Published online Jun 28, 2023. doi: 10.3748/wjg.v29.i24.3871
Peer-review started: March 26, 2023
First decision: April 26, 2023
Revised: May 6, 2023
Accepted: May 30, 2023
Article in press: May 30, 2023
Published online: June 28, 2023
Processing time: 94 Days and 9.2 Hours
Stereotactic body radiotherapy (SBRT) and programmed cell death 1 inhibitors have shown potential in treating hepatocellular carcinoma (HCC) in retrospective studies.
To evaluate the efficacy of combining SBRT with sintilimab for patients with recurrent or oligometastatic HCC.
This trial involved patients with recurrent or oligometastatic HCC intravenously treated with SBRT plus sintilimab every 3 wk for 12 mo or until disease pro
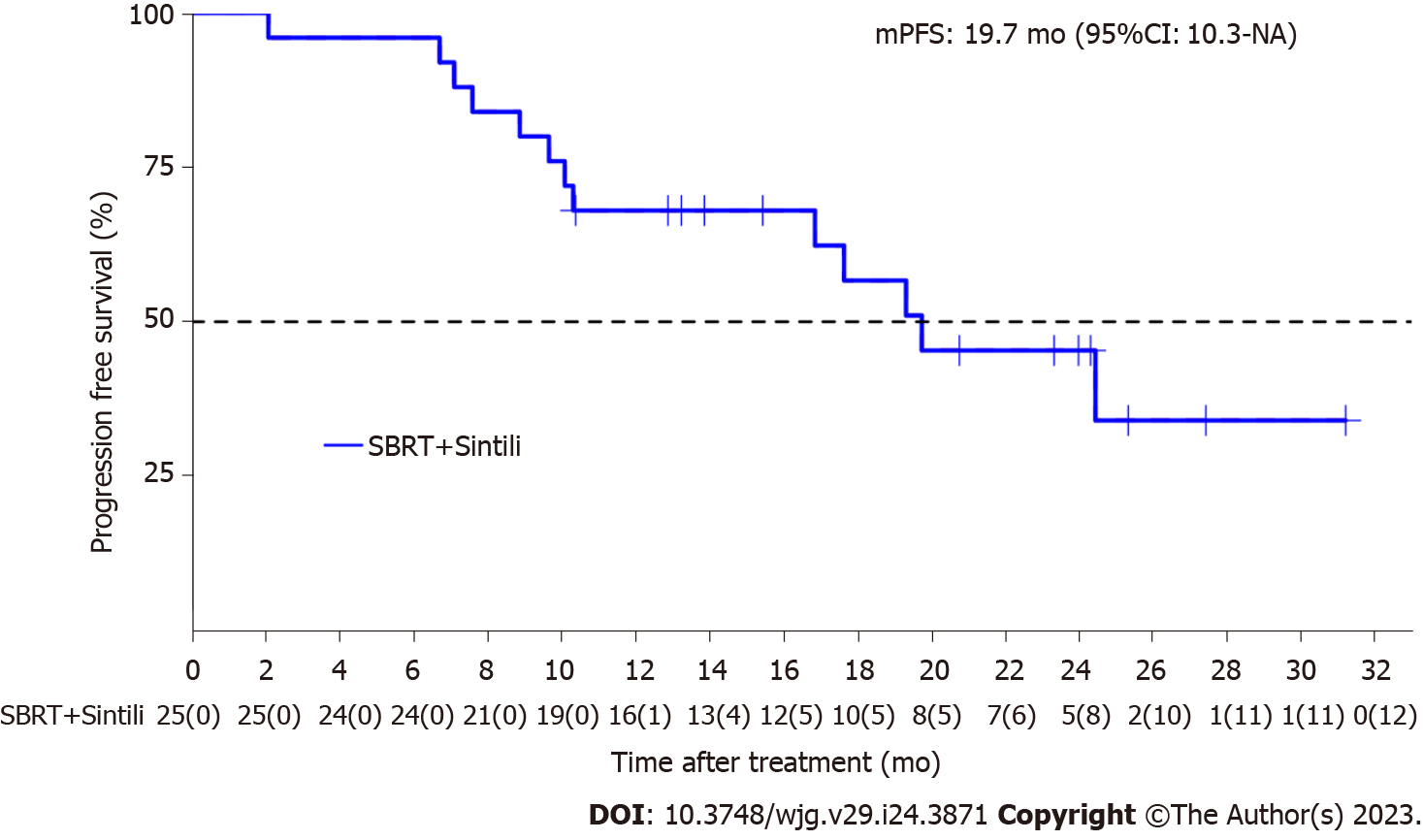
Twenty-five patients were enrolled from August 14, 2019, to August 23, 2021. The median treatment duration was 10.2 (range, 0.7-14.6) months. SBRT was delivered at a median dose of 54 (range, 48-60) Gy in 6 (range, 6-10) fractions. The median follow-up time was 21.9 (range, 10.3-39.7) mo, and 32 targeted lesions among 25 patients were evaluated for treatment response according to the Response Evaluation Criteria in Solid Tumors version 1.1. The median PFS was 19.7 mo [95% confidence interval (CI): 16.9-NA], with PFS rates of 68% (95%CI: 52-89) and 45.3% (95%CI: 28-73.4) at 12 and 24 mo, respectively. The median overall survival (OS) was not reached, with OS rates of 91.5% (95%CI: 80.8-100.0) and 83.2% (95%CI: 66.5-100.0) at 12 and 24 mo, respectively. The 1- and 2-year local control rate were 100% and 90.9% (95%CI: 75.4%-100.0%), respectively. The confirmed objective response rate and disease control rate was 96%, and 96%, respectively. Most adverse events were graded as 1 or 2, and grade 3 adverse events were observed in three patients.
SBRT plus sintilimab is an effective, well-tolerated treatment regimen for patients with recurrent or oligometastatic HCC.
Core Tip: In this single-arm phase II trial, stereotactic body radiotherapy (SBRT) plus sintilimab showed outstanding progression-free survival and objective response rate in the treatment of recurrent or oligometastatic hepatocellular carcinoma. Moreover, the combined use of SBRT and sintilimab is safe and tolerable. Patients can recover rapidly from radiotherapy-induced decreases in lymphocyte counts. This result suggests that SBRT combined with sintilimab may be a new treatment option for patients with recurrent or oligometastatic hepatocellular carcinoma.
- Citation: Chen YX, Yang P, Du SS, Zhuang Y, Huang C, Hu Y, Zhu WC, Yu YY, Liu TS, Zeng ZC. Stereotactic body radiotherapy combined with sintilimab in patients with recurrent or oligometastatic hepatocellular carcinoma: A phase II clinical trial. World J Gastroenterol 2023; 29(24): 3871-3882
- URL: https://www.wjgnet.com/1007-9327/full/v29/i24/3871.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i24.3871
Liver cancer is the sixth most prevalent cancer, with the fourth highest mortality rate. China accounts for nearly half of all hepatocellular carcinoma (HCC) cases globally[1,2]. Surgery is an important curative therapy for HCC. The 5-year recurrence rate of HCC has reached 60%-70% after surgical resection and active postoperative management[3-5]. In addition to intrahepatic recurrence, the most common sites of metastasis are the lung, lymph node, and bone[6]. Oligometastasis is defined as a transition point between localized disease and widespread metastasis and has been applied to several solid tumors[7]. Although systemic treatment is the standard of care for patients with HCC presenting with extrahepatic metastases, effective treatments for oligometastatic HCC remain unknown[8]. Studies have demonstrated the efficacy of stereotactic body radiotherapy (SBRT) for HCC, with a high local control rate and acceptable toxicity[9-11]. However, the treatment effect of SBRT is determined by lesions in the irradiated field. Therefore, the development of additional treatment regimens to further reduce the recurrence out of the radiation volume and improve the prognosis of HCC is urgently required.
The combination of programmed cell death 1 (PD-1) inhibitors and targeted antiangiogenic treatment is considered the standard first-line treatment regimen for advanced HCC[12,13]. Immunotherapy, as a systematic treatment, has a long tail effect. It can provide a lasting survival benefit by generating memory T cells and exercise better control of tumor cells throughout the body[14]. The upregulation of programmed death ligand-1 (PD-L1) is associated with radioresistance in patients with HCC after radiotherapy, and the use of PD-1/PD-L1 inhibitors can kill tumor cells through the immune response effect. Thus, the recurrence rate can be further reduced owing to the potential synergistic effect of combined radiotherapy and PD-1 inhibitors[15,16].
Sintilimab is a highly selective, fully human immunoglobulin G4 monoclonal antibody against PD-1, and the combined use of sintilimab and bevacizumab is the first-line treatment for advanced HCC in China[13]. A retrospective cohort study included 76 patients with intermediate-stage HCC who presented with trans-arterial chemoembolization (TACE) refractoriness and found that progression-free survival (PFS) and overall survival (OS) were improved after the use of SBRT combined with a PD-1 inhibitor[17], but these results were not verified in clinical trials. Thus, the current phase II trial aimed to assess the efficacy and safety of SBRT plus sintilimab in patients with recurrent or oligometastatic HCC.
This was a single-arm, open-label, single-center, phase II trial registered with ClinicalTrials.gov (NCT03857815). This study enrolled adult patients (aged between 18 and 75 years) with diagnostic criteria for HCC between August 14, 2019, and August 23, 2021, at Zhongshan Hospital, Fudan University. Patients with Child–Pugh A and an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–1 were eligible for this study. Other main inclusion criteria were as follows: (1) Patients with the number of lesions between one and five, which is suitable for SBRT; (2) patients without a history of radiotherapy; and (3) patients with adequate hematological and organ functions. Patients presenting with fibrolamellar HCC, sarcomatoid HCC, and cholangiocarcinoma and with a history of liver transplantation and other acute or chronic conditions were ineligible. The eligibility criteria are detailed in the study protocol and are shown in the supplementary file.
The Medical Ethics Committee of Zhongshan Hospital, Fudan University, approved this study (B2019-010R), which was conducted in accordance with the standards of Good Clinical Practice and the Declaration of Helsinki and Istanbul. All patients consented to participate after being informed of the study’s purpose.
All authors had access to the study data and reviewed and approved the final manuscript.
After enrollment, all patients underwent SBRT combined with sintilimab for 12 mo or until disease progression. For SBRT, the gross tumor volume (GTV) was defined as computed tomography (CT), magnetic resonance imaging (MRI), or CT/MRI/positron emission tomography fusion images, and all lesions were included. The internal target volume (ITV) was constructed based on four-dimensional CT to delineate the GTV of each time phase and was generated. The planning target volume was defined as the ITV plus a margin of 3 mm. The patients underwent daily on-board megavoltage CT for imaging guidance. The patients received a total dose of 48–60 Gy in 5–10 fractions 5 times per week. Patients received 200 mg sintilimab intravenously once every 3 wk for 12 mo or until disease progression. Tumor imaging assessments were performed every 6 wk (± 7 d) from the first day of sintilimab treatment and every 12 wk (± 7 d) after 48 wk. After the initial evaluation of imaging progression by the investigator, if the participant was clinically stable, medication was continued, and imaging was repeated 4-6 wk later to confirm disease progression, or treatment was continued if no progression was confirmed.
The primary endpoint was PFS, which was defined as the time from the start of treatment to the date of progressive disease or death, whichever occurred first. The secondary endpoints included OS (the time from receipt of the study drug to death), local control rate, objective response rate [ORR, the proportion of patients who achieved complete response (CR) or partial response (PR) as their best overall response (BOR)], disease control rate (DCR, the proportion of patients who achieved CR, PR, or stable disease as their BOR), and adverse events. All outcomes were assessed by blinded independent central reviewers and investigators according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. The details of the adverse event assessment are shown in the study protocol.
With reference to historical controls[18], patients with HCC with oligometastases or recurrence were treated with SBRT, with a median PFS period of approximately 8 mo. Assuming a median PFS period of 14.8 mo with SBRT plus sintilimab, a sample size of 25 patients would be required to provide a power of 80% with a one-sided alpha level of 0.05, assuming a drop rate of 10%. The Kaplan-Meier method was used for survival analysis. Descriptive summaries were provided for all other efficacy and safety endpoints. Unpaired T Test was used to explore the differences in the counts of biomarkers between groups. The P values for biomarkers between groups were two-sided, with P < 0.05 being considered statistically significant. Statistical analyses were performed using R 4.0.5.
The baseline characteristics of the enrolled patients are shown in Table 1. Twenty-five patients were enrolled in this study. Twenty-four (96%) patients were infected with hepatitis B. The liver function of all patients was Child-Pugh class A. Nineteen (76%) patients had intrahepatic recurrence, 6 (24%) patients showed extrahepatic recurrence, and 2 patients showed both intra- and extrahepatic recurrences. Tumors 1, 2, and 3 were reported in 19, 5, and 1 patient(s), respectively. All the patients underwent surgery, ablation, or TACE previously. The median treatment duration was 10.2 (range, 0.7-14.6) months, and SBRT was delivered at a median dose of 54 (range, 48–60) Gy in 6 (range, 6–10) fractions.
| Characteristics | n = 25 |
| Age, years, median (range) | 64 (33-71) |
| Male sex, n (%) | 24 (96) |
| Etiology, n (%) | |
| Hepatitis B infection | 24 (96) |
| Others | 1 (4) |
| ECOG performance status, n (%) | |
| 0 | 19 (76) |
| 1 | 6 (24) |
| Child-Pugh class A, n (%) | 25 (100) |
| BCLC C stage, n (%) | 25 (100) |
| Site of recurrence, n (%) | |
| Intrahepatic | 19 (76) |
| Extrahepatic | 6 (24) |
| Both Intra-Extrahepatic | 2 (8) |
| Target tumor size, cm, median (range) | 2.0 (1.0-7.4) |
| Tumor number, n (%) | |
| 1 | 19 (76) |
| 2 | 5 (25) |
| 3 | 1 (4) |
| Serum AFP ≥ 400 ng/mL, n (%) | 3 (12) |
| Prior therapy, n (%) | |
| Surgery | 17 (68) |
| Ablation | 9 (36) |
| TACE | 13 (52) |
The median follow-up time was 21.9 (range, 10.3–39.7) months, and 32 lesions in 25 patients were evaluated for treatment response according to RECIST version 1.1. The median PFS period was 19.7 [95% confidence interval (CI): 16.9-NA; Figure 1] mo, and the PFS rates at 12 and 24 mo were 68% (95%CI: 52-89) and 45.3% (95%CI: 28-73.4), respectively.
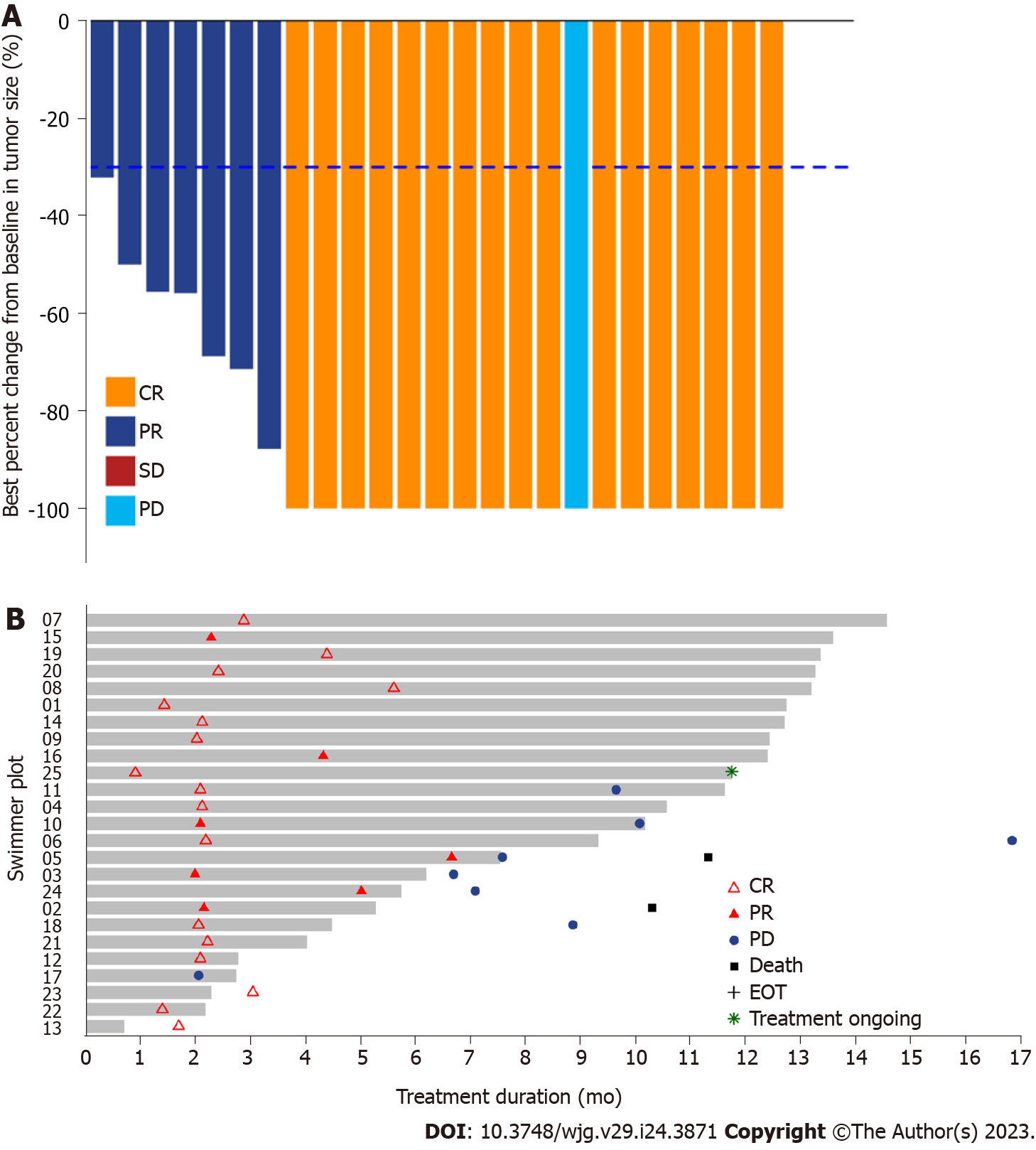
The median OS was not reached, and three events of death were observed, with 91.5% (95%CI: 80.8-100.0) and 83.2% (95%CI: 66.5-100.0) OS rates at 12 and 24 mo, respectively. Moreover, the 1- and 2-year local control rates were 100% and 90.9% (95%CI: 75.4%-100.0%), respectively (Table 2). The ORR was 96% (24/25) according to RECIST version 1.1, with 17 achieving CR and 7 achieving PR after sintilimab plus SBRT therapy. After one cycle of treatment, all target liver lesions disappeared, although new lesions appeared in the lungs of one patient, which was judged as PD. The best change in the target tumor size is shown in Figure 2A. One patient achieved PR after two cycles of sintilimab plus SBRT therapy. However, the patient underwent a self-determined liver transplant (protocol violation reported) and died 5 mo after surgery due to liver failure caused by liver transplantation (Figure 2B). Similarly, the DCR for patients with HCC treated with SBRT combined with sintilimab was 96% (Table 2). The median time to response was 2.1 (range, 0.9-6.6) months for the 24 responders, whereas the median duration of response was 16.9 mo (95%CI: 8.18-NA).
| Variable | n = 25 |
| Best overall response, n (%) | |
| Complete response | 17 (68) |
| Partial response | 7 (28) |
| Stable disease | 0 |
| Progressive disease | 1 (4) |
| Objective response rate, n (%) | 24 (96) |
| Disease control rate, n (%) | 24 (96) |
| 1 yr local control rate, n (%) | 25 (100) |
All the patients received at least one dose of the study treatment and were included in the safety analysis. The details of the adverse events in patients with HCC treated with SBRT combined with sintilimab are shown in Table 3. In total, 22 (88.0%) patients reported treatment-related adverse events (TRAEs), and grade 1 and 2 adverse events were observed in 17 (68%) and 14 (56%) patients, respectively. The most common TRAE was decreased lymphocyte count (probably caused by radiotherapy). The lymphocyte count decreased significantly after the second treatment (Supple
| Treatment-related adverse events | Any grade | Grade 1 | Grade 2 | Grade 3 |
| All adverse events, n (%) | 22 (88) | 17 (68) | 14 (56) | 3 (12) |
| Decreased lymphocyte count | 14 (56) | 7 (28) | 7 (28) | 0 |
| Increased GGT | 7 (28) | 3 (12) | 3 (12) | 1 (4) |
| Decreased platelet count | 5 (20) | 0 | 4 (16) | 1 (4) |
| Increased blood bilirubin | 5 (20) | 4 (16) | 1 (4) | 0 |
| Rash | 4 (16) | 2 (8) | 2 (8) | 0 |
| Increased ALT | 2 (8) | 1 (4) | 1 (4) | 0 |
| Increased AST | 2 (8) | 2 (8) | 0 | 0 |
| Myositis | 2 (8) | 0 | 1 (4) | 1 (4) |
| Abnormal liver function | 2 (8) | 1 (4) | 1 (4) | 0 |
| Paronychia | 2 (8) | 2 (8) | 0 | 0 |
| Jaundice | 2 (8) | 0 | 2 (8) | 0 |
| Decreased white blood cell count | 1 (4) | 1 (4) | 0 | 0 |
| Decreased neutrophil count | 1 (4) | 1 (4) | 0 | 0 |
| Ascites | 1 (4) | 0 | 1 (4) | 0 |
| Arthritis | 1 (4) | 0 | 1 (4) | 0 |
| Pneumonia | 1 (4) | 0 | 1 (4) | 0 |
| Myocarditis | 1 (4) | 1 (4) | 0 | 0 |
| Dry eye | 1 (4) | 1 (4) | 0 | 0 |
Four patients presented with serious adverse events. One patient had grade 1 myocarditis, possibly due to immunotherapy; one had grade 2 immune pneumonia; and one patient had viral hepatitis, which was evaluated to be independent of the treatment regimen. One patient experienced upper gastrointestinal bleeding owing to cirrhosis of liver. All four patients recovered after medical treatment. The study treatment was interrupted due to emergent adverse events in 3 (12%) of the 25 patients, including 1 patient with myocarditis, 1 with upper gastrointestinal bleeding, and 1 with arthritis. Patients should continue treatment after recovery from adverse reactions; however, all three patients refused to resume use. Permanent discontinuation due to adverse events occurred in three patients, including one patient with pneumonia (grade 2), one with elevated bilirubin level (grade 3), and one with ascites (grade 2).
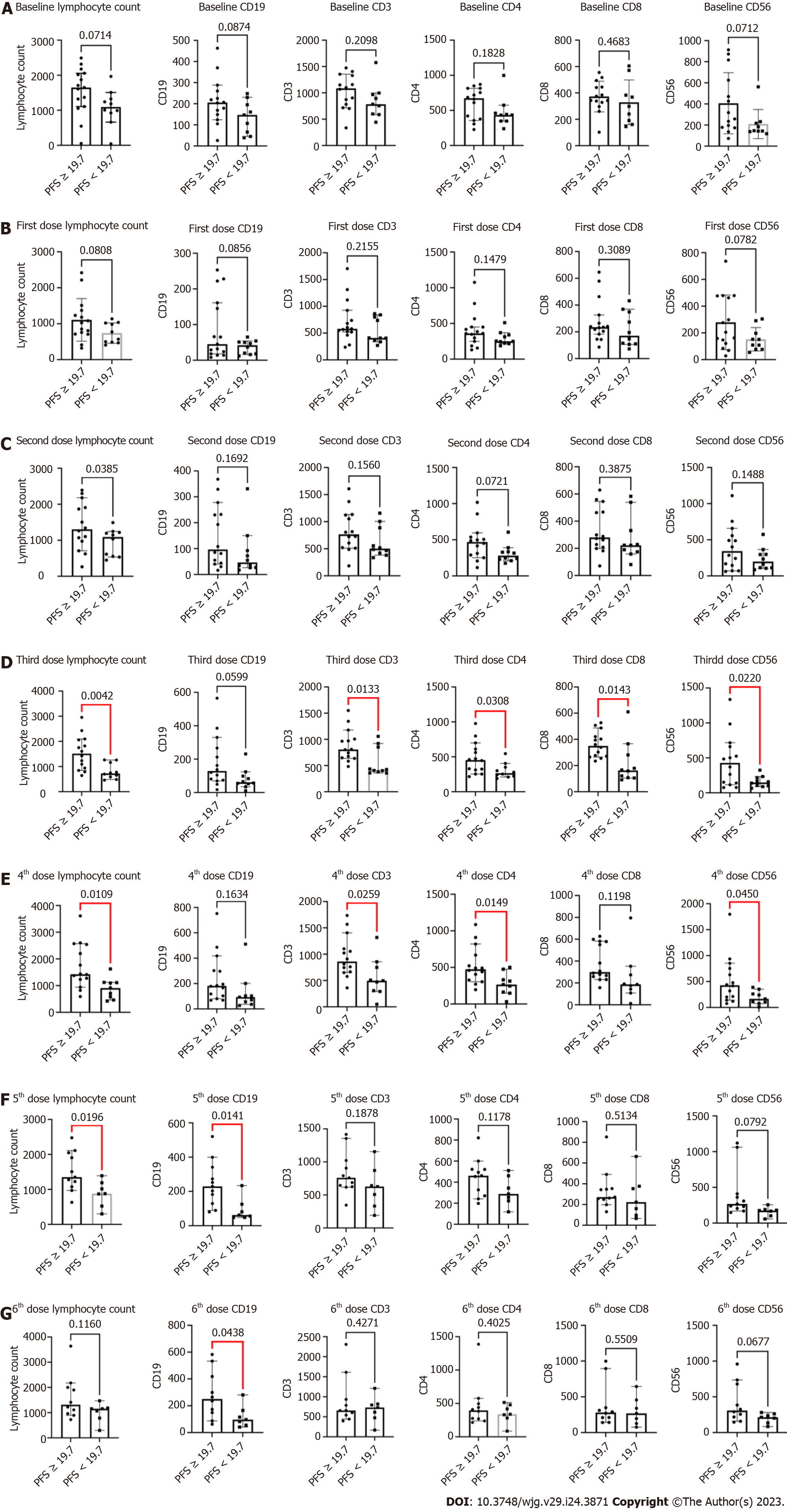
This study also explored biomarkers that could be used to predict efficacy. In this study, blood samples from patients after each treatment were collected and analyzed for lymphocyte counts and their subset populations [CD19+ B cells, CD3+, CD4+, CD8+ T cells, and CD15+CD56+ natural killer (NK) cells] by flow cytometry. The median PFS period (19.7 mo) was applied as a cutoff value, and there were no significant differences between PFS ≥ 19.7 and PFS < 19.7 mo for lymphocyte count, CD19+ B, CD3+, CD4+, CD8+ T, and CD15+CD56+ NK cells at baseline. PFS period ≥ 19.7 mo was associated with higher lymphocyte counts and CD3+, CD4+, CD8+ T, and CD15+CD56+ NK cells compared to PFS period < 19.7 mo after three treatments. These significant differences persisted after the four treatments, except for CD8+ T cells. Furthermore, the difference between PFS ≥ 19.7 and PFS < 19.7 mo after five treatments disappeared for most biomarkers, whereas significant differences remained for lymphocyte count and CD19+ B cells. Finally, PFS ≥ 19.7 mo persisted and was associated with elevated number of CD19+ B cells (Figure 3).
To the best of our knowledge, this is the first prospective study of SBRT in combination with PD-1 inhibitor for recurrent or oligometastatic HCC. This study demonstrated that SBRT delivered at a median dose of 54 Gy in 6 fractions plus sintilimab achieved a remarkable PFS period of 19.7 mo and an outstanding ORR of 96% in such patients. Although the median OS was not reached, the OS rates at 12 and 24 mo were 91.5% and 83.2%, respectively. Moreover, the local control rates at 12 and 24 mo were 100% and 90.9%, respectively, whereas the DCR after treatment was 96%. Furthermore, TRAEs in most patients were mild and well tolerated.
The enrolled patients in our study were diagnosed with recurrent or oligometastatic HCC and belonged to the advanced-stage HCC population. Currently, the standard systemic treatment for advanced HCC is PD-1/PD-L1 inhibitors in combination with bevacizumab, such as sintilimab plus bevacizumab and atezolizumab plus bevacizumab. The median PFS period of the experimental groups in Orient-32 and Imbrave150 were 4.6 and 6.8 mo, respectively[13,19]. In our study, 19.7 mo of median PFS (mPFS) period showed a beneficial tendency with SBRT and sintilimab. Notably, the population characteristics of our study were different from these two phase-III studies. The proportion of patients with vessel invasion or extrahepatic metastasis was > 60% in both Orient-32 and Imbrave150. Moreover, patients accounted for approximately 40% of the total population with both ECOG PS of 1 andα-fetoprotein (AFP) ≥ 400 ng/mL. However, patients with few metastases or relapses were enrolled in our study, and the median number of metastases was 1. Additionally, most of them had ECOG PS 0 (76%), and patients with AFP ≥ 400 ng/mL only accounted for 12%. Given the above, the high proportion of patients with good physical condition and the low proportion of patients with AFP ≥ 400 ng/mL could be explained by the remarkable mPFS period in this study. Moreover, it can also be considered the main possible reason that patients with recurrent or oligometastatic HCC (tumor lesions ≤ 5) benefit from combination therapy of SBRT plus immunotherapy.
Oligometastasis was defined as ≤ 5 metastatic lesions, and SBRT was recommended for patients with oligometastasis and many other cancers. However, no prospective clinical studies have reported the treatment strategies for patients with recurrent or oligometastatic HCC. Several retrospective clinical studies on patients with recurrent or oligometastatic HCC demonstrated that after SBRT, the 1-year OS and local control rates were 55%-84% and 77%-94%, respectively[5,20-23]. In the current study, the 1-year OS and local control rates were 91.5% and 100%, respectively. Although comparisons between studies need to be made cautiously, in terms of benefit trends, it seems that SBRT plus sintilimab has more survival benefits. This encouraging therapeutic effect can be interpreted as radiation activating the immune system by inducing tumor cell death, which can promote cytokine release and damage-associated molecular patterns. The tumor-specific T lymphocytes are then primed and trafficked into the tumor microenvironment, which can enhance the efficacy of PD-1 inhibitors[24]. Another retrospective study of SBRT plus PD-1 inhibitors for patients with Barcelona Clinic Liver Cancer intermediate-stage HCC with a median PFS period of up to 19.6 mo also verified the combination efficacy[17]. It should be emphasized that this retrospective study included patients with intermediate-stage HCC, which theoretically represent a better prognosis.
In addition, our study reported that the ORR after sintilimab and SBRT was 96%, and only one patient had liver lesions that disappeared with pulmonary metastasis. In previous retrospective studies on HCC with recurrence or oligometastases, the ORR of SBRT in this subset was 59%-85%. The authors clearly stated that out-of-field recurrence was the major cause of disease progression[5,20-23]. Sintilimab, as a systemic immunotherapy, can inhibit tumor cell escape and reactivate T cells, thereby reducing out-field recurrence.
In terms of safety, the combination of sintilimab and SBRT was well tolerated. Most TRAEs were of grades 1-2. Grade 3 TRAEs were observed in only 12% of the patients. A decreased lymphocyte count a common AE associated with radiotherapy. The results of monitoring changes in lymphocyte counts showed that decreased lymphocyte counts gradually recovered, and previous reports indicated that recovery from reduced lymphocyte count is faster in patients with HCC treated with SBRT than in those treated with conventional RT.
Biomarker-related analyses have mainly explored the relevance of lymphocytes and their subsets to efficacy. The results showed that patients with a longer PFS had higher lymphocyte counts and CD19+ B, CD3+, CD4+, CD8+ T, and CD15+CD56+ NK cells after four to five prior therapies. This is consistent with the result of a previous biomarker explorative study on SBRT for lung cancer, suggesting that lymphocyte counts and subsets can be potential biomarkers for predicting efficacy[25]. The significant difference gradually disappeared after the fifth to sixth treatments, which is likely related to the reduced sample size due to the increasing number of patients with disease progression and loss of follow-up. This needs to be confirmed in further studies with larger sample sizes.
This study has some limitations. The current study was designed as a phase II trial with no parallel control group. Moreover, the sample size was small, and stratified analyses according to patient characteristics were not performed. Finally, the history of prior therapy varied across the enrolled patients, which could have affected the prognosis of HCC.
In this single-arm phase II trial, SBRT plus sintilimab showed outstanding PFS and ORR in the treatment of recurrent or oligometastatic HCC. Moreover, the combined use of SBRT and sintilimab is safe and tolerable. Patients can recover rapidly from radiotherapy-induced decreases in lymphocyte counts. Considering mostly included patients (96%) infected with hepatitis B, this result suggests that SBRT combined with sintilimab may be a new treatment option for patients with recurrent or oligometastatic HCC and infected with hepatitis B. Further large-scale, parallel-control randomized controlled trials should be conducted to compare the effects of SBRT plus sintilimab with other treatments, including SBRT without immunotherapy or radioembolization delivers doses to the tumor greater than 100 Gy.
Hepatocellular carcinoma (HCC) accounts for the third-leading cause of cancer-related deaths, and only 30%-40% of patients were diagnosed at early stages. The oligometastatic HCC indicated the metastasis of HCC was not widespread, and some patients may obtain benefits for curative treatments.
Stereotactic body radiotherapy (SBRT) and programmed cell death 1 (PD-1) inhibitors are widely used for HCC, whether the use of SBRT combined with a PD-1 inhibitor could offer superior benefits for oligometastatic HCC remained unclear.
To assess the efficacy and safety of combining SBRT with sintilimab for patients with recurrent or oligometastatic HCC.
Patients with recurrent or oligometastatic HCC were involved and treated with combining SBRT with sintilimab for 12 mo or disease progression. The primary endpoint was progression-free survival (PFS), while the secondary endpoints included overall survival (OS), local control rate, objective response rate (ORR), disease control rate (DCR), and adverse events.
The median PFS was 19.7 mo [95% confidence interval (CI): 16.9-NA], while the median OS was not reached. Moreover, the 1- and 2-year local control rate were 100% and 90.9% (95%CI: 75.4%-100.0%), while the ORR and DCR were 96%, and 96%, respectively. Only 3 patients reported grade 3 adverse events, including increased gamma-glutamyl transpeptidase level, decreased platelet count, and myositis.
SBRT plus sintilimab can serve as an effective, well-tolerated treatment regimen for patients with recurrent or oligometastatic HCC.
Further large-scale, parallel-control randomized controlled trials should be performed to verify the therapeutic effects of SBRT plus sintilimab for patients with recurrent or oligometastatic HCC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lykoudis PM, United Kingdom; Manfredi S, France S-Editor: Fan JR L-Editor: A P-Editor: Liu JH
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64230] [Article Influence: 16057.5] [Reference Citation Analysis (174)] |
| 2. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4235] [Cited by in RCA: 11376] [Article Influence: 3792.0] [Reference Citation Analysis (4)] |
| 3. | Zou H, Zhu CZ, Wang C, Wang ZS, Ma X, Han B, Wu LQ. Recurrence of Barcelona Clinic Liver Cancer Stage A Hepatocellular Carcinoma After Hepatectomy. Am J Med Sci. 2017;354:262-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Lee SU, Yoon SM, Cheng JC, Kim TH, Kim BH, Park JH, Jung J, Tsai CL, Chiang Y, Park JW. Multi-Institutional Retrospective Study of Radiotherapy for Hepatocellular Carcinoma in the Caudate Lobe. Front Oncol. 2021;11:646473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 5. | Yao E, Chen J, Zhao X, Zheng Y, Wu X, Han F, Huang H, Liang P, Liu J, Wu F, Lin L. Efficacy of Stereotactic Body Radiotherapy for Recurrent or Residual Hepatocellular Carcinoma after Transcatheter Arterial Chemoembolization. Biomed Res Int. 2018;2018:5481909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Li C, Wang H, Chen R, Zhang H, Xu Y, Zhang B, Li Y, Zhang C, Yang Y, Wang X, Li X. Outcomes and recurrence patterns following curative hepatectomy for hepatocellular carcinoma patients with different China liver cancer staging. Am J Cancer Res. 2022;12:907-921. [PubMed] |
| 7. | Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol. 2011;8:378-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 715] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 8. | Nowak AK, Chow PK, Findlay M. Systemic therapy for advanced hepatocellular carcinoma: a review. Eur J Cancer. 2004;40:1474-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 133] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Kang JK, Kim MS, Cho CK, Yang KM, Yoo HJ, Kim JH, Bae SH, Jung DH, Kim KB, Lee DH, Han CJ, Kim J, Park SC, Kim YH. Stereotactic body radiation therapy for inoperable hepatocellular carcinoma as a local salvage treatment after incomplete transarterial chemoembolization. Cancer. 2012;118:5424-5431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 245] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 10. | Andolino DL, Johnson CS, Maluccio M, Kwo P, Tector AJ, Zook J, Johnstone PA, Cardenes HR. Stereotactic body radiotherapy for primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2011;81:e447-e453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 321] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 11. | Bujold A, Massey CA, Kim JJ, Brierley J, Cho C, Wong RK, Dinniwell RE, Kassam Z, Ringash J, Cummings B, Sykes J, Sherman M, Knox JJ, Dawson LA. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol. 2013;31:1631-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 593] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 12. | Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, Yau T, Garrido M, Chan SL, Knox J, Daniele B, Ebbinghaus SW, Chen E, Siegel AB, Zhu AX, Cheng AL; KEYNOTE-240 investigators. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J Clin Oncol. 2020;38:193-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1365] [Cited by in RCA: 1336] [Article Influence: 267.2] [Reference Citation Analysis (0)] |
| 13. | Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, Li Q, Lu Y, Chen Y, Guo Y, Chen Z, Liu B, Jia W, Wu J, Wang J, Shao G, Zhang B, Shan Y, Meng Z, Gu S, Yang W, Liu C, Shi X, Gao Z, Yin T, Cui J, Huang M, Xing B, Mao Y, Teng G, Qin Y, Xia F, Yin G, Yang Y, Chen M, Wang Y, Zhou H, Fan J; ORIENT-32 study group. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol. 2021;22:977-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 663] [Article Influence: 165.8] [Reference Citation Analysis (1)] |
| 14. | Chen H, Li Z, Qiu L, Dong X, Chen G, Shi Y, Cai L, Liu W, Ye H, Zhou Y, Ouyang J, Cai Z, Liu X. Personalized neoantigen vaccine combined with PD-1 blockade increases CD8(+) tissue-resident memory T-cell infiltration in preclinical hepatocellular carcinoma models. J Immunother Cancer. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 15. | Li W, Yan J, Tian H, Li B, Wang G, Sang W, Zhang Z, Zhang X, Dai Y. A platinum@polymer-catechol nanobraker enables radio-immunotherapy for crippling melanoma tumorigenesis, angiogenesis, and radioresistance. Bioact Mater. 2023;22:34-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 16. | Kreidieh M, Zeidan YH, Shamseddine A. The Combination of Stereotactic Body Radiation Therapy and Immunotherapy in Primary Liver Tumors. J Oncol. 2019;2019:4304817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Xiang YJ, Wang K, Zheng YT, Feng S, Yu HM, Li XW, Cheng X, Cheng YQ, Feng JK, Zhou LP, Meng Y, Zhai J, Shan YF, Cheng SQ. Effects of Stereotactic Body Radiation Therapy Plus PD-1 Inhibitors for Patients With Transarterial Chemoembolization Refractory. Front Oncol. 2022;12:839605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Huang WY, Jen YM, Lee MS, Chang LP, Chen CM, Ko KH, Lin KT, Lin JC, Chao HL, Lin CS, Su YF, Fan CY, Chang YW. Stereotactic body radiation therapy in recurrent hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2012;84:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 146] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 19. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 4646] [Article Influence: 929.2] [Reference Citation Analysis (2)] |
| 20. | Gerum S, Heinz C, Belka C, Walter F, Paprottka P, De Toni EN, Roeder F. Stereotactic body radiation therapy (SBRT) in patients with hepatocellular carcinoma and oligometastatic liver disease. Radiat Oncol. 2018;13:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Matoba M, Tsuchiya H, Kondo T, Ota K. Stereotactic body radiotherapy delivered with IMRT for oligometastatic regional lymph node metastases in hepatocellular carcinoma: a single-institutional study. J Radiat Res. 2020;61:776-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Su TS, Liang P, Lu HZ, Liang J, Gao YC, Zhou Y, Huang Y, Tang MY, Liang JN. Stereotactic body radiation therapy for small primary or recurrent hepatocellular carcinoma in 132 Chinese patients. J Surg Oncol. 2016;113:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 23. | Jo IY, Park HC, Kim ES, Yeo SG, Kim M, Seong J, Kim JW, Kim TH, Yoon WS, Jeong BK, Kim SH, Lee JH. Stereotactic ablative radiotherapy for pulmonary oligometastases from primary hepatocellular carcinoma: a multicenter and retrospective analysis (KROG 17-08). Jpn J Clin Oncol. 2022;52:616-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Romano E, Honeychurch J, Illidge TM. Radiotherapy-Immunotherapy Combination: How Will We Bridge the Gap Between Pre-Clinical Promise and Effective Clinical Delivery? Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 25. | Liu C, Hu Q, Xu B, Hu X, Su H, Li Q, Zhang X, Yue J, Yu J. Peripheral memory and naïve T cells in non-small cell lung cancer patients with lung metastases undergoing stereotactic body radiotherapy: predictors of early tumor response. Cancer Cell Int. 2019;19:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |