Published online Jun 28, 2023. doi: 10.3748/wjg.v29.i24.3883
Peer-review started: March 25, 2023
First decision: April 21, 2023
Revised: May 11, 2023
Accepted: May 31, 2023
Article in press: May 31, 2023
Published online: June 28, 2023
Processing time: 94 Days and 13.4 Hours
Laparoscopic and endoscopic cooperative surgery is a safe, organ-sparing surgery that achieves full-thickness resection with adequate margins. Recent studies have demonstrated the safety and efficacy of these procedures. However, these techniques are limited by the exposure of the tumor and mucosa to the peritoneal cavity, which could lead to viable cancer cell seeding and the spillage of gastric juice or enteric liquids into the peritoneal cavity. Non-exposed endoscopic wall-inversion surgery (NEWS) is highly accurate in determining the resection margins to prevent intraperitoneal contamination because the tumor is inverted into the visceral lumen instead of the peritoneal cavity. Accurate intraoperative asse
To determine the safety and feasibility of NEWS in early gastric and colon cancers and of adding rapid intraoperative lymph node (LN) assessment with OSNA.
The patient-based experiential portion of our investigations was conducted at the General and Oncological Surgery Unit of the St. Giuseppe Moscati Hospital (Avellino, Italy). Patients with early-stage gastric or colon cancer (diagnosed via endoscopy, endoscopic ultrasound, and computed tomography) were included. All lesions were treated by NEWS procedure with intraoperative OSNA assay between January 2022 and October 2022. LNs were examined intraoperatively with OSNA and postoperatively with conventional histology. We analyzed patient demographics, lesion features, histopathological diagnoses, R0 resection (negative margins) status, adverse events, and follow-up results. Data were collected prospectively and analyzed retrospectively.
A total of 10 patients (5 males and 5 females) with an average age of 70.4 ± 4.5 years (range: 62-78 years) were enrolled in this study. Five patients were diagnosed with gastric cancer. The remaining 5 patients were diagnosed with early-stage colon cancer. The mean tumor diameter was 23.8 ± 11.6 mm (range: 15-36 mm). The NEWS procedure was successful in all cases. The mean procedure time was 111.5 ± 10.7 min (range: 80-145 min). The OSNA assay revealed no LN metastases in any patients. Histologically complete resection (R0) was achieved in 9 patients (90.0%). There was no recurrence during the follow-up period.
NEWS combined with sentinel LN biopsy and OSNA assay is an effective and safe technique for the removal of selected early gastric and colon cancers in which it is not possible to adopt conventional endoscopic resection techniques. This procedure allows clinicians to acquire additional information on the LN status intraoperatively.
Core Tip: The treatment efficacy of non-exposed endoscopic wall-inversion surgery combined with sentinel lymph node (LN) biopsy and intraoperative one-step nucleic acid amplification assay remains to be fully evaluated in early gastric and colon cancers. The patients included in the experiential portion of our investigations were diagnosed with early gastric and colon cancers that were not amenable to conventional endoscopic resection techniques. We found that this approach provides essential details on LN status intraoperatively and we discuss the related literature to help guide future research studies and clinical care.
- Citation: Crafa F, Vanella S, Morante A, Catalano OA, Pomykala KL, Baiamonte M, Godas M, Antunes A, Costa Pereira J, Giaccaglia V. Non-exposed endoscopic wall-inversion surgery with one-step nucleic acid amplification for early gastrointestinal tumors: Personal experience and literature review. World J Gastroenterol 2023; 29(24): 3883-3898
- URL: https://www.wjgnet.com/1007-9327/full/v29/i24/3883.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i24.3883
The most effective treatment for patients with resectable gastrointestinal submucosal tumors (SMTs), including gastrointestinal stromal tumors[1-4] and early gastrointestinal cancer[5], is complete surgical resection. Segmental resection is acceptable based on oncologic principles[6-8]. Endoscopic submucosal dissection (ESD) is a well-studied treatment for adenomas and early cancer of the gastrointestinal tract[9]. In some cases, it has low rates of microscopic resections with a negative margin (R0). ESD in lesions involving the muscularis propria remains controversial due to the increased risk of perforation[10-13]. Adverse events have also been described in lesions located in anatomical positions that are difficult to access.
Previous studies reported that laparoscopic wedge resection for gastric submucosal tumors (SMTs) was oncologically feasible and safe with a decrease in blood loss and length of hospital stay[14-16] compared to other classic gastric resections. Using the conventional laparoscopic approach, it is difficult to establish the exact line of resection for intraluminally developing gastrointestinal tumors. With limited or overabundant resections, there is a risk of having postoperative functional problems or infiltrated resection margins with postoperative recurrence[17,18]. Endoscopic control of the resection margin is essential for safe local resection in early gastrointestinal cancers[19]. Narrow-band imaging (NBI) is a digital optical method of image-enhanced endoscopy that enhances the vessel and surface structure of colorectal lesions using 415 nm and 540 nm wavelength filters[20-22]. Several NBI classifications for colorectal lesions have been proposed. These classifications were unified as the NBI International Colorectal Endoscopic (NICE) classification for non-magnifying endoscopy in 2009 and the Japan NBI Expert Team (JNET) classification for magnifying endoscopy in 2014[20,23-27].
NICE was divided into three groups based on histology: Hyperplastic or sessile serrated polyp (type 1), adenoma (type 2), and deep submucosal invasive cancer (type 3). There are also three criteria: Surface pattern, vase pattern and colour[26,27]. The Japanese endoscopists separated the type 2 NICE category into type 2A (low-grade adenoma) and type 2B (high-grade adenoma and submucosal cancer) using magnifying endoscopy. They developed the JNET classification as an advanced version of the NICE classification. When magnifying endoscopy is available, the JNET classification is better at selecting the correct therapeutic strategy based on precise diagnosis[20].
To overcome the limitations of endoscopic resections and wedge resections, Hiki et al[28] combined the ESD technique to properly determine the resection margin with laparoscopic full-thickness excision, developing a hybrid technique of laparoscopic endoscopic cooperative surgery (LECS). The LECS allows to preserve the vascularization, the innervation of the wall, and the organs' functionality with a better postoperative quality of life for the patient.
To expand the indication of LECS, some modified LECS procedures were developed, such as non-exposed endoscopic wall-inversion surgery (NEWS)[29-36]. Currently, the LECS, already widely studied for stomach lesions, is also used for lesions of other organs, such as the duodenum[37], the rectum and the colon[38,39]. This technique allows the lesion to be approached simultaneously from the inside endoscopically and externally laparoscopically, allowing a limited resection that respects oncological principles[40-44].
Diagnostic imaging assessment of lymphadenopathy in gastric and colorectal cancers (CRCs) is challenging. Individual imaging modalities face specific intrinsic limitations, which negatively impact detection and it does not allow a correct evaluation of important lymph node (LN) details. Therefore we only have the size criterion available to determine lymphadenopathies[45]. A new semi-automated diagnostic method called one-step nucleic acid amplification (OSNA) has been developed to detect LN metastases. OSNA is based on reverse transcription–loop-mediated isothermal amplification[46,47] to amplify cytokeratin 19 (CK19) mRNA. The OSNA test has been used successfully in numerous malignancies[48]. Yaguchi et al[49] defined the CK19 mRNA cutoff to identify LN metastases with OSNA assay in gastric cancer[49-52]. Kumagai et al[50] showed that OSNA could be advantageously used to diagnose LN metastases in advanced gastric cancer.
CK19 is an epithelial marker. When it is identified in LNs from patients with CRC, it is highly suggestive of LN metastases[53-55]. The OSNA assay can assess LN metastasis in various cancers expressing CK19, including sentinel LNs in breast cancer and other cancers[56-59].
In this study, we determined the safety and feasibility of NEWS in early gastric and colon cancers and adding rapid intraoperative LN assessment with OSNA.
A cohort study approach was taken, relying on data from a prospectively maintained database of consecutive patients undergoing elective NEWS for early gastric and colon cancers at St. Giuseppe Moscati Hospital of Avellino, Italy. The database included preoperative, intraoperative and postoperative data. Inclusion criteria were: (1) Adult age; (2) diagnosis with early gastric or colon cancer; and (3) eligibility for the NEWS procedure. Exclusion criterion was: Allergy to indocyanine green (ICG). In total, 10 patients were included in this study.
This study was carried out in continuity with our previous study[59] and conducted according to the ethical principles of the Institution following the Declaration of Helsinki and with approval by the Institutional Review Board of the St. Giuseppe Moscati Hospital (Approval No. 201801). Written informed consent was granted by study participants for the publication of this article.
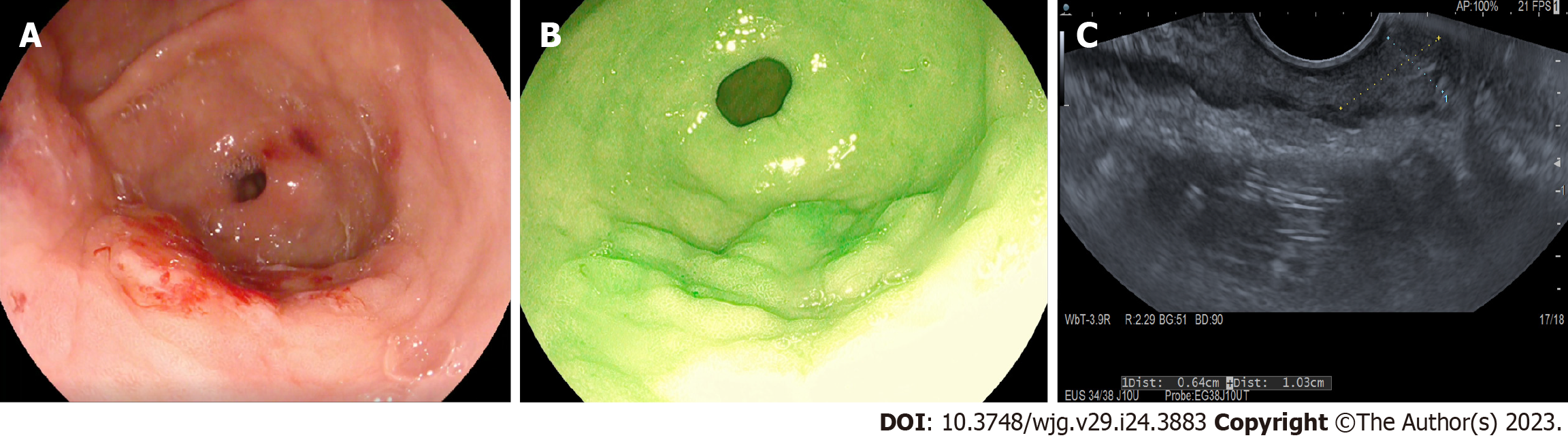
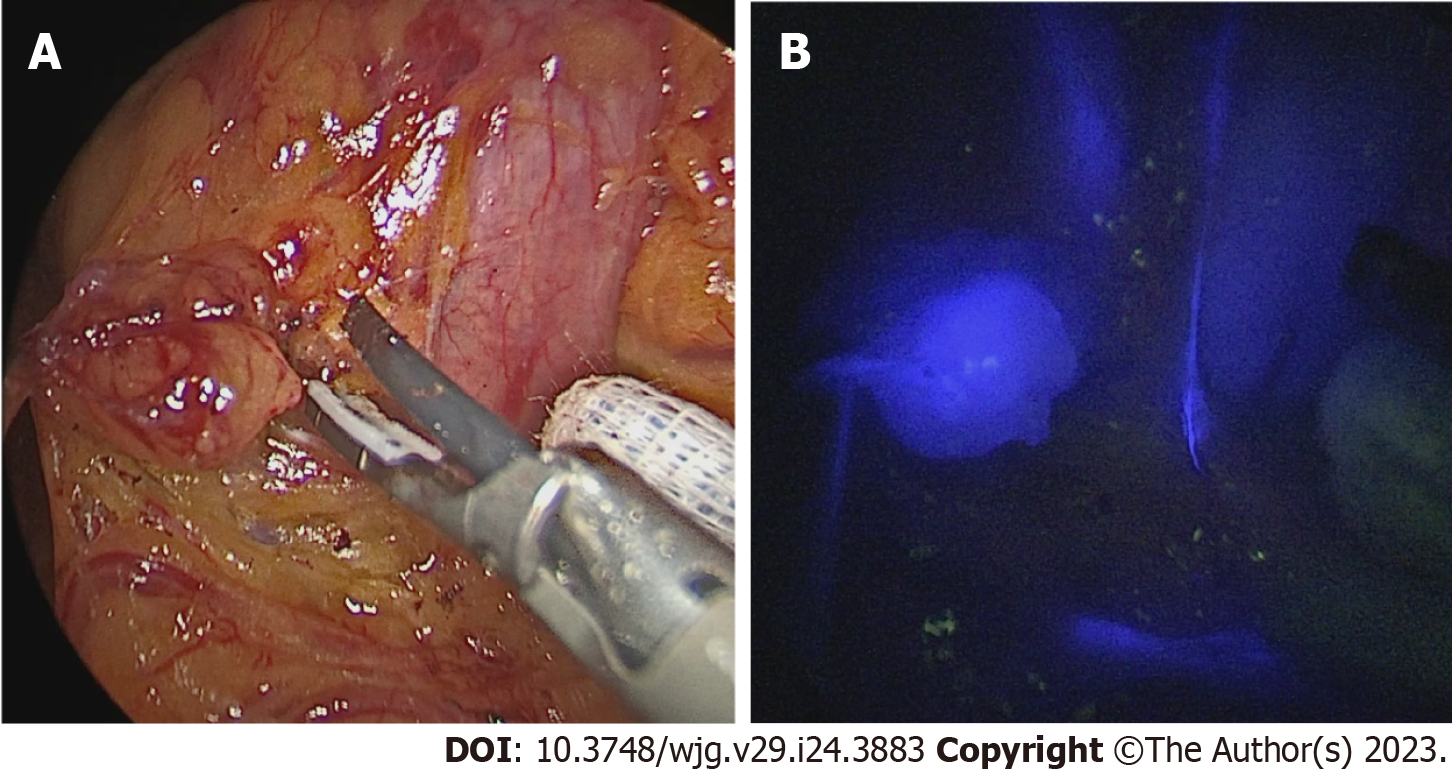
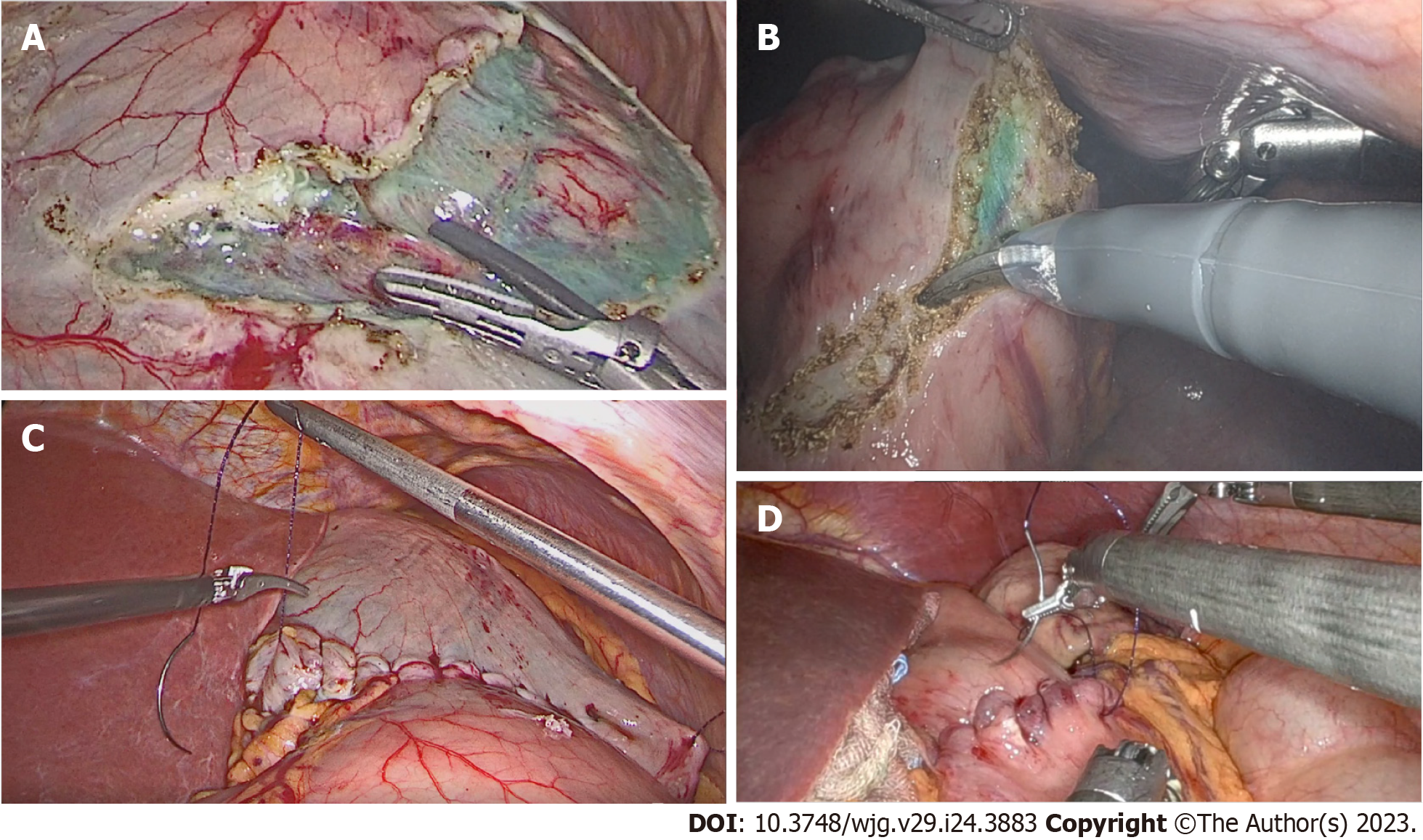
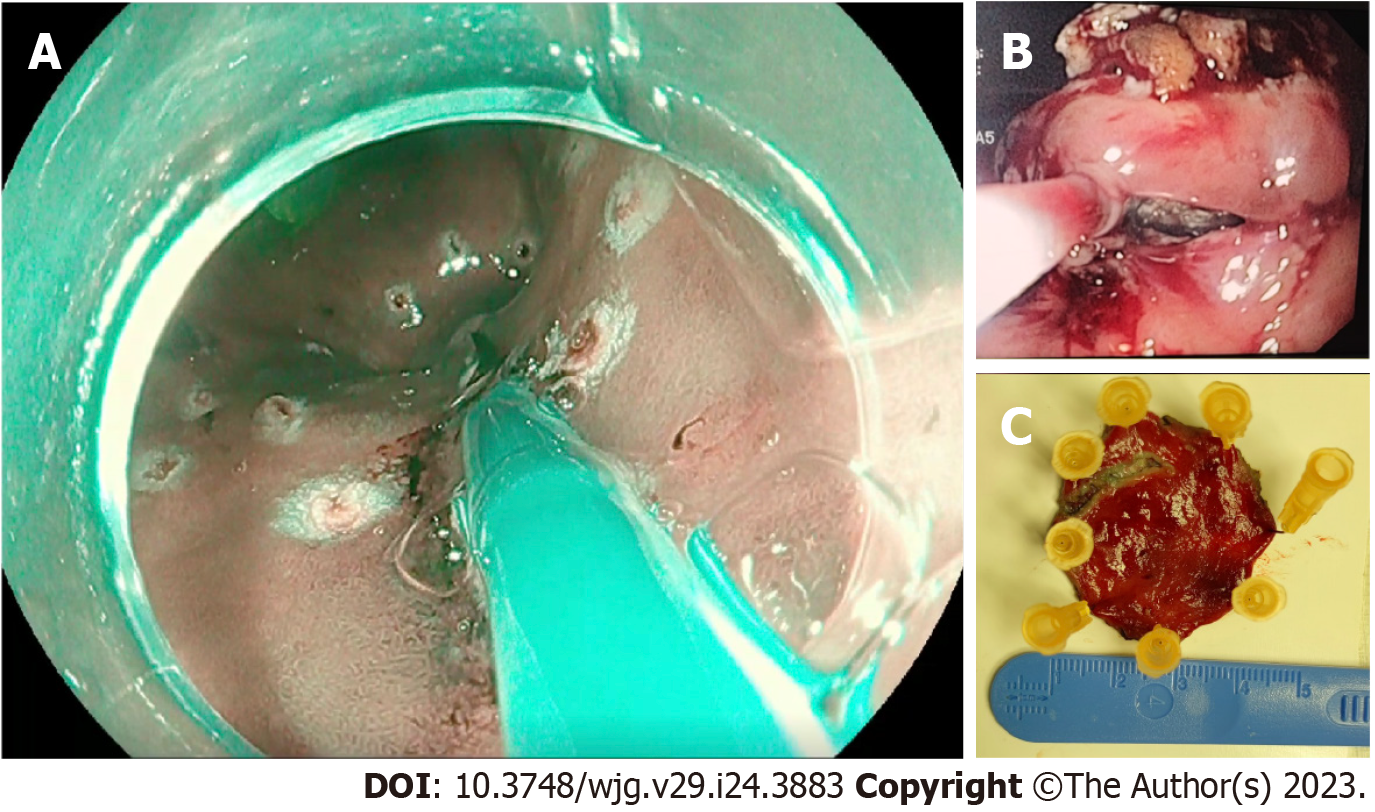
Immediately after access to the abdominal cavity by laparotomy or laparoscopy, a flexible endoscope was passed to the tumor site (Figure 1) to allow a 0.5 mL submucosal injection of ICG (2.5–5.0 mg/mL) at four points around the tumor (Figure 2). The nodal basin was then examined under near-infrared (NIR) illumination (Karl Storz SE & Co., Tuttlingen, Germany). In the case of the stomach, we proceeded to the exeresis of identifiable nodes at one or two nodal basins (Figure 3). In contrast, in the case of the colon, any identifiable sentinel LNs were excised and submitted immediately to the pathology department. LNs harvested in this way were cleaned of fat. LNs weighing between 0.05 g and 0.60 g or with a cross diameter of less than 8 mm were exclusively processed using the OSNA technique. LNs weighing more than 0.60 g or with diameter over 8 mm were dissected and analyzed using the OSNA technique and hematoxylin and eosin (H&E). In the latter case, LNs were divided at 2-mm intervals, and nonadjacent blocks were alternatively subjected to definitive histopathological examination with H&E or OSNA. Our technique for preparation of materials for OSNA and interpretation of results has already been described in our previous study[59] (Figure 4). The patient’s total tumor load (TTL) resulted from the sum of all CK19 mRNA tumor copies/μL of each LN.
OSNA results with CK19 mRNA above 250 copies/μL were designated positive, and those with fewer than 250 copies/μL were considered negative[59]. The result of the sentinel LN analysis was intraoperatively communicated to the surgeon. All patients underwent the NEWS procedure.
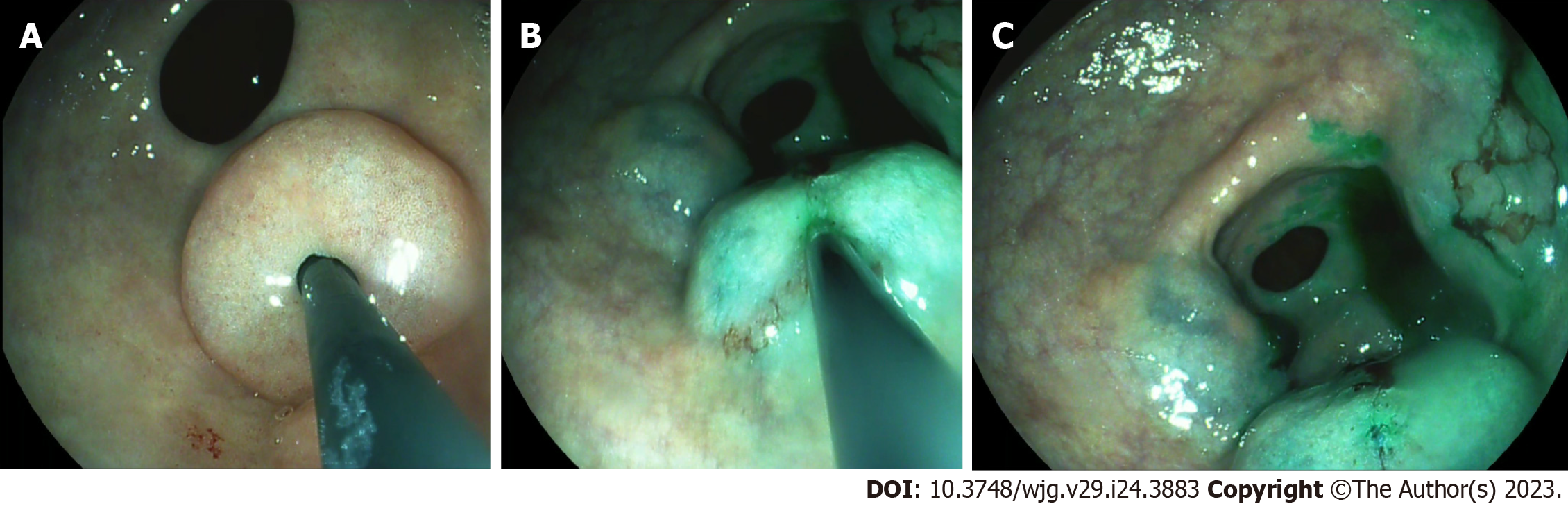
In this procedure, mucosal markings are placed around the tumor followed by serosal markings via laparoscope under endoscopic navigation. A longitudinal seromuscular incision is performed laparoscopically around the serosal markings, taking special care not to create a full-thickness opening in the wall. The pending vessels of the submucosa are coagulated laparoscopically. The seromuscular layers are sutured transversely (Figure 5) to avoid postoperative strictures, particularly in patients with colon cancer, and the lesion is inserted into the inside of the lumen. Finally, circumferential mucosal and submucosal tissue incisions are made endoscopically around the inverted tumor (Figure 6). The resected tumor is retrieved, and the mucosal defect is sutured with several endoscopic clips.
Patients with early gastric cancer and early colon cancer were encouraged to begin drinking and eating on postoperative day 1 after the NEWS procedure. If the postoperative course was uneventful, then patients could be discharged on postoperative day 1 or 2.
Subsequently, the records of each patient were discussed in a multidisciplinary conference with surgeons, pathologists, and oncologists, considering the definitive examination of the surgical specimen and the result of the sentinel LN analysis. The final histology of the surgical specimen and harvested LNs were always concluded based on the H&E analysis.
A total of 10 patients (5 males and 5 females) with an average age of 70.4 ± 4.5 years (range: 62-78 years) were observed in this study. Five patients were diagnosed with early-stage gastric cancer, and five patients were diagnosed with early-stage colon cancer. The mean tumor diameter was 23.8 ± 11.6 mm (range: 15-36 mm). The patient characteristics are shown in Table 1.
| Variables | Results |
| Sex | |
| Male | 5 (50) |
| Female | 5 (50) |
| Age | 70.4 ± 4.5 (62-78) |
| BMI | 23.7 ± 2.1 (20.2-26.5) |
| ASA physical status classification | |
| I | 0 |
| II | 5 |
| III | 5 |
| IV | 0 |
| V | 0 |
| Tumor size in mm | 23.8 ± 11.6 (10-36) |
| Tumor location | |
| Stomach | 5 |
| Colon | 5 |
| Operation time in min | 111.5 ± 10.7 (80-145) |
| Negative margin | 9/10 |
| Number of nodes | 2.5 ± 2.2 (1-5) |
| Postoperative complications | 1/10 |
| Recurrence | 0 |
| Follow-up period in mo | 6.3 ± 4.2 |
The NEWS procedure was successful in all cases. The mean procedure time was 111.5 ± 10.7 min (range: 80-145 min). The OSNA assay revealed no LN metastases in any patient. The diagnostic accuracy in predicting the LN status based on the sentinel LN concept by OSNA compared with the postoperative histological examination was 100%. Complete histological resection (R0) was achieved in 9 (90.0%) patients. The average post-procedure length of hospitalization was 3.1 ± 4.8 d (range: 1-8 d). There was no recurrence during the follow-up. The mean follow-up was 6.3 ± 4.2 mo.
There was only 1 patient who experienced a complication. In the gastric cancer group, 1 patient was treated conservatively for intrabdominal fluid collection (Table 2). One patient who underwent the removal of a lesion affecting the proximal transverse colon presented positive focal margins after histological examination. Therefore, he underwent a right hemicolectomy. The definitive histological examination showed no residual tumor foci or LN metastases (Table 3).
| Variables | Results |
| Number | 5 |
| Tumor size in mm | 23.5 ± 12.1 (10-36) |
| Primary site | |
| Body | 3 (60) |
| Antrum | 2 (40) |
| Operation time in min | 110.5 ± 10.9 (80-145) |
| Complete resection | 5/5 |
| Complications | 1/5 |
| Number of lymph nodes | 2.6 ± 2.2 (1-4) |
| Length of hospitalization in d | 3.6 ± 4.2 (1-8) |
| Recurrence | 0 |
| Variables | Results |
| Tumor number | 5 |
| Tumor size in mm | 24.0 ± 10.6 (18-35) |
| Primary site | |
| Right colon | 2 (40) |
| Left colon | 3 (60) |
| Operation time in min | 112.5 ± 10.6 (105-120) |
| Complete resection | 4/5 |
| Complications | 0/5 |
| Number of lymph nodes | 2.4 ± 2.7 (1-5) |
| Length of hospitalization in d | 2.3 ± 1.7 (1-4) |
| Recurrence | 0 |
The average LN count was 2.5 ± 2.2, ranging from 1 to 5 nodes per patient. Five patients had early gastric cancer, and five had early CRC confirmed by final histology.
In this article, we investigated the possibility of adding to the NEWS (for early gastrointestinal cancers) an intraoperative study of LNs using fluorescence and ICG to identify the LN basin of the tumor and OSNA for the analysis of the excised LNs. We also performed a literature review on the use of NEWS in early gastrointestinal cancers, on sentinel LN and LN basin studies in gastric and colonic cancers, and on the intraoperative use of OSNA.
NEWS has been reported as a novel full-thickness resection technique without wall perforation. It is primarily used to treat early gastrointestinal cancer[30,32-34,60,61]. An advantage of NEWS is that all the gastric or intestinal layers can be resected precisely under direct visualization by laparoscopy and endoscopy. As a result, wall resection is limited and oncological principles are respected. The size of the tumor is one of the main limitations of NEWS. Lesion size should be 30 mm or less as the resected specimen is retrieved perorally or transanally. The LECS concept initially adopted for gastric lesions can also be applied to colorectal tumors[38]. The appropriate indications of LECS for colorectal tumors are: (1) Intramucosal carcinoma (Tis) and adenoma with high-grade atypia accompanied by a severe degree of fibrosis in the submucosal layer (tumor recurrence after endoscopic or surgical resection); (2) Tis and adenoma with high-grade atypia involving the appendix or diverticulum; or (3) intraluminal or intramural growth-type SMTs. LECS for the colon is a safe and feasible procedure[62,63]. LECS for colorectal tumors has a 0% rate for either residual or local recurrence.
Currently, modified LECS procedures can be applied to cases of early gastrointestinal cancer (within the indication for endoscopic mucosal resection/ESD) that would be technically difficult to treat with endoscopic mucosal resection/ESD. Innovative organ-preserving procedures such as transanal minimally invasive surgery, LECS, and modified LECS allow adequate resection of early-stage tumors without extensive interventions[64].
The LN stage remains crucial for oncological treatments. Identifying LN metastases in gastric or CRC is still unsatisfactory despite improvements in imaging techniques[65-67]. Sentinel LN biopsy for early gastric cancer is reportedly helpful when deciding whether to perform LN dissection, and LECS combined with sentinel LN dissection has been attempted for early gastric cancer as well[31,68-70]. The sentinel LN is the first LN that receives lymphatic drainage from the primary tumor and is a predictor of the pathological status of all other LNs. Miwa[71] studied lymphatic basin dissection in gastric cancer as a method of sentinel LN biopsy. The specific lymphatic system for gastric cancer is stained by a dye tracer injected near the gastric lesion and drained into the lymphatic system. The lymphatic system is then dissected en bloc, and the sentinel LN analysis is performed.
Multiple reports have investigated lymphatic basin dissection as a specific sentinel LN biopsy in stomach cancer[72-75]. The lymphatic basin identified by dye mapping is excised en bloc, and sentinel LNs are analyzed ex vivo after dissection of the basin and sent for rapid intraoperative diagnosis. If, after sentinel LN biopsy, a positive LN is found, D2 gastrectomy is added. If LNs are negative for metastases, further dissection is avoided, gastric vasculature outside the basin is preserved, and the gastric resection area is minimized[76-79].
In recent years, a number of clinical trials in Japan and South Korea have shown that the safety and therapeutic effect of sentinel LN navigation surgery in early gastric cancer are acceptable[74,80-84]. A multicenter study in Japan showed that the detection rate of sentinel LNs in early gastric cancer reached 97.5%, with 93% sensitivity and 99% accuracy[75]. A multicenter randomized controlled trial (SENORITA) conducted in South Korea showed that the sentinel LN biopsy group did not have non-inferiority to the radical gastrectomy group for 3-year disease-free survival. However, the 3-year disease-free survival and 3-year overall survival rates did not differ after rescue surgery in recurrence/metachronous gastric cancer cases. The sentinel LN biopsy group had a better long-term quality of life and nutrition than the radical gastrectomy group[76,77].
Goto et al[34] reported the use of NEWS for early gastric cancer in combination with sentinel LN navigation surgery (SNNS). The lymphatic basin is an essential concept in SNNS.
Recent developments in the OSNA test allow for rapidly identifying metastases over the entire LN. The OSNA assay, already extensively studied in breast cancer[56], is under evaluation as an alternative diagnostic test for identifying secondary localizations of sentinel LNs in gastric cancer[65]. In the study by Yaguchi et al[49], the agreement rate between the OSNA test and H&E stain was 94.4%, and the sensitivity and specificity were 88.9 and 96.8%, respectively, when the CK19 mRNA cutoff value is 250 copies/μL. The multicenter study by Kumagai et al[50] showed that the OSNA test has the same precision as the 2 mm interval histological examination in detecting LN metastases in the case of stomach cancer. Shoji et al[85] showed that single-tracer sentinel LN mapping by ICG fluorescence imaging with intraoperative diagnosis by OSNA assay is feasible and safe.
Joosten et al[86] in 1999 introduced the concept of sentinel LN in the CRC to reduce false negative results and study the importance of LN involvement. The main advantage of sentinel LN mapping in CRC is identifying nodes with an increased risk of metastasis so that they undergo further testing.
The OSNA test can also detect colorectal metastases in LNs based on CK19 levels within 20 min of removal[87-89]. Until recently, it was predominantly used to evaluate the entire LN basin, but its rapidity could make it a functional intraoperative test to guide decision-making.
Vogelaar et al[90] compared OSNA with a single H&E stain and multilevel fine pathology (immunohistochemistry with pan-cytokeratin antibody staining) to identify metastases in the sentinel LN of colon cancer patients. OSNA and fine pathological examination were superior to a single H&E stain. Additionally, combining the two methods observed a 46.5% upstaging rate. The study by Yamamoto et al[91] shows that if the sum of CK19 mRNA is higher, the number of histologically positive LNs increases. The median CK19 mRNA value was significantly lower if there were fewer than three metastatic regional LNs than in patients with more than four positive LNs.
The median TTL values of pN0, pN1 (1-3 LN positive), and pN2 (4 or more LN positive) were 1550 copies/μL (300-320000 copies/μL), 24050 copies/μL (250-890000 copies/μL) and 90600 copies/μL (7700-1635100 copies/μL), respectively. The TTL increases with the advancing LN stage. In the study by Aldecoa et al[92], TTL correlated with pT stage (P = 0.01) and tumor size (P < 0.01) in low-grade tumors. In this study, classic high-risk factors correlated with TTL in patients with stage I-II colon cancer. These results showed that the sum of CK19 mRNA obtained by the OSNA method is comparable with the current pathology diagnosis system. These studies pave the way for a new molecular staging using OSNA based on the amount of CK19mRNA rather than the number of LN metastases. Furthermore, TTL has been suggested to be related to a poor prognosis, worse disease-free survival, and other CRC risk factors[45,93,94], such as pN, pT, tumor grade, male sex, tumor size, and lymph vascular invasion.
In the literature, the association between the use of fluorescence to search for colorectal LNs and the use of OSNA to provide an accurate and rapid assessment of the oncological status of these specific LNs has been shown, which can give information on the entire LN basin. In the study by Shimada et al[58], there is a high agreement between OSNA and histopathology. For this reason, the OSNA test can be considered a convenient, objective and valuable alternative to the current pathological method for detecting LN metastases.
Some studies have used intraoperative OSNA testing to evaluate its diagnostic accuracy and elapsed time between surgery and postoperative adjuvant chemotherapy[59,95,96]. A rapid intraoperative test, such as OSNA (which takes approximately 40 min[95]), to obtain information on the status of LNs in the tumor drainage basin can overcome the limitations of preoperative imaging in clinically LN-negative patients. The OSNA test, thanks to its intraoperative diagnostic accuracy and rapidity, may be indispensable for safely performing minimal resections in patients with early-stage gastrointestinal cancer. With more experience using this technology and further refinement of the technique, ICG analysis could be used in conjunction with OSNA intraoperatively to identify LN metastases, which would influence surgical decision-making.
In our previous study[37] we described the advantages and limitations of the OSNA technique. Some authors have proposed that positive CK19 on immunohistochemistry in primary tumors should be a prerequisite for OSNA use[37,73].
To our knowledge, this is the first study investigating the utility of intraoperative OSNA testing in the assessment of sentinel LN in patients with early-stage gastrointestinal cancer undergoing the NEWS procedure.
This article included a single-center pilot study. As such, the number of patients collected was small and the selected patients did not have LN metastases. Therefore, it was not possible to validate the sentinel LN technique.
At present, two methods are predominantly used in the detection of sentinel LNs; it involves injecting a radioisotope with a gamma probe or a dye. Hot LNs are identified by radioisotope uptake, and sentinel LNs are colored blue or green by dye after injection around the tumor with a radioisotope colloid or dye. The currently established double-tracer method (dye and radioisotope tracers) described in several studies[97-99] appears to increase the sensitivity of identifying true sentinel LNs. We used only one tracer method in this study. ICG has hypoallergenic potential, deep detection depth, high sensitivity, and stable signal. Furthermore, tight regulation and costs of radioactive substances limit the widespread use of the probe-guided method.
The SNNS concept has been established in early gastric cancer but is still controversial in CRC. Colorectal sentinel LN basins are still not well defined. The usefulness of sentinel LN basin dissection for curative resection needs to be investigated. In this situation, the effectiveness of SNNS combined with OSNA could not be determined in CRC.
NEWS is a feasible and safe technique for organ-sparing surgery in selected patients in centers with a high experience in endoscopy, laparoscopy, and robotic surgery. ICG-NIR lymphangiography in combination with sentinel LN OSNA analysis is feasible and may allow intraoperative prediction of LN status in patients with early colon or gastric cancer treated with the NEWS procedure. Furthermore, its implementation allows more precise staging. ICG-NIR lymphangiography and OSNA could be used to plan personalized surgery and lymphadenectomy in patients with early-stage cancers. Prospective multicenter studies with large populations of patient cohorts are needed to provide definitive conclusions.
There are more and more studies in the literature concerning endoscopic and surgical resections for organ preservation in early gastrointestinal neoplasms, respecting oncological principles. Lymph node (LN) study with one-step nucleic acid amplification (OSNA) is also the subject of numerous studies.
Organ-sparing endoscopic and imaging techniques do not currently allow for an accurate LN study. Using LN biopsy with rapid intraoperative results during the laparoscopic and endoscopic cooperative approach can add important information.
This article aims to stimulate studies that can add further information on LN status in patients with early gastrointestinal cancer, which, if treated only with endoscopic technique or with modified Laparoscopic and endoscopic cooperative surgery, would have no additional information on LN status beyond radiological ones. However, our study is the first to evaluate the utility of intraoperative OSNA assay in assessing SN in patients with early-stage gastrointestinal cancer undergoing the Non-exposed endoscopic wall-inversion surgery (NEWS).
This pilot study with a literature review is based on data collected prospectively from a database of patients undergoing elective NEWS for early gastrointestinal cancer at St. Giuseppe Moscati Hospital of Avellino, Italy. The database included preoperative, operative, and postoperative data. Inclusion criteria included adult patients with early gastric and colonic cancer eligible for the NEWS procedure. Exclusion criteria included participants with an allergy to any indocyanine green (ICG).
A total of 10 patients were enrolled in this study, which included 5 gastric and 5 colonic early-stage cancers. The NEWS procedure was successful in all cases. The OSNA assay revealed no LN metastasis in all patients. The diagnostic accuracy in predicting the LN status based on the SN concept by OSNA compared with the postoperative histological examination was 100%. Histologically complete resection (R0) was achieved in 9 (90.0%) patients. There was no recurrence during the follow-up. An intrabdominal fluid collection treated conservatively was observed in 1 (10.0%) patient of the gastric group. One patient who underwent the removal of a lesion affecting the proximal transverse colon presented positive focal margins on a definitive histological examination. Therefore he subsequently underwent a right hemicolectomy. The definitive histological examination showed no residual tumour foci or LN metastases.The mean follow-up was 6.3 ± 4.2 mo. There was no recurrence during the follow-up period. Ours is a single-centre pilot study. The number of patients collected is small. The selected patients were all patients without LN metastasis; therefore, it is not possible to validate the sentinel node technique.
Our study is the first to analyze the utility of intraoperative OSNA assay in sentinel node and nodal basin assessment in patients with early-stage gastrointestinal cancer undergoing the NEWS procedure. NEWS is a feasible and safe technique for organ-sparing surgery in selected patients. Additionally, implementing the NEWS association with the intraoperative study with OSNA will allow for more precise staging.
OSNA and ICG near-infrared lymphangiography could be used to develop customized surgery and lymphadenectomy in patients with early cancers. Prospective multicenter studies with large populations of patient cohorts are needed to provide definitive conclusions.
We thank the colleagues of the Department of Surgery and Histopathology of the "San Giuseppe Moscati" Hospital of National Importance and High Specialty for carefully reading and revising the manuscript.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Goto O, Japan; Guo X, China S-Editor: Fan JR L-Editor: A P-Editor: Zhang XD
| 1. | Koo DH, Ryu MH, Kim KM, Yang HK, Sawaki A, Hirota S, Zheng J, Zhang B, Tzen CY, Yeh CN, Nishida T, Shen L, Chen LT, Kang YK. Asian Consensus Guidelines for the Diagnosis and Management of Gastrointestinal Stromal Tumor. Cancer Res Treat. 2016;48:1155-1166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 147] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 2. | Nishida T, Hirota S, Yanagisawa A, Sugino Y, Minami M, Yamamura Y, Otani Y, Shimada Y, Takahashi F, Kubota T; GIST Guideline Subcommittee. Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan: English version. Int J Clin Oncol. 2008;13:416-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 321] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 3. | Iwahashi M, Takifuji K, Ojima T, Nakamura M, Nakamori M, Nakatani Y, Ueda K, Ishida K, Naka T, Ono K, Yamaue H. Surgical management of small gastrointestinal stromal tumors of the stomach. World J Surg. 2006;30:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Onimaru M, Inoue H, Ikeda H, Abad MRA, Quarta Colosso BM, Shimamura Y, Sumi K, Deguchi Y, Ito H, Yokoyama N. Combination of laparoscopic and endoscopic approaches for neoplasia with non-exposure technique (CLEAN-NET) for gastric submucosal tumors: updated advantages and limitations. Ann Transl Med. 2019;7:582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1327] [Article Influence: 60.3] [Reference Citation Analysis (4)] |
| 6. | Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6210] [Cited by in RCA: 8636] [Article Influence: 539.8] [Reference Citation Analysis (0)] |
| 7. | Goh C, Burke JP, McNamara DA, Cahill RA, Deasy J. Endolaparoscopic removal of colonic polyps. Colorectal Dis. 2014;16:271-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Nakajima T, Saito Y, Tanaka S, Iishi H, Kudo SE, Ikematsu H, Igarashi M, Saitoh Y, Inoue Y, Kobayashi K, Hisasbe T, Matsuda T, Ishikawa H, Sugihara K. Current status of endoscopic resection strategy for large, early colorectal neoplasia in Japan. Surg Endosc. 2013;27:3262-3270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 182] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 9. | Chung IK, Lee JH, Lee SH, Kim SJ, Cho JY, Cho WY, Hwangbo Y, Keum BR, Park JJ, Chun HJ, Kim HJ, Kim JJ, Ji SR, Seol SY. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc. 2009;69:1228-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 476] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 10. | Chun SY, Kim KO, Park DS, Lee IJ, Park JW, Moon SH, Baek IH, Kim JH, Park CK, Kwon MJ. Endoscopic submucosal dissection as a treatment for gastric subepithelial tumors that originate from the muscularis propria layer: a preliminary analysis of appropriate indications. Surg Endosc. 2013;27:3271-3279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | He Z, Sun C, Wang J, Zheng Z, Yu Q, Wang T, Chen X, Liu W, Wang B. Efficacy and safety of endoscopic submucosal dissection in treating gastric subepithelial tumors originating in the muscularis propria layer: a single-center study of 144 cases. Scand J Gastroenterol. 2013;48:1466-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Białek A, Wiechowska-Kozłowska A, Pertkiewicz J, Polkowski M, Milkiewicz P, Karpińska K, Ławniczak M, Starzyńska T. Endoscopic submucosal dissection for treatment of gastric subepithelial tumors (with video). Gastrointest Endosc. 2012;75:276-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 13. | Guo JT, Zhang JJ, Wu YF, Liao Y, Wang YD, Zhang BZ, Wang S, Sun SY. Endoscopic full-thickness resection using an over-the-scope device: A prospective study. World J Gastroenterol. 2021;27:725-736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Choi SM, Kim MC, Jung GJ, Kim HH, Kwon HC, Choi SR, Jang JS, Jeong JS. Laparoscopic wedge resection for gastric GIST: long-term follow-up results. Eur J Surg Oncol. 2007;33:444-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 15. | Matsuhashi N, Osada S, Yamaguchi K, Okumura N, Tanaka Y, Imai H, Sasaki Y, Nonaka K, Takahashi T, Futamura M, Yoshida K. Long-term outcomes of treatment of gastric gastrointestinal stromal tumor by laparoscopic surgery: review of the literature and our experience. Hepatogastroenterology. 2013;60:2011-2015. [PubMed] |
| 16. | Melstrom LG, Phillips JD, Bentrem DJ, Wayne JD. Laparoscopic versus open resection of gastric gastrointestinal stromal tumors. Am J Clin Oncol. 2012;35:451-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Matsuda T, Hiki N, Nunobe S, Aikou S, Hirasawa T, Yamamoto Y, Kumagai K, Ohashi M, Sano T, Yamaguchi T. Feasibility of laparoscopic and endoscopic cooperative surgery for gastric submucosal tumors (with video). Gastrointest Endosc. 2016;84:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Hu J, Or BH, Hu K, Wang ML. Comparison of the post-operative outcomes and survival of laparoscopic versus open resections for gastric gastrointestinal stromal tumors: A multi-center prospective cohort study. Int J Surg. 2016;33 Pt A:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Eisenberg D, Bell R. Intraoperative endoscopy: a requisite tool for laparoscopic resection of unusual gastrointestinal lesions--a case series. J Surg Res. 2009;155:318-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Sano Y, Teramoto A, Iwatate M. Magnifying Endoscopy: Image-Enhanced Endoscopy Focused on JNET Classification—Narrow-Band Imaging (NBI). In: Tanaka, S., Saitoh, Y. (eds) Endoscopic Management of Colorectal T1(SM) Carcinoma. Singapore: Springer; 2020: 17-23. |
| 21. | Yang Q, Liu Z, Sun H, Jiao F, Zhang B, Chen J. A narrative review: narrow-band imaging endoscopic classifications. Quant Imaging Med Surg. 2023;13:1138-1163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 22. | Gono K, Obi T, Yamaguchi M, Ohyama N, Machida H, Sano Y, Yoshida S, Hamamoto Y, Endo T. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J Biomed Opt. 2004;9:568-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 611] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 23. | Sano Y, Horimatsu T, Fu KI, Katagiri A, Muto M, Ishikawa H. Magnifying observation of microvascular architecture of colorectal lesions using a narrow-band imaging system. Dig Endosc. 2006;18:S44-S51. [RCA] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Sano Y, Tanaka S, Kudo SE, Saito S, Matsuda T, Wada Y, Fujii T, Ikematsu H, Uraoka T, Kobayashi N, Nakamura H, Hotta K, Horimatsu T, Sakamoto N, Fu KI, Tsuruta O, Kawano H, Kashida H, Takeuchi Y, Machida H, Kusaka T, Yoshida N, Hirata I, Terai T, Yamano HO, Kaneko K, Nakajima T, Sakamoto T, Yamaguchi Y, Tamai N, Nakano N, Hayashi N, Oka S, Iwatate M, Ishikawa H, Murakami Y, Yoshida S, Saito Y. Narrow-band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Dig Endosc. 2016;28:526-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 402] [Article Influence: 44.7] [Reference Citation Analysis (1)] |
| 25. | Iwatate M, Sano Y, Tanaka S, Kudo SE, Saito S, Matsuda T, Wada Y, Fujii T, Ikematsu H, Uraoka T, Kobayashi N, Nakamura H, Hotta K, Horimatsu T, Sakamoto N, Fu KI, Tsuruta O, Kawano H, Kashida H, Takeuchi Y, Machida H, Kusaka T, Yoshida N, Hirata I, Terai T, Yamano HO, Nakajima T, Sakamoto T, Yamaguchi Y, Tamai N, Nakano N, Hayashi N, Oka S, Ishikawa H, Murakami Y, Yoshida S, Saito Y; Japan NBI Expert Team (JNET). Validation study for development of the Japan NBI Expert Team classification of colorectal lesions. Dig Endosc. 2018;30:642-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 26. | Hewett DG, Kaltenbach T, Sano Y, Tanaka S, Saunders BP, Ponchon T, Soetikno R, Rex DK. Validation of a simple classification system for endoscopic diagnosis of small colorectal polyps using narrow-band imaging. Gastroenterology. 2012;143:599-607.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 417] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 27. | Hayashi N, Tanaka S, Hewett DG, Kaltenbach TR, Sano Y, Ponchon T, Saunders BP, Rex DK, Soetikno RM. Endoscopic prediction of deep submucosal invasive carcinoma: validation of the narrow-band imaging international colorectal endoscopic (NICE) classification. Gastrointest Endosc. 2013;78:625-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 321] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 28. | Hiki N, Yamamoto Y, Fukunaga T, Yamaguchi T, Nunobe S, Tokunaga M, Miki A, Ohyama S, Seto Y. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc. 2008;22:1729-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 340] [Article Influence: 18.9] [Reference Citation Analysis (2)] |
| 29. | Nunobe S, Hiki N, Gotoda T, Murao T, Haruma K, Matsumoto H, Hirai T, Tanimura S, Sano T, Yamaguchi T. Successful application of laparoscopic and endoscopic cooperative surgery (LECS) for a lateral-spreading mucosal gastric cancer. Gastric Cancer. 2012;15:338-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 30. | Goto O, Mitsui T, Fujishiro M, Wada I, Shimizu N, Seto Y, Koike K. New method of endoscopic full-thickness resection: a pilot study of non-exposed endoscopic wall-inversion surgery in an ex vivo porcine model. Gastric Cancer. 2011;14:183-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (1)] |
| 31. | Goto O, Takeuchi H, Kawakubo H, Sasaki M, Matsuda T, Matsuda S, Kigasawa Y, Kadota Y, Fujimoto A, Ochiai Y, Horii J, Uraoka T, Kitagawa Y, Yahagi N. First case of non-exposed endoscopic wall-inversion surgery with sentinel node basin dissection for early gastric cancer. Gastric Cancer. 2015;18:434-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 32. | Mitsui T, Goto O, Shimizu N, Hatao F, Wada I, Niimi K, Asada-Hirayama I, Fujishiro M, Koike K, Seto Y. Novel technique for full-thickness resection of gastric malignancy: feasibility of nonexposed endoscopic wall-inversion surgery (news) in porcine models. Surg Laparosc Endosc Percutan Tech. 2013;23:e217-e221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | Mitsui T, Niimi K, Yamashita H, Goto O, Aikou S, Hatao F, Wada I, Shimizu N, Fujishiro M, Koike K, Seto Y. Non-exposed endoscopic wall-inversion surgery as a novel partial gastrectomy technique. Gastric Cancer. 2014;17:594-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 34. | Goto O, Takeuchi H, Kawakubo H, Matsuda S, Kato F, Sasaki M, Fujimoto A, Ochiai Y, Horii J, Uraoka T, Kitagawa Y, Yahagi N. Feasibility of non-exposed endoscopic wall-inversion surgery with sentinel node basin dissection as a new surgical method for early gastric cancer: a porcine survival study. Gastric Cancer. 2015;18:440-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 35. | Inoue H, Ikeda H, Hosoya T, Yoshida A, Onimaru M, Suzuki M, Kudo SE. Endoscopic mucosal resection, endoscopic submucosal dissection, and beyond: full-layer resection for gastric cancer with nonexposure technique (CLEAN-NET). Surg Oncol Clin N Am. 2012;21:129-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 143] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 36. | Kikuchi S, Nishizaki M, Kuroda S, Tanabe S, Noma K, Kagawa S, Shirakawa Y, Kato H, Okada H, Fujiwara T. Nonexposure laparoscopic and endoscopic cooperative surgery (closed laparoscopic and endoscopic cooperative surgery) for gastric submucosal tumor. Gastric Cancer. 2017;20:553-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 37. | Irino T, Nunobe S, Hiki N, Yamamoto Y, Hirasawa T, Ohashi M, Fujisaki J, Sano T, Yamaguchi T. Laparoscopic-endoscopic cooperative surgery for duodenal tumors: a unique procedure that helps ensure the safety of endoscopic submucosal dissection. Endoscopy. 2015;47:349-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 38. | Fukunaga Y, Tamegai Y, Chino A, Ueno M, Nagayama S, Fujimoto Y, Konishi T, Igarashi M. New technique of en bloc resection of colorectal tumor using laparoscopy and endoscopy cooperatively (laparoscopy and endoscopy cooperative surgery - colorectal). Dis Colon Rectum. 2014;57:267-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Hiki N, Nunobe S. Laparoscopic endoscopic cooperative surgery (LECS) for the gastrointestinal tract: Updated indications. Ann Gastroenterol Surg. 2019;3:239-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (1)] |
| 40. | Ntourakis D, Mavrogenis G. Cooperative laparoscopic endoscopic and hybrid laparoscopic surgery for upper gastrointestinal tumors: Current status. World J Gastroenterol. 2015;21:12482-12497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 41. | ESMO / European Sarcoma Network Working Group. Gastrointestinal stromal tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23 Suppl 7:vii49-vii55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 42. | Yada T, Yokoi C, Uemura N. The current state of diagnosis and treatment for early gastric cancer. Diagn Ther Endosc. 2013;2013:241320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 43. | Aisu Y, Yasukawa D, Kimura Y, Hori T. Laparoscopic and endoscopic cooperative surgery for gastric tumors: Perspective for actual practice and oncological benefits. World J Gastrointest Oncol. 2018;10:381-397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 44. | Tamegai Y, Fukunaga Y, Suzuki S, Lim DNF, Chino A, Saito S, Konishi T, Akiyoshi T, Ueno M, Hiki N, Muto T. Laparoscopic and endoscopic cooperative surgery (LECS) to overcome the limitations of endoscopic resection for colorectal tumors. Endosc Int Open. 2018;6:E1477-E1485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 45. | Crafa F, Vanella S, Catalano OA, Pomykala KL, Baiamonte M. Role of one-step nucleic acid amplification in colorectal cancer lymph node metastases detection. World J Gastroenterol. 2022;28:4019-4043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 46. | Güller U, Zettl A, Worni M, Langer I, Cabalzar-Wondberg D, Viehl CT, Demartines N, Zuber M. Molecular investigation of lymph nodes in colon cancer patients using one-step nucleic acid amplification (OSNA): a new road to better staging? Cancer. 2012;118:6039-6045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 47. | Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28:E63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5533] [Cited by in RCA: 5670] [Article Influence: 226.8] [Reference Citation Analysis (0)] |
| 48. | Tamaki Y. One-step nucleic acid amplification (OSNA): where do we go with it? Int J Clin Oncol. 2017;22:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 49. | Yaguchi Y, Sugasawa H, Tsujimoto H, Takata H, Nakabayashi K, Ichikura T, Ono S, Hiraki S, Sakamoto N, Horio T, Kumano I, Otomo Y, Mochizuki H, Yamamoto J, Hase K. One-step nucleic acid amplification (OSNA) for the application of sentinel node concept in gastric cancer. Ann Surg Oncol. 2011;18:2289-2296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 50. | Kumagai K, Yamamoto N, Miyashiro I, Tomita Y, Katai H, Kushima R, Tsuda H, Kitagawa Y, Takeuchi H, Mukai M, Mano M, Mochizuki H, Kato Y, Matsuura N, Sano T. Multicenter study evaluating the clinical performance of the OSNA assay for the molecular detection of lymph node metastases in gastric cancer patients. Gastric Cancer. 2014;17:273-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 51. | Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, Repici A, Vieth M, De Ceglie A, Amato A, Berr F, Bhandari P, Bialek A, Conio M, Haringsma J, Langner C, Meisner S, Messmann H, Morino M, Neuhaus H, Piessevaux H, Rugge M, Saunders BP, Robaszkiewicz M, Seewald S, Kashin S, Dumonceau JM, Hassan C, Deprez PH. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:829-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 928] [Article Influence: 92.8] [Reference Citation Analysis (0)] |
| 52. | Maehata T, Goto O, Takeuchi H, Kitagawa Y, Yahagi N. Cutting edge of endoscopic full-thickness resection for gastric tumor. World J Gastrointest Endosc. 2015;7:1208-1215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 53. | Schem C, Maass N, Bauerschlag DO, Carstensen MH, Löning T, Roder C, Batic O, Jonat W, Tiemann K. One-step nucleic acid amplification-a molecular method for the detection of lymph node metastases in breast cancer patients; results of the German study group. Virchows Arch. 2009;454:203-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 54. | Tsujimoto M, Nakabayashi K, Yoshidome K, Kaneko T, Iwase T, Akiyama F, Kato Y, Tsuda H, Ueda S, Sato K, Tamaki Y, Noguchi S, Kataoka TR, Nakajima H, Komoike Y, Inaji H, Tsugawa K, Suzuki K, Nakamura S, Daitoh M, Otomo Y, Matsuura N. One-step nucleic acid amplification for intraoperative detection of lymph node metastasis in breast cancer patients. Clin Cancer Res. 2007;13:4807-4816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 336] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 55. | Visser M, Jiwa M, Horstman A, Brink AA, Pol RP, van Diest P, Snijders PJ, Meijer CJ. Intra-operative rapid diagnostic method based on CK19 mRNA expression for the detection of lymph node metastases in breast cancer. Int J Cancer. 2008;122:2562-2567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 56. | Tamaki Y, Akiyama F, Iwase T, Kaneko T, Tsuda H, Sato K, Ueda S, Mano M, Masuda N, Takeda M, Tsujimoto M, Yoshidome K, Inaji H, Nakajima H, Komoike Y, Kataoka TR, Nakamura S, Suzuki K, Tsugawa K, Wakasa K, Okino T, Kato Y, Noguchi S, Matsuura N. Molecular detection of lymph node metastases in breast cancer patients: results of a multicenter trial using the one-step nucleic acid amplification assay. Clin Cancer Res. 2009;15:2879-2884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 162] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 57. | Castellano I, Macrì L, Deambrogio C, Balmativola D, Bussone R, Ala A, Coluccia C, Sapino A. Reliability of whole sentinel lymph node analysis by one-step nucleic acid amplification for intraoperative diagnosis of breast cancer metastases. Ann Surg. 2012;255:334-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 58. | Shimada A, Takeuchi H, Nishi T, Mayanagi S, Fukuda K, Suda K, Nakamura R, Wada N, Kawakubo H, Nakahara T, Kameyama K, Kitagawa Y. Utility of the one-step nucleic acid amplification assay in sentinel node mapping for early gastric cancer patients. Gastric Cancer. 2020;23:418-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 59. | Esposito F, Noviello A, Moles N, Coppola Bottazzi E, Baiamonte M, Macaione I, Ferbo U, Lepore M, Miro A, Crafa F. Sentinel Lymph Node Analysis in Colorectal Cancer Patients Using One-Step Nucleic Acid Amplification in Combination With Fluorescence and Indocyanine Green. Ann Coloproctol. 2019;35:174-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 60. | Min JS, Seo KW, Jeong SH. Choice of LECS Procedure for Benign and Malignant Gastric Tumors. J Gastric Cancer. 2021;21:111-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 61. | Matsuda T, Nunobe S, Ohashi M, Hiki N. Laparoscopic endoscopic cooperative surgery (LECS) for the upper gastrointestinal tract. Transl Gastroenterol Hepatol. 2017;2:40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 62. | Lee SW, Garrett KA, Shin JH, Trencheva K, Sonoda T, Milsom JW. Dynamic article: long-term outcomes of patients undergoing combined endolaparoscopic surgery for benign colon polyps. Dis Colon Rectum. 2013;56:869-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 63. | Yan J, Trencheva K, Lee SW, Sonoda T, Shukla P, Milsom JW. Treatment for right colon polyps not removable using standard colonoscopy: combined laparoscopic-colonoscopic approach. Dis Colon Rectum. 2011;54:753-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 64. | deBeche-Adams T, Hassan I, Haggerty S, Stefanidis D. Transanal Minimally Invasive Surgery (TAMIS): a clinical spotlight review. Surg Endosc. 2017;31:3791-3800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 65. | Sakuragi M, Togashi K, Konishi F, Koinuma K, Kawamura Y, Okada M, Nagai H. Predictive factors for lymph node metastasis in T1 stage colorectal carcinomas. Dis Colon Rectum. 2003;46:1626-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 90] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 66. | Dighe S, Purkayastha S, Swift I, Tekkis PP, Darzi A, A'Hern R, Brown G. Diagnostic precision of CT in local staging of colon cancers: a meta-analysis. Clin Radiol. 2010;65:708-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 67. | Choi AH, Nelson RA, Schoellhammer HF, Cho W, Ko M, Arrington A, Oxner CR, Fakih M, Wong J, Sentovich SM, Garcia-Aguilar J, Kim J. Accuracy of computed tomography in nodal staging of colon cancer patients. World J Gastrointest Surg. 2015;7:116-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 68. | Takeuchi H, Goto O, Yahagi N, Kitagawa Y. Function-preserving gastrectomy based on the sentinel node concept in early gastric cancer. Gastric Cancer. 2017;20:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 69. | Eom BW, Kim CG, Kook MC, Yoon HM, Ryu KW, Kim YW, Rho JY, Kim YI, Lee JY, Choi IJ. Non-exposure Simple Suturing Endoscopic Full-thickness Resection with Sentinel Basin Dissection in Patients with Early Gastric Cancer: the SENORITA 3 Pilot Study. J Gastric Cancer. 2020;20:245-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 70. | Kim SG, Eom BW, Yoon HM, Kim CG, Kook MC, Kim YW, Ryu KW. Recent updates and current issues of sentinel node navigation surgery for early gastric cancer. Chin J Cancer Res. 2021;33:142-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 71. | Miwa K. [Sentinel node concept and its application for cancer surgery]. Nihon Geka Gakkai Zasshi. 2000;101:307-310. [PubMed] |
| 72. | Kinami S, Nakamura N, Tomita Y, Miyata T, Fujita H, Ueda N, Kosaka T. Precision surgical approach with lymph-node dissection in early gastric cancer. World J Gastroenterol. 2019;25:1640-1652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 73. | Kinami S, Fujimura T, Ojima E, Fushida S, Ojima T, Funaki H, Fujita H, Takamura H, Ninomiya I, Nishimura G, Kayahara M, Ohta T, Yoh Z. PTD classification: proposal for a new classification of gastric cancer location based on physiological lymphatic flow. Int J Clin Oncol. 2008;13:320-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 74. | Kinami S, Nakamura N, Miyashita T, Kitakata H, Fushida S, Fujimura T, Iida Y, Inaki N, Ito T, Takamura H. Life prognosis of sentinel node navigation surgery for early-stage gastric cancer: Outcome of lymphatic basin dissection. World J Gastroenterol. 2021;27:8010-8030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 75. | Kitagawa Y, Takeuchi H, Takagi Y, Natsugoe S, Terashima M, Murakami N, Fujimura T, Tsujimoto H, Hayashi H, Yoshimizu N, Takagane A, Mohri Y, Nabeshima K, Uenosono Y, Kinami S, Sakamoto J, Morita S, Aikou T, Miwa K, Kitajima M. Sentinel node mapping for gastric cancer: a prospective multicenter trial in Japan. J Clin Oncol. 2013;31:3704-3710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 233] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 76. | Kim YW, Min JS, Yoon HM, An JY, Eom BW, Hur H, Lee YJ, Cho GS, Park YK, Jung MR, Park JH, Hyung WJ, Jeong SH, Kook MC, Han M, Nam BH, Ryu KW. Laparoscopic Sentinel Node Navigation Surgery for Stomach Preservation in Patients With Early Gastric Cancer: A Randomized Clinical Trial. J Clin Oncol. 2022;40:2342-2351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 77. | An JY, Min JS, Hur H, Lee YJ, Cho GS, Park YK, Jung MR, Park JH, Hyung WJ, Jeong SH, Kim YW, Yoon HM, Eom BW, Kook MC, Han MR, Nam BH, Ryu KW; SEntinel Node ORIented Tailored Approach (SENORITA) Study Group. Laparoscopic sentinel node navigation surgery versus laparoscopic gastrectomy with lymph node dissection for early gastric cancer: short-term outcomes of a multicentre randomized controlled trial (SENORITA). Br J Surg. 2020;107:1429-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 78. | Huang Y, Pan M, Deng Z, Ji Y, Chen B. How useful is sentinel lymph node biopsy for the status of lymph node metastasis in cT1N0M0 gastric cancer? A systematic review and meta-analysis. Updates Surg. 2021;73:1275-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 79. | Ohi M, Toiyama Y, Omura Y, Ichikawa T, Yasuda H, Okugawa Y, Fujikawa H, Okita Y, Yoshiyama S, Hiro J, Araki T, Kusunoki M. Possibility of limited gastrectomy for early gastric cancer located in the upper third of the stomach, based on the distribution of sentinel node basins. Surg Today. 2019;49:529-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 80. | Isozaki H, Matsumoto S, Murakami S. Survival outcomes after sentinel node navigation surgery for early gastric cancer. Ann Gastroenterol Surg. 2019;3:552-560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 81. | Takahashi N, Nimura H, Fujita T, Mitsumori N, Shiraishi N, Kitano S, Satodate H, Yanaga K. Laparoscopic sentinel node navigation surgery for early gastric cancer: a prospective multicenter trial. Langenbecks Arch Surg. 2017;402:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 82. | Feng M, Wei J, Ji K, Zhang Y, Yang H, Wu X, Zhang J, Bu Z, Ji J. Characteristics of lymph node stations/basins metastasis and construction and validation of a preoperative combination prediction model that accurately excludes lymph node metastasis in early gastric cancer. Chin J Cancer Res. 2022;34:519-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 83. | Arigami T, Natsugoe S, Uenosono Y, Mataki Y, Ehi K, Higashi H, Arima H, Yanagida S, Ishigami S, Hokita S, Aikou T. Evaluation of sentinel node concept in gastric cancer based on lymph node micrometastasis determined by reverse transcription-polymerase chain reaction. Ann Surg. 2006;243:341-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 84. | Shimizu Y, Takeuchi H, Sakakura Y, Saikawa Y, Nakahara T, Mukai M, Kitajima M, Kitagawa Y. Molecular detection of sentinel node micrometastases in patients with clinical N0 gastric carcinoma with real-time multiplex reverse transcription-polymerase chain reaction assay. Ann Surg Oncol. 2012;19:469-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 85. | Shoji Y, Kumagai K, Kamiya S, Ida S, Nunobe S, Ohashi M, Yoshimizu S, Horiuchi Y, Yoshio T, Ishiyama A, Hirasawa T, Osako T, Yamamoto N, Fujisaki J, Sano T, Hiki N. Prospective feasibility study for single-tracer sentinel node mapping by ICG (indocyanine green) fluorescence and OSNA (one-step nucleic acid amplification) assay in laparoscopic gastric cancer surgery. Gastric Cancer. 2019;22:873-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 86. | Joosten JJ, Strobbe LJ, Wauters CA, Pruszczynski M, Wobbes T, Ruers TJ. Intraoperative lymphatic mapping and the sentinel node concept in colorectal carcinoma. Br J Surg. 1999;86:482-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 168] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 87. | Croner RS, Schellerer V, Demund H, Schildberg C, Papadopulos T, Naschberger E, Stürzl M, Matzel KE, Hohenberger W, Schlabrakowski A. One step nucleic acid amplification (OSNA) - a new method for lymph node staging in colorectal carcinomas. J Transl Med. 2010;8:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 88. | Yamamoto H, Sekimoto M, Oya M, Yamamoto N, Konishi F, Sasaki J, Yamada S, Taniyama K, Tominaga H, Tsujimoto M, Akamatsu H, Yanagisawa A, Sakakura C, Kato Y, Matsuura N. OSNA-based novel molecular testing for lymph node metastases in colorectal cancer patients: results from a multicenter clinical performance study in Japan. Ann Surg Oncol. 2011;18:1891-1898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 89. | Yamamoto N, Daito M, Hiyama K, Ding J, Nakabayashi K, Otomo Y, Tsujimoto M, Matsuura N, Kato Y. An optimal mRNA marker for OSNA (One-step nucleic acid amplification) based lymph node metastasis detection in colorectal cancer patients. Jpn J Clin Oncol. 2013;43:264-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 90. | Vogelaar FJ, Reimers MS, van der Linden RL, van der Linden JC, Smit VT, Lips DJ, van de Velde CJ, Bosscha K. The diagnostic value of one-step nucleic acid amplification (OSNA) for sentinel lymph nodes in colon cancer patients. Ann Surg Oncol. 2014;21:3924-3930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 91. | Yamamoto H, Tomita N, Inomata M, Furuhata T, Miyake Y, Noura S, Kato T, Murata K, Hayashi S, Igarashi S, Itabashi M, Kameoka S, Matsuura N. OSNA-Assisted Molecular Staging in Colorectal Cancer: A Prospective Multicenter Trial in Japan. Ann Surg Oncol. 2016;23:391-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 92. | Aldecoa I, Atares B, Tarragona J, Bernet L, Sardon JD, Pereda T, Villar C, Mendez MC, Gonzalez-Obeso E, Elorriaga K, Alonso GL, Zamora J, Planell N, Palacios J, Castells A, Matias-Guiu X, Cuatrecasas M. Molecularly determined total tumour load in lymph nodes of stage I-II colon cancer patients correlates with high-risk factors. A multicentre prospective study. Virchows Arch. 2016;469:385-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 93. | Itabashi M, Yamamoto H, Tomita N, Inomata M, Murata K, Hayashi S, Miyake Y, Igarashi S, Kato T, Noura S, Furuhata T, Ozawa H, Takemasa I, Yasui M, Takeyama H, Okamura S, Ohno Y, Matsuura N. Lymph Node Positivity in One-Step Nucleic Acid Amplification is a Prognostic Factor for Postoperative Cancer Recurrence in Patients with Stage II Colorectal Cancer: A Prospective, Multicenter Study. Ann Surg Oncol. 2020;27:1077-1083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 94. | Rakislova N, Montironi C, Aldecoa I, Fernandez E, Bombi JA, Jimeno M, Balaguer F, Pellise M, Castells A, Cuatrecasas M. Lymph node pooling: a feasible and efficient method of lymph node molecular staging in colorectal carcinoma. J Transl Med. 2017;15:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 95. | Snook KL, Layer GT, Jackson PA, de Vries CS, Shousha S, Sinnett HD, Nigar E, Singhal H, Chia Y, Cunnick G, Kissin MW; OSNA Study Group. Multicentre evaluation of intraoperative molecular analysis of sentinel lymph nodes in breast carcinoma. Br J Surg. 2011;98:527-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 96. | Marhic A, Tremblay JF, Kaci R, André T, Eveno C, Pocard M. Molecular analysis of sentinel lymph node in colon carcinomas by one-step nucleic acid amplification (OSNA) reduces time to adjuvant chemotherapy interval. Dig Liver Dis. 2017;49:924-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 97. | Li Z, Li X, Zhu X, Ai S, Guan W, Liu S. Tracers in Gastric Cancer Surgery. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 98. | Eom BW, Yoon HM, Min JS, Cho I, Park JH, Jung MR, Hur H, Kim YW, Park YK, Nam BH, Ryu KW; Sentinel Node Oriented Tailored Approach (SENORITA) Study Group. Prospective Multicenter Feasibility Study of Laparoscopic Sentinel Basin Dissection after Endoscopic Submucosal Dissection for Early Gastric Cancer: SENORITA 2 Trial Protocol. J Gastric Cancer. 2019;19:157-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 99. | Ichikura T, Chochi K, Sugasawa H, Yaguchi Y, Sakamoto N, Takahata R, Kosuda S, Mochizuki H. Individualized surgery for early gastric cancer guided by sentinel node biopsy. Surgery. 2006;139:501-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |