and TMPRSS2. This suggests that the bile duct epithelium may be able to support pseudoparticle invasion. Despite a higher number of SARS-CoV2 virus receptors and a higher risk of infection of bile duct epithelia, COVID-19 does not follow a cholestatic pattern[5].
Studies conducted before the COVID-19 pandemic indicated that patients with hepatitis C virus (HCV)-related cirrhosis had 30 times higher ACE2 receptor expression on hepatocytes than healthy individuals[6]. The overexpression of ACE2 and TMPRSS2 has also been documented in obesity and nonalcoholic steatohepatitis patients, but not in patients with steatosis alone[7]. ACE2 is an interferon-inducible gene found in human respiratory epithelia, possibly SARS-CoV2 hepatotropism can be potentiated by the effects of systemic inflammation on hepatocytes and can lead to hepatocyte injury[8,9].
Additionally, ACE2 receptors are found in intestinal epithelia/enterocytes and SARS-CoV2 RNA has been documented by polymerase chain reaction in stool up to one week after recovery from respiratory illness. The latest data suggests that viral protein and RNA are found in intestinal biopsies for several months after resolution of respiratory illness[5,10].
In a study from Italy, postmortem wedged liver biopsy samples from 48 patients dying from severe COVID-19 were examined[11]. The results revealed vascular abnormalities such as sinusoidal and partial to complete portal venous microthromboses in almost 100% of samples. Additionally, mild portal inflammation, portal fibrosis, microvesicular and macrovesicular steatosis were documented in 66%, 60%, and 50% of patients, respectively. The latter finding is probably related to pre-existing liver disease such as NAFLD, as suggested by the presence of metabolic risk factors which were more prevalent in this patient group. Electron microscopy of these biopsies also revealed potential coronavirus-like particles, mitochondrial edema, and apoptosis of hepatocytes. However, comprehensive proteomic analysis of autopsy tissue from 19 patients with COVID-19 did not find signs of viral replication[12].
Furthermore, proteomic profiling revealed disrupted oxidative phosphorylation, fatty acid oxidation, and up-regulated immunological activators and profibrotic pathways. It is possible that hepatic steatosis, coagulative necrosis, and multi-organ dysfunction were all linked to mitochondrial dysfunction, dysregulated oxidative phosphorylation, etc[13].
Despite higher SARS-CoV2 receptor expression on cholangiocytes and SECs, liver function derangement is usually in the form of a mild elevation in liver enzymes [1-2 upper limit of normal (ULN)][14-16].
Singh et al[17] showed that the presence of pre-existing liver illness has no effect on the incidence of liver enzyme elevations, although patients with pre-existing liver disease had a higher mortality rate.
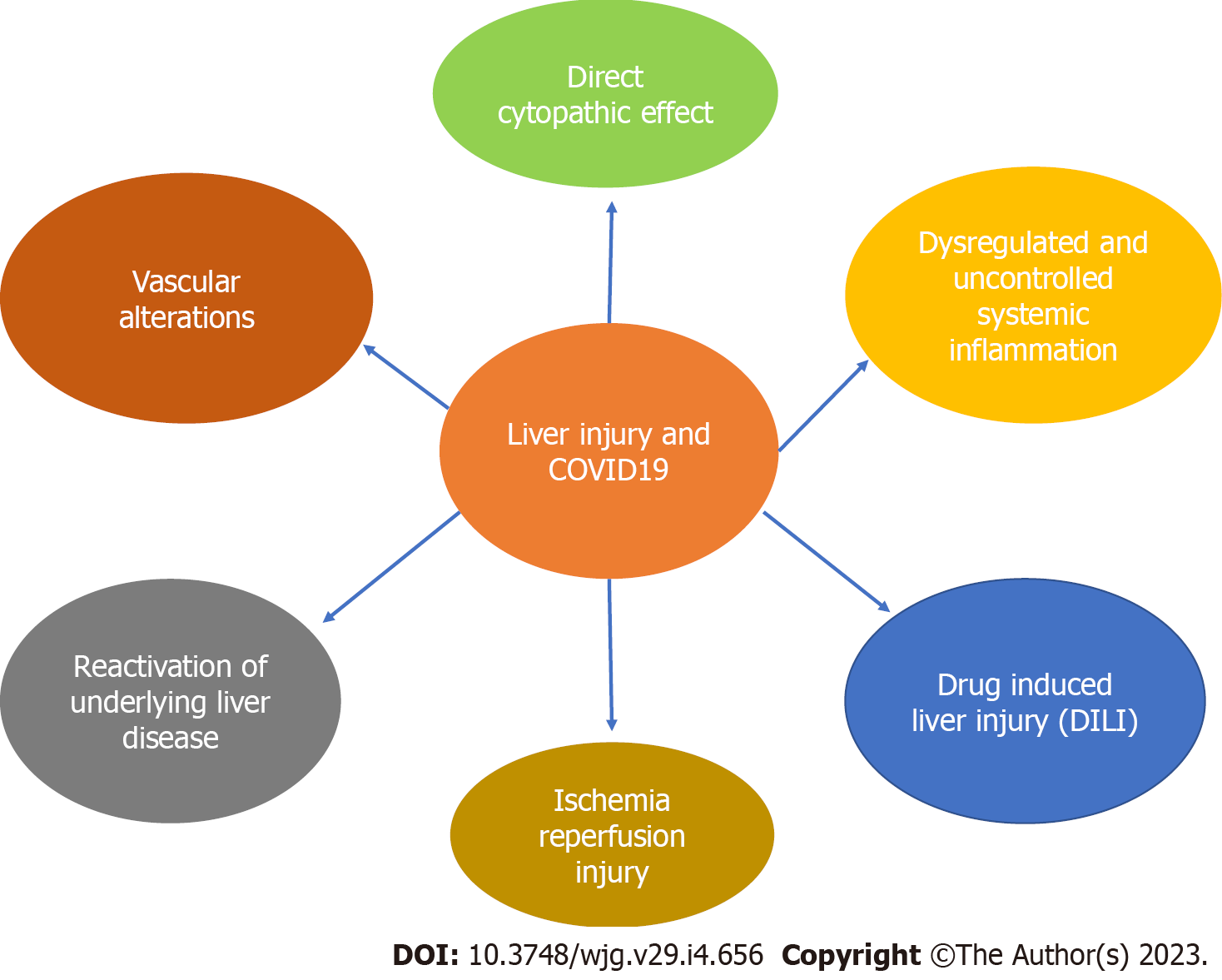
In COVID-19, SARS-CoV2 induces a systemic inflammatory response and the release of cytokines. The predominant molecules are interleukin-6 and tumor necrosis factor alpha (TNF-alpha). Elevated cytokines result in hepatocyte inflammation and injury with liver ischemia, hypoxia, worsening of already existing chronic liver disease (CLD) and/or toxicity of medications used to treat the illness (Figure 1). Hepatic congestion as well as potential direct infection of hepatocytes although uncommon may also result in the release of transaminases[18].
However, indicators of muscle breakdown or systemic inflammation did not correlate with serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels in hospitalised COVID-19 patients[19,20].
As AST level was frequently observed to surpass ALT level throughout the course of COVID-19, this was similar to patients with alcoholic liver disease, ischemic hepatitis and cirrhosis compared to a traditional hepatocellular pattern where ALT level is greater than AST[19]. Possibly, COVID-19 related mitochondrial dysfunction results in hepatic steatosis and altered hepatic perfusion is the result of sinusoidal microthrombosis[11,21-23].
Respiratory epithelia involvement by SARS-CoV2 leads to defective oxygenation and the release of cytokines causes peripheral vasodilation and reduced tissue perfusion; the resultant perfusion and oxygenation defect causes systemic hypoxia which is a contributory factor in hepatocyte injury[24].
Early in the pandemic as no definitive treatment was available, experimental therapies in the form of drugs such as tocilizumab, remdesivir and lopinavir-ritonavir were used, which are known to cause hepatic injury[25-29]. Remdesivir was documented to cause elevations in liver enzymes in different studies[30,31]. Tocilizumab was well known for its risk of hepatitis B virus (HBV) reactivation and screening of hepatitis B and hepatitis C was advised before its use.
Ponziani et al[32] and Yip et al[33] showed that elevations in liver enzymes were associated with an increased incidence of shock, ICU admissions and invasive ventilation. However, these studies could be biased as hospitalized patients with severe disease undergo intensive monitoring of liver function (which increases the chances of detecting liver injury) as compared to home isolated patients with mild disease due to quarantine.
Some studies have suggested that there is no apparent correlation between liver function derangement and mortality[34,35]. Others have suggested an increased risk of death in patients with ALT levels > ULN[16,36,37].
According to Bangash et al[38], elevated liver transaminases linked to COVID-19 are more likely caused by severity of the disease, in which the host's reaction and iatrogenic factors such as medication and invasive ventilation cause bystander liver injury and thus explain its link to mortality in a manner similar to that of sepsis[38]. Because of this, clinicians must focus more on these factors than just elevated aminotransferases especially in patients with no pre-existing liver disease.
In the early days of the COVID-19 pandemic, the hepatology community worked fast to establish the risk of SARS-CoV2 acquisition and harmful COVID-19 outcome in pre-existing CLD. According to data from major case series and population-level electronic health records during the first global spike, patients with CLD were not overrepresented, indicating that these diseases did not make patients more susceptible to infection[15,39]. In fact, a significant North American study discovered that people with cirrhosis had a decreased probability of SARS-CoV2 positivity, probably due to improved awareness, testing, and patient adherence to public health recommendations for home isolation and quarantine. However, it is now evident that individuals with cirrhosis are more likely to experience negative COVID-19 outcomes after infection, including mortality. Multiple lines of evidence, such as findings from the international registries SECURE-Cirrhosis and COVID-Hep[40], sizable observational cohorts such as the COVID-Cirrhosis-CHESS group[41], and population-level data, have all been used to support this. These registries were created early in the pandemic and interestingly, due to the emergence of the new Delta and Omicron variants as well as the introduction of vaccines, the relation between COVID-19 and liver will continue to evolve.
In a large registry cohort of 729 patients from 29 countries, it was discovered that mortality in individuals with cirrhosis after SARS-CoV2 infection was 32% overall, with case fatality increasing gradually with each Child-Pugh (CP) class (CLD without cirrhosis: 8%, CP-A: 19%, CP-B: 35%, CP-C: 51%)[42]. The rates of invasive mechanical ventilation, renal replacement treatment, and intensive care unit (ICU) hospitalisation all showed similar stepwise trajectories. Additionally, after adjusting for age and comorbidities, patients with decompensated cirrhosis (CP-B and CP-C) had a considerably higher probability of dying than patients without cirrhosis who tested positive for SARS-CoV2. Reports of elevated COVID-19 mortality in cirrhosis have been confirmed in two Asian-only registries[43] and in numerous multicenter cohort studies conducted in various geographic locations[44-46]. Iavarone et al[44] observed a 30-d mortality of 30% in Northern Italy during the early stages of the pandemic, which was much greater than a historical cohort of patients with cirrhosis hospitalised with bacterial infection[44]. Decompensated cirrhosis was also reported as an independent risk factor of death in CLD patients across 21 North American institutions[45]. Additionally, individuals with hepatocellular carcinoma (HCC) had a seven-fold higher chance of dying from COVID-19 than cirrhotic patients without HCC, indicating that this population may be particularly vulnerable to the side effects of SARS-CoV2 infection. A retrospective French cohort of > 259000 COVID-19 inpatients, including > 15000 with pre-existing CLD, showed that patients with decompensated cirrhosis had a higher adjusted risk of COVID-19 mortality[47]. This was in contrast to the findings in a nationwide Swedish cohort, which failed to identify a connection between cirrhosis and COVID-19 related mortality[48]. Cirrhosis overall, and decompensated cirrhosis in particular, should be considered a risk factor for death and severe COVID-19.
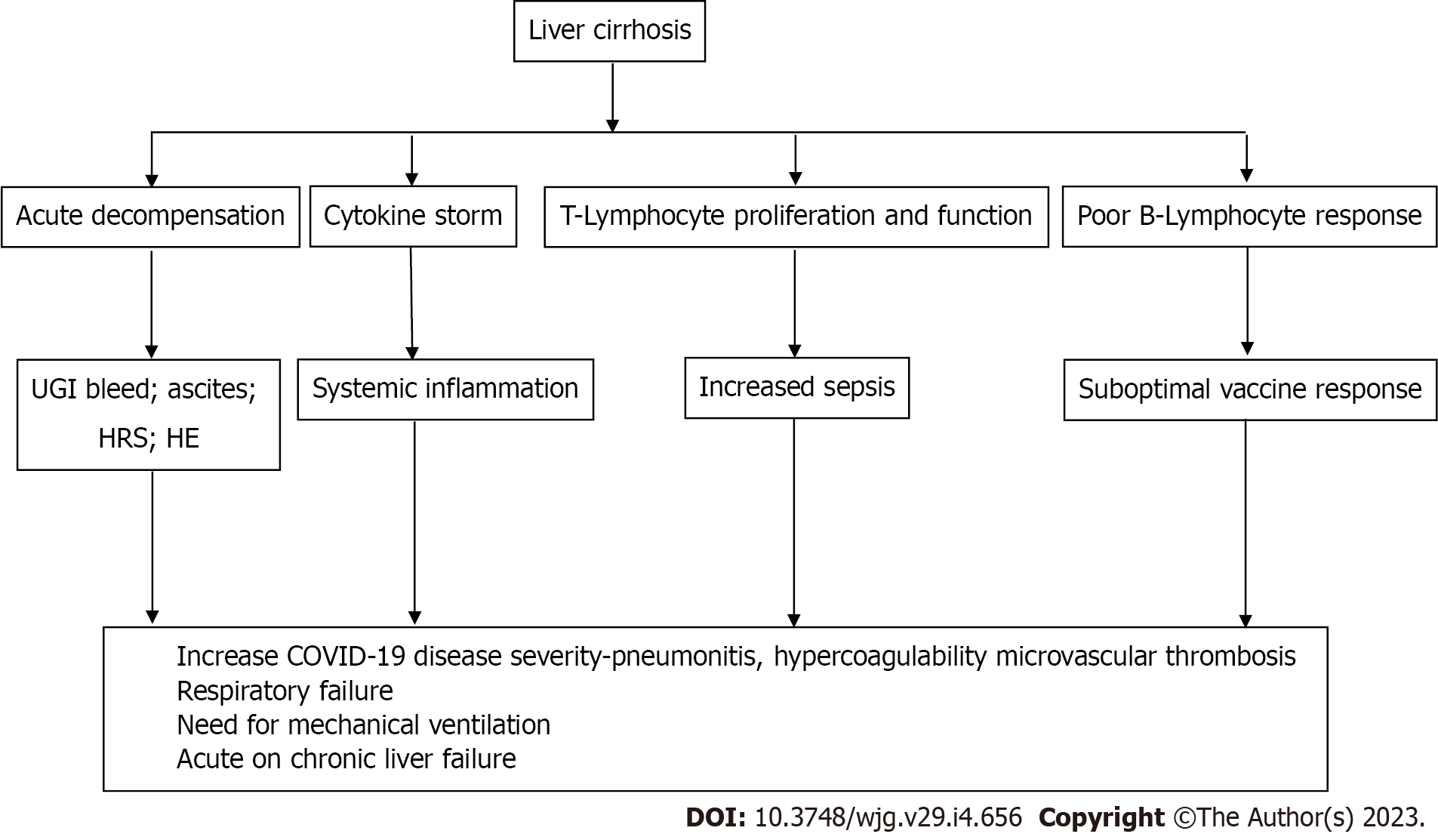
There are various characteristics related to the clinical course of COVID-19 in cirrhotic individuals. First, up to 46% of patients can present with acute hepatic decompensation, usually with new or worsening ascites and/or hepatic encephalopathy (HE)[42]. This can occur between 20% and 58% of the time even in the absence of the usual COVID-19 respiratory symptoms[42,44]. Patients with CLD present with gastrointestinal symptoms more frequently than matched controls[42]. This is linked to a more severe disease trajectory[45], a phenomenon that is widespread in society[49] and is connected to increased intestinal permeability, electrolyte imbalance, and systemic inflammatory load, and is documented in up to 12% to 50%[42-44,46] of patients with COVID-19 and decompensated cirrhosis. In the context of COVID-19, a number of well-known prognostic scoring models have been used to assess cirrhosis, with the CLIF-C ACLF score and CLIF organ failure scores surpassing Model for End-stage Liver Disease, North American Consortium for the Study of End-stage Liver Disease, and CP scores in the international and Latin American cohorts, respectively[42,50]. Actually, the likelihood of recovery rapidly decreases as organ support requirements increase. For instance, patients with CP-C cirrhosis have a mere 21% probability of surviving if admitted to the ICU, and decreases to 10% if mechanical breathing is necessary[42].
Despite the fact that SARS-CoV2 infection causes immediate hepatic decompensation, respiratory failure (71%) and problems related to the liver (19%) are the primary causes of death in individuals with cirrhosis[42]. Hepatic dysfunction and lung damage are likely linked by a number of overlapping pathways (Figure 2), including immunological dysfunction brought on by cirrhosis, coagulopathy, and altered pulmonary dynamics due to ascites and HE[51]. Given that the composition of the gut microbiota has been demonstrated to influence how the host immune system reacts to COVID-19, it is conceivable that intestinal permeability and dysbiosis linked to cirrhosis may also have a negative effect[52,53].
Although COVID-19 in patients with cirrhosis is linked to a significant immediate risk of death, rates of mortality and re-admission at 90 d appear equivalent to patients with cirrhosis alone in those who survive the initial shock[54]. Therefore, it appears that SARS-CoV2 infection does not accelerate the progression of liver disease beyond the course of cirrhosis after the acute infective period. However, up to 4 mo after recovering from acute COVID-19, hepatic MRI alterations, including enhanced T1 signaling, raised fat fraction, and hepatomegaly, have been found in 10% to 28% of otherwise healthy people[55,56]. In both patients with and without underlying CLD, it is unknown what these radiological characteristics following COVID-19 mean clinically long-term. Furthermore, although this remains unexplored and is not considered in the current investigations, these hepatic abnormalities might not be exclusive to COVID-19 and might also be present in individuals recovering from other severe systemic insults.
It is crucial to note that studies undertaken in the years before COVID-19 vaccination and the appearance of viral variants like Delta and Omicron are largely responsible for our knowledge of the disease course in individuals with COVID-19 and cirrhosis. CLD can affect 1% to 11% of people with SARS-CoV2 infection[57]. Numerous liver cirrhosis patients have been shown to have drunk alcohol in an ineffective effort to ward off coronavirus infection, raising the risk of alcoholic hepatitis[58].
Implications of COVID-19 include increased mortality associated with severe COVID-19, increased risk of hepatic decompensation, and decreased routine and HCC surveillance.
Although the acute mortality associated with COVID-19 in patients with cirrhosis is substantial, the rates of death and readmission at 90 d are equivalent to those in patients with cirrhosis alone in those who survived the initial insult[54]. Therefore, SARS-CoV2 infection does not appear to accelerate the course of liver disease beyond the typical history of cirrhosis after the acute infective period.
It is well known that infections put people at risk of decompensation (worsening ascites, encephalopathy, or acute kidney injury), and in the case of COVID-19, which is characterized by significant cytokine activation, cytokine-induced hepatocyte apoptosis and necrosis in the presence of decreased liver reserve may result in hepatic decompensation. To rule out COVID-19 as a possible cause, patients with cirrhosis who exhibit decompensation should be evaluated.
As there are many etiologies (part of a systemic illness, immune mediated, direct SARS-CoV-2 infection, viral hepatitis, drug-induced, and ischemic hepatic injury) which can cause derangement of liver function tests, one of which is chronic HBV and HCV infection, it is always important to identify these underlying infections[59,60].
Prednisolone and tocilizumab have been used in the treatment of COVID-19, which are known to increase the likelihood of HBV reactivation and flare-up alongside HCV flare-up. When starting COVID-19-related therapy in those with advanced liver disease brought on by HBV and HCV, care must be taken[59,60]. Although the risk/benefit of an intervention is likely to weigh strongly when dealing with COVID-19, established criteria in such cases need to be followed to limit the risk of hepatic decompensation (Table 1).
| Ref. | Country | Study design | Study population | Sample size | Outcome |
| HBV | |||||
| Anugwom et al[70], 2021 | China | Letter | Peer reviewed articles with confirmed COVID-19 and HBV information | 2054; HBV | Inverse relation of HBV with COVID-19 |
| Kang et al[71], 2021 | Korea | Retrospective, nationwide case-control study | Korean National Health Insurance Service COVID database | 7723; HBV | Underlying chronic hepatitis B with COVID-19 severity (adjusted OR 0.65; 95%CI: 0.57-0.74) |
| HCV | |||||
| Richardson et al[15], 2020 | United States | Case series | With confirmed COVID-19 and information on HCV infection | 5700 | HCV infections in < 0.1% (n = 3) of COVID-19 patients |
| Ronderos et al[72], 2021 | United States | Retrospective single-center | With confirmed COVID-19 and information on HCV infection | 1193; HCV | HCV infection predictor of in hospital mortality |
| NAFLD | |||||
| Ji et al[62], 2020 | China | Retrospective | With confirmed COVID-19 and information on NAFLD status | 202; NAFLD (n = 76) | HSI with disease progression (OR 6.4; 95%CI: 1.5-31.2) |
| Targher et al[64], 2020 | China | Prospective observational | Laboratory confirmed COVID-19 | 310; NAFLD (n = 94) | FIB-4 (adjusted OR 1.90, 95%CI: 1.33 to 2.72) or NFS (adjusted OR 2.57, 95%CI: 1.73 to 3.82) with COVID-19 severity |
| Lopez-Mendez et al[65], 2021 | Mexico | Retrospective | Medical records of hospitalized COVID-19 | 155; liver fibrosis (n = 69) | FIB-4 with risk of ICU admission (OR 1.74, 95%CI: 1.74-2.68; P = 0.023); mortality (OR 6.45, 95%CI: 2.01-20.83, P = 0.002) |
| Sachdeva et al[73], 2020 | India | Systemic review | - | 8142; NAFLD (n = 833) | Pooled adjusted 2.358 (95%CI: 1.902-2.923) with severity of COVID-19 |
| Mahamid et al[74], 2021 | Israel | Retrospective case-control | Medical records of COVID-19 | 71; NAFLD (n = 22) | OR 3.57 (95%CI: 1.22-14.48) with severity of disease |
| Hashemi et al[75], 2020 | United States | Multicentre retrospective | Laboratory confirmed COVID-19 | 363; NAFLD (n = 55) | aOR 2.30 (95%CI: 1.27-4.17) with ICU admission |
| Yao et al[76], 2021 | China | Retrospective | Laboratory confirmed COVID-19 | 86; NAFLD (n = 38) | OR 11.057 (95%CI: 1.193-102.439, P = 0.034) with severe COVID-19 |
| Li et al[77], 2022 | China and United States | Observational; 2-sample Mendelian randomization | Laboratory confirmed COVID-19 | 8267; NAFLD (n = 136) | OR 0.97 (95%CI: 0.88-1.08, P = 0.61) with COVID-19 |
| BCS | |||||
| Espinoza et al[78], 2021 | Brazil | Case report | Laboratory confirmed COVID-19 | - | Thrombosis of an abdominal vessel should be considered as a differential diagnosis in patients with undefined abdominal pain and elevated liver biochemical tests |
| Sh Hassan et al[79], 2021 | Saudi Arabia | Case report | Laboratory confirmed COVID-19 | - | Thromboembolic events could be the first manifestation of COVID-19 |
Risk factors in the general population for COVID-19 morbidity and mortality include advancing age, obesity, and diabetes[6]. With regard to how NAFLD affects the course of COVID-19, significant differences have been found in various studies. These differences may be attributable to problems in distinguishing the impact of NAFLD from other metabolic comorbidities due to the confounding effect of viral-induced steatosis or due to different diagnostic criteria. The latter point is especially crucial as the hepatology community at large struggles with the proposed classification modifications from NAFLD to metabolic dysfunction-associated liver disease[39]. Studies have shown that obesity is associated with increased severity and mortality in COVID-19. On the other hand, obese patients have a higher prevalence of diabetes, NAFLD, dyslipidemia, hypertension and metabolic syndrome. In a retrospective series of 202 patients with SARS-CoV2 infection, NAFLD was identified as a risk factor for progressive COVID-19, abnormal liver enzyme levels, and extended viral shedding times[61]. A study of 327 participants revealed an association between NAFLD and the likelihood of severe COVID-19 in people under 60 years of age[62]. Similar to this, MRI results from 287 SARS-CoV2 patients (79 positive, 208 negative) showed that obese patients with a concurrent liver fat fraction of less than 10% were three times more likely to develop symptoms of laboratory-confirmed COVID-19 (available as a non-peer-reviewed Preprint only)[63].
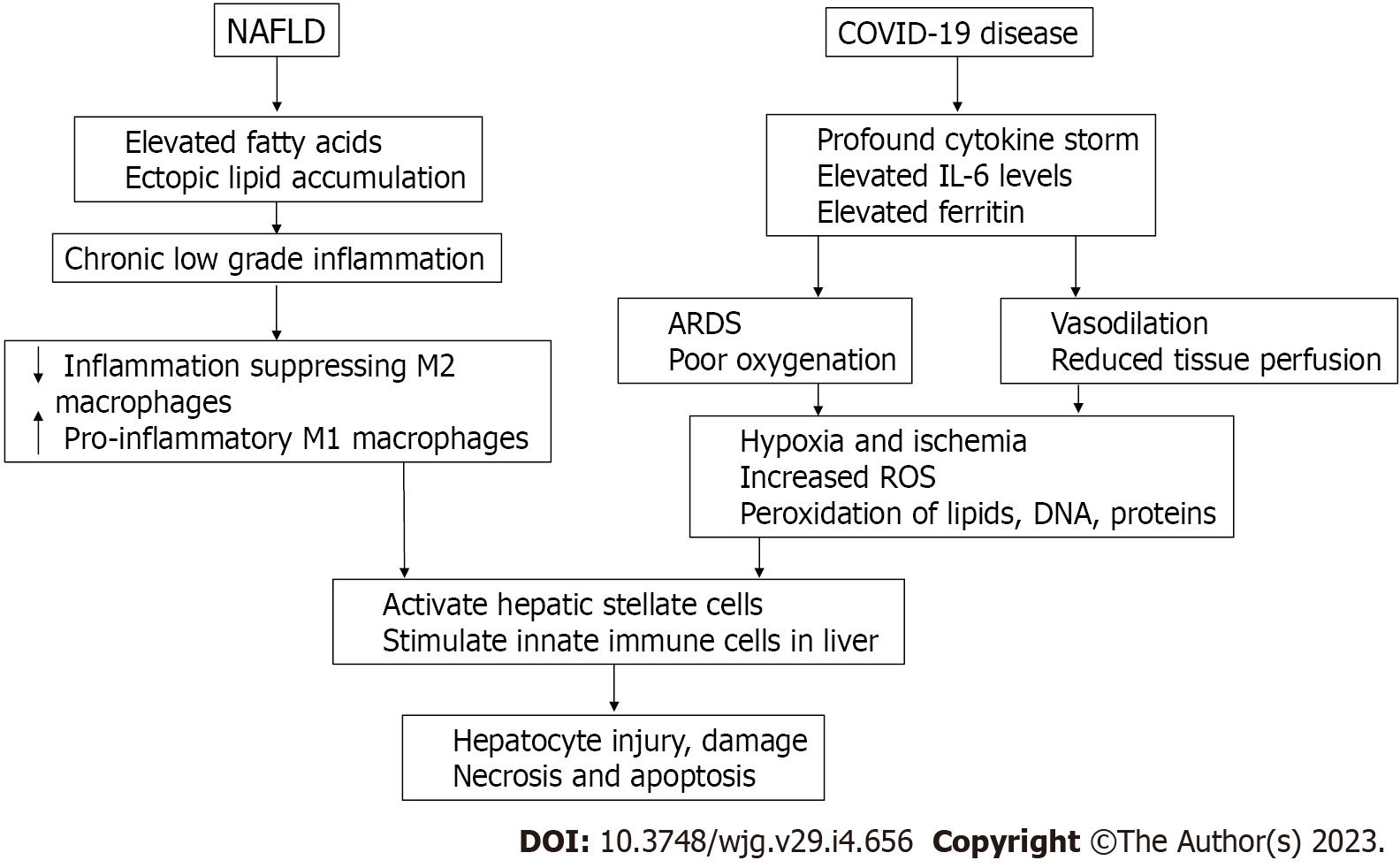
The chronic low-grade inflammation in NAFLD shifts macrophages from M2 to M1 phenotype and causes activation of hepatic stellate cells and the innate immune system which in collaboration with profound systemic inflammation in COVID-19 leads to hepatocyte injury, necrosis, and apoptosis (Figure 3).
Targher et al[64] reported high fibrosis-4 and NAFLD fibrosis scores with increased COVID-19 severity. Similarly, Lopez-Mendez et al[65] showed steatosis and fibrosis to be linked to increased ICU admissions. However, due to the constraints of isolation, quarantine and adequate manpower, there was a lack of detailed history and tissue histology; therefore, we do not have comparative studies of liver steatosis, steatohepatitis and fibrosis in relation to COVID-19 severity. The COVID-19 pandemic severely affected hepatology services in terms of early diagnosis, surveillance programs, implementation of hepatitis B and C eradication programs, etc (Table 2).
| Decrease | Increase |
| OPD follow-up and care | Inhospital admission |
| HBV treatment | Alcohol intake |
| HCV community level programs | HCC incidence |
| HCC surveillance and screening | Acute on chronic liver failure |
| UGI endoscopy | Gastrointestinal bleeding especially variceal bleeding |
| Liver transplantation | Unhealthy lifestyle |
| NAFLD/MAFLD |
Very little is known regarding the results of COVID-19 in individuals with autoimmune hepatitis (AIH), a rare form of CLD. The study by Marjot et al[66] in October 2020, included more than 1700 participants, and aimed to describe the course of COVID-19 and risk of unfavorable outcomes in 70 individuals with AIH. It was shown that despite the potential reporting of individuals with more severe liver disease, AIH does not significantly increase susceptibility to negative outcomes following SARS-CoV2 infection after several comparisons of non-AIH CLD and non-CLD cohorts. In contrast to the use of immunosuppressive agents, for which no adverse effects were found, age and the severity of baseline liver disease continue to be the most significant drivers of outcome in this patient group[45]. This should reassure patients and medical professionals, and support suggestions that immunosuppressive agents should not be frequently changed or stopped during COVID-19.
There are few case reports of secondary sclerosing cholangitis in patients with severe COVID-19 and histologic changes due to cholangiocyte injury and cholangiopathy. These patients had a protracted course and significant liver-related morbidity. Essentially, this condition was noted after recovery of COVID-19; therefore, it was called post COVID-19 cholangiopathy[67].
Recently, long-term sequelae of COVID-19 have been identified with symptoms of fatigue, insomnia, body ache and cognitive dysfunction. Persistence of viral antigens in gut epithelia have been documented[68]. It is possible that these persistent antigens cause immune dysfunction and low-grade persistent inflammation which manifests in various ways. It could be a basis for immune perturbation in post COVID-19. Its effect on liver in the post COVID era will be an area for research.
The effects of mRNA vaccines for COVID-19 prevention have been implicated in the causation of “immune mediated hepatitis” due to the production of antibodies against the spike protein of SARS-CoV2 virus[69]. It will be interesting in the near future to detect autoimmune hepatitis or immune mediated hepatitis prevalence in the community.
During COVID-19, liver enzymes may be mildly elevated and generally recover without treatment. The presence of NAFLD has been linked to increased COVID-19 severity and ICU admissions. Different studies have shown the variable impact of NAFLD on COVID-19 related mortality. In patients with chronic hepatitis B and hepatitis C, a mild COVID-19 course is well tolerated, whereas in moderate-severe COVID-19 requiring steroids and/or tocilizumab, the risk of viral flare and worsening of liver disease is present. Patients with compensated cirrhosis are at increased risk of decompensation after COVID-19. In decompensated cirrhosis, the trajectory of COVID-19 severity and mortality rises with worsening Child-Pugh scores. With emerging evidence of persistent gut viral antigens capable of stimulating the immune system, we should be vigilant for postacute COVID-19 syndrome.
I am thankful to Dr. Sandeep Goyal PhD for his assistance in manuscript writing and framing.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Association for the Study of Liver Diseases, No. 226223; Indian National Association for the Study of Liver Diseases, No. 1310.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Marchesini G, Italy; Prikhodko V, Russia S-Editor: Chen YL L-Editor: Webster JR P-Editor: Chen YL
| 1. | Beyerstedt S, Casaro EB, Rangel ÉB. COVID-19: angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur J Clin Microbiol Infect Dis. 2021;40:905-919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 601] [Cited by in RCA: 497] [Article Influence: 124.3] [Reference Citation Analysis (0)] |
| 2. | Pirola CJ, Sookoian S. SARS-CoV-2 virus and liver expression of host receptors: Putative mechanisms of liver involvement in COVID-19. Liver Int. 2020;40:2038-2040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 3. | Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3643] [Cited by in RCA: 4149] [Article Influence: 197.6] [Reference Citation Analysis (0)] |
| 4. | Zhao B, Ni C, Gao R, Wang Y, Yang L, Wei J, Lv T, Liang J, Zhang Q, Xu W, Xie Y, Wang X, Yuan Z, Zhang R, Lin X. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell. 2020;11:771-775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 312] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 5. | Gaebler C, Wang Z, Lorenzi JCC, Muecksch F, Finkin S, Tokuyama M, Cho A, Jankovic M, Schaefer-Babajew D, Oliveira TY, Cipolla M, Viant C, Barnes CO, Bram Y, Breton G, Hägglöf T, Mendoza P, Hurley A, Turroja M, Gordon K, Millard KG, Ramos V, Schmidt F, Weisblum Y, Jha D, Tankelevich M, Martinez-Delgado G, Yee J, Patel R, Dizon J, Unson-O'Brien C, Shimeliovich I, Robbiani DF, Zhao Z, Gazumyan A, Schwartz RE, Hatziioannou T, Bjorkman PJ, Mehandru S, Bieniasz PD, Caskey M, Nussenzweig MC. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591:639-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 972] [Cited by in RCA: 1249] [Article Influence: 312.3] [Reference Citation Analysis (0)] |
| 6. | Paizis G, Tikellis C, Cooper ME, Schembri JM, Lew RA, Smith AI, Shaw T, Warner FJ, Zuilli A, Burrell LM, Angus PW. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut. 2005;54:1790-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 281] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 7. | Fondevila MF, Mercado-Gómez M, Rodríguez A, Gonzalez-Rellan MJ, Iruzubieta P, Valentí V, Escalada J, Schwaninger M, Prevot V, Dieguez C, Crespo J, Frühbeck G, Martinez-Chantar ML, Nogueiras R. Obese patients with NASH have increased hepatic expression of SARS-CoV-2 critical entry points. J Hepatol. 2021;74:469-471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 8. | Chua RL, Lukassen S, Trump S, Hennig BP, Wendisch D, Pott F, Debnath O, Thürmann L, Kurth F, Völker MT, Kazmierski J, Timmermann B, Twardziok S, Schneider S, Machleidt F, Müller-Redetzky H, Maier M, Krannich A, Schmidt S, Balzer F, Liebig J, Loske J, Suttorp N, Eils J, Ishaque N, Liebert UG, von Kalle C, Hocke A, Witzenrath M, Goffinet C, Drosten C, Laudi S, Lehmann I, Conrad C, Sander LE, Eils R. COVID-19 severity correlates with airway epithelium-immune cell interactions identified by single-cell analysis. Nat Biotechnol. 2020;38:970-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 795] [Article Influence: 159.0] [Reference Citation Analysis (0)] |
| 9. | Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, Cao Y, Yousif AS, Bals J, Hauser BM, Feldman J, Muus C, Wadsworth MH 2nd, Kazer SW, Hughes TK, Doran B, Gatter GJ, Vukovic M, Taliaferro F, Mead BE, Guo Z, Wang JP, Gras D, Plaisant M, Ansari M, Angelidis I, Adler H, Sucre JMS, Taylor CJ, Lin B, Waghray A, Mitsialis V, Dwyer DF, Buchheit KM, Boyce JA, Barrett NA, Laidlaw TM, Carroll SL, Colonna L, Tkachev V, Peterson CW, Yu A, Zheng HB, Gideon HP, Winchell CG, Lin PL, Bingle CD, Snapper SB, Kropski JA, Theis FJ, Schiller HB, Zaragosi LE, Barbry P, Leslie A, Kiem HP, Flynn JL, Fortune SM, Berger B, Finberg RW, Kean LS, Garber M, Schmidt AG, Lingwood D, Shalek AK, Ordovas-Montanes J; HCA Lung Biological Network. Electronic address: lung-network@humancellatlas.org; HCA Lung Biological Network. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell. 2020;181:1016-1035.e19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1890] [Cited by in RCA: 1795] [Article Influence: 359.0] [Reference Citation Analysis (0)] |
| 10. | Zuo T, Liu Q, Zhang F, Lui GC, Tso EY, Yeoh YK, Chen Z, Boon SS, Chan FK, Chan PK, Ng SC. Depicting SARS-CoV-2 faecal viral activity in association with gut microbiota composition in patients with COVID-19. Gut. 2021;70:276-284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 263] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 11. | Sonzogni A, Previtali G, Seghezzi M, Grazia Alessio M, Gianatti A, Licini L, Morotti D, Zerbi P, Carsana L, Rossi R, Lauri E, Pellegrinelli A, Nebuloni M. Liver histopathology in severe COVID 19 respiratory failure is suggestive of vascular alterations. Liver Int. 2020;40:2110-2116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 218] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 12. | Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, Li X, Xu P, Zhang L, Zhao L, Cao Y, Kang J, Yang J, Li L, Liu X, Li Y, Nie R, Mu J, Lu F, Zhao S, Lu J, Zhao J. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 457] [Article Influence: 91.4] [Reference Citation Analysis (0)] |
| 13. | Nie X, Qian L, Sun R, Huang B, Dong X, Xiao Q, Zhang Q, Lu T, Yue L, Chen S, Li X, Sun Y, Li L, Xu L, Li Y, Yang M, Xue Z, Liang S, Ding X, Yuan C, Peng L, Liu W, Yi X, Lyu M, Xiao G, Xu X, Ge W, He J, Fan J, Wu J, Luo M, Chang X, Pan H, Cai X, Zhou J, Yu J, Gao H, Xie M, Wang S, Ruan G, Chen H, Su H, Mei H, Luo D, Zhao D, Xu F, Zhu Y, Xia J, Hu Y, Guo T. Multi-organ proteomic landscape of COVID-19 autopsies. Cell. 2021;184:775-791.e14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 358] [Cited by in RCA: 314] [Article Influence: 78.5] [Reference Citation Analysis (0)] |
| 14. | Sultan S, Altayar O, Siddique SM, Davitkov P, Feuerstein JD, Lim JK, Falck-Ytter Y, El-Serag HB; AGA Institute. Electronic address: ewilson@gastro.org. AGA Institute Rapid Review of the Gastrointestinal and Liver Manifestations of COVID-19, Meta-Analysis of International Data, and Recommendations for the Consultative Management of Patients with COVID-19. Gastroenterology. 2020;159:320-334.e27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 309] [Cited by in RCA: 293] [Article Influence: 58.6] [Reference Citation Analysis (1)] |
| 15. | Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW; the Northwell COVID-19 Research Consortium, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6024] [Cited by in RCA: 6518] [Article Influence: 1303.6] [Reference Citation Analysis (0)] |
| 16. | Phipps MM, Barraza LH, LaSota ED, Sobieszczyk ME, Pereira MR, Zheng EX, Fox AN, Zucker J, Verna EC. Acute Liver Injury in COVID-19: Prevalence and Association with Clinical Outcomes in a Large U.S. Cohort. Hepatology. 2020;72:807-817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 272] [Article Influence: 54.4] [Reference Citation Analysis (2)] |
| 17. | Singh S, Khan A. Clinical Characteristics and Outcomes of Coronavirus Disease 2019 Among Patients With Preexisting Liver Disease in the United States: A Multicenter Research Network Study. Gastroenterology. 2020;159:768-771.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 270] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 18. | Jothimani D, Venugopal R, Abedin MF, Kaliamoorthy I, Rela M. COVID-19 and the liver. J Hepatol. 2020;73:1231-1240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 364] [Cited by in RCA: 358] [Article Influence: 71.6] [Reference Citation Analysis (1)] |
| 19. | Bloom PP, Meyerowitz EA, Reinus Z, Daidone M, Gustafson J, Kim AY, Schaefer E, Chung RT. Liver Biochemistries in Hospitalized Patients With COVID-19. Hepatology. 2021;73:890-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 143] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 20. | Buckholz AP, Kaplan A, Rosenblatt RE, Wan D. Clinical Characteristics, Diagnosis, and Outcomes of 6 Patients With COVID-19 Infection and Rhabdomyolysis. Mayo Clin Proc. 2020;95:2557-2559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, White KM, O'Meara MJ, Rezelj VV, Guo JZ, Swaney DL, Tummino TA, Hüttenhain R, Kaake RM, Richards AL, Tutuncuoglu B, Foussard H, Batra J, Haas K, Modak M, Kim M, Haas P, Polacco BJ, Braberg H, Fabius JM, Eckhardt M, Soucheray M, Bennett MJ, Cakir M, McGregor MJ, Li Q, Meyer B, Roesch F, Vallet T, Mac Kain A, Miorin L, Moreno E, Naing ZZC, Zhou Y, Peng S, Shi Y, Zhang Z, Shen W, Kirby IT, Melnyk JE, Chorba JS, Lou K, Dai SA, Barrio-Hernandez I, Memon D, Hernandez-Armenta C, Lyu J, Mathy CJP, Perica T, Pilla KB, Ganesan SJ, Saltzberg DJ, Rakesh R, Liu X, Rosenthal SB, Calviello L, Venkataramanan S, Liboy-Lugo J, Lin Y, Huang XP, Liu Y, Wankowicz SA, Bohn M, Safari M, Ugur FS, Koh C, Savar NS, Tran QD, Shengjuler D, Fletcher SJ, O'Neal MC, Cai Y, Chang JCJ, Broadhurst DJ, Klippsten S, Sharp PP, Wenzell NA, Kuzuoglu-Ozturk D, Wang HY, Trenker R, Young JM, Cavero DA, Hiatt J, Roth TL, Rathore U, Subramanian A, Noack J, Hubert M, Stroud RM, Frankel AD, Rosenberg OS, Verba KA, Agard DA, Ott M, Emerman M, Jura N, von Zastrow M, Verdin E, Ashworth A, Schwartz O, d'Enfert C, Mukherjee S, Jacobson M, Malik HS, Fujimori DG, Ideker T, Craik CS, Floor SN, Fraser JS, Gross JD, Sali A, Roth BL, Ruggero D, Taunton J, Kortemme T, Beltrao P, Vignuzzi M, García-Sastre A, Shokat KM, Shoichet BK, Krogan NJ. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459-468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3145] [Cited by in RCA: 3188] [Article Influence: 637.6] [Reference Citation Analysis (0)] |
| 22. | Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, Chen H, Ding X, Zhao H, Zhang H, Wang C, Zhao J, Sun X, Tian R, Wu W, Wu D, Ma J, Chen Y, Zhang D, Xie J, Yan X, Zhou X, Liu Z, Wang J, Du B, Qin Y, Gao P, Qin X, Xu Y, Zhang W, Li T, Zhang F, Zhao Y, Li Y. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. N Engl J Med. 2020;382:e38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1532] [Cited by in RCA: 1606] [Article Influence: 321.2] [Reference Citation Analysis (0)] |
| 23. | Díaz LA, Idalsoaga F, Cannistra M, Candia R, Cabrera D, Barrera F, Soza A, Graham R, Riquelme A, Arrese M, Leise MD, Arab JP. High prevalence of hepatic steatosis and vascular thrombosis in COVID-19: A systematic review and meta-analysis of autopsy data. World J Gastroenterol. 2020;26:7693-7706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (1)] |
| 24. | Papic N, Pangercic A, Vargovic M, Barsic B, Vince A, Kuzman I. Liver involvement during influenza infection: perspective on the 2009 influenza pandemic. Influenza Other Respir Viruses. 2012;6:e2-e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 25. | Olry A, Meunier L, Délire B, Larrey D, Horsmans Y, Le Louët H. Drug-Induced Liver Injury and COVID-19 Infection: The Rules Remain the Same. Drug Saf. 2020;43:615-617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 26. | Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. 2020;382:1787-1799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3386] [Cited by in RCA: 3627] [Article Influence: 725.4] [Reference Citation Analysis (0)] |
| 27. | Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, Zhou G, Gou J, Qu J, Sun Y, Liu Y, He Q, Chen J, Liu L, Xu L. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73:566-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 623] [Cited by in RCA: 661] [Article Influence: 132.2] [Reference Citation Analysis (0)] |
| 28. | Muhović D, Bojović J, Bulatović A, Vukčević B, Ratković M, Lazović R, Smolović B. First case of drug-induced liver injury associated with the use of tocilizumab in a patient with COVID-19. Liver Int. 2020;40:1901-1905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 29. | Mahamid M, Mader R, Safadi R. Hepatotoxicity of tocilizumab and anakinra in rheumatoid arthritis: management decisions. Clin Pharmacol. 2011;3:39-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh MD, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC; ACTT-1 Study Group Members. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020;383:1813-1826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5711] [Cited by in RCA: 5119] [Article Influence: 1023.8] [Reference Citation Analysis (0)] |
| 31. | Montastruc F, Thuriot S, Durrieu G. Hepatic Disorders With the Use of Remdesivir for Coronavirus 2019. Clin Gastroenterol Hepatol. 2020;18:2835-2836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 32. | Ponziani FR, Del Zompo F, Nesci A, Santopaolo F, Ianiro G, Pompili M, Gasbarrini A; “Gemelli against COVID-19” group. Liver involvement is not associated with mortality: results from a large cohort of SARS-CoV-2-positive patients. Aliment Pharmacol Ther. 2020;52:1060-1068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 33. | Yip TC, Lui GC, Wong VW, Chow VC, Ho TH, Li TC, Tse YK, Hui DS, Chan HL, Wong GL. Liver injury is independently associated with adverse clinical outcomes in patients with COVID-19. Gut. 2021;70:733-742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 123] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 34. | Weber S, Hellmuth JC, Scherer C, Muenchhoff M, Mayerle J, Gerbes AL. Liver function test abnormalities at hospital admission are associated with severe course of SARS-CoV-2 infection: a prospective cohort study. Gut. 2021;70:1925-1932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 35. | Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020;40:2095-2103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 310] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 36. | Yadav DK, Singh A, Zhang Q, Bai X, Zhang W, Yadav RK, Zhiwei L, Adhikari VP, Liang T. Involvement of liver in COVID-19: systematic review and meta-analysis. Gut. 2021;70:807-809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 37. | Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, Zhang XJ, Cai J, Lin L, Ouyang S, Wang X, Yang C, Cheng X, Liu W, Li H, Xie J, Wu B, Luo H, Xiao F, Chen J, Tao L, Cheng G, She ZG, Zhou J, Wang H, Lin J, Luo P, Fu S, Ye P, Xiao B, Mao W, Liu L, Yan Y, Chen G, Huang X, Zhang BH, Yuan Y. Longitudinal Association Between Markers of Liver Injury and Mortality in COVID-19 in China. Hepatology. 2020;72:389-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 309] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 38. | Bangash MN, Patel JM, Parekh D, Murphy N, Brown RM, Elsharkawy AM, Mehta G, Armstrong MJ, Neil D. SARS-CoV-2: Is the liver merely a bystander to severe disease? J Hepatol. 2020;73:995-996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 39. | Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, Cockburn J, McDonald HI, MacKenna B, Tomlinson L, Douglas IJ, Rentsch CT, Mathur R, Wong AYS, Grieve R, Harrison D, Forbes H, Schultze A, Croker R, Parry J, Hester F, Harper S, Perera R, Evans SJW, Smeeth L, Goldacre B. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4343] [Cited by in RCA: 4209] [Article Influence: 841.8] [Reference Citation Analysis (0)] |
| 40. | Moon AM, Webb GJ, Aloman C, Armstrong MJ, Cargill T, Dhanasekaran R, Genescà J, Gill US, James TW, Jones PD, Marshall A, Mells G, Perumalswami PV, Qi X, Su F, Ufere NN, Barnes E, Barritt AS, Marjot T. High mortality rates for SARS-CoV-2 infection in patients with pre-existing chronic liver disease and cirrhosis: Preliminary results from an international registry. J Hepatol. 2020;73:705-708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 203] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 41. | Qi X, Liu Y, Wang J, Fallowfield JA, Li X, Shi J, Pan H, Zou S, Zhang H, Chen Z, Li F, Luo Y, Mei M, Liu H, Wang Z, Li J, Yang H, Xiang H, Liu T, Zheng MH, Liu C, Huang Y, Xu D, Kang N, He Q, Gu Y, Zhang G, Shao C, Liu D, Zhang L, Kawada N, Jiang Z, Wang F, Xiong B, Takehara T, Rockey DC; COVID-Cirrhosis-CHESS Group. Clinical course and risk factors for mortality of COVID-19 patients with pre-existing cirrhosis: a multicentre cohort study. Gut. 2021;70:433-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (1)] |
| 42. | Marjot T, Moon AM, Cook JA, Abd-Elsalam S, Aloman C, Armstrong MJ, Pose E, Brenner EJ, Cargill T, Catana MA, Dhanasekaran R, Eshraghian A, García-Juárez I, Gill US, Jones PD, Kennedy J, Marshall A, Matthews C, Mells G, Mercer C, Perumalswami PV, Avitabile E, Qi X, Su F, Ufere NN, Wong YJ, Zheng MH, Barnes E, Barritt AS 4th, Webb GJ. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J Hepatol. 2021;74:567-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 384] [Article Influence: 96.0] [Reference Citation Analysis (0)] |
| 43. | Sarin SK, Choudhury A, Lau GK, Zheng MH, Ji D, Abd-Elsalam S, Hwang J, Qi X, Cua IH, Suh JI, Park JG, Putcharoen O, Kaewdech A, Piratvisuth T, Treeprasertsuk S, Park S, Wejnaruemarn S, Payawal DA, Baatarkhuu O, Ahn SH, Yeo CD, Alonzo UR, Chinbayar T, Loho IM, Yokosuka O, Jafri W, Tan S, Soo LI, Tanwandee T, Gani R, Anand L, Esmail ES, Khalaf M, Alam S, Lin CY, Chuang WL, Soin AS, Garg HK, Kalista K, Batsukh B, Purnomo HD, Dara VP, Rathi P, Al Mahtab M, Shukla A, Sharma MK, Omata M; APASL COVID Task Force, APASL COVID Liver Injury Spectrum Study (APCOLIS Study-NCT 04345640). Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; The APCOLIS Study (APASL COVID-19 Liver Injury Spectrum Study). Hepatol Int. 2020;14:690-700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 216] [Article Influence: 43.2] [Reference Citation Analysis (1)] |
| 44. | Iavarone M, D'Ambrosio R, Soria A, Triolo M, Pugliese N, Del Poggio P, Perricone G, Massironi S, Spinetti A, Buscarini E, Viganò M, Carriero C, Fagiuoli S, Aghemo A, Belli LS, Lucà M, Pedaci M, Rimondi A, Rumi MG, Invernizzi P, Bonfanti P, Lampertico P. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol. 2020;73:1063-1071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 279] [Cited by in RCA: 276] [Article Influence: 55.2] [Reference Citation Analysis (2)] |
| 45. | Kim D, Adeniji N, Latt N, Kumar S, Bloom PP, Aby ES, Perumalswami P, Roytman M, Li M, Vogel AS, Catana AM, Wegermann K, Carr RM, Aloman C, Chen VL, Rabiee A, Sadowski B, Nguyen V, Dunn W, Chavin KD, Zhou K, Lizaola-Mayo B, Moghe A, Debes J, Lee TH, Branch AD, Viveiros K, Chan W, Chascsa DM, Kwo P, Dhanasekaran R. Predictors of Outcomes of COVID-19 in Patients With Chronic Liver Disease: US Multi-center Study. Clin Gastroenterol Hepatol. 2021;19:1469-1479.e19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 174] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 46. | Bajaj JS, Garcia-Tsao G, Biggins SW, Kamath PS, Wong F, McGeorge S, Shaw J, Pearson M, Chew M, Fagan A, de la Rosa Rodriguez R, Worthington J, Olofson A, Weir V, Trisolini C, Dwyer S, Reddy KR. Comparison of mortality risk in patients with cirrhosis and COVID-19 compared with patients with cirrhosis alone and COVID-19 alone: multicentre matched cohort. Gut. 2021;70:531-536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 176] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 47. | Mallet V, Beeker N, Bouam S, Sogni P, Pol S; Demosthenes research group. Prognosis of French COVID-19 patients with chronic liver disease: A national retrospective cohort study for 2020. J Hepatol. 2021;75:848-855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 48. | Simon TG, Hagström H, Sharma R, Söderling J, Roelstraete B, Larsson E, Ludvigsson JF. Risk of severe COVID-19 and mortality in patients with established chronic liver disease: a nationwide matched cohort study. BMC Gastroenterol. 2021;21:439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 49. | Mao R, Qiu Y, He JS, Tan JY, Li XH, Liang J, Shen J, Zhu LR, Chen Y, Iacucci M, Ng SC, Ghosh S, Chen MH. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:667-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 741] [Cited by in RCA: 755] [Article Influence: 151.0] [Reference Citation Analysis (0)] |
| 50. | Mendizabal M, Ridruejo E, Piñero F, Anders M, Padilla M, Toro LG, Torre A, Montes P, Urzúa A, Gonzalez Ballerga E, Silveyra MD, Michelato D, Díaz J, Peralta M, Pages J, García SR, Gutierrez Lozano I, Macias Y, Cocozzella D, Chavez-Tapia N, Tagle M, Dominguez A, Varón A, Vera Pozo E, Higuera-de la Tijera F, Bustios C, Conte D, Escajadillo N, Gómez AJ, Tenorio L, Castillo Barradas M, Schinoni MI, Bessone F, Contreras F, Nazal L, Sanchez A, García M, Brutti J, Cabrera MC, Miranda-Zazueta G, Rojas G, Cattaneo M, Castro-Narro G, Rubinstein F, Silva MO. Comparison of different prognostic scores for patients with cirrhosis hospitalized with SARS-CoV-2 infection. Ann Hepatol. 2021;25:100350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 51. | Marjot T, Webb GJ, Barritt AS 4th, Moon AM, Stamataki Z, Wong VW, Barnes E. COVID-19 and liver disease: mechanistic and clinical perspectives. Nat Rev Gastroenterol Hepatol. 2021;18:348-364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 278] [Cited by in RCA: 272] [Article Influence: 68.0] [Reference Citation Analysis (2)] |
| 52. | Yeoh YK, Zuo T, Lui GC, Zhang F, Liu Q, Li AY, Chung AC, Cheung CP, Tso EY, Fung KS, Chan V, Ling L, Joynt G, Hui DS, Chow KM, Ng SSS, Li TC, Ng RW, Yip TC, Wong GL, Chan FK, Wong CK, Chan PK, Ng SC. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70:698-706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 995] [Cited by in RCA: 866] [Article Influence: 216.5] [Reference Citation Analysis (0)] |
| 53. | Scarpellini E, Fagoonee S, Rinninella E, Rasetti C, Aquila I, Larussa T, Ricci P, Luzza F, Abenavoli L. Gut Microbiota and Liver Interaction through Immune System Cross-Talk: A Comprehensive Review at the Time of the SARS-CoV-2 Pandemic. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 54. | Bajaj JS, Garcia-Tsao G, Wong F, Biggins SW, Kamath PS, McGeorge S, Chew M, Pearson M, Shaw J, Kalluri A, Fagan A, Olofson A, Moini M, de la Rosa Rodriguez R, Reddy KR. Cirrhosis Is Associated With High Mortality and Readmissions Over 90 Days Regardless of COVID-19: A Multicenter Cohort. Liver Transpl. 2021;27:1343-1347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 55. | Raman B, Cassar MP, Tunnicliffe EM, Filippini N, Griffanti L, Alfaro-Almagro F, Okell T, Sheerin F, Xie C, Mahmod M, Mózes FE, Lewandowski AJ, Ohuma EO, Holdsworth D, Lamlum H, Woodman MJ, Krasopoulos C, Mills R, McConnell FAK, Wang C, Arthofer C, Lange FJ, Andersson J, Jenkinson M, Antoniades C, Channon KM, Shanmuganathan M, Ferreira VM, Piechnik SK, Klenerman P, Brightling C, Talbot NP, Petousi N, Rahman NM, Ho LP, Saunders K, Geddes JR, Harrison PJ, Pattinson K, Rowland MJ, Angus BJ, Gleeson F, Pavlides M, Koychev I, Miller KL, Mackay C, Jezzard P, Smith SM, Neubauer S. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine. 2021;31:100683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 427] [Cited by in RCA: 400] [Article Influence: 100.0] [Reference Citation Analysis (0)] |
| 56. | Dennis A, Wamil M, Alberts J, Oben J, Cuthbertson DJ, Wootton D, Crooks M, Gabbay M, Brady M, Hishmeh L, Attree E, Heightman M, Banerjee R, Banerjee A; COVERSCAN study investigators. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open. 2021;11:e048391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 270] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 57. | Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1348] [Cited by in RCA: 1295] [Article Influence: 259.0] [Reference Citation Analysis (4)] |
| 58. | Shalbafan M, Khademoreza N. What we can learn from COVID-19 outbreak in Iran about the importance of alcohol use education. Am J Drug Alcohol Abuse. 2020;46:385-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 59. | Sun J, Aghemo A, Forner A, Valenti L. COVID-19 and liver disease. Liver Int. 2020;40:1278-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 219] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 60. | Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5228] [Cited by in RCA: 5785] [Article Influence: 1157.0] [Reference Citation Analysis (2)] |
| 61. | Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan JG, Grønbæk H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2883] [Cited by in RCA: 2837] [Article Influence: 567.4] [Reference Citation Analysis (1)] |
| 62. | Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, Lau G. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J Hepatol. 2020;73:451-453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 408] [Article Influence: 81.6] [Reference Citation Analysis (2)] |
| 63. | Zhou YJ, Zheng KI, Wang XB, Yan HD, Sun QF, Pan KH, Wang TY, Ma HL, Chen YP, George J, Zheng MH. Younger patients with MAFLD are at increased risk of severe COVID-19 illness: A multicenter preliminary analysis. J Hepatol. 2020;73:719-721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 64. | Targher G, Mantovani A, Byrne CD, Wang XB, Yan HD, Sun QF, Pan KH, Zheng KI, Chen YP, Eslam M, George J, Zheng MH. Risk of severe illness from COVID-19 in patients with metabolic dysfunction-associated fatty liver disease and increased fibrosis scores. Gut. 2020;69:1545-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 161] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 65. | Lopez-Mendez I, Aquino-Matus J, Gall SM, Prieto-Nava JD, Juarez-Hernandez E, Uribe M, Castro-Narro G. Association of liver steatosis and fibrosis with clinical outcomes in patients with SARS-CoV-2 infection (COVID-19). Ann Hepatol. 2021;20:100271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 66. | Marjot T, Buescher G, Sebode M, Barnes E, Barritt AS 4th, Armstrong MJ, Baldelli L, Kennedy J, Mercer C, Ozga AK, Casar C, Schramm C; contributing Members and Collaborators of ERN RARE-LIVER/COVID-Hep/SECURE-Cirrhosis, Moon AM, Webb GJ, Lohse AW. SARS-CoV-2 infection in patients with autoimmune hepatitis. J Hepatol. 2021;74:1335-1343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 67. | Roth NC, Kim A, Vitkovski T, Xia J, Ramirez G, Bernstein D, Crawford JM. Post-COVID-19 Cholangiopathy: A Novel Entity. Am J Gastroenterol. 2021;116:1077-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 129] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 68. | Zollner A, Koch R, Jukic A, Pfister A, Meyer M, Rössler A, Kimpel J, Adolph TE, Tilg H. Postacute COVID-19 is Characterized by Gut Viral Antigen Persistence in Inflammatory Bowel Diseases. Gastroenterology. 2022;163:495-506.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 200] [Article Influence: 66.7] [Reference Citation Analysis (0)] |
| 69. | Zin Tun GS, Gleeson D, Al-Joudeh A, Dube A. Immune-mediated hepatitis with the Moderna vaccine, no longer a coincidence but confirmed. J Hepatol. 2022;76:747-749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 64] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 70. | Anugwom CM, Aby ES, Debes JD. Inverse Association Between Chronic Hepatitis B Infection and Coronavirus Disease 2019 (COVID-19): Immune Exhaustion or Coincidence? Clin Infect Dis. 2021;72:180-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 71. | Kang SH, Cho DH, Choi J, Baik SK, Gwon JG, Kim MY. Association between chronic hepatitis B infection and COVID-19 outcomes: A Korean nationwide cohort study. PLoS One. 2021;16:e0258229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 72. | Ronderos D, Omar AMS, Abbas H, Makker J, Baiomi A, Sun H, Mantri N, Choi Y, Fortuzi K, Shin D, Patel H, Chilimuri S. Chronic hepatitis-C infection in COVID-19 patients is associated with in-hospital mortality. World J Clin Cases. 2021;9:8749-8762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 73. | Sachdeva S, Khandait H, Kopel J, Aloysius MM, Desai R, Goyal H. NAFLD and COVID-19: a Pooled Analysis. SN Compr Clin Med. 2020;2:2726-2729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 74. | Mahamid M, Nseir W, Khoury T, Mahamid B, Nubania A, Sub-Laban K, Schifter J, Mari A, Sbeit W, Goldin E. Nonalcoholic fatty liver disease is associated with COVID-19 severity independently of metabolic syndrome: a retrospective case-control study. Eur J Gastroenterol Hepatol. 2021;33:1578-1581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 75. | Hashemi N, Viveiros K, Redd WD, Zhou JC, McCarty TR, Bazarbashi AN, Hathorn KE, Wong D, Njie C, Shen L, Chan WW. Impact of chronic liver disease on outcomes of hospitalized patients with COVID-19: A multicentre United States experience. Liver Int. 2020;40:2515-2521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 117] [Article Influence: 23.4] [Reference Citation Analysis (2)] |
| 76. | Yao R, Zhu L, Wang J, Liu J, Xue R, Xue L, Liu L, Li C, Zhao H, Cheng J, Huang S, Li Y, Zhao XA, Zhu C, Li M, Huang R, Wu C. Risk of severe illness of COVID-19 patients with NAFLD and increased NAFLD fibrosis scores. J Clin Lab Anal. 2021;35:e23880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 77. | Li J, Tian A, Zhu H, Chen L, Wen J, Liu W, Chen P. Mendelian Randomization Analysis Reveals No Causal Relationship Between Nonalcoholic Fatty Liver Disease and Severe COVID-19. Clin Gastroenterol Hepatol. 2022;20:1553-1560.e78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 78. | Espinoza JAL, Júnior JE, Miranda CH. Atypical COVID-19 presentation with Budd-Chiari syndrome leading to an outbreak in the emergency department. Am J Emerg Med. 2021;46:800.e5-800.e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 79. | Sh Hassan AA, Alsaleh ME, Al Zaher FA, Almajed FA, Alkhudhair AM, Alali MM, Alzayer HA, Alolayan AJ. Budd-Chiari Syndrome: A Case Report of a Rare Presentation of COVID-19. Cureus. 2021;13:e12554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |