Published online Jan 14, 2023. doi: 10.3748/wjg.v29.i2.367
Peer-review started: October 1, 2022
First decision: November 5, 2022
Revised: November 9, 2022
Accepted: December 21, 2022
Article in press: December 21, 2022
Published online: January 14, 2023
Processing time: 96 Days and 17.9 Hours
The pandemics of coronavirus disease 2019 (COVID-19) and non-alcoholic fatty liver disease (NAFLD) coexist. Elevated liver function tests are frequent in COVID-19 and may influence liver damage in NAFLD, while preexisting liver damage from NAFLD may influence the course of COVID-19. However, the prognostic relevance of this interaction, though, is unclear. Obesity is a risk factor for the presence of NAFLD as well as a severe course of COVID-19. Cohort studies reveal conflicting results regarding the influence of NAFLD presence on COVID-19 illness severity. Striking molecular similarities of cytokine pathways in both diseases, including postacute sequelae of COVID-19, suggest common pathways for chronic low-activity inflammation. This review will summarize existing data regarding the interaction of both diseases and discuss possible mechanisms of the influence of one disease on the other.
Core Tip: The “colliding” pandemics of coronavirus disease 2019 (COVID-19) and non-alcoholic fatty liver disease (NAFLD) influence each other in several ways. Molecular similarities of cytokine pathways in both diseases including postacute sequelae of COVID-19 (PASC) may be responsible for amplification of chronic low-active inflammation. While there are conflicting data regarding the clinical influence of NAFLD on acute COVID-19 and vice versa, further research is necessary to study the long-term influence of COVID-19 hygienic measures and PASC on NAFLD.
- Citation: Dietrich CG, Geier A, Merle U. Non-alcoholic fatty liver disease and COVID-19: Harmless companions or disease intensifier? World J Gastroenterol 2023; 29(2): 367-377
- URL: https://www.wjgnet.com/1007-9327/full/v29/i2/367.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i2.367
The prevalence of non-alcoholic fatty liver disease (NAFLD) has increased rapidly over the past 30 years, particularly in Western countries. This is due to a lifestyle with hypercaloric diets and obesity leading to a concomitant increase in metabolic syndrome[1]. It is estimated that approximately 30% of people in Western countries have NAFLD, and approximately 5% have non-alcoholic steatohepatitis (NASH), the inflammatory variant of fatty liver[2]. NAFLD and NASH represent chronic liver diseases with high morbidity and potential mortality.
The coronavirus disease 2019 (COVID-19) pandemic began in Wuhan, China, in late 2019. From there, the disease spread rapidly throughout the world. To date, over 500 million people have contracted COVID-19 and over 6 million people have died from it[3]. Despite effective vaccination, it is foreseeable that the coronavirus cannot be eradicated. While vaccination protects against a severe course, it cannot completely prevent infection and minor disease. To that extent, COVID-19 is likely to persist in the world as a disease, its severity depending on the prevailing variants, and to impact the population and preexisting concomitant diseases in an individual. After acute COVID-19 resolves, a proportion of COVID-19 patients suffer from postacute sequelae of COVID-19 (PASC) also named “long COVID” - as a range of new, returning, or ongoing health problems people can experience four or more weeks following initial severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection[4,5]. Therefore, in addition to acute COVID-19, postacute sequelae from COVID-19 are an emerging global health crisis, and there are hints that metabolic factors and a chronic inflammatory state (both characteristics of NAFLD) predispose patients to PASC[6].
While NAFLD is a noninfectious disease whose pandemic spread depends on people’s lifestyle, especially dietary habits, COVID-19 has an acute course due to its nature as an infectious disease. This leads to waves of infection that have prompted epidemic hygiene countermeasures to contain the infection, especially in the past 2 years (2020 and 2021). Lockdowns have occurred in numerous countries to restrict the mobility of people and thus prevent the spread of the coronavirus. The harsh isolation and lockdown measures in the past 2 years under the more pathogenic previous variants had significant sociological and psychological effects. Thus, in addition to the direct viral effects of COVID-19 on the liver, there are also indirect effects on the liver or liver disease, which may play an important role in the further development of these diseases. As COVID-19 can still cause renewed lockdowns and isolation measures in the future, for example, when more lethal mutations arise again or due to newly emerging infectious diseases, such effects should also be modeled and taken into account in the future.
NAFLD and COVID-19 can be referred to as “syndemic”[7]. They represent, in a sense, “colliding pandemics”[8] due to the various possible interactions, which have different dynamics but some molecular and pathogenetic commonalities. These effects are summarized in this review and the current state of the evidence is evaluated. Between August and September 2022, we searched PubMed using the terms coronavirus, COVID-19, SARS-CoV-2, NAFLD, fatty liver, NASH, MAFLD. We analyzed all retrieved abstracts and obtained the full papers, if the study was dedicated to the connection between COVID-19 and NAFLD.
NAFLD covers a wide spectrum of severity, ranging from bland fatty liver without any inflammation (NAFL) and with little or no tendency to progress all the way to NASH with inflammatory reactions and hepatocyte damage with or without fibrosis. A total of 5% to 20% of patients with NAFLD develop NASH, which undergoes a further transition to higher-grade fibrosis and eventually liver cirrhosis in 10% to 20% of cases[9]. These clinical features of NAFLD are the background for chronic low inflammatory activity of the disease. Intestinal barrier dysfunction plays a major role in triggering and amplifying these inflammatory processes, leading to translocation of bacteria or bacterial components into the portal circulation and induction of hepatic inflammation[10]. Obesity induced by an unhealthy lifestyle (insufficient exercise and hypercaloric diet) leads to increased secretion of proinflammatory leptin, interleukin (IL)-6, and tumor necrosis factor (TNF)-α from peripheral adipose tissue, while secretion of adiponectin, an inhibitor of human stellate cell proliferation, is decreased[11]. The massive disruption of lipid metabolism due to the disturbed balance between lipolysis, oxidation, secretion, and uptake of lipids between adipose tissue and liver contributes to hepatic steatosis as well as lipotoxicity, affecting key cellular elements such as the endoplasmic reticulum or mitochondrial function[12]. In terms of a vicious cycle, hepatic metabolic pathways (especially β-oxidation) are dysregulated and further reinforce the imbalance in lipid metabolism[13] and thus lipotoxicity. Activation of human stellate cells and cytokine production by Kupffer cells follows, with IL-1β, TNF-α, IL-6, interferon (IFN)-γ, nuclear factor-kappaB, and reactive oxygen species being key extracellular and/or intracellular proinflammatory mediators that maintain chronic low-activity inflammation and induce the development of fibrosis[14,15].
Interestingly, several of these factors also appear to play important roles in COVID-19 pathogenesis in the context of systemic inflammatory response syndrome. IL-1β, TNF-α, IL-6, and IFN-γ are elevated during acute COVID-19 disease[16], and IL-6 in particular may be considered a central cytokine for the hepatic effects of COVID-19 due to its principal role in the negative acute phase response. After acute COVID-19 resolves, chronic systemic inflammatory responses may persist in patients with sequelae after acute disease, although the exact molecular drivers of PASC are largely unknown. Recently, Schultheiß et al[17] showed that PASC is associated with chronic elevation of IL-1β, TNF-α, and IL-6 levels. Phetsouphanh et al[18] even demonstrated elevation of IFN-γ (and other proinflammatory cytokines) in patients 4 mo after SARS-CoV-2 infection, irrespective of whether they had PASC symptoms.
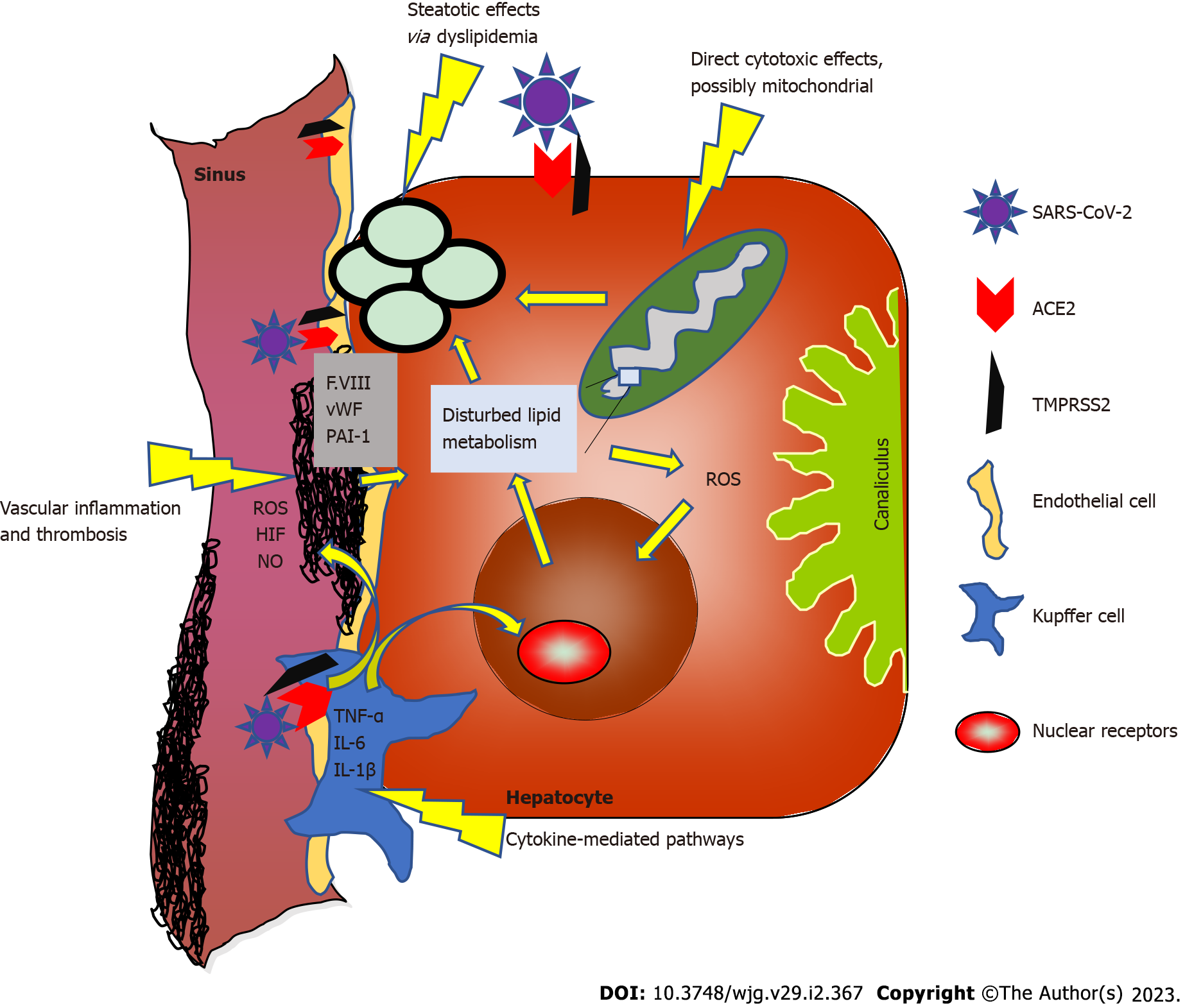
It is therefore straightforward to speculate that low-activity NAFLD inflammation may be amplified or exacerbated by the acute phase of COVID-19 and chronic systemic inflammatory responses in at least some patients after acute COVID-19, resulting in interactions between the two diseases at the molecular level (see Figure 1).
Because COVID-19 is an aerosol-transmitted disease, clinical symptoms of respiratory disease have always been the primary clinical focus. However, many case series and clinical studies show that COVID-19 also has systemic effects. These include vascular inflammation, thrombosis, and other organ involvement. Liver inflammation is therefore only part of a systemic inflammatory component of SARS-CoV-2 and is almost never the primary clinical symptom.
In a comprehensive review, it was shown that COVID-19 leads to elevations in liver enzymes in approximately 17%-58% of patients[19]. Elevations of transaminases (“hepatitis”) dominate, and cholestatic constellations are much less frequent, suggesting predominantly hepatocytic damage[19]. Frequently, this concomitant COVID hepatitis is clinically inapparent. In a recent meta-analysis involving over 77000 patients, the prevalence of clinically overt liver damage was shown to be correlated with the severity of COVID-19. In this analysis, liver damage was described in 40%-47% of severe COVID-19 cases, whereas patients with a milder course were affected in only 10% on average[20]. COVID-19 may trigger acute-on-chronic liver failure in patients with liver cirrhosis due to NAFLD[21]. In contrast, severe hepatic inflammation with impairment of liver function does not seem to occur in patients without advanced preexisting fibrosis[22].
Intracellular uptake of SARS-CoV-2 requires binding of the virus with the spike protein to angiotensin-converting enzyme 2 (ACE2). Further molecular interactions with transmembrane serine protease 2, among others, lead to priming of the S protein and internalization of the virus and its genetic material into the cell[23].
Hepatic tropism of SARS-CoV-2 has been shown recently[24]. However, the exact mechanism of infection of the liver is unclear. Although ACE2 protein expression was observed in the liver, this expression is predominantly located in Kupffer cells (and only at relatively low levels in hepatocytes). In line with that, the SARS-CoV-2 spike protein could be detected in Kupffer and parenchymal cells (for example, hepatocytes)[24,25]. As a potential mechanism of hepatocyte infection, alternative hepatocyte cell entry facilitators are under discussion, e.g., high-density lipoprotein scavenger receptor class B member 1[24] and the asialoglycoprotein receptor[26].
Several mechanisms of liver cell damage are conceivable (see also Figure 1). A direct cytotoxic effect does not appear to be the dominant mechanism of damage in the normal liver. Healthy hepatocytes express almost no ACE2, whereas in liver cirrhosis, ACE2 expression and activity are significantly higher[27]. These molecular regulatory mechanisms tend to argue against direct cytotoxic effects of SARS-CoV-2 in healthy liver but could explain why cirrhotic patients are more susceptible to (further) liver damage or more severe COVID-19 disease overall.
Inflamed hepatocytes (as well as other somatic cells) may exhibit mitochondrial dysfunction in NAFLD or NASH[28,29], which in turn favors ACE2 upregulation[30] and could support viral infection. Conversely, COVID-19 also appears to directly affect mitochondrial function[31]; thus, these effects may amplify each other and induce a more severe course of both diseases.
Another pathogenetic mechanism is the additional fat storage in hepatocytes triggered by SARS-CoV-2. COVID-19 causes dyslipidemia[32] and autopsy studies show a high proportion of steatosis in COVID-19 patients[33-35]. However, it remains unclear in many cases whether steatosis was already preexisting or triggered upon infection. NAFLD patients apparently express ACE2 and various serine proteases at higher levels in the liver[36]; thus, preexisting steatosis may promote COVID-19-induced damage. In turn, COVID-19 may exacerbate steatosis. Whether these dynamics are quantitatively significant effects must remain open for now and deserves future research.
The most significant hypothesis of hepatocyte injury based on clinical characteristics concerns inflammatory cytokine-mediated pathways[37], with commonalities between acutely mediated COVID-19 effects and NAFLD-mediated chronic liver inflammation (especially IL-6; see above). ACE2 expression on Kupffer cells[38] may be the origin of virus-mediated, locally amplified liver inflammation.
Autopsy studies also support possible vascular-associated mechanisms for COVID-19-mediated liver injury[35,39]. One early series of postmortem liver biopsies from patients with COVID-19 reported portal or sinusoidal vascular thrombosis in at least 50% of patients[39]. Patients with COVID-19 exhibit coagulopathy and endotheliopathy, characterized by elevated levels of von Willebrand factor and factor VIII[40]. As factor VIII is produced primarily by liver sinusoidal endothelial cells (LSECs)[41], hypercoagulable LSECs might play an additional role in COVID-19-related liver injury. Of note, endotheliopathy has been reported to be sustained following COVID-19[42], suggesting not only acute but also long-term interactions and consequences of endothelial-mediated inflammation with chronic liver diseases such as NAFLD. Another direct link between COVID-19 and NAFLD may be via plasminogen activator inhibitor 1 (PAI-1). PAI-1 has been shown to be elevated among COVID-19 patients[43]. This role of PAI-1 in COVID-19 liver injury is potentially interesting, especially in NAFLD patients, as elevated PAI-1 has been associated with NAFLD and NASH[44]; therefore, COVID-19-induced PAI-1 elevation may aggravate NAFLD.
The mechanisms described above partly overlap or influence each other, as indicated by the arrows in Figure 1. The pathogenic significance of these molecular associations is not clear in most cases. There are many hints to suggest that the effects of COVID-19 on the liver and especially NAFLD are multifactorial and may also differ individually. Despite these different possible mechanisms, severe liver injury and liver failure are rare even in prediseased liver patients[22].
Numerous studies of varying quality have addressed the risk for morbidity and mortality of COVID-19 in subjects with NAFLD (Table 1). The studies are extraordinarily heterogeneous, making reliable conclusions difficult. Numerous studies were published only as letters to the editor, not as full articles. Almost all studies are retrospective and include low numbers of patients. Definitions of NAFLD/metabolic syndrome-associated fatty liver disease (MAFLD) vary considerably; in some cases, only blood-based surrogate scores for steatosis and liver fibrosis (such as the hepatic steatosis index, NAFLD fibrosis score, or Dallas steatosis index) were applied. Imaging with ultrasound or computed tomography (CT) is mostly used as the criterion for the presence of fatty liver. Biopsy-proven NAFLD is a rare exception. Importantly, data on alcohol consumption are lacking in many studies. No statements are found on inflammatory activity of fatty liver (i.e., on the presence of NASH). Definitions of severe COVID progression are also inconsistent. Studies that define NAFLD only by scores or by imaging (ultrasound, CT) during the course of hospitalization for COVID-19 cannot provide information about the presence of fatty liver before COVID-19 emergence. Control groups almost invariably contain fewer patients with classic metabolic factors, such as diabetes mellitus and obesity, than the respective NAFLD groups; this metabolic imbalance of study groups cannot easily be controlled by multivariate analysis.
| Ref. | Study type and number of NAFLD patients | Results | Appraisal |
| Zhou et al[45], 2020 | Retrospective, matched cohorts, n = 55 per group | More severe COVID-19 in MAFLD OR = 4.07 | Poor matching regarding metabolic status, more male pat in MAFLD group |
| Targher et al[46], 2020 | Retrospective, cohort study n = 94 (216 w/o MAFLD) | More severe COVID-19 with higher FIB-4 or NFS | No matching, no full paper |
| Ji et al[47], 2020 | Retrospective, cohort study n = 202 | NAFLD 87 % in progressive COVID-19 (n = 39) vs 26 % in stable COVID-19 (n = 163) | Comorbidities highly different between groups, no full paper, NAFLD definition only via HSI |
| Hashemi et al[48], 2020 | Retrospective, CLD cohort with 55 NAFLD patients (294 w/o CLD/NAFLD) | Presence of CLD and NAFLD higher risk for mechanical ventilation (OR = 2.15) and ICU admission (OR = 2.3), cirrhosis risk factor for mortality | Imbalance in metabolic status, NAFLD diagnosis relying on prior imaging |
| Huang et al[49], 2020 | Retrospective, cohort n = 86 (194 w/o NAFLD) | Only higher ALT in NAFLD patients, course of disease comparable to controls | NAFLD only defined by HSI, imbalance in metabolic status |
| Forlano et al[50], 2020 | Retrospective, cohort n = 61 (132 w/o NAFLD) | NAFLD pat with higher CRP, younger age. Fibrosis or cirrhosis no risk for more severe COVID-19 | Only hospitalized patients, higher BMI in NAFLD, diagnosis by imaging (US or CT) |
| Lopez-Mendez et al[51], 2021 | Retrospective, cohort study n = 66 (89 w/o steatosis) | Presence of steatosis (and/or liver fibrosis) not related to severity or mortality of COVID-19 | Steatosis only defined by HSI, imbalance on metabolic status |
| Zheng et al[52], 2020 | Retrospective, cohort study n = 66 (45 with and 21 w/o obesity) | Obesity risk factor for COVID severity in MAFLD patients (OR = 6.3) | Diagnosis of MAFLD by CT and clinical criteria, no controls w/o MAFLD, no full paper |
| Zhou et al[53], 2020 | Retrospective, cohort study n = 93 (out of 327 total patients) | Younger MAFLD patients with relatively higher risk for severe COVID | No full paper, small number of older patients, CT data |
| Valenti et al[54], 2020 | Retrospective, United Kingdom Biobank cohort (Mendelian randomization), total n > 500000 | No evidence for NAFLD as risk factor for severe COVID-19 | Data errors possible, partly little characterization of patients, no full paper |
| Mahamid et al[55], 2021 | Retrospective, cohort study n = 22 (49 w/o MAFLD) | 8/22 with severe COVID-19 vs 5/49 w/o MAFLD | CT data, large differences in metabolic status between groups |
| Chen et al[56], 2021 | Retrospective, cohort study n = 178 (164 w/o hepatic steatosis) | More intubation and vasopressors in steatosis, but lower mortality | Only hospitalized patients, HSI or imaging, rel. high percentage of steatosis in cohort, metabolic status not balanced |
| Gao et al[57], 2021 | Retrospective, matched cohorts, n = 65 | OR = 4.07 for severe COVID-19 only in non-diabetic patients | Poor matching regarding metabolic status, NAFLD diagnosis by CT and clinical criteria, duplicate patients with Zhou et al[45] |
| Marjot et al[58], 2021 | Retrospective CLD cohort with 322 NAFLD patients | No higher mortality for NAFLD patients in multivariate analysis | Control group matched only to complete CLD cohort, not specifically to NAFLD patients. Unclear definition of NAFLD |
| Parlak et al[59], 2021 | Retrospective, cohort study n = 55 (288 w/o fatty liver) | Presence of fatty liver risk factor (OR = 3.9) for severe COVID-19 | CT data, no data regarding BMI, no data comparison NAFLD vs non-NAFLD |
| Mushtaq et al[60], 2021 | Retrospective, cohort study n = 320 (269 w/o NAFLD) | NAFLD predictor for mild or moderate liver injury, but not for disease severity or mortality | NAFLD only defined by HIS, imbalance on metabolic status, no full paper |
| Campos-Murguía et al[61], 2021 | Retrospective, cohort study n = 176 (256 w/o MAFLD) | Liver fibrosis, not MAFLD alone, predictor for severity and mortality of COVID-19 | CT data, relatively good obesity matching to controls |
| Kim et al[62], 2021 | Retrospective, CLD cohort with 456 NAFLD patients | NAFLD no risk factor for severe course or mortality of COVID-19 | No control cohort w/o liver disease, tertiary centers only, NAFLD ICD-diagnosis |
| Simon et al[63], 2021 | Large Swedish CLD cohort (total n = 42320), biopsy confirmed, with unclear number of NAFLD patients | CLD presence as risk factor for hospitalization, but not for severe COVID (including cirrhosis) | Historic cohort with possible drop-outs, underlying CLD in controls may have been missed |
| Roca-Fernández et al[64], 2021 | United Kingdom Biobank cohort, with prospective data on infection and hospitalization for COVID | Fatty liver with increased risk for testing COVID-positive, obesity and fatty liver with higher risk for hospitalization, but not obesity alone | Data errors possible, little characterization of patients, small number of patients with severe COVID |
| Ziaee et al[65], 2021 | Retrospective Iranian cohort n = 218 (357 patients w/o NAFLD, additional control group w/o COVID) | Fatty liver significant more prevalent in COVID group compared to control group (38% vs 9%). Longer hospital stay and larger pulmonal involvement in NAFLD patients | Very low percentage of fatty liver in control group. Control group with missing data |
| Liu et al[66], 2022 | COVID-19 HGI and United Kingdom Biobank cohorts (Mendelian randomization), retrospective data, total n > 2500000 | No evidence for NAFLD as risk factor for severe COVID-19 | Data errors possible, little characterization of patients, no full paper |
| Chang et al[67], 2022 | South Korean COVID-19 cohort with FLI score (total n = 3122) | Highest FLI tertile with higher risk for severe COVID-19, but not for higher mortality | No NAFLD-specific case definition, FLI score tertile cutoff low |
| Vrsaljko et al[68], 2022 | Prospective cohort study n = 120 (96 w/o NAFLD) | NAFLD with higher risk for severe COVID-19 including pulmonary thrombosis | No data regarding fibrosis |
| Tripon et al[69], 2022 | Retrospective French cohort n = 311 (408 w/o NAFLD) | NAFLD with higher risk for hospitalization, high FIB-4 with higher risk for severe COVID-19 | NAFLD only defined by NFS, important data missing in cohort patients |
| Moctezuma-Velázquez et al[70], 2022 | Retrospective Mexican cohort n = 359 (111 w/o NAFLD) | NAFLD associated with mortality, ICU admission and mechanical ventilation, but CT-determined liver steatosis was not | NAFLD definition based on DSI, small number of control patients, only hospitalized patients |
| Okuhama et al[71], 2022 | Retrospective Japanese cohort n = 89 (133 w/o fatty liver) | Fatty liver associated with severe COVID-19 | CT data, no data regarding dyslipidemia, only hospitalized patients |
These conditions do not allow confident conclusions to be drawn at this time. Presently, the data suggest that NAFLD alone is not a relevant risk factor for severe COVID-19 progression or mortality. In particular, registry studies from large liver collectives with different etiologies tend not to support a special role for NAFLD[54,63,64,66]. However, available studies also show that the presence of liver fibrosis or cirrhosis is associated with a higher risk of severe COVID-19 disease[61,69]. In this respect, the increase in risk for a more severe course of COVID-19 may not be attributed to NAFLD per se but rather to advanced liver disease irrespective of the underlying etiology in general.
Disease hygiene measures in the context of the infectious waves probably represent an important factor in the influence of the COVID-19 pandemic on NAFLD. The psychosocial impact of the COVID-19 pandemic resulted in measurable exacerbations of metabolic comorbidities of NAFLD. A United States cohort study examined 111 NAFLD patients and found decreases in physical activity in 51%, weight increase in 34%, and increases in alcohol consumption in 5% during the COVID-19 pandemic[72]. A Spanish study screened over 6000 workers for metabolic factors and found significant increases in body mass index, insulin resistance, and low-density lipoprotein during the pandemic. The average fatty liver index (FLI) as a surrogate for NAFLD increased from 25.2 to 33 in this study[73]. Another Spanish study showed a decrease in physical activity during lockdown with a consecutive increase in FLI and worsening of metabolic status[74]. In an Italian cohort study, 48% of 357 NAFLD patients gained weight during lockdown, and this weight gain was associated with abandonment of a Mediterranean diet and decreased physical activity in univariate analysis and various multivariate models. Interestingly, in PNPLA3-GG polymorphism patients, this genotype represented the only favoring factor for weight gain[75]. A Japanese study examined 973 patients with health checks in 2018 and 2020. In this study, the absolute number of MAFLD patients increased from 261 to 305; however, as the authors identified predominantly higher alcohol consumption as a risk factor for this development, there is actually a definition problem of MAFLD in the strict sense[76]. Overall, these studies show a decrease in physical activity and an increase in weight in the general population. It can be assumed, though not yet clearly shown, that this favors the de novo development or exacerbation of steatosis and inflammation in NAFLD, that fibrosis in turn may be further advanced and that the prognosis of the liver disease overall is thus worsened at the end. Long-term studies into these effects of pandemic-associated lifestyle changes are necessary.
NAFLD and COVID-19 have both taken a pandemic course in their own ways. Whereas infectious disease essentially causes short-term disease, NAFLD represents a chronic pandemic. Interestingly, the molecular mechanisms of inflammation are similar, although NAFLD is more of a chronic low-activity inflammation while COVID-19 is an acute inflammatory condition. However, in NAFLD patients with ongoing PASC both conditions may chronically interact with unknown mutual effects.
Obesity certainly represents an important unifying clinical factor of both diseases, as obesity is an important risk factor for the development of NAFLD and the severe course of COVID-19. In contrast, the presence of NAFLD per se does not appear to be a relevant risk factor for particularly severe COVID-19. The effects of COVID-19 on liver disease are more complex and still poorly understood. While the direct viral effect on NAFLD may be limited, probably because of the short duration of the acute viral infection, the individual effects associated with lockdowns and isolation are potential risk factors for disease progression due to a reported decrease in physical activity together with an increase in obesity. European Association for the study of the liver position papers provide valuable recommendations for liver patients after the outbreak of the pandemic, including specific recommendations for NAFLD patients[77,78].
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: EASL, 13336
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Messias LHD, Brazil; Nooripour R, Iran S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3179] [Article Influence: 353.2] [Reference Citation Analysis (4)] |
| 2. | Estes C, Anstee QM, Arias-Loste MT, Bantel H, Bellentani S, Caballeria J, Colombo M, Craxi A, Crespo J, Day CP, Eguchi Y, Geier A, Kondili LA, Kroy DC, Lazarus JV, Loomba R, Manns MP, Marchesini G, Nakajima A, Negro F, Petta S, Ratziu V, Romero-Gomez M, Sanyal A, Schattenberg JM, Tacke F, Tanaka J, Trautwein C, Wei L, Zeuzem S, Razavi H. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. 2018;69:896-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 1308] [Article Influence: 186.9] [Reference Citation Analysis (0)] |
| 3. | Chan FHM, Ataide R, Richards JS, Narh CA. Contrasting Epidemiology and Population Genetics of COVID-19 Infections Defined by Multilocus Genotypes in SARS-CoV-2 Genomes Sampled Globally. Viruses. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Groff D, Sun A, Ssentongo AE, Ba DM, Parsons N, Poudel GR, Lekoubou A, Oh JS, Ericson JE, Ssentongo P, Chinchilli VM. Short-term and Long-term Rates of Postacute Sequelae of SARS-CoV-2 Infection: A Systematic Review. JAMA Netw Open. 2021;4:e2128568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 545] [Cited by in RCA: 704] [Article Influence: 176.0] [Reference Citation Analysis (0)] |
| 5. | Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, Cook JR, Nordvig AS, Shalev D, Sehrawat TS, Ahluwalia N, Bikdeli B, Dietz D, Der-Nigoghossian C, Liyanage-Don N, Rosner GF, Bernstein EJ, Mohan S, Beckley AA, Seres DS, Choueiri TK, Uriel N, Ausiello JC, Accili D, Freedberg DE, Baldwin M, Schwartz A, Brodie D, Garcia CK, Elkind MSV, Connors JM, Bilezikian JP, Landry DW, Wan EY. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3262] [Cited by in RCA: 3000] [Article Influence: 750.0] [Reference Citation Analysis (0)] |
| 6. | Scherer PE, Kirwan JP, Rosen CJ. Post-acute sequelae of COVID-19: A metabolic perspective. Elife. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 7. | Di Ciaula A, Krawczyk M, Filipiak KJ, Geier A, Bonfrate L, Portincasa P. Noncommunicable diseases, climate change and iniquities: What COVID-19 has taught us about syndemic. Eur J Clin Invest. 2021;51:e13682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Moore JB. COVID-19, childhood obesity, and NAFLD: colliding pandemics. Lancet Gastroenterol Hepatol. 2022;7:499-501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Weiß J, Rau M, Geier A. Non-alcoholic fatty liver disease: epidemiology, clinical course, investigation, and treatment. Dtsch Arztebl Int. 2014;111:447-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 10. | Mouries J, Brescia P, Silvestri A, Spadoni I, Sorribas M, Wiest R, Mileti E, Galbiati M, Invernizzi P, Adorini L, Penna G, Rescigno M. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J Hepatol. 2019;71:1216-1228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 459] [Article Influence: 76.5] [Reference Citation Analysis (0)] |
| 11. | Adolph TE, Grander C, Grabherr F, Tilg H. Adipokines and Non-Alcoholic Fatty Liver Disease: Multiple Interactions. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 159] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 12. | Geng Y, Faber KN, de Meijer VE, Blokzijl H, Moshage H. How does hepatic lipid accumulation lead to lipotoxicity in non-alcoholic fatty liver disease? Hepatol Int. 2021;15:21-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 239] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 13. | Hughey CC, Puchalska P, Crawford PA. Integrating the contributions of mitochondrial oxidative metabolism to lipotoxicity and inflammation in NAFLD pathogenesis. Biochim Biophys Acta Mol Cell Biol Lipids. 2022;1867:159209. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Bessone F, Razori MV, Roma MG. Molecular pathways of nonalcoholic fatty liver disease development and progression. Cell Mol Life Sci. 2019;76:99-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 418] [Article Influence: 69.7] [Reference Citation Analysis (0)] |
| 15. | Wang H, Mehal W, Nagy LE, Rotman Y. Immunological mechanisms and therapeutic targets of fatty liver diseases. Cell Mol Immunol. 2021;18:73-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 136] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 16. | Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodríguez L. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 552] [Cited by in RCA: 776] [Article Influence: 155.2] [Reference Citation Analysis (0)] |
| 17. | Schultheiß C, Willscher E, Paschold L, Gottschick C, Klee B, Henkes SS, Bosurgi L, Dutzmann J, Sedding D, Frese T, Girndt M, Höll JI, Gekle M, Mikolajczyk R, Binder M. The IL-1β, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19. Cell Rep Med. 2022;3:100663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 246] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 18. | Phetsouphanh C, Darley DR, Wilson DB, Howe A, Munier CML, Patel SK, Juno JA, Burrell LM, Kent SJ, Dore GJ, Kelleher AD, Matthews GV. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat Immunol. 2022;23:210-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 606] [Article Influence: 202.0] [Reference Citation Analysis (0)] |
| 19. | Chen H, Chen Q. COVID-19 Pandemic: Insights into Interactions between SARS-CoV-2 Infection and MAFLD. Int J Biol Sci. 2022;18:4756-4767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 20. | Chen Z, Peng Y, Wu X, Pang B, Yang F, Zheng W, Liu C, Zhang J. Comorbidities and complications of COVID-19 associated with disease severity, progression, and mortality in China with centralized isolation and hospitalization: A systematic review and meta-analysis. Front Public Health. 2022;10:923485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 21. | Satapathy SK, Roth NC, Kvasnovsky C, Hirsch JS, Trindade AJ, Molmenti E, Barish M, Hirschwerk D, Da BL, Bernstein D; Northwell Health COVID-19 Research Consortium. Risk factors and outcomes for acute-on-chronic liver failure in COVID-19: a large multi-center observational cohort study. Hepatol Int. 2021;15:766-779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Sobotka LA, Esteban J, Volk ML, Elmunzer BJ, Rockey DC; North American Alliance for the Study of Digestive Manifestation of COVID-19*. Acute Liver Injury in Patients Hospitalized with COVID-19. Dig Dis Sci. 2022;67:4204-4214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 23. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271-280.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11946] [Cited by in RCA: 14272] [Article Influence: 2854.4] [Reference Citation Analysis (0)] |
| 24. | Wanner N, Andrieux G, Badia-I-Mompel P, Edler C, Pfefferle S, Lindenmeyer MT, Schmidt-Lauber C, Czogalla J, Wong MN, Okabayashi Y, Braun F, Lütgehetmann M, Meister E, Lu S, Noriega MLM, Günther T, Grundhoff A, Fischer N, Bräuninger H, Lindner D, Westermann D, Haas F, Roedl K, Kluge S, Addo MM, Huber S, Lohse AW, Reiser J, Ondruschka B, Sperhake JP, Saez-Rodriguez J, Boerries M, Hayek SS, Aepfelbacher M, Scaturro P, Puelles VG, Huber TB. Molecular consequences of SARS-CoV-2 liver tropism. Nat Metab. 2022;4:310-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 122] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 25. | Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, Li X, Xu P, Zhang L, Zhao L, Cao Y, Kang J, Yang J, Li L, Liu X, Li Y, Nie R, Mu J, Lu F, Zhao S, Lu J, Zhao J. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 457] [Article Influence: 91.4] [Reference Citation Analysis (0)] |
| 26. | Collins DP, Steer CJ. Binding of the SARS-CoV-2 Spike Protein to the Asialoglycoprotein Receptor on Human Primary Hepatocytes and Immortalized Hepatocyte-Like Cells by Confocal Analysis. Hepat Med. 2021;13:37-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Paizis G, Tikellis C, Cooper ME, Schembri JM, Lew RA, Smith AI, Shaw T, Warner FJ, Zuilli A, Burrell LM, Angus PW. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut. 2005;54:1790-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 281] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 28. | Koliaki C, Szendroedi J, Kaul K, Jelenik T, Nowotny P, Jankowiak F, Herder C, Carstensen M, Krausch M, Knoefel WT, Schlensak M, Roden M. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 2015;21:739-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 766] [Article Influence: 76.6] [Reference Citation Analysis (0)] |
| 29. | Yu L, Zhang X, Ye S, Lian H, Wang H, Ye J. Obesity and COVID-19: Mechanistic Insights From Adipose Tissue. J Clin Endocrinol Metab. 2022;107:1799-1811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 30. | Zhao Q, Zhou X, Kuiper R, Curbo S, Karlsson A. Mitochondrial dysfunction is associated with lipid metabolism disorder and upregulation of angiotensin-converting enzyme 2. PLoS One. 2022;17:e0270418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 31. | Romão PR, Teixeira PC, Schipper L, da Silva I, Santana Filho P, Júnior LCR, Peres A, Gonçalves da Fonseca S, Chagas Monteiro M, Lira FS, Andrey Cipriani Frade M, Comerlato J, Comerlato C, Sant'Anna FH, Bessel M, Abreu CM, Wendland EM, Dorneles GP. Viral load is associated with mitochondrial dysfunction and altered monocyte phenotype in acute severe SARS-CoV-2 infection. Int Immunopharmacol. 2022;108:108697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 32. | Bruzzone C, Bizkarguenaga M, Gil-Redondo R, Diercks T, Arana E, García de Vicuña A, Seco M, Bosch A, Palazón A, San Juan I, Laín A, Gil-Martínez J, Bernardo-Seisdedos G, Fernández-Ramos D, Lopitz-Otsoa F, Embade N, Lu S, Mato JM, Millet O. SARS-CoV-2 Infection Dysregulates the Metabolomic and Lipidomic Profiles of Serum. iScience. 2020;23:101645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 170] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 33. | Santana MF, Guerra MT, Hundt MA, Ciarleglio MM, Pinto RAA, Dutra BG, Xavier MS, Lacerda MVG, Ferreira AJ, Wanderley DC, Borges do Nascimento IJ, Araújo RFA, Pinheiro SVB, Araújo SA, Leite MF, Ferreira LCL, Nathanson MH, Vieira Teixeira Vidigal P. Correlation Between Clinical and Pathological Findings of Liver Injury in 27 Patients With Lethal COVID-19 Infections in Brazil. Hepatol Commun. 2022;6:270-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 34. | Bidari Zerehpoosh F, Sabeti S, Bahrami-Motlagh H, Mokhtari M, Naghibi Irvani SS, Torabinavid P, Esmaeili Tarki F, Amirdosara M, Rezaei O, Mostafazadeh B, Hajiesmaeili M, Rabiei MM, Alavi Darazam I. Post-mortem Histopathologic Findings of Vital Organs in Critically Ill Patients with COVID-19. Arch Iran Med. 2021;24:144-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Tabary M, Khanmohammadi S, Araghi F, Dadkhahfar S, Tavangar SM. Pathologic features of COVID-19: A concise review. Pathol Res Pract. 2020;216:153097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 36. | Meijnikman AS, Bruin S, Groen AK, Nieuwdorp M, Herrema H. Increased expression of key SARS-CoV-2 entry points in multiple tissues in individuals with NAFLD. J Hepatol. 2021;74:748-749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 37. | Li D, Ding X, Xie M, Tian D, Xia L. COVID-19-associated liver injury: from bedside to bench. J Gastroenterol. 2021;56:218-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 38. | Song X, Hu W, Yu H, Zhao L, Zhao Y, Zhao X, Xue HH. Little to no expression of angiotensin-converting enzyme-2 on most human peripheral blood immune cells but highly expressed on tissue macrophages. Cytometry A. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 83] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 39. | Sonzogni A, Previtali G, Seghezzi M, Grazia Alessio M, Gianatti A, Licini L, Morotti D, Zerbi P, Carsana L, Rossi R, Lauri E, Pellegrinelli A, Nebuloni M. Liver histopathology in severe COVID 19 respiratory failure is suggestive of vascular alterations. Liver Int. 2020;40:2110-2116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 218] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 40. | McConnell MJ, Kawaguchi N, Kondo R, Sonzogni A, Licini L, Valle C, Bonaffini PA, Sironi S, Alessio MG, Previtali G, Seghezzi M, Zhang X, Lee AI, Pine AB, Chun HJ, Fernandez-Hernando C, Qing H, Wang A, Price C, Sun Z, Utsumi T, Hwa J, Strazzabosco M, Iwakiri Y. Liver injury in COVID-19 and IL-6 trans-signaling-induced endotheliopathy. J Hepatol. 2021;75:647-658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 41. | Shahani T, Covens K, Lavend'homme R, Jazouli N, Sokal E, Peerlinck K, Jacquemin M. Human liver sinusoidal endothelial cells but not hepatocytes contain factor VIII. J Thromb Haemost. 2014;12:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 144] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 42. | Fogarty H, Townsend L, Morrin H, Ahmad A, Comerford C, Karampini E, Englert H, Byrne M, Bergin C, O'Sullivan JM, Martin-Loeches I, Nadarajan P, Bannan C, Mallon PW, Curley GF, Preston RJS, Rehill AM, McGonagle D, Ni Cheallaigh C, Baker RI, Renné T, Ward SE, O'Donnell JS; Irish COVID-19 Vasculopathy Study (iCVS) investigators. Persistent endotheliopathy in the pathogenesis of long COVID syndrome. J Thromb Haemost. 2021;19:2546-2553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 213] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 43. | Zuo Y, Warnock M, Harbaugh A, Yalavarthi S, Gockman K, Zuo M, Madison JA, Knight JS, Kanthi Y, Lawrence DA. Plasma tissue plasminogen activator and plasminogen activator inhibitor-1 in hospitalized COVID-19 patients. Sci Rep. 2021;11:1580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 164] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 44. | Chang ML, Hsu CM, Tseng JH, Tsou YK, Chen SC, Shiau SS, Yeh CT, Chiu CT. Plasminogen activator inhibitor-1 is independently associated with non-alcoholic fatty liver disease whereas leptin and adiponectin vary between genders. J Gastroenterol Hepatol. 2015;30:329-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 45. | Zhou YJ, Zheng KI, Wang XB, Sun QF, Pan KH, Wang TY, Ma HL, Chen YP, George J, Zheng MH. Metabolic-associated fatty liver disease is associated with severity of COVID-19. Liver Int. 2020;40:2160-2163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 46. | Targher G, Mantovani A, Byrne CD, Wang XB, Yan HD, Sun QF, Pan KH, Zheng KI, Chen YP, Eslam M, George J, Zheng MH. Risk of severe illness from COVID-19 in patients with metabolic dysfunction-associated fatty liver disease and increased fibrosis scores. Gut. 2020;69:1545-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 161] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 47. | Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, Lau G. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J Hepatol. 2020;73:451-453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 408] [Article Influence: 81.6] [Reference Citation Analysis (2)] |
| 48. | Hashemi N, Viveiros K, Redd WD, Zhou JC, McCarty TR, Bazarbashi AN, Hathorn KE, Wong D, Njie C, Shen L, Chan WW. Impact of chronic liver disease on outcomes of hospitalized patients with COVID-19: A multicentre United States experience. Liver Int. 2020;40:2515-2521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 117] [Article Influence: 23.4] [Reference Citation Analysis (2)] |
| 49. | Huang R, Zhu L, Wang J, Xue L, Liu L, Yan X, Huang S, Li Y, Zhang B, Xu T, Li C, Ji F, Ming F, Zhao Y, Cheng J, Wang Y, Zhao H, Hong S, Chen K, Zhao XA, Zou L, Sang D, Shao H, Guan X, Chen X, Chen Y, Wei J, Zhu C, Wu C. Clinical Features of Patients With COVID-19 With Nonalcoholic Fatty Liver Disease. Hepatol Commun. 2020;4:1758-1768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 50. | Forlano R, Mullish BH, Mukherjee SK, Nathwani R, Harlow C, Crook P, Judge R, Soubieres A, Middleton P, Daunt A, Perez-Guzman P, Selvapatt N, Lemoine M, Dhar A, Thursz MR, Nayagam S, Manousou P. In-hospital mortality is associated with inflammatory response in NAFLD patients admitted for COVID-19. PLoS One. 2020;15:e0240400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 51. | Lopez-Mendez I, Aquino-Matus J, Gall SM, Prieto-Nava JD, Juarez-Hernandez E, Uribe M, Castro-Narro G. Association of liver steatosis and fibrosis with clinical outcomes in patients with SARS-CoV-2 infection (COVID-19). Ann Hepatol. 2021;20:100271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 52. | Zheng KI, Gao F, Wang XB, Sun QF, Pan KH, Wang TY, Ma HL, Chen YP, Liu WY, George J, Zheng MH. Letter to the Editor: Obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. 2020;108:154244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 233] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 53. | Zhou YJ, Zheng KI, Wang XB, Yan HD, Sun QF, Pan KH, Wang TY, Ma HL, Chen YP, George J, Zheng MH. Younger patients with MAFLD are at increased risk of severe COVID-19 illness: A multicenter preliminary analysis. J Hepatol. 2020;73:719-721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 54. | Valenti L, Jamialahmadi O, Romeo S. Lack of genetic evidence that fatty liver disease predisposes to COVID-19. J Hepatol. 2020;73:709-711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 55. | Mahamid M, Nseir W, Khoury T, Mahamid B, Nubania A, Sub-Laban K, Schifter J, Mari A, Sbeit W, Goldin E. Nonalcoholic fatty liver disease is associated with COVID-19 severity independently of metabolic syndrome: a retrospective case-control study. Eur J Gastroenterol Hepatol. 2021;33:1578-1581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 56. | Chen VL, Hawa F, Berinstein JA, Reddy CA, Kassab I, Platt KD, Hsu CY, Steiner CA, Louissaint J, Gunaratnam NT, Sharma P. Hepatic Steatosis Is Associated with Increased Disease Severity and Liver Injury in Coronavirus Disease-19. Dig Dis Sci. 2021;66:3192-3198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 57. | Gao F, Zheng KI, Wang XB, Yan HD, Sun QF, Pan KH, Wang TY, Chen YP, George J, Zheng MH. Metabolic associated fatty liver disease increases coronavirus disease 2019 disease severity in nondiabetic patients. J Gastroenterol Hepatol. 2021;36:204-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 85] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 58. | Marjot T, Moon AM, Cook JA, Abd-Elsalam S, Aloman C, Armstrong MJ, Pose E, Brenner EJ, Cargill T, Catana MA, Dhanasekaran R, Eshraghian A, García-Juárez I, Gill US, Jones PD, Kennedy J, Marshall A, Matthews C, Mells G, Mercer C, Perumalswami PV, Avitabile E, Qi X, Su F, Ufere NN, Wong YJ, Zheng MH, Barnes E, Barritt AS 4th, Webb GJ. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J Hepatol. 2021;74:567-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 384] [Article Influence: 96.0] [Reference Citation Analysis (0)] |
| 59. | Parlak S, Çıvgın E, Beşler MS, Kayıpmaz AE. The effect of hepatic steatosis on COVID-19 severity: Chest computed tomography findings. Saudi J Gastroenterol. 2021;27:105-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 60. | Mushtaq K, Khan MU, Iqbal F, Alsoub DH, Chaudhry HS, Ata F, Iqbal P, Elfert K, Balaraju G, Almaslamani M, Al-Ejji K, AlKaabi S, Kamel YM. NAFLD is a predictor of liver injury in COVID-19 hospitalized patients but not of mortality, disease severity on the presentation or progression - The debate continues. J Hepatol. 2021;74:482-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 61. | Campos-Murguía A, Román-Calleja BM, Toledo-Coronado IV, González-Regueiro JA, Solís-Ortega AA, Kúsulas-Delint D, Cruz-Contreras M, Cruz-Yedra N, Cubero FJ, Nevzorova YA, Martínez-Cabrera CF, Moreno-Guillén P, Lozano-Cruz OA, Chapa-Ibargüengoitia M, Gulías-Herrero A, Aguilar-Salinas CA, Ruiz-Margáin A, Macías-Rodríguez RU. Liver fibrosis in patients with metabolic associated fatty liver disease is a risk factor for adverse outcomes in COVID-19. Dig Liver Dis. 2021;53:525-533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 62. | Kim D, Adeniji N, Latt N, Kumar S, Bloom PP, Aby ES, Perumalswami P, Roytman M, Li M, Vogel AS, Catana AM, Wegermann K, Carr RM, Aloman C, Chen VL, Rabiee A, Sadowski B, Nguyen V, Dunn W, Chavin KD, Zhou K, Lizaola-Mayo B, Moghe A, Debes J, Lee TH, Branch AD, Viveiros K, Chan W, Chascsa DM, Kwo P, Dhanasekaran R. Predictors of Outcomes of COVID-19 in Patients With Chronic Liver Disease: US Multi-center Study. Clin Gastroenterol Hepatol. 2021;19:1469-1479.e19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 184] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 63. | Simon TG, Hagström H, Sharma R, Söderling J, Roelstraete B, Larsson E, Ludvigsson JF. Risk of severe COVID-19 and mortality in patients with established chronic liver disease: a nationwide matched cohort study. BMC Gastroenterol. 2021;21:439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 64. | Roca-Fernández A, Dennis A, Nicholls R, McGonigle J, Kelly M, Banerjee R, Banerjee A, Sanyal AJ. Hepatic Steatosis, Rather Than Underlying Obesity, Increases the Risk of Infection and Hospitalization for COVID-19. Front Med (Lausanne). 2021;8:636637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 65. | Ziaee A, Azarkar G, Ziaee M. Role of fatty liver in coronavirus disease 2019 patients' disease severity and hospitalization length: a case-control study. Eur J Med Res. 2021;26:115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 66. | Liu D, Zhang Q, Bai P, Zhao J. Assessing causal relationships between COVID-19 and non-alcoholic fatty liver disease. J Hepatol. 2022;76:740-742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 67. | Chang Y, Jeon J, Song TJ, Kim J. Association between the fatty liver index and the risk of severe complications in COVID-19 patients: a nationwide retrospective cohort study. BMC Infect Dis. 2022;22:384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 68. | Vrsaljko N, Samadan L, Viskovic K, Mehmedović A, Budimir J, Vince A, Papic N. Association of Nonalcoholic Fatty Liver Disease With COVID-19 Severity and Pulmonary Thrombosis: CovidFAT, a Prospective, Observational Cohort Study. Open Forum Infect Dis. 2022;9:ofac073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 69. | Tripon S, Bilbault P, Fabacher T, Lefebvre N, Lescuyer S, Andres E, Schmitt E, Garnier-KepKA S, Borgne PL, Muller J, Merdji H, Chaffraix F, Mutter D, Baumert TF, Meziani F, Doffoel M. Abnormal liver tests and non-alcoholic fatty liver disease predict disease progression and outcome of patients with COVID-19. Clin Res Hepatol Gastroenterol. 2022;46:101894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 70. | Moctezuma-Velázquez P, Miranda-Zazueta G, Ortiz-Brizuela E, Garay-Mora JA, González-Lara MF, Tamez-Torres KM, Román-Montes CM, Díaz-Mejía BA, Pérez-García E, Villanueva-Reza M, Chapa-Ibargüengoitia M, Uscanga-Domínguez L, Sifuentes-Osornio J, Ponce-de-León A, Kershenobich-Stalnikowitz D, Mota-Ayala B, Moctezuma-Velázquez C. NAFLD determined by Dallas Steatosis Index is associated with poor outcomes in COVID-19 pneumonia: a cohort study. Intern Emerg Med. 2022;17:1355-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 71. | Okuhama A, Hotta M, Ishikane M, Kawashima A, Miyazato Y, Terada M, Yamada G, Kanda K, Inada M, Sato L, Sato M, Akiyama Y, Suzuki T, Nakamoto T, Nomoto H, Ide S, Nakamura K, Saito S, Kinoshita N, Yamamoto K, Morioka S, Ujiie M, Hayakawa K, Kustuna S, Shida Y, Tajima T, Teruya K, Funato Y, Yamamoto M, Izumi S, Hojo M, Sugiyama H, Ohmagari N. Fatty liver on computed tomography scan on admission is a risk factor for severe coronavirus disease. J Infect Chemother. 2022;28:217-223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 72. | Kim RG, Medina SP, Magee C, Khalili M. Fatty Liver and the Coronavirus Disease 2019 Pandemic: Health Behaviors, Social Factors, and Telemedicine Satisfaction in Vulnerable Populations. Hepatol Commun. 2022;6:1045-1055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 73. | López-González ÁA, Altisench Jané B, Masmiquel Comas L, Arroyo Bote S, González San Miguel HM, Ramírez Manent JI. Impact of COVID-19 Lockdown on Non-Alcoholic Fatty Liver Disease and Insulin Resistance in Adults: A before and after Pandemic Lockdown Longitudinal Study. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 74. | Mascaró CM, Bouzas C, Montemayor S, García S, Mateos D, Casares M, Gómez C, Ugarriza L, Borràs PA, Martínez JA, Tur JA. Impact of Physical Activity Differences Due to COVID-19 Pandemic Lockdown on Non-Alcoholic Fatty Liver Parameters in Adults with Metabolic Syndrome. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 75. | Cinque F, Cespiati A, Lombardi R, Costantino A, Maffi G, Alletto F, Colavolpe L, Francione P, Oberti G, Fatta E, Bertelli C, Sigon G, Dongiovanni P, Vecchi M, Fargion S, Fracanzani AL. Interaction between Lifestyle Changes and PNPLA3 Genotype in NAFLD Patients during the COVID-19 Lockdown. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 76. | Fujii H, Nakamura N, Fukumoto S, Kimura T, Nakano A, Nadatani Y, Tauchi Y, Nishii Y, Takashima S, Kamada Y, Watanabe T, Kawada N. Lifestyle changes during the coronavirus disease 2019 pandemic impact metabolic dysfunction-associated fatty liver disease. Liver Int. 2022;42:995-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 77. | Boettler T, Marjot T, Newsome PN, Mondelli MU, Maticic M, Cordero E, Jalan R, Moreau R, Cornberg M, Berg T. Impact of COVID-19 on the care of patients with liver disease: EASL-ESCMID position paper after 6 months of the pandemic. JHEP Rep. 2020;2:100169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 121] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 78. | Marjot T, Eberhardt CS, Boettler T, Belli LS, Berenguer M, Buti M, Jalan R, Mondelli MU, Moreau R, Shouval D, Berg T, Cornberg M. Impact of COVID-19 on the liver and on the care of patients with chronic liver disease, hepatobiliary cancer, and liver transplantation: An updated EASL position paper. J Hepatol. 2022;77:1161-1197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |