Published online May 28, 2023. doi: 10.3748/wjg.v29.i20.3216
Peer-review started: March 10, 2023
First decision: April 14, 2023
Revised: April 21, 2023
Accepted: May 4, 2023
Article in press: May 4, 2023
Published online: May 28, 2023
Processing time: 76 Days and 22.2 Hours
Inflammatory myofibroblastic tumor (IMT) is a relatively rare tumor. The global incidence of IMT is less than 1%. There is no specific clinical manifestation. It usually occurs in the lungs, but the pancreas is not the predilection site.
We present a case of a male patient, 51 years old, who was diagnosed with a pancreatic neck small mass on ultrasound one year ago during a physical examination. As he had no clinical symptoms and the mass was relatively small, he did not undergo treatment. However, the mass was found to be larger on review, and he was referred to our hospital. Since the primal clinical diagnosis was pancreatic neuroendocrine tumor, the patient underwent surgical treatment. However, the case was confirmed as pancreatic IMT by postoperative pathology.
Pancreatic IMT is relatively rare and easily misdiagnosed. We can better under-stand and correctly diagnose this disease by this case report.
Core Tip: Inflammatory myofibroblastic tumor (IMT) is a rare mesenchymal tumor composed of spindle-shaped myofibroblasts accompanied by a mixed inflammatory infiltrate, and is particularly rare in the pancreas. The diagnosis of pancreatic IMT is made on the basis of histopathology and immunohistochemistry. Different lesions exhibit diverse biological characteristics, and there are several surgical treatment protocols. This case emphasizes the importance of correct preoperative diagnosis of IMT and reminds us to broaden our thinking in relation to the diagnosis of pancreatic lesions.
- Citation: Liu JB, Gu QB, Liu P. Inflammatory myofibroblastic tumor of the pancreatic neck misdiagnosed as neuroendocrine tumor: A case report. World J Gastroenterol 2023; 29(20): 3216-3221
- URL: https://www.wjgnet.com/1007-9327/full/v29/i20/3216.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i20.3216
Inflammatory myofibroblastic tumor (IMT) is a rare mesenchymal tumor. This concept of IMT was first put forward by Pettinato et al[1] in 1990. According to the current World Health Organisation (WHO) guidelines, IMTs are typically low-grade neoplasms with occasional malignant potential[2]. An IMT can occur anywhere at any age. This report describes a case of pancreatic IMT.
A 51-year-old male patient was found to have a pancreatic mass more than one year ago.
The patient found a small pancreatic tumor in a physical examination one year ago. However, two months ago, the follow-up of the physical examination center found that the tumor became bigger, then he was referred to our hospital. There were no clinical symptoms throughout the course of the disease.
The patient had a history of hypertension, hyperglycemia, hyperlipidemia, thyroid nodule, prediabetes, and urticaria.
The patient had no family history of malignant tumors, psychological, or genetic disorders.
The physical examination did not reveal any obvious abnormalities.
Uric acid and triglyceride levels were elevated. Other blood parameters and tumor markers (carcinoembryonic antigen and cancer antigen 19-9) levels were within the normal range.
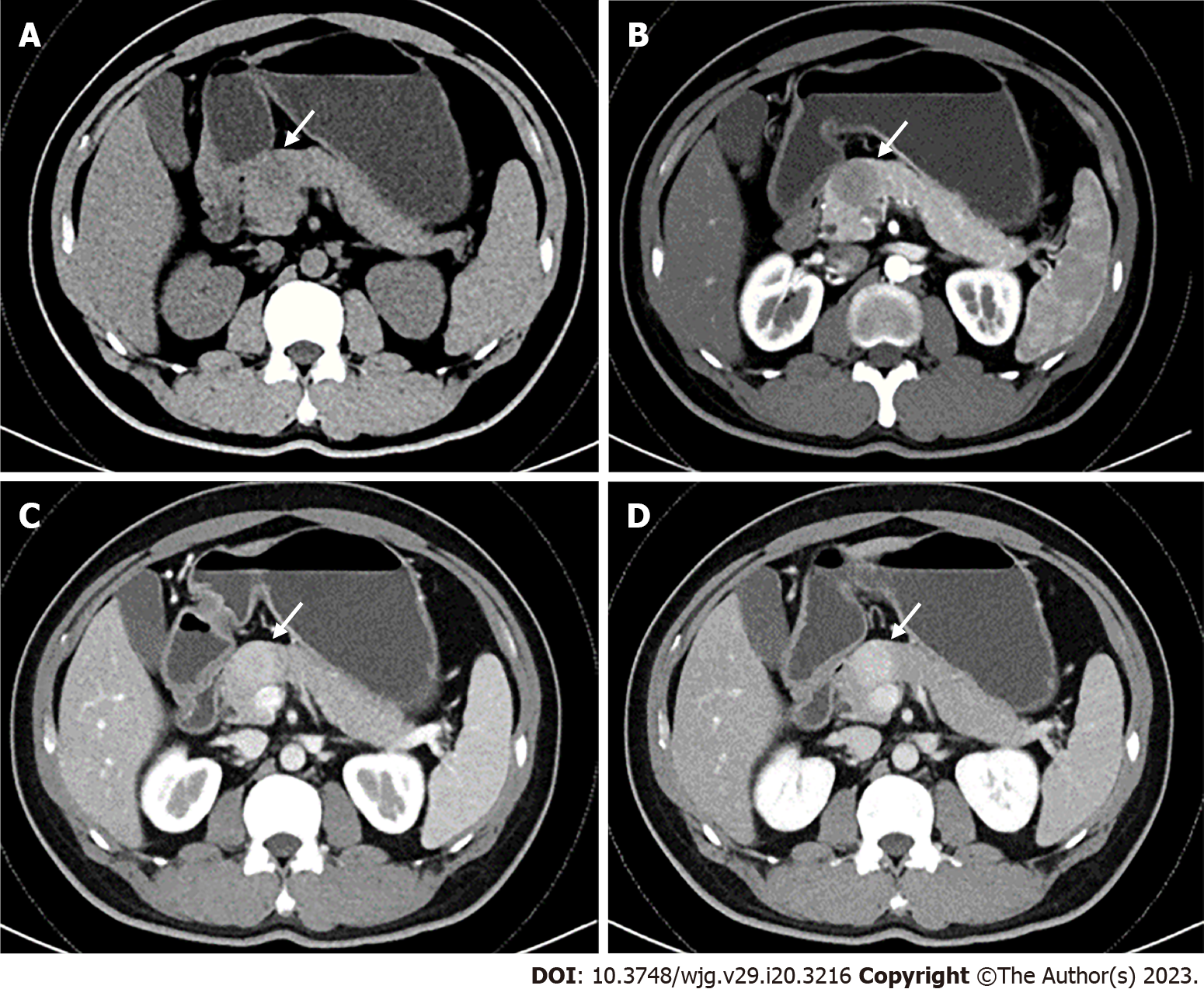
An abdominal contrast-enhanced computed tomography (CECT) scan showed a round, well-defined, low-density mass 3.5 cm in diameter in the neck of the pancreas. On the CECT scan, the mass showed lower attenuation than the normal pancreatic parenchyma in the pre-contrast phase and arterial phase, and heterogeneous hyperenhancement in the portal venous phase (Figure 1). A pancreatic neuroendocrine tumour was strongly suspected.
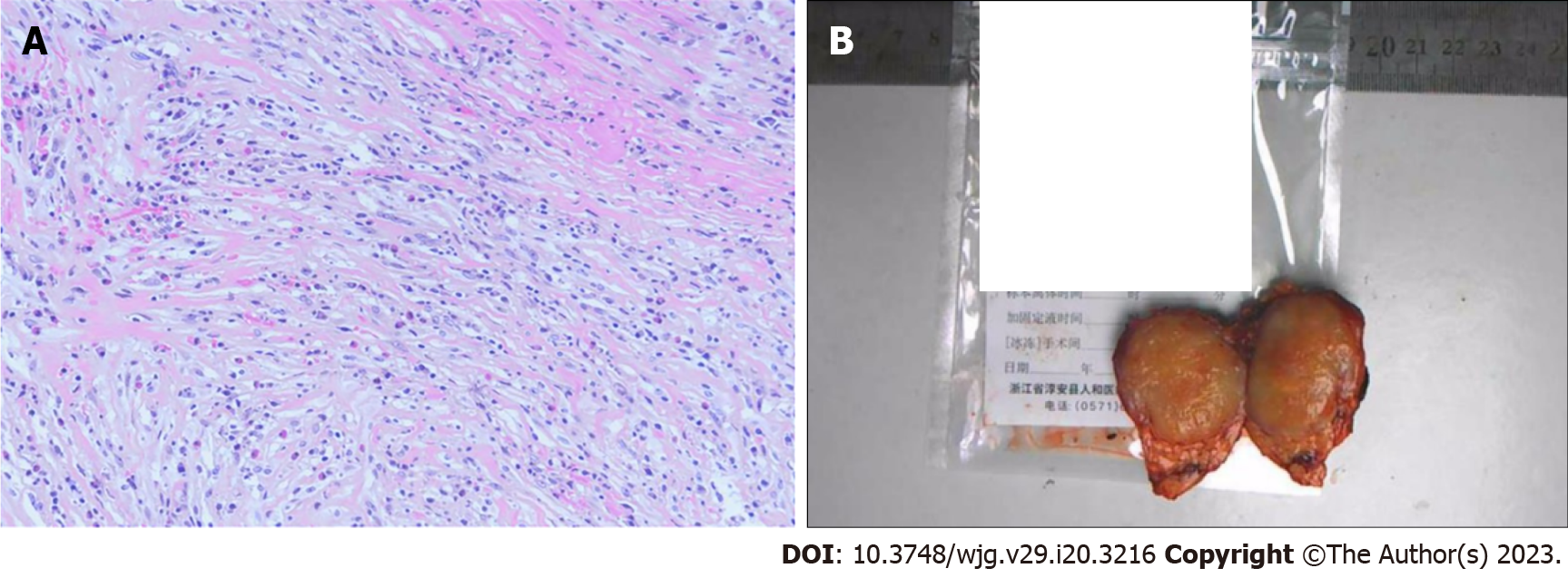
The patient was diagnosed with IMT of the pancreas by postoperative pathology (Figure 2).
After completion of preoperative investigations, a laparoscopic middle pancreatectomy was performed.
Postoperative pancreatic leakage occurred in the patient. However, he was discharged in good clinical condition after 40 d. No apparent events were observed at the 2-mo postoperative follow-up.
IMT is a rare mesenchymal tumor. Due to its rarity and the fact that the etiology is unknown, there are only a few cases reported. It has been revealed that IMT may have gene rearrangement with anaplastic lymphoma kinase (ALK)[3], and ALK positivity was also associated with a higher recurrence and less chance of distant metastasis[4]. A recent literature review by Chen et al[5] in 2021, included 30 patients with IMT occurring in the pancreas. The reported mean age of the patients was 40 years (range, 0-82 years) with an obvious male preponderance. The tumor was mostly located in the head of the pancreas (21/30 patients). In this series, abdominal pain was the most frequent symptom followed by jaundice. Only five cases of asymptomatic pancreatic IMT have been reported in the medical literature[5-9]. Our patient had no clinical symptoms and the pancreatic mass was found on physical examination. Among the previously reported cases of pancreatic IMT, many were initially misdiagnosed as pancreatic cancer, while the present case was misdiagnosed as pancreatic neuroendocrine tumor. Therefore, it is meaningful to collect more cases and information to obtain reliable diagnosis and treatment methods for IMT.
As the low incidence of pancreatic IMT, there is no specific clinical manifestations has been established. It commonly manifests as abdominal pain or jaundice, and can sometimes be asymptomatic. Although IMT is considered a low-grade tumor, one case of pulmonary metastasis has been reported[10].
At present, pancreatic IMT is mainly diagnosed by histopathology and immunohistochemistry[11]. Under the light microscope, the tumour tissue in this case consisted mainly of spindle-shaped myofibroblasts accompanied by a mixed inflammatory infiltrate. The histopathological findings of this case were consistent with previous reports. Myofibroblasts in IMT stain positive for alpha-smooth muscle antigen (SMA), vimentin, and fibronectin, and stain negative for desmin and caldesmon[12]. In this case, immunohistochemistry report showed positive for IgG4, SMA and actin, and negative for desmin, ALK, CD30, S-100, and Catenin B, similar to those in the literature.
To date, pancreatic IMT is easily misdiagnosed as pancreatic neuroendocrine tumour and pancreatic cancer due to the lack of specific imaging features, and its diagnosis is unclear in clinical practice. A circular low-density mass was observed in the pancreatic IMT reported here. After enhancement, the arterial mass is mildly enhanced, the venous phase is significantly enhanced, and the density is progressively higher than that of the surrounding normal pancreas.
However, in some cases, CECT did not show an enhancing mass and was suspected to be ductal adenocarcinoma, which was ultimately confirmed by pathology to be pancreatic IMT. Hence, the radiologic features of pancreatic IMT require further data.
Pancreatic IMT needs to be differentiated from pancreatic neuroendocrine tumor, pancreatic cancer, and solid-pseudopapillary neoplasm. Pancreatic neuroendocrine tumor is uncommon and has no gender predilection[13]. Additionally, the mass shows high enhancement in the arterial or portal phases due to the rich capillary network in the stroma. Pancreatic cancer is typically seen in patients over 60 years old, with a slight male predominance. It is a hypovascular mass with extensive fibrosis on histopathologic examination[14]. Typical ductal adenocarcinomas appear as poorly defined masses with extensive surrounding desmoplastic reaction and they enhance poorly compared to adjacent normal pancreatic tissue. The presence of abrupt pancreatic duct cutoff, upstream pancreatic duct dilatation, upstream pancreatic parenchymal atrophy, and decreased enhancement in the distal pancreatic parenchyma favors a diagnosis of malignancy[15]. Solid-pseudopapillary neoplasms of the pancreas are cystic-solid masses with a complete capsule. They are prone to bleeding, necrosis and calcification. The solid portion shows enhancement in the arterial phase and can continue to enhance in the delayed phase. However, the imaging features of pancreatic IMT from some pancreatic lesions overlap.
In the present case, a wrong diagnosis was made for several reasons. First, the patient had no clinical manifestations, and the clinical history and laboratory indices are unremarkable. Second, the preoperative imaging findings were difficult to distinguish from a pancreatic neuroendocrine tumour. The border of the lesion was distinct, with persistent hyperenhancement of the mass, and typical double duct sign and vascular involvement were not observed. Accordingly, preliminary diagnosis of imaging was limited to benign mass. In general, IMT of the pancreas is lack of characteristic in terms of clinic-radiological features. Therefore, for more accurate diagnosis and treatment of pancreatic IMT, there is a need to obtain more meaningful information.
Given the rarity of pancreatic IMT, this case can contribute to further understanding of the etiology, mechanism, imaging characteristics of this disease. Obtaining a better understanding of all aspects of this disease will help provide a more precise diagnosis.
We are very grateful to Dr. Liu Yu (Hunan Provincial People’s Hospital, First Affiliated Hospital of Hunan Normal University) for providing professional guidance to the pathological analysis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kamer E, Turkey; Kumar M, India; Yarmahmoodi F, Iran S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Pettinato G, Manivel JC, De Rosa N, Dehner LP. Inflammatory myofibroblastic tumor (plasma cell granuloma). Clinicopathologic study of 20 cases with immunohistochemical and ultrastructural observations. Am J Clin Pathol. 1990;94:538-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 260] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 2. | Choi JH, Ro JY. The 2020 WHO Classification of Tumors of Soft Tissue: Selected Changes and New Entities. Adv Anat Pathol. 2021;28:44-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 248] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 3. | Zhu L, Li J, Liu C, Ding W, Lin F, Guo C, Liu L. Pulmonary inflammatory myofibroblastic tumor vs IgG4-related inflammatory pseudotumor: differential diagnosis based on a case series. J Thorac Dis. 2017;9:598-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol. 2007;31:509-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 627] [Cited by in RCA: 621] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 5. | Chen ZT, Lin YX, Li MX, Zhang T, Wan DL, Lin SZ. Inflammatory myofibroblastic tumor of the pancreatic neck: A case report and review of literature. World J Clin Cases. 2021;9:6418-6427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Pungpapong S, Geiger XJ, Raimondo M. Inflammatory myofibroblastic tumor presenting as a pancreatic mass: a case report and review of the literature. JOP. 2004;5:360-367. [PubMed] |
| 7. | Yamamoto H, Watanabe K, Nagata M, Tasaki K, Honda I, Watanabe S, Soda H, Takenouti T. Inflammatory myofibroblastic tumor (IMT) of the pancreas. J Hepatobiliary Pancreat Surg. 2002;9:116-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Dulundu E, Sugawara Y, Makuuchi M. Inflammatory myofibroblastic tumor of the pancreas--a case report. Biosci Trends. 2007;1:167-169. [PubMed] |
| 9. | Berhe S, Goldstein S, Thompson E, Hackam D, Rhee DS, Nasr IW. Challenges in Diagnosis and Management of Pancreatic Inflammatory Myofibroblastic Tumors in Children. Pancreas. 2019;48:e27-e29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Walsh SV, Evangelista F, Khettry U. Inflammatory myofibroblastic tumor of the pancreaticobiliary region: morphologic and immunocytochemical study of three cases. Am J Surg Pathol. 1998;22:412-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | A G H, Kumar S, Singla S, Kurian N. Aggressive Inflammatory Myofibroblastic Tumor of Distal Pancreas: A Diagnostic and Surgical Challenge. Cureus. 2022;14:e22820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 12. | Chan JK, Cheuk W, Shimizu M. Anaplastic lymphoma kinase expression in inflammatory pseudotumors. Am J Surg Pathol. 2001;25:761-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 163] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 13. | Lewis RB, Lattin GE Jr, Paal E. Pancreatic endocrine tumors: radiologic-clinicopathologic correlation. Radiographics. 2010;30:1445-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 159] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 14. | Frampas E, Morla O, Regenet N, Eugène T, Dupas B, Meurette G. A solid pancreatic mass: tumour or inflammation? Diagn Interv Imaging. 2013;94:741-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Tamada T, Ito K, Kanomata N, Sone T, Kanki A, Higaki A, Hayashida M, Yamamoto A. Pancreatic adenocarcinomas without secondary signs on multiphasic multidetector CT: association with clinical and histopathologic features. Eur Radiol. 2016;26:646-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |