Published online Oct 7, 2023. doi: 10.3748/wjg.v29.i37.5327
Peer-review started: June 28, 2023
First decision: August 15, 2023
Revised: August 23, 2023
Accepted: September 12, 2023
Article in press: September 12, 2023
Published online: October 7, 2023
Processing time: 89 Days and 3.7 Hours
Non-alcoholic fatty liver disease (NAFLD) is the leading cause of chronic liver disease. The prevalence and disease burden of NAFLD are projected to exponentially increase resulting in significant healthcare expenditures and lower health-related quality of life. To date, there are no approved pharmacotherapies for NAFLD or non-alcoholic steatohepatitis (NASH). Semaglutide has glycemic and weight loss benefits that may be advantageous for patients with NAFLD.
To investigate the efficacy and safety of semaglutide in patients with NAFLD.
MEDLINE, CENTRAL, and EMBASE were searched from inception to May 1, 2023, to identify eligible randomized controlled trials (RCTs). Meta-analysis was performed using random effects model expressing continuous outcomes as mean differences (MD) or standardized MDs (SMD), and dichotomous outcomes as odds ratios (OR) with 95% confidence intervals (CI). Statistical heterogeneity was assessed using the Cochran’s Q test and I2 statistic.
Three RCTs involving 458 patients were included. Semaglutide increased the likelihood of NASH resolution (OR: 3.18, 95%CI: 1.70, 5.95; P < 0.001), impro
Semaglutide is effective in the treatment of NAFLD while maintaining a well-tolerated safety profile. Future studies are required to evaluate its effects on fibrosis regression and different phases of NAFLD.
Core Tip: Semaglutide demonstrates significant histologic improvements, with a higher likelihood of non-alcoholic steatohepatitis resolution and improved steatosis, lobular inflammation, and hepatocellular ballooning, but it does not significantly improve fibrosis stage compared to placebo. Furthermore, semaglutide results in radiologic improvements in liver stiffness and steatosis, liver enzymes, as well as cardiometabolic effects on body weight and HgA1c, while maintaining a well-tolerated safety profile.
- Citation: Zhu K, Kakkar R, Chahal D, Yoshida EM, Hussaini T. Efficacy and safety of semaglutide in non-alcoholic fatty liver disease. World J Gastroenterol 2023; 29(37): 5327-5338
- URL: https://www.wjgnet.com/1007-9327/full/v29/i37/5327.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i37.5327
Non-alcoholic fatty liver disease (NAFLD) is the leading cause of chronic liver disease, with an estimated worldwide prevalence of 32.4%[1,2]. The prevalence and disease burden of NAFLD are projected to exponentially increase, with mathematical models forecasting a 168% increase in the incidence of decompensated cirrhosis and a 178% increase in NAFLD-related deaths between 2015 and 2030. These projections highlight the significant healthcare expenditures and lower health-related quality of life associated with the disease[3-5].
NAFLD is a spectrum of liver disease characterized by hepatic steatosis in the absence of excessive alcohol consumption[6]. The majority of patients with NAFLD have NAFL, of which approximately 20% will develop non-alcoholic steatohepatitis (NASH) and have a risk of further progression to cirrhosis, hepatocellular carcinoma, and end-stage liver disease[3]. Although lean NAFLD is increasingly recognized, the majority of patients with NAFLD have one or more components of metabolic syndrome, which is also independently strongly associated with fibrosis progression[7,8].
GLP-1 receptor agonists (RAs) offer promising therapeutic options in NAFLD due to their beneficial glycemic and weight loss effects. GLP-1 receptors have been detected on human hepatocytes, and it is hypothesized that their activation by GLP-1 RAs can have positive effects on hepatic steatosis, lipotoxicity, fatty acid oxidation, and cytokines involved in hepatic inflammation and fibrosis[9,10]. Moreover, GLP-1 RAs may have indirect hepatoprotective benefits through increased insulin secretion in response to hyperglycemia, decreased glucagon secretion, delayed gastric emptying, and significant weight loss[11,12].
Among the GLP-1 RAs, semaglutide has demonstrated the greatest glycemic and weight loss benefits[13]. In a recent phase three trial of patients with overweight or obesity, semaglutide showed a significant decrease in body weight by 14.9% compared to 2.4% with placebo[14]. Additionally, semaglutide has shown reduced rates of major adverse cardiovascular events and a lower risk of adverse renal outcomes in patients with type 2 diabetes (T2DM)[15]. It has since been approved for the treatment of T2DM and chronic weight management.
Several randomized clinical trials have also demonstrated the beneficial effects of semaglutide in patients with NAFLD. A previous systematic review with meta-analysis was conducted to assess the impact of semaglutide on biochemical and radiologic measures of NAFLD[16]. However, more than 85% of the study’s patients had diabetes or obesity rather than confirmed NAFLD. Additionally, no histological outcomes were reported, which are considered the gold standard for diagnosing and managing NAFLD. Since its publication, a recent randomized controlled trial (RCT) by Loomba et al[17] has been performed, focusing on semaglutide in patients with NASH and compensated cirrhosis. The purpose of this systematic review and meta-analysis is to provide an updated review on the efficacy and safety of semaglutide, focusing on patients with NAFLD, in order to more specifically reflect the NAFLD population and expand the current understanding of semaglutide in NAFLD.
The systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines and was registered prospectively on the PROSPERO database (ID: CRD42023422487). Two independent reviewers (K.Z. and R.K.) evaluated the titles and abstracts of all identified studies based on predetermined inclusion and exclusion criteria (Supplementary Table 1). Any discrepancies were resolved through discussion with a third reviewer (T.H.).
Multiple databases, including MEDLINE, CENTRAL, EMBASE, and grey literature sources such as Clinicaltrials.gov and the World Health Organization International Clinical Trials Registries, were searched from inception to May 1, 2023 using a predefined search strategy (Supplementary Table 2). Additionally, a forward and backward citation search was performed on eligible studies using CitationChaser.
Two reviewers (K.Z. and R.K.) independently extracted the data using predetermined data collection forms. Any discrepancies were resolved through consultation with a third reviewer (T.H.).
The primary outcomes of interest for this study were histological improvement in NAFLD activity score, resolution of NASH with no worsening of liver fibrosis, and improvement in liver fibrosis without worsening of NASH, as defined by the NASH Clinical Research Network Criteria (CRN).
The secondary outcomes of interest included radiologic improvement in liver stiffness and steatosis, measured using either magnetic resonance elastography (MRE) or Fibroscan; changes in liver enzymes [alanine aminotransferase (ALT) and aspartate aminotransferase (AST)]; cardiometabolic parameters such as body weight, HgA1c, total cholesterol, non-HDL cholesterol, and LDL cholesterol; as well as adverse events, including gastrointestinal-related side effects and serious adverse events.
The risk of bias was independently conducted by two reviewers (K.Z. and R.K.) using the Cochrane risk-of-bias 2 tool for randomized trials. Due to the limited number of included studies, a funnel plot was not generated to assess publication bias. Finally, the quality of the evidence was evaluated using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework.
To address missing data, attempts were made to contact the study authors for the necessary information. In cases where the data could not be obtained, relevant values were extracted from figures, using the PlotDigitizer software, following Cochrane methodology. If the data was unavailable anywhere in the study, it was excluded from the analysis. Missing standard deviations (SD) for continuous outcomes were estimated using the available standard error, 95% confidence interval (CI), or
Continuous outcomes were presented as mean differences (MD) in change scores with corresponding 95%CI. The DerSimonian and Laird random-effects model was used given expected clinical and methodological diversity between the included studies. When outcomes were measured on different scales that could not be converted to a common scale, such as Fibroscan and MRE measurements, they were reported as standardized MDs (SMD) using Hedges’ G. A correlation coefficient of r = 0.4, which is consistent with previous meta-analyses of liraglutide in NAFLD, was utilized[19].
Dichotomous outcomes were expressed as odds ratios (OR) with 95%CI. Statistical heterogeneity was assessed using Cochran’s Q statistic and I2 statistic, where P < 0.10 and I2 > 50% were considered significant indicators of heterogeneity. The significance level for all other statistical tests was set at P < 0.05. RevMan version 5.4 (Copenhagen, Denmark) was used for all statistical analyses.
Sensitivity analysis was conducted using the leave-one-out method to evaluate the influence of each individual study on the overall estimate. Additional sensitivity analysis was performed comparing the outcomes using a fixed-effect model vs random-effect model. Subgroup analysis was performed by stratifying participants within the included studies based on their T2DM status. However, due to the limited number of included studies, additional sensitivity and subgroup analyses were not performed.
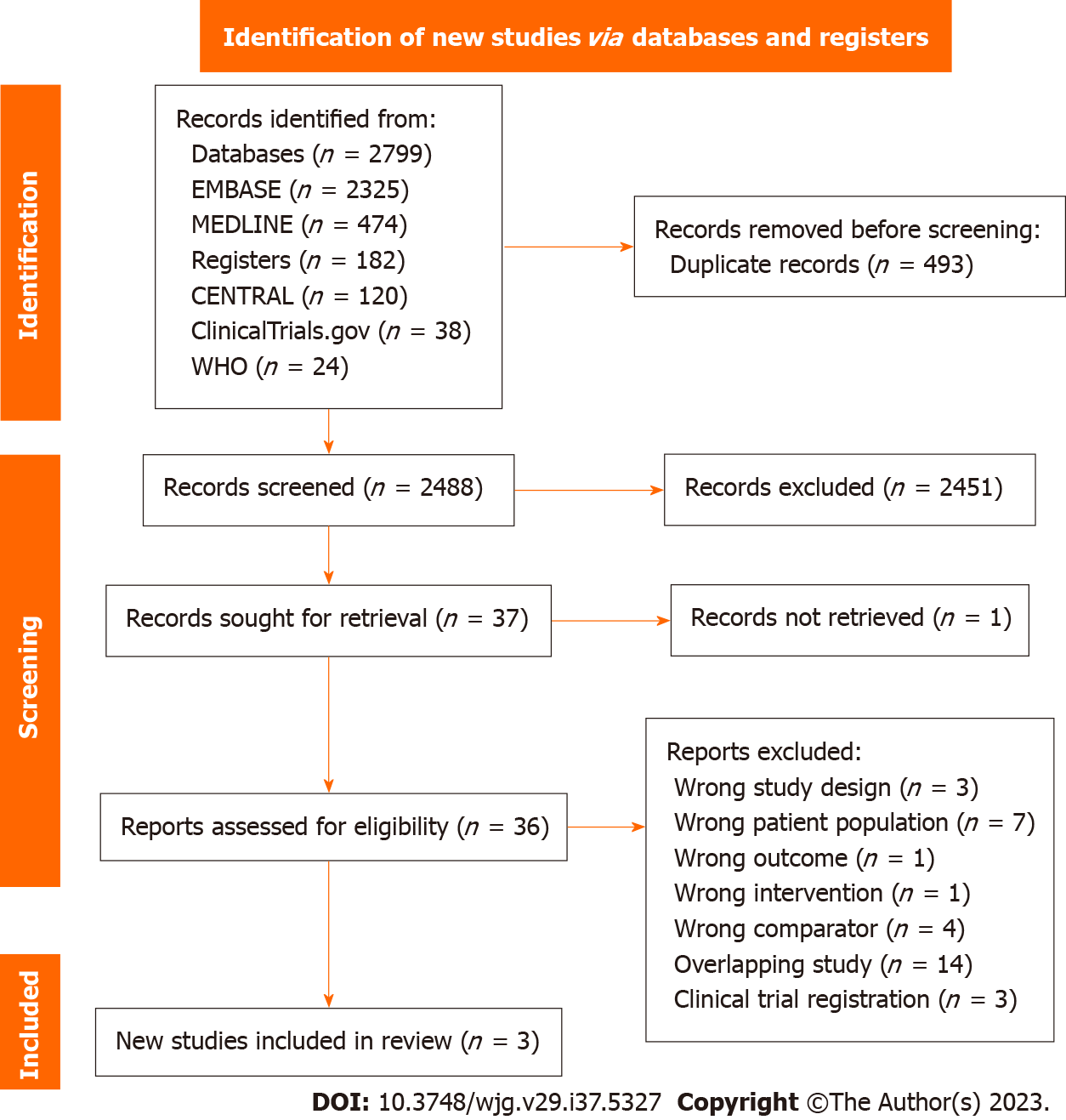
A total of 2981 potentially eligible studies were identified, of which 493 duplicates were removed prior to screening. An additional 2451 studies were excluded based on titles and abstracts screening. Full text was obtained for 36 out of the 37 eligible studies. From these, we identified three studies that met our inclusion criteria and were included in the meta-analysis[17,20,21] (Figure 1).
The study characteristics of the included summaries are outlined in Table 1. A total of 458 patients were included, with 321 receiving semaglutide and 137 receiving placebo. All studies were RCTs ranging from 48 wk to 72 wk in duration. Various doses of semaglutide were utilized. Newsome et al[20] compared daily doses of 0.1 mg, 0.2 mg, and 0.4 mg of semaglutide, whereas Flint et al[21] and Loomba et al[17] used daily doses of 0.4 mg and weekly doses of 2.4 mg, respectively. Furthermore, Flint et al[21] focused exclusively on patients with NAFL, while the other two studies only included patients with biopsy confirmed NASH. Both Newsome et al[20] and Loomba et al[17] conducted histological assessments. All studies included patients with and without T2DM.
| Ref. | Location sponsor | Study design | Sample size (n) | Demographics (%) | Intervention/comparator(s) | Outcomes assessed |
| Newsome et al[20], 2021 | 16 countrie, 143 sites; Novo Nordisk | MC, DB, four-arm parallel-group RCT; duration: 72 wk; randomization: 3:3:3:1:1:1 | 320 | Age (SD): 55.0 (10.6); male/female: 125(39)/195(61); T2DM: 199 (62) | Semaglutide: 0.1 mg SQ OD (n = 80); 0.2 mg SQ OD (n = 78); 0.4 mg SQ OD (n = 82); and placebo (n = 80) | Primary: Resolution of NASH; secondary: Liver fibrosis stage, total and component of NAS, ALT, AST, liver stiffness, liver steatosis, cardiometabolic parameters, adverse events |
| Flint et al[21], 2021 | Germany, 2 sites; Novo Nordisk | Two-centre, DB, two-arm parallel-group RCT; duration: 72 wk; randomization: 1:1 | 67 | Age (SD): 60.0 (9.3); male/female: 47(70)/20(30); T2DM: 49 (73) | Semaglutide: 0.4 mg SQ OD (n = 34); placebo (n = 33) | Primary: Liver stiffness MRE at week 48; secondary: Liver stiffness at week 24 and 72, liver steatosis, ALT, AST, cardiometabolic parameters, adverse events |
| Loomba et al[17], 2023 | 5 countries, 38 sites; Novo Nordisk | MC, DB, two-arm-parallel group RCT; duration: 48 wk; randomization: 2:1 | 71 | Age (SD): 59.5 (8.0); male/female: 22(31)/49(61); T2DM: 53 (75) | Semaglutide: 2.4 mg SQ qw (n = 47); Placebo (n = 24) | Primary: Liver fibrosis stage; secondary: Liver stiffness, liver steatosis, NASH resolution, total and component of NAS, ALT, AST, cardiometabolic parameters, adverse events |
The methodological qualities of the studies are summarized in the appendix, Supplementary Figure 1. The trial of Newsome et al[20] was classified as having a low risk of bias, while the other trials raised some concerns. Specifically, the study of Loomba et al[17] had concerns related to randomization, as a higher proportion of patients in the semaglutide group had an Ishak fibrosis score of 6, while more patients receiving placebo had a score of 4 or 5. Additionally, baseline measurements of MRE, hepatic collagen proportion, liver enzymes, and pro-C3 were slightly higher in the semaglutide group. Flint et al[21] raised concerns regarding missing data, as seven out of 34 patients in the semaglutide group discontinued treatment, and no sensitivity analysis was performed, or analysis conducted to address the resulting bias. The quality of the evidence was evaluated using the GRADE framework as illustrated in Table 2.
| Outcomes | Anticipated absolute effects1 (95%CI) | Relative effect (95%CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with placebo | Risk with semaglutide | ||||
| Resolution of NASH with no worsening of liver fibrosis assessed with: Liver biopsy | 183 per 1000 | 416 per 1000 (276 to 571) | OR 3.18 (1.70 to 5.95) | 301 (2 RCTs) | +++O: Moderate2 |
| Improvement in liver fibrosis stage without worsening of NASH assessed with: Liver biopsy | 317 per 1000 | 248 per 1000 (65 to 613) | OR 0.71 (0.15 to 3.41) | 301 (2 RCTs) | ++OO: Low2,3,4 |
| Liver stiffness assessed with: MRI-PDFF or Fibroscan | - | SMD 0.48 lower (0.86 lower to 0.11 lower) | - | 350 (3 RCTs) | ++++: High |
| Liver steatosis assessed with: MRE | The mean liver steatosis ranged from -0.57% to -2.57% | MD 4.96 % lower (9.92 lower to 0.01 higher) | - | 138 (2 RCTs) | +++O: Moderate3 |
| ALT | The mean ALT ranged from 1.90 U/L to -11.22 U/L | MD 14.06 U/L lower (22.06 lower to 6.07 lower) | - | 458 (3 RCTs) | ++++: High |
| AST | The mean AST ranged from 1.50 U/K to -5.76 U/K | MD 11.44 U/K lower (17.23 lower to 5.65 lower) | - | 458 (3 RCTs) | ++++: High |
| Serious adverse events | 109 per 1000 | 147 per 1000 (84 to 244) | OR 1.40 (0.75 to 2.62) | 456 (3 RCTs) | +++O: Moderate2,4 |
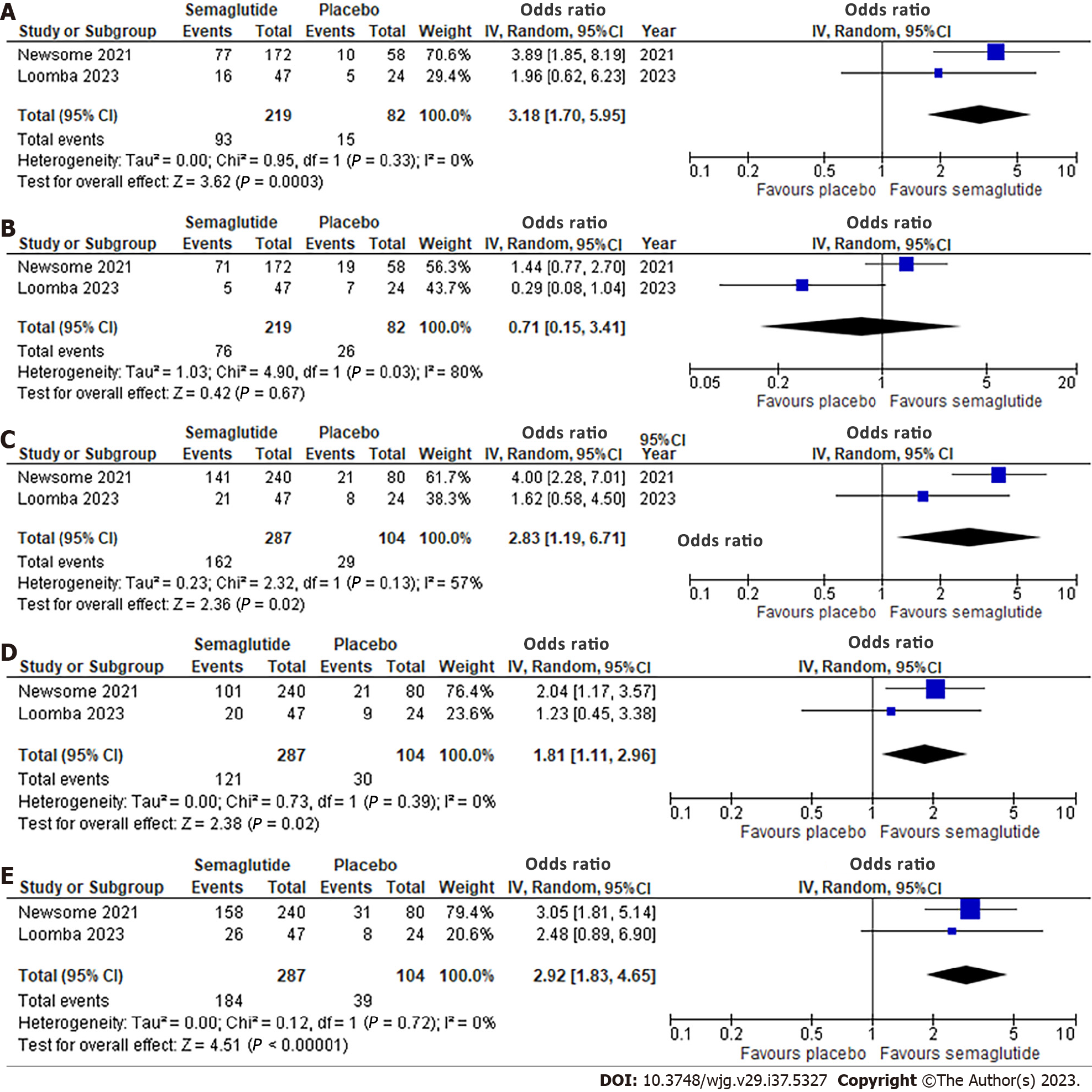
Effect of semaglutide on histological parameters: Two studies, involving 391 patients, evaluated histological outcomes (Figure 2). Semaglutide was associated with a significantly higher likelihood of NASH resolution with no worsening of liver fibrosis (OR: 3.18, 95%CI: 1.70, 5.95; I2 = 0%) (Figure 2A). However, there was no significant improvement in liver fibrosis stage without worsening of NASH (OR: 0.71, 95%CI: 0.15, 3.41; I2 = 80%) (Figure 2B). Significant improvements were observed in all NAFLD Activity Score (NAS) components with OR 2.83 (95%CI: 1.19, 6.71; I2 = 57%) for steatosis, 1.81 (95%CI: 1.11, 2.96; I2 = 0%) for lobular inflammation, and 2.92 (95%CI: 1.83, 4.65; I2 = 0%) for hepatocellular ballooning (Figure 2C-E). Significant heterogeneity was noted for improvement in liver fibrosis stage.
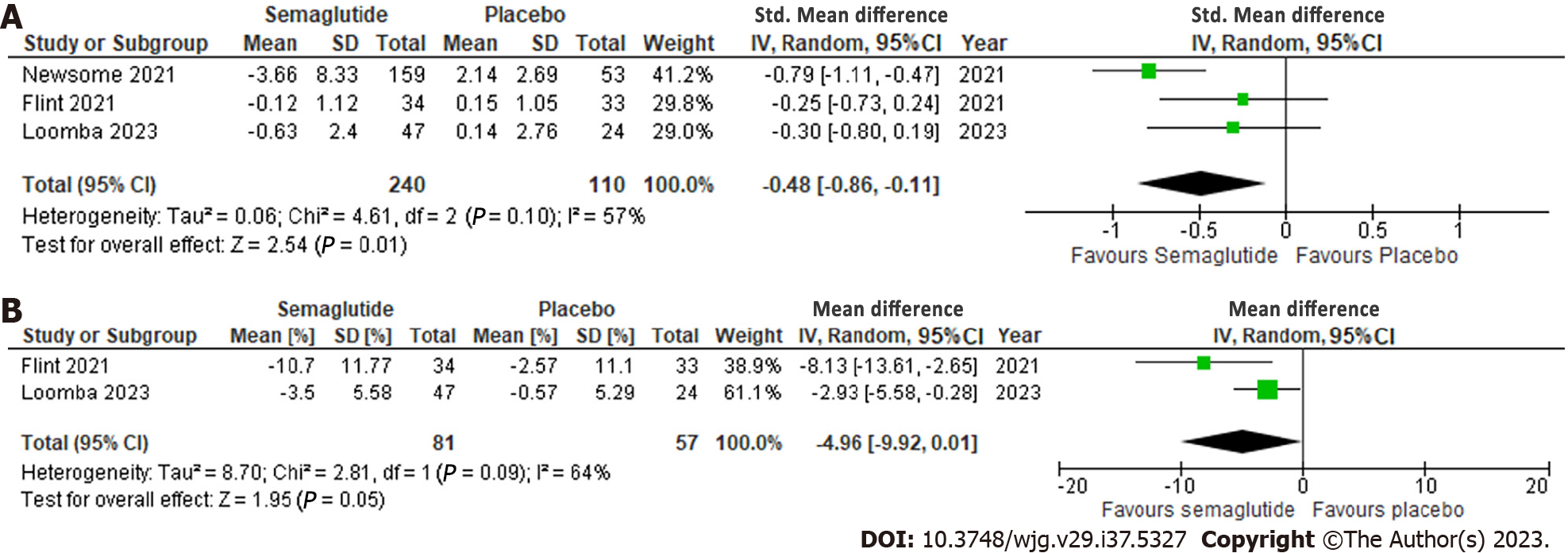
Effect of semaglutide on radiologic parameters: All three studies reported radiologic parameters and semaglutide demonstrated a significant reduction in liver stiffness on MRE or Fibroscan, with a standardized MD of -0.48 (95%CI: -0.86, -0.11; I2 = 57%) (Figure 3A). Additionally, a significant reduction in liver steatosis on MRI proton density fat fraction (MRI-PDFF) was observed, with a MD of -4.96% (95%CI: -9.92, 0.01; I2 = 64%), although significant heterogeneity was noted (Figure 3B).
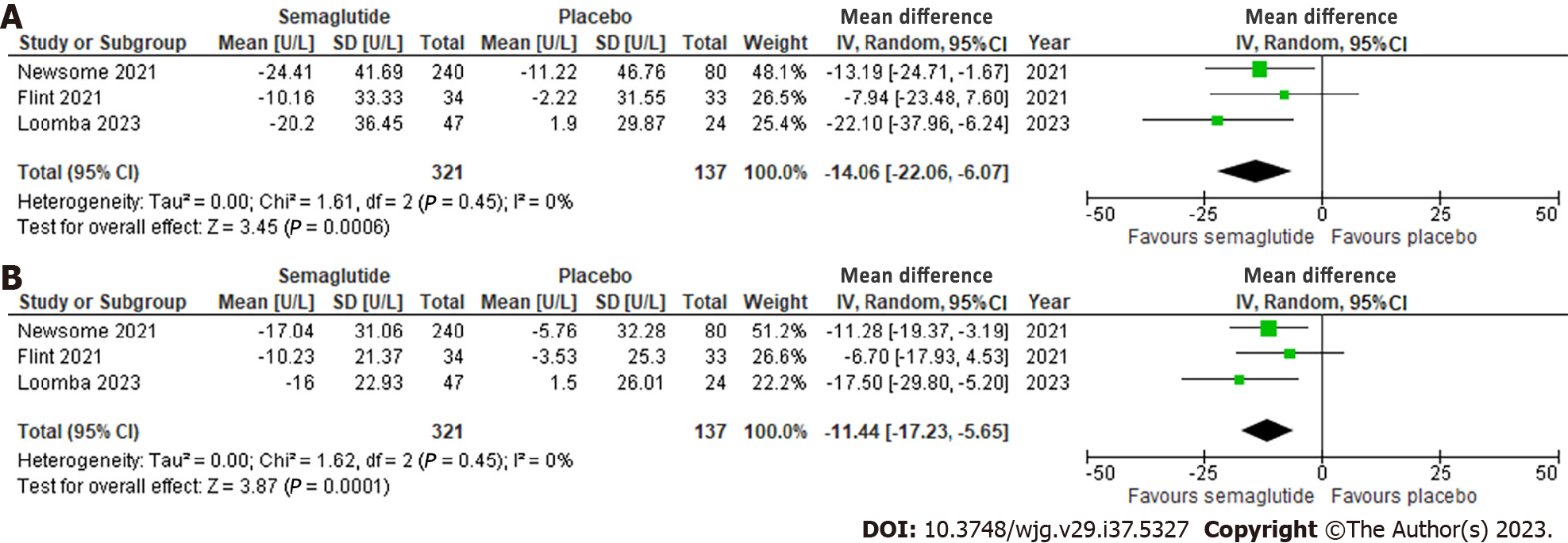
Effect of semaglutide on liver enzymes: All three studies evaluated ALT and AST, which showed a significant reduction of 14.06 U/L (95%CI: -22.06, -6.07; I2 = 0%) and 11.44 U/L (95%CI: -17.23, -5.65; I2 = 0%), respectively, compared to placebo. No significant heterogeneity was observed (Figure 4).
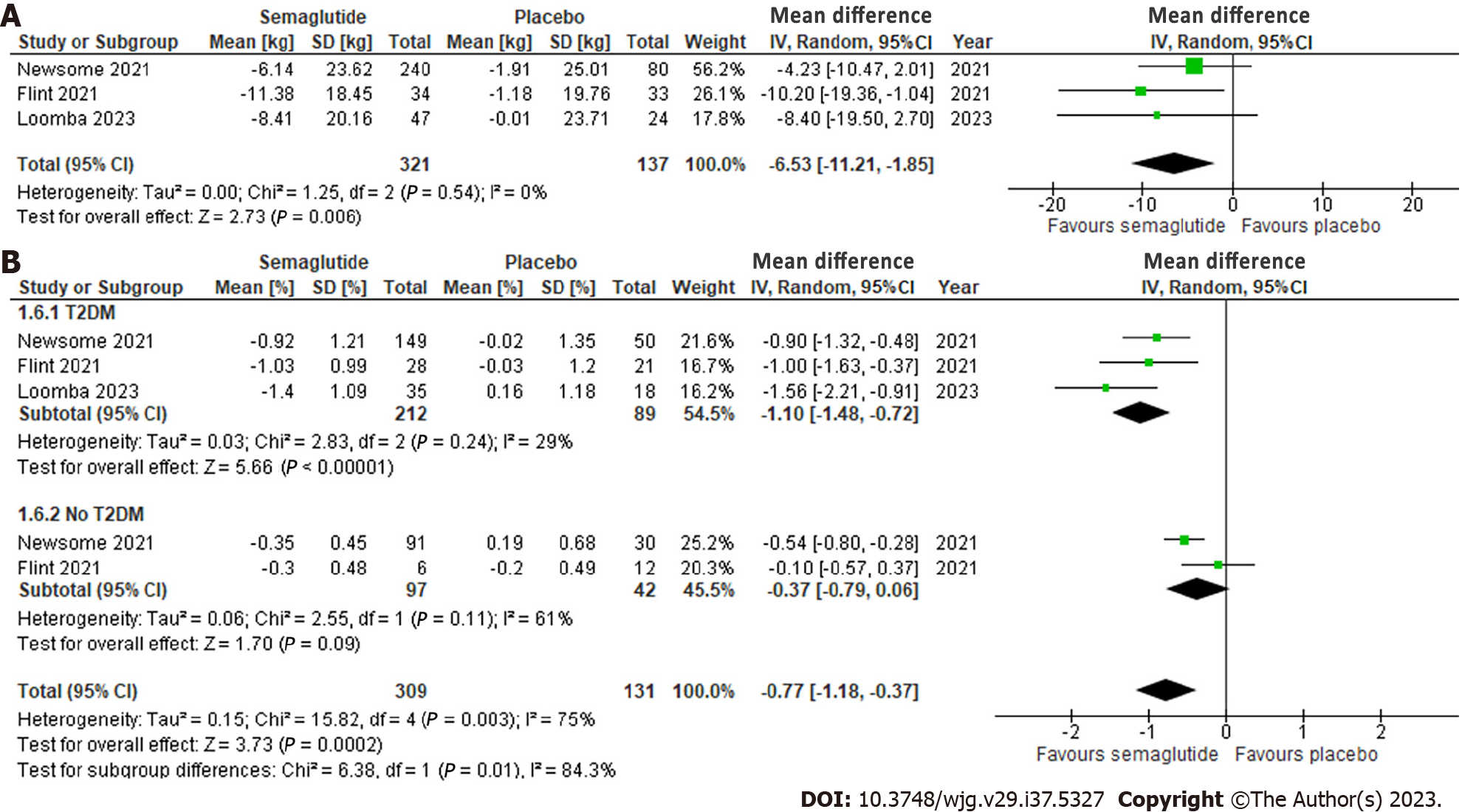
Effect of semaglutide on cardiometabolic parameters: All three studies evaluated total body weight, which revealed a significant reduction of 6.53 kg (95%CI: -11.21, -1.85; I2 = 0%) compared to placebo, with no significant heterogeneity observed (Figure 5A).
Overall, semaglutide also significantly decreased HgA1c by 0.77% (95%CI: -1.18, -0.37; I2 = 75%) compared to placebo (Figure 5B). Subgroup analysis of participants within the studies, stratified by T2DM status, showed a significant reduction in HgA1c among patients with T2DM (MD: -1.10%, 95%CI: -1.48, -0.72; I2 = 29%), but not among those without (MD: -0.37%, 95%CI: -0.79, 0.06; I2 = 61%). Heterogeneity was no longer significant with the subgroup analysis.
Regarding lipid panel results, semaglutide was not associated with a significant difference in triglycerides (MD: -24.03 mg/dL, 95%CI: -60.94, 12.88; I2 = 42%), total cholesterol (MD: -7.31 mg/dL, 95%CI: -51.66, 37.03; I2 = 87%), non-HDL cholesterol (MD: -7.52 mg/dL, 95%CI: -49.32, 34.27; I2 = 84%), and LDL cholesterol (MD: -4.72 mg/dL, 95%CI: -56.23, 46.79; I2 = 92%), although significant heterogeneity was observed (Supplementary Figure 2).
Adverse events with semaglutide: Semaglutide was associated with a significantly higher occurrence of gastrointestinal-related side effects compared to placebo (OR: 3.72, 95%CI: 1.68, 8.23; I2 = 49%) (Figure 6A). However, the overall risk of serious adverse events was comparable between the two groups (OR: 1.40, 95%CI: 0.75, 2.62; I2 = 0%) (Figure 6B).
Each article was individually excluded to examine the influence of each study on the overall effect-size estimate (Supplementary Table 3). Most of the outcomes remained unchanged; however, when Newsome et al[20] was removed, the effect size for resolution of NASH, steatosis, lobular inflammation, and hepatocellular ballooning became non-significant. The effect estimates for liver stiffness when restricted to MRE and excluding Fibroscan as used in the study by Newsome et al[20], also became non-significant. However, there was no longer any heterogeneity observed.
Regarding cardiometabolic outcomes, the significant effect of semaglutide on body weight was no longer sustained when the trial by Flint et al[21] was excluded. Furthermore, when Newsome et al[20] was removed, there was a significant decrease in triglycerides, total cholesterol, and non-HDL cholesterol. Additionally, the exclusion of Newsome et al[20] led to a significant reduction in LDL-cholesterol, whereas the exclusion of Loomba et al[17] resulted in significant increases. No significant differences were observed in the sensitivity analysis when comparing the fixed-effect model to the random-effect model (Supplementary Table 4).
In this systematic review and meta-analysis, semaglutide demonstrated significant histologic improvements, with a higher likelihood of NASH resolution and improved NAS components, but it did not significantly improve fibrosis stage compared to placebo. Furthermore, semaglutide resulted in radiologic improvements in liver stiffness and steatosis, liver enzymes, as well as cardiometabolic effects on body weight and HgA1c, while maintaining a well-tolerated safety profile.
In the systematic review with meta-analysis on the impact of semaglutide on biochemical and radiologic measures of NAFLD conducted by Dutta et al[16], the majority of included patients did not have confirmed NAFLD. Out of the four RCTs included, only two involved patients with NAFLD. The other two trials focused on the cardiovascular outcomes of semaglutide in patients with type 2 diabetes and the efficacy of semaglutide in weight loss for patients with obesity. Since these two trials did not separately report outcomes for the NAFLD subgroup, they were not included in our systematic review and meta-analysis. Furthermore, the meta-analysis did not include any histological outcomes, which are considered the gold standard for NAFLD diagnosis and management. In contrast, our paper exclusively focuses on the NAFLD population, reports histological outcomes, and provides an updated review that includes the recent RCT conducted by Loomba et al[17].
Previous studies have demonstrated an association between histological resolution of NASH and decrease in NAS components with improvement in fibrosis stage[22,23]. However, despite improvements in other histologic outcomes, our meta-analysis did not observe an improvement in liver fibrosis. Of note, Loomba et al[17] had an imbalance in baseline characteristics, with a higher proportion of patients in the semaglutide group exhibiting higher grade fibrosis and non-invasive markers of inflammation compared to the placebo group. This imbalance may have reduced the treatment effect estimate and resulted in the observed heterogeneity. Furthermore, the proportion of patients with improved fibrosis in the placebo group in the trial of Newsome et al[20] (33%) was higher than that reported in the LEAN trial (14%) or the pooled placebo outcomes of 23 RCTs involving patients with NAFLD (21%), possibly contributing to the non-significant treatment effect estimate[24,25]. Despite no difference in the improvement of fibrosis, Newsome’s trial observed that a smaller proportion of patients in the semaglutide group (5%) experienced worsening of fibrosis compared to the placebo group (19%), suggesting a potential benefit[20]. It is possible that a longer follow-up time may be required to achieve improvements in fibrosis, particularly since most of the patients had advanced fibrosis, and the timeline for improvement in fibrosis remains unclear.
Although liver biopsy remains the gold standard for diagnosing and staging NAFLD, MRE and MRI-PDFF are effective non-invasive assessments of liver stiffness and steatosis, respectively. MRI-PDFF is significantly associated with histological NASH CRN steatosis grade, independent of age, sex, and other NASH parameters[26]. This correlation has been demonstrated in several other studies[27,28]. Both MRE and MRI-PDFF are considered more accurate than ultrasound-based transient elastography in detecting fibrosis and steatosis, respectively, and remain effective in patients with obesity, which is a common comorbidity in patients with NAFLD[29].
However, the accuracy of MRI-PDFF is limited by the extent of hepatic fibrosis. Permutt et al[30] demonstrated that the association between MRI and histology-determined steatosis remained relatively stable at fibrosis stages 0-3 but significantly dropped at stage 4. Similarly, Idilman et al[27] showed that the correlation between liver biopsy and MRI-determined steatosis was less pronounced when fibrosis was present (r = 0.60) than when fibrosis was absent (r = 0.86). In contrast, liver stiffness measured using MRE is less influenced by fibrosis and provides a more accurate prediction of liver fibrosis in patients with more advanced fibrosis[31]. The decreased reproducibility and accuracy of MRI PDFF in higher fibrosis stages may explain the substantial heterogeneity observed in liver steatosis in our study, especially considering that the population of the included studies had advanced fibrosis. Heterogeneity in liver stiffness, although not statistically significant, became negligible when Fibroscan was removed and only MRE was utilized to measure liver stiffness.
Semaglutide showed a significant association with moderate decreases in ALT and AST, which have previously been correlated with histologic response, fibrosis regression, and reduced progression in NAFLD[22,32]. However, both the LEAN trial and a meta-analysis of liraglutide in NAFLD did not observe significant reductions in liver enzymes[24,33]. Despite being in the same drug class with similar mechanisms of action, there may be intrinsic differences between effect of semaglutide and liraglutide. Furthermore, our study demonstrated a significant improvement in HgA1c and a reduction of 6.53 kg in body weight in the semaglutide compared to placebo group. The superior metabolic outcomes associated with semaglutide, compared to liraglutide, may lead to a more effective reduction in hepatocellular stress and injury, resulting in a significant decrease in liver enzymes. Vilar-Gomez et al[34] previously demonstrated that patients with weight losses ≥ 10% had the highest rates of NAS reduction, NASH resolution and fibrosis regression. Therefore, semaglutide appears to be particularly beneficial for patients with both NAFLD and features of metabolic syndrome.
The safety profile of semaglutide was comparable to that reported in the recent phase 3 trial with once-weekly dosing in patients with obesity and trials from the SUSTAIN (Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes) programs[14,35]. Gastrointestinal-related side effects, including nausea, constipation, vomiting, and abdominal pain, were significantly more prevalent in patients receiving semaglutide compared to placebo. These side effects are well-documented among the GLP-1 RA class. In all RCTs included in our review, adverse events mostly occurred during treatment initiation or the dose-escalation period. These symptoms become less pronounced with gradual up-titration and are often self-limiting, subsiding after a few weeks. Furthermore, the risks of serious adverse events were not different between semaglutide and placebo.
Our study has several limitations. The included RCTs are clinically heterogeneous from each other, with patients across the spectrum of NAFLD. Flint et al[21] recruited patients with NAFL, whereas Newsome and Loomba’s trials involved patients with NASH and NASH-related cirrhosis, respectively. The heterogeneous patient population limits the applicability of the results, as treatment and response across the spectrum of NAFLD may differ. Additionally, a range of doses of semaglutide was used across the trials, including 0.1 mg, 0.2 mg, 0.4 mg once daily, and 2.4 mg weekly. However, this is less of a concern as once-weekly dosing has been shown to be comparably effective in the obesity population, and unpublished data suggests similar plasma concentrations to daily dosing[14,17]. Furthermore, histologic outcomes were only reported for patients with NASH or NASH-related cirrhosis, but not for patients with NAFL. Therefore, the effect of semaglutide on histologic outcomes in the NAFL population remains unclear. Lastly, the limited number of eligible studies restricted our ability to perform subgroup analysis comparing outcomes in NAFL, NASH, and NASH-related cirrhosis populations, or meta-regression to investigate heterogeneity and the effect of covariates on the effect sizes.
In conclusion, our meta-analysis of RCTs demonstrates that semaglutide has beneficial histologic, radiologic, liver enzyme, and cardiometabolic effects in patients with NAFLD, with a well-tolerated safety profile. Semaglutide is particularly beneficial for patients with NAFLD and features of metabolic syndrome, given its notable effects on lowering HbA1c and promoting weight loss. However, the results are limited by the small number of included studies and clinical heterogeneity, which restricts the generalizability these findings across the spectrum of NAFLD. Additional RCTs with larger sample sizes and longer durations are required to characterize the effects of semaglutide on fibrosis regression and its role in the different phases of NAFLD.
Non-alcoholic fatty liver disease (NAFLD) is the leading cause of chronic liver disease. The prevalence and disease burden of NAFLD are projected to exponentially increase resulting in significant healthcare expenditures and lower health-related quality of life. To date, there are no approved pharmacotherapies for NAFLD or non-alcoholic steatohepatitis (NASH).
Several randomized clinical trials have demonstrated the beneficial effects of semaglutide in patients with NAFLD. Prior systematic review with meta-analysis assessing the impact of semaglutide did not report histological outcomes and were not focused on a NAFLD specific population.
This study aimed to review the efficacy and safety of semaglutide, focusing on patients with NAFLD, in order to more specifically reflect the NAFLD population and expand the current understanding of semaglutide in NAFLD.
MEDLINE, CENTRAL, EMBASE, and grey literature sources were searched from inception to May 1, 2023, to identify eligible randomized controlled trials (RCTs) using a predefined search strategy. Predetermined outcomes were extracted, and quality assessment was performed using the Cochrane risk-of-bias 2 tool and GRADE framework. Meta-analysis was performed using random effects model expressing continuous outcomes as mean differences (MD) or standardized MDs (SMD), and dichotomous outcomes as odds ratios (OR) with 95% confidence intervals (CI). Statistical heterogeneity was assessed using the Cochran’s Q test and I2statistic.
A total of three RCTs involving 458 patients were included. Semaglutide increased the likelihood of NASH resolution (OR: 3.18, 95%CI: 1.70, 5.95; P < 0.001), improvement in steatosis (OR: 2.83, 95%CI: 1.19, 6.71; P = 0.03), lobular inflammation (OR: 1.81, 95%CI: 1.11, 2.96; P = 0.02), and hepatocellular ballooning (OR: 2.92, 95%CI: 1.83, 4.65; P < 0.001), but not fibrosis stage (OR: 0.71, 95%CI: 0.15, 3.41; P = 0.67). Radiologically, semaglutide reduced liver stiffness (SMD: -0.48, 95%CI: -0.86, -0.11; P = 0.01) and steatosis (MD: -4.96%, 95%CI: -9.92, 0.01; P = 0.05). It also reduced ALT (MD: -14.06 U/L, 95%CI: -22.06, -6.07; P < 0.001) and AST (MD: -11.44 U/L, 95%CI: -17.23, -5.65; P < 0.001).
Semaglutide led to improved cardiometabolic outcomes, including decreased HgA1c (MD: -0.77%, 95%CI: -1.18, -0.37; P < 0.001) and weight loss (MD: -6.53 kg, 95%CI: -11.21, -1.85; P = 0.006), but increased the occurrence of GI-related side effects (OR: 3.72, 95%CI: 1.68, 8.23; P = 0.001). Overall risk of serious adverse events was similar compared to placebo (OR: 1.40, 95%CI: 0.75, 2.62; P < 0.29).
Semaglutide demonstrated significant histologic improvements, with a higher likelihood of NASH resolution and improved NAS components, but it did not significantly improve fibrosis stage compared to placebo. Furthermore, semaglutide resulted in radiologic improvements in liver stiffness and steatosis, liver enzymes, as well as cardiometabolic effects on body weight and HgA1c, while maintaining a well-tolerated safety profile.
Additional RCTs with larger sample sizes and longer durations are required to characterize the effects of semaglutide on fibrosis regression and its role in the different phases of NAFLD.
The authors thank Dr. Lorri Puil for her advice and assistance with the statistical analysis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Canada
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Du Y, China; Morozov S, Russia; Ren WR, China S-Editor: Chen YL L-Editor: A P-Editor: Yu HG
| 1. | Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, Swain MG, Congly SE, Kaplan GG, Shaheen AA. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7:851-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 1178] [Article Influence: 392.7] [Reference Citation Analysis (1)] |
| 2. | Cheemerla S, Balakrishnan M. Global Epidemiology of Chronic Liver Disease. Clin Liver Dis (Hoboken). 2021;17:365-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 317] [Article Influence: 79.3] [Reference Citation Analysis (0)] |
| 3. | Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1028] [Cited by in RCA: 1704] [Article Influence: 243.4] [Reference Citation Analysis (0)] |
| 4. | Golabi P, Otgonsuren M, Cable R, Felix S, Koenig A, Sayiner M, Younossi ZM. Non-alcoholic Fatty Liver Disease (NAFLD) is associated with impairment of Health Related Quality of Life (HRQOL). Health Qual Life Outcomes. 2016;14:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 5. | Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, Racila A, Hunt S, Beckerman R. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64:1577-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 915] [Article Influence: 101.7] [Reference Citation Analysis (0)] |
| 6. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ; American Gastroenterological Association; American Association for the Study of Liver Diseases; American College of Gastroenterologyh. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1226] [Cited by in RCA: 1355] [Article Influence: 104.2] [Reference Citation Analysis (4)] |
| 7. | Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263-2273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1508] [Cited by in RCA: 1755] [Article Influence: 175.5] [Reference Citation Analysis (0)] |
| 8. | Ma J, Hwang SJ, Pedley A, Massaro JM, Hoffmann U, Chung RT, Benjamin EJ, Levy D, Fox CS, Long MT. Bi-directional analysis between fatty liver and cardiovascular disease risk factors. J Hepatol. 2017;66:390-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 162] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 9. | Gupta NA, Mells J, Dunham RM, Grakoui A, Handy J, Saxena NK, Anania FA. Glucagon-like peptide-1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology. 2010;51:1584-1592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 412] [Cited by in RCA: 408] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 10. | Dichtel LE. The Glucagon-Like Peptide-1 Receptor Agonist, Semaglutide, for the Treatment of Nonalcoholic Steatohepatitis. Hepatology. 2021;74:2290-2292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Davies MJ, Aroda VR, Collins BS, Gabbay RA, Green J, Maruthur NM, Rosas SE, Del Prato S, Mathieu C, Mingrone G, Rossing P, Tankova T, Tsapas A, Buse JB. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;45:2753-2786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 827] [Article Influence: 275.7] [Reference Citation Analysis (1)] |
| 12. | Targher G, Mantovani A, Byrne CD. Mechanisms and possible hepatoprotective effects of glucagon-like peptide-1 receptor agonists and other incretin receptor agonists in non-alcoholic fatty liver disease. Lancet Gastroenterol Hepatol. 2023;8:179-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 75] [Reference Citation Analysis (0)] |
| 13. | Nauck MA, Meier JJ. MANAGEMENT OF ENDOCRINE DISEASE: Are all GLP-1 agonists equal in the treatment of type 2 diabetes? Eur J Endocrinol. 2019;181:R211-R234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 153] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 14. | Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, McGowan BM, Rosenstock J, Tran MTD, Wadden TA, Wharton S, Yokote K, Zeuthen N, Kushner RF; STEP 1 Study Group. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med. 2021;384:989-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 2192] [Article Influence: 548.0] [Reference Citation Analysis (0)] |
| 15. | Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsbøll T; SUSTAIN-6 Investigators. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2016;375:1834-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3025] [Cited by in RCA: 4071] [Article Influence: 452.3] [Reference Citation Analysis (1)] |
| 16. | Dutta D, Kumar M, Shivaprasad KS, Kumar A, Sharma M. Impact of semaglutide on biochemical and radiologic measures of metabolic-dysfunction associated fatty liver disease across the spectrum of glycaemia: A meta-analysis. Diabetes Metab Syndr. 2022;16:102539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Loomba R, Abdelmalek MF, Armstrong MJ, Jara M, Kjær MS, Krarup N, Lawitz E, Ratziu V, Sanyal AJ, Schattenberg JM, Newsome PN; NN9931-4492 investigators. Semaglutide 2·4 mg once weekly in patients with non-alcoholic steatohepatitis-related cirrhosis: a randomised, placebo-controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2023;8:511-522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 241] [Article Influence: 120.5] [Reference Citation Analysis (0)] |
| 18. | Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. J Clin Epidemiol. 2006;59:7-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 995] [Cited by in RCA: 1157] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 19. | Kalogirou MS, Patoulias D, Haidich AB, Akriviadis E, Sinakos E. Liraglutide in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis of randomized controlled trials. Clin Res Hepatol Gastroenterol. 2021;45:101568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, Sanyal AJ, Sejling AS, Harrison SA; NN9931-4296 Investigators. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N Engl J Med. 2021;384:1113-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 1208] [Article Influence: 302.0] [Reference Citation Analysis (0)] |
| 21. | Flint A, Andersen G, Hockings P, Johansson L, Morsing A, Sundby Palle M, Vogl T, Loomba R, Plum-Mörschel L. Randomised clinical trial: semaglutide versus placebo reduced liver steatosis but not liver stiffness in subjects with non-alcoholic fatty liver disease assessed by magnetic resonance imaging. Aliment Pharmacol Ther. 2021;54:1150-1161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 135] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 22. | Kleiner DE, Brunt EM, Wilson LA, Behling C, Guy C, Contos M, Cummings O, Yeh M, Gill R, Chalasani N, Neuschwander-Tetri BA, Diehl AM, Dasarathy S, Terrault N, Kowdley K, Loomba R, Belt P, Tonascia J, Lavine JE, Sanyal AJ; Nonalcoholic Steatohepatitis Clinical Research Network. Association of Histologic Disease Activity With Progression of Nonalcoholic Fatty Liver Disease. JAMA Netw Open. 2019;2:e1912565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 277] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 23. | Brunt EM, Kleiner DE, Wilson LA, Sanyal AJ, Neuschwander-Tetri BA; Nonalcoholic Steatohepatitis Clinical Research Network. Improvements in Histologic Features and Diagnosis Associated With Improvement in Fibrosis in Nonalcoholic Steatohepatitis: Results From the Nonalcoholic Steatohepatitis Clinical Research Network Treatment Trials. Hepatology. 2019;70:522-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 24. | Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, Hazlehurst JM, Guo K; LEAN trial team, Abouda G, Aldersley MA, Stocken D, Gough SC, Tomlinson JW, Brown RM, Hübscher SG, Newsome PN. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1469] [Article Influence: 163.2] [Reference Citation Analysis (1)] |
| 25. | Han MAT, Altayar O, Hamdeh S, Takyar V, Rotman Y, Etzion O, Lefebvre E, Safadi R, Ratziu V, Prokop LJ, Murad MH, Noureddin M. Rates of and Factors Associated With Placebo Response in Trials of Pharmacotherapies for Nonalcoholic Steatohepatitis: Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2019;17:616-629.e26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (1)] |
| 26. | Tang A, Tan J, Sun M, Hamilton G, Bydder M, Wolfson T, Gamst AC, Middleton M, Brunt EM, Loomba R, Lavine JE, Schwimmer JB, Sirlin CB. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology. 2013;267:422-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 422] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 27. | Idilman IS, Aniktar H, Idilman R, Kabacam G, Savas B, Elhan A, Celik A, Bahar K, Karcaaltincaba M. Hepatic steatosis: quantification by proton density fat fraction with MR imaging versus liver biopsy. Radiology. 2013;267:767-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 308] [Article Influence: 25.7] [Reference Citation Analysis (1)] |
| 28. | Bannas P, Kramer H, Hernando D, Agni R, Cunningham AM, Mandal R, Motosugi U, Sharma SD, Munoz del Rio A, Fernandez L, Reeder SB. Quantitative magnetic resonance imaging of hepatic steatosis: Validation in ex vivo human livers. Hepatology. 2015;62:1444-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 29. | Park CC, Nguyen P, Hernandez C, Bettencourt R, Ramirez K, Fortney L, Hooker J, Sy E, Savides MT, Alquiraish MH, Valasek MA, Rizo E, Richards L, Brenner D, Sirlin CB, Loomba R. Magnetic Resonance Elastography vs Transient Elastography in Detection of Fibrosis and Noninvasive Measurement of Steatosis in Patients With Biopsy-Proven Nonalcoholic Fatty Liver Disease. Gastroenterology. 2017;152:598-607.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 542] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 30. | Permutt Z, Le TA, Peterson MR, Seki E, Brenner DA, Sirlin C, Loomba R. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease - MRI accurately quantifies hepatic steatosis in NAFLD. Aliment Pharmacol Ther. 2012;36:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 281] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 31. | Jayakumar S, Middleton MS, Lawitz EJ, Mantry PS, Caldwell SH, Arnold H, Mae Diehl A, Ghalib R, Elkhashab M, Abdelmalek MF, Kowdley KV, Stephen Djedjos C, Xu R, Han L, Mani Subramanian G, Myers RP, Goodman ZD, Afdhal NH, Charlton MR, Sirlin CB, Loomba R. Longitudinal correlations between MRE, MRI-PDFF, and liver histology in patients with non-alcoholic steatohepatitis: Analysis of data from a phase II trial of selonsertib. J Hepatol. 2019;70:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 159] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 32. | Loomba R, Sanyal AJ, Kowdley KV, Terrault N, Chalasani NP, Abdelmalek MF, McCullough AJ, Shringarpure R, Ferguson B, Lee L, Chen J, Liberman A, Shapiro D, Neuschwander-Tetri BA. Factors Associated With Histologic Response in Adult Patients With Nonalcoholic Steatohepatitis. Gastroenterology. 2019;156:88-95.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 33. | Song T, Jia Y, Li Z, Wang F, Ren L, Chen S. Effects of Liraglutide on Nonalcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Diabetes Ther. 2021;12:1735-1749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 34. | Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, Friedman SL, Diago M, Romero-Gomez M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology. 2015;149:367-78.e5; quiz e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1181] [Cited by in RCA: 1625] [Article Influence: 162.5] [Reference Citation Analysis (1)] |
| 35. | Aroda VR, Ahmann A, Cariou B, Chow F, Davies MJ, Jódar E, Mehta R, Woo V, Lingvay I. Comparative efficacy, safety, and cardiovascular outcomes with once-weekly subcutaneous semaglutide in the treatment of type 2 diabetes: Insights from the SUSTAIN 1-7 trials. Diabetes Metab. 2019;45:409-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 172] [Article Influence: 28.7] [Reference Citation Analysis (0)] |