Published online Jan 14, 2023. doi: 10.3748/wjg.v29.i2.257
Peer-review started: September 12, 2022
First decision: November 15, 2022
Revised: November 29, 2022
Accepted: December 21, 2022
Article in press: December 21, 2022
Published online: January 14, 2023
Processing time: 115 Days and 5.6 Hours
The new coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified in December 2019, in Wuhan, China. The virus was rapidly spread worldwide, causing coronavirus disease 2019 (COVID-19) pandemic. Although COVID-19 is presented, usually, with typical respiratory symptoms (i.e., dyspnea, cough) and fever, extrapulmonary manifestations are also encountered. Liver injury is a common feature in patients with COVID-19 and ranges from mild and temporary elevation of liver enzymes to severe liver injury and, even, acute liver failure. The pathogenesis of liver damage is not clearly defined; multiple mechanisms contribute to liver disorder, including direct cytopathic viral effect, cytokine storm and immune-mediated hepatitis, hypoxic injury, and drug-induced liver toxicity. Patients with underlying chronic liver disease (i.e., cirrhosis, non-alcoholic fatty liver disease, alcohol-related liver disease, hepatocellular carcinoma, etc.) may have greater risk to develop both severe COVID-19 and further liver deterioration, and, as a consequence, certain issues should be considered during disease management. The aim of this review is to present the prevalence, clinical manifestation and pathophysiological mechanisms of liver injury in patients with SARS-CoV-2 infection. Moreover, we overview the association between chronic liver disease and SARS-CoV-2 infection and we briefly discuss the management of liver injury during COVID-19.
Core Tip: Liver injury is a common feature in coronavirus disease 2019 (COVID-19) patients and was associated with disease severity and prognosis. Multiple pathophysiological mechanisms are responsible for liver injury, including direct viral effect, cytokine storm, hypoxia and drug hepatotoxicity, however, further research is needed, in order, for them, to be clearly defined. Patients with underlying chronic liver disease may be more susceptible to severe acute respiratory syndrome coronavirus 2 infection; nevertheless, evidence is still limited. It is necessary to know the mechanisms of liver injury, the clinical manifestations and the effect of COVID-19 in underlying liver disease, in order to design appropriate management programs.
- Citation: Papagiouvanni I, Kotoulas SC, Pataka A, Spyratos DG, Porpodis K, Boutou AK, Papagiouvannis G, Grigoriou I, Vettas C, Goulis I. COVID-19 and liver injury: An ongoing challenge. World J Gastroenterol 2023; 29(2): 257-271
- URL: https://www.wjgnet.com/1007-9327/full/v29/i2/257.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i2.257
In December 2019, severe acute respiratory syndrome coronavirus 2, (SARS-CoV-2), causing respiratory infection in humans, was detected in Wuhan, China[1]. The new coronavirus was spread worldwide, resulting in coronavirus disease 2019 (COVID-19) outbreak. On March 11, 2020, the World Health Organization declared COVID-19 as a global pandemic[2]. As of September 2022, over 603 million confirmed cases and over 6.4 million deaths have been reported worldwide[3].
Most COVID-19 patients present with typical respiratory symptoms (i.e., cough, dyspnea) and fever. However, abnormal liver function is often developed in patients with COVID-19, and liver injury has been related with severe disease[4,5]. Liver damage ranges from mild asymptomatic elevation of liver enzymes to severe liver injury, while a few cases of acute liver failure have also been reported[6,7].
The aim of this review is to present the prevalence and clinical manifestations of liver injury in COVID-19, to overview the potential pathophysiological mechanisms leading to liver damage and to summarize the existing literature for patients with COVID-19 and underlying chronic liver disease. Furthermore, the management of liver complications during SARS-CoV-2 infection is also briefly discussed.
Numerous studies have focused on liver injury induced by COVID-19 infection. However, the definition of liver injury in COVID-19 patients has not been clearly established yet. Some researchers defined it, as any increase of liver enzymes above the upper limit of normal (ULN), while others, as an increase, at least 2 or 3 times above the ULN[8-12]. Moreover, the different statistical time points across the studies, could also affect the incidence of liver injury[8]. As a consequence, the prevalence of liver damage varies across studies. Wang et al[13] conducted a retrospective study and found that the 41% of 156 COVID-19 patients had abnormal liver function, while, Fan et al[10] demonstrated that 55 out of 148 patients (37.2%) had elevated liver enzymes on admission. In a recent retrospective study of 228 patients, without chronic liver disease, 29.4% had abnormal liver function on admission; the rate increased to 56.3% during hospitalization[14]. Cai et al[15] defining liver injury as alanine transaminase (ALT) or aspartate aminotransferase (AST) 3 times higher than ULN or alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), total bilirubin (TBIL) 2 times higher than ULN, observed that 41% of patients had abnormal liver tests and 5% had liver injury on admission. During hospitalization, patients with abnormal liver tests and patients with liver injury increased to 76.3% and 21.5%, respectively. Ding et al[16], also, demonstrated the same trend of liver function, in a large retrospective cohort study of 2073 patients. On admission, 46.2% and 5.1% had abnormal liver tests and liver injury, respectively. Yet, during hospitalization, the incidence increased to 61.8% and 14.3%, respectively. Across several meta-analyses, the pooled prevalence of liver injury ranged between 19% and 27.4%[4,5,17]. Kulkarni et al[18], in their meta-analysis, found that the pooled incidence of abnormal liver enzymes at initial presentation, only slightly increased during the course of disease (from 23.1% to 24.4%). Wu et al[19] observed a similar trend; the pooled incidence of elevated liver tests on admission and during hospitalization was 27.2% and 36%, respectively.
Liver injury has been associated with severe COVID-19 disease[4,5,19-21]. Chen et al[22] demo
In most cases, liver injury is presented as elevated liver enzymes without specific symptoms and signs. The elevation of AST, ALT and/or TBIL is a very common manifestation in COVID-19 patients, while increased GGT and/or ALP is a less usual feature, observed in later stages of the disease[6,7]. The elevation of the aminotransferases is usually mild; their level is mostly < 5 times ULN[26]. Furthermore, liver injury in COVID-19 has been noted to be transient, while hepatic biochemical tests return to normal within 2-3 wk[6]. Severe liver injury, with aminotransferases > 20 times ULN, has been observed in 0.1% of COVID-19 patients on admission and in 2% during hospitalization, while acute liver failure, induced by COVID-19, has been reported in extremely rare cases[27,28]. Febrile hepatitis, acute cholecystitis and hepatic artery thrombosis are, also, rare clinical presentations of COVID-19[29-31]. Moreover, in some cases reports, it is suggested that SARS-CoV-2 triggered a de novo development of immune-mediated liver disease, such as autoimmune hepatitis and primary bile cholangitis[32-35]. Interestingly, cholangiopathy, characterized by cholestasis and structural abnormalities of bile duct, has been reported in post-COVID-19 patients, who recovered from severe and critical disease[36,37].
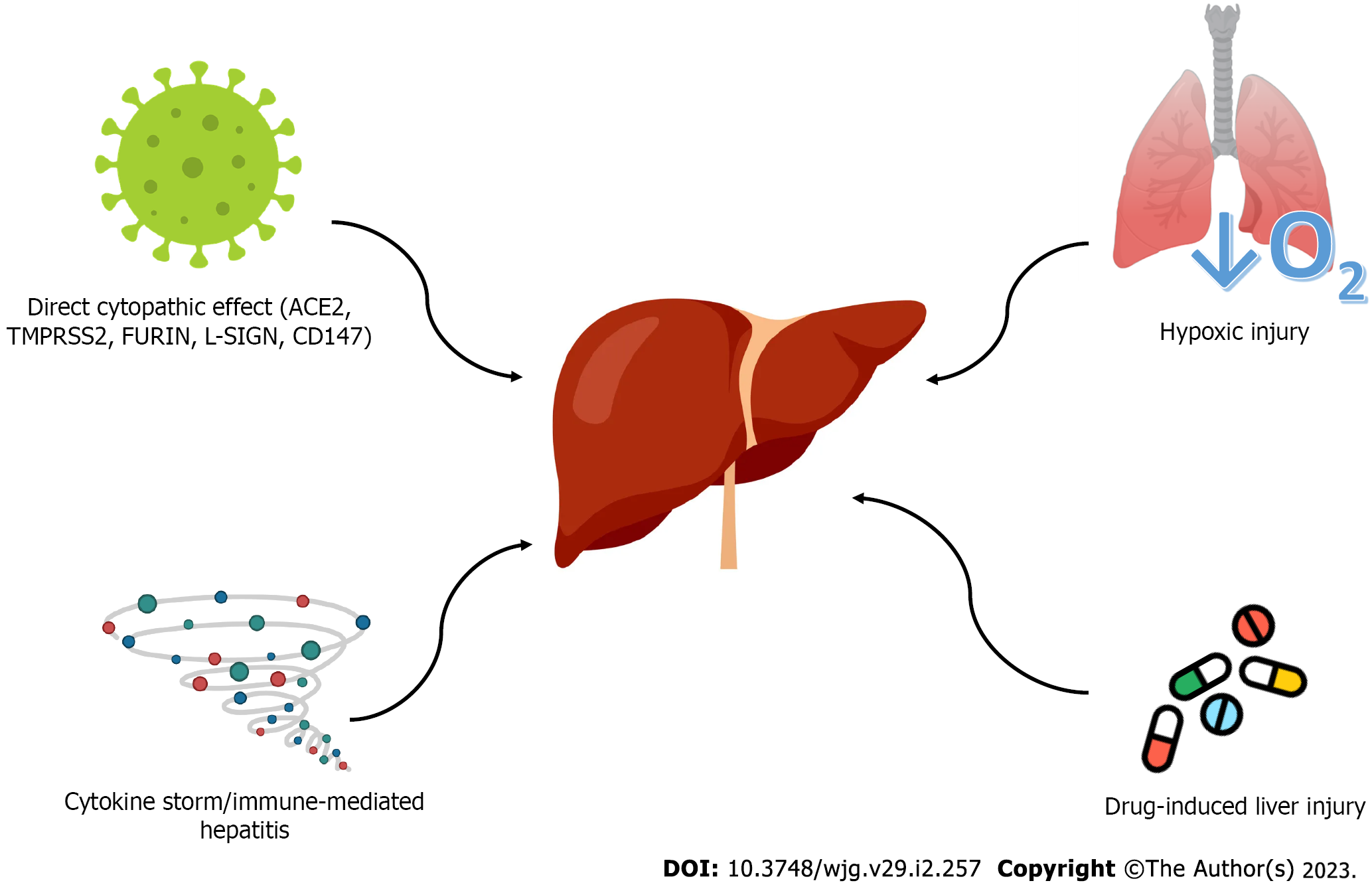
The pathogenesis of live injury in COVID-19 disease is still unclear. According to the available literature, the underlying mechanisms of liver injury are multifactorial and mainly, include direct viral cytopathic damage, immune-mediated hepatitis, caused by cytokine storm, hypoxia and ischemic injury and drug-induced liver toxicity. The possible pathophysiologic mechanisms of liver injury are presented in Figure 1.
Liver is a potential target of direct SARS-CoV-2 infection. Existing literature suggests that the new coronavirus could be detected in the liver and indicates typical histological lesions related to viral infection[38]. Indeed, a series of small sample size studies demonstrated that SARS-CoV-2 RNA and viral particles are detectable in the liver of patients with COVID-19[13,39-43]. Furthermore, in a recent cohort study of 45 autopsy cases, virus RNA was detected in 69% of cases[44].
SARS-CoV-2 uses angiotensin-converting enzyme 2 (ACE2) to invade into host cells, while cell entry is facilitated by transmembrane serine protease 2 (TMPRSS2) and paired basic amino acid cleaving enzyme (FURIN)[45,46]. Single-cell RNA sequencing analysis revealed that ACE2 is expressed among different cell types in liver; in parallel, TMPRSS2 and FURIN are, also, expressed in liver cells[47-49]. The above evidence indicates that liver tissue could be susceptible to COVID-19 infection. Yet, the expression of ACE2 in bile duct cells is 20-fold higher than the expression level in hepatocytes[50]. Despite the high expression of ACE2 in cholangiocytes, which would be associated with cholestatic injury (i.e., elevated levels of GGT and ALP), most studies found that hepatocellular damage is the most common pattern in COVID-19 patients (i.e., elevated levels of ALT and AST)[17,51,52]. Therefore, alternative molecular pathways for liver infection cannot be excluded. The liver/Lymph node-specific intercellular adhesion molecule-3-grabbing integrin, a liver-specific capture receptor, and CD147, a receptor highly expressed in inflamed and/or pathogen-infected tissues, have been proposed as alternative receptors or enhancer factors, mediating in the SARS-CoV-2 cellular entry in the liver. Moreover, antibody-dependent enhancement may be responsible for liver infection[53]. Instead of neutralizing the virus completely, suboptimal non-neutralizing antibodies, attached to Fc receptor, promote viral entry into the liver cells[53]. In addition, existing evidence suggests that inflammatory signals [i.e., interleukin-6 (IL-6), type 1 interferon] and hypoxia, related to SARS-CoV-2 infection, could result to hepatocyte regeneration, compensatory hyperplasia and upregulated expression of ACE2, leading to potentially increased hepatic susceptibility to SARS-Cov-2[45,53].
Despite that virus particles have been observed in hepatocytes and molecular pathways of virus invasion have been suggested, further evidence is needed to clearly establish the role of direct viral infection in liver injury.
COVID-19 infection can trigger uncontrolled immune response, called cytokine storm, which is characterized by exaggerated activation of immune cells and massive production of inflammatory mediators[54,55]. Indeed, pro-inflammatory cytokines [i.e., IL-1β, IL-2, IL-6, IL-8, tumor necrosis factor-α, interferon-α (IFN-α), IFN-γ, granulocyte-macrophage colony-stimulating factor] were increased in severe COVID-19 disease[56]. Cytokine storm generates a process leading to tissue damage and even multiorgan failure[57]. Due to its anatomical location, liver is highly exposed to circulating cytokines, and thus, prone to inflammatory-mediated injury[58]. Furthermore, viral-induced CD8+ T cells provoke the activation of Kupfer cells, resulting to T cell-mediated hepatitis[58].
Several studies have demonstrated a correlation between liver injury and increased levels of inflammatory mediators in COVID-19 patients. In a recent cohort study of 192 patients, increased IL-6 and IL-10 Levels and decreased number of CD4+ T cells were identified as independent risk factors for severe liver injury[59]. Likewise, in another retrospective cohort study, inflammatory markers, such as IL-6, CRP and ferritin, were significantly higher in patients with liver injury[60]. Huang et al[61], conducting a retrospective study of 2623 patients, found a positive correlation between IL-6 and liver enzymes (i.e., AST, ALT, and GGT), indicating that COVID-19-induced cytokine storm leads to hepatotoxicity. In addition to that, Liao et al[62], suggested that, apart from IL-6, IL-2 and IL17A were also key inflammatory factors triggering liver damage.
The liver is a highly aerobic organ, and, thus, it is remarkably susceptible to hypoxia[38]. Patients with COVID-19 can be complicated with respiratory failure, acute heart failure and systemic stress, causing low oxygen saturation level and/or decreased systemic arterial pressure. As a consequence, arterial perfusion and oxygenation of the liver can be reduced, leading to hepatic ischemia and hypoxia-reperfusion injury[38,63]. Furthermore, systemic inflammatory response, through microvascular dysfunction and microthrombosis, could worsen liver hypoxia[38]. Hepatic venous congestion, caused by heart failure, or high positive end-respiratory pressure, used in patients with respiratory failure, can, also, lead to hypoxic damage in the liver cells[58].
Hypoxic injury involves a biphasic process; ischemic cell damage and reperfusion-associated inflammatory response. Lipid accumulation, glycogen consumption, mitochondrial damage and increased reactive oxygen species and their peroxidation products lead to cell death, during ischemia[53]. Following ischemic injury, reperfusion induces activation of immune response and release of pro-inflammatory cytokines, resulting in further cell damage[53].
In a retrospective cohort study, hepatocellular injury pattern in COVID-19 patients was associated with hypoxia[64]. Likewise, Fu et al[65], in a more recent multicenter retrospective study, confirmed that patients with hypoxia were more likely to have abnormal liver function.
The liver plays a crucial role in drug metabolism. Several drug metabolites induce liver cell apoptosis/necrosis and can lead to liver damage. Drug-induced liver injury (DILI) is often detected by liver enzymes tests, using the following thresholds: (1) ALT > 5 times ULN; (2) ALP > 2 times ULN; and (3) ALT > 3 times ULN and TBL > 2 times ULN[66]. Based on ALT/ALP ratio, DILI pattern can be defined as hepatocellular, cholestatic or mixed. DILI can also be intrinsic, which is dose-dependent and predictable, or idiosyncratic, which is unpredictable, with variable latency period[66]. Concerning prognosis, DILI ranges from mild to severe or even fatal, with approximately 10% of patients requiring liver transplantation[66].
At present, many drugs have been used to treat COVID-19 patients, such as corticosteroids, antiviral agents, immunoregulatory factors and antibiotics, leading to potential hepatotoxicity. Systemic corticosteroids, especially dexamethasone, were widely prescribed to both outpatients and hospitalized patients with COVID-19. Despite that, the prolonged use of corticosteroids is related to side effects (i.e., infections, hyperglycemia), DILI is uncommon[67]. Corticosteroids have been associated with liver steatosis, hepatomegaly, worsening non-alcoholic fatty liver disease (NAFLD) and exacerbating HBV re-activation, however, existing literature is limited[67,68]. With regards to COVID-19, Yip et al[69] found that the use of corticosteroids was an independent factor of liver injury. However, this association could be explained by the fact that patients with more severe disease received corticosteroids.
Remdesivir is an inhibitor of viral RNA-dependent RNA polymerases, used in COVID-19 disease. Among its side effects, remdesivir can cause hepatotoxicity, manifested as elevated AST and ALT[67]. In most studies, 10%-50% of patients developed mild-to-moderate increase of aminotransferases, while levels > 5 times ULN were reported in 9% of patients in clinical trials[70]. Subsequently, remdesivir is contraindicated in patients with ALT > 5 times ULN or severe liver dysfunction[71]. The elevation of aminotransferases is generally reversible without clinically apparent hepatic dysfunction[67].
Tocilizumab, a humanized anti-interleukin-6 receptor (IL-6R) monoclonal antibody, is indicated in hospitalized COVID-19 patients with rapid respiratory deterioration[67]. Elevation of aminotransferases has been reported, but it is generally transient, dose-dependent, without significant liver complications[67,72]. Anakinra, an IL-1 inhibitor, has, also, been used in severe COVID-19, but hepatotoxicity is an extremely uncommon side effect. In addition to that, liver enzymes levels did not significantly differ between anakinra and placebo in clinical trials[67,73].
More recently, nirmatrelvir/ritonavir has been prescribed in COVID-19 patients, early in the course of infection, as a post-exposure protection. In clinical trials, elevation of aminotransferases was uncommon or mild in nirmatrelvir/ritonavir group and did not differ from placebo group. However, clinical data are still limited and further evidence is needed[74].
Table 1 presents the most studied drugs for COVID-19 and the existing evidence concerning their hepatotoxicity.
| Drug | Mechanism of action | Characteristics of LI | Risk of DILI | DILI pattern |
| Corticosteroids[126] | Anti-inflammatory | Hepatomegaly, steatosis; triggering/worsening NAFLD; reactivation HBV (prolonged administration) | Low | Hepatocellular or mixed |
| Remdesivir[70] | Antiviral; active inhibitor of viral RNA-dependent RNA polymerases | Mild-to-moderate ALT and AST elevations; Elevation > 5 times ULN in 9% (resolved with discontinuation) | Moderate | Hepatocellular |
| Tocilizumab[72] | Anti-IL-6 receptor monoclonal antibody | Elevation of ALT and AST; no reports of severe LI or HBV reactivation (in COVID-19 trials) | Moderate | Hepatocellular |
| Anakinra[73] | IL-1 inhibitor | ALT elevation in < 1%; No association with HBV reactivation | Low | Hepatocellular |
| Nirmatrelvir/ritonavir[74] | Antiviral; Inhibitor of the main protease of SARS-CoV-2/protease inhibitor and potent inhibitor of the enzyme CYP 3A4 | Mild ALT and AST elevation; no reports of clinical apparent LI; limited data | Low | Hepatocellular |
| Molnupiravir[127] | Antiviral; prodrug of the ribonucleoside analogue N-hydroxycytidine | Mild ALT and AST elevation; no reports of clinical apparent LI; limited data | Low | Hepatocellular |
| Low-molecular-weight heparins[128] | Anticoagulant | Mild ALT and AST elevation; LI with rapid onset and rapid recovery, without clinical symptoms | Low | Hepatocellular |
| NSAIDs[129] | Anti-inflammatory | Mild, transient and asymptomatic elevation of liver enzymes; more common in obese patients with comorbidities; reports of acute hepatitis (idiosyngratic, prolonged administration) | Moderate | Hepatocellular, cholestatic or mixed |
| Acetaminophen[130] | Analgesic and antipyretic | Dose-dependent; transient and asymptomatic elevation of ALT and AST; acute hepatitis and/or acute liver failure in overdose | High | Hepatocellular |
Most studies have not provided sufficient data about the prevalence of underlying chronic liver disease (CLD) in COVID-19 patients. However, in a meta-analysis of 73 studies including 24299 COVID-19 patients, the pooled prevalence of CLD was estimated to be at 3%[75]. Patients with CLD may, already, have liver damage and SARS-Cov-2 infection is an additional “hit” to the liver, leading to further liver functional impairment[76]. Although patients with stable CLD, without cirrhosis, are not more susceptible to severe COVID-19, those with cirrhosis, alcoholic liver disease (ALD), hepatocellular carcinoma (HCC) and NAFLD may be in a greater risk for severe disease with liver injury and poor outcome[6,76-78].
Patients with cirrhosis may be more susceptible to SARS-CoV-2 infection, due to their immunodeficient status, referred as cirrhosis-associated immune dysfunction[79]. In several studies, COVID-19 patients with cirrhosis presented worse prognosis, compared to patients without cirrhosis[80-85]. In a large multicenter study, including 745 COVID-19 patients with CLD (386 with and 359 without cirrhosis), cirrhotic patients exhibited higher mortality rate, compared to those without cirrhosis (32% vs 8%, P < 0.001)[82]. Mortality was correlated with the stage of liver cirrhosis; 19% in Child- Pugh class A, 35% in class B, and 51% in class C. A similar trend was also observed in the rates of ICU admission, mechanical ventilation and renal replacement therapy. In the same study, it was noted, that the main cause of death was respiratory failure (71%) followed by liver complications[82].
Moreover, COVID-19 patients with cirrhosis are in increased risk for acute decompensation and acute-on-chronic liver failure (ACLF)[78]. Sarin et al[86], conducting a multicenter cohort study, found that 20% of patients with compensated cirrhosis developed acute decompensation or ACLF during COVID-19 disease, while 57% of patients with decompensated cirrhosis had further liver complications. Acute decompensation is a common clinical feature in cirrhotic patients during SARS-CoV-2 infection, usually presented as new or worsening ascites or hepatic encephalopathy[82]. Interestingly, liver complications can also be developed and in the absence of typical symptoms of respiratory system[82,85].
Patients with NAFLD usually have other comorbidities, such as diabetes mellitus, obesity, hypertension and chronic cardiac disease, which are common risk factors for severe COVID-19[77]. Consequently, it is challenging to define an independent effect of NAFLD on COVID-19 and evidence from concomitant studies is controversial. More particularly, some studies did not prove an association between NAFLD and worse COVID-19 outcomes[87-89]. On the other hand, numerous observational studies demo
Although the existing evidence is limited, few studies demonstrated that ALD is related to increased COVID-19 mortality. In a multicenter cohort study of 867 COVID-19 patients, reported that ALD is an independent risk factor of higher mortality[100]. Likewise, Marjot et al[82] identified independent association between ALD and COVID-19 mortality. Mallet et al[101], also, found that ALD is a risk factor of day-30 mortality after COVID-19. The exact mechanism leading to the aforementioned correlation is not clear. However, ALD-related immune dysregulation and low nutritional status may have a negative impact on the course of SARS-CoV-2 infection[7,79].
The influence of viral hepatitis on COVID-19 severity and COVID-19-related liver injury has not been clearly established. COVID-19 patients with chronic hepatitis B (CHB) may have prolonged virus shedding and infection[48]. Furthermore, during SARS-CoV infection, replication of hepatitis B virus (HBV) was found to be enhanced, inducing more severe liver injury; similar enhancement could be noted during SARS-CoV-2 infection[102]. Wang et al[103], in a retrospective cohort study of 437 patients, found that those with co-infection SARS-CoV-2/HBV had higher risk of severe disease and mortality. Likewise, Zou et al[104] reported that COVID-19 patients with CHB and liver injury were more prone to poor outcomes. Nevertheless, other studies did not demonstrate the above associations. Chen et al[105] found no difference in terms of liver function and disease severity between COVID-19 patients with HBV and those without co-infection. Guan et al[106] also suggested that CHB does not affect COVID-19 outcome, as only one of 23 patients with CHB developed severe disease. In addition, Yip et al[107] demonstrated that current and past HBV infection were not related to higher risk of liver injury or mortality.
Due to extended use of immunosuppressive drugs for COVID-19 treatment (i.e., tocilizumab), potential re-activation of HBV should be taken into consideration. Although the immunosuppressive therapies are short-term and results of clinical trials are contradictory, there are some clinical case reports of HBV re-activation in COVID-19 patients after administration of these immunosuppressive agents[108].
Of note, COVID-19 pandemic has disrupted the progress in the global hepatitis C virus (HCV) elimination program, resulting in delays in diagnosis and HCV therapy, which could extend the direct COVID-19-related morbidity and mortality in these patients[109].
Although immunosuppressive therapy, used in patients with autoimmune liver diseases, could be associated with higher risk of severe disease, there is no evidence that patients with autoimmune hepatitis (AIH), primary biliary cholangitis (PBC) or primary sclerosing cholangitis (PSC) are more prone to SARS-CoV-2 infection[6,77]. In a phone-based survey, there was no difference in percentage of COVID-19 diagnosis in patients with autoimmune liver diseases and the general population. Most of patients reported a favorable disease outcome in the same survey[110]. Data derived from three multinational registries (SECURE-Cirrhosis, COVID-Hep and ERN RARE-LIVER) revealed that patients with AIH had increased risk of hospitalization compared to patients with other CLD, but there was no difference in adverse outcome, including ICU admission and death, despite the immunosuppressive treatment[111]. However, a recent retrospective study of 254 patients with COVID-19 and AIH demonstrated that baseline treatment with corticosteroids or azathioprine was associated with COVID-19 severity[112]. Evidence for patients with PBC and PSC is limited and no defined association with COVID-19 severity has been established yet[7].
COVID-19 patients with may have a high risk for poor outcomes. Due to chemotherapy/immunotherapy, HCC patients are immunosuppressed, and, subsequently, vulnerable to severe SARS-CoV-2 infection[102]. Furthermore, most HCC patients have an underlying CLD (i.e., cirrhosis, ALD etc.), and as a result, they are already identified as a high-risk group[102]. However, the corresponding literature is limited. A small retrospective study of 28 cancer patients with COVID-19, including 2 HCC patients) found that these patients had worse prognosis compared to general population[113].
Different types of SARS-CoV-2 vaccines have been developed, such as mRNA vaccines, adenoviral-vectored vaccines and inactivated vaccines. In general, patients with CLD may exhibit lower immune response to vaccination; according to previous studies, rate of seroconversion after HBV vaccine and cell-mediated immunity were reduced in cirrhotic patients[114,115]. Regarding efficacy, although trials of both mRNA vaccines included few patients with underlying CLD, they reported significant efficacy in the subgroup with coexisting comorbidities[116,117]. Of note, in a large retrospective cohort study of cirrhotic patients, a single mRNA vaccine dose appeared to reduce not only rates of SARS-CoV-2 infection, but also, rates of hospitalization and mortality[118]. With regard to safety, none of vaccine contain living virus, and subsequently, they can be used even in immunosuppressed patients[119]. Moreover, no significant liver-associated side effects have been reported in the vaccinated population[120]. Given that benefits outweigh the potential risks, European Association for the Study of the Liver (EASL) and American Association for the Study of Liver Diseases (AASLD) recommend that patients with CLD should be vaccinated against SARS-CoV-2[121,122].
Liver injury in COVID-19 is usually mild and resolves spontaneously without any special treatment[77]. If present, hypoxia and circulatory failure should be regulated with standard symptomatic support (i.e., oxygen therapy, intravenous fluids) in order to prevent further liver damage[45,123]. If liver injury persists, underlying chronic liver disease should be suspected[124]. With regard to DILI, there are no specific management guidelines. Discontinuation or dose’s reduction of suspected medication is the most effective treatment in case of DILI, as the only available antidote is N-acetylcysteine for acetaminophen overdose[67]. In the case of severe COVID-19, benefits and risks have to be weighed in order to decide discontinuation of systematic treatment. This dilemma hardly arises for pharmaceutical agents which need short administration, such as remdesivir and tocilizumab[67]. Standard guidelines and supportive therapy should be followed for management of acute liver failure[67].
Regarding chronic liver diseases, comprehensive recommendations related to COVID-19 mana
Liver abnormalities are common in COVID-19 patients, especially in patients with severe and critical disease. The pathogenesis of liver injury may be multifactorial involving direct cytopathic viral effect, inflammatory storm, hypoxic/hypoperfusion injury and drug hepatotoxicity. Liver injury is usually mild and transient; however, some cases of severe liver injury and acute liver failure have been reported. Although, patients with stable chronic liver disease are not more vulnerable to SARS-CoV-2 infection, patients with cirrhosis, ALD, NAFLD and HCC have higher risk for severe COVID-19 and liver damage. Specific management issues should be taken into consideration during COVID-19 treatment in patients with underlying CLD. Further investigation is needed in order to clarify the association between SARS-CoV-2 and liver dysfunction, in terms of prognosis, pathophysiology and treatment.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shaikh TG, Pakistan; Zhou C, China S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Borges do Nascimento IJ, Cacic N, Abdulazeem HM, von Groote TC, Jayarajah U, Weerasekara I, Esfahani MA, Civile VT, Marusic A, Jeroncic A, Carvas Junior N, Pericic TP, Zakarija-Grkovic I, Meirelles Guimarães SM, Luigi Bragazzi N, Bjorklund M, Sofi-Mahmudi A, Altujjar M, Tian M, Arcani DMC, O'Mathúna DP, Marcolino MS. Novel Coronavirus Infection (COVID-19) in Humans: A Scoping Review and Meta-Analysis. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 367] [Cited by in RCA: 328] [Article Influence: 65.6] [Reference Citation Analysis (2)] |
| 2. | Cucinotta D, Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020;91:157-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2623] [Reference Citation Analysis (0)] |
| 3. | World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available from: https://covid19.who.int. |
| 4. | Kumar-M P, Mishra S, Jha DK, Shukla J, Choudhury A, Mohindra R, Mandavdhare HS, Dutta U, Sharma V. Coronavirus disease (COVID-19) and the liver: a comprehensive systematic review and meta-analysis. Hepatol Int. 2020;14:711-722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 111] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 5. | Mao R, Qiu Y, He JS, Tan JY, Li XH, Liang J, Shen J, Zhu LR, Chen Y, Iacucci M, Ng SC, Ghosh S, Chen MH. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:667-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 741] [Cited by in RCA: 754] [Article Influence: 150.8] [Reference Citation Analysis (0)] |
| 6. | Ekpanyapong S, Bunchorntavakul C, Reddy KR. COVID-19 and the Liver: Lessons Learnt from the EAST and the WEST, A Year Later. J Viral Hepat. 2022;29:4-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 7. | Dufour JF, Marjot T, Becchetti C, Tilg H. COVID-19 and liver disease. Gut. 2022;71:2350-2362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 8. | Deng ML, Chen YJ, Yang ML, Liu YW, Chen H, Tang XQ, Yang XF. COVID-19 combined with liver injury: Current challenges and management. World J Clin Cases. 2021;9:3487-3497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 9. | Ye Z, Song B. COVID-19 Related Liver Injury: Call for International Consensus. Clin Gastroenterol Hepatol. 2020;18:2848-2851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561-1566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 559] [Cited by in RCA: 556] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 11. | Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020;40:2095-2103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 310] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 12. | Hundt MA, Deng Y, Ciarleglio MM, Nathanson MH, Lim JK. Abnormal Liver Tests in COVID-19: A Retrospective Observational Cohort Study of 1,827 Patients in a Major U.S. Hospital Network. Hepatology. 2020;72:1169-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 197] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 13. | Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, Li X, Xu P, Zhang L, Zhao L, Cao Y, Kang J, Yang J, Li L, Liu X, Li Y, Nie R, Mu J, Lu F, Zhao S, Lu J, Zhao J. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 457] [Article Influence: 91.4] [Reference Citation Analysis (0)] |
| 14. | Wang J, Zhu L, Xue L, Liu L, Yan X, Huang S, Zhang B, Xu T, Li C, Ji F, Ming F, Zhao Y, Cheng J, Shao H, Chen K, Zhao XA, Sang D, Zhao H, Guan X, Chen X, Chen Y, Liu J, Huang R, Zhu C, Wu C. Risk factors of liver injury in patients with coronavirus disease 2019 in Jiangsu, China: A retrospective, multi-center study. J Med Virol. 2021;93:3305-3311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, Zhou G, Gou J, Qu J, Sun Y, Liu Y, He Q, Chen J, Liu L, Xu L. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73:566-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 623] [Cited by in RCA: 661] [Article Influence: 132.2] [Reference Citation Analysis (0)] |
| 16. | Ding ZY, Li GX, Chen L, Shu C, Song J, Wang W, Wang YW, Chen Q, Jin GN, Liu TT, Liang JN, Zhu P, Zhu W, Li Y, Zhang BH, Feng H, Zhang WG, Yin ZY, Yu WK, Yang Y, Zhang HQ, Tang ZP, Wang H, Hu JB, Liu JH, Yin P, Chen XP, Zhang B; Tongji Multidisciplinary Team for Treating COVID-19 (TTTC). Association of liver abnormalities with in-hospital mortality in patients with COVID-19. J Hepatol. 2021;74:1295-1302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 109] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 17. | Wan J, Wang X, Su S, Zhang Y, Jin Y, Shi Y, Wu K, Liang J. Digestive symptoms and liver injury in patients with coronavirus disease 2019 (COVID-19): A systematic review with meta-analysis. JGH Open. 2020;4:1047-1058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Kulkarni AV, Kumar P, Tevethia HV, Premkumar M, Arab JP, Candia R, Talukdar R, Sharma M, Qi X, Rao PN, Reddy DN. Systematic review with meta-analysis: liver manifestations and outcomes in COVID-19. Aliment Pharmacol Ther. 2020;52:584-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 183] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 19. | Wu Y, Li H, Guo X, Yoshida EM, Mendez-Sanchez N, Levi Sandri GB, Teschke R, Romeiro FG, Shukla A, Qi X. Incidence, risk factors, and prognosis of abnormal liver biochemical tests in COVID-19 patients: a systematic review and meta-analysis. Hepatol Int. 2020;14:621-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 20. | Xin S, Xu J, Yu Y. Abnormal Liver Function Tests of Patients with Coronavirus Disease 2019 in Mainland China: A Systematic Review and Meta- Analysis. J Gastrointestin Liver Dis. 2020;29:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Mohammed SA, Eid KM, Anyiam FE, Wadaaallah H, Muhamed MAM, Morsi MH, Dahman NBH. Liver injury with COVID-19: laboratory and histopathological outcome-systematic review and meta-analysis. Egypt Liver J. 2022;12:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Chen LY, Chu HK, Bai T, Tu SJ, Wei Y, Li ZL, Hu LL, Zhu R, Zhang L, Han CQ, Xiao L, He Q, Song J, Liu WH, Zhu QJ, Chen H, Yang L, Hou XH. Liver damage at admission is an independent prognostic factor for COVID-19. J Dig Dis. 2020;21:512-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 23. | Huang H, Chen S, Li H, Zhou XL, Dai Y, Wu J, Zhang J, Shao L, Yan R, Wang M, Wang J, Tu Y, Ge M. The association between markers of liver injury and clinical outcomes in patients with COVID-19 in Wuhan. Aliment Pharmacol Ther. 2020;52:1051-1059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14766] [Article Influence: 2953.2] [Reference Citation Analysis (0)] |
| 25. | Xie H, Zhao J, Lian N, Lin S, Xie Q, Zhuo H. Clinical characteristics of non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: A retrospective study. Liver Int. 2020;40:1321-1326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 213] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 26. | Elmunzer BJ, Spitzer RL, Foster LD, Merchant AA, Howard EF, Patel VA, West MK, Qayed E, Nustas R, Zakaria A, Piper MS, Taylor JR, Jaza L, Forbes N, Chau M, Lara LF, Papachristou GI, Volk ML, Hilson LG, Zhou S, Kushnir VM, Lenyo AM, McLeod CG, Amin S, Kuftinec GN, Yadav D, Fox C, Kolb JM, Pawa S, Pawa R, Canakis A, Huang C, Jamil LH, Aneese AM, Glamour BK, Smith ZL, Hanley KA, Wood J, Patel HK, Shah JN, Agarunov E, Sethi A, Fogel EL, McNulty G, Haseeb A, Trieu JA, Dixon RE, Yang JY, Mendelsohn RB, Calo D, Aroniadis OC, LaComb JF, Scheiman JM, Sauer BG, Dang DT, Piraka CR, Shah ED, Pohl H, Tierney WM, Mitchell S, Condon A, Lenhart A, Dua KS, Kanagala VS, Kamal A, Singh VK, Pinto-Sanchez MI, Hutchinson JM, Kwon RS, Korsnes SJ, Singh H, Solati Z, Willingham FF, Yachimski PS, Conwell DL, Mosier E, Azab M, Patel A, Buxbaum J, Wani S, Chak A, Hosmer AE, Keswani RN, DiMaio CJ, Bronze MS, Muthusamy R, Canto MI, Gjeorgjievski VM, Imam Z, Odish F, Edhi AI, Orosey M, Tiwari A, Patwardhan S, Brown NG, Patel AA, Ordiah CO, Sloan IP, Cruz L, Koza CL, Okafor U, Hollander T, Furey N, Reykhart O, Zbib NH, Damianos JA, Esteban J, Hajidiacos N, Saul M, Mays M, Anderson G, Wood K, Mathews L, Diakova G, Caisse M, Wakefield L, Nitchie H, Waljee AK, Tang W, Zhang Y, Zhu J, Deshpande AR, Rockey DC, Alford TB, Durkalski V; North American Alliance for the Study of Digestive Manifestations of COVID-19. Digestive Manifestations in Patients Hospitalized With Coronavirus Disease 2019. Clin Gastroenterol Hepatol. 2021;19:1355-1365.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 27. | Khawaja J, Bawa A, Omer H, Ashraf F, Zulfiqar P. COVID-19 Infection Presenting as an Isolated Severe Acute Liver Failure. Cureus. 2022;14:e24873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 28. | Sobotka LA, Esteban J, Volk ML, Elmunzer BJ, Rockey DC; North American Alliance for the Study of Digestive Manifestation of COVID-19*. Acute Liver Injury in Patients Hospitalized with COVID-19. Dig Dis Sci. 2022;67:4204-4214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 29. | Filippidis P, van Ouwenaller F, Cerutti A, Geiger-Jacquod A, Sempoux C, Pantaleo G, Moradpour D, Lamoth F. Case Report: SARS-CoV-2 as an unexpected causal agent of predominant febrile hepatitis. F1000Res. 2021;10:400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 30. | Antunes de Brito CA, de Oliveira Filho JRB, Marques DT, Lencastre MDC, de Almeida JR, Lopes EP. COVID-19 and Hepatic Artery Thrombosis: A Case Report. Am J Case Rep. 2021;22:e932531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Balaphas A, Gkoufa K, Meyer J, Peloso A, Bornand A, McKee TA, Toso C, Popeskou SG. COVID-19 can mimic acute cholecystitis and is associated with the presence of viral RNA in the gallbladder wall. J Hepatol. 2020;73:1566-1568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 32. | Bartoli A, Gitto S, Sighinolfi P, Cursaro C, Andreone P. Primary biliary cholangitis associated with SARS-CoV-2 infection. J Hepatol. 2021;74:1245-1246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 33. | Kabaçam G, Wahlin S, Efe C. Autoimmune hepatitis triggered by COVID-19: A report of two cases. Liver Int. 2021;41:2527-2528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 34. | Hong JK, Chopra S, Kahn JA, Kim B, Khemichian S. Autoimmune hepatitis triggered by COVID-19. Intern Med J. 2021;51:1182-1183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 35. | Macías-Rodríguez RU, Ruiz-Margáin A, Román-Calleja BM, Espin-Nasser ME, Flores-García NC, Torre A, Galicia-Hernández G, Rios-Torres SL, Fernández-Del-Rivero G, Orea-Tejeda A, Lozano-Cruz OA. Effect of non-alcoholic beer, diet and exercise on endothelial function, nutrition and quality of life in patients with cirrhosis. World J Hepatol. 2020;12:1299-1313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Roth NC, Kim A, Vitkovski T, Xia J, Ramirez G, Bernstein D, Crawford JM. Post-COVID-19 Cholangiopathy: A Novel Entity. Am J Gastroenterol. 2021;116:1077-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 129] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 37. | Rojas M, Rodríguez Y, Zapata E, Hernández JC, Anaya JM. Cholangiopathy as part of post-COVID syndrome. J Transl Autoimmun. 2021;4:100116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 38. | Zhang X, Yu Y, Zhang C, Wang H, Zhao L, Su Y, Yang M. Mechanism of SARS-CoV-2 Invasion into the Liver and Hepatic Injury in Patients with COVID-19. Mediterr J Hematol Infect Dis. 2022;14:e2022003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 39. | Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, Chilla S, Heinemann A, Wanner N, Liu S, Braun F, Lu S, Pfefferle S, Schröder AS, Edler C, Gross O, Glatzel M, Wichmann D, Wiech T, Kluge S, Pueschel K, Aepfelbacher M, Huber TB. Multiorgan and Renal Tropism of SARS-CoV-2. N Engl J Med. 2020;383:590-592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1254] [Cited by in RCA: 1424] [Article Influence: 284.8] [Reference Citation Analysis (0)] |
| 40. | Sonzogni A, Previtali G, Seghezzi M, Grazia Alessio M, Gianatti A, Licini L, Morotti D, Zerbi P, Carsana L, Rossi R, Lauri E, Pellegrinelli A, Nebuloni M. Liver histopathology in severe COVID 19 respiratory failure is suggestive of vascular alterations. Liver Int. 2020;40:2110-2116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 218] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 41. | Tian S, Xiong Y, Liu H, Niu L, Guo J, Liao M, Xiao SY. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33:1007-1014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 576] [Cited by in RCA: 656] [Article Influence: 131.2] [Reference Citation Analysis (0)] |
| 42. | Chornenkyy Y, Mejia-Bautista M, Brucal M, Blanke T, Dittmann D, Yeldandi A, Boike JR, Lomasney JW, Nayar R, Jennings LJ, Pezhouh MK. Liver Pathology and SARS-CoV-2 Detection in Formalin-Fixed Tissue of Patients With COVID-19. Am J Clin Pathol. 2021;155:802-814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 43. | Kaltschmidt B, Fitzek ADE, Schaedler J, Förster C, Kaltschmidt C, Hansen T, Steinfurth F, Windmöller BA, Pilger C, Kong C, Singh K, Nierhaus A, Wichmann D, Sperhake J, Püschel K, Huser T, Krüger M, Robson SC, Wilkens L, Schulte Am Esch J. Hepatic Vasculopathy and Regenerative Responses of the Liver in Fatal Cases of COVID-19. Clin Gastroenterol Hepatol. 2021;19:1726-1729.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 44. | Wanner N, Andrieux G, Badia-I-Mompel P, Edler C, Pfefferle S, Lindenmeyer MT, Schmidt-Lauber C, Czogalla J, Wong MN, Okabayashi Y, Braun F, Lütgehetmann M, Meister E, Lu S, Noriega MLM, Günther T, Grundhoff A, Fischer N, Bräuninger H, Lindner D, Westermann D, Haas F, Roedl K, Kluge S, Addo MM, Huber S, Lohse AW, Reiser J, Ondruschka B, Sperhake JP, Saez-Rodriguez J, Boerries M, Hayek SS, Aepfelbacher M, Scaturro P, Puelles VG, Huber TB. Molecular consequences of SARS-CoV-2 liver tropism. Nat Metab. 2022;4:310-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 122] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 45. | Cai Y, Ye LP, Song YQ, Mao XL, Wang L, Jiang YZ, Que WT, Li SW. Liver injury in COVID-19: Detection, pathogenesis, and treatment. World J Gastroenterol. 2021;27:3022-3036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 46. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271-280.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11946] [Cited by in RCA: 14269] [Article Influence: 2853.8] [Reference Citation Analysis (0)] |
| 47. | Qi F, Qian S, Zhang S, Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. 2020;526:135-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 523] [Cited by in RCA: 741] [Article Influence: 148.2] [Reference Citation Analysis (0)] |
| 48. | Elnaggar M, Abomhya A, Elkhattib I, Dawoud N, Doshi R. COVID-19 and liver diseases, what we know so far. World J Clin Cases. 2022;10:3969-3980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 49. | Pirola CJ, Sookoian S. SARS-CoV-2 virus and liver expression of host receptors: Putative mechanisms of liver involvement in COVID-19. Liver Int. 2020;40:2038-2040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 50. | Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, Zhou J, Shi G, Fang N, Fan J, Cai J, Lan F. Specific ACE2 Expression in Cholangiocytes May Cause Liver Damage After 2019-nCoV Infection. BioRxiv. 2020;931766. |
| 51. | Wijarnpreecha K, Ungprasert P, Panjawatanan P, Harnois DM, Zaver HB, Ahmed A, Kim D. COVID-19 and liver injury: a meta-analysis. Eur J Gastroenterol Hepatol. 2021;33:990-995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 52. | Del Zompo F, De Siena M, Ianiro G, Gasbarrini A, Pompili M, Ponziani FR. Prevalence of liver injury and correlation with clinical outcomes in patients with COVID-19: systematic review with meta-analysis. Eur Rev Med Pharmacol Sci. 2020;24:13072-13088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 53. | Li D, Ding X, Xie M, Tian D, Xia L. COVID-19-associated liver injury: from bedside to bench. J Gastroenterol. 2021;56:218-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 54. | Hu B, Huang S, Yin L. The cytokine storm and COVID-19. J Med Virol. 2021;93:250-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 470] [Cited by in RCA: 1029] [Article Influence: 205.8] [Reference Citation Analysis (0)] |
| 55. | Zanza C, Romenskaya T, Manetti AC, Franceschi F, La Russa R, Bertozzi G, Maiese A, Savioli G, Volonnino G, Longhitano Y. Cytokine Storm in COVID-19: Immunopathogenesis and Therapy. Medicina (Kaunas). 2022;58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 172] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 56. | Yalcin AD, Yalcin AN. Future perspective: biologic agents in patients with severe COVID-19. Immunopharmacol Immunotoxicol. 2021;43:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 57. | Kim JS, Lee JY, Yang JW, Lee KH, Effenberger M, Szpirt W, Kronbichler A, Shin JI. Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics. 2021;11:316-329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 322] [Cited by in RCA: 310] [Article Influence: 77.5] [Reference Citation Analysis (0)] |
| 58. | Idalsoaga F, Ayares G, Arab JP, Díaz LA. COVID-19 and Indirect Liver Injury: A Narrative Synthesis of the Evidence. J Clin Transl Hepatol. 2021;9:760-768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 59. | Zhan K, Liao S, Li J, Bai Y, Lv L, Yu K, Qiu L, Li C, Yuan G, Zhang A, Mei Z. Risk factors in patients with COVID-19 developing severe liver injury during hospitalisation. Gut. 2021;70:628-629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 60. | Da BL, Kushner T, El Halabi M, Paka P, Khalid M, Uberoi A, Lee BT, Perumalswami PV, Rutledge SM, Schiano TD, Friedman SL, Saberi B. Liver Injury in Patients Hospitalized with Coronavirus Disease 2019 Correlates with Hyperinflammatory Response and Elevated Interleukin-6. Hepatol Commun. 2021;5:177-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 61. | Huang W, Li C, Wang Z, Wang H, Zhou N, Jiang J, Ni L, Zhang XA, Wang DW. Decreased serum albumin level indicates poor prognosis of COVID-19 patients: hepatic injury analysis from 2,623 hospitalized cases. Sci China Life Sci. 2020;63:1678-1687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 106] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 62. | Liao S, Zhan K, Gan L, Bai Y, Li J, Yuan G, Cai Y, Zhang A, He S, Mei Z. Inflammatory cytokines, T lymphocyte subsets, and ritonavir involved in liver injury of COVID-19 patients. Signal Transduct Target Ther 2020; 5: 255.. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 63. | McGrowder DA, Miller F, Anderson Cross M, Anderson-Jackson L, Bryan S, Dilworth L. Abnormal Liver Biochemistry Tests and Acute Liver Injury in COVID-19 Patients: Current Evidence and Potential Pathogenesis. Diseases. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 64. | Chu H, Bai T, Chen L, Hu L, Xiao L, Yao L, Zhu R, Niu X, Li Z, Zhang L, Han C, Song S, He Q, Zhao Y, Zhu Q, Chen H, Schnabl B, Yang L, Hou X. Multicenter Analysis of Liver Injury Patterns and Mortality in COVID-19. Front Med (Lausanne). 2020;7:584342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 65. | Fu Y, Zhu R, Bai T, Han P, He Q, Jing M, Xiong X, Zhao X, Quan R, Chen C, Zhang Y, Tao M, Yi J, Tian D, Yan W. Clinical Features of Patients Infected With Coronavirus Disease 2019 With Elevated Liver Biochemistries: A Multicenter, Retrospective Study. Hepatology. 2021;73:1509-1520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 66. | European Association for the Study of the Liver; Clinical Practice Guideline Panel: Chair; EASL Governing Board Representative. EASL Clinical Practice Guidelines: Drug-induced liver injury. J Hepatol. 2019;70:1222-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 715] [Cited by in RCA: 669] [Article Influence: 111.5] [Reference Citation Analysis (0)] |
| 67. | Gabrielli M, Franza L, Esperide A, Gasparrini I, Gasbarrini A, Franceschi F, On Behalf Of Gemelli Against Covid. Liver Injury in Patients Hospitalized for COVID-19: Possible Role of Therapy. Vaccines (Basel). 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 68. | Ortiz GX, Lenhart G, Becker MW, Schwambach KH, Tovo CV, Blatt CR. Drug-induced liver injury and COVID-19: A review for clinical practice. World J Hepatol. 2021;13:1143-1153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 69. | Yip TC, Lui GC, Wong VW, Chow VC, Ho TH, Li TC, Tse YK, Hui DS, Chan HL, Wong GL. Liver injury is independently associated with adverse clinical outcomes in patients with COVID-19. Gut. 2021;70:733-742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 123] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 70. | Remdesivir. 2022 Feb 3. In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012. [PubMed] |
| 71. | Aleem A, Kothadia JP. Remdesivir. 2022 Sep 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. [PubMed] |
| 72. | Tocilizumab. 2021 May 11. In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012. [PubMed] |
| 73. | Anakinra. 2020 Apr 20. In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012. [PubMed] |
| 74. | Paxlovid. 2022 Jan 31. In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012. [PubMed] |
| 75. | Kovalic AJ, Satapathy SK, Thuluvath PJ. Prevalence of chronic liver disease in patients with COVID-19 and their clinical outcomes: a systematic review and meta-analysis. Hepatol Int. 2020;14:612-620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 99] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 76. | Shousha HI, Ramadan A, Lithy R, El-Kassas M. Patterns of liver profile disturbance in patients with COVID-19. World J Clin Cases. 2022;10:2063-2071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 77. | Ozkurt Z, Çınar Tanrıverdi E. COVID-19: Gastrointestinal manifestations, liver injury and recommendations. World J Clin Cases. 2022;10:1140-1163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (7)] |
| 78. | Nasa P, Alexander G. COVID-19 and the liver: What do we know so far? World J Hepatol. 2021;13:522-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 79. | Spearman CW, Aghemo A, Valenti L, Sonderup MW. COVID-19 and the liver: A 2021 update. Liver Int. 2021;41:1988-1998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 80. | Singh S, Khan A. Clinical Characteristics and Outcomes of Coronavirus Disease 2019 Among Patients With Preexisting Liver Disease in the United States: A Multicenter Research Network Study. Gastroenterology. 2020;159:768-771.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 270] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 81. | Bajaj JS, Garcia-Tsao G, Biggins SW, Kamath PS, Wong F, McGeorge S, Shaw J, Pearson M, Chew M, Fagan A, de la Rosa Rodriguez R, Worthington J, Olofson A, Weir V, Trisolini C, Dwyer S, Reddy KR. Comparison of mortality risk in patients with cirrhosis and COVID-19 compared with patients with cirrhosis alone and COVID-19 alone: multicentre matched cohort. Gut. 2021;70:531-536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 176] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 82. | Marjot T, Moon AM, Cook JA, Abd-Elsalam S, Aloman C, Armstrong MJ, Pose E, Brenner EJ, Cargill T, Catana MA, Dhanasekaran R, Eshraghian A, García-Juárez I, Gill US, Jones PD, Kennedy J, Marshall A, Matthews C, Mells G, Mercer C, Perumalswami PV, Avitabile E, Qi X, Su F, Ufere NN, Wong YJ, Zheng MH, Barnes E, Barritt AS 4th, Webb GJ. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J Hepatol. 2021;74:567-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 384] [Article Influence: 96.0] [Reference Citation Analysis (0)] |
| 83. | Shalimar, Elhence A, Vaishnav M, Kumar R, Pathak P, Soni KD, Aggarwal R, Soneja M, Jorwal P, Kumar A, Khanna P, Singh AK, Biswas A, Nischal N, Dar L, Choudhary A, Rangarajan K, Mohan A, Acharya P, Nayak B, Gunjan D, Saraya A, Mahapatra S, Makharia G, Trikha A, Garg P. Poor outcomes in patients with cirrhosis and Corona Virus Disease-19. Indian J Gastroenterol. 2020;39:285-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 84. | Moon AM, Webb GJ, Aloman C, Armstrong MJ, Cargill T, Dhanasekaran R, Genescà J, Gill US, James TW, Jones PD, Marshall A, Mells G, Perumalswami PV, Qi X, Su F, Ufere NN, Barnes E, Barritt AS, Marjot T. High mortality rates for SARS-CoV-2 infection in patients with pre-existing chronic liver disease and cirrhosis: Preliminary results from an international registry. J Hepatol. 2020;73:705-708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 203] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 85. | Iavarone M, D'Ambrosio R, Soria A, Triolo M, Pugliese N, Del Poggio P, Perricone G, Massironi S, Spinetti A, Buscarini E, Viganò M, Carriero C, Fagiuoli S, Aghemo A, Belli LS, Lucà M, Pedaci M, Rimondi A, Rumi MG, Invernizzi P, Bonfanti P, Lampertico P. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol. 2020;73:1063-1071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 279] [Cited by in RCA: 276] [Article Influence: 55.2] [Reference Citation Analysis (2)] |
| 86. | Sarin SK, Choudhury A, Lau GK, Zheng MH, Ji D, Abd-Elsalam S, Hwang J, Qi X, Cua IH, Suh JI, Park JG, Putcharoen O, Kaewdech A, Piratvisuth T, Treeprasertsuk S, Park S, Wejnaruemarn S, Payawal DA, Baatarkhuu O, Ahn SH, Yeo CD, Alonzo UR, Chinbayar T, Loho IM, Yokosuka O, Jafri W, Tan S, Soo LI, Tanwandee T, Gani R, Anand L, Esmail ES, Khalaf M, Alam S, Lin CY, Chuang WL, Soin AS, Garg HK, Kalista K, Batsukh B, Purnomo HD, Dara VP, Rathi P, Al Mahtab M, Shukla A, Sharma MK, Omata M; APASL COVID Task Force, APASL COVID Liver Injury Spectrum Study (APCOLIS Study-NCT 04345640). Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; The APCOLIS Study (APASL COVID-19 Liver Injury Spectrum Study). Hepatol Int. 2020;14:690-700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 216] [Article Influence: 43.2] [Reference Citation Analysis (1)] |
| 87. | Valenti L, Jamialahmadi O, Romeo S. Lack of genetic evidence that fatty liver disease predisposes to COVID-19. J Hepatol. 2020;73:709-711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 88. | Mushtaq K, Khan MU, Iqbal F, Alsoub DH, Chaudhry HS, Ata F, Iqbal P, Elfert K, Balaraju G, Almaslamani M, Al-Ejji K, AlKaabi S, Kamel YM. NAFLD is a predictor of liver injury in COVID-19 hospitalized patients but not of mortality, disease severity on the presentation or progression - The debate continues. J Hepatol. 2021;74:482-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 89. | Liu D, Zhang Q, Bai P, Zhao J. Assessing causal relationships between COVID-19 and non-alcoholic fatty liver disease. J Hepatol. 2022;76:740-742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 90. | Mahamid M, Nseir W, Khoury T, Mahamid B, Nubania A, Sub-Laban K, Schifter J, Mari A, Sbeit W, Goldin E. Nonalcoholic fatty liver disease is associated with COVID-19 severity independently of metabolic syndrome: a retrospective case-control study. Eur J Gastroenterol Hepatol. 2021;33:1578-1581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 91. | Hashemi N, Viveiros K, Redd WD, Zhou JC, McCarty TR, Bazarbashi AN, Hathorn KE, Wong D, Njie C, Shen L, Chan WW. Impact of chronic liver disease on outcomes of hospitalized patients with COVID-19: A multicentre United States experience. Liver Int. 2020;40:2515-2521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 117] [Article Influence: 23.4] [Reference Citation Analysis (2)] |
| 92. | Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, Lau G. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J Hepatol. 2020;73:451-453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 408] [Article Influence: 81.6] [Reference Citation Analysis (2)] |
| 93. | Zhou YJ, Zheng KI, Wang XB, Yan HD, Sun QF, Pan KH, Wang TY, Ma HL, Chen YP, George J, Zheng MH. Younger patients with MAFLD are at increased risk of severe COVID-19 illness: A multicenter preliminary analysis. J Hepatol. 2020;73:719-721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 106] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 94. | Targher G, Mantovani A, Byrne CD, Wang XB, Yan HD, Sun QF, Pan KH, Zheng KI, Chen YP, Eslam M, George J, Zheng MH. Risk of severe illness from COVID-19 in patients with metabolic dysfunction-associated fatty liver disease and increased fibrosis scores. Gut. 2020;69:1545-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 161] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 95. | Singh A, Hussain S, Antony B. Non-alcoholic fatty liver disease and clinical outcomes in patients with COVID-19: A comprehensive systematic review and meta-analysis. Diabetes Metab Syndr. 2021;15:813-822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 96. | Hegyi PJ, Váncsa S, Ocskay K, Dembrovszky F, Kiss S, Farkas N, Erőss B, Szakács Z, Hegyi P, Pár G. Metabolic Associated Fatty Liver Disease Is Associated With an Increased Risk of Severe COVID-19: A Systematic Review With Meta-Analysis. Front Med (Lausanne). 2021;8:626425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 97. | Sachdeva S, Khandait H, Kopel J, Aloysius MM, Desai R, Goyal H. NAFLD and COVID-19: a Pooled Analysis. SN Compr Clin Med. 2020;2:2726-2729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 98. | Gao F, Zheng KI, Wang XB, Yan HD, Sun QF, Pan KH, Wang TY, Chen YP, George J, Zheng MH. Metabolic associated fatty liver disease increases coronavirus disease 2019 disease severity in nondiabetic patients. J Gastroenterol Hepatol. 2021;36:204-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 85] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 99. | Bramante C, Tignanelli CJ, Dutta N, Jones E, Tamariz L, Clark JM, Usher M, Metlon-Meaux G, Ikramuddin S. Non-alcoholic fatty liver disease (NAFLD) and risk of hospitalization for Covid-19. medRxiv. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 100. | Kim D, Adeniji N, Latt N, Kumar S, Bloom PP, Aby ES, Perumalswami P, Roytman M, Li M, Vogel AS, Catana AM, Wegermann K, Carr RM, Aloman C, Chen VL, Rabiee A, Sadowski B, Nguyen V, Dunn W, Chavin KD, Zhou K, Lizaola-Mayo B, Moghe A, Debes J, Lee TH, Branch AD, Viveiros K, Chan W, Chascsa DM, Kwo P, Dhanasekaran R. Predictors of Outcomes of COVID-19 in Patients With Chronic Liver Disease: US Multi-center Study. Clin Gastroenterol Hepatol. 2021;19:1469-1479.e19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 184] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 101. | Mallet V, Beeker N, Bouam S, Sogni P, Pol S; Demosthenes research group. Prognosis of French COVID-19 patients with chronic liver disease: A national retrospective cohort study for 2020. J Hepatol. 2021;75:848-855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 102. | Dawood DRM, Salum GM, El-Meguid MA. The Impact of COVID-19 on Liver Injury. Am J Med Sci. 2022;363:94-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 103. | Wang J, Lu Z, Jin M, Wang Y, Tian K, Xiao J, Cai Y, Zhang X, Chen T, Yao Z, Yang C, Deng R, Zhong Q, Deng X, Chen X, Yang XP, Wei G, Wang Z, Tian J, Chen XP. Clinical characteristics and risk factors of COVID-19 patients with chronic hepatitis B: a multi-center retrospective cohort study. Front Med. 2022;16:111-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 104. | Zou X, Fang M, Li S, Wu L, Gao B, Gao H, Ran X, Bian Y, Li R, ShanshanYu, Ling J, Li D, Tian D, Huang J. Characteristics of Liver Function in Patients With SARS-CoV-2 and Chronic HBV Coinfection. Clin Gastroenterol Hepatol. 2021;19:597-603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 105. | Chen L, Huang S, Yang J, Cheng X, Shang Z, Lu H, Cheng J. Clinical characteristics in patients with SARS-CoV-2/HBV co-infection. J Viral Hepat. 2020;27:1504-1507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 106. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 18875] [Article Influence: 3775.0] [Reference Citation Analysis (7)] |
| 107. | Yip TC, Wong VW, Lui GC, Chow VC, Tse YK, Hui VW, Liang LY, Chan HL, Hui DS, Wong GL. Current and Past Infections of HBV Do Not Increase Mortality in Patients With COVID-19. Hepatology. 2021;74:1750-1765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 108. | Baroiu L, Anghel L, Laurențiu Tatu A, Iancu AV, Dumitru C, Leșe AC, Drăgănescu M, Năstase F, Niculeț E, Fotea S, Nechita A, Voinescu DC, Stefanopol AI. Risk of hepatitis B reactivation: From biologic therapies for psoriasis to immunosuppressive therapies for COVID-19 (Review). Exp Ther Med. 2022;23:385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 109. | Blach S, Kondili LA, Aghemo A, Cai Z, Dugan E, Estes C, Gamkrelidze I, Ma S, Pawlotsky JM, Razavi-Shearer D, Razavi H, Waked I, Zeuzem S, Craxi A. Impact of COVID-19 on global HCV elimination efforts. J Hepatol. 2021;74:31-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 195] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 110. | Di Giorgio A, Nicastro E, Speziani C, De Giorgio M, Pasulo L, Magro B, Fagiuoli S, D' Antiga L. Health status of patients with autoimmune liver disease during SARS-CoV-2 outbreak in northern Italy. J Hepatol. 2020;73:702-705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 111. | Marjot T, Buescher G, Sebode M, Barnes E, Barritt AS 4th, Armstrong MJ, Baldelli L, Kennedy J, Mercer C, Ozga AK, Casar C, Schramm C; contributing Members and Collaborators of ERN RARE-LIVER/COVID-Hep/SECURE-Cirrhosis, Moon AM, Webb GJ, Lohse AW. SARS-CoV-2 infection in patients with autoimmune hepatitis. J Hepatol. 2021;74:1335-1343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 112. | Efe C, Lammert C, Taşçılar K, Dhanasekaran R, Ebik B, Higuera-de la Tijera F, Calışkan AR, Peralta M, Gerussi A, Massoumi H, Catana AM, Purnak T, Rigamonti C, Aldana AJG, Khakoo N, Nazal L, Frager S, Demir N, Irak K, Melekoğlu-Ellik Z, Kacmaz H, Balaban Y, Atay K, Eren F, Alvares-da-Silva MR, Cristoferi L, Urzua Á, Eşkazan T, Magro B, Snijders R, Barutçu S, Lytvyak E, Zazueta GM, Demirezer-Bolat A, Aydın M, Heurgue-Berlot A, De Martin E, Ekin N, Yıldırım S, Yavuz A, Bıyık M, Narro GC, Kıyıcı M, Akyıldız M, Kahramanoğlu-Aksoy E, Vincent M, Carr RM, Günşar F, Reyes EC, Harputluoğlu M, Aloman C, Gatselis NK, Üstündağ Y, Brahm J, Vargas NCE, Güzelbulut F, Garcia SR, Aguirre J, Anders M, Ratusnu N, Hatemi I, Mendizabal M, Floreani A, Fagiuoli S, Silva M, Idilman R, Satapathy SK, Silveira M, Drenth JPH, Dalekos GN, N Assis D, Björnsson E, Boyer JL, Yoshida EM, Invernizzi P, Levy C, Montano-Loza AJ, Schiano TD, Ridruejo E, Wahlin S. Effects of immunosuppressive drugs on COVID-19 severity in patients with autoimmune hepatitis. Liver Int. 2022;42:607-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 113. | Zhang L, Zhu F, Xie L, Wang C, Wang J, Chen R, Jia P, Guan HQ, Peng L, Chen Y, Peng P, Zhang P, Chu Q, Shen Q, Wang Y, Xu SY, Zhao JP, Zhou M. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31:894-901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 819] [Cited by in RCA: 1016] [Article Influence: 203.2] [Reference Citation Analysis (0)] |
| 114. | Aggeletopoulou I, Davoulou P, Konstantakis C, Thomopoulos K, Triantos C. Response to hepatitis B vaccination in patients with liver cirrhosis. Rev Med Virol. 2017;27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 115. | Cheong HJ, Song JY, Park JW, Yeon JE, Byun KS, Lee CH, Cho HI, Kim TG, Kim WJ. Humoral and cellular immune responses to influenza vaccine in patients with advanced cirrhosis. Vaccine. 2006;24:2417-2422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 116. | Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T; COVE Study Group. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384:403-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7073] [Cited by in RCA: 7568] [Article Influence: 1892.0] [Reference Citation Analysis (1)] |
| 117. | Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC; C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603-2615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10556] [Cited by in RCA: 10701] [Article Influence: 2140.2] [Reference Citation Analysis (1)] |
| 118. | John BV, Deng Y, Scheinberg A, Mahmud N, Taddei TH, Kaplan D, Labrada M, Baracco G, Dahman B. Association of BNT162b2 mRNA and mRNA-1273 Vaccines With COVID-19 Infection and Hospitalization Among Patients With Cirrhosis. JAMA Intern Med. 2021;181:1306-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 119. | Motamedi H, Ari MM, Dashtbin S, Fathollahi M, Hossainpour H, Alvandi A, Moradi J, Abiri R. An update review of globally reported SARS-CoV-2 vaccines in preclinical and clinical stages. Int Immunopharmacol. 2021;96:107763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 120. | Sharma A, Patnaik I, Kumar A, Gupta R. COVID-19 Vaccines in Patients With Chronic Liver Disease. J Clin Exp Hepatol. 2021;11:720-726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 121. | Cornberg M, Buti M, Eberhardt CS, Grossi PA, Shouval D. EASL position paper on the use of COVID-19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipients. J Hepatol. 2021;74:944-951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 174] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 122. | Fix OK, Blumberg EA, Chang KM, Chu J, Chung RT, Goacher EK, Hameed B, Kaul DR, Kulik LM, Kwok RM, McGuire BM, Mulligan DC, Price JC, Reau NS, Reddy KR, Reynolds A, Rosen HR, Russo MW, Schilsky ML, Verna EC, Ward JW, Fontana RJ; AASLD COVID-19 Vaccine Working Group. American Association for the Study of Liver Diseases Expert Panel Consensus Statement: Vaccines to Prevent Coronavirus Disease 2019 Infection in Patients With Liver Disease. Hepatology. 2021;74:1049-1064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 138] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 123. | Kayaaslan B, Guner R. COVID-19 and the liver: A brief and core review. World J Hepatol. 2021;13:2013-2023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 124. | Fix OK, Hameed B, Fontana RJ, Kwok RM, McGuire BM, Mulligan DC, Pratt DS, Russo MW, Schilsky ML, Verna EC, Loomba R, Cohen DE, Bezerra JA, Reddy KR, Chung RT. Clinical Best Practice Advice for Hepatology and Liver Transplant Providers During the COVID-19 Pandemic: AASLD Expert Panel Consensus Statement. Hepatology. 2020;72:287-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 422] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 125. | Boettler T, Marjot T, Newsome PN, Mondelli MU, Maticic M, Cordero E, Jalan R, Moreau R, Cornberg M, Berg T. Impact of COVID-19 on the care of patients with liver disease: EASL-ESCMID position paper after 6 months of the pandemic. JHEP Rep. 2020;2:100169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 121] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 126. | Corticosteroids. 2021 May 7. In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012. [PubMed] |
| 127. | Molnupiravir. 2022 Jan 31. In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012. [PubMed] |
| 128. | Low Molecular Weight Heparins. 2017 Nov 13. In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012. [PubMed] |