Published online Jun 21, 2023. doi: 10.3748/wjg.v29.i23.3645
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial.
Peer-review started: February 9, 2023
First decision: March 9, 2023
Revised: March 16, 2023
Accepted: May 4, 2023
Article in press: May 4, 2023
Published online: June 21, 2023
Processing time: 127 Days and 1 Hours
The prognostic assessment of patients after surgical resection of gastric cancer (GC) patients is critical. However, the role of the circadian clock gene NPAS2 expression in GC remains unknown.
To explore the relationship between NPAS2 and the survival prognosis of GC patients and clarify its role in evaluating GC prognosis.
The tumor tissues and clinical data of 101 patients with GC were collected retrospectively. Immunohistochemical staining (IHC) was used to detect the expression of NPAS2 protein in GC and adjacent tissues. Univariate and multivariate Cox regression analysis was used to determine the independent prognostic factors of GC, and a nomogram prediction model was established. The receiver operating characteristic (ROC) curve, the ROC area under the curve, the calibration curve, and C-index were used to evaluate the predictive effectiveness of the model. Kaplan Meier analysis was used to compare the risk stratification of subgroups according to the median score in the nomogram model of each patient.
Microarray IHC analysis showed that the positive rate of NPAS2 protein expression in GC tissues was 65.35%, which was significantly higher than 30.69% in adjacent tissues. The high expression of NPAS2 was correlated with tumor-node-metastasis (TNM) stage (P < 0.05), pN stage (P < 0.05), metastasis (P < 0.05), venous invasion (P < 0.05), lymphatic invasion (P < 0.05), and lymph node positive (P < 0.05) of GC. Kaplan Meier survival analysis showed that the 3-year overall survival (OS) of patients with high NPAS2 expression was significantly shortened (P < 0.0001). Univariate and multivariate COX regression analysis showed that TNM stage (P = 0.009), metastasis (P = 0.009), and NPAS2 expression (P = 0.020) were independent prognostic factors of OS in GC patients for 3 years. The nomogram prediction model based on independent prognostic factors has a C-Index of 0.740 (95%CI: 0.713-0.767). Furthermore, subgroup analysis showed that the 3-year OS time of the high-risk group was significantly lower than that of the low-risk group (P < 0.0001).
NPAS2 is highly expressed in GC tissues and is closely related to worse OS in patients. Therefore, the evaluation of NPAS2 expression may be a potential marker for GC prognosis evaluation. Notably, the nomogram model based on NPAS2 can improve the accuracy of GC prognosis prediction and assist clinicians in postoperative patient management and decision-making.
Core Tip: The prognostic assessment of patients after surgical resection of gastric cancer (GC) is critical. However, the role of NPAS2 expression in GC remains unknown. Our study identified for the first time that high NPAS2 protein expression was associated with poor overall survival prognosis in GC patients and was an independent risk factor for patients after radical resection of GC. We constructed a nomogram prediction model by combining NPAS2 and other clinically independent risk factors, thus improving the predictive accuracy of GC prognosis.
- Citation: Cao XM, Kang WD, Xia TH, Yuan SB, Guo CA, Wang WJ, Liu HB. High expression of the circadian clock gene NPAS2 is associated with progression and poor prognosis of gastric cancer: A single-center study. World J Gastroenterol 2023; 29(23): 3645-3657
- URL: https://www.wjgnet.com/1007-9327/full/v29/i23/3645.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i23.3645
Gastric cancer (GC) ranks fifth and fourth in the incidence and mortality of global cancer, respectively, and is more likely to occur in men[1,2]. The etiology of GC remains unclear, involving multiple cell types and complex molecular mechanisms[3]. Despite recent improvements in the diagnosis and treatment of GC, the overall treatment outcome remains poor[4]. Notably, radical gastrectomy is the primary treatment method for GC. However, postoperative recurrence rates and mortality remain high, and their mortality rates are closely associated with peritoneal, hematologic, and lymph node metastases in GC[5]. Therefore, there is an urgent need to identify new markers to predict the malignant biological behavior and prognosis of GC to guide the treatment strategy and improve the clinical outcome of GC.
Biological rhythm is a regulatory system in almost all living things. It produces 24-h periodic rhythm changes in many essential behaviors and physiological processes[6], including the sleep-wake cycle, body temperature cycle, hormone secretion, heart rate, blood pressure, excretion, etc.[7,8]. The destruction of normal biological rhythm adversely affects mammalian physiology[9]. The role and mechanism of biological rhythm-related genes in tumors, and the design of tumor treatment methods targeting biological rhythm genes, are one of the hot spots in the current cancer research field[10]. NPAS2, also known as MOP4, is the largest core circadian gene found so far, with a length of about 176.68Kb, located on human chromosome 2 (2q11.2), encoding proteins belonging to the basic helix loop helix PAS domain of transcription factors[11]. NPAS2 is mainly present in the forebrain of mammals and is also expressed in some peripheral organs such as the liver and skin[12]. Additionally, it is an important part of the positive circadian rhythm feedback circuit[8]. Furthermore, Studies have confirmed that NPAS2 is associated with various rhythm-related diseases, such as cancer, diabetes, depression, sleep disorders, obesity, etc.[13].
Recent studies have shown that low expression of NPAS2 accelerates cell growth and tumor cell cycle progression and plays a unique and critical role in cancer progression[14]. The low expression of NPAS2 promotes the proliferation and invasion of colorectal cancer cells. Additionally, it increases the wound-healing ability of colorectal cancer cells, indicating that NPAS2 has a key tumor inhibitory effect. Additionally, its high expression is positively correlated with overall survival (OS)[15]. However, some studies have found that NPAS2, as an oncogene, promotes the proliferation of hepatocellular carcinoma (HCC) cells and participates in the critical process of hepatocellular carcinogenesis. Its high expression is related to the invasive clinical characteristics and low OS of HCC patients[16]. It promotes the survival of HCC cells by up-regulating the expression of mitotic cycle 25A and inhibiting mitochondrial-dependent intrinsic apoptosis[16]. These results have also been observed in acute myeloid leukemia[17]. In contrast, there are few studies on the expression and role of NPAS2 in GC at home and abroad, and there is a significant lack of experimental research. Therefore, the expression level of NPAS2 in GC and its role in the occurrence and development of GC in different signal pathways are controversial and need to be further studied.
This study intends to detect the expression level of NPAS2 in GC and paraneoplastic tissues by using tissue microarray immunohistochemical staining to establish a nomogram prediction model based on NPAS2 and other clinicopathologically independent risk factors. Furthermore, we aim to investigate the effect of NPAS2 on the prognosis of GC and its value in the prognosis prediction of GC patients.
This study was approved by the Medical Ethics Committee of the Second Hospital of Lanzhou University. Informed consent was obtained from all patients, and all specimens were pseudonymous. The tumor tissues, precancerous tissues, and clinical data of 101 patients with primary GC resected by surgery from July to October 2019 in the second Hospital of Lanzhou University were collected and followed up by standard outpatient clinic and telephone. The inclusion criteria of the study were as follows: (1) Histopathological diagnosis of gastric adenocarcinoma; (2) age ≥ 18 years old; (3) patients with primary GC underwent surgical resection; (4) complete pathological, treatment, surgical and follow-up data; and (5) the patient gave written informed consent. Exclusion criteria: (1) Death before discharge; (2) preoperative radiotherapy and chemotherapy or neoadjuvant chemotherapy; (3) multiple cancers; and (4) gastric stump cancer. 10% neutral formalin fixation and routine paraffin-embedded GC and paraffin-embedded tissues were confirmed by pathology, and all pathology was independently reviewed by two pathologists. The patients were followed up every 3 mo in the first 2 years and every 6 mo in the third year. Follow-up included laboratory examination, chest X-ray examination, and abdominal computed tomography (CT) examination. The follow-up period was 36 mo, and OS was defined as from the date of the first operation to the end of follow-up or the time of death.
One hundred one paraffin-embedded primary GC tissues and adjacent tissues were fixed with formalin and then serially sectioned on a microtome with a thickness of 4 μm. After drying, hematoxylin and eosin staining (HE) were performed. A professional pathologist selected and marked the representative positions of HE-stained pathological sections and corresponding wax blocks according to the view under the light microscope. The tissue chip wax blocks were made by a drilling mechanism and sliced with a thickness of 4μm. After being dewaxed in xylene and washed in gradient ethanol, the slices were immersed in a pressure tank containing 800 mL of 0.1% sodium citrate buffer solution (pH 6.0) and boiled for 3 min, followed by cooling. Following the manufacturer's instructions, we then conducted a streptavidin-Biotin Complex assay (Fuzhou Maxin Biotechnology Development Company). The slides were then incubated overnight with the primary antibody against NPAS2 at 4 ℃ (diluted, 1:200, rabbit anti-human NPAS2 polyclonal antibody, Biossantibodies, China). Color development, hematoxylin re-staining, differentiation, blueing, and dehydration were performed prior to sealing.
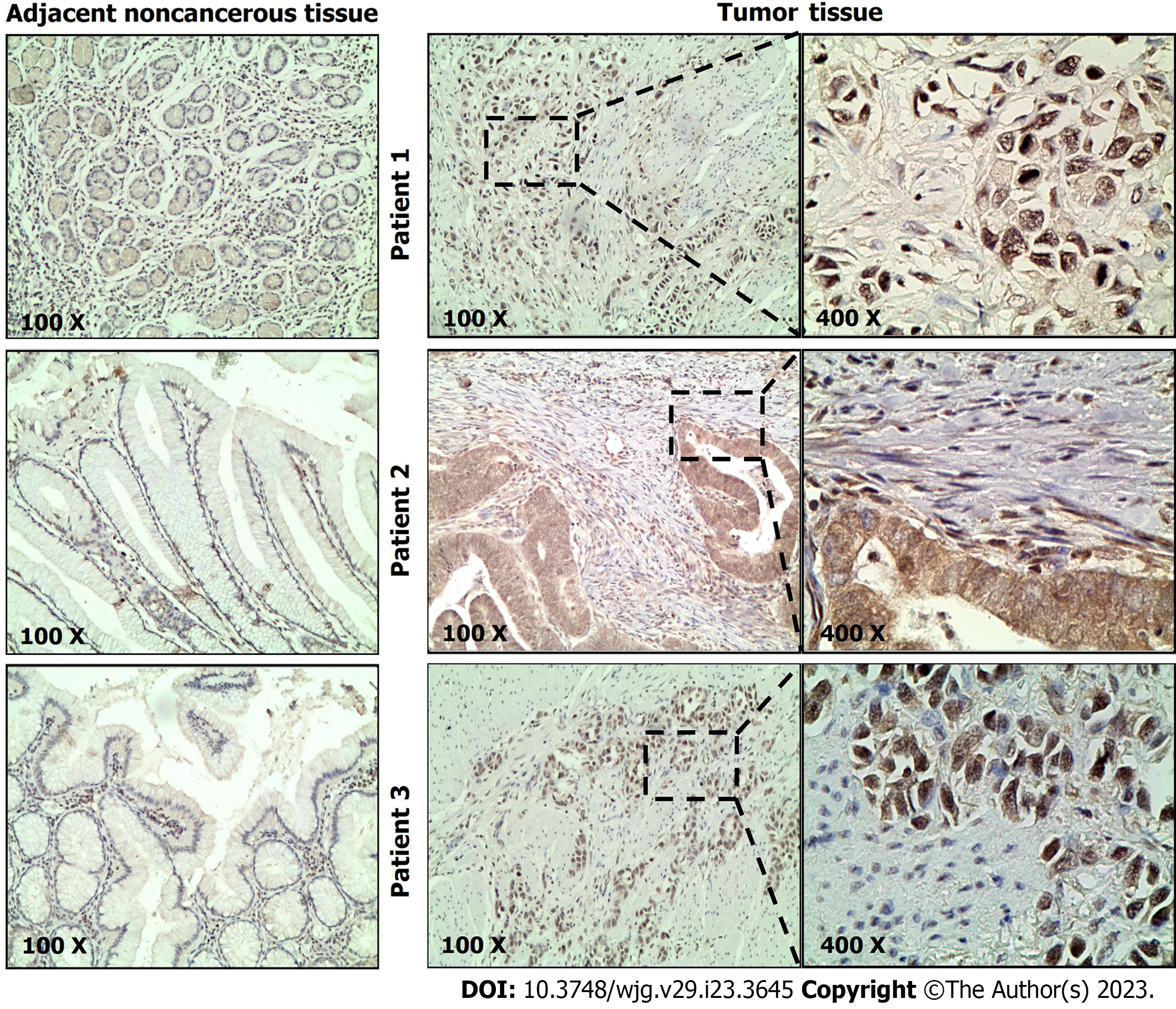
The complete immunohistochemical sections of 101 cases were observed under the microscope. NPAS2 was mainly expressed in the cytoplasm and nucleus, and the positive cells were yellow and brown granular. Two professional pathologists scored the section staining and did not know the clinicopathological factors and clinical outcome of the patient. The positive results were judged by the semi-quantitative integration method. Five high-power visual fields (400 ×) were selected for each chip on each slide under the microscope, and cells' positive rate and staining intensity were observed. (1) The score of cell positive rate: 0 indicates that the proportion of positive cells is less than 5%. The score of 5%-25% is 1, 26%-50% is 2, 51%-75% is 3, and more than 75% is 4; and (2) Staining intensity score: 0 = no coloring, 1 = light yellow, 2 = brown, 3 = brown. The immunostaining score was obtained by multiplying the positive rate of cells with the result of staining intensity. 0 was negative, 1 to 4 was weakly positive, 5 to 8 was moderately positive, and 9 to 12 was strongly positive. In this study, negative and weak positive was defined as low expression of NPAS2, while medium positive and strong positive was defined as high expression.
All data were analyzed using R software (Version 4.1.1, http://www.r-project.org) and SPSS (Version 26). Classification variables are represented by percentage (%), chi-square test, and continuity correction. Pearson's chi-squared test was used to evaluate the correlation between NPAS2 expression and clinicopathological factors. Survival analysis was performed by Kaplan-Meier curve and log-rank test. Univariate and multivariate Cox analysis determined the independent prognostic factors of OS and established the Cox proportional hazards model, and the hazard ratios (HRs) and 95% confidence intervals (CIs) were subsequently calculated. "Survival" and "surminer" R package were used for survival and Cox analysis. The "rms" package drew a nomogram model based on independent risk factors to predict OS. Furthermore, the receiver operating characteristic (ROC) curve, aera under the curve (AUC), calibration curve, and C-index were used to evaluate its prediction performance. In this study, P < 0.05 was considered to be statistically significant.
To explore the prognostic value of NPAS2 in GC, we performed immunohistochemical staining on 101 GC tissues collected. Strong or moderate positive immunostaining of NPAS2 protein was observed in the nucleus and cytoplasm of tumor cells in GC tissues. In contrast, weak positive or negative immunostaining was mainly detected in adjacent tissues. Representative positive staining results of NPAS2 showed that the positive rate of NPAS2 expression in GC tissues was 65.35% (66/101) (Figure 1), while the positive rate in corresponding adjacent tissues was 30.69% (31/101).
Of the 101 patients with GC, 65 (64.36%) died during follow-up. Table 1 summarizes the correlation between clinicopathological characteristics and NPAS2, showing that high expression of NPAS2 is associated with clinical progression in GC patients. The high expression of NPAS2 was significantly correlated with tumor-node-metastasis (TNM) stage (P = 0.001), pN stage (P = 0.009), metastasis (P = 0.032), venous invasion (P = 0.012), lymphatic invasion (P = 0.001) and lymph node positivity (P = 0.001).
| Characteristic | Low expression of NPAS2 | High expression of NPAS2 | P value |
| Number | 35 | 66 | |
| Age, n (%) | 0.599 | ||
| < 60 | 21 (60.0) | 36 (54.5) | |
| ≥ 60 | 14 (40.0) | 30 (45.5) | |
| Sex, n (%) | 0.626 | ||
| Female | 7 (20.0) | 16 (24.2) | |
| Male | 28 (80.0) | 51 (75.8) | |
| TNM stage, n (%) | 0.001 | ||
| I-II | 25 (71.4) | 11 (16.7) | |
| III-IV | 10 (28.6) | 55 (83.3) | |
| pT stage, n (%) | 0.351 | ||
| T1-T2 | 6 (17.1) | 29 (82.9) | |
| T3-T4 | 7 (10.6) | 59 (89.4) | |
| pN stage, n (%) | 0.009 | ||
| N0 | 16 (45.7) | 12 (18.2) | |
| N1-N3 | 19 (54.3) | 54 (81.8) | |
| Metastasis, n (%) | 0.032 | ||
| M0 | 35 (100.0) | 58 (87.9) | |
| M1 | 0 (0.0) | 8 (12.1) | |
| Tumor size, n (%) | 0.626 | ||
| < 5 | 15 (42.9) | 25 (37.9) | |
| ≥ 5 | 20 (57.1) | 41 (62.1) | |
| Tumor location, n (%) | 0.611 | ||
| Antrum | 14 (40.0) | 33 (50.0) | |
| Body | 9 (25.7) | 13 (19.7) | |
| Cardia | 12 (34.3) | 20 (30.3) | |
| Venous invasion, n (%) | 0.012 | ||
| Negative | 9 (25.7) | 5 (7.6) | |
| Positive | 26 (74.3) | 61 (92.4) | |
| Neural invasion, n (%) | 0.736 | ||
| Negative | 15 (42.9) | 26 (39.4) | |
| Positive | 20 (57.1) | 40 (60.6) | |
| Lymphatic invasion, n (%) | 0.001 | ||
| Negative | 22 (62.9) | 12 (18.2) | |
| Positive | 13 (37.1) | 54 (81.8) | |
| Positive lymph node, n (%) | 0.001 | ||
| Negative | 22 (62.9) | 14 (21.2) | |
| Positive | 13 (37.1) | 52 (78.8) | |
| Differentiated degree, n (%) | 0.16 | ||
| Poorly | 27 (77.1) | 58 (87.9) | |
| Moderately and highly | 8 (22.9) | 8 (12.1) | |
| Lauren’s Classification, n (%) | 0.569 | ||
| Intestinal | 23 (65.7) | 47 (71.2) | |
| Diffuse | 12 (34.3) | 19 (28.8) |
The prognostic value of NPAS2 expression in GC was further investigated by Kaplan-Meier analysis and log-rank test. The results demonstrated that GC patients with high NPAS2 expression were significantly associated with shorter OS (P < 0.001) (Figure 2). Univariate Cox regression analysis showed that the TNM stage of GC patients (HR = 7.07, 95%CI: 3.47-14.41, P < 0.001), pN stage (HR = 1.72, 95%CI: 1.38-2.15, P < 0.001), metastasis (HR = 4.85, 95%CI: 2.24-10.46, P < 0.001), venous invasion (HR = 5.01, 95%CI: 1.57-15.98, P = 0.007), neural invasion (HR = 1.73, 95%CI: 1.03-2.9, P = 0.037), lymphatic invasion (HR = 3.53, 95%CI: 1.88-6.64, P < 0.001), lymph node positivity (HR = 3.35, 95%CI: 1.82-6.18, P < 0.001), and high expression of NPAS2 (HR = 5.13, 95%CI: 2.6-10.13, P < 0.001) were significantly correlated with OS. Multivariate Cox regression analysis showed that the TNM stage (HR = 3.61, 95%CI: 1.37-9.49, P = 0.009), metastasis (HR = 3.07, 95%CI: 1.33-7.1, P = 0.009), and high expression of NPAS2 (HR = 2.43, 95%CI: 1.15-5.13, P = 0.020) were independent risk factors for predicting OS (Table 2). Therefore, these collective data suggest that the expression level of NPAS2 has a good predictive value in the prognosis of GC patients.
| Characteristics | Univariate analysis | Multivariate analysis | ||
| Hazard ratio (95%CI) | P value | Hazard ratio (95%CI) | P value | |
| Age | ||||
| < 60 | 1 | |||
| ≥ 60 | 1.33 (0.82-2.17) | 0.25 | ||
| Gender | ||||
| Female | 1 | |||
| Male | 1.13 (0.63-2.05) | 0.679 | ||
| TNM stage | ||||
| I-II | 1 | |||
| III-IV | 7.07 (3.47-14.41) | < 0.001 | 3.61 (1.37-9.49) | 0.009 |
| pT stage | ||||
| T1-T2 | 1 | |||
| T3-T4 | 1.87 (0.81-4.35) | 0.143 | ||
| pN stage | ||||
| N0 | 1 | |||
| N1-N3 | 1.72 (1.38-2.15) | < 0.001 | 1.66 (0.68-4.07) | 0.265 |
| Metastasis | ||||
| M0 | 1 | |||
| M1 | 4.85 (2.24-10.46) | < 0.001 | 3.07 (1.33-7.1) | 0.009 |
| Tumor size | ||||
| < 5 | 1 | |||
| ≥ 5 | 1.63 (0.97-2.72) | 0.063 | ||
| Tumor location | ||||
| Antrum | 1 | |||
| Body | 0.92 (0.53-1.60) | 0.76 | ||
| Cardia | 1.13 (0.58-2.23) | 0.72 | ||
| Venous invasion | ||||
| Negative | 1 | |||
| Positive | 5.01 (1.57-15.98) | 0.007 | 1.69 (0.49-5.84) | 0.409 |
| Neural invasion | ||||
| Negative | 1 | |||
| Positive | 1.73 (1.03-2.9) | 0.037 | 1.71 (0.98-3) | 0.06 |
| Lymphatic invasion | ||||
| Negative | 1 | |||
| Positive | 3.53 (1.88-6.64) | < 0.001 | 0.39 (0.09-1.77) | 0.225 |
| Positive lymph node | ||||
| Negative | ||||
| Positive | 3.35 (1.82-6.18) | < 0.001 | 2.23 (0.58-8.49) | 0.241 |
| Differentiated degree | ||||
| Moderately and highly | 1 | |||
| Poorly | 0.62 (0.29-1.30) | 0.203 | ||
| Lauren’s Classification | ||||
| Diffuse type | 1 | |||
| Intestinal type | 0.97 (0.57-1.64) | 0.908 | ||
| 0.97 (0.57-1.64) | ||||
| NPAS2 expression | ||||
| Low | 1 | |||
| High | 5.13 (2.6-10.13) | < 0.001 | 2.43 (1.15-5.13) | 0.020 |
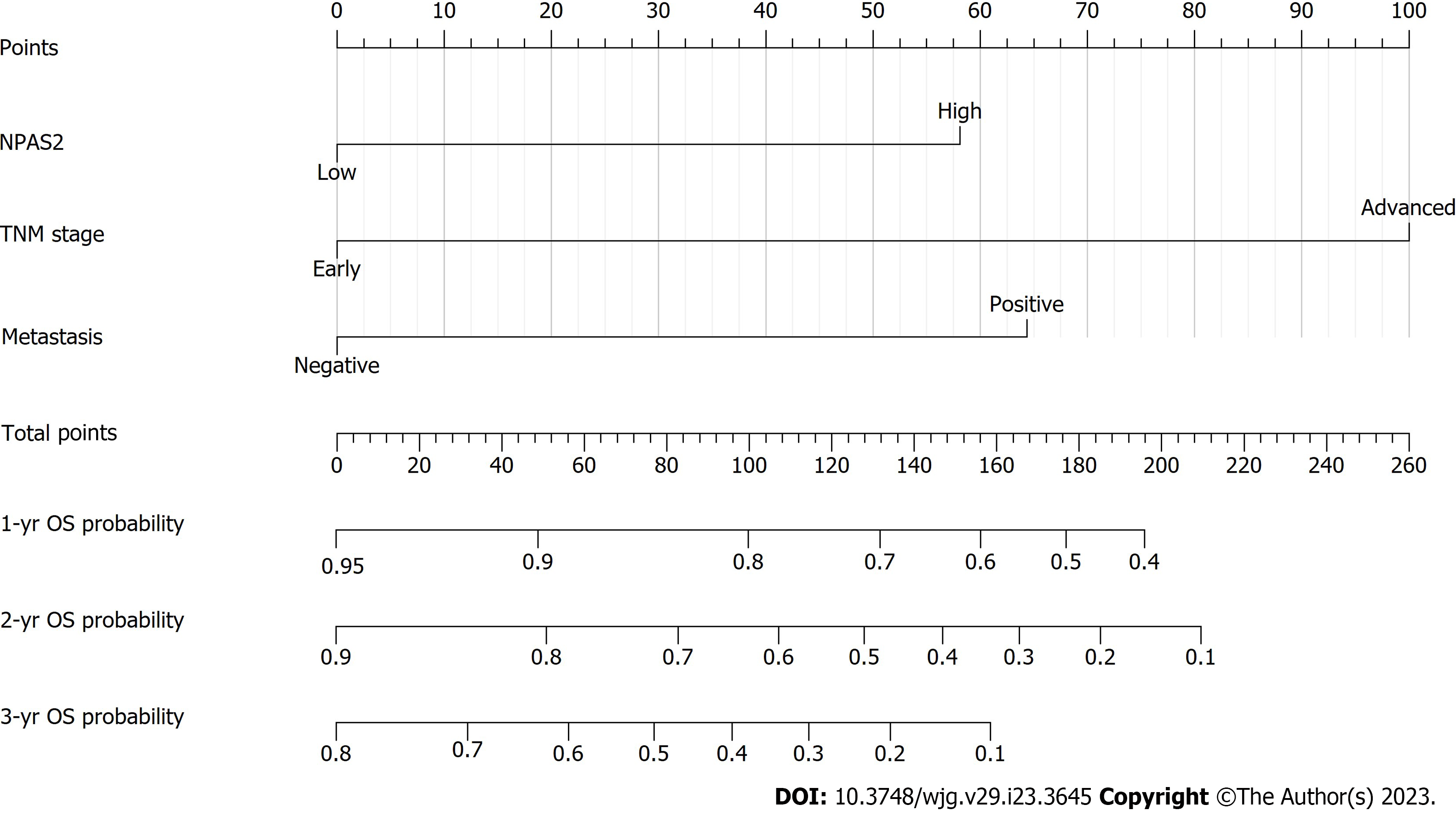
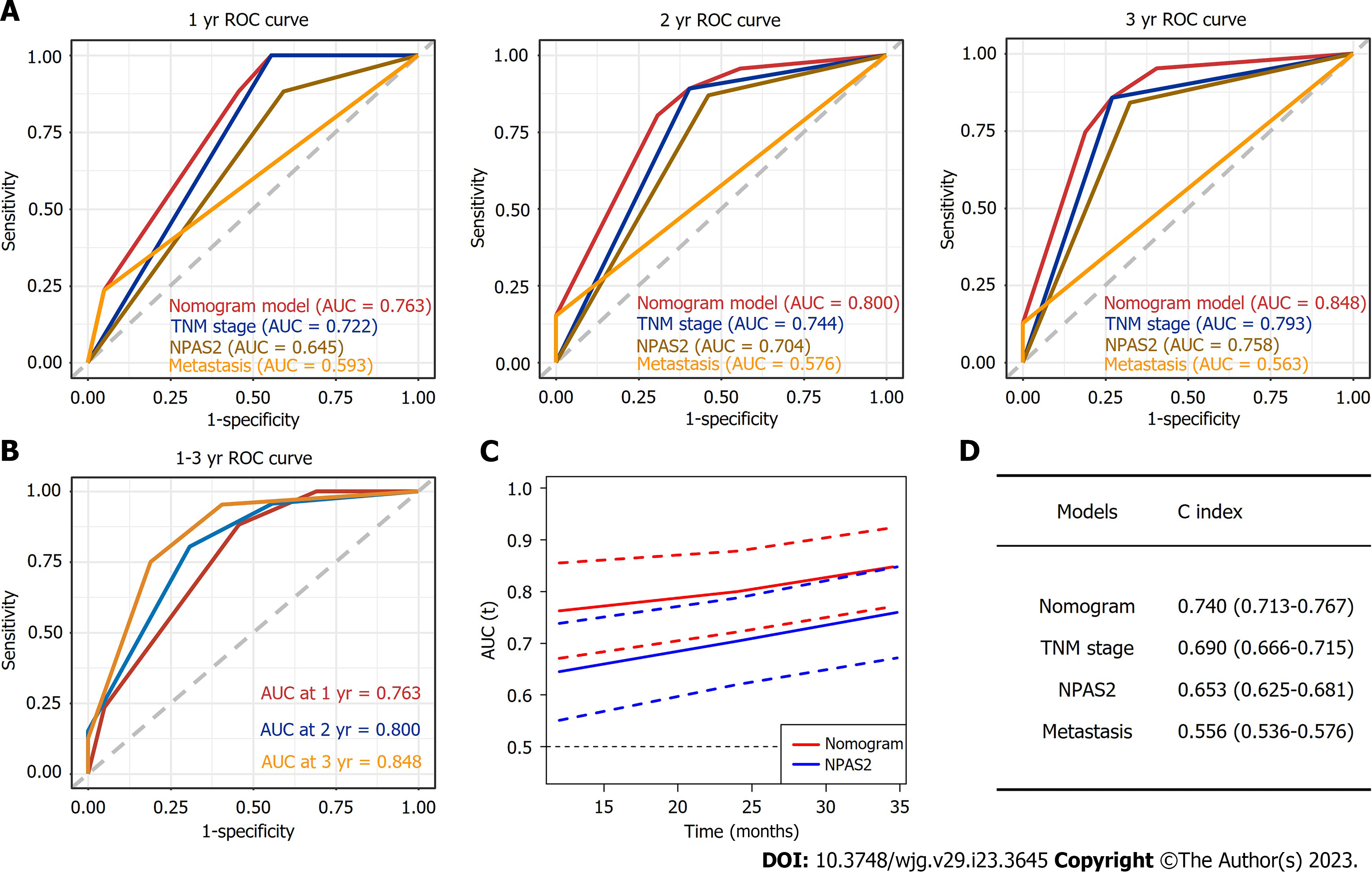
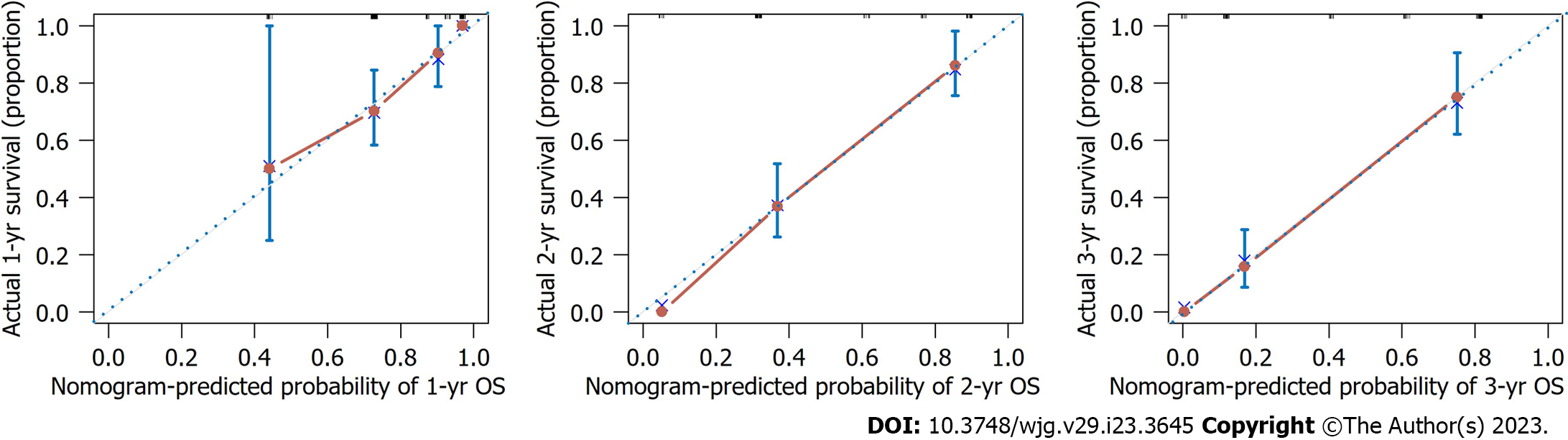
To obtain the best prediction model, TNM stage and metastasis were determined as independent prognostic factors for GC according to multivariate Cox regression analysis and combined with NAPS2 high expression. Together, these were used as a combined prediction model for predicting OS at 1, 2, and 3 years after GC resection was constructed. The prediction model was visualized by nomogram (Figure 3). The 1-year, 2-year, and 3-year ROC curves of the Nomogram prediction model and other independent factors (Figure 4A) and the Time-dependent ROC curve were used to show the model's prediction performance (Figure 4C). The AUC of the nomogram prediction model for 1, 2, and 3 years is 0.763, 0.800, and 0.848, respectively (Figure 4B). Additionally, the C-index of each model shows the prediction performance of different models (Figure 4D), and the nomogram has the best prediction performance with a C-index of 0.740 (95%CI: 0.713-0.767). Finally, the prediction accuracy of the nomogram was verified by calibration curves, and the results showed that the combined nomogram model's 1-year, 2-year, and 3-year survival rates based on NPAS2 were in good agreement with the actual survival probability (Figure 5).
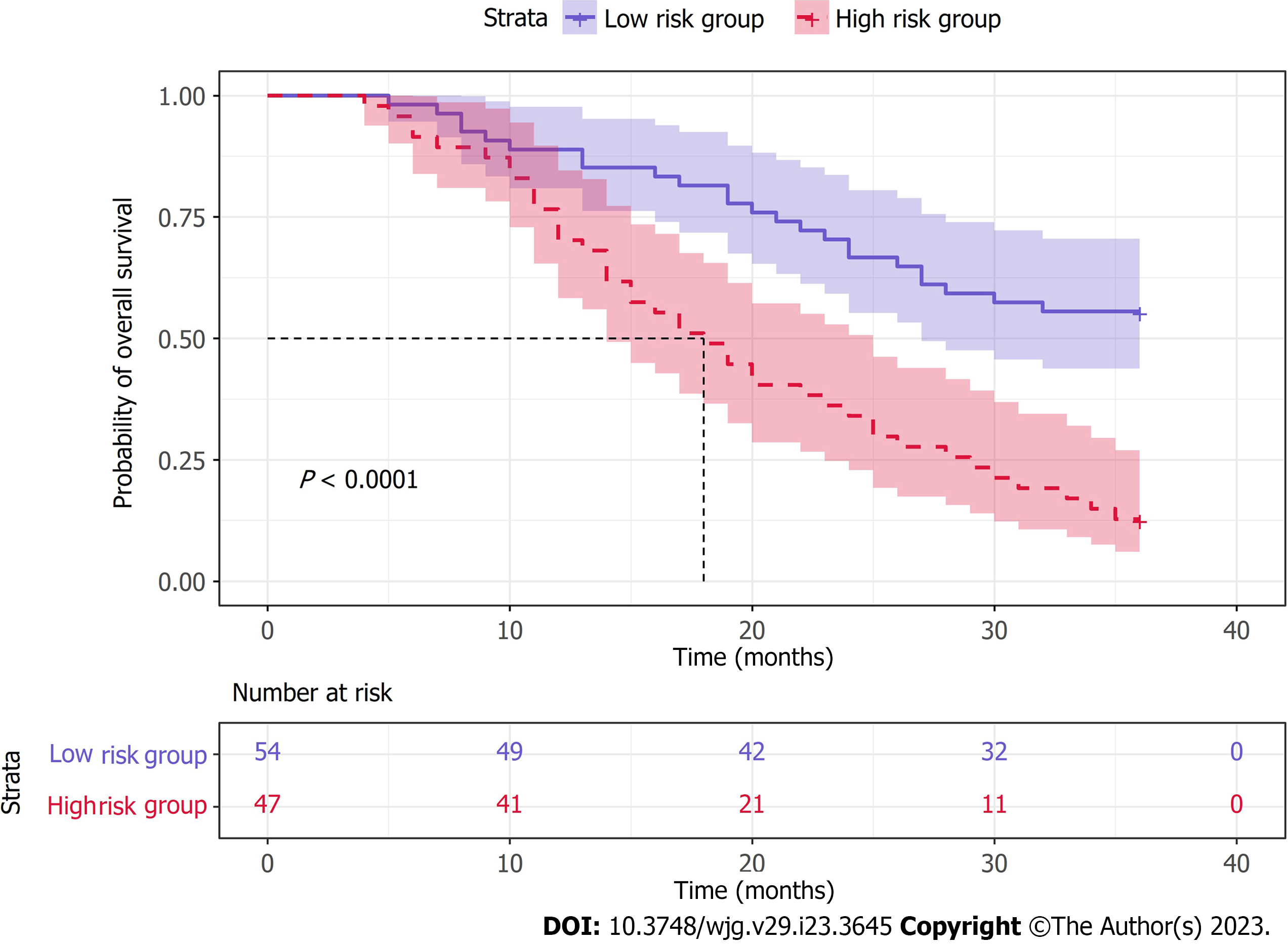
To evaluate the positive effect of the nomogram prediction model in different subgroups, we divided all patients into a low-risk subgroup (score < 158) and a high-risk subgroup (score ≥ 158) according to the median nomogram score. Our results showed that patients in the high-risk group had significantly lower OS than those in the low-risk group (P < 0.0001) (Figure 6).
Epidemiological studies have shown that the disruption of normal circadian rhythm may increase the risk of a variety of cancers[18], such as breast cancer[19], lung cancer[20], bladder cancer[21], prostate cancer[22], and liver cancer[23]. Notably, women on night shifts have a higher risk of breast cancer[19]. Likewise, breast cancer patients with irregular circadian rhythms have a worse prognosis because of changes in the expression of NPAS2 in breast cancer tissues[19]. Additionally, the disruption of biorhythm can promote the occurrence of lung cancer, which is a promising diagnostic marker for patients with lung adenocarcinoma and an independent prognostic marker for non-small cell lung cancer[24]. Notably, NPAS2 regulates the expression of several genes as markers of bladder cancer and further reduces the migration ability of bladder cancer cells[21]. The development of prostate cancer depends on androgen levels, and NPAS2 may interact with dihydrotestosterone, thus affecting the androgen receptor-dependent signaling pathway leading to prostate cancer[25]. Importantly, NPAS2 plays a role in reprogramming glucose metabolism and the progression of liver cancer, suggesting that NPAS2 may be an important therapeutic target to normalize the abnormal glucose metabolism that leads to the advancement of HCC[23]. Furthermore, NPAS2 plays different roles in different tumors, which can be understood that the expression level and function of NPAS2 are tumor-specific. The exact molecular mechanism of the change of NPAS2 expression in GC remains unclear. Moreso, there are no reports on the correlation between NPAS2 expression and clinicopathological data of GC patients.
The tissue microarray results in this study showed that NPAS2 was expressed in GC and precancerous tissues and was primarily located in the nucleus and cytoplasm. The results showed that the expression was high in 65.35% of GC tissues and low in 34.65% of GC tissues. To the best of our knowledge, our experimental data demonstrate the role of NPAS2 in the diagnosis and prognosis of GC for the first time. The survival analysis showed that the high expression of NPAS2 in GC was significantly correlated with the shorter OS (P < 0.001). Univariate and multivariate Cox analysis also showed that the high expression of NPAS2 was an independent risk factor for GC. Furthermore, the ROC curve shows that NPAS2 is valuable in predicting the prognosis of GC. Therefore, the expression level of NPAS2 in GC tissue directly affects the OS of GC patients and may be used as a tumor-promoting factor to affect the occurrence and development of GC, which is a new prognostic biomarker. Various animal and in vitro studies have found that NPAS2 can regulate the expression of oncogenes (such as c-myc), tumor suppressor genes (such as P53 and P21), and transcription factors[26], thereby regulating cell cycle, apoptosis, DNA damage and repair systems, invasion, metastasis, carcinogen metabolism, and detoxification[27]. Notably, DNA repair maintains genetic stability and protects DNA from internal and external stimuli. Once human biorhythm genes are mutated (due to spontaneous mutations, prolonged late nights, or other environmental factors), it may lead to irreversible disorders and loss of DNA repair capacity[27].
The American Joint Commission on the Cancer TNM staging system is widely used to monitor the prognosis of patients with GC[28]. Our study analyzed the clinicopathological factors affecting OS in patients with GC, and our results showed that TNM stage and metastasis were independent risk factors for poor survival outcomes. Although the TNM stage and metastasis of GC patients have been widely used in the formulation of the treatment plan and the evaluation of prognosis, with the increasing number of GC patients, more studies and related factors are needed to evaluate the survival and prognosis of these patients more accurately and individually. Our study combined three independent risk factors of NPAS2 and TNM stage and metastasis to establish a Cox regression model for predicting 3-year OS in patients with GC. Compared with the TNM stage alone, it has more predictive power and can be used as a more convenient and accurate tool to predict the prognosis of patients with GC following gastrectomy. Therefore, the accurate prognosis prediction by nomogram score is of great significance for postoperative management and diagnosis and treatment of patients.
However, we can not solve the causal relationship in this study. Therefore, further basic and clinical research is needed to explore these aspects. Our advantage is that this is the first study to show that the increased expression of NPAS2 may be related to the malignant biological behavior and poor prognosis of patients with GC. Of course, our research has the following noted limitations. First, the sample size of our study is small, and there may be selection bias. Secondly, this study is a single-center retrospective study, so it is necessary to conduct a multicenter prospective study to verify it further. Finally, we used immunohistochemical staining to detect the expression of NPAS2 in the center and periphery of each GC tissue. However, considering the heterogeneity, the expression level of NPAS2 in the sampling site may not represent whole tumor regions.
Based on these investigations, further exploration of the molecular mechanism and influence of NPAS2 in the occurrence and development of GC will help to promote its clinical application. The established nomogram prediction model provides a potential objective clinical prediction tool to assist clinicians in predicting the prognosis of GC patients and making postoperative management and clinical decisions.
In conclusion, our study found that high expression of NAPS2 is associated with poor prognosis of OS in patients with GC and is an independent risk factor for patients after radical resection of GC. We further constructed a nomogram model by combining NAPS2 and other independent risk factors, thus improving the accuracy of predicting the prognosis of GC.
The circadian clock gene NPAS2 expression is associated with multiple tumor prognosis, its role in gastric cancer (GC) is unknown.
NPAS2 can improve the accuracy of GC prognosis prediction and assist clinicians in postoperative patient management and decision-making.
This study aimed to explore the relationship between the circadian clock gene NPAS2 and the survival prognosis of GC patients and clarify its role in evaluating GC prognosis.
Immunohistochemical staining was used to detect the expression of NPAS2 protein in GC and adjacent tissues. Univariate and multivariate Cox regression analysis was used to determine the independent prognostic factors of GC, and a nomogram prediction model was established. The receiver operating characteristic curve and area under the curve, the calibration curve, and C-index were used to evaluate the predictive effectiveness of the model.
The NPAS2 expression were independent prognostic factors of overall survival in GC patients for 3 years.
NPAS2 is highly expressed in GC and is closely related to worse overall survival in patients. Therefore, the evaluation of NPAS2 expression may be a potential marker for GC prognosis evaluation.
Based on NPAS2 expression, clinicians can predict patient prognosis and guide clinical decision making and follow up.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Benna C, Italy; Papadia C, United Kingdom; Qin SS, China S-Editor: Yan JP L-Editor: A P-Editor: Chen YX
| 1. | Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, Bray F. Cancer statistics for the year 2020: An overview. Int J Cancer. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2411] [Cited by in RCA: 2944] [Article Influence: 736.0] [Reference Citation Analysis (7)] |
| 2. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64442] [Article Influence: 16110.5] [Reference Citation Analysis (176)] |
| 3. | Lei ZN, Teng QX, Tian Q, Chen W, Xie Y, Wu K, Zeng Q, Zeng L, Pan Y, Chen ZS, He Y. Signaling pathways and therapeutic interventions in gastric cancer. Signal Transduct Target Ther. 2022;7:358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 160] [Article Influence: 53.3] [Reference Citation Analysis (1)] |
| 4. | Ugai T, Sasamoto N, Lee HY, Ando M, Song M, Tamimi RM, Kawachi I, Campbell PT, Giovannucci EL, Weiderpass E, Rebbeck TR, Ogino S. Is early-onset cancer an emerging global epidemic? Current evidence and future implications. Nat Rev Clin Oncol. 2022;19:656-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 280] [Article Influence: 93.3] [Reference Citation Analysis (0)] |
| 5. | Gwee YX, Chia DKA, So J, Ceelen W, Yong WP, Tan P, Ong CJ, Sundar R. Integration of Genomic Biology Into Therapeutic Strategies of Gastric Cancer Peritoneal Metastasis. J Clin Oncol. 2022;40:2830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 6. | Shostak A. Circadian Clock, Cell Division, and Cancer: From Molecules to Organism. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 7. | Milev NB, Reddy AB. Circadian redox oscillations and metabolism. Trends Endocrinol Metab. 2015;26:430-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Franzoni A, Markova-Car E, Dević-Pavlić S, Jurišić D, Puppin C, Mio C, De Luca M, Petruz G, Damante G, Pavelić SK. A polymorphic GGC repeat in the NPAS2 gene and its association with melanoma. Exp Biol Med (Maywood). 2017;242:1553-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Liu H, Gao Y, Hu S, Fan Z, Wang X, Li S. Bioinformatics Analysis of Differentially Expressed Rhythm Genes in Liver Hepatocellular Carcinoma. Front Genet. 2021;12:680528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Sancar A, Van Gelder RN. Clocks, cancer, and chronochemotherapy. Science. 2021;371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 170] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 11. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13205] [Article Influence: 1467.2] [Reference Citation Analysis (3)] |
| 12. | Renthlei Z, Gurumayum T, Borah BK, Trivedi AK. Daily expression of clock genes in central and peripheral tissues of tree sparrow (Passer montanus). Chronobiol Int. 2019;36:110-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Roenneberg T, Merrow M. The Circadian Clock and Human Health. Curr Biol. 2016;26:R432-R443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 610] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 14. | Zhang J, Lv H, Ji M, Wang Z, Wu W. Low circadian clock genes expression in cancers: A meta-analysis of its association with clinicopathological features and prognosis. PLoS One. 2020;15:e0233508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Qiu MJ, Liu LP, Jin S, Fang XF, He XX, Xiong ZF, Yang SL. Research on circadian clock genes in common abdominal malignant tumors. Chronobiol Int. 2019;36:906-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Yuan P, Li J, Zhou F, Huang Q, Zhang J, Guo X, Lyu Z, Zhang H, Xing J. NPAS2 promotes cell survival of hepatocellular carcinoma by transactivating CDC25A. Cell Death Dis. 2017;8:e2704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 17. | Song B, Chen Y, Liu Y, Wan C, Zhang L, Zhang W. NPAS2 regulates proliferation of acute myeloid leukemia cells via CDC25A-mediated cell cycle progression and apoptosis. J Cell Biochem. 2019;120:8731-8741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Kiessling S, Beaulieu-Laroche L, Blum ID, Landgraf D, Welsh DK, Storch KF, Labrecque N, Cermakian N. Enhancing circadian clock function in cancer cells inhibits tumor growth. BMC Biol. 2017;15:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 134] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 19. | Nagata C, Tamura T, Wada K, Konishi K, Goto Y, Nagao Y, Ishihara K, Yamamoto S. Sleep duration, nightshift work, and the timing of meals and urinary levels of 8-isoprostane and 6-sulfatoxymelatonin in Japanese women. Chronobiol Int. 2017;34:1187-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Gao LW, Wang GL. Comprehensive bioinformatics analysis identifies several potential diagnostic markers and potential roles of cyclin family members in lung adenocarcinoma. Onco Targets Ther. 2018;11:7407-7415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Iyyanki T, Zhang B, Wang Q, Hou Y, Jin Q, Xu J, Yang H, Liu T, Wang X, Song F, Luan Y, Yamashita H, Chien R, Lyu H, Zhang L, Wang L, Warrick J, Raman JD, Meeks JJ, DeGraff DJ, Yue F. Subtype-associated epigenomic landscape and 3D genome structure in bladder cancer. Genome Biol. 2021;22:105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 22. | Yu CC, Chen LC, Chiou CY, Chang YJ, Lin VC, Huang CY, Lin IL, Chang TY, Lu TL, Lee CH, Huang SP, Bao BY. Genetic variants in the circadian rhythm pathway as indicators of prostate cancer progression. Cancer Cell Int. 2019;19:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Yuan P, Yang T, Mu J, Zhao J, Yang Y, Yan Z, Hou Y, Chen C, Xing J, Zhang H, Li J. Circadian clock gene NPAS2 promotes reprogramming of glucose metabolism in hepatocellular carcinoma cells. Cancer Lett. 2020;469:498-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 24. | He Y, Wang G, Wang Q, Zhao Z, Gan L, Yang S, Wang Y, Guo S, An J, Zhang J, Zhang Z, Zhou F. Genetic variants in NPAS2 gene and clinical outcomes of resectable non-small-cell lung cancer. Future Oncol. 2021;17:795-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Mukhopadhyay NK, Ferdinand AS, Mukhopadhyay L, Cinar B, Lutchman M, Richie JP, Freeman MR, Liu BC. Unraveling androgen receptor interactomes by an array-based method: discovery of proto-oncoprotein c-Rel as a negative regulator of androgen receptor. Exp Cell Res. 2006;312:3782-3795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Yang SL, Ren QG, Wen L, Hu JL, Wang HY. Research progress on circadian clock genes in common abdominal malignant tumors. Oncol Lett. 2017;14:5091-5098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | LeVan TD, Xiao P, Kumar G, Kupzyk K, Qiu F, Klinkebiel D, Eudy J, Cowan K, Berger AM. Genetic Variants in Circadian Rhythm Genes and Self-Reported Sleep Quality in Women with Breast Cancer. J Circadian Rhythms. 2019;17:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Lu J, Zheng ZF, Xie JW, Wang JB, Lin JX, Chen QY, Cao LL, Lin M, Tu RH, Huang CM, Zheng CH, Li P. Is the 8th Edition of the AJCC TNM Staging System Sufficiently Reasonable for All Patients with Noncardia Gastric Cancer? A 12,549-Patient International Database Study. Ann Surg Oncol. 2018;25:2002-2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |