Published online Aug 28, 2023. doi: 10.3748/wjg.v29.i32.4815
Peer-review started: June 5, 2023
First decision: July 8, 2023
Revised: July 22, 2023
Accepted: August 9, 2023
Article in press: August 9, 2023
Published online: August 28, 2023
Processing time: 80 Days and 23.2 Hours
The robotic liver resection (RLR) has been increasingly applied in recent years and its benefits shown in some aspects owing to the technical advancement of robotic surgical system, however, controversies still exist. Based on the foundation of the previous consensus statement, this new consensus document aimed to update clinical recommendations and provide guidance to improve the outcomes of RLR clinical practice. The guideline steering group and guideline expert group were formed by 29 international experts of liver surgery and evidence-based medicine (EBM). Relevant literature was reviewed and analyzed by the evidence evaluation group. According to the WHO Handbook for Guideline Development, the Guidance Principles of Development and Amendment of the Guidelines for Clinical Diagnosis and Treatment in China 2022, a total of 14 recommendations were generated. Among them were 8 recommendations formulated by the GRADE method, and the remaining 6 recommendations were formulated based on literature review and experts’ opinion due to insufficient EBM results. This international experts consensus guideline offered guidance for the safe and effective clinical practice and the research direction of RLR in future.
Core Tip: The robotic liver resection (RLR) has been increasingly applied in recent years. Based on the foundation of the previous consensus statement, this new consensus guideline document aimed to update clinical recommendations and provide guidance to improve the outcomes of RLR clinical practice. The guideline steering group and guideline expert group were formed by 29 international experts of liver surgery and evidence-based medicine. Relevant literature was reviewed and analyzed by the evidence evaluation group. According to the WHO Handbook for Guideline Development, the Guidance Principles of Development and Amendment of the Guidelines for Clinical Diagnosis and Treatment in China 2022, a total of 14 recommendations were generated.
- Citation: Liu R, Abu Hilal M, Wakabayashi G, Han HS, Palanivelu C, Boggi U, Hackert T, Kim HJ, Wang XY, Hu MG, Choi GH, Panaro F, He J, Efanov M, Yin XY, Croner RS, Fong YM, Zhu JY, Wu Z, Sun CD, Lee JH, Marino MV, Ganpati IS, Zhu P, Wang ZZ, Yang KH, Fan J, Chen XP, Lau WY. International experts consensus guidelines on robotic liver resection in 2023. World J Gastroenterol 2023; 29(32): 4815-4830
- URL: https://www.wjgnet.com/1007-9327/full/v29/i32/4815.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i32.4815
Laparoscopic liver resection (LLR) was first introduced in the 1990s for treatment of benign and malignant tumors[1,2]. Over the past few decades, laparoscopic hepatectomy has developed rapidly and gained widespread acceptance around the world. In 2008, the Louisville Statement indicated the feasibility of laparoscopic hepatectomy[3], and the second international consensus conference in Morioka recommended LLR as a standard practice in specific types of hepatectomy[4]. After that, the Southampton consensus guidelines promoted the safe expansion of LLR and improved patient care after LLR[5]. Since then, laparoscopic techniques have occupied an important place in hepatobiliary surgery. At the same time, with the approval of clinical application and technique developments of robotic surgery systems, robotic hepatectomy has gradually been used in clinical practice. Experience in robotic liver resection (RLR) has been reported in many parts of the world and its feasibility has gradually been identified by clinical studies[6-8]. The robotic system is superior in providing three-dimensional magnified field of vision and robotic arms offer flexibility and tremor filter to surgeons to overcome the shortages of conventional laparoscopic hepatectomy. However, the high cost of the mainstream models and the lack of surgical instruments and experience limit the application of RLR.
With the advent of advanced technique and with experience accumulation, the indications of RLR have been expanded. The robotic system can be used in almost all types of liver resection, including minor hepatectomy, major hepatectomy, donor hepatectomy, and complex liver resections[9-16]. In 2018, an international consensus statement on robotic hepatectomy provided recommendations for RLR based on relevant research and experts’ opinions, which contribute to standardization of robotic hepatectomy[17]. However, the recommendation grades were relatively low due to the lack of high-quality evidence. Furthermore, some important issues, like cost-effectiveness of RLR, learning curve of RLR, and outcomes of RLR for difficult liver segments, remain controversial. Available data derive from case series, case-comparative studies, reviews and meta-analyses have been published in recent years, and some new theories also provide new views and perspectives on RLR[18-25].
Based on the foundation of the previous statement, the 2023 international consensus statement on RLR aimed to update clinical recommendations and provide guidance to improve the outcomes of RLR clinical practice. We invited a team of robotic surgeon experts to provide their views related to RLR. Based on the related topics, we searched the online databases for RLR studies and the GRADE system was used to grade the evidence. The final consensus was reached by using a combination of clinical evidence and experts’ opinions with the aim to improve the clinical outcome of RLR.
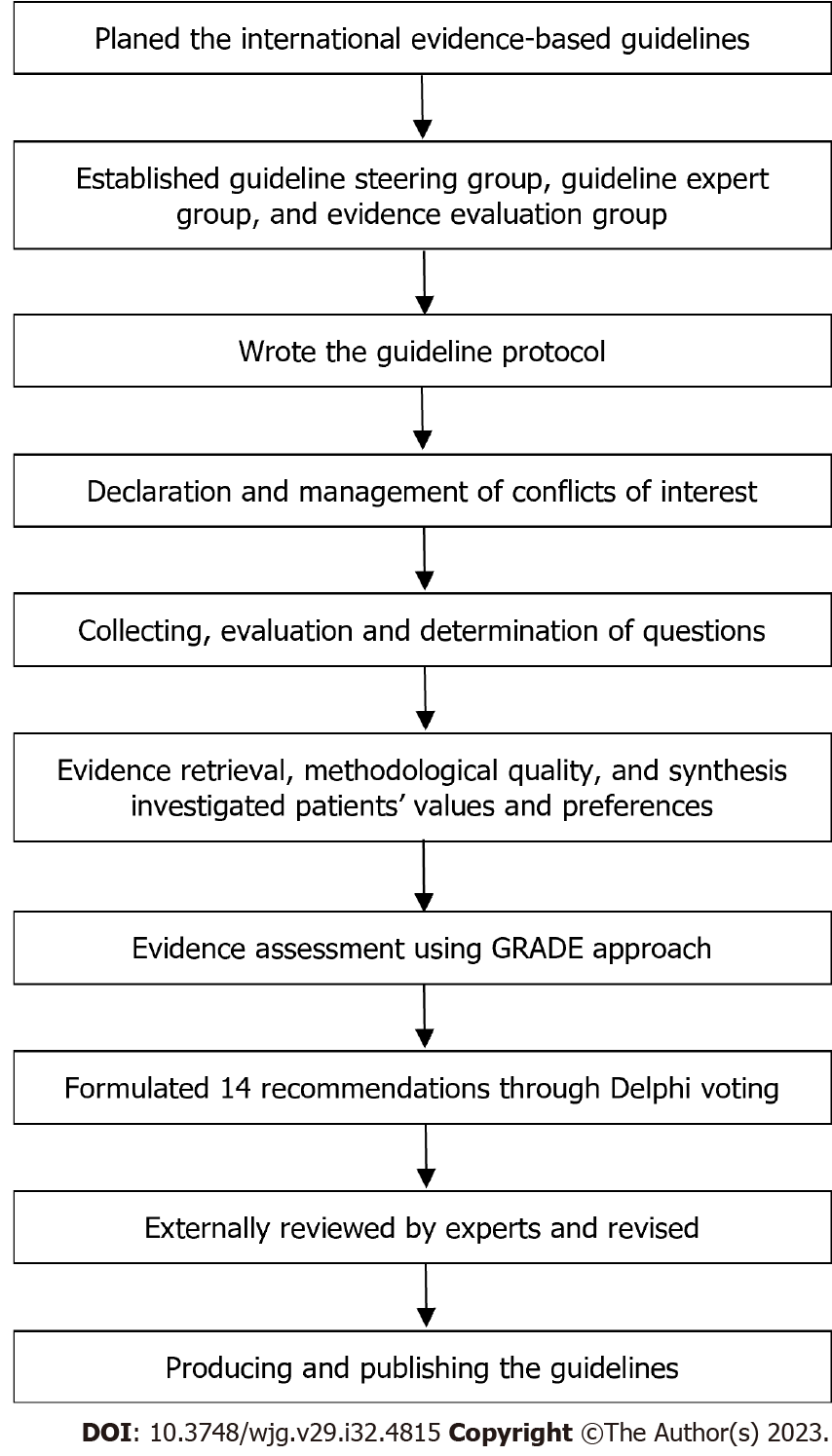
This international evidence-based guideline is based on the WHO Handbook for Guideline Development and refers to the Guidance Principles of Development and Amendment of the Guidelines for Clinical Diagnosis and Treatment in China 2022. Reporting of the guideline follows the Appraisal of Guidelines, Research, and Evaluation (AGREE II)[26]. A flow chart describes the process and steps of the guideline development (Figure 1).
The guideline development group included the Steering Committee, Consensus Expert Group, and Evidence Evaluation Group. The Steering Committee and Consensus Expert Group consisted of 29 members from different countries or regions. Literature review, questionnaires, and expert discussions were implemented to initially identify clinical questions. All members of the Steering Committee and Consensus Expert Group were encouraged to submit suggestions and add potential questions. Each question was then evaluated and confirmed by the Steering Committee through meetings or emails. The Evidence Evaluation Group performed a literature search, literature screening, method quality evaluation, and data extraction, and thus formed the body of evidence.
PubMed, Cochrane Library, Web of Science, Embase, Scottish Intercollegiate Guidelines Network, WHO, National Guideline Clearing-house, Guidelines International Network, and National Institute for Health and Care Excellence databases were systematically searched from inception to September 5, 2022, to retrieval potential eligible studies. There were no regional and language restrictions, but it was limited to human studies. The Evidence Evaluation Group evaluated titles, abstracts, and full texts to identify the eligible publications based on inclusion and exclusion criteria approved by the Steering Committee. The eligible literature included clinical guidelines, systematic reviews and meta-analyses, randomized controlled trials (RCT), non-RCT, cohort studies, case-comparative studies, and case reports or case series that were considered for inclusion when necessary. Information on the included studies was extracted according to a pre-designed extraction form. A Measure Tool to Assess Systematic Reviews (AMSTAR II) was used to evaluate the methodological quality of the included systematic reviews and meta-analyses[27]. The revised Cochrane Risk of Bias Tool and the Newcastle-Ottawa Scale were performed to assess the methodological quality of their corresponding types of clinical studies[28,29]. All the above processes were completed by two independent terms of the Evidence Evaluation Group. If there are differences, they will be resolved by discussing or consulting third parties.
According to the WHO Handbook for Guideline Development, the Evidence Evaluation Group evaluated the need for an update or rapid development of systematic review[30]. The method of GRADE was used to assess the quality of the evidence[31]. Based on the GRADE approach, clinical outcomes, patients’ benefit, and economic evaluation, the Steering Committee and Consensus Expert Group used the GRADE decision form and Delphi voting to formulate the strength of recommendations. The consensus was considered to have been reached when 80% of the experts approved a proposal. Before submitting the manuscript for publication, the final draft was reviewed and approved by all members of the guideline development group. This evidence-based guideline is expected to be updated again in 2026.
In general, the indications of the RLR are similar to those of LLR and open liver resection (OLR). RLR can be applied for treating various liver diseases, including hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma (ICC), colorectal liver metastases (CRLM), benign tumors of liver, and living donor hepatectomy. RLR is associated with the following advantages over OLR and LLR in the majority of the currently available studies: less intraoperative blood loss, shorter postoperative hospital stay, less overall complications, and lower pain intensity after surgery[21,22,32-45]. However, in complex hepatectomies such as large tumor size or proximity of tumor to vital vascular structures, RLR should be performed with caution and by highly experienced surgeons (clinical recommendation: Expert agreement 100%).
In a prospective study using propensity score matching, Zhu et al[21] compared the short-term perioperative outcomes and long-term survival outcomes of RLR, LLR, and OLR in patients with BCLC stage 0-A hepatocellular carcinoma. The results showed that there were no significant differences among the three interventions except for a shorter hospital stay in the minimally invasive group (RLR and LLR)[21]. Hu et al[33] evaluated the clinical efficacy of robotic, laparoscopic, and open liver resection for giant liver haemangiomas (> 10 cm in diameter). They reported robotic hemihepatectomy to be associated with less intraoperative blood loss, better postoperative recovery and lower pain score than OLR. When compared with laparoscopic hemihepatectomy, robotic hemihepatectomy was associated with significantly less intraoperative blood loss and a shorter operative time[33]. Masetti et al[46] analyzed the short-term outcomes of patients with CRLM comparing from a multicenter study who underwent RLR vs LLR. The results showed RLR and LLR to be comparable in postoperative overall complication rates, intraoperative blood loss, conversion rates, operation time, and hospital stay, but RLR showed a reduced rate of R1 resection margins compared with LLR. Masetti et al[46] found the effect size of RLR to be increased for posterosuperior lesions and difficult procedures, which may be due to RLR being able to offer some technical advantages over conventional laparoscopy to improve the short-term outcomes of these patients.
Recommendation: RLR is safe and feasible for HCC, as it is associated with lower overall complication rates than LLR and OLR and a shorter hospital stay than OLR, although it has a longer operative time than LLR and OLR. Other perioperative outcomes are comparable among the three interventions. Regarding oncologic outcomes, limited evidence suggested there is also no significant difference.
Level of evidence: Low level of recommendation: Weak (Grade 2C). Expert agreement: 96.55%.
Two systematic reviews[47,48] and 15 publications[21,35,37,43,49-59] were included to evaluate the perioperative and oncologic outcomes of robotic vs laparoscopic or open hepatectomy for treatment of HCC patients, respectively. When compared with LLR for HCC patients, the pooled results indicated that RLR had a lower overall complications rate (RR = 0.72, 95%CI: 0.57 to 0.90; P = 0.003), lower minor complication (Clavien-Dindo I-II) rate (RR = 0.72, 95%CI: 0.54 to 0.96; P = 0.030), and longer operative time (SMD = 0.66, 95%CI: 0.22 to 1.10; P = 0.003)[21,50,52-57]. When compared with OLR for HCC patients, RLR was associated with a shorter hospital stay (SMD = -0.42, 95%CI: -0.57 to -0.28; P < 0.00001), lower overall complication rate (RR = 0.61, 95%CI: 0.45 to 0.84; P = 0.002), lower minor complication rate (RR = 0.59, 95%CI: 0.42 to 0.84; P = 0.003), and longer operative time (SMD = 0.82, 95%CI: 0.18 to 1.46; P = 0.01)[21,35,37,43,49,51,58,59]. For other outcomes, RLR showed similar results when compared with LLR or OLR, including estimated blood loss, transfusion rate, severe complication (Clavien-Dindo III-IV), and 90-d mortality. Thus, RLR was comparable to OLR and LLR in feasibility and safety for patients with HCC.
Regarding oncologic outcomes, the three interventions showed comparable R0 resection rates and similar short- and long-term oncological outcomes[21,35,37,43,49,51,53-56,58,59]. Also, a recently published study with a large sample size suggested that robotics may be associated with improved overall survival[54]. The study reported that the RLR group had significantly higher 1- , 3- , and 5- year overall survival rates of 92%, 75%, and 63% compared with the LLR group of 86%, 60%, and 45%, respectively (all P < 0.01)[54]. Based on the currently available studies, the implementation of RLR is feasible and safe and is associated with similar oncologic outcomes. Prospective studies are recommended to further evaluate whether the safety and efficacy of RLR can be affected by tumor size, resection complexity, and the quality of the underlying liver parenchyma.
Recommendation: Currently, there is insufficient evidence to compare the safety and feasibility between RLR and LLR for treatment of ICC. Limited evidence suggests that RLR has less intraoperative blood loss, shorter hospital stay, and better overall survival than OLR.
Level of evidence: Very low. Level of recommendation: Weak (Grade 2D). Expert agreement: 86.21%.
Two comparative studies were reported to evaluate the safety and feasibility of robotic vs open hepatectomy for ICC[36,60]. The pooled results suggested that RLR had less intraoperative blood loss (SMD = -0.75, 95%CI: -1.46 to -0.05; P = 0.04) and shorter hospital stay (SMD = -0.31, 95%CI: -0.54 to -0.09; P = 0.006) than OLR[36,60]. There were no significant differences in other perioperative outcomes between the two groups. Limited evidence suggests RLR may improve the outcomes of ICC when compared to OLR, but we need to carefully interpret these findings. Shapera et al[60] suggested that patients after RLR or OLR had similar resection margins, and median overall survival was similar in patients with any resection margin distance. Hamad et al[36] compared short- and long-term outcomes between RLR and OLR for patients with ICC, and they reported that patients who underwent RLR had shorter hospital stays and similar long-term risk of death between the two groups. These results suggested RLR for ICC could shorten hospital stay without compromising oncological outcomes such as negative margins, postoperative mortality, and long-term survival[36]. As there have been few comparative studies focusing on ICC and there is no meta-analysis on the short- and long-term clinical outcomes of RLR vs LLR or OLR for ICC, higher levels of evidence are urgently needed to answer this question.
Recommendation: RLR is safe and feasible for patients with CRLM, since it is associated with a lower conversion rate but longer hospital stay than that of LLR. Limited evidence suggests no significant difference in all the perioperative outcomes between RLR and OLR in patients with CRLM. Oncologic outcomes with limited evidence suggested there was also no significant difference between RLR vs LLR and RLR vs OLR.
Level of evidence: Very low. Level of recommendation: Weak (Grade 2D). Expert agreement: 89.66%.
Studies on RLR vs LLR or OLR for CRLM have been extensively published in the past three years[45,46,61-63]. The evidence suggested RLR to have a lower conversion rate (RR = 0.42, 95%CI: 0.23 to 0.77; P = 0.005) and longer hospital stays (SMD = 0.19, 95%CI: 0.03 to 0.35; P = 0.020) than LLR for patients with CRLM, and there were no significant difference in operation time, estimated blood loss, intraoperative blood transfusion rates, R0 resection, postoperative morbidity, and overall survival[45,46,61,62]. In addition, limited evidence showed there were no significant differences in operation time, estimated blood loss, R0 resection, hospital stays, overall complications, minor and major complications, postoperative 30-d mortality, and survival outcomes between RLR and OLR for patients with CRLM[61,63]. With appropriate expertise and experience, robotic-assisted surgery can be considered to be an alternative minimally invasive approach to CRLM resections[64,65].
Currently, there is insufficient evidence on patients who underwent simultaneous robotic-assisted resections of CRLM and the primary tumor. A conference abstract on one RCT in comparing RLR and OLR for simultaneous resections suggested a longer operating time in the robotic arm, and patients who underwent RLR had less blood loss, less Clavien-Dindo III-IV complications, a shorter time to pass first flatus, and a shorter hospital stay when compared to open surgery[40]. A recent systematic review reported that all patients who underwent robot-assisted R0 resection had no perioperative deaths[65]. However, when patients who underwent simultaneous major hepatectomy combined with complex colorectal surgery, the operative risks were increased, leading to poorer perioperative outcomes with increased length of stay, morbidity, and mortality, and unfavorable patient recovery due to delay in the initiation of subsequent adjuvant therapy[5,65].
Recommendation: Robotic living donor hepatectomy can be a safe and feasible alternative to open and laparoscopic approach. Robotic living donor hepatectomy has a longer operative time than that of OLR and LLR, but a shorter hospital stay compared with OLR. The other donor and recipient outcomes were reported to be comparable among the three interventions.
Level of evidence: Very low. Level of recommendation: Weak (Grade 2D). Expert agreement: 96.55%.
We systematically reviewed 4 studies that investigated the safety and feasibility of robotic and open living donor hepatectomy, with a total of 972 patients being included in the meta-analysis[41,66-68]. The pooled results suggested that RLR had lower postoperative peak serum bilirubin (SMD = -0.59, 95%CI: -0.81 to -0.37; P < 0.0001), shorter postoperative hospital stays (SMD = -0.53, 95%CI: -0.90 to -0.17; P = 0.004) and a longer operative time (SMD = 1.45, 95%CI: 0.66 to 2.25; P = 0.003) compared to the open group[41,66-68]. There were no significant differences in terms of other donor and recipient outcomes. Rho et al[41] evaluated the clinical and perioperative outcomes of robotic living donor right hepatectomy from carried out on 52 consecutive cases patients and compared with patients who underwent comparison with open (n = 62) and laparoscopic (n = 118) donor hepatectomy. They reported that although RLR was associated with a longer operation time, the mean estimated blood loss was significantly lower compared with LLR and OLR, and donor satisfaction (body image and cosmetic appearance scores) was higher in RLR than that of LLR[41].
Large incisions resulting in large scars are an ongoing concern for surgeons performing open living donor hepatectomy. For robotic living donor hepatectomy, there is a better body image, improved cosmetic appearance, and fewer wound-related complications[41]. Living donor hepatectomy is considered to be the pinnacle of hepatobiliary surgery, which requires assurance of donor survival, good graft status and minimization of associated complications[66]. Based on the currently available studies, RLR reduced postoperative pain, resulted in rapid donor recovery and with similar postoperative complications[41,66-68]. However, studies comparing RLR with LLR are insufficient[41]. In summary, RLR for living donor hepatectomy is feasible and safe when performed by surgeons with both excellent knowledge of liver anatomy and long experience of open living donor hepatectomy[41,66]. However, biliary complications are a problem that should never be ignored. The combination of robotic-assisted procedures and indocyanine green (ICG) fluoroscopy is recommended for precise division and fine suturing of the divided bile ducts[41,66].
Recommendation: For minor hepatectomy, the safety and feasibility of RLR are comparable to that of LLR and OLR. Robotic minor hepatectomy was reported to have a longer operative time than LLR, but there was less overall complication. RLR resulted in a shorten hospital stay and decreased overall morbidity compared to the open approach. The other perioperative outcomes were comparable among the three interventions.
Level of evidence: Low. Level of recommendation: Weak (Grade 2C). Expert agreement: 96.55%.
Minor hepatectomy with fewer than 3 adjacent hepatic segmental resections is the most commonly carried out procedure in RLR. As a minimally invasive surgery, the benefits of the robotic approach for minor hepatectomy have been controversial[36,37,49,51,69-72]. An updated meta-analysis based on new evidence was conducted by the Evidence Evaluation Group on 13 retrospective cohort studies with 735 robotic and 1362 laparoscopic minor hepatectomies[12,22,34,53,73-81]. The results showed that the operative time was significantly longer (SMD = 0.44, 95%CI: 0.19 to 0.69; P = 0.0006) with the robotic approach, but it had less overall complication (RR = 0.76, 95%CI: 0.61 to 0.95; P = 0.01) than LLR. No statistically significant differences were observed in estimated blood loss, conversion rate, blood transfusion rate, postoperative hospital stays, 90-d mortality, and R0 resection. Based on the currently available studies and expert opinions, RLH was associated with a longer operation time when compared with LLH because of the additional time required to dock and undock the robot, and the large extent to exchange instruments required by the robot-assisted procedures[34,69,70].
We also systematically reviewed 6 studies to investigate the safety and feasibility between RLR and OLR[9,38,71,73,82,83]. The results showed that robotic minor hepatectomy had a shorter postoperative hospital stay (SMD = -0.91, 95%CI: - 1.27 to -0.55; P < 0.00001), less overall complications (RR = 0.39, 95%CI: 0.23 to 0.64; P = 0.0002), and less severe complications (RR = 0.28, 95%CI: 0.08 to 0.97; P = 0.04) when compared to open surgery. There were no significant differences in terms of operation time, estimated blood loss, intra-operative blood transfusion rate, and readmission rate, and R0 resection for malignancy between robotic and open minor hepatectomies. Liver resections for difficult-located segments (1, 4a, 7, and 8) are recommended to be defined as technical major hepatectomy.
Recommendation: For major hepatectomy, robotic hepatectomy is as safe and feasible as laparoscopic and open hepatectomy. Compared with LLR, RLR was significantly better in estimated blood loss and conversion rate. In comparison with OLR, the estimated blood loss and hospital stay of RLR are significantly better than those of OLR, but there is a longer operation time in the RLR group.
Level of evidence: Low. Level of recommendation: Weak (Grade 2C). Expert agreement: 93.10%.
Chong et al[32] compared the efficacy and safety of 989 individuals, including 220 who underwent robotic and 769 who underwent laparoscopic right and extended right hepatectomy (RH/ERH). They reported a lower open conversion rate and a shorter postoperative hospital stay for robotic RH/ERH. We systematically reviewed 10 studies that investigated the safety and feasibility of robotic and laparoscopic major hepatectomy[22,32,33,39,84-89]. The results showed that robotic major hepatectomy resulted in less estimated blood loss (SMD = -0.52, 95%CI: -0.88 to -0.16; P = 0.005) and a lower conversion rate (RR = 0.44, 95%CI: 0.29 to 0.67; P = 0.0001) compared to laparoscopic surgery. There were no significant differences between robotic and laparoscopic major hepatectomies in transfusion rate, R0 resection, readmission, mortality within 90 d, overall complications, mild/severe complication, bile leakage and liver failure rates.
Hamad et al[36] investigated 1876 patients who underwent open (n = 1804) and robotic assisted (n = 72) resection between 2004 and 2017. The results showed that the patients who underwent RLR had a shorter length of hospital stays yet there was no difference in 30-d readmission or 90-d mortality[36]. Lee et al[43] matched 36 patients each in the robotic and open group. They found that operative time was significantly longer but the postoperative hospital stays was significantly shorter in the robotic group[43]. An updated meta-analysis based on the latest evidence was conducted by the Evidence Evaluation Group on 8 retrospective cohort studies. The pooled results suggested the estimated blood loss and hospital stay of the robotic group to be significantly shorter than the open group, but there was a longer operation time in the robotic group[22,33,37,43,49,51,71,72].
We also focused on the safety and feasibility of RLR in technically “major” resections (segment 1, 4a, 7, and 8). Liver resection on segments 1, 4a, 7, and 8 is relatively more technically difficult owing to a large parenchymal transection plane with proximity to critical structures and major vessels[90-92]. RLR on difficult liver segments has been reported to be a safe and feasible procedure, and many experienced surgeons tend to perform robotic resections on these selected patients. In a recent international multicenter retrospective study comparing robotic vs laparoscopic right posterior sectionectomy, the author reported RLR to be associated with reduced blood loss and lower open conversion rates than LLR, and suggested that RLR and LLR could be performed in expert centers with good outcomes in well selected patients[93]. A study reported a patient with hepatocellular carcinoma which involved segments 4 and 8 to undergo robotic central bisectionectomy. The outcomes indicated that robotic central bisectionectomy could be performed safely using proper exposure techniques and an appropriate combination of several useful technical tips[16]. A multicenter study reported that robotic right posterior sectionectomy could be performed in expert centers with less blood loss and lower conversion rates in well-selected patients[93,94]. Another study indicated that RLR on segments 1, 4a, 7, and 8 showed similar surgical outcomes, including blood loss, hospital stay, R0 negative margin rate, and morbidity, when compared with laparoscopic liver resection[95]. The application of robotic approach has also been reported for caudate lobectomy, including Spiegelian lobectomy, isolated partial and complete caudate lobectomy[96,97]. However, most studies reported only a few cases with the potential of selection bias, thus limiting the application of the results. It is necessary to conduct high-quality clinical studies in the future to further clarify the impact of robotic hepatectomy on difficult liver segments.
Recommendation: As the policy on medical expense and definition of cost are different in the literature, the real cost of three interventions should be calculated and compared based on a standard method in the future. Limited evidence suggests the total cost of RLR to be higher than LLR, but there were no significant differences between RLR and OLR. The cost-effectiveness of the three interventions should be synthetically evaluated based on many factors, including direct and indirect costs, hidden benefits from favorable clinical outcomes and local social and economic situations.
Level of evidence: Very low. Level of recommendation: Weak (Grade 2D). Expert agreement: 89.66%.
The most common concern about cost-effectiveness analysis of RLR is simply on the total cost, hospitalization costs, and readmission costs. One systematic review and meta-analysis and nine additional updated original studies were included to answer this question[9,34,42,84,98-103]. The resynthesized evidence suggests that the total costs (SMD = 1.15, 95%CI: 0.24 to 2.07; P = 0.01) and hospitalization costs (SMD = 0.96, 95%CI: 0.50 to 1.41; P < 0.0001) of RLR is higher compared to LLR[34,42,75,78,104]. However, no statistically significant differences were observed regarding total costs, hospitalization costs, and readmission costs between RLR and OLR[9,42,44,68,101]. In addition, total costs were assessed for different type of liver resection. For the included studies which investigated RLR vs LLR, these studies were more focused on the cost-effectiveness in minor liver resections, and the results showed that RLR had higher total costs than LLR (SMD = 1.22, 95%CI: 0.60 to 1.83; P < 0.0001)[34,75,78]. When various types of pathologies in patients were included in the comparison between RLR and OLR, including complex hepatolithiasis, donor right hepatectomy, minor and major resections, these confounders were introduced to become an important reason to decrease the level of evidence[9,44,68,103]. According to the currently available studies, even though the higher total costs of RLR were closely associated with more operating room supplies[34,68,73,79,80,84], the higher total costs were mainly due to longer hospital stays and more treatment of complications in the laparoscopic and open group than the robotic group[9,71,100,105,106]. The favorable perioperative outcomes in RLR should be weighed against the higher operative costs when compared with laparoscopic or open approaches[98].
Robotic surgical systems have evolved rapidly in recent years, and the trends in the total cost and operative cost are worth future investigating. Although the costs of RLR may differ among countries or regions due to differences in medical policy or insurance premium, a serious obstacle to the widespread use of robotic surgical system is the large indirect cost[34,78-80]. Furthermore, a comprehensive evaluation of the cost-effectiveness should also consider the hidden benefits from favorable clinical outcomes associated with robotic hepatectomy to include patient’s psychological benefits and social benefits.
In the setting of cirrhotic patients, similar to LLR, RLR could also be performed in to selected patients. Currently, there are insufficient studies focusing on the application of RLR on cirrhotic patients (clinical recommendation: Expert agreement 100%).
The improvement of surgical techniques, perioperative management, and patient selection improved the surgical outcome of patients with cirrhosis, and the overall improvement of surgical techniques as represented by the minimally invasive approach improved the outcomes of perioperative management in patients with cirrhosis[107-109]. Minimally invasive hepatectomy for cirrhotic patients has been reported to be associated with less complications, shorter postoperative hospital stay, and similar outcomes in operative time, estimated blood loss and postoperative complications when compared with non-cirrhotic patients, and laparoscopic surgery may provide potential benefits in reducing the incidence of postoperative ascites and liver failure[5,110]. Only a few patients were included in these studies and the outcome of robotic hepatectomy in patients with cirrhosis were not evaluated separately[47]. It is necessary to conduct more research on RLR for liver cancer patients with cirrhosis to further evaluate the safety and efficacy of the robotic approach.
For lesions located close to major vascular and biliary structures, especially for deeply located lesions, parenchyma-sparing liver resection should be performed by using the robotic approach to rely on the delicate dissection offered by the stable and flexible movements of the robotic arms, as an alternative approach to major liver resection. Compared to robotic major liver resection, robotic parenchyma-sparing liver resection could potentially increase resectability of these lesions. However, as this is a technically demanding procedure, it should be performed by experienced surgeons on well-selected patients (clinical recommendation: Expert agreement 100%).
For malignant liver lesion with a small tumor size but in close proximity to important vessels such as the hepatocaval confluence, or the bifurcation of the primary or secondary Glissonean pedicles, the conventional approach is to use major resection to remove the vessel in its entirety along with the liver tissues it supplies or drains. However, in patients with a poor liver function and insufficient liver remnant volume, the safety of major resection is difficult to ensure, resulting in a reduced operative resection rate. Parenchyma-sparing liver resection addresses this dilemma to some extent. Removal of the tumor off the adjacent vascular structures or combined vascular resection and reconstruction could increase the resection rate of the tumor. In contrast to the laparoscopic approach with its intrinsic drawbacks including the fulcrum effect and rigid instruments, the robotic system could offer stable and flexible movements of the instruments, which facilitate the operative procedures to achieve a higher level of difficulty. RLR for treatment of centrally located lesions has been reported and shown to be safe and feasible in small case series[111,112]. In addition, with the help of intraoperative ultrasound, it has been shown that the robotic approach allowed optimal access to all liver segments and facilitated parenchymal-sparing surgery for lesions located in the posterosuperior segments or in contact with main liver vessels, not only for colorectal liver metastasis but also for HCC[113]. Robotic parenchyma-sparing liver resection for lesions located close to major vascular and biliary structures allows for sufficient liver remnant to be retained, thus allowing patients to start their postoperative adjuvant therapy early, which is important in improving patients’ survival in today’s rapidly evolving of comprehensive treatment.
Robotic first- or second-stage associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) is an optional strategy for treatment of primary and metastatic liver cancer in patients with insufficient residual liver volume. Due to the complexity of ALPPS surgery and the high morbidity rate, the benefit of robotic ALPPS is unclear on the curative effect of the initially unresectable liver cancer, as there have been rapidly evolving developments in locoregional and systemic therapies. Robotic ALPPS must be evaluated with caution before operation and should only be performed in highly selected patients (clinical recommendation: Expert agreement 100%).
ALPPS has been introduced to treat patients with advanced liver tumors and insufficient residual liver volumes for more than 10 years. However, there is still a lot of controversies on the value of this approach due to its high mortality rate[114,115]. Minimally invasive approach for ALPPS has been applied for the purpose of reducing mortality and improving recovery of patients. Most of the ALPPS procedures were used for the first-stage[116]. A systematic review of 27 patients who underwent ALPPS reported the potential benefit of using the minimally invasive ALPPS in reducing morbidity and mortality[117]. However, there was selective bias in this study and the sample size was small, which compromised the robustness of the conclusions. Previous studies with small case numbers showed that the robotic approach could be used for the first-stage ALPPS for HCC, CRLM and ICC[118-120]. Complex robotic ALPPS procedures (e.g., robotic ALPPS for patients with portal vein thrombosis, robotic ALPPS with simultaneous left colectomy) have also been reported to be safe and feasible carried out by experienced surgeons[121-124]. After the long period of development of ALPPS and its modified approaches, the role of ALPPS should be revisited due to its complexity in treating patients with advanced stage of malignancy and the high morbidity rate, especially when facing the major developments in locoregional and systemic therapies[125-128]. Although the robotic ALPPS is technically feasible, the oncological and health economic benefits are still unclear. The efficacy of robotic ALPPS still needs to be evaluated through high-quality clinical researches.
The case number required to surmount the learning curve for RLR has been reported to be lower than that for LLR. The case number required to surmount the learning curve of RLR varied among different studies[12,32,129-134]. The surgeons’ experience in LLR could have a significant influence on the learning curve of RLR. About 25 consecutive cases are needed for an experienced surgeon to surmount the learning curve of major RLR and 15 cases for minor RLR (clinical recommendation: Expert agreement 100%).
Because of the enhanced surgical dexterity offered by the robotic system, robotic hepatectomy may have a shorter learning curve compared to laparoscopic hepatectomy. However, the large experience in LLR surgeons had before embarking on robotic liver surgery could have an impact on the learning curve[100,135]. Chua et al[136] reviewed the literature reported up to July 2019 pertaining to the learning curves in minimally invasive hepatectomy (MIH) by searching PubMed and Scopus databases. Forty studies were included to explore quantitatively the learning curve for MIH. They reported the case number required to surmount the learning curve for RLR was 25 consecutive cases (range: 16-50)[136].
Liu et al[131], by analyzing the learning curve of 100 robotic left and right hemihepatectomy (RLH/RRH) in terms of operative time, reported that the learning process was completed in the RLH group after an initial phase of 35 cases, which was shorter than the RRH group (n = 45). Given that, mobilization and resection of the left liver are easier to perform than the right liver, RLH might be the first choice for beginners.
Ban and Iwate reported on the difficulty scoring system for LLR which was externally validated for RLR. The two difficulty scoring systems are currently recommended. A difficulty scoring system exclusively for RLR should be established by further studies (clinical recommendation: Expert agreement 100%).
By predicting the complexity of different surgeries, the difficulty scoring system (DSS) can recommend suitable patients to surgeons at their corresponding learning stages. Current DSS scores on the basis of the weighted combination of predicted factors that affect the surgeries’ complexity include tumor size, position, number of the tumor, extent of liver resection and liver function[137,138].
However, the scoring system for RLR remains to be designed. Linn et al[137] summarized 11 types of DSS for LLR based on previously reported studies and conducted a meta-analysis. The results showed that 5 DSS (including Ban DSS, Iwate DSS, Hasegawa DSS, IMM DSS, and Southampton DSS) could be used for predicting the difficulty of LLR surgeries[138-141]. Though no DSS has significant advantages, Ban and Iwate DSS were externally validated for RLR[137,138].
Intraoperative ultrasonography (IOUS) and indocyanine green (ICG) imaging has been used for tumor locating and surgical margin delineation in RLR. Surgeons are supposed to master these techniques and choose the suitable navigation tools to increase the safety of RLR (clinical recommendation: Expert agreement 100%).
Studies have supported the importance of IOUS in liver operations[142,143]. Based on the experience of IOUS in 110 consecutive patients, Zhu et al[144] put forward a standardized 4-step IOUS agreement to ensure the safety of RLR operation. Moreover, when using IOUS to examine all the patients, they found 11 patients (10%) had extra lesions, and 7 of these patients (63.64%) underwent improved surgical strategies[144]. However, IOUS has the limitations to accurately show irregular hepatic segmental demarcation and anatomical structure, while ICG imaging has become a supplementary tool for IOUS due to its ability on real-time 3D recognition of tumor margins and to guide liver transection plane. Wakabayashi et al[145] reviewed articles related to ICG imaging in liver resection and showed that the combination of IOUS and ICG imaging can increase the safety of liver resection, but the timing and dose administration of ICG remain uncertain. Furthermore, Liu and his team reported the application of ICG using “four-zone three-phase” fluorescence imaging in robot-assisted anatomical hepatectomy in which the liver was divided into 4 anatomical zones include the “tumor zone”, “peritumor zone”, “ischemia zone”, and “reserved liver zone”[146]. The ICG “four-zone three-phase” fluorescence imaging could accurately locate most tumors, clearly display the liver resection plane in a real-time manner and achieved the precision and standardization of anatomical hepatectomy.
The application of augmented reality (AR) technology can increase the accuracy of tumor positioning and surgical margin delineation. At present, only a few cases have been reported on the application of AR technology in RLR. Further technological advance and studies on evaluation of AR technology in clinical applications remain to be done.
This international experts consensus guideline offered guidance for the safe and effective clinical practice and the research direction of RLR in future. This evidence-based guideline is expected to be updated again in 2026 and further randomized controlled trials are needed to validate these recommendations.
We thank all the external reviewers for giving responses to the consensus; and all Members of the Evidence Evaluation Group: Shao-Ming Song and Lin Zhu (The First School of Clinical Medicine, Lanzhou University, Lanzhou 730000, China), Gui-Neng Zeng and Yue Li (School of Medicine, Nankai University, Tianjin 300300, China), Zhao-Da Deng and Zhi-Yuan Yao (Medical School of Chinese PLA, Beijing 100000, China).
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Goja S, India; Montasser IF, Egypt; Morise Z, Japan S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Reich H, McGlynn F, DeCaprio J, Budin R. Laparoscopic excision of benign liver lesions. Obstet Gynecol. 1991;78:956-958. [PubMed] |
| 2. | Katkhouda N, Fabiani P, Benizri E, Mouiel J. Laser resection of a liver hydatid cyst under videolaparoscopy. Br J Surg. 1992;79:560-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Buell JF, Cherqui D, Geller DA, O'Rourke N, Iannitti D, Dagher I, Koffron AJ, Thomas M, Gayet B, Han HS, Wakabayashi G, Belli G, Kaneko H, Ker CG, Scatton O, Laurent A, Abdalla EK, Chaudhury P, Dutson E, Gamblin C, D'Angelica M, Nagorney D, Testa G, Labow D, Manas D, Poon RT, Nelson H, Martin R, Clary B, Pinson WC, Martinie J, Vauthey JN, Goldstein R, Roayaie S, Barlet D, Espat J, Abecassis M, Rees M, Fong Y, McMasters KM, Broelsch C, Busuttil R, Belghiti J, Strasberg S, Chari RS; World Consensus Conference on Laparoscopic Surgery. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg. 2009;250:825-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1249] [Cited by in RCA: 1142] [Article Influence: 71.4] [Reference Citation Analysis (0)] |
| 4. | Wakabayashi G, Cherqui D, Geller DA, Buell JF, Kaneko H, Han HS, Asbun H, OʼRourke N, Tanabe M, Koffron AJ, Tsung A, Soubrane O, Machado MA, Gayet B, Troisi RI, Pessaux P, Van Dam RM, Scatton O, Abu Hilal M, Belli G, Kwon CH, Edwin B, Choi GH, Aldrighetti LA, Cai X, Cleary S, Chen KH, Schön MR, Sugioka A, Tang CN, Herman P, Pekolj J, Chen XP, Dagher I, Jarnagin W, Yamamoto M, Strong R, Jagannath P, Lo CM, Clavien PA, Kokudo N, Barkun J, Strasberg SM. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg. 2015;261:619-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 403] [Reference Citation Analysis (0)] |
| 5. | Abu Hilal M, Aldrighetti L, Dagher I, Edwin B, Troisi RI, Alikhanov R, Aroori S, Belli G, Besselink M, Briceno J, Gayet B, D'Hondt M, Lesurtel M, Menon K, Lodge P, Rotellar F, Santoyo J, Scatton O, Soubrane O, Sutcliffe R, Van Dam R, White S, Halls MC, Cipriani F, Van der Poel M, Ciria R, Barkhatov L, Gomez-Luque Y, Ocana-Garcia S, Cook A, Buell J, Clavien PA, Dervenis C, Fusai G, Geller D, Lang H, Primrose J, Taylor M, Van Gulik T, Wakabayashi G, Asbun H, Cherqui D. The Southampton Consensus Guidelines for Laparoscopic Liver Surgery: From Indication to Implementation. Ann Surg. 2018;268:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 518] [Cited by in RCA: 495] [Article Influence: 70.7] [Reference Citation Analysis (0)] |
| 6. | Choi GH, Chong JU, Han DH, Choi JS, Lee WJ. Robotic hepatectomy: the Korean experience and perspective. Hepatobiliary Surg Nutr. 2017;6:230-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 7. | Goja S, Singh MK, Vohra V, Soin AS. Robotic Left Hepatectomy: a Case Report (First Reported Case of Robotic Hepatectomy in India). Indian J Surg. 2015;77:338-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Kam JH, Goh BK, Chan CY, Wong JS, Lee SY, Cheow PC, Chung AY, Ooi LL. Robotic hepatectomy: initial experience of a single institution in Singapore. Singapore Med J. 2016;57:209-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Stewart C, Wong P, Warner S, Raoof M, Singh G, Fong Y, Melstrom L. Robotic minor hepatectomy: optimizing outcomes and cost of care. HPB (Oxford). 2021;23:700-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Sucandy I, Jacoby H, Crespo K, Syblis C, App S, Ignatius J, Ross S, Rosemurgy A. A Single Institution's Experience With Robotic Minor and Major Hepatectomy. Am Surg. 2023;89:1387-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Sucandy I, Luberice K, Lippert T, Castro M, Krill E, Ross S, Rosemurgy A. Robotic Major Hepatectomy: An Institutional Experience and Clinical Outcomes. Ann Surg Oncol. 2020;27:4970-4979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | O'Connor VV, Vuong B, Yang ST, DiFronzo A. Robotic Minor Hepatectomy Offers a Favorable Learning Curve and May Result in Superior Perioperative Outcomes Compared with Laparoscopic Approach. Am Surg. 2017;83:1085-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Chen PD, Wu CY, Hu RH, Ho CM, Lee PH, Lai HS, Lin MT, Wu YM. Robotic liver donor right hepatectomy: A pure, minimally invasive approach. Liver Transpl. 2016;22:1509-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 14. | Broering DC, Elsheikh Y, Alnemary Y, Zidan A, Elsarawy A, Saleh Y, Alabbad S, Sturdevant M, Wu YM, Troisi RI. Robotic Versus Open Right Lobe Donor Hepatectomy for Adult Living Donor Liver Transplantation: A Propensity Score-Matched Analysis. Liver Transpl. 2020;26:1455-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 15. | Camerlo A, Delayre T, Fara R. Robotic central hepatectomy for hepatocarcinoma by glissonean approach (with video). Surg Oncol. 2021;36:82-83. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Rojas AE, Paterakos P, Choi SH. Robotic Central Bisectionectomy for Centrally Located Hepatic Malignant Tumor. Ann Surg Oncol. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 17. | Liu R, Wakabayashi G, Kim HJ, Choi GH, Yiengpruksawan A, Fong Y, He J, Boggi U, Troisi RI, Efanov M, Azoulay D, Panaro F, Pessaux P, Wang XY, Zhu JY, Zhang SG, Sun CD, Wu Z, Tao KS, Yang KH, Fan J, Chen XP. International consensus statement on robotic hepatectomy surgery in 2018. World J Gastroenterol. 2019;25:1432-1444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 107] [Cited by in RCA: 150] [Article Influence: 25.0] [Reference Citation Analysis (2)] |
| 18. | Chong CC, Fuks D, Lee KF, Zhao JJ, Choi GH, Sucandy I, Chiow AKH, Marino MV, Gastaca M, Wang X, Lee JH, Efanov M, Kingham TP, D'Hondt M, Troisi RI, Choi SH, Sutcliffe RP, Chan CY, Lai ECH, Park JO, Di Benedetto F, Rotellar F, Sugioka A, Coelho FF, Ferrero A, Long TCD, Lim C, Scatton O, Liu Q, Schmelzle M, Pratschke J, Cheung TT, Liu R, Han HS, Tang CN, Goh BKP; International Robotic and Laparoscopic Liver Resection study group investigators. Propensity Score-Matched Analysis Comparing Robotic and Laparoscopic Right and Extended Right Hepatectomy. JAMA Surg. 2022;157:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 76] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 19. | Varghese CT, Chandran B, Sudhindran S. Robotic Donor Hepatectomy-Safety in Novelty Is the Essence. JAMA Surg. 2021;156:1171-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Muaddi H, Hafid ME, Choi WJ, Lillie E, de Mestral C, Nathens A, Stukel TA, Karanicolas PJ. Clinical Outcomes of Robotic Surgery Compared to Conventional Surgical Approaches (Laparoscopic or Open): A Systematic Overview of Reviews. Ann Surg. 2021;273:467-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 121] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 21. | Zhu P, Liao W, Zhang WG, Chen L, Shu C, Zhang ZW, Huang ZY, Chen YF, Lau WY, Zhang BX, Chen XP. A Prospective Study Using Propensity Score Matching to Compare Long-term Survival Outcomes After Robotic-assisted, Laparoscopic, or Open Liver Resection for Patients With BCLC Stage 0-A Hepatocellular Carcinoma. Ann Surg. 2023;277:e103-e111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 58] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 22. | Tsung A, Geller DA, Sukato DC, Sabbaghian S, Tohme S, Steel J, Marsh W, Reddy SK, Bartlett DL. Robotic versus laparoscopic hepatectomy: a matched comparison. Ann Surg. 2014;259:549-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 257] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 23. | Ji WB, Wang HG, Zhao ZM, Duan WD, Lu F, Dong JH. Robotic-assisted laparoscopic anatomic hepatectomy in China: initial experience. Ann Surg. 2011;253:342-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 140] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 24. | Liu R, Liu Q, Wang F. Prognosis control surgery: From theory to practice. Zhongguo Kexue Jianbao. 2019;64:1137-48. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 25. | Liu R, Wang Y, Zhang XP. Revisiting human liver anatomy: dynamic watershed theory. Hepatobiliary Surg Nutr. 2021;10:139-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182:E839-E842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1714] [Cited by in RCA: 2462] [Article Influence: 164.1] [Reference Citation Analysis (0)] |
| 27. | Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson E, Henry DA. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3100] [Cited by in RCA: 5683] [Article Influence: 710.4] [Reference Citation Analysis (0)] |
| 28. | Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6581] [Cited by in RCA: 15243] [Article Influence: 2540.5] [Reference Citation Analysis (0)] |
| 29. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8858] [Cited by in RCA: 12656] [Article Influence: 843.7] [Reference Citation Analysis (0)] |
| 30. | Elliott JH, Synnot A, Turner T, Simmonds M, Akl EA, McDonald S, Salanti G, Meerpohl J, MacLehose H, Hilton J, Tovey D, Shemilt I, Thomas J; Living Systematic Review Network. Living systematic review: 1. Introduction-the why, what, when, and how. J Clin Epidemiol. 2017;91:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 365] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 31. | Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ; GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11058] [Cited by in RCA: 14919] [Article Influence: 877.6] [Reference Citation Analysis (0)] |
| 32. | Beard RE, Tsung A. Minimally Invasive Approaches for Surgical Management of Primary Liver Cancers. Cancer Control. 2017;24:1073274817729234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Hu M, Chen K, Zhang X, Li C, Song D, Liu R. Robotic, laparoscopic or open hemihepatectomy for giant liver haemangiomas over 10 cm in diameter. BMC Surg. 2020;20:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 34. | Zhu L, Liu Y, Hu M, Zhao Z, Li C, Zhang X, Tan X, Wang F, Liu R. Comparison of robotic and laparoscopic liver resection in ordinary cases of left lateral sectionectomy. Surg Endosc. 2022;36:4923-4931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Zhang XP, Xu S, Hu MG, Zhao ZM, Wang ZH, Zhao GD, Li CG, Tan XL, Liu R. Short- and long-term outcomes after robotic and open liver resection for elderly patients with hepatocellular carcinoma: a propensity score-matched study. Surg Endosc. 2022;36:8132-8143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 36. | Hamad A, Ansari A, Li Y, Shen C, Cloyd J, Pawlik TM, Ejaz A. Short- and long-term outcomes following robotic and open resection for intrahepatic cholangiocarcinoma: A national cohort study. Surg Oncol. 2022;43:101790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 37. | Chen PD, Wu CY, Hu RH, Chou WH, Lai HS, Liang JT, Lee PH, Wu YM. Robotic Versus Open Hepatectomy for Hepatocellular Carcinoma: A Matched Comparison. Ann Surg Oncol. 2017;24:1021-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 38. | Nota CL, Woo Y, Raoof M, Boerner T, Molenaar IQ, Choi GH, Kingham TP, Latorre K, Borel Rinkes IHM, Hagendoorn J, Fong Y. Robotic Versus Open Minor Liver Resections of the Posterosuperior Segments: A Multinational, Propensity Score-Matched Study. Ann Surg Oncol. 2019;26:583-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 39. | Sucandy I, Rayman S, Lai EC, Tang CN, Chong Y, Efanov M, Fuks D, Choi GH, Chong CC, Chiow AKH, Marino MV, Prieto M, Lee JH, Kingham TP, D'Hondt M, Troisi RI, Choi SH, Sutcliffe RP, Cheung TT, Rotellar F, Park JO, Scatton O, Han HS, Pratschke J, Wang X, Liu R, Goh BKP; International Robotic, Laparoscopic Liver Resection Study Group Investigators. Robotic Versus Laparoscopic Left and Extended Left Hepatectomy: An International Multicenter Study Propensity Score-Matched Analysis. Ann Surg Oncol. 2022;29:8398-8406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 40. | Xu J, Wei Y, Chang W. Robot-assisted procedure vs open surgery for simultaneous resection of colorectal cancer with liver metastases: short-term outcomes of a randomized controlled study. Ann Oncol. 2017;28:x42. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 41. | Rho SY, Lee JG, Joo DJ, Kim MS, Kim SI, Han DH, Choi JS, Choi GH. Outcomes of Robotic Living Donor Right Hepatectomy From 52 Consecutive Cases: Comparison With Open and Laparoscopy-assisted Donor Hepatectomy. Ann Surg. 2022;275:e433-e442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 42. | Cortolillo N, Patel C, Parreco J, Kaza S, Castillo A. Nationwide outcomes and costs of laparoscopic and robotic vs. open hepatectomy. J Robot Surg. 2019;13:557-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 43. | Lee KF, Chong C, Cheung S, Wong J, Fung A, Lok HT, Lo E, Lai P. Robotic versus open hemihepatectomy: a propensity score-matched study. Surg Endosc. 2021;35:2316-2323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 44. | Shu J, Wang XJ, Li JW, Bie P, Chen J, Zheng SG. Robotic-assisted laparoscopic surgery for complex hepatolithiasis: a propensity score matching analysis. Surg Endosc. 2019;33:2539-2547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 45. | Beard RE, Khan S, Troisi RI, Montalti R, Vanlander A, Fong Y, Kingham TP, Boerner T, Berber E, Kahramangil B, Buell JF, Martinie JB, Vrochides D, Shen C, Molinari M, Geller DA, Tsung A. Long-Term and Oncologic Outcomes of Robotic Versus Laparoscopic Liver Resection for Metastatic Colorectal Cancer: A Multicenter, Propensity Score Matching Analysis. World J Surg. 2020;44:887-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 46. | Masetti M, Fallani G, Ratti F, Ferrero A, Giuliante F, Cillo U, Guglielmi A, Ettorre GM, Torzilli G, Vincenti L, Ercolani G, Cipressi C, Lombardi R, Aldrighetti L, Jovine E. Minimally invasive treatment of colorectal liver metastases: does robotic surgery provide any technical advantages over laparoscopy? A multicenter analysis from the IGoMILS (Italian Group of Minimally Invasive Liver Surgery) registry. Updates Surg. 2022;74:535-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 47. | Murtha-Lemekhova A, Fuchs J, Hoffmann K. Innovation for the Sake of Innovation? How Does Robotic Hepatectomy Compare to Laparoscopic or Open Resection for HCC-A Systematic Review and Meta-Analysis. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 48. | Hu Y, Guo K, Xu J, Xia T, Wang T, Liu N, Fu Y. Robotic versus laparoscopic hepatectomy for malignancy: A systematic review and meta-analysis. Asian J Surg. 2021;44:615-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 49. | Sucandy I, Shapera E, Syblis CC, Crespo K, Przetocki VA, Ross SB, Rosemurgy AS. Propensity score matched comparison of robotic and open major hepatectomy for malignant liver tumors. Surg Endosc. 2022;36:6724-6732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 50. | Wu YM, Hu RH, Lai HS, Lee PH. Robotic-assisted minimally invasive liver resection. Asian J Surg. 2014;37:53-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 51. | Yang HY, Rho SY, Han DH, Choi JS, Choi GH. Robotic major liver resections: Surgical outcomes compared with open major liver resections. Ann Hepatobiliary Pancreat Surg. 2021;25:8-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 52. | Yu S, Yuan G, Lu S, Li J, Tang B, Zhong F, Su H, He S. Application of da Vinci robot and laparoscopy on repeat hepatocellular carcinoma. J Minim Access Surg. 2022;18:378-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 53. | Balzano E, Bernardi L, Tincani G, Ghinolfi D, Melandro F, Bronzoni J, Meli S, Arenga G, Biancofiore G, Crocetti L, De Simone P. Implementing a robotic liver resection program does not always require prior laparoscopic experience. Surg Endosc. 2022;36:3317-3322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 54. | Duong LM, Cai H, Shrubsole MJ, Bailey CE, Idrees K, Shu XO. Outcomes of robotic-assisted liver surgery versus laparoscopic liver surgery for treatment of stage I hepatocellular carcinoma. Cancer. 2022;128:762-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 55. | Lai EC, Tang CN. Long-term Survival Analysis of Robotic Versus Conventional Laparoscopic Hepatectomy for Hepatocellular Carcinoma: A Comparative Study. Surg Laparosc Endosc Percutan Tech. 2016;26:162-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 56. | Lim C, Goumard C, Salloum C, Tudisco A, Napoli N, Boggi U, Azoulay D, Scatton O. Outcomes after 3D laparoscopic and robotic liver resection for hepatocellular carcinoma: a multicenter comparative study. Surg Endosc. 2021;35:3258-3266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 57. | Magistri P, Tarantino G, Guidetti C, Assirati G, Olivieri T, Ballarin R, Coratti A, Di Benedetto F. Laparoscopic versus robotic surgery for hepatocellular carcinoma: the first 46 consecutive cases. J Surg Res. 2017;217:92-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 58. | Pesi B, Bencini L, Moraldi L, Tofani F, Batignani G, Bechi P, Farsi M, Annecchiarico M, Coratti A. Robotic Versus Open Liver Resection in Hepatocarcinoma: Surgical and Oncological Outcomes. Surg Laparosc Endosc Percutan Tech. 2021;31:468-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 59. | Wang WH, Kuo KK, Wang SN, Lee KT. Oncological and surgical result of hepatoma after robot surgery. Surg Endosc. 2018;32:3918-3924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 60. | Shapera EA, Ross S, Syblis C, Crespo K, Rosemurgy A, Sucandy I. Analysis of Oncological Outcomes After Robotic Liver Resection for Intrahepatic Cholangiocarcinoma. Am Surg. 2023;89:2399-2412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 61. | Gumbs AA, Lorenz E, Tsai TJ, Starker L, Flanagan J, Benedetti Cacciaguerra A, Yu NJ, Bajul M, Chouillard E, Croner R, Abu Hilal M. Study: International Multicentric Minimally Invasive Liver Resection for Colorectal Liver Metastases (SIMMILR-CRLM). Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 62. | Rahimli M, Perrakis A, Schellerer V, Gumbs A, Lorenz E, Franz M, Arend J, Negrini VR, Croner RS. Robotic and laparoscopic liver surgery for colorectal liver metastases: an experience from a German Academic Center. World J Surg Oncol. 2020;18:333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 63. | Shapera E, Ross S, Crespo K, Syblis C, Przetocki V, Rosemurgy A, Sucandy I. Analysis of surgical approach and tumor distance to margin after liver resection for colorectal liver metastasis. J Robot Surg. 2022;16:1427-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 64. | Rocca A, Scacchi A, Cappuccio M, Avella P, Bugiantella W, De Rosa M, Costa G, Polistena A, Codacci-Pisanelli M, Amato B, Carbone F, Ceccarelli G. Robotic surgery for colorectal liver metastases resection: A systematic review. Int J Med Robot. 2021;17:e2330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (1)] |
| 65. | Machairas N, Dorovinis P, Kykalos S, Stamopoulos P, Schizas D, Zoe G, Terra A, Nikiteas N. Simultaneous robotic-assisted resection of colorectal cancer and synchronous liver metastases: a systematic review. J Robot Surg. 2021;15:841-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 66. | Amma BSPT, Mathew JS, Varghese CT, Nair K, Mallick S, Chandran B, Menon RN, Gopalakrishnan U, Balakrishnan D, George PS, Vayoth SO, Sudhindran S. Open to robotic right donor hepatectomy: A tectonic shift in surgical technique. Clin Transplant. 2022;36:e14775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 67. | Lincango Naranjo EP, Garces-Delgado E, Siepmann T, Mirow L, Solis-Pazmino P, Alexander-Leon H, Restrepo-Rodas G, Mancero-Montalvo R, Ponce CJ, Cadena-Semanate R, Vargas-Cordova R, Herrera-Cevallos G, Vallejo S, Liu-Sanchez C, Prokop LJ, Ziogas IA, Vailas MG, Guerron AD, Visser BC, Ponce OJ, Barbas AS, Moris D. Robotic Living Donor Right Hepatectomy: A Systematic Review and Meta-Analysis. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 68. | Yeow M, Soh S, Starkey G, Perini MV, Koh YX, Tan EK, Chan CY, Raj P, Goh BKP, Kabir T. A systematic review and network meta-analysis of outcomes after open, mini-laparotomy, hybrid, totally laparoscopic, and robotic living donor right hepatectomy. Surgery. 2022;172:741-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 69. | Nota CL, Rinkes IHB, Molenaar IQ, van Santvoort HC, Fong Y, Hagendoorn J. Robot-assisted laparoscopic liver resection: a systematic review and pooled analysis of minor and major hepatectomies. HPB (Oxford). 2016;18:113-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 70. | Wang JM, Li JF, Yuan GD, He SQ. Robot-assisted versus laparoscopic minor hepatectomy: A systematic review and meta-analysis. Medicine (Baltimore). 2021;100:e25648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 71. | Daskalaki D, Gonzalez-Heredia R, Brown M, Bianco FM, Tzvetanov I, Davis M, Kim J, Benedetti E, Giulianotti PC. Financial Impact of the Robotic Approach in Liver Surgery: A Comparative Study of Clinical Outcomes and Costs Between the Robotic and Open Technique in a Single Institution. J Laparoendosc Adv Surg Tech A. 2017;27:375-382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 72. | Valverde A, Abdallah S, Danoussou D, Goasguen N, Jouvin I, Oberlin O, Lupinacci RM. Transitioning From Open to Robotic Liver Resection. Results of 46 Consecutive Procedures Including a Majority of Major Hepatectomies. Surg Innov. 2021;28:309-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 73. | Croner RS, Perrakis A, Hohenberger W, Brunner M. Robotic liver surgery for minor hepatic resections: a comparison with laparoscopic and open standard procedures. Langenbecks Arch Surg. 2016;401:707-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 74. | Kadam P, Sutcliffe RP, Scatton O, Sucandy I, Kingham TP, Liu R, Choi GH, Syn NL, Gastaca M, Choi SH, Chiow AKH, Marino MV, Efanov M, Lee JH, Chong CC, Tang CN, Cheung TT, Pratschke J, Wang X, Campos RR, Ivanecz A, Park JO, Rotellar F, Fuks D, D'Hondt M, Han HS, Troisi RI, Goh BKP; International Robotic and Laparoscopic Liver Resection Study Group Investigators. An international multicenter propensity-score matched and coarsened-exact matched analysis comparing robotic versus laparoscopic partial liver resections of the anterolateral segments. J Hepatobiliary Pancreat Sci. 2022;29:843-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 75. | Kim JK, Park JS, Han DH, Choi GH, Kim KS, Choi JS, Yoon DS. Robotic versus laparoscopic left lateral sectionectomy of liver. Surg Endosc. 2016;30:4756-4764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 76. | Lai EC, Yang GP, Tang CN. Robot-assisted laparoscopic liver resection for hepatocellular carcinoma: short-term outcome. Am J Surg. 2013;205:697-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 77. | Lee KF, Cheung YS, Chong CC, Wong J, Fong AK, Lai PB. Laparoscopic and robotic hepatectomy: experience from a single centre. ANZ J Surg. 2016;86:122-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 78. | Mejia A, Cheng SS, Vivian E, Shah J, Oduor H, Archarya P. Minimally invasive liver resection in the era of robotics: analysis of 214 cases. Surg Endosc. 2020;34:339-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 79. | Packiam V, Bartlett DL, Tohme S, Reddy S, Marsh JW, Geller DA, Tsung A. Minimally invasive liver resection: robotic versus laparoscopic left lateral sectionectomy. J Gastrointest Surg. 2012;16:2233-2238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 80. | Salloum C, Lim C, Lahat E, Gavara CG, Levesque E, Compagnon P, Azoulay D. Robotic-Assisted Versus Laparoscopic Left Lateral Sectionectomy: Analysis of Surgical Outcomes and Costs by a Propensity Score Matched Cohort Study. World J Surg. 2017;41:516-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 81. | Tranchart H, Ceribelli C, Ferretti S, Dagher I, Patriti A. Traditional versus robot-assisted full laparoscopic liver resection: a matched-pair comparative study. World J Surg. 2014;38:2904-2909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 82. | Lee KF, Fong AK, Chong CC, Cheung SY, Wong J, Lai PB. Robotic Liver Resection For Primary Hepatolithiasis: Is It Beneficial? World J Surg. 2016;40:2490-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 83. | Morel P, Jung M, Cornateanu S, Buehler L, Majno P, Toso C, Buchs NC, Rubbia-Brandt L, Hagen ME. Robotic versus open liver resections: A case-matched comparison. Int J Med Robot. 2017;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 84. | Cai JP, Chen W, Chen LH, Wan XY, Lai JM, Yin XY. Comparison between robotic-assisted and laparoscopic left hemi-hepatectomy. Asian J Surg. 2022;45:265-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 85. | Fruscione M, Pickens R, Baker EH, Cochran A, Khan A, Ocuin L, Iannitti DA, Vrochides D, Martinie JB. Robotic-assisted versus laparoscopic major liver resection: analysis of outcomes from a single center. HPB (Oxford). 2019;21:906-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 86. | Lorenz E, Arend J, Franz M, Rahimli M, Perrakis A, Negrini V, Gumbs AA, Croner RS. Robotic and laparoscopic liver resection-comparative experiences at a high-volume German academic center. Langenbecks Arch Surg. 2021;406:753-761. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 87. | Marino MV, Shabat G, Guarrasi D, Gulotta G, Komorowski AL. Comparative Study of the Initial Experience in Performing Robotic and Laparoscopic Right Hepatectomy with Technical Description of the Robotic Technique. Dig Surg. 2019;36:241-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 88. | Spampinato MG, Coratti A, Bianco L, Caniglia F, Laurenzi A, Puleo F, Ettorre GM, Boggi U. Perioperative outcomes of laparoscopic and robot-assisted major hepatectomies: an Italian multi-institutional comparative study. Surg Endosc. 2014;28:2973-2979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 89. | Wang ZZ, Tang WB, Hu MG, Zhao ZM, Zhao GD, Li CG, Tan XL, Zhang X, Lau WY, Liu R. Robotic vs laparoscopic hemihepatectomy: A comparative study from a single center. J Surg Oncol. 2019;120:646-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 90. | Chin KM, Linn YL, Cheong CK, Koh YX, Teo JY, Chung AYF, Chan CY, Goh BKP. Minimally invasive versus open right anterior sectionectomy and central hepatectomy for central liver malignancies: a propensity-score-matched analysis. ANZ J Surg. 2021;91:E174-E182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 91. | Cho CW, Rhu J, Kwon CHD, Choi GS, Kim JM, Joh JW, Koh KC, Kim GS. Short-Term Outcomes of Totally Laparoscopic Central Hepatectomy and Right Anterior Sectionectomy for Centrally Located Tumors: A Case-Matched Study with Propensity Score Matching. World J Surg. 2017;41:2838-2846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 92. | Jeung IH, Choi SH, Kim S, Kwon SW. Laparoscopic Central Bisectionectomy and Right Anterior Sectionectomy Using Two Retraction Methods: Technical Aspects with Video. World J Surg. 2019;43:3120-3127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 93. | Chiow AKH, Fuks D, Choi GH, Syn N, Sucandy I, Marino MV, Prieto M, Chong CC, Lee JH, Efanov M, Kingham TP, Choi SH, Sutcliffe RP, Troisi RI, Pratschke J, Cheung TT, Wang X, Liu R, D'Hondt M, Chan CY, Tang CN, Han HS, Goh BKP; International Robotic and Laparoscopic Liver Resection Study Group collaborators. International multicentre propensity score-matched analysis comparing robotic versus laparoscopic right posterior sectionectomy. Br J Surg. 2021;108:1513-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 94. | Patriti A, Cipriani F, Ratti F, Bartoli A, Ceccarelli G, Casciola L, Aldrighetti L. Robot-assisted versus open liver resection in the right posterior section. JSLS. 2014;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 95. | Montalti R, Scuderi V, Patriti A, Vivarelli M, Troisi RI. Robotic versus laparoscopic resections of posterosuperior segments of the liver: a propensity score-matched comparison. Surg Endosc. 2016;30:1004-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 96. | Zhao ZM, Yin ZZ, Pan LC, Hu MG, Tan XL, Liu R. Robotic isolated partial and complete hepatic caudate lobectomy: A single institution experience. Hepatobiliary Pancreat Dis Int. 2020;19:435-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 97. | Zhao ZM, Yin ZZ, Pan LC, Jiang N, Tan XL, Chen X, Liu R. Robotic anatomic isolated complete caudate lobectomy: Left-side approach and techniques. Asian J Surg. 2021;44:269-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 98. | Ziogas IA, Evangeliou AP, Mylonas KS, Athanasiadis DI, Cherouveim P, Geller DA, Schulick RD, Alexopoulos SP, Tsoulfas G. Economic analysis of open versus laparoscopic versus robotic hepatectomy: a systematic review and meta-analysis. Eur J Health Econ. 2021;22:585-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 99. | Aziz H, Hanna K, Lashkari N, Ahmad NU, Genyk Y, Sheikh MR. Hospitalization Costs and Outcomes of Open, Laparoscopic, and Robotic Liver Resections. Am Surg. 2022;88:2331-2337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 100. | D'Hondt M, Devooght A, Willems E, Wicherts D, De Meyere C, Parmentier I, Provoost A, Pottel H, Verslype C. Transition from laparoscopic to robotic liver surgery: clinical outcomes, learning curve effect, and cost-effectiveness. J Robot Surg. 2023;17:79-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 101. | Hawksworth J, Llore N, Holzner ML, Radkani P, Meslar E, Winslow E, Satoskar R, He R, Jha R, Haddad N, Fishbein T. Robotic Hepatectomy Is a Safe and Cost-Effective Alternative to Conventional Open Hepatectomy: a Single-Center Preliminary Experience. J Gastrointest Surg. 2021;25:825-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 102. | Miller HP, Hakim A, Kellish A, Wozniak M, Gaughan J, Sensenig R, Atabek UM, Spitz FR, Hong YK. Cost-Benefit Analysis of Robotic vs. Laparoscopic Hepatectomy: A Propensity-Matched Retrospective Cohort Study of American College of Surgeons National Surgical Quality Improvement Program Database. Am Surg. 2022;88:2886-2892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 103. | Shapera E, Sucandy I, Syblis C, Crespo K, Ja'Karri T, Ross S, Rosemurgy A. Cost analysis of robotic versus open hepatectomy: Is the robotic platform more expensive? J Robot Surg. 2022;16:1409-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 104. | Yu YD, Kim KH, Jung DH, Namkoong JM, Yoon SY, Jung SW, Lee SK, Lee SG. Robotic versus laparoscopic liver resection: a comparative study from a single center. Langenbecks Arch Surg. 2014;399:1039-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 105. | Sham JG, Richards MK, Seo YD, Pillarisetty VG, Yeung RS, Park JO. Efficacy and cost of robotic hepatectomy: is the robot cost-prohibitive? J Robot Surg. 2016;10:307-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 106. | Wu CY, Chen PD, Chou WH, Liang JT, Huang CS, Wu YM. Is robotic hepatectomy cost-effective? In view of patient-reported outcomes. Asian J Surg. 2019;42:543-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 107. | Coletta D, De Padua C, Parrino C, De Peppo V, Oddi A, Frigieri C, Grazi GL. Laparoscopic Liver Surgery: What Are the Advantages in Patients with Cirrhosis and Portal Hypertension? Systematic Review and Meta-Analysis with Personal Experience. J Laparoendosc Adv Surg Tech A. 2020;30:1054-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 108. | Dong KS, Liang BY, Zhang ZY, Zhang EL, Yang G, Xia SL, Chen XP, Huang ZY. Histologic severity of liver cirrhosis: A key factor affecting surgical outcomes of hepatocellular carcinoma in patients with portal hypertension. Asian J Surg. 2019;42:981-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 109. | Lin CH, Ho CM, Wu CH, Liang PC, Wu YM, Hu RH, Lee PH, Ho MC. Minimally invasive surgery versus radiofrequency ablation for single subcapsular hepatocellular carcinoma ≤ 2 cm with compensated liver cirrhosis. Surg Endosc. 2020;34:5566-5573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 110. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6058] [Article Influence: 865.4] [Reference Citation Analysis (3)] |
| 111. | Birgin E, Hartwig V, Rasbach E, Seyfried S, Rahbari M, Reeg A, Jentschura SL, Téoule P, Reißfelder C, Rahbari NN. Minimally invasive mesohepatectomy for centrally located liver lesions-a case series. Surg Endosc. 2022;36:8935-8942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 112. | Hawksworth J, Radkani P, Filice R, Aguirre O, Nguyen B, Fishbein T, Winslow E. Robotic Central Hepatectomy and Right Anterior Sectionectomy: Minimally Invasive Parenchyma Sparing Surgery for Central Liver Tumors. J Gastrointest Surg. 2023;27:407-410. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 113. | Casciola L, Patriti A, Ceccarelli G, Bartoli A, Ceribelli C, Spaziani A. Robot-assisted parenchymal-sparing liver surgery including lesions located in the posterosuperior segments. Surg Endosc. 2011;25:3815-3824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 114. | Au KP, Chan ACY. Current status of associating liver partition with portal vein ligation for staged hepatectomy: Comparison with two-stage hepatectomy and strategies for better outcomes. World J Gastroenterol. 2019;25:6373-6385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 115. | Lang H. ALPPS - Beneficial or detrimental? Surg Oncol. 2020;33:249-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 116. | Michal K, Sau M, Tamara GMH, Long JR. A better route to ALPPS: minimally invasive vs open ALPPS. Surg Endosc. 2020;34:2379-2389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 117. | Melandro F, Giovanardi F, Hassan R, Larghi Laureiro Z, Ferri F, Rossi M, Mennini G, Pawlik TM, Lai Q. Minimally Invasive Approach in the Setting of ALPPS Procedure: a Systematic Review of the Literature. J Gastrointest Surg. 2019;23:1917-1924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 118. | Vicente E, Quijano Y, Ielpo B, Fabra I. First ALPPS procedure using a total robotic approach. Surg Oncol. 2016;25:457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 119. | Krishnamurthy J, Naragund AV, Mahadevappa B. First Ever Robotic Stage One ALPPS Procedure in India: for Colorectal Liver Metastases. Indian J Surg. 2018;80:269-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 120. | Machado MAC, Surjan RC, Makdissi F. Robotic ALPPS. Ann Surg Oncol. 2020;27:1174-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 121. | Di Benedetto F, Assirati G, Magistri P. Full robotic ALPPS for HCC with intrahepatic portal vein thrombosis. Int J Med Robot. 2020;16:e2087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 122. | Masetti M, Lombardi R, Romboli A, Jovine E. Fully Robotic ALPPS and Simultaneous Left Colectomy for Synchronous Colorectal Liver Metastases. J Laparoendosc Adv Surg Tech A. 2020;30:1106-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 123. | Hu MG, Wang J, Yin ZZ, Liu R. First two-stage robotic ALPPS in HCC patients with hepatic vein invasion: a step-by-step procedure from a clinical case. World J Surg Oncol. 2021;19:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 124. | Di Benedetto F, Magistri P. First Case of Full Robotic ALPPS for Intrahepatic Cholangiocarcinoma. Ann Surg Oncol. 2021;28:865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 125. | Schadde E, Ardiles V, Robles-Campos R, Malago M, Machado M, Hernandez-Alejandro R, Soubrane O, Schnitzbauer AA, Raptis D, Tschuor C, Petrowsky H, De Santibanes E, Clavien PA; ALPPS Registry Group. Early survival and safety of ALPPS: first report of the International ALPPS Registry. Ann Surg. 2014;260:829-36; discussion 836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 349] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 126. | Schadde E, Raptis DA, Schnitzbauer AA, Ardiles V, Tschuor C, Lesurtel M, Abdalla EK, Hernandez-Alejandro R, Jovine E, Machado M, Malago M, Robles-Campos R, Petrowsky H, Santibanes ED, Clavien PA. Prediction of Mortality After ALPPS Stage-1: An Analysis of 320 Patients From the International ALPPS Registry. Ann Surg. 2015;262:780-5; discussion 785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 179] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 127. | Rimassa L, Finn RS, Sangro B. Combination immunotherapy for hepatocellular carcinoma. J Hepatol. 2023;79:506-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 172] [Reference Citation Analysis (0)] |
| 128. | Sadeghi S, Bejjani A, Finn RS. Systemic Therapy for Primary Liver Tumors: Cholangiocarcinoma and Hepatocellular Carcinoma. Surg Oncol Clin N Am. 2019;28:695-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 129. | Zhu P, Liao W, Ding ZY, Chen L, Zhang WG, Zhang BX, Chen XP. Learning Curve in Robot-Assisted Laparoscopic Liver Resection. J Gastrointest Surg. 2019;23:1778-1787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 130. | Varghese CT, Chandran B, Gopalakrishnan U, Nair K, Mallick S, Mathew JS, Sivasankara Pillai Thankamony Amma B, Balakrishnan D, Othiyil Vayoth S, Sudhindran S. Extended criteria donors for robotic right hepatectomy: A propensity score matched analysis. J Hepatobiliary Pancreat Sci. 2022;29:874-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 131. | Liu Q, Zhang T, Hu M, Zhao Z, Zhao G, Li C, Zhang X, Lau WY, Liu R. Comparison of the learning curves for robotic left and right hemihepatectomy: A prospective cohort study. Int J Surg. 2020;81:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 132. | Gravetz A, Sucandy I, Wilfong C, Patel N, Spence J, Ross S, Rosemurgy A. Single-Institution Early Experience and Learning Curve with Robotic Liver Resections. Am Surg. 2019;85:115-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 133. | Efanov M, Alikhanov R, Tsvirkun V, Kazakov I, Melekhina O, Kim P, Vankovich A, Grendal K, Berelavichus S, Khatkov I. Comparative analysis of learning curve in complex robot-assisted and laparoscopic liver resection. HPB (Oxford). 2017;19:818-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 134. | Chen PD, Wu CY, Hu RH, Chen CN, Yuan RH, Liang JT, Lai HS, Wu YM. Robotic major hepatectomy: Is there a learning curve? Surgery. 2017;161:642-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 129] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 135. | Goh BKP, Teo JY, Lee SY, Kam JH, Cheow PC, Jeyaraj P, Chow PKH, Ooi L, Chung AYF, Chan CY. Critical appraisal of the impact of individual surgeon experience on the outcomes of laparoscopic liver resection in the modern era: collective experience of multiple surgeons at a single institution with 324 consecutive cases. Surg Endosc. 2018;32:1802-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 136. | Chua D, Syn N, Koh YX, Goh BKP. Learning curves in minimally invasive hepatectomy: systematic review and meta-regression analysis. Br J Surg. 2021;108:351-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 91] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 137. | Linn YL, Wu AG, Han HS, Liu R, Chen KH, Fuks D, Soubrane O, Cherqui D, Geller D, Cheung TT, Edwin B, Aldrighetti L, Abu Hilal M, Troisi RI, Wakabayashi G, Goh BKP; International Robotic and Laparoscopic Liver Resection Study Group Investigators. Systematic review and meta-analysis of difficulty scoring systems for laparoscopic and robotic liver resections. J Hepatobiliary Pancreat Sci. 2023;30:36-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 138. | Wakabayashi G. What has changed after the Morioka consensus conference 2014 on laparoscopic liver resection? Hepatobiliary Surg Nutr. 2016;5:281-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 203] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 139. | Hasegawa Y, Wakabayashi G, Nitta H, Takahara T, Katagiri H, Umemura A, Makabe K, Sasaki A. A novel model for prediction of pure laparoscopic liver resection surgical difficulty. Surg Endosc. 2017;31:5356-5363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 113] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 140. | Kawaguchi Y, Fuks D, Kokudo N, Gayet B. Difficulty of Laparoscopic Liver Resection: Proposal for a New Classification. Ann Surg. 2018;267:13-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 185] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 141. | Halls MC, Berardi G, Cipriani F, Barkhatov L, Lainas P, Harris S, D'Hondt M, Rotellar F, Dagher I, Aldrighetti L, Troisi RI, Edwin B, Abu Hilal M. Development and validation of a difficulty score to predict intraoperative complications during laparoscopic liver resection. Br J Surg. 2018;105:1182-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 146] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 142. | Castaing D, Emond J, Kunstlinger F, Bismuth H. Utility of operative ultrasound in the surgical management of liver tumors. Ann Surg. 1986;204:600-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 98] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 143. | Ferrero A, Lo Tesoriere R, Russolillo N, Viganò L, Forchino F, Capussotti L. Ultrasound-guided laparoscopic liver resections. Surg Endosc. 2015;29:1002-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 144. | Zhu P, Liao W, Ding ZY, Luo HC, Zhang BH, Zhang WG, Zhang W, Zhang ZG, Zhang BX, Chen XP. Intraoperative ultrasonography of robot-assisted laparoscopic hepatectomy: initial experiences from 110 consecutive cases. Surg Endosc. 2018;32:4071-4077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 145. | Wakabayashi T, Cacciaguerra AB, Abe Y, Bona ED, Nicolini D, Mocchegiani F, Kabeshima Y, Vivarelli M, Wakabayashi G, Kitagawa Y. Indocyanine Green Fluorescence Navigation in Liver Surgery: A Systematic Review on Dose and Timing of Administration. Ann Surg. 2022;275:1025-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 97] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 146. | Zhang X, Xu S, Zhao Z. Application of ICG “four-zone three-phase” fluorescence imaging in robot-assisted anatomical hepatectomy. Chin J Hepat Surg. 2021;10:191-6. |